Abstract
During the SARS-CoV-2 pandemic, a variety of dermatological conditions were reported by physicians. Given the context, these lesions have been labeled as secondary to SARS-CoV-2 infection. We report the case of a recurrence of herpes zoster in a patient hospitalized with an SARS-CoV-2 infection. The rash occurred on the 15th day of hospitalization while the patient was recovering from a severe form. Local swab showed the presence of varicella-zoster virus within the vesicles. Dermatological symptoms secondary to COVID-19 have been frequently described. This is the first case that demonstrates the recurrence of herpes zoster during a SARS-CoV-2 infection.
Key Words: COVID-19, SARS-CoV-2, varicella-zoster virus, VZV, chickenpox, herpes zoster
We describe a patient with COVID-19 and disseminated herpes zoster. This case is, to our knowledge, the first case of clinical reactivation of varicella-zoster virus (VZV) during a SARS-CoV-2 infection.
An 80-year-old woman with a history of hypertension and aortic aneurysm, presented with fever, cough, and dyspnea, was hospitalized in the internal medicine ward. COVID-19 was diagnosed in the patient on the basis of Reverse transcriptase-polymerase chain reaction testing that detected SARS-CoV-2 infection, and computed tomography scan showed critical involvement with pulmonary embolism.
Initial vital signs were as follows: blood pressure, 145/98 mm Hg; heart rate, 110 bpm; oxygen saturation, 94% on 15 L with non-rebreathing mask; respiratory rate 30/min; and temperature, 37.7°C.
Initial pertinent laboratory results on admission were as follows: C-reactive protein, 373 mg/L; total lymphocytes count, 720/mm3; ASAT, 176 UI/L; Alanine aminotransferase, 96 UI/L; serum ferritin, 1400 μg/L.
The initial treatment was curative anticoagulation and off-label Anakinra (anti IL-1 receptor inhibitor) for a total of 10 days. However, shortly after her admission, the illness subsequently progressed, warranting the initiation of noninvasive ventilation. After several days of noninvasive ventilation, her clinical condition stabilized, and a very gradual decrease in oxygen therapy was initiated.
Meanwhile, the biological inflammatory parameters regressed (C-reactive protein, 45 mg/L; ferritin, 707 μg/L).
On the 15th day of hospitalization, 8 days after stopping Anakinra, the patient presented with a diffuse pruritic vesicular rash, including the palms of the hands and soles of the feet. Some of the vesicles were surrounded by urticaria with excoriation lesions. In the following days, lesions of different ages were found (Fig. 1).
FIGURE 1.
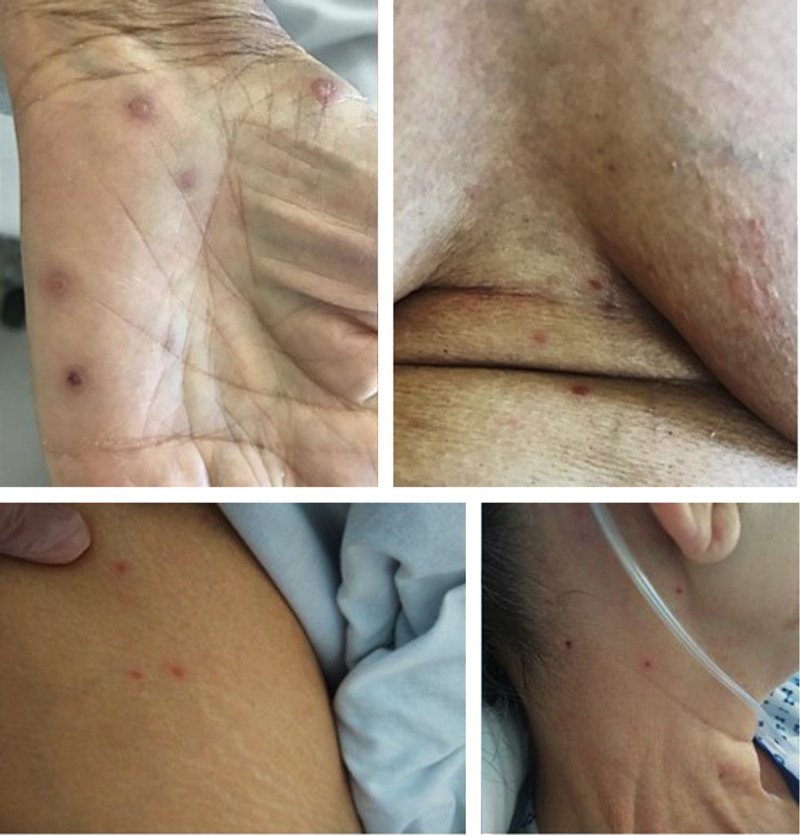
Vesicular lesions of different age and topography.
The biologic workup did not reveal hypereosinophilia or an increase in the inflammatory syndrome. On the other hand, we noted a reappearance of lymphopenia (total lymphocytes count, 630/mm3) although it had corrected itself during hospitalization.
The medical file reports a history of chickenpox during childhood and single dermatome, nonrecurrent herpes zoster in adulthood.
Vesicles were swabbed for PCR or RT-PCR testing for detection of SARS-CoV-2, VZV, and herpes simplex virus 1–2 using different swabs. Only the result of the PCR for detection of VZV came back positive, suggesting that VZV viremia is responsible for this rash.
Symptomatic treatment was instituted with local disinfection of the lesions. Clinical improvement occurred spontaneously within 8 days.
Similar lesions have been described in the context of SARS-CoV-2 infection but without associated microbiological documentation.1
This VZV infection is probably secondary to SARS-CoV-2 infection although it may be secondary to Anakinra treatment. However, treatment had been interrupted 8 days before and the VZV rashes described on Anakinra are either concomitant with treatment or due to infectious contagion, which is not the case for this patient.2–4 In addition, Mourgues et al5 showed that the administration of anti-interleukin did not affect the VZV viral load of treated patients.
A pathophysiological hypothesis for this multidermatomal herpes zoster recurrence could be the involvement of CD4 and CD8 T cells in the SARS-CoV-2 infection.6,7
Thus, the immunity acquired during the first VZV infection is reduced or even ineffective, allowing the virus to spread in the body, as seen in immunodeficient patients.7 On the one hand, it has been observed that the absolute number of lymphocytes decreased during SARS-CoV-2 infections in proportion to the severity of the disease.8 This coincides with the significant drop in lymphocyte count at the time of the rash seen in this patient. On the other hand, it can be assumed that the lymphocyte recruitment generated by the infection and triggering the classical immune mechanisms9 could reduce the effectiveness of acquired immunity against VZV.
The presence of VZV within the vesicles suggests that there has been viremia,10 as opposed to common zoster where the rash is present in 1 or 2 adjacent dermatomes and nonsystemic, which seems atypical and could testify to the depth of temporary immunosuppression caused by SARS-CoV-2.
Finally, the microbiological documentation of this rash is an argument for the hypothesis that the skin damage occurring in the context of SARS-CoV-2 infections is secondary to this infection and linked to immunological phenomena rather than directly related to this virus, which so far has not been directly objectified in skin lesions.
However, these elements deserve to be confirmed by dedicated immunological analyses.
As far as we know, this is the first case of diffuse vesicular rash post–COVID-19 with positive VZV PCR. This highlights the need for increased vigilance regarding the risk of contamination of people susceptible to severe VZV infection, such as immunodeficient patients or pregnant women.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Contributor Information
Nina Deluca, Email: nina.deluca@live.fr.
Annabelle Mahé, Email: amahe@hpsj.fr.
Erwan Lelorc'h, Email: elelorch@hpsj.fr.
Sidonie Hubert, Email: shubert@hpsj.fr.
Elodie Ménage, Email: emenage@hpsj.fr.
Marie-Françoise Borie, Email: mborie@hpsj.fr.
Philippe Azria, Email: pazria@hpsj.fr.
Charlotte Fite, Email: cfite@hpsj.fr.
Benoit Pilmis, Email: bpilmis@hpsj.fr.
Jean-Jacques Mourad, Email: jjmourad@ghpsj.fr.
REFERENCES
- 1.Marzano AV Genovese G Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg S Wynne K Omoyinmi E, et al. Efficacy and safety of anakinra for undifferentiated autoinflammatory diseases in children: a retrospective case review. Rheumatol Adv Pract. 2019;3(1):rkz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lequerré T Quartier P Rosellini D, et al. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset still disease: preliminary experience in France. Ann Rheum Dis. 2008;67(3):302–308. [DOI] [PubMed] [Google Scholar]
- 4.Rigante D Ansuini V Berrettini A, et al. Exposition to chickenpox of two children with autoinflammatory syndromes under treatment with anakinra. Rheumatol Int. 2008;28(8):793–796. [DOI] [PubMed] [Google Scholar]
- 5.Mourgues C Henquell C Tatar Z, et al. Surveillance de la charge virale des virus Epstein-Barr (EBV), cytomégalovirus (CMV) et de la varicelle (VZV) chez les patients traités par tocilizumab pour la polyarthrite rhumatoïde. Joint Bone Spine. 2016;83(4):412–415. [DOI] [PubMed] [Google Scholar]
- 6.Wang F Nie J Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang M Guo Y Luo Q, et al. T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of Coronavirus Disease 2019. J Infect Dis. 2020;222(2):198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay SL Guo A Pergam SA, et al. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2019;ciz1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F Hou H Luo Y, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(10):e137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satyaprakash AK Tremaine AM Stelter AA, et al. Viremia in acute herpes zoster. J Infect Dis. 2009;200(1):26–32. [DOI] [PubMed] [Google Scholar]


