Abstract
We present a case of a 65-year-old woman with a persistently positive nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 PCR who developed new complications of coronavirus disease 2019 (COVID-19) 63 days from illness onset. She presented with intermittent fevers, fluctuating disorientation, gait instability, diffuse corticospinal tract signs, and acute venous thromboembolism. No alternate diagnosis was identified. This case highlights the potential for prolonged SARS-CoV-2 PCR positivity and persistent multisystem complications (particularly neurological), even after several months of initial COVID-19 diagnosis.
Key Words: COVID-19, SARS-CoV-2, persistent detection, neurological manifestations
Since December of 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged causing pandemic outbreaks of coronavirus disease 2019 (COVID-19) with greater than 15.8 million cases and over 641,000 deaths worldwide.1 The most characteristic presentation of COVID-19 includes fever and respiratory symptoms with fatal outcomes attributed to respiratory complications.2 Multisystem organ involvement of COVID-19 portends a poor prognosis.3 Some of the first complications reported outside of the pulmonary system included thromboembolic events suggesting COVID-19 induced coagulopathy. However, there is a lack of consensus on the appropriate anticoagulation strategy to mitigate the risk of hypercoagulability.2,3 Neurological complications have also been described.4 The diagnosis of COVID-19 is typically made by the detection of SARS-CoV-2 RNA in nasopharyngeal samples.2 The longest duration of SARS-CoV-2 positivity is not known at this time but like SARS-CoV-1, SARS-CoV-2 RNA viral shedding may correlate with illness severity and warrants further investigation.5 We present a case of a 65-year-old woman with persistently positive SARS-CoV-2 PCR by nasopharyngeal swab who developed neurological and thromboembolic complications longer than 60 days after symptom onset of a mild to moderate presentation of COVID-19.
CASE REPORT
A 65-year-old woman presented early in the pandemic with 10 days of dyspnea, fever, dry cough, myalgias, and poor appetite after a suspected SARS-CoV-2 exposure at a church event. She had a history of chronic obstructive pulmonary disease and required nocturnal supplemental oxygen. Before admission, she was empirically treated with oseltamivir despite a negative test for Influenza because 2 of her grandchildren had been diagnosed with the flu. Her symptoms progressed which prompted further evaluation. On the initial evaluation, her oxygen saturation was 91% while breathing ambient air, temperature 100.2°F, heart rate was 121 beats per minute, and respiratory rate of 20 breaths per minute. Physical examination was significant for decreased breath sounds on lung auscultation.
Initial laboratory studies revealed lymphopenia and procalcitonin of 0.26 ng/mL. A complete metabolic panel was within normal limits. Chest X-ray showed bilateral infiltrates (Fig. 1). She was empirically started on ceftriaxone and azithromycin and placed on supplemental oxygen because of acute respiratory insufficiency superimposed on her chronic respiratory failure. As SARS-CoV-2 PCR testing became more widely available, a nasopharyngeal sample was sent and subsequently returned positive. There was concern for disease progression 2 days after admission because of increasing oxygen requirements and worsening cough. A computed tomography chest revealed bilateral infiltrates superimposed upon a background of diffuse emphysema (Fig. 2). She gradually improved with supportive treatment and was weaned down to 2 L per minute of supplemental oxygen. She was discharged 10 days later to self-quarantine at home.
FIGURE 1.
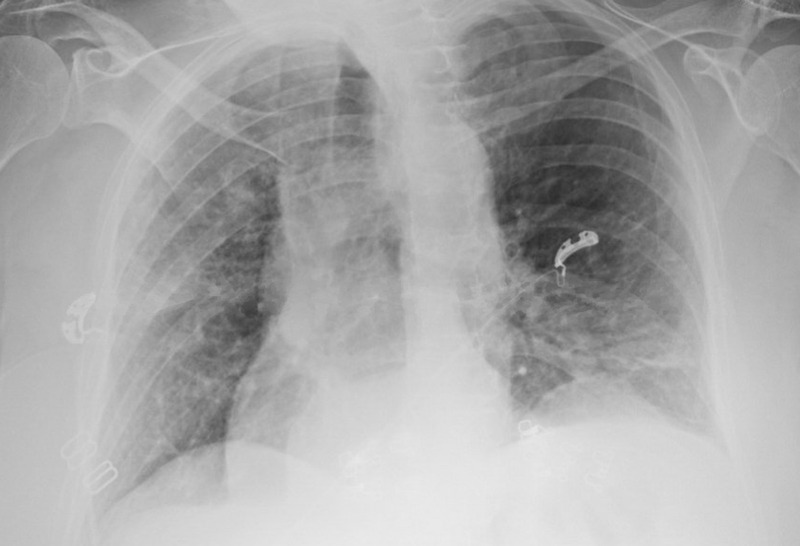
Chest X-ray with patchy infiltrates in the left lower and right upper lobes.
FIGURE 2.
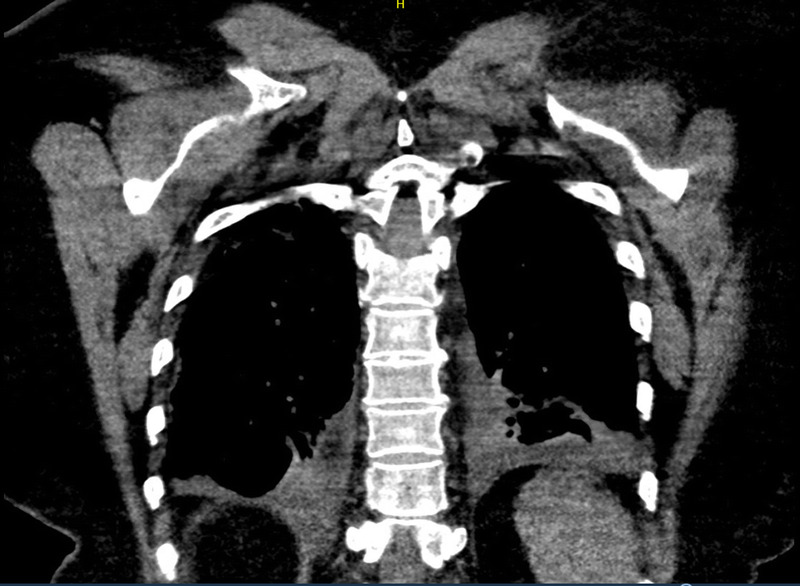
Computed tomography chest coronal view demonstrating worsening interstitial and alveolar infiltrates superimposed upon a background of diffuse emphysema.
She had residual symptoms of intermittent confusion, gait instability, and fatigue after discharge from the hospital. She was evaluated through a telemedicine visit by her pulmonologist 49 days after initial symptoms, where she described dysgeusia and recurrent near-falls, none of which were present before her symptoms of COVID-19. The patient's daughter noted fluctuating disorientation. She presented to an outpatient orthopedic visit 55 days after symptom onset with an acute left proximal humerus fracture after a fall. Vital signs were significant for a fever of 101°F. She was prescribed oxycodone-acetaminophen for the pain, but this contributed to the patient's confusion. The fevers persisted at home, and she subsequently developed right lower extremity swelling and calf pain around 59 days from initial symptom onset. She experienced another fall which prompted further evaluation 63 days from initial onset of COVID-19 symptoms (Fig. 3).
FIGURE 3.
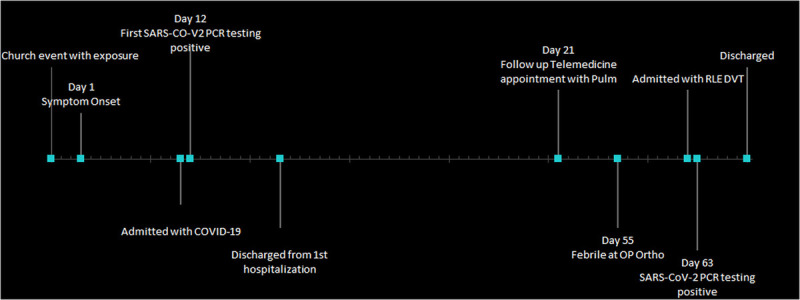
Timeline of clinical course.
Vital signs were significant for temperature 100.4°F, the heart rate was 107 beats per minute, and the respiratory rate was 24 breaths per minute. Physical examination was notable for bronchial breath sounds and right lower extremity swelling and tenderness to palpation. Laboratory studies revealed complete blood count and complete metabolic panel within normal limits and an elevated d-dimer of 2110 ng/mL. Venous duplex ultrasound revealed deep venous thrombosis involving the right distal popliteal vein extending into the proximal calf vein. Blood cultures were negative. The SARS-CoV-2 nasopharyngeal swab PCR testing was positive 63 days from initial symptom onset. She was admitted and placed on low molecular weight heparin. The inpatient neurology service was consulted. Neurological examination was notable for disorientation to time and diffuse hyperreflexia, including bilateral Hoffmann signs and right up-going toe. Magnetic resonance imaging of the brain and cervical spine were unremarkable. Screening laboratories for alternate causes of myelopathy (including B12, ceruloplasmin, vitamin E, and antinuclear antibody) were normal. Lumbar puncture was considered but ultimately deferred due to patient apprehension and inability to test for CSF SARS-CoV-2 RNA at our institution. Her mentation returned to normal on hospital day 5 and she was discharged home on apixaban.
DISCUSSION
There have been no confirmed cases of re-infection with SARS-CoV-2.6 Although this possibility cannot be completely excluded in our patient, re-infection seemed unlikely because she had remained in home isolation since her initial hospital discharge. However, asymptomatic secondary transmission within her household could not be ruled out because the rest of her family did not undergo testing.7 At the time of this report, the longest duration of persistently positive nasopharyngeal swabs for SARS-CoV-2 has ranged from 42 to 82 days from symptom onset.8,9 Of those with persistent or new symptoms, majority had fatigue and respiratory complaints unlike our patient who developed new thrombotic and neurological complications.10
Hypercoagulability in COVID-19
Hypercoagulability including venous, arterial, and microvascular thrombotic events have been reported in patients with severe SARS-CoV-2 infection.11 The pathogenesis is yet to be determined but there are likely several contributory factors including the severe inflammatory response and direct injury of endothelial cells by SARS-CoV-2.12 The reported incidence of thrombotic events in patients with severe COVID-19 infection has ranged from 20% to 43%, and the data suggest higher rates of mortality in such patients.12,13 As a result, most institutional treatment protocols for COVID-19 recommend therapeutic-intensity dosed anticoagulation for critically ill patients during hospitalization and after discharge, although the optimal duration is unknown.14
The data on the incidence of thrombotic events in less critically ill patients like ours are sparse. The general consensus is to begin prophylactic anticoagulation in these patients unless contraindications exist. It remains to be determined if there is a benefit to using higher-intensity thromboprophylaxis based on D-dimer cutoff values in some patients during hospitalization. In addition, it is unclear if post-discharge thromboprophylaxis is warranted in patients with mild to moderate COVID-19 and for how long. Further research on the incidence of venous thromboembolism in patients with COVID-19 should clarify the duration of susceptibility to hypercoagulation.
Neurological Complications of COVID-19
Neurological manifestations of COVID-19 are multitudinous and implicate both the central and peripheral nervous systems. A retrospective observational case series of 214 consecutively hospitalized patients in China with COVID-19 showed that 36.8% of patients in this cohort exhibited neurological symptoms.15 Central nervous system involvement may include encephalopathy, acute cerebrovascular events, and meningoencephalitis.16 Encephalopathy may range from mild confusion to delirium and coma.17 Virus-induced systemic hypercoagulability and endothelial injury may explain the increased risk of large-vessel acute ischemic stroke in patients with acute COVID-19.18 Alarmingly, COVID-19-associated large vessel acute ischemic strokes are being seen in patients younger than 50 years.19 Meningoencephalitis may present with meningismus, neuropsychiatric changes, and seizures.20 Pathogenic mechanisms are not fully understood at this time, but leading theories include direct viral invasion and parainfectious immune-mediated inflammatory responses.21 Magnetic resonance imaging brain may be normal or may show a combination of leptomeningeal enhancement, limbic encephalitis, hemorrhagic encephalitis, acute disseminated encephalomyelitis, or cytotoxic lesions of the corpus callosum.22 Acute necrotizing encephalopathy, a unique form of encephalitis, has been reported with COVID-19 and is characterized by symmetric bilateral thalamic lesions.23,24 As with our patient, nonspecific signs of upper motor neuron dysfunction (such as diffuse hyperreflexia) may be present.4
The most common peripheral nervous system manifestation of COVID-19 is Guillain-Barré syndrome, generally presenting with progressive ascending weakness and areflexia.25 Distal paresthesias and peripheral facial nerve palsies may also be present.
Treatment of severe neurological manifestations of COVID-19 is primarily aimed at modulating the immune system. High-dose corticosteroids, plasma exchange, and intravenous immunoglobulin (IVIg) have been used with varying success.16 At our institution, a patient with concurrent acute COVID-19 and the Miller-Fischer variant of Guillain-Barré syndrome (characterized by gait instability, sensory ataxia, areflexia, and sensorineural deafness) improved significantly after administration of IVIg. Another patient hospitalized at our facility with COVID-19–related encephalitis showed clinical improvement and radiographic lesion stability with initiation of high-dose methylprednisolone and IVIg.
It is yet to be determined if these neurological features are limited to the acute phase of severe SARS-CoV-2 infection and the duration of susceptibility. We postulate that our patient's intermittent confusion, gait instability, recurrent falls, and corticospinal tract involvement are consequences of COVID-19 given reports of neurological manifestations of COVID-19 in the literature and exclusion of alternative diagnoses with imaging and serologic studies.
Our patient developed intermittent fevers, corticospinal tract signs with resultant falls, venous thromboembolism, and had detectable SARS-CoV-2 RNA 63 days after symptom onset of nonsevere COVID-19. Whether this represents persistence, recrudescence, or reinfection remains to be determined. From a public health perspective, the lack of correlation between a prolonged positive RT-PCR test and growth of viral culture has led to debates about infectivity and the risk of transmission. While it is thought that the risk of transmission is low after 10 days, the implications of persistently detectable SARS-CoV-2 RNA in patients are unknown.26,27 Our case highlights the potential for persistence of neurological symptoms with prolonged detection of SARS-CoV-2 RNA. With the looming prospect of SARS-CoV-2 becoming seasonal like influenza, we must elucidate the clinical ramifications of persistent infection in individual patients.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Contributor Information
Amaka Awoniyi, Email: amaka.awoniyi@wellstar.org.
Zachery Rohm, Email: zachrohm@gmail.com.
Neha Paranjape, Email: neha.paranjape@wellstar.org.
REFERENCES
- 1.COVID-19 Dashboard . Johns Hopkins University and Medicine Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed July 25, 2020.
- 2.“Management of Patients with Confirmed 2019-NCoV”. Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 20 May 2020,www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
- 3.Wichmann D Sperhake JP Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J Kremer S Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y Yan LM Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/s1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkcaldy RD, King BA, Brooks JT. COVID-19 and postinfection immunity: limited evidence, many remaining questions. JAMA. 2020;323:2245–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W Zhang B Lu J, et al. The characteristics of household transmission of COVID-19. Clin Infect Dis. 2020;ciaa450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao AT Tong YX Gao C, et al. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020;127:104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korea Centers for Disease Control and Prevention . Findings from Investigation and Analysis of re-positive cases. Available at: https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=367267&nPage=1external icon. Accessed July 22, 2020.
- 10.Carfi A Bernabei R Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok FA Kruip MJHA van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magro C Mulvey JJ Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms J Tacquard C Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID-19 and VTE/Anticoagulation: Frequently Asked Questions. Updated May 18, 2020. Accessed May 22, 2020. American Society of Hematology. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation.
- 15.Mao L Jin H Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghannam M Alshaer Q Al-Chalabi M, et al. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg RK, Paliwal VK, Gupta A. Encephalopathy in patients with COVID-19: A review. J Med Virol. 2020;10.1002/jmv.26207. doi: 10.1002/jmv.26207. [DOI] [PubMed] [Google Scholar]
- 18.Valderrama EV Humbert K Lord A, et al. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020;51(7):e124–e127. [DOI] [PubMed] [Google Scholar]
- 19.Oxley TJ Mocco J Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard-Valnet R Pizzarotti B Anichini A, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020; 10.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellul MA Benjamin L Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020; S1474–4422(20)30221–0. doi:10.1016/S1474-4422(20)30221-0;19:767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremer S Lersy F Anheim M, et al. Neurologic and neuroimaging findings in COVID-19 patients: a retrospective multicenter study. Neurology. 2020: 10.1212. [DOI] [PubMed] [Google Scholar]
- 23.Poyiadji N Shahin G Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyoshi K Hamada Y Yamada H, et al. Acute necrotizing encephalopathy associated with hemophagocytic syndrome. Pediatr Neurol. 2006;34(4):315–318. [DOI] [PubMed] [Google Scholar]
- 25.Alberti P Beretta S Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wölfel R Corman VM Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. [DOI] [PubMed] [Google Scholar]
- 27.Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (interim guidance). 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. Accessed May 18, 2020.


