The current coronavirus disease 2019 (COVID-19) pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) coronavirus, which infects the cells by interaction of its envelope S1 spike protein (S1) with ACE2 (angiotensin-converting enzyme 2).1 ACE2 is a carboxypeptidase and a negative regulator of the renin-angiotensin system, reducing the levels of Ang II (angiotensin II) and its pathological actions in cardiovascular diseases. Early on, conflicting views were expressed regarding the use of AT1R (angiotensin type 1 receptor) blockers in patients with COVID-19,2 and incertitude remains regarding the role of the renin-angiotensin system in SARS-CoV-2 infection.
We previously reported that cellular ACE2 activity is strictly dependent on its plasma membrane localization. The enzyme is internalized by Ang II, and this effect depends on AT1R expression.3 In HEK293T cells, which do not endogenously express AT1R, treatment with S1 (10–300 ng/mL) failed to induce ACE2 internalization (Figure [A], top) and consequently, we detected no decrease in enzymatic activity (Figure [B], left). Upon AT1R transfection ACE2 internalization was observed following Ang II and S1 exposure (Figure [A], bottom). The effects of Ang II and S1 were not additive, similar reduction in ACE2 activity being observed after simultaneous addition of both treatments (29.6±2.5%). Like for Ang II, S1-driven internalization depended on ACE2/AT1R ratio (Figure [B]). Pretreatment with the typical AT1R blocker losartan (1 μmol/L) or the β-arrestin biased AT1R ligand TRV0274 (1 μmol/L) fully prevented the effects of Ang II or S1. These drugs have no effect on ACE2 activity in the absence of AT1R (data not shown).
Figure.
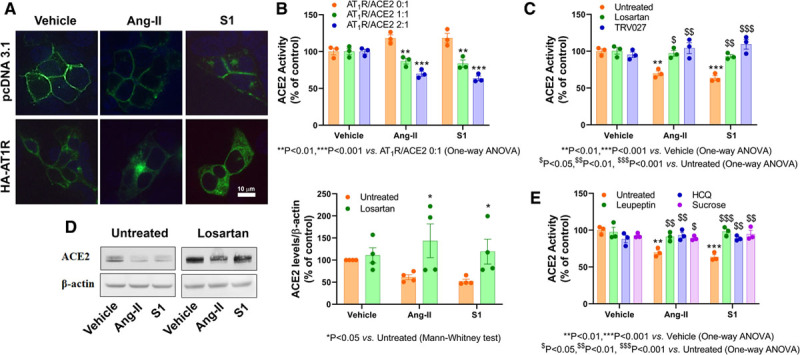
The effects of Ang II (angiotensin II) and S1 spike protein (S1) on ACE2 (angiotensin-converting enzyme 2) in HEK293T cells. A, Subcellular localization of ACE2 (green) in HEK293T cells cotransfected with 0.5 μg GFP (green fluorescent protein)-tagged ACE2 and 1 μg pcDNA3.1 (control vector, top) or HA-tagged AT1R (angiotensin type 1 receptor; bottom) after vehicle (left), Ang II (1 μmol/L, middle) or S1 (30 ng/mL, right) treatment for 4 h. B, ACE2 activity in HEK293T cells cotransfected with GFP-tagged ACE2 (0.5 μg) and HA-tagged AT1R (0, 0.5, and 1 μg). The total transfected cDNA was kept at 1.5 μg by addition of necessary amounts of pcDNA3.1. ACE2 activity was determined in vehicle-treated cells or after 4 h (corresponding to maximal effect on enzyme activity) treatment with Ang II (1 μmol/L) or S1 (30 ng/mL). C, Pretreatment (30 min) with the AT1R antagonist losartan (1 μmol/L,) or β-arrestin biased agonist TRV027 (100 nmol/L) blocked Ang II– or S1-mediated reduction of ACE2 activity in HEK293T cells transfected with 0.5 μg GFP-ACE2 and 1 μg AT1R. D, Effects of treatment for 18 h (corresponding to maximal effect on protein levels) with Ang II (1 μmol/L) or S1 (30 ng/mL) on total ACE2 cellular levels determined by Western blotting. E, Pretreatment (30 min each) with losartan (1 μmol/L), HCQ (hydroxychloroquine; 100 μmol/L) or sucrose (100 mmol/L) blocks the decrease of ACE2 activity induced by Ang II (1 μmol/L) or S1 (30 ng/mL). The data are presented as mean ±SEM with n=3–4 from at least 3 independent transfections. The experiments were performed as described previously5 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7456754/).
As observed for Ang II, S1-induced ACE2 internalization resulted in enzyme degradation (Figure [D]), an effect also prevented by losartan. Accordingly, the lysosomal inhibitor leupeptin prevented the loss of ACE2 activity (Figure [E]) and its degradation (data not shown). Hydroxychloroquine (100 μmol/L), which inhibits lysosomal fusion to endosomes and the activity of lysosomal enzymes, reversed the effects of Ang II and S1 on ACE2 activity (Figure [E]). The use of sucrose as a hyperosmolar agent prevented the loss of ACE2 activity (Figure [E]), thus confirming the importance of clathrin-coated pits in ACE2 internalization.
In summary, this is the first demonstration that S1 binding to ACE2 induces enzyme internalization through clathrin-coated pits, followed by lysosomal degradation, as previously reported by our group for Ang II.3 This process requires AT1R expression and higher AT1R levels potentiate the internalization of S1, component of SARS-CoV-2 envelope. One can envision that S1 stimulates the formation of AT1R/ACE2 complexes which are required for enzyme internalization, but further experiments are required to validate this hypothesis.
Blockade of lysosomal function by leupeptin or hydroxychloroquine fully prevented the effects of S1 on ACE2 internalization and degradation. Importantly from a clinical point of view, our cellular experiments strongly indicate that losartan, an extensively used antihypertensive drug, blocked ACE2 internalization induced by S1. Therefore, unlike initial concerns,2 the use of AT1R blockers in COVID-19 may produce unexpected benefits. However, clinical studies are necessary to confirm these in vitro results.
Acknowledgments
The use of RCMI core facilities at Howard University is gratefully acknowledged.
Sources of Funding
This work was supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) HL150592 Grant (to C.M. Filipeanu and E. Lazartigues), Merit Award (BX004294) from the US Department of Veterans Affairs (to E. Lazartigues), and bridge funds from Howard University (to C.M. Filipeanu).
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ACE2
- angiotensin-converting enzyme 2
- Ang II
- angiotensin II
- AT1R
- angiotensin type 1 receptor
- COVID-19
- coronavirus disease 2019
For Sources of Funding and Disclosures, see page e43.
Contributor Information
Blessing O. Ogunlade, Email: blessing.ogunlade@bison.howard.edu.
Eric Lazartigues, Email: elazar@lsuhsc.edu.
References
- 1.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020; 581:215–220. doi: 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- 2.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020; 75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014; 64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho-Galvão A, Ogunlade B, Xu J, Silva-Alves CRA, Mendes-Júnior LG, Guimarães DD, Cruz JC, Queiroz TM, Balarini CM, Braga VA, et al. Central administration of TRV027 improves baroreflex sensitivity and vascular reactivity in spontaneously hypertensive rats. Clin Sci (Lond). 2018; 132:1513–1527. doi: 10.1042/CS20180222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogunlade B, Guidry JJ, Mukerjee S, Sriramula S, Lazartigues E, Filipeanu CM. The actin bundling protein fascin-1 as an ACE2-accessory protein [published online August 31, 2020]. Cell Mol Neurobiol. doi: 10.1007/s10571-020-00951-x. https://link.springer.com/article/10.1007/s10571-020-00951-x [DOI] [PMC free article] [PubMed] [Google Scholar]


