Abstract
Patients with chronic kidney disease (CKD) exhibit an elevated cardiovascular risk manifesting as coronary artery disease, heart failure, arrhythmias, and sudden cardiac death. Although the incidence and prevalence of cardiovascular events is already significantly higher in patients with early CKD stages (CKD stages 1–3) compared with the general population, patients with advanced CKD stages (CKD stages 4–5) exhibit a markedly elevated risk. Cardiovascular rather than end-stage kidney disease (CKD stage 5) is the leading cause of death in this high-risk population. CKD causes a systemic, chronic proinflammatory state contributing to vascular and myocardial remodeling processes resulting in atherosclerotic lesions, vascular calcification, and vascular senescence as well as myocardial fibrosis and calcification of cardiac valves. In this respect, CKD mimics an accelerated aging of the cardiovascular system. This overview article summarizes the current understanding and clinical consequences of cardiovascular disease in CKD.
Keywords: arrhythmias, cardiovascular disease, chronic kidney disease, clinical aspects, heart failure, sudden cardiac, death
Richard Bright, a British physician, was the first to report the association of chronic kidney disease (CKD) with cardiovascular disease (CVD).1 Patients with CKD exhibit a pronounced risk for cardiovascular events: 50% of all patients with CKD stage 4 to 5 have CVD,2 and cardiovascular mortality accounts for ≈40% to 50% of all deaths in patients with advanced CKD (stage 4) as well as end-stage kidney disease (stage 5), compared with 26% in controls with normal kidney function3,4 (Figure 1). In addition to the high risk for fatal atherosclerosis-related complications such as myocardial infarction and stroke, cardiovascular death also results from heart failure (HF) and fatal arrhythmias, particularly in advanced CKD stages. In >70 studies in nondialyzed subjects with CKD, correction for classical and even less classical cardiovascular risk factors, such as hypertension, diabetes, and dyslipidemia, did not neutralize the impact of CKD on cardiovascular risk.6 This review summarizes the current knowledge of CVD in patients with CKD, clinical consequences, and treatment options of CVD in CKD (Figure 2). Given space limitations, we will not cover special situations such as extrarenal involvement in vasculitides or the association of autosomal dominant polycystic kidney disease with vascular abnormalities such as intracranial, aortic, or coronary artery aneurysms as well as aortic dissection.7
Figure 1.
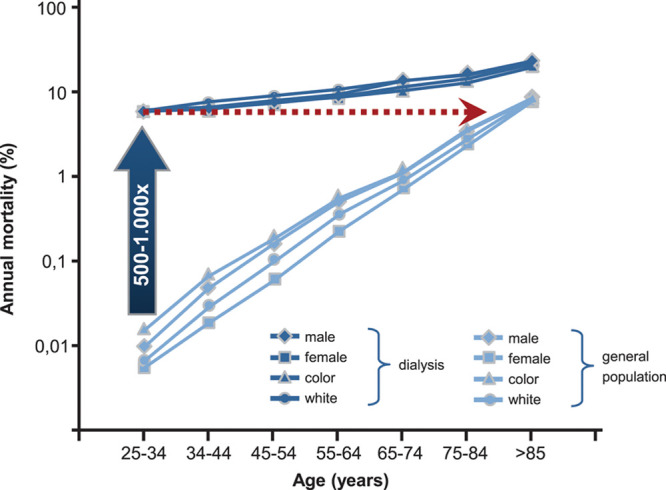
Cardiovascular mortality in the general population and in patients with end-stage kidney disease. In 25- to 34-year-old patients with end-stage kidney disease, annual mortality is increased 500- to 1000-fold and corresponds to that of the ≈85-year-old general population. Adapted from Foley et al.5
Figure 2.
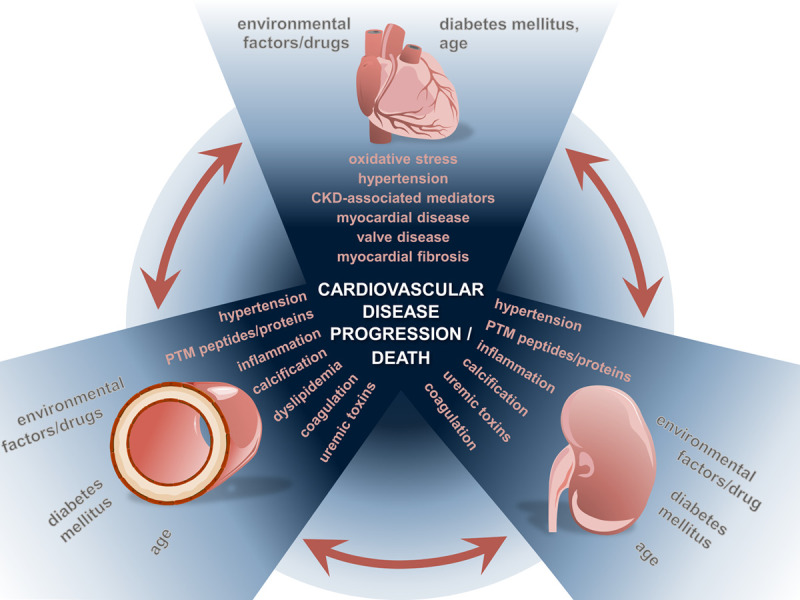
Interaction of cardiovascular disease (CVD) and chronic kidney disease (CKD). Various mediators and mechanisms in vascular disease, heart failure, and CKD contribute to the progression of CVD and influence the prognosis of patients. PTM indicates post-translational modification.
Epidemiology and prognosis
The definition and classification of CKD was introduced by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative in 2002,8 and the international guideline group Kidney Disease Improving Global Outcomes in 2004.9 Kidney damage refers to kidney abnormalities observed during clinical evaluation indicating a reduction in kidney function.9,10 Chronic kidney disease is defined as abnormalities in kidney damage or glomerular filtration rate <60 mL/min/1.73 m2 that have been present for >3 months and have an impact on health.8,11 Kidney damage in many kidney diseases can be ascertained by the presence of albuminuria, defined as albumin-to-creatinine ratio >30 mg/g (Figure 3). Because there is an increasing evidence indicating a continuous relationship between albuminuria and cardiorenal risk in the renal and nonrenal population,13,14 albuminuria is considered a prognostic marker for cardiovascular or renal risk, or both.15 Higher levels of albuminuria indicate a graded increase in risk for mortality independent of eGFR.12,16
Figure 3.
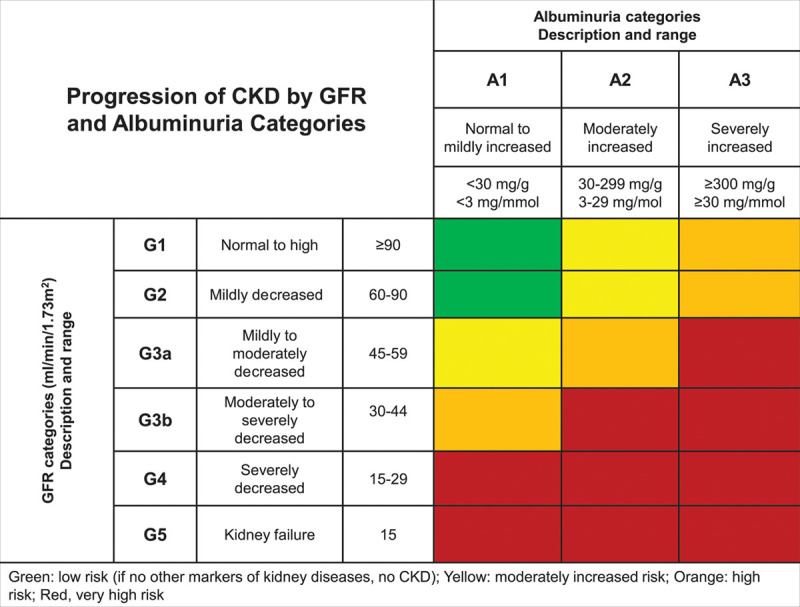
Classification and prognosis of chronic kidney disease (CKD) from 2012 KDIGO (Kidney Disease Improving Global Outcomes) guidelines. GFR indicates glomerular filtration rate. Adapted from the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group.12
CKD is increasingly recognized as a global public health problem17 imposing huge medical and financial burdens on societies and health care systems with an estimated prevalence of 13.4% globally.18 The worldwide rise in the prevalence of CKD is accompanied by an increase in patients reaching CKD stage 5 requiring kidney replacement therapy. Currently, about 3 million patients are receiving kidney replacement therapy for CKD stage 5D worldwide out of 10 million who would qualify for kidney replacement therapy; these numbers are expected to grow by 50% to 100% until 203019,20 (Figure 4). Reasons for the increasing incidence and prevalence of advanced CKD, among others, include aging populations, increasing prevalence of type 2 diabetes and hypertension,22 and a low detection rate and therapeutic inertia in the early stages of CKD.8,23,24
Figure 4.
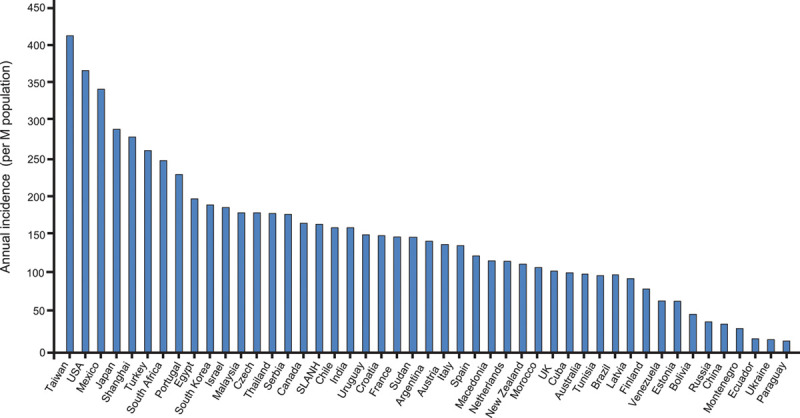
Annual incidence rates of end-stage kidney disease in different countries. Adapted from Jha et al.21
Despite the fact that health care resources allocated for the treatment of CKD have significantly increased in recent years, patients with CKD still exhibit a dramatically reduced life expectancy, with a loss of 25 years of life at advanced stages compared with individuals with normal kidney function.25,26 Worldwide, CKD accounted for 2 968 600 (1.1%) of disability-adjusted life-years and 2 546 700 (1.3%) of life-years lost in 2012.4 A meta-analysis of the association between nondialysis-dependent CKD and the risk for all-cause and cardiovascular mortality involving 1 371 990 patients demonstrated an exponential increase in absolute risk for death with decreasing kidney function even after adjustment for other established risk factors.27 A meta-analysis of cohort studies involving >1.4 million individuals28,29 yielded an association of not only low eGFR but also higher albuminuria with cardiovascular disease (Figure 5).30 Thus, the risk of developing CVD in patients with CKD surpasses the risk of reaching end-stage kidney disease, and therefore, CKD must be considered one of the strongest risk factors for the development of CVD.27
Figure 5.
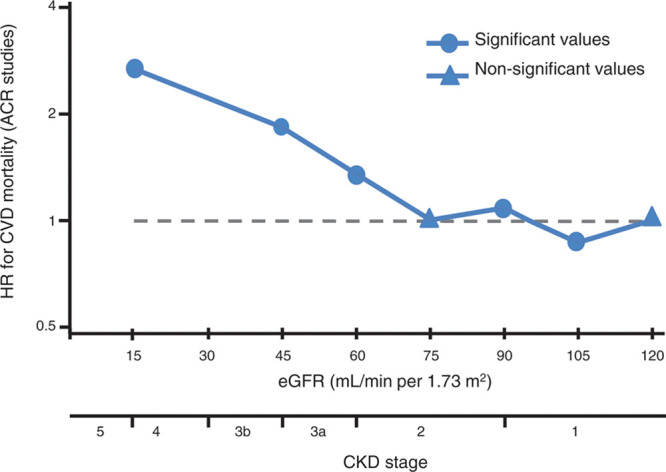
Independent association of kidney function with cardiovascular mortality. ACR indicates albumin-to-creatinine ratio; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; and HR, hazard ratio. Adapted and modified from Gansevoort et al.30
Pathophysiology of CVD in CKD
In general, in addition to traditional risk factors, 2 major mechanisms are thought to contribute to the development of CVD in CKD. On the one hand, the kidney can release hormones,31–34 enzymes, and cytokines35–37 in response to kidney injury or kidney insufficiency, which leads to characteristic changes in the vasculature. On the other hand, CKD-associated mediators as well as hemodynamic alterations contribute to cardiac damage,38 as discussed in the following sections.
Traditional Risk Factors of Vascular Disease in CKD
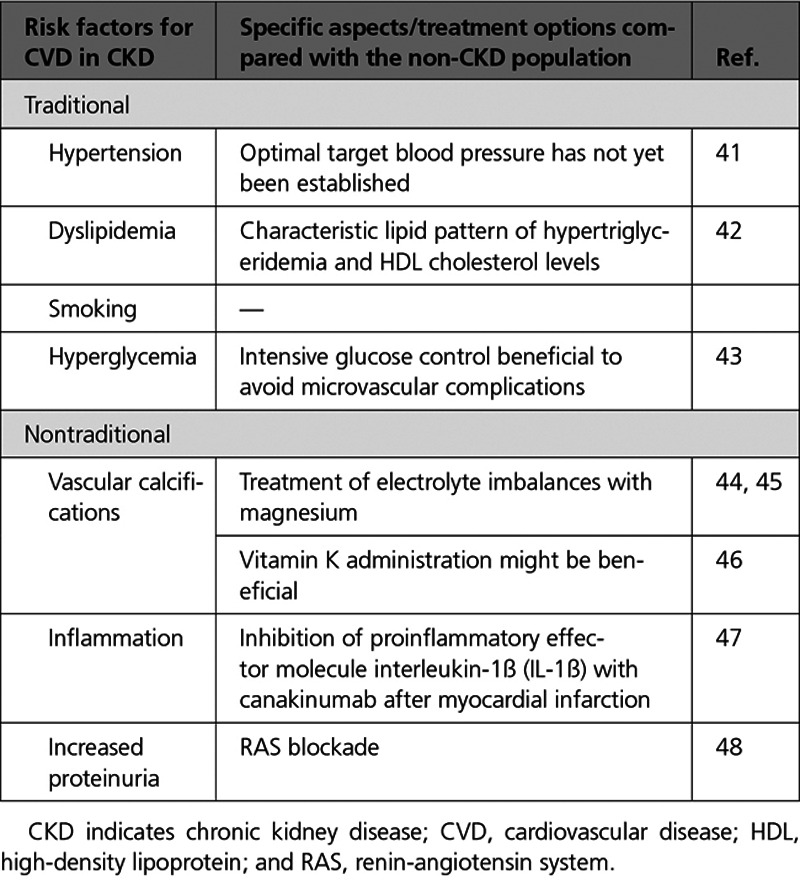
Traditional cardiovascular risk factors are highly prevalent in patients with CKD, and their contribution to atherosclerotic vascular disease is particularly important in earlier CKD stages.39,40 Among others, hypertension, insulin resistance/diabetes, dyslipidemia, and smoking contribute not only to atherosclerotic cardiovascular and cerebrovascular sequelae (Table) but also to CKD progression because of their effect on large (eg, kidney artery stenosis) and smaller (eg, nephrosclerosis) kidney vessels.49,50 In addition, some of these effects also seem to contribute to the recently described association of CKD with abdominal aortic aneurysms.51
Table.
Traditional and Nontraditional Risk Factors for CVD in CKD
Hypertension
The elevated cardiovascular risk in CKD cannot solely be explained by the presence of traditional risk factors as shown by data from the ARIC (Atherosclerosis Risk In Communities) and CHS (Cardiovascular Health Study) trials.52 In addition, the specific aspects of CKD have not fully been addressed in studies targeting the modification of these risk factors. However, treatment of hypertension is beneficial in CKD, as recently corroborated by results of the SPRINT trial (Systolic Blood Pressure Intervention Trial), but the optimal target blood pressure in patients with CKD has not yet been established.41
Diabetes
Hyperglycemia is strongly associated with the development of both CKD and CVD. However, improvement in glycemic control in type 2 diabetes mainly contributes to a reduction in microvascular events such as nephropathy, although various studies failed to show a significant effect on macrovascular events; for example, the ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation”) demonstrated in ≈11 000 patients with type 2 diabetes that intensive glucose control compared with standard therapy leads to a reduction in the combined outcome of major macrovascular and microvascular events, but this effect was mainly driven by a reduction in nephropathy with no significant effect on macrovascular events43; the ACCORD trial (Action to Control Cardiovascular Risk in Diabetes)53 was not able to demonstrate that treatment targeting nearly normal glycemic control reduces the risk of cardiovascular events in ≈10 000 patients with type 2 diabetes, and intensive versus standard glucose control in patients with poorly controlled type 2 diabetes had no significant effect on the rates of major cardiovascular events, death, or microvascular complications in VADT (Veterans Affairs Diabetes Trial),54 including 1791 patients.
Moreover, data for lifestyle modifications are mostly observational and extrapolated from non-CKD trials. This fact has been clearly exposed by a recent meta-analysis reporting that randomized trials conducted between 2006 to 2014 were less likely to exclude patients with CKD than those between 1985 to 2005 (46% versus 56%).55 However, this apparently encouraging trend is not sufficient to close the gap of evidence in patients with CKD.
Dyslipidemia
In addition, patients with CKD exhibit a characteristic lipid pattern of hypertriglyceridemia and low high-density lipoprotein (HDL) cholesterol levels, but mostly normal low-density lipoprotein cholesterol levels. Recent clinical evidence suggests that vascular effects of HDL can be heterogeneous in different conditions, and that progressive kidney dysfunction dramatically changes the composition and quality of blood lipids, particularly HDL and triglyceride-rich lipoproteins, in favor of a more atherogenic profile.42 Adverse endothelial effects of HDL are also detectable in children with CKD, in whom cardiovascular risk factors such as smoking, hypertension, diabetes, and dyslipidemia were not yet present.56 Several factors modify the composition of the HDL particle in CKD, including uremic toxins, increased oxidative stress, and the proinflammatory microenvironment. These factors contribute to a pronounced remodeling of HDL particles, altering the proteome and lipidome composition of HDL and inducing posttranslational modifications of HDL’s protein cargo. Furthermore, the accumulation of uremic toxins such as symmetrical dimethylarginine in advancing CKD plays a key role in the functional changes of HDL.42
Last, increased albuminuria or proteinuria is a potent risk factor for CVD in both diabetic and nondiabetic patients with CKD (Figure 3), and the incidence of cardiovascular events decreases with the institution of antiproteinuric measures, in particular renin-angiotensin system (RAS) blockade. However, the pathomechanistic link between albuminuria and CVD may not be a direct one, as systemic but particularly intrarenal hemodynamic effects of RAS blockers affect progression of CKD and thus indirectly of CVD. Therefore, the data in support of RAS blockers in albuminuric patients are reasonably strong for preventing progression of CKD and less so for CVD protection.48
Nontraditional Risk Factors of Vascular Disease in CKD
Vascular Calcification
Vascular smooth muscle cells are the cellular components of the medial layer of the vessels, which can switch from a contractile phenotype to a more synthetic phenotype caused by hemodynamic changes observed in CKD.57 Resulting cardiovascular calcifications are markedly accelerated in patients with CKD, and even children with advanced CKD frequently exhibit vascular calcifications.58 The histological prevalence of vascular calcifications in radial arteries was 45-fold greater in patients with CKD compared with those without CKD.59 In addition to CKD, several common comorbidities, in particular diabetes, further enhance the progression of calcification.
Calcification of central arterial vessels contributes to increased pulse wave velocity, earlier reflection of the pulse wave, increased cardiac afterload, and thus HF.60 Resulting hemodynamic alterations induce left ventricular hypertrophy associated with a decrease in coronary perfusion.61,62 A particularly severe form of vascular calcification is uremic calcific arteriolopathy (calciphylaxis), which is caused by calcium deposition in the media of the dermo-hypodermic arterioles63 leading to skin necrosis and carries a high mortality rate.64 The exact mechanism of uremic calcific arteriolopathy is unclear: previously, an increase in the calcium-phosphorus product was thought to cause calcification65 leading to uremic calcific arteriolopathy, but it becomes increasingly clear that calcification involves active cellular processes, not just passive mineralization, because of an increase in calcium-phosphorus concentrations.66 However, hemodynamic consequences of medial calcification seem to have an exacerbated risk for left ventricular hypertrophy.67
Calcification of cardiac valves, in particular the aortic valve, is a frequent cause of valvular stenosis requiring intervention. The extent and progression rate of vascular calcifications in CKD herald a poor prognosis.68 However, the first data raise the hypothesis that repleting patients with vitamin K can retard the progression of valvular calcification46; still, negative trials have also been published on this topic.69
In addition, electrolyte imbalances like dysmagnesemia are common in patients with CKD70 and contribute to poor patient outcome,71 and therefore, electrolyte imbalances are potential targets for managing coronary artery calcification. In particular, magnesium, frequently reduced in serum in CKD,70 has recently gained interest because of the inhibitory effect on vascular calcification44,45: magnesium interferes with hydroxyapatite crystal formation and can halt vascular calcification progress in advanced CKD.72
Inflammation
Inflammation is a key process observed in patients with CKD, and CKD is considered a systemic inflammatory disease with many causes73,74 and has been shown to predict the long-term risk of developing CKD.74 Proinflammatory circulatory mediators progressively increase as kidney function declines.75 Proinflammatory processes in CKD patients comprise, among others, a variety of infections including periodontal disease, oxidative stress caused by accumulation of advanced glycation end products, metabolic acidosis, reduced cytokine clearance, insulin resistance, posttranslational modifications of blood-borne molecules such as lipoproteins, and epigenetic factors.73
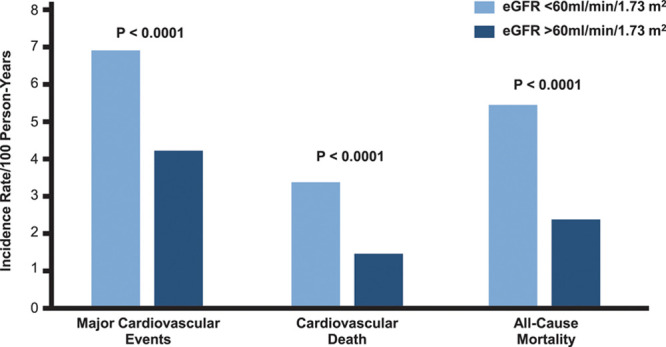
In accordance, the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) focusing on ≈10 000 stable postmyocardial infarction patients with high-sensitivity C-reactive protein demonstrated a benefit of inhibition of proinflammatory effector molecule interleukin-1β (IL-1β) with the antibody canakinumab, which was larger in patients with eGFR <60 mL/min/1.73 m2 than in those with eGFR >60 mL/min/1.73 m247 (Figure 6). However, further studies are needed to firmly establish the pathophysiological mechanisms and potential treatment options for inflammation in patients with CKD.
Figure 6.
Independent association of kidney function with cardiovascular mortality. ACR, albumin-to-creatinine ratio; and eGFR, estimated glomerular filtration rate. Adapted and modified from Gansevoort et al.30
Myocardial Alterations in CKD
Patients with CKD exhibit characteristic changes in the myocardium with pathological myocardial fibrosis with collagen deposition between capillaries and cardiomyocytes and cardiac hypertrophy the hallmarks of uremic cardiomyopathy.76 Left ventricular hypertrophy (LVH) is present in about one-third of all patients with CKD, increasing up to 70% to 80% in patients with end-stage kidney disease. The presence of LVH is an independent predictor of survival in patients with CKD, even in those with early-stage CKD. Three main mechanisms are considered to contribute to LVH in CKD: (1) afterload- and (2) preload-related factors as well as (3) nonafterload, nonpreload-related factors.77 Afterload-related factors include abnormal arterial stiffness, increased systemic arterial resistance, and systolic hypertension, leading to an initial concentric LVH.78 Continuous left ventricular overload subsequently leads to maladaptive changes and cardiomyocyte death, which in turn result in an eccentric hypertrophy and subsequent left ventricular dilatation, systolic dysfunction, and reduced ejection fraction (EF).79 Preload-related factors in the pathophysiology of LVH comprise the expansion of intravascular volume in CKD leading to volume overload, length extension of myocardial cells, and eccentric or asymmetrical left ventricular remodeling.78 Nonafterload, nonpreload-related factors include intracellular mediators and pathways contributing to progressive LVH.80 Essential mechanisms in this context are activation of peroxisome proliferator-activated receptors, stimulation of small G-proteins or the mechanistic target of rapamycin pathway, as well as metabolic changes such as decreased fatty acid oxidation. The second hallmark of uremic cardiomyopathy besides LVH is the development of myocardial fibrosis occurring independently of LVH itself.76 Cardiac fibrosis in patients with CKD is characterized by diffuse collagen deposition between capillaries and cardiomyocytes funneling into the maladaptive ventricular hypertrophy with subsequent dilatation of the heart.
Furthermore, there is an epidemiological collinearity of the prevalence and incidence of CKD with aortic and mitral valve disease.81 Valve disease has a strong impact on the outcome in patients with CKD.82 Early CKD stages 1 to 3 are associated with enhanced calcifications of valves and coronary arteries.83 Heart valve calcification occurs in stage 5 CKD in up to 88% to 99% of patients, increasing from 40% of patients in CKD stage 3,84 and the final destruction of valves occurs at a 10-fold higher rate in patients with CKD compared with patients without CKD. Valvular disease in patients with CKD is accelerated by comorbidities like diabetes, arterial hypertension, hyperlipidemia, anemia and ongoing infections of valves, and malnutrition, as well as hypercalcemia, hyperphosphatemia, and hyperparathyroidism.85
Therapy of cardiovascular disease in CKD
Treatment of Vascular Disease in Patients With CKD
Control of traditional risk factors as well as antiplatelet therapy are cornerstones to reduce cardiovascular risk. As such, current guidelines recommend to lower systolic blood pressure to a range of 130 to 139 mm Hg in patients with diabetic or nondiabetic CKD, and renin-angiotensin-aldosterone inhibitors are first-line agents in CKD.86 Given the only moderate effect of glucose control on macrovascular events, hemoglobin A1c targets should be individualized, and side effects such as hypoglycemia should be avoided, in particular in CKD, because hypoglycemic episodes are associated with an increase in mortality in this group of patients. Data from large cardiovascular outcome trials with glucose-lowering sodium-glucose cotransporter 2 (SGLT2) inhibitors or GLP-1 receptor agonists have shown a significant reduction in cardiovascular events in patients with type 2 diabetes at high cardiovascular risk. Thus, various guidelines recommend treatment with these agents in CKD and non-CKD patients with CVD or multiple cardiovascular risk factors.
The effect of lipid-lowering strategies on CV risk reduction in CKD seems to be dependent on the severity of CKD. As such, the SHARP study (Study of Heart and Renal Protection)87 examined the effect of simvastatin 20 mg/d versus simvastatin 20 mg/d plus ezetimibe in 9438 patients with advanced chronic kidney disease without a history of myocardial infarction or coronary revascularization and found a significant 17% relative reduction of the primary end point of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization.88 In contrast, both the 4D study (Deutsche Diabetes Dialysis Study)89 and the AURORA study (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events) failed to show a significant reduction of cardiovascular death, nonfatal MI, and nonfatal stroke by atorvastatin or rosuvastatin, respectively, versus placebo in patients with hemodialysis.90 These data suggest that the cardiovascular benefit of lipid-lowering therapies is attenuated in subjects with low glomerular filtration rate and limited/absent in patients with end-stage renal disease on hemodialysis.91
In patients with coronary artery disease without CKD, antiplatelet therapy is well established to reduce cardiovascular risk, but in CKD, the prognostic benefit is less clear. Moreover, these drugs increase the risk of bleeding events in patients with CKD, possibly outweighing the potential benefit.92
The ISCHEMIA-CKD trial (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches-Chronic Kidney Disease) assessed an invasive or conservative care approach in patients with stable CAD and CKD. A total of 777 patients with advanced kidney disease and moderate or severe ischemia on stress testing were randomly assigned to initial invasive strategy consisting of coronary angiography and revascularization (if appropriate) added to medical therapy or an initial conservative strategy consisting of medical therapy alone and angiography reserved for those in whom medical therapy had failed. After a median follow-up of 2.2 years, there was difference for the primary composite end point of death or nonfatal myocardial infarction between groups. However, the invasive strategy was associated with a significantly higher incidence of stroke than the conservative strategy and with a higher incidence of death or initiation of dialysis.93 In addition, groups did not differ with respect to angina-related health status.94
Interestingly, a large registry study in patients with acute myocardial infarction showed that patients with CKD were less likely to receive statins, β-blockers, and antiplatelet therapy compared with those without CKD, suggesting that patients with CKD still receive fewer evidence-based therapies, which may as well contribute to substantially higher mortality rates.95
Treatment of Patients With HF and CKD
Current therapeutic options in patients with HF are largely on the basis of cardiovascular outcome trials, which assessed the effect of both medical and interventional therapy to reduce morbidity and mortality. However, patients with CKD have been excluded in most clinical HF studies, and recommendations for patients with CKD have to be extrapolated from subgroup analyses. There is to date no treatment option available that convincingly reduced morbidity and mortality in patients with HF and preserved EF (left ventricular EF ≥50%) or moderately impaired left-ventricular function (left ventricular EF 40%–49%) in CKD.
However, at the stage of symptomatically reduced EF (HFrEF; left ventricular EF <40%), therapy with angiotensin-converting enzyme (ACE) inhibitors and β-blockers is recommended as first-line therapy. ACE inhibitors have been shown to reduce morbidity and mortality in numerous large randomized trials. A clear benefit of ACE inhibitors in patients with CKD stage 1 to 3 has been suggested, but few data are available in patients with advanced CKD stages. In the Swedish Cardiac Insufficiency Registry, a total of 2410 patients with HFrEF and CKD (serum creatinine 2.5 mg/dL or creatinine clearance <30 mL/min) with or without RAS inhibitor were studied.96 Propensity score matching was used to compare 602 patients with and without angiotensin1-receptor blockers or ACE inhibitors. In patients with RAS inhibition, total mortality was significantly lower at 1 year compared with patients without RAS inhibition (hazard ratio, 0.76 [95% CI, 0.67–0.86]).96
On the basis of large randomized studies showing a reduction in total mortality, β-blockers are also recommended as first-line therapy in parallel to renin-angiotensin-aldosterone inhibitors to counteract sudden cardiac death and progression of HF in patients with HFrEF.97–99 A meta-analysis of intervention studies with β-blockers in patients with CKD stage 3 to 5 clearly demonstrated that these patients benefit from this therapy,100 suggesting that β-blockers are equally effective in patients with CKD as in non-CKD patients. Recent data underline the benefits of a β-blocker therapy in patients with CKD (CKD stages 3–4) with HF, left ventricular EF <50%, and sinus rhythm.101 If patients with HFrEF are still symptomatic despite treatment with ACE inhibitors and β-blockers, and if the left ventricular EF is ≤35%, administration of mineralocorticoid receptor antagonists (MRAs) is indicated, but with particular caution in patients with advanced CKD. Spironolactone and eplerenone improved the prognosis of patients with HFrEF, and this therapy is effective in patients with HF and CKD stages 1 to 3.102 In the DOHAS study (Dialysis Outcomes Heart Failure Aldactone Study), 309 patients with CKD stage 5D were randomized to either 25 mg spironolactone per day or to standard of care only.103 Compared with the control group, the combined primary end point of mortality and cardio- or cerebrovascular hospitalization was significantly reduced in the spironolactone group (hazard ratio, 0.40 [95% CI, 0.20–0.81]). However, cardiovascular efficacy and safety of spironolactone are still uncertain in CKD stage 5. In recent placebo-controlled trials, spironolactone appeared safe in carefully monitored maintenance CKD stage 5 patient cohorts but it did not affect cardiovascular parameters like diastolic function104 or left ventricular mass, ambulatory blood pressure, left ventricular EF, 6-minute walk test distance, or New York Heart Association class.105 Because spironolactone increased the frequency of moderate—albeit not severe—hyperkalemia in patients with CKD stage 4 to 5,104,105 MRAs formally are still contraindicated in advanced CKD. The ongoing ALCHEMIST trial (ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial) examines the effect of aldosterone on cardiovascular outcome (including HF) in chronic hemodialysis patients. Novel therapeutic strategies with potassium binders may provide an additional option for patients with hyperkalemia.
Diuretics are indicated at New York Heart Association II stage with fluid retention, and generally in New York Heart Association stage III to IV patients, to reduce the risk of decompensation, but no data demonstrating a prognostic benefit of diuretics on mortality are available.
If patients on combination therapy with ACE inhibitors (or angiotensin1-receptor blockers), β-blockers, and MRAs continue to be symptomatic and the ACE inhibitor (or angiotensin1-receptor blocker) was well tolerated, the administration of an angiotensin receptor/neprilysin inhibitor is recommended. Neprilysin inhibitors, such as sacubitril, a relatively new class of drugs, inhibit the enzyme neprilysin, thus prolonging the half-life of vasoactive peptides such as BNP (B-type natriuretic peptide); sacubitril is given in combination with valsartan. For this substance, LCZ 696 (sacubitril and valsartan), a reduction in overall mortality, cardiovascular mortality, and hospitalization compared with enalapril was demonstrated, and this effect was also seen in patients with CKD stages 3 to 5.106 Thus, angiotensin receptor/neprilysin inhibitors seem effective in patients with HF and CKD.
Therapy with the channel inhibitor ivabradine may be considered once maximally tolerated β-blocker therapy is in place. The evidence for this recommendation is derived from the SHIFT trial (Systolic Heart Failure Treatment with the I[f] Inhibitor Ivabradine Trial), which showed a significant reduction in the combined primary end point of cardiovascular mortality or heat failure hospitalization compared with placebo in patients treated with ivabradine.107 The incidence of the primary end point was similar in both patients with (CKD stages 3–5) and without CKD.108
Prevention of Sudden Cardiac Death and Arrhythmias in CKD
More than two-thirds of mortality in advanced CKD stages are a result of sudden cardiac death109,110 (Figure 6). Sudden cardiac death refers to the unexpected natural death from a cardiac cause within 1 hour after onset of symptoms in a person who has no lethal underlying disease. Sudden cardiac death is mainly caused by ventricular arrhythmias.111,112 The rate of sudden cardiac death is 59 deaths in 1000 patient-years in the CKD stage 5D population, whereas it is 1 death in 1000 patient-years in the general population.113
Patients with CKD not only show an increased risk of sudden cardiac death but also have clear differences from the general population in terms of the pathophysiology and cause of sudden cardiac death. In the general population, >80% of sudden cardiac deaths are associated with coronary heart disease.114 Despite the fact that patients with CKD stage 5D have a high incidence of coronary heart disease, the rate of sudden cardiac death is disproportionately high compared with the incidence of coronary heart disease in these patients (Figure 7). Moreover, even a complete revascularization can only partially reduce the risk of sudden cardiac death in patients with CKD.116 According to the current state of knowledge, components of the myocardium, the blood vessels, and the blood as a whole add up to the high risk in these patients. In addition, dialysis itself is a risk factor for sudden cardiac death, with the highest risk of sudden cardiac death within the first 12 hours after dialysis and after a long dialysis-free interval. Potential mechanisms include volume and sudden electrolyte shifts after dialysis as well as volume overload and electrolyte disturbance.117 Accordingly, patients with peritoneal dialysis seem to exhibit a lower risk for sudden cardiac death. To date, noninvasive strategies such as assessment of heart rate variability, late potentials, QT dispersion, or wave alternans failed to adequately predict sudden cardiac death risk in patients with dialysis.118
Figure 7.
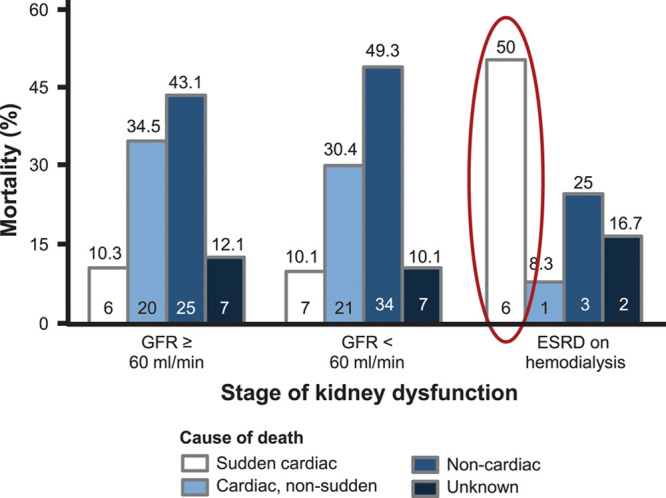
Cause-specific mortality according to varying levels of kidney dysfunction. For the 3 categories of kidney dysfunction, cause-specific mortality is depicted. Sudden cardiac death was the major cause of death in patients with end-stage renal disease (ESRD) on dialysis (50.0% vs 10.1% [glomerular filtration rate {GFR} <60 mL/min] vs 10.3% [GFR ≥60 mL/min], χ2 P=0.010). Number at the top of each bar is the mortality rate; number within the bar is the n per group. The unknown category was reserved for those patients whose cause of death could not be determined. Adapted from Cheema et al.115
Compared with drug therapies (eg, antiarrhythmic agents), implantable cardioverter-defibrillators (ICDs) lead to a significant reduction in mortality in cardiovascular patients as primary and secondary prevention, but patients with CKD were again mostly excluded in these studies. A meta-analysis of the effectiveness and importance of implantation of ICDs showed that ICD patients with CKD exhibit an increased mortality, and therefore the value of ICD implantation in this group has been questioned.119
Despite the small number of dialysis patients in clinical studies, current guidelines also recommend primary prophylactic ICD implantation if the EF is ≤35%. To what extent dialysis patients with an EF >35% have an increased risk of arrhythmia and may benefit from primary prophylactic ICD implantation is also currently unclear.
Therapy of Valve Disease in CKD
Guideline recommendations for patients with CKD do not differ much from patients without CKD in approaches to treat valve disease.81 In-hospital mortality can rise up to 21% in patients with CKD stage 5.120 CKD is a predictor for acute kidney injury and death after valve surgery.121 Therefore, the Society of Thoracic Surgeons Score, EuroSCORE-II (European System for Cardiac Operative Risk Evaluation), or logistic EuroSCORE have incorporated kidney function as 1 parameter.81 In patients with low perioperative risk (EuroSCORE-II <4% or logEuroSCORE <10%), surgical aortic valve replacement is recommended.
Transcatheter aortic valve implantation is recommended as a safe and effective treatment option in patients <75 years old at elevated operative risk (Society of Thoracic Surgeons Score >4%). Recent data suggest that in patients at low risk, the all-cause mortality after 24 months decreases by 12% and stroke incidence by 19% compared with surgical aortic valve replacement, which was independent of the preoperative risk before the intervention.122 Recently published prospective randomized trials comparing transcatheter aortic valve implantation and surgical aortic valve replacement in patients without CKD showed a superiority of interventional valve treatment compared with operative valve treatment.123,124 However, impaired kidney function affects mortality and risk for dialysis after transcatheter aortic valve implantation.125 Long-term risk for death and need for introduction of kidney replacement therapy were increased by 51% and 56%, respectively.126 Nevertheless, acute kidney injury after transcatheter aortic valve implantation (7%) was less prevalent than surgical aortic valve replacement (12%).127
Surgical treatment of mitral valve incompetence with valve reconstruction is superior to valve replacement.81 Recently, reconstruction of mitral valves in functional mitral incompetence with the MitraClip system has shown superior results128 with a reduction of hospitalization caused by cardiac decompensation in 2 years (hazard ratio, 0.54; P<0.001) and extensive reduction of all-cause death129 compared with optimal medical treatment. CKD is associated with adverse outcomes in mitral valve interventions. In patients with CKD stage 1 to 2, mortality was 13%; at CKD stage 3, 19%; and CKD stage 4 to 5, 33%.130 There was a slight improvement of kidney function by 4.8 mL/min/1.73 m2 in CKD stage 4 to 5127 after valve replacement, indicating that valve improvement and improvement in myocardial performance might impact kidney function. This improvement was associated with decreased therapy cost and in-hospital treatment duration.127
Because valve disease is a common comorbidity in patients with CKD, after echocardiographic evaluation, the decision to treat valve disease with surgery or intervention should be on the basis of the temporary guidelines of the American Heart Association, American College of Cardiology, and European Society of Cardiology. In general, the degree of CKD is associated with increased adverse outcomes risk after interventions and surgery as well as bearing an enhanced intermediate and long-term risk, in particular in patients >75 years of age. In the latter group, aortic valve transfemoral aortic valve implantation should be considered the superior method to be used.
Novel therapeutic approaches
Although CKD is one of the most common comorbidities in CVD, few specific treatment options are available for the high-risk population of patients with advanced CKD.131 Finding a balance between the optimization of clinical outcomes in CKD and CVD still requires validation in large prospective, multicenter clinical studies. SGLT2 inhibitors, currently used to treat patients with type 2 diabetes, have shown unprecedented cardiovascular as well as kidney protective effects. In cardiovascular outcome studies such as EMPA REG OUTCOME ([Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) with empagliflozin in >7000 cardiovascular type 2 diabetic patients, the primary end point and cardiovascular mortality were significantly reduced in the SGLT2 group.132 A positive effect on cardiovascular morbidity was also demonstrated in cardiovascular outcome studies with canagliflozin and dapagliflozin.133,134
In these studies, the secondary outcome of “HF-related hospitalizations” was less frequent, suggesting a class effect of SGLT2 inhibitors. The use of canagliflozin (CANVAS)133 and empagliflozin (EMPA REG OUTCOME),132 in patients with type 2 diabetes, both confirmed the reduction of albuminuria progression by 27% to 38% and the preservation of eGFR, even in advanced CKD stages. Recently, CREDENCE became the first phase III study with an SGLT2 inhibitor in type 2 diabetic patients with CKD (n=4400) with a combined primary kidney end point135: within 2 and a half years, canagliflozin significantly reduced the risk of kidney replacement therapy, doubling serum creatinine and death caused by kidney insufficiency by 33%. In addition, most recently, DAPA-CKD, a dedicated trial in patients with CKD (with or without type 2 diabetes), was published. In this placebo-controlled trial, dapagliflozin led to a significant reduction in the primary composite end point of sustained ≥50% eGFR decline, renal or cardiovascular death, hospitalization for heart failure, as well as a reduction in all-cause mortality independent of the presence of diabetes.136 Initial findings indicate that SGLT2 inhibitors improve kidney function by regulating kidney sodium reabsorption, the resulting glomerular hyperfiltration, and hypertension. On the basis of the promising effects of SGLT2 inhibitors on HF-related end points, various cardiovascular outcomes trials directly assess the efficacy of these agents in HF populations. DAPA-HF (Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction)—the first study among them to reports results—examined the effect of dapagliflozin in HFrEF patients with or without diabetes enrolling patients with an eGFR down to 30 mL/min/1.73 m2. Dapagliflozin significantly reduced HF hospitalization, cardiovascular death, and all-cause mortality in patients with and without diabetes.137 In EMPEROR-reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), a trial enrolling HFrEF patients with or without diabetes with an eGFR down to 20 mL/min/1.73 m2, empagliflozin significantly reduced the composite end point of time to first event of adjudicated cardiovascular death or adjudicated hospitalization for heart failure.138 Potential mechanisms explaining the beneficial effects of SGLT2 inhibitors in patients with HF or CKD include hemodynamic as well as metabolic effects.139 In addition, SGLT2 inhibitors may selectively reduce interstitial fluid, and this may limit the reflex neurohumoral stimulation that occurs in response to intravascular volume contraction with traditional diuretics.140
MRAs reduce the aldosterone-mediated proinflammatory effects that are involved in the fibrotic remodeling processes. The new selective nonsteroidal MRA finerenone also blocks the damaging effects of the overactivated aldosterone system. In contrast with the MRAs spironolactone and eplerenone, finerenone is equally distributed in myocardial and kidney tissue. Finerenone binds to the same ligand domain but to different amino acids, leading to a different expression pattern of cardiac genes compared with spironolactone and eplerenone. Finerenone also reduced cardiac fibrosis and inflammation more than eplerenone in animal experiments at a comparable dose.
In the phase II ARTS trial (Arterial Revascularization Therapies Study) with >450 patients with CKD and congestive HF, finerenone reduced the urinary albumin-creatinine ratio and NT-proBNP (N-terminal pro-BNP) as potently as spironolactone with significantly lower rates of deteriorating kidney function and hyperkalemia.141 Similarly, in phase IIb, with >800 patients with type 2 diabetes, finerenone reduced albumin—creatinine ratio in urine by up to 38% and was well tolerated.142 The incidence of severe adverse events, including a 30% glomerular filtration rate decrease, was similar to placebo. Study cessation because of hyperkalemia was rare. In the phase III trials FIDELIO (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) and FIGARO (Finerenone in Reducing CV Mortality and Morbidity in Diabetic Kidney Disease), >13 000 type-2 diabetic patients with CKD are currently being tested to determine whether finerenone can reduce cardiovascular morbidity and mortality or prevent progression of kidney disease. The completion of the studies is expected in May 2020 (FIDELIO) and July 2021 (FIGARO), respectively.
Conclusions
Patients with CKD have high cardiovascular risk, with cardiovascular death being the leading cause of death. Several novel therapies to decrease the risk of cardiovascular diseases in CKD are in clinical development or have been already established, raising the hope that cardiovascular risk in patients with CKD may be modifiable in the future. Still, the lack of data from large cardiovascular outcome trials in the high-risk group of patients with CKD should be a call for action to ensure that novel therapeutic options are assessed in dedicated trials in the CKD population, in particular in those with advanced CKD, thus paving the way toward a more evidence-based approach to reduce cardiovascular risk in CKD.
Sources of Funding
This work is funded by the Deutsche Forschungsgemeinschaft (German Research Foundation); by the Transregional Collaborative Research Center (TRR 219; Project-ID 322900939) to J.J. (subproject C-03), J.F. (subproject C-01, M-01), D.F. (subproject C-07, Z-01), M.B. (subproject S-02), and N.M. (subproject M-03, M-05); and by a grant of the CORONA-foundation to N.M. and J.J..
Disclosures
Dr Jankowski has given lectures for Bayer and Fresenius Medica Care. In addition, he holds 4 patents on the topic of the article and is the inventor of an additional, already sold patent to Baxter. Dr Floege has received consultancy fees from Amgen, Bayer, Calliditas, Retrophin, Omeros, and Vifor-Fresenius. In addition, he serves on Data Safety Monitoring Boards of NovoNordisk and Visterra. Dr Fliser has given lectures for Amgen, Boehringer Ingelheim, AstraZeneca, and Vifor and has served as an advisor for Amgen, Astellas, Boehringer Ingelheim, AstraZeneca, FMC, and Vifor. Dr Böhm reports fees for lectures and scientific advice from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor outside the submitted work. In addition, Dr Böhm served in trial leadership for Boehringer Ingelheim, AstraZeneca, Medtronic, and ReCor. Dr Marx has given lectures for Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dohm, Bristol Myers Squibb, AstraZeneca, Lilly, and NovoNordisk; has received unrestricted research grants from Boehringer Ingelheim; and has served as an advisor for Bayer, Boehringer Ingelheim, Sanofi-Aventis, MSD, BMS, AstraZeneca, and NovoNordisk. In addition, Dr Marx served in trial leadership for Boehringer Ingelheim and NovoNordisk. Dr Marx declines all personal compensation from pharmaceutical or device companies.
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Contributor Information
Joachim Jankowski, Email: jjankowski@ukaachen.de.
Jürgen Floege, Email: juergen.floege@rwth-aachen.de.
Danilo Fliser, Email: danilo.fliser@uks.eu.
Michael Böhm, Email: Michael.Boehm@uks.eu.
References
- 1.Bright R. Cases and observations, illustrative of renal disease, accompanied with the secretion of albuminous urine. Guy’s Hosp Trans. 1836338–379 [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, Hague N, New J, Farmer CK. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72:92–99. doi: 10.1038/sj.ki.5002273 [DOI] [PubMed] [Google Scholar]
- 3.Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M; Alberta Kidney Disease Network. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;325 suppl 3S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470 [DOI] [PubMed] [Google Scholar]
- 6.Vanholder R, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, et al. Uremic toxicity: present state of the art. Int J Artif Organs. 2001;24:695–725. doi: 10.1177/039139880102401004 [PubMed] [Google Scholar]
- 7.Kuo IY, Chapman AB. Polycystins, ADPKD, and cardiovascular disease. Kidney Int Rep. 2020;5:396–406. doi: 10.1016/j.ekir.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266 [PubMed] [Google Scholar]
- 9.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013 [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.76 [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011). 2013;3:19–62. doi: 10.1038/kisup.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D’Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132 [DOI] [PubMed] [Google Scholar]
- 15.Mulè G, Castiglia A, Cusumano C, Scaduto E, Geraci G, Altieri D, Di Natale E, Cacciatore O, Cerasola G, Cottone S. Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv Exp Med Biol. 2017;956:279–306. doi: 10.1007/5584_2016_85 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 17.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0 [DOI] [PubMed] [Google Scholar]
- 18.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Society of Nephrology. ISN Global Health Atlas. 2019. Accessed February 25, 2021. https://www.theisn.org/initiatives/global-kidney-health-atlas
- 20.United States Renal Data System, Annual Data Report. 2018. Accessed February 25, 2021. https://wwwusrdsorg/2018/view/v2_01aspx
- 21.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 22.Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12:73–81. doi: 10.1038/nrneph.2015.173 [DOI] [PubMed] [Google Scholar]
- 23.Ma I, Guo M, Muruve D, Benediktsson H, Naugler C. Sociodemographic associations with abnormal estimated glomerular filtration rate (eGFR) in a large Canadian city: a cross-sectional observation study. BMC Nephrol. 2018;19:198. doi: 10.1186/s12882-018-0991-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12:1713–1720 [DOI] [PubMed] [Google Scholar]
- 25.Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallappallil M, Friedman EA, Delano BG, McFarlane SI, Salifu MO. Chronic kidney disease in the elderly: evaluation and management. Clin Pract (Lond). 2014;11:525–535. doi: 10.2217/cpr.14.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085 [DOI] [PubMed] [Google Scholar]
- 28.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, et al. ; Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536 [DOI] [PubMed] [Google Scholar]
- 30.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 31.Buglioni A, Burnett JC., Jr. Pathophysiology and the cardiorenal connection in heart failure. Circulating hormones: biomarkers or mediators. Clin Chim Acta. 2015;443:3–8. doi: 10.1016/j.cca.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briet M, Barhoumi T, Mian MO, Sierra C, Boutouyrie P, Davidman M, Bercovitch D, Nessim SJ, Frisch G, Paradis P, et al. Effects of recombinant human erythropoietin on resistance artery endothelial function in stage 4 chronic kidney disease. J Am Heart Assoc. 2013;2:e000128. doi: 10.1161/JAHA.113.000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onal EM, Sag AA, Sal O, Yerlikaya A, Afsar B, Kanbay M. Erythropoietin mediates brain-vascular-kidney crosstalk and may be a treatment target for pulmonary and resistant essential hypertension. Clin Exp Hypertens. 2017;39:197–209. doi: 10.1080/10641963.2016.1246565 [DOI] [PubMed] [Google Scholar]
- 34.Nasrallah R, Hassouneh R, Hébert RL. PGE2, kidney disease, and cardiovascular risk: beyond hypertension and diabetes. J Am Soc Nephrol. 2016;27:666–676. doi: 10.1681/ASN.2015050528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan K, Sethi SK. Biomarkers in cardiorenal syndromes. Transl Res. 2014;164:122–134. doi: 10.1016/j.trsl.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 36.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–1228. doi: 10.1002/cphy.c130040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agharazii M, St-Louis R, Gautier-Bastien A, Ung RV, Mokas S, Larivière R, Richard DE. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am J Hypertens. 2015;28:746–755. doi: 10.1093/ajh/hpu225 [DOI] [PubMed] [Google Scholar]
- 38.Fujii H, Goto S, Fukagawa M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins. 2018;10:202–220. doi: 10.3390/toxins10050202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, Mallamaci F, Massy ZA, Rossignol P, Vanholder R, et al. ; Board of the EURECA-m Working Group of ERA-EDTA. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843. doi: 10.1016/S0140-6736(14)60384-6 [DOI] [PubMed] [Google Scholar]
- 40.Major RW, Cheng MRI, Grant RA, Shantikumar S, Xu G, Oozeerally I, Brunskill NJ, Gray LJ. Cardiovascular disease risk factors in chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2018;13:e0192895. doi: 10.1371/journal.pone.0192895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roehm B, Weiner DE. Blood pressure targets and kidney and cardiovascular disease: same data but discordant guidelines. Curr Opin Nephrol Hypertens. 2019;28:245–250. doi: 10.1097/MNH.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zewinger S, Kleber ME, Rohrer L, Lehmann M, Triem S, Jennings RT, Petrakis I, Dressel A, Lepper PM, Scharnagl H, et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur Heart J. 2017;38:1597–1607. doi: 10.1093/eurheartj/ehx118 [DOI] [PubMed] [Google Scholar]
- 43.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. ; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 44.Ter Braake AD, Shanahan CM, de Baaij JHF. Magnesium counteracts vascular calcification: passive interference or active modulation? Arterioscler Thromb Vasc Biol. 2017;37:1431–1445. doi: 10.1161/ATVBAHA.117.309182 [DOI] [PubMed] [Google Scholar]
- 45.Salem S, Bruck H, Bahlmann FH, Peter M, Passlick-Deetjen J, Kretschmer A, Steppan S, Volsek M, Kribben A, Nierhaus M, et al. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am J Nephrol. 2012;35:31–39. doi: 10.1159/000334742 [DOI] [PubMed] [Google Scholar]
- 46.Brandenburg VM, Reinartz S, Kaesler N, Krüger T, Dirrichs T, Kramann R, Peeters F, Floege J, Keszei A, Marx N, et al. Slower progress of aortic valve calcification with vitamin K supplementation: results from a prospective interventional proof-of-concept study. Circulation. 2017;135:2081–2083. doi: 10.1161/CIRCULATIONAHA.116.027011 [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71:2405–2414. doi: 10.1016/j.jacc.2018.03.490 [DOI] [PubMed] [Google Scholar]
- 48.KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. 2016. Accessed February 25, 2021. https://kdigo.org/wp-con-tent/uploads/2016/10/KDIGO-2012-Blood-Pressure-Guideline-English.pdf
- 49.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet, 2017. 2017. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention [Google Scholar]
- 51.Matsushita K, Kwak L, Ballew SH, Grams ME, Selvin E, Folsom AR, Coresh J, Tang W. Chronic kidney disease measures and the risk of abdominal aortic aneurysm. Atherosclerosis. 2018;279:107–113. doi: 10.1016/j.atherosclerosis.2018.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. doi: 10.1016/j.jacc.2007.03.037 [DOI] [PubMed] [Google Scholar]
- 53.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:844–846. doi: 10.1161/CIRCULATIONAHA.110.960138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. ; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 55.Maini R, Wong DB, Addison D, Chiang E, Weisbord SD, Jneid H. Persistent underrepresentation of kidney disease in randomized, controlled trials of cardiovascular disease in the contemporary era. J Am Soc Nephrol. 2018;29:2782–2786. doi: 10.1681/ASN.2018070674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shroff R, Speer T, Colin S, Charakida M, Zewinger S, Staels B, Chinetti-Gbaguidi G, Hettrich I, Rohrer L, O’Neill F, et al. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol. 2014;25:2658–2668. doi: 10.1681/ASN.2013111212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunet P, Gondouin B, Duval-Sabatier A, Dou L, Cerini C, Dignat-George F, Jourde-Chiche N, Argiles A, Burtey S. Does uremia cause vascular dysfunction? Kidney Blood Press Res. 2011;34:284–290. doi: 10.1159/000327131 [DOI] [PubMed] [Google Scholar]
- 58.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003 [DOI] [PubMed] [Google Scholar]
- 59.O’Neill WC, Lomashvili KA. Recent progress in the treatment of vascular calcification. Kidney Int. 2010;78:1232–1239. doi: 10.1038/ki.2010.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guérin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–1021. doi: 10.1093/ndt/15.7.1014 [DOI] [PubMed] [Google Scholar]
- 61.London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J. 1999;1383 pt 2220–224. doi: 10.1016/s0002-8703(99)70313-3 [DOI] [PubMed] [Google Scholar]
- 62.Gauthier-Bastien A, Ung RV, Larivière R, Mac-Way F, Lebel M, Agharazii M. Vascular remodeling and media calcification increases arterial stiffness in chronic kidney disease. Clin Exp Hypertens. 2014;36:173–180. doi: 10.3109/10641963.2013.804541 [DOI] [PubMed] [Google Scholar]
- 63.Shetty M, Chowdhury Y, Yegneswaran B. Calcific uremic arteriolopathy. Cleve Clin J Med. 2018;85:584–585. doi: 10.3949/ccjm.85a.18009 [DOI] [PubMed] [Google Scholar]
- 64.Brandenburg VM, Evenepoel P, Floege J, Goldsmith D, Kramann R, Massy Z, Mazzaferro S, Schurgers LJ, Sinha S, Torregrosa V, et al. ; ERA-EDTA Working Group on CKD-MBD and EUCALNET. Lack of evidence does not justify neglect: how can we address unmet medical needs in calciphylaxis? Nephrol Dial Transplant. 2016;31:1211–1219. doi: 10.1093/ndt/gfw025 [DOI] [PubMed] [Google Scholar]
- 65.Shaker JL, Deftos L. Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossman A, Hershman JM, Hofland J, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Purnell J, Singer F, Stratakis CA, Trence DL, Wilson DP, eds. Calcium and Phosphate Homeostasis. 2018 Jan 19. In: Endotext [Internet]. 2000–. South Dartmouth (MA): MDText.com, Inc. [Google Scholar]
- 66.Ellis CL, O’Neill WC. Questionable specificity of histologic findings in calcific uremic arteriolopathy. Kidney Int. 2018;94:390–395. doi: 10.1016/j.kint.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 67.Shobeiri N, Pang J, Adams MA, Holden RM. Cardiovascular disease in an adenine-induced model of chronic kidney disease: the temporal link between vascular calcification and haemodynamic consequences. J Hypertens. 2013;31:160–168. doi: 10.1097/HJH.0b013e32835b15bb [DOI] [PubMed] [Google Scholar]
- 68.Raggi P. Cardiovascular disease: coronary artery calcification predicts risk of CVD in patients with CKD. Nat Rev Nephrol. 2017;13:324–326. doi: 10.1038/nrneph.2017.61 [DOI] [PubMed] [Google Scholar]
- 69.De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, De Surgeloose D, Van Hoenacker P, Van Vlem B, Verbeke F. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie Study. J Am Soc Nephrol. 2020;31:186–196. doi: 10.1681/ASN.2019060579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Floege J. Magnesium in CKD: more than a calcification inhibitor? J Nephrol. 2015;28:269–277. doi: 10.1007/s40620-014-0140-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dhondup T, Qian Q. Electrolyte and acid-base disorders in chronic kidney disease and end-stage kidney failure. Blood Purif. 2017;43:179–188. doi: 10.1159/000452725 [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi Y, Hamano T, Obi Y, Monden C, Oka T, Yamaguchi S, Matsui I, Hashimoto N, Matsumoto A, Shimada K, et al. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol. 2019;30:1073–1085. doi: 10.1681/ASN.2018111150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, et al. ; European Renal and Cardiovascular Medicine (EURECA-m) Working Group of the European Renal Association – European Dialysis Transplantation Association (ERA-EDTA). The systemic nature of CKD. Nat Rev Nephrol. 2017;13:344–358. doi: 10.1038/nrneph.2017.52 [DOI] [PubMed] [Google Scholar]
- 74.Amdur RL, Feldman HI, Dominic EA, Anderson AH, Beddhu S, Rahman M, Wolf M, Reilly M, Ojo A, Townsend RR, et al. ; CRIC Study Investigators. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC Study. Am J Kidney Dis. 2019;73:344–353. doi: 10.1053/j.ajkd.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kielstein JT, Veldink H, Martens-Lobenhoffer J, Haller H, Perthel R, Lovric S, Lichtinghagen R, Kliem V, Bode-Böger SM. Unilateral nephrectomy causes an abrupt increase in inflammatory mediators and a simultaneous decrease in plasma ADMA: a study in living kidney donors. Am J Physiol Renal Physiol. 2011;301:F1042–F1046. doi: 10.1152/ajprenal.00640.2010 [DOI] [PubMed] [Google Scholar]
- 76.Alhaj E, Alhaj N, Rahman I, Niazi TO, Berkowitz R, Klapholz M. Uremic cardiomyopathy: an underdiagnosed disease. Congest Heart Fail. 2013;19:E40–E45. doi: 10.1111/chf.12030 [DOI] [PubMed] [Google Scholar]
- 77.Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 20094 suppl 1S79–S91. doi: 10.2215/CJN.04860709 [DOI] [PubMed] [Google Scholar]
- 78.Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med. 2015;5:254–266. doi: 10.1159/000435838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Little WC. Heart failure with a normal left ventricular ejection fraction: diastolic heart failure. Trans Am Clin Climatol Assoc. 2008;119:93–99. discussion 99 [PMC free article] [PubMed] [Google Scholar]
- 80.Segall L, Nistor I, Covic A. Heart failure in patients with chronic kidney disease: a systematic integrative review. Biomed Res Int. 2014;2014:937398. doi: 10.1155/2014/937398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 82.Wang Z, Jiang A, Wei F, Chen H. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: a meta-analysis. BMC Cardiovasc Disord. 2018;18:12. doi: 10.1186/s12872-018-0747-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abd Alamir M, Radulescu V, Goyfman M, Mohler ER, III, Gao YL, Budoff MJ; CRIC Study Investigators. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: results from CRIC study. Atherosclerosis. 2015;242:117–122. doi: 10.1016/j.atherosclerosis.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rong S, Qiu X, Jin X, Shang M, Huang Y, Tang Z, Yuan W. Risk factors for heart valve calcification in chronic kidney disease. Medicine (Baltimore). 2018;97:e9804. doi: 10.1097/MD.0000000000009804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urena-Torres P, D’Marco L, Raggi P, Garcia-Moll X, Brandenburg V, Mazzaferro S, Lieber A, Guirado L, Bover J. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant. 2019;1:1–8 [DOI] [PubMed] [Google Scholar]
- 86.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 87.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, et al. ; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, et al. ; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545 [DOI] [PubMed] [Google Scholar]
- 90.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-RiskaAURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177 [DOI] [PubMed] [Google Scholar]
- 91.Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157:263–275. doi: 10.7326/0003-4819-157-4-201208210-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, Copetti M, Graziano G, Tognoni G, Jardine M, et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;156:445–459. doi: 10.7326/0003-4819-156-6-201203200-00007 [DOI] [PubMed] [Google Scholar]
- 93.Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, et al. ; ISCHEMIA-CKD Research Group. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382:1608–1618. doi: 10.1056/NEJMoa1915925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spertus JA, Jones PG, Maron DJ, Mark DB, O’Brien SM, Fleg JL, Reynolds HR, Stone GW, Sidhu MS, Chaitman BR, et al. ; ISCHEMIA-CKD Research Group. Health status after invasive or conservative care in coronary and advanced kidney disease. N Engl J Med. 2020;382:1619–1628. doi: 10.1056/NEJMoa1916374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Washam JB, Herzog CA, Beitelshees AL, Cohen MG, Henry TD, Kapur NK, Mega JL, Menon V, Page RL, II, Newby LK; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, Council on Functional Genomics and Translational Biology, Council on the Kidney in Cardiovascular Disease, and Council on Quality of Care and Outcomes Research. Pharmacotherapy in chronic kidney disease patients presenting with acute coronary syndrome: a scientific statement from the American Heart Association. Circulation. 2015;131:1123–1149. doi: 10.1161/CIR.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 96.Edner M, Benson L, Dahlström U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: a prospective propensity score-matched cohort study. Eur Heart J. 2015;36:2318–2326. doi: 10.1093/eurheartj/ehv268 [DOI] [PubMed] [Google Scholar]
- 97.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101 [DOI] [PubMed] [Google Scholar]
- 98.Packer M, Poole-Wilson PA, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Rydén L, Thygesen K, Uretsky BF. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation. 1999;100:2312–2318. doi: 10.1161/01.cir.100.23.2312 [DOI] [PubMed] [Google Scholar]
- 99.Packer M, Miller AB. Can physicians always explain the results of clinical trials? A case study of amlodipine in heart failure. Am J Cardiol. 1999;84:1L–2L. doi: 10.1016/s0002-9149(99)00358-6 [DOI] [PubMed] [Google Scholar]
- 100.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161. doi: 10.1016/j.jacc.2011.04.041 [DOI] [PubMed] [Google Scholar]
- 101.Kotecha D, Gill SK, Flather MD, Holmes J, Packer M, Rosano G, Böhm M, McMurray JJV, Wikstrand J, Anker SD, et al. ; Beta-Blockers in Heart Failure Collaborative Group. Impact of renal impairment on beta-blocker efficacy in patients with heart failure. J Am Coll Cardiol. 2019;74:2893–2904. doi: 10.1016/j.jacc.2019.09.059 [DOI] [PubMed] [Google Scholar]
- 102.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa100949221073363 [Google Scholar]
- 103.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, Yakushigawa T, Sugiyama H, Shimada Y, Nojima Y, Shio N. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63:528–536. doi: 10.1016/j.jacc.2013.09.056 [DOI] [PubMed] [Google Scholar]
- 104.Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, Anderson AH, Hung AM, Mehrotra R, Sharma S, et al. ; Hemodialysis Novel Therapies Consortium. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int. 2019;95:973–982. doi: 10.1016/j.kint.2018.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hammer F, Malzahn U, Donhauser J, Betz C, Schneider MP, Grupp C, Pollak N, Störk S, Wanner C, Krane V; MiREnDa Study Group. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney Int. 2019;95:983–991. doi: 10.1016/j.kint.2018.11.025 [DOI] [PubMed] [Google Scholar]
- 106.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. ; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 107.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1 [DOI] [PubMed] [Google Scholar]
- 108.Voors AA, van Veldhuisen DJ, Robertson M, Ford I, Borer JS, Böhm M, Komajda M, Swedberg K, Tavazzi L; SHIFT investigators. The effect of heart rate reduction with ivabradine on renal function in patients with chronic heart failure: an analysis from SHIFT. Eur J Heart Fail. 2014;16:426–434. doi: 10.1002/ejhf.59 [DOI] [PubMed] [Google Scholar]
- 109.Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, Massy ZA, Mallamaci F, Valdivielso JM, Malyszko J, et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol. 2018;14:727–749. doi: 10.1038/s41581-018-0072-9 [DOI] [PubMed] [Google Scholar]
- 110.Genovesi S, Boriani G, Covic A, Vernooij RWM, Combe C, Burlacu A, Davenport A, Kanbay M, Kirmizis D, Schneditz D, et al. Sudden cardiac death in dialysis patients: different causes and management strategies. Nephrol Dial Transplant. 2019;1:1–10. doi: 10.1093/ndt/gfz182 [DOI] [PubMed] [Google Scholar]
- 111.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7 [DOI] [PubMed] [Google Scholar]
- 112.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Green D, Roberts PR, New DI, Kalra PA. Sudden cardiac death in hemodialysis patients: an in-depth review. Am J Kidney Dis. 2011;57:921–929. doi: 10.1053/j.ajkd.2011.02.376 [DOI] [PubMed] [Google Scholar]
- 114.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69:2268–2273. doi: 10.1038/sj.ki.5000446 [DOI] [PubMed] [Google Scholar]
- 115.Cheema A, Singh T, Kanwar M, Chilukuri K, Maria V, Saleem F, Johnson K, Frank J, Pires L, Hassan S. Chronic kidney disease and mortality in implantable cardioverter-defibrillator recipients. Cardiol Res Pract. 2010;2010:989261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x [DOI] [PubMed] [Google Scholar]
- 117.Perl J, Chan CT. Timing of sudden death relative to the hemodialysis procedure. Nat Clin Pract Nephrol. 2006;2:668–669. doi: 10.1038/ncpneph0345 [DOI] [PubMed] [Google Scholar]
- 118.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ; DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489 [DOI] [PubMed] [Google Scholar]
- 119.Korantzopoulos P, Liu T, Li L, Goudevenos JA, Li G. Implantable cardioverter defibrillator therapy in chronic kidney disease: a meta-analysis. Europace. 2009;11:1469–1475. doi: 10.1093/europace/eup282 [DOI] [PubMed] [Google Scholar]
- 120.Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients in the United States with prosthetic heart valves: should ACC/AHA practice guidelines on valve selection be modified? Circulation. 2002;105:1336–1341. doi: 10.1161/hc1102.100075 [DOI] [PubMed] [Google Scholar]
- 121.Fernando M, Paterson HS, Byth K, Robinson BM, Wolfenden H, Gracey D, Harris D. Outcomes of cardiac surgery in chronic kidney disease. J Thorac Cardiovasc Surg. 2014;148:2167–2173. doi: 10.1016/j.jtcvs.2013.12.064 [DOI] [PubMed] [Google Scholar]
- 122.Siontis GCM, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F, Pilgrim T, Petrinic T, Nikolakopoulou A, Salanti G, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J. 2019;40:3143–3153. doi: 10.1093/eurheartj/ehz275 [DOI] [PubMed] [Google Scholar]
- 123.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. ; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 124.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 125.Patel KK, Shah SY, Arrigain S, Jolly S, Schold JD, Navaneethan SD, Griffin BP, Nally JV, Desai MY. Characteristics and outcomes of patients with aortic stenosis and chronic kidney disease. J Am Heart Assoc. 2019;8:e009980. doi: 10.1161/JAHA.118.009980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Makki N, Lilly SM. Advanced chronic kidney disease: relationship to outcomes post-TAVR, a meta-analysis. Clin Cardiol. 2018;41:1091–1096. doi: 10.1002/clc.22993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Doshi R, Shlofmitz E, Shah J, Meraj P. Comparison of transcatheter mitral valve repair versus surgical mitral valve repair in patients with advanced kidney disease (from the National Inpatient Sample). Am J Cardiol. 2018;121:762–767. doi: 10.1016/j.amjcard.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 128.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. ; COAPT Investigators. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
- 129.Taramasso M, Pozzoli A, Guidotti A, Nietlispach F, Inderbitzin DT, Benussi S, Alfieri O, Maisano F. Percutaneous tricuspid valve therapies: the new frontier. Eur Heart J. 2017;38:639–647. doi: 10.1093/eurheartj/ehv766 [DOI] [PubMed] [Google Scholar]
- 130.Shah B, Vemulapalli S, Manandhar P, Amoroso N, Ruiz-Maya T, Staniloae C, Saric M, Williams M. Comparison after transcatheter mitral valve repair in patients with chronic kidney disease: an analysis of 5,241 patients in the United States. J Am Coll Cardiol. 2018;71:A1980 [Google Scholar]
- 131.Carubelli V, Metra M, Lund LH. Negotiating renal dysfunction when treating patients with heart failure. Expert Rev Cardiovasc Ther. 2018;16:113–122. doi: 10.1080/14779072.2018.1422178 [DOI] [PubMed] [Google Scholar]
- 132.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 133.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 134.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 135.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 136.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. ; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 137.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 138.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 139.Lan NSR, Fegan PG, Yeap BB, Dwivedi G. The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Fail. 2019;6:927–935. doi: 10.1002/ehf2.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7 [DOI] [PubMed] [Google Scholar]
- 141.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, et al. ; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884–894. doi: 10.1001/jama.2015.10081 [DOI] [PubMed] [Google Scholar]


