Abstract
Background
In patients with COVID-19, granulocyte-macrophage colony stimulating factor (GM-CSF) might be a mediator of the hyperactive inflammatory response associated with respiratory failure and death. We aimed to evaluate whether mavrilimumab, a monoclonal antibody to the GM-CSF receptor, would improve outcomes in patients with COVID-19 pneumonia and systemic hyperinflammation.
Methods
This investigator-initiated, multicentre, double-blind, randomised trial was done at seven hospitals in the USA. Inclusion required hospitalisation, COVID-19 pneumonia, hypoxaemia, and a C-reactive protein concentration of more than 5 mg/dL. Patients were excluded if they required mechanical ventilation. Patients were randomly assigned (1:1) centrally, with stratification by hospital site, to receive mavrilimumab 6 mg/kg as a single intravenous infusion, or placebo. Participants and all clinical and research personnel were masked to treatment assignment. The primary endpoint was the proportion of patients alive and off supplemental oxygen therapy at day 14. The primary outcome and safety were analysed in the intention-to-treat population. This trial is registered at ClinicalTrials.gov, NCT04399980, NCT04463004, and NCT04492514.
Findings
Between May 28 and Sept 15, 2020, 40 patients were enrolled and randomly assigned to mavrilimumab (n=21) or placebo (n=19). A trial of 60 patients was planned, but given slow enrolment, the study was stopped early to inform the natural history and potential treatment effect. At day 14, 12 (57%) patients in the mavrilimumab group were alive and off supplemental oxygen therapy compared with nine (47%) patients in the placebo group (odds ratio 1·48 [95% CI 0·43–5·16]; p=0·76). There were no treatment-related deaths, and adverse events were similar between groups.
Interpretation
There was no significant difference in the proportion of patients alive and off oxygen therapy at day 14, although benefit or harm of mavrilimumab therapy in this patient population remains possible given the wide confidence intervals, and larger trials should be completed.
Funding
Kiniksa Pharmaceuticals.
Introduction
For people infected with SARS-CoV-2, the cause of COVID-19, there are few effective treatments.1, 2, 3 Early disease manifestations are related to viral replication and immune cell-mediated death in respiratory epithelial cells. As viral replication wanes after the first week of infection, a subset of patients develop a hyperactive immune response that perpetuates lung injury, and most deaths occur in patients with heightened systemic inflammation who develop acute respiratory distress syndrome.4, 5 Granulocyte-macrophage colony stimulating factor (GM-CSF) might contribute to the hyperinflammatory state in patients with COVID-19 because it is elevated in bronchoalveolar lavage of patients with COVID-19 who develop acute respiratory distress syndrome, is substantially increased in the serum of those who die from COVID-19, and is a master regulator of pro-inflammatory cytokine responses in the lung.6, 7, 8, 9, 10, 11
In the lungs, GM-CSF activates alveolar macrophages to promote clearance of respiratory microbes through production of pro-inflammatory cytokines, but the resultant feed-forward inflammatory loop might promote further damage.9, 10, 11, 12, 13 In a large, randomised trial, broad immunosuppression with dexamethasone improved mortality in patients with severe or critical COVID-19 pneumonia,2 supporting the importance of hyperinflammation in adverse outcomes. Observational studies have also suggested a potential benefit with GM-CSF antagonism in patients with COVID-19 pneumonia and heightened systemic inflammation.14, 15
Research in context.
Evidence before this study
A dysregulated immune response contributes to adverse outcomes in some patients with COVID-19. To ameliorate this heightened inflammatory response, different cytokine pathways have been targeted. Within the lungs, granulocyte-macrophage colony stimulating factor (GM-CSF) might be central in this inappropriate innate immune response. Mavrilimumab is a monoclonal antibody that binds to the α subunit of the GM-CSF receptor and blocks intracellular signalling downstream of GM-CSF. We searched PubMed using the search terms “COVID-19”, “SARS-CoV-2”, “mavrilimumab”, and “GM-CSF” for primary research published between Jan 1 and March 28, 2020, with no language restrictions and found no results.
Added value of this study
To our knowledge, MASH-COVID is the first placebo-controlled study of mavrilimumab in patients with COVID-19 pneumonia and heightened systemic inflammation. In a double-blind, randomised trial of 40 adults, the proportion free from supplemental oxygen at day 14 was 57% with mavrilimumab versus 47% with placebo. At day 28, 95% of patients in the mavrilimumab group were alive and without respiratory failure versus 79% in the placebo group; mortality at day 28 was 5% in the mavrilimumab groups versus 16% in the placebo group. Adverse events were similar in both groups, and no safety concerns were noted.
Implications of all the available evidence
Mavrilimumab was not associated with significant improvements in clinical outcomes in this small study of patients with severe COVID-19 pneumonia and increased inflammation, although findings are hypothesis-generating and larger trials should be completed based on these results.
Mavrilimumab is a monoclonal antibody that binds to the α subunit of the GM-CSF receptor and blocks intracellular signalling of GM-CSF.16 Based on this mechanism of action, the putative role of increased GM-CSF in adverse outcomes from COVID-19, and encouraging results from an observational study of patients with COVID-19 pneumonia and hyperinflammation treated with mavrilimumab, we aimed to test the hypothesis that treatment with mavrilimumab would lead to better clinical outcomes in patients with COVID-19 pneumonia, hypoxaemia, and hyperinflammation. The primary hypothesis was that a higher proportion of patients treated with mavrilimumab would not require oxygen at day 14, compared with patients treated with placebo. The secondary hypothesis was that patients treated with mavrilimumab would have improved survival and more freedom from respiratory failure at day 28. A favourable signal for efficacy from this study could inform a larger trial.
Methods
Study design and participants
The MASH-COVID study is an investigator-initiated, multicentre, double-blind, randomised, placebo-controlled trial that was done at seven hospitals in the USA (three referral centres and four community hospitals; appendix p 1).
Inclusion required inpatient hospitalisation for COVID-19; documented COVID-19 pneumonia defined as a positive upper respiratory tract specimen for SARS-CoV-2 with associated abnormalities or infiltrates on chest x-ray or chest CT; active fever or documented fever within 48 h or antipyretic use; hypoxaemia, defined as a room air oxygen saturation of less than 92% or requirement of supplemental oxygen; and a C-reactive protein concentration greater than 5 mg/dL. Notable exclusion criteria included age younger than 18 years, absolute neutrophil count less than 1500/mm3, home oxygen therapy, mechanical ventilation, uncontrolled systemic bacterial infection, and onset of symptoms more than 14 days before hospital admission and enrolment (full inclusion and exclusion criteria are listed in the appendix pp 1–3).
The design and conduct of the study were approved by the US Food and Drug Administration. The trial was done according to the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice Guidelines. The trial was overseen by a data monitoring committee with details described in a separate charter. The data monitoring committee assessed safety and made no formal assessment of efficacy. The protocol was approved by the Institutional Review Board at each site, and written informed consent was obtained from all patients or their legally authorised representative. Data management was coordinated by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research; Cleveland, OH, USA). Data were entered into a secure REDCap Cloud database, and analysis was done by C5Research. The study protocol is in the appendix pp 21–59.
Randomisation and masking
Patients were randomly assigned (1:1) to receive mavrilimumab or placebo. Randomisation was centralised through REDCap Cloud with stratification by hospital site. The participants and all clinical and research personnel were masked to treatment assignment, except for a research pharmacist who prepared the mavrilimumab infusion or equal volume infusion of diluent for placebo. This research pharmacist did not participate in the administration of the infusion. Irrespective of their participation in the study, enrolled patients received COVID-19 therapies considered appropriate by their clinicians.
Procedures
Mavrilimumab 6 mg/kg was administered as a single intravenous infusion. For placebo, an equal volume of diluent was given intravenously via the same infusion pump that was used for mavrilimumab. The investigator was required to discontinue treatment if continuation would negatively affect a participant's wellbeing. After study discontinuation, the participant would remain in the study unless consent was withdrawn. Reasons for study discontinuation could include patient or surrogate request, pregnancy, use of prohibited treatment (appendix p 2), any safety risk to the patient, and any laboratory abnormalities that in the judgment of the investigator would prevent the patient from continuing. Following enrolment, adverse events and clinical status including the ordinal scale were assessed daily until discharge as well as at day 7, 14, 21, 28, and 60. Information on concomitant medications was collected at baseline and daily until day 7, and then at days 14, 21, and 28. Concomitant medications included antiviral drugs related to COVID-19, corticosteroids, convalescent plasma, other immunosuppressive agents, and antimicrobial drugs related to non-COVID-19 infections. Follow-up laboratory testing was done according to clinical standard of care, in alignment with institutional policies for caregiver safety and conservation of personal protective equipment, and accordingly was not uniform across all patients and sites. Follow-up laboratory testing was also not done after patient discharge.
Outcomes
The primary efficacy outcome was the proportion of patients alive and off supplemental oxygen therapy at day 14 after infusion of mavrilimumab or placebo. The secondary endpoints were the proportion of patients alive at day 28, and the proportion of patients alive and without respiratory failure at day 28. Respiratory failure was defined as a requirement for mechanical ventilation, non-invasive ventilation, or high-flow oxygen. Primary and secondary outcomes were assessed in the intention-to-treat population. An assessment for interaction between treatment group and corticosteroid or remdesivir use was prespecified. The first exploratory endpoint was the time to clinical improvement up to day 14 or day 28, defined as time from randomisation to an improvement of two points on a seven-category ordinal scale or discharge from the hospital. The ordinal scale was modified from an original model proposed by WHO by removing the category uninfected, combining categories of ventilation and ventilation plus additional organ support, and substituting resumption of normal activities with need for oxygen therapy.17 The modified categories were (1) not hospitalised and not on supplemental oxygen; (2) not hospitalised but on supplemental oxygen; (3) hospitalised, not requiring supplemental oxygen; (4) hospitalised, requiring supplemental oxygen; (5) hospitalised, requiring nasal high-flow oxygen or non-invasive mechanical ventilation, or both; (6) hospitalised, requiring invasive mechanical ventilation, extracorporeal membrane oxygenation, or both; and (7) death. The other exploratory endpoints were proportion of patients in each category of the ordinal scale at day 7, 14, 21, and 28; mortality at day 60; mortality at day 14; need for mechanical ventilation; duration of hospitalisation; changes in the ratio of arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) at day 3, 5, 7, 14, 21, and 28, or until discharge; change in the Sequential Organ Failure Assessment (SOFA) score at day 7, 14, 21, and 28, or until discharge; reduction in C-reactive protein concentration at day 7 and 14; and time to negative SARS-CoV-2 RNA concentrations in oropharyngeal or nasopharyngeal swabs. After randomisation, subsequent daily PaO2 to FiO2 ratios were selected as the lowest value over the 24 h period beginning at midnight. All exploratory outcomes were assessed in all patients for whom follow-up data were available.
Safety and adverse events were assessed by the principal investigator during the hospital admission. The principal investigator assessed all new clinical diagnoses and abnormal laboratory results to determine whether they were adverse events, and whether they were serious adverse events, treatment-related adverse events, or both. The severity of the event was also assessed. After patient discharge, safety and adverse events were assessed by research personnel during telemedicine visits.
Statistical analysis
Estimates of the efficacy of mavrilimumab in this patient population are scarce and, based on preliminary case-control data of patients with severe COVID-19 pneumonia treated with mavrilimumab,15 it was estimated that 40% of patients in the placebo group would meet the primary endpoint compared with 80% of patients treated with mavrilimumab. Therefore, a sample size of 30 patients per group (60 total) would provide 80% power to detect this difference using a two-sided α error of 0·05. However, due to slow enrolment after the first surge of COVID-19, the study was concluded after enrolment of 40 patients, in the interest of having a more accurate measure of event rate in the control group and an estimate of effect size in the treatment group. Between-group comparisons were done with t tests, Wilcoxon rank sum tests, χ2 tests, and Fisher exact tests, as appropriate. The magnitude of effect for the primary and secondary endpoints is expressed using the odds ratio (OR) with 95% CI. The time to clinical improvement was assessed with a log-rank test between mavrilimumab and placebo. Adjusted recovery rate ratios (RRRs) with 95% CIs were calculated from a Cox proportional hazards model. The RRR is similar to the hazard ratio (HR) in survival analysis except for the beneficial outcome of clinical improvement; therefore, an RRR greater than 1 indicates clinical improvement. HRs for mortality were also calculated from a Cox proportional hazards model. No adjustments were made for multiple hypothesis testing. Given the early termination of the study, nominal p values are descriptive, and all results should be considered hypothesis-generating. QW and KEW did the statistical analyses. Analyses were done using SAS version 9.4. The statistical analysis plan is in the appendix pp 4–20. This trial is registered at ClinicalTrials.gov, NCT04399980, NCT04463004, and NCT04492514.
Role of the funding source
The funder of the study provided the study drug, facilitated formation of the investigator consortium, assisted with study design and interpretation of the data, and had a role in editing the report. The investigator consortium and the funder conceived of the study. The funder of the study had no role in data analysis or data collection.
Results
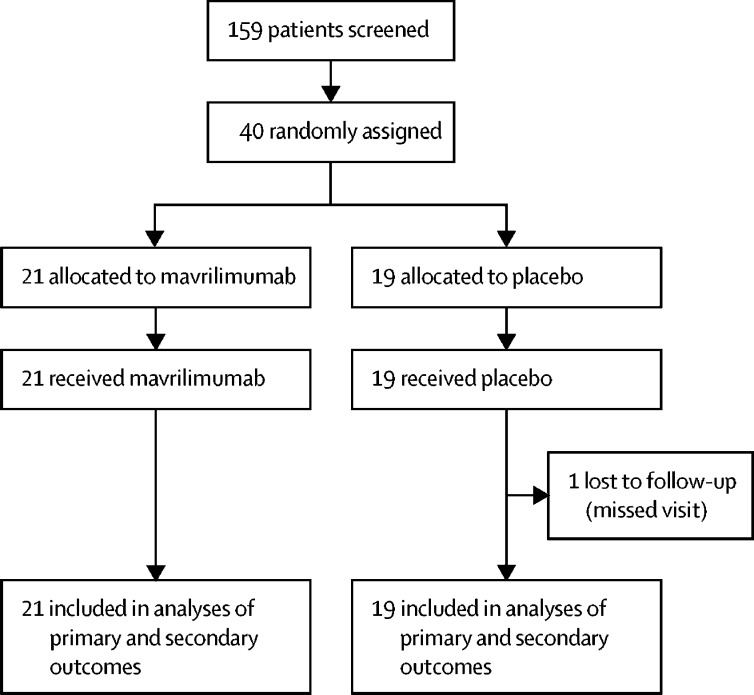
Between May 28 and Sept 15, 2020, 40 patients were enrolled and randomly assigned to a treatment group (mavrilimumab n=21, placebo n=19; figure 1 ). All patients completed the allocated intervention. One patient in the placebo group missed visits following hospital discharge, although their vital status was known at study completion. 26 (65%) patients were men, 16 (40%) were African-American, and comorbidities were common (table 1 ). Patients were randomly assigned a median of 2 days (IQR 1–3) after hospital admission. At enrolment, all patients were hypoxaemic, half required nasal high-flow oxygen or non-invasive ventilation, and inflammatory markers were substantially elevated (table 1). Similar numbers of patients in the mavrilimumab and placebo groups received COVID-19 directed therapies. Before random assignment, 26 (65%) of 40 patients were treated with corticosteroids, including 14 (67%) of 21 patients who received mavrilimumab and 12 (63%) of 19 patients who received placebo. After random assignment, an additional five patients were started on corticosteroids (two in the mavrilimumab group, three in the placebo group). For patients enrolled before July 1, 2020, three (25%) of 12 patients across both groups were treated with corticosteroids. After publication of the RECOVERY trial in July, 2020,2 all 28 (100%) subsequently enrolled patients across both groups were treated with corticosteroids. Before random assignment, 30 (75%) of 40 patients were treated with remdesivir, including 16 (76%) of 21 patients who received mavrilimumab and 14 (74%) of 19 patients who received placebo. After random assignment, three patients received remdesivir (two in the mavrilimumab group, one in the placebo group); ten patients received convalescent plasma (four in the mavrilimumab group, six in the placebo group); and two patients received tocilizumab (one in each group).
Figure 1.
Trial profile
Table 1.
Baseline characteristics of patients in the intention-to-treat population
| Total (n=40) | Mavrilimumab group (n=21) | Placebo group (n=19) | ||
|---|---|---|---|---|
| Age, years | 56·7 (44·9–68·7) | 54·8 (49·7–68·1) | 59·0 (41·0–69·3) | |
| Sex | ||||
| Male | 26 (65%) | 14 (67%) | 12 (63%) | |
| Female | 14 (35%) | 7 (33%) | 7 (37%) | |
| Race | ||||
| African-American | 16 (40%) | 8 (38%) | 8 (42%) | |
| White | 19 (48%) | 11 (52%) | 8 (42%) | |
| Other | 5 (13%) | 2 (10%) | 3 (16%) | |
| Ethnicity | ||||
| Hispanic or Latino | 6 (15%) | 3 (14%) | 3 (16%) | |
| Body-mass index, kg/m2 | 32·7 (29·0–38·1) | 30·3 (27·2–41·1) | 32·7 (30·5–35·5) | |
| Comorbidities | ||||
| Diabetes | 17 (43%) | 8 (38%) | 9 (47%) | |
| Hypertension | 22 (55%) | 10 (48%) | 12 (63%) | |
| Hyperlipidaemia | 18 (45%) | 7 (33%) | 11 (58%) | |
| Coronary artery disease | 4 (10%) | 1 (5%) | 3 (16%) | |
| Stroke | 1 (3%) | 0 | 1 (5%) | |
| Chronic obstructive pulmonary disease | 3 (8%) | 3 (14%) | 0 | |
| Chronic kidney disease | 3 (8%) | 1 (5%) | 2 (11%) | |
| Current or former smoker | 11 (28%) | 7 (33%) | 4 (21%) | |
| Time from symptom onset to hospitalisation, days | 7 (4–8) | 5 (2–8) | 7 (5–8) | |
| Time from symptom onset to random assignment, days | 9 (6–11) | 9 (6–10) | 9 (7–11) | |
| Dyspnoea | 32 (80%) | 19 (90%) | 13 (68%) | |
| Temperature, °C | 37·0 (36·7–37·3) | 37·3 (36·7–37·8) | 37·0 (36·6–37·1) | |
| Hospitalised requiring nasal high-flow oxygen, non-invasive ventilation, or both | 20 (50%) | 10 (48%) | 10 (53%) | |
| Hospitalised requiring supplemental oxygen | 20 (50%) | 11 (52%) | 9 (47%) | |
| Baseline PaO2 to FiO2 ratio | 137 (88–193) | 138 (83–172) | 136 (103–221) | |
| Baseline SOFA score | 2 (2–3) | 2 (2–3) | 2 (2–3) | |
| C-reactive protein concentration, mg/dL* | 13·1 (9·8–18·8) | 14·0 (9·9–18·8) | 12·3 (9·4–19·4) | |
| Lymphocyte count, thousand cells per μL† | 1·1 (0·7–1·3) | 1·0 (0·8–1·2) | 1·1 (0·6–1·3) | |
| Ferritin, ng/mL‡ | 1040 (486–1860) | 1122 (410–2523) | 1000 (499–1728) | |
| D-dimer, ng/mL§ | 890 (430–1270) | 860 (470–1200) | 900 (410–1270) | |
Data are median (IQR) or n (%). Percentages might not total 100% due to rounding. FiO2=fractional inspired oxygen. PaO2=arterial oxygen partial pressure. SOFA=Sequential Organ Failure Assessment.
Reference range 0·0–0·4 mg/dL.
Reference range 1·0–4·0 thousand cells per μL.
Reference range 14·7–205·1 ng/mL.
Reference range <500 ng/mL.
There was no significant difference between the groups in the proportion of patients who were alive and off supplemental oxygen therapy by day 14 (OR 1·48 [95% CI 0·43–5·16]; table 2 ). There was no significant interaction between treatment group and proportion of patients who were alive and off supplemental oxygen therapy with corticosteroid use (OR 1·40 [0·30–6·62]) or remdesivir use (3·21 [0·70–14·74]). At day 28, there was no significant difference between the groups in the proportion of patients who were alive and without respiratory failure (OR 5·33 [0·54–52·7]; table 2). There was no significant interaction between treatment group and the proportion of patients who were alive and without respiratory failure with corticosteroid use (OR 1·29 [0·26–6·27]) or remdesivir use (1·71 [0·40–7·29]). By day 28, one (5%) patient treated with mavrilimumab had died compared with three (16%) patients treated with placebo (HR 3·72 [0·39–35·79; table 2).
Table 2.
Efficacy outcomes
| Mavrilimumab group (n=21) | Placebo group (n=19) | p value | |
|---|---|---|---|
| Primary endpoint | |||
| Patients alive and off supplemental oxygen therapy at day 14 | 12 (57%) | 9 (47%) | 0·76* |
| Secondary endpoints | |||
| Patients alive and without respiratory failure at day 28 | 20 (95%) | 15 (79%) | 0·43* |
| Mortality at day 28 | 1 (5%) | 3 (16%) | 0·22† |
| Exploratory endpoints | |||
| Mortality at day 60 | 1 (5%) | 4 (21%) | 0·11† |
| Duration of hospitalisation, days | 7·5 (6·0–11·0) | 8·0 (6·0–10·0) | 0·92‡ |
Data are n (%) or median (IQR).
p value from generalised Wilcoxon test.
p value from log-rank test.
p value from Wilcoxon rank-sum test.
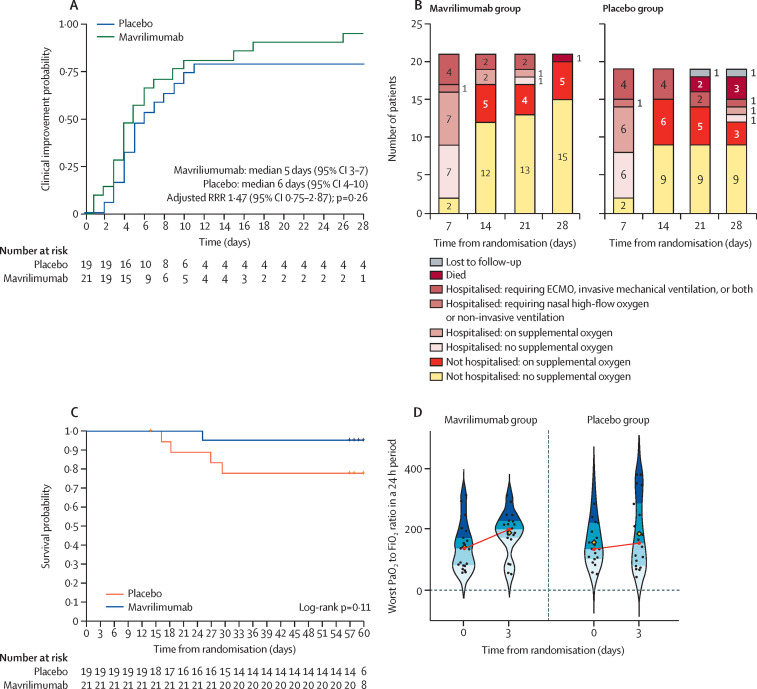
There was no significant difference in time to clinical improvement up to day 28 for patients treated with mavrilimumab compared with placebo (median 5 days [95% CI 3–7] vs 6 days [4–10]; RRR 1·47 [95% CI 0·75–2·87]; p=0·26; figure 2A ). By day 28, 15 (71%) of 21 patients who received mavrilimumab had been discharged from hospital and were no longer on supplemental oxygen compared with nine (50%) of 18 patients who received placebo, as one patient in the placebo group was lost to follow-up (figure 2B). At day 60, one (5%) patient treated with mavrilimumab had died compared with four (21%) patients treated with placebo (HR 5·0 [95% CI 0·56–45·07]; table 2; figure 2C). All five deaths occurred in patients who deteriorated and required invasive ventilation. Except for one patient in the placebo group, deaths occurred in patients who were on high-flow oxygen or non-invasive ventilation at baseline. In the placebo group, two deaths occurred in patients with a baseline PaO2 to FiO2 ratio of more than 200, one death occurred in a patient with a baseline PaO2 to FiO2 ratio of 100–200, and one death occurred in a patient with a baseline PaO2 to FiO2 ratio of less than 100. For the patient who received mavrilimumab and died, the baseline PaO2 to FiO2 ratio was less than 100.
Figure 2.
Exploratory outcomes
(A) Kaplan-Meier curves for clinical improvement up to day 28. (B) Proportion of patients in each category of the ordinal scale during follow-up at 28 days. (C) Kaplan-Meier curves for survival up to day 60. (D) PaO2 to FiO2 ratio at baseline and at day 3. The yellow diamond represents the mean, and the red circle represents the median. The first, second, third, and fourth quartiles are blue shaded. At day 3, PaO2 to FiO2 ratios were available for 20 of 21 patients who received mavrilimumab and 18 of 19 patients who received placebo. The median baseline PaO2 to FiO2 ratio for patients who received mavrilimumab was 138 and increased to 200 at day 3. The median baseline PaO2 to FiO2 ratio for patients who received placebo was 136 and increased to 155 at day 3. ECMO=extracorporeal membrane oxygenation. FiO2=fractional inspired oxygen. PaO2=arterial oxygen partial pressure. RRR=recovery rate ratio.
During the study, five (24%) of 21 patients who received mavrilimumab required invasive mechanical ventilation compared with four (21%) of 19 patients who received placebo (OR 1·2 [95% CI 0·26–5·21]). The median duration of mechanical ventilation was 12 days (IQR 9–18) in patients who received mavrilimumab compared with 17 days (11–25) in patients who received placebo.
At day 3, the PaO2 to FiO2 ratio increased by median 36 (IQR 6 to 89) in 20 patients who received mavrilimumab compared with median 11 (−25 to 74) in 18 patients who received placebo (figure 2D). Because this study was done during a time of limited resources due to the pandemic, laboratory testing was done according to clinical care, and patients did not return to the hospital once they were discharged. Results for the PaO2 to FiO2 ratio after day 3, change in SOFA score, reduction in C-reactive protein concentration, and time to negative SARS-CoV-2 RNA concentrations are therefore not reported due to missing data.
All patients completed the infusion without reaction, and there were no treatment related deaths. In the intention to treat population (n=40), adverse events and abnormal laboratory values were similar between the two groups (table 3 ). There were no infusion reactions, and no patient developed neutropenia. In addition, no patients developed bacteraemia. Bacterial pneumonia was diagnosed in one patient who received placebo (5%) and two patients who received mavrilimumab (10%).
Table 3.
Adverse events and selected laboratory safety data in the intention-to-treat population
| Mavrilimumab group (n=21) | Placebo group (n=19) | |
|---|---|---|
| Any serious adverse event | 5 (24%) | 4 (21%) |
| Circulatory shock | 2 (10%) | 1 (5%) |
| Acute kidney injury | 4 (19%) | 3 (16%) |
| Bacterial pneumonia | 2 (10%) | 1 (5%) |
| Bacteraemia | 0 | 0 |
| Neutropenia | 0 | 0 |
| Alanine aminotransferase more than 3 times normal value | 5 (24%) | 3 (16%) |
| Aspartate aminotransferase more than 3 times normal value | 6 (29%) | 4 (21%) |
Discussion
The MASH-COVID study was designed as an early signal of efficacy trial to assess whether GM-CSF inhibition with mavrilimumab would improve clinical outcomes in patients with severe COVID-19 pneumonia and increased systemic inflammation. There was no significant difference between the groups for the primary endpoint, although patients who received mavrilimumab were numerically more likely to be alive and off oxygen at day 14 than were patients who received placebo. In addition, by day 28, patients who received mavrilimumab were numerically more likely to be alive and without respiratory failure than were patients who received placebo. In comparison with the initial statistical analysis plan, more patients in the placebo group than anticipated were off supplemental oxygen therapy by day 14, the relative risk reduction with mavrilimumab was lower than predicted, and enrolment was stopped due to slower than expected recruitment after the first surge of COVID-19, with the intent of gathering an earlier estimate of effect size to inform larger studies. There were no safety concerns noted with mavrilimumab in this trial, even with a high proportion of patients on concomitant corticosteroids.
GM-CSF is produced mainly at sites of inflammation via cell types such as epithelial cells, fibroblasts, endothelial cells, macrophages, dendritic cells, T cells, and neutrophils, increasing the inflammatory reaction via cytokine pathways that have been termed the colony stimulating factor network.9, 12, 18 In patients with COVID-19, GM-CSF plasma concentrations are elevated compared with healthy control samples,6 and GM-CSF-activated macrophages produce pro-inflammatory cytokines, including tumour necrosis factor (TNF), IL-1β, IL-6, IL-23, and IL-12.18, 19 Mavrilimumab is an anti-GM-CSF-Rα monoclonal antibody (human isoform IgG4) that has been shown to inhibit the GM-CSF signalling axis in humans and to improve clinical outcomes in phase 2 trials in patients with rheumatoid arthritis and giant cell arteritis.7, 8, 20, 21 With regard to mitigation of the aberrant immune response in the setting of COVID-19 pneumonia, it is unclear whether blockade of GM-CSF in the lung is required in addition to abolition of signalling in the periphery. Preclinical data suggested that a dose higher than that needed to achieve 100% receptor occupancy in circulation (3 mg/kg) might be required to achieve therapeutic inhibitory concentrations in the lung.22, 23 Therefore, a dose of 6 mg/kg administered intravenously was selected to assess the pharmacodynamic effects in the lung to inhibit the inappropriate innate immune response and reduce further lung injury.
Results from an observational study by De Luca and colleagues15 of patients hospitalised with COVID-19 pneumonia and systemic hyperinflammation suggested a possible benefit in patients who received mavrilimumab.15 Specifically, during 28-day follow-up, none of the 13 patients who received mavrilimumab died compared with seven of the 26 participants in the historical control group. All the patients who received mavrilimumab and 65% of patients in the control group showed clinical improvement. Patient age and C-reactive protein elevations were similar between De Luca and colleagues' study and the MASH-COVID trial. However, in our study, mean baseline PaO2 to FiO2 ratio was lower: 138 in the placebo group of MASH-COVID compared with 217 in the control group of the observational study. In MASH-COVID, 79% of patients in the placebo group were alive and without respiratory failure at day 28. Although speculative, despite more severe hypoxaemia in the MASH-COVID than in the study by De Luca and colleagues, the better than expected outcomes in the placebo group of MASH-COVID might reflect improvements in patient care and efficacy of concomitant medications, including corticosteroids and remdesivir, as opposed to background therapy of lopinavir–ritonavir and hydroxychloroquine used in the observational study.
Several randomised trials have evaluated therapies that inhibit the innate immune response in patients with COVID-19,2, 3, 24, 25 although understanding of the potential efficacy of these therapies is incomplete in terms of the severity of illness most likely to respond, the magnitude of systemic hyperinflammation necessary to derive a benefit, the timing and duration of the intervention, the breadth of immunosuppression required, and the role of concomitant therapies. With respect to the degree of illness, patients with more severe hypoxaemia might be more likely to respond to therapies that inhibit the innate immune response. Data from the RECOVERY Collaborative group showed that patients with COVID-19 requiring invasive mechanical ventilation derived the greatest benefit from dexamethasone, followed by non-intubated patients receiving oxygen, whereas patients who did not require oxygen at baseline did not accrue a survival benefit.2 In the BACC Bay study, tocilizumab was not effective in preventing intubation or death in patients with COVID-19, but patients requiring more than 10 L nasal cannula oxygen were excluded from the study.24 Conversely, in the EMPACTA trial, although there was no difference in survival, patients with COVID-19 who received tocilizumab were less likely to require invasive ventilation than those who did not, and approximately a quarter were receiving high-flow oxygen or non-invasive ventilation at baseline.25 Moreover, data from the ADAPTIVE and RECOVERY platforms showed better outcomes with IL-6 antagonists in some patients with COVID-19, although peer-reviewed data from the RECOVERY trial are not yet published.26, 27
The degree of systemic inflammation is also likely to be important, and in a systematic review, circulating concentrations of IL-6 in many patients with COVID-19 were lower than in patients with acute respiratory distress syndrome or septic shock.28 Therefore, the wide spectrum of disease severity in patients with COVID-19 is important to recognise, and there are probably different patterns of immunopathology within COVID-19.29 In particular, trials with tocilizumab and sarilumab that did not require patients to have increased systemic inflammation for inclusion might not have targeted the ideal patient population (NCT04320615, NCT04327388). The breadth of immunosuppression required might also be crucial, and there might be a benefit to upstream antagonism of GM-CSF.18 The role of concomitant therapies should also be emphasised. Data from the ACTT-2 study group showed that combination treatment with baricitinib, a Janus kinase inhibitor, plus remdesivir shortened time to recovery, particularly among patients with COVID-19 receiving high-flow oxygen or non-invasive ventilation.3 However, only a minority of patients in that study were receiving corticosteroids. Therefore, whether targeted therapies provide benefit beyond dexamethasone, which is widely available and inexpensive, is an important clinical question. Such investigations might require an adjustment in sample size due to lower-than-expected event rates in the control group and a related smaller benefit of add-on therapies. Nevertheless, despite the benefits of dexamethasone, the mortality of patients with severe and critical COVID-19 disease is unacceptably high. Given the widespread use of dexamethasone, there are also concerns about the use of dual anti-inflammatory medications and the potential for more profound immunosuppression in a patient population at risk for secondary infections. Therefore, future studies could also consider formally evaluating the potential efficacy of GM-CSF antagonism in patients who have disease progression despite dexamethasone, a clinical scenario applicable to some patients enrolled in the MASH-COVID study.
The MASH-COVID study has notable limitations. As emphasised, the study is small, hypothesis-generating, and subject to type II error. Due to slow recruitment, enrolment was stopped after 40 of a planned 60 patients, in part to assess for an efficacy signal to inform a larger trial (NCT04447469). Likewise, although an advantage of our study is high background corticosteroid and remdesivir use, the interaction analyses with treatment and outcome were underpowered. In addition, exploratory analyses evaluating changes in inflammatory markers were initially planned, but collection of laboratory test results varied between patients. However, this investigator-initiated study was done during a time of resource limitation with an emphasis on protection of health-care workers and conservation of personal protective equipment. Therefore, blood draws and patient contact were restricted to clinical care. Moreover, given infection control considerations, patients did not return to the hospital for laboratory testing after discharge. In addition, a C-reactive protein concentration of more than 5 mg/dL was required for inclusion, although more marked elevations or use of other inflammatory markers such as ferritin or lactate dehydrogenase might be useful in identifying future patients most likely to benefit from mavrilimumab. The balance of baseline characteristics and other treatments is inherently difficult in small trials, and this is especially relevant with evolving therapeutic approaches to COVID-19. For example, a minority of patients were treated with corticosteroids before publication of the dexamethasone data from the RECOVERY collaborative group, whereas all patients recruited after publication received corticosteroids, although an interaction between corticosteroids and outcomes was not observed in MASH-COVID.2
Mavrilimumab did not show a statistically significant increase in the proportion of patients free of supplemental oxygen at day 14 among those with severe COVID-19 pneumonia, hypoxaemia, and systemic hyperinflammation, although this positive outcome was numerically more likely in patients treated with mavrilimumab. By day 28, patients who received mavrilimumab were also numerically more likely to be alive and without respiratory failure. Based on these hypothesis-generating results, larger trials should be completed.
Data sharing
The statistical analysis plan (appendix pp 4–20) and the study protocol (appendix pp 21–59) are available. Other study documents, including individual patient level data, are not available.
Acknowledgments
Acknowledgments
This study was done with financial assistance from Kiniksa Pharmaceuticals.
Contributors
All authors participated in reviewing and editing the manuscript and approved the submitted draft. All authors critically reviewed the manuscript and had final responsibility for the decision to submit for publication. PCC, QW, and KEW have verified the underlying data and had access to all the data in the study. PCC, AA, KH, SYC, PR, AD, TSW, JFP, and BCT conceived and designed the study. PCC, AA, KH, CM, JM, SYC, CCS, BVT, AB, AV, BC, QW, KEW, PR, AD, TSW, JFP, and BCT collected, analysed and interpreted the data. PCC, AA, KH, and JFP drafted the manuscript. PCC, QW, and KEW did the statistical analysis. PCC, AA, KH, SYC, CCS, AB, AV, BC, QW, KEW, PR, AD, TSW, JFP, and BCT critically revised the manuscript for important intellectual content. PCC supervised the study.
Declaration of interests
PCC has served on scientific advisory committees for Sobi and Kiniksa pharmaceuticals, and has received an investigator-initiated grant from Novartis pharmaceuticals, all outside the submitted work; PCC also received an investigator-initiated grant from Kiniksa to conduct the submitted work. AA has received research support from and served as an advisor to Kiniksa, outside the submitted work. SYC has served as a consultant for Pure Tech and has received support as a speaker for La Jolla Pharmaceuticals, outside the submitted work. AB and AV have received a travel grant from Kiniksa Pharmaceuticals and receive honoraria from Effetti (Milan, Italy) to collaborate on the Inflammology medical website, outside the submitted work. BVT has received grants from Kiniksa Pharmaceuticals, outside the submitted work. JFP is an employee and stockholder of Kiniksa Pharmaceuticals, and is an inventor on patent applications related to mavrilimumab. BCT has received personal fees from Kiniksa Pharmaceuticals, outside the submitted work. All other authors declare no competing interests.
Contributor Information
MASH-COVID study group:
Deborah Gladish, Karen Myers, Yuki Kuramochi, Christina Sewell, Craig Balog, Denise Kosty Sweeny, Jill Kandrac, Stephanie Spencer, Alice Goyanes, Debasis Sahoo, Siddharth Dugar, Robier Aguillon Prada, Dave Nichols, Jeannie Celiberti, Annie Partisano, Fang Fang, Jennifer Coehlo, Randy Perrin, Brian Mandell, Steven Gordon, Herbert Wiedemann, James Young, Joan Greer, Ai-Chen Ho, Any Ladd, Virginia Mihalick, Alison Montpetit, Joyce O'Brine, Catherine Owen, Mary Pal, Anna Priday, Yub Raj Sedhai, George Wohlford, Nicole Hummel, and Leslie Korbee
Supplementary Material
References
- 1.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang FM, Lee KM, Teijaro JR, Becher B, Hamilton JA. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Fu B, Zheng X, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiomi A, Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kritas SK, Ronconi G, Caraffa A, Gallenga CE, Ross R, Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. 2020;34:9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 11.Hue S, Beldi-Ferchiou A, Bendib I, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202:1509–1519. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1178–L1188. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temesgen Z, Assi M, Shweta FNU, et al. GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin Proc. 2020;95:2382–2394. doi: 10.1016/j.mayocp.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Luca G, Cavalli G, Campochiaro C, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2:e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burmester GR, Feist E, Sleeman MA, Wang B, White B, Magrini F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann Rheum Dis. 2011;70:1542–1549. doi: 10.1136/ard.2010.146225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO R&D blueprint COVID-19 therapeutic trial synopsis. http://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/
- 18.Mehta P, Porter JC, Manson JJ, et al. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir Med. 2020;8:822–830. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonaventura A, Vecchié A, Wang TS, et al. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor α monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679–689. doi: 10.1002/art.40420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cid MC, Unizony S, Pupim L, et al. Mavrilimumab (anti GM-CSF receptor α monoclonal antibody) reduces time to flare and increases sustained remission in a phase 2 trial of patients with giant Cell arteritis. ACR Convergence 2020; virtual; Nov 9, 2020 (abstr L06).
- 22.Wang B, Lau YY, Liang M, et al. Mechanistic modeling of antigen sink effect for mavrilimumab following intravenous administration in patients with rheumatoid arthritis. J Clin Pharmacol. 2012;52:1150–1161. doi: 10.1177/0091270011412964. [DOI] [PubMed] [Google Scholar]
- 23.Campbell J, Nys J, Eghobamien L, Cohen ES, Robinson MJ, Sleeman MA. Pulmonary pharmacodynamics of an anti-GM-CSFRα antibody enables therapeutic dosing that limits exposure in the lung. MAbs. 2016;8:1398–1406. doi: 10.1080/19420862.2016.1215790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2100433. published online Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.02.11.21249258. published online Feb 11. (preprint). [DOI] [Google Scholar]
- 28.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The statistical analysis plan (appendix pp 4–20) and the study protocol (appendix pp 21–59) are available. Other study documents, including individual patient level data, are not available.