Abstract
BACKGROUND
Trauma-induced coagulopathy (TIC) substantially contributes to mortality in bleeding trauma patients.
OBJECTIVE
The aim of the study was to administer fibrinogen concentrate in the prehospital setting to improve blood clot stability in trauma patients bleeding or presumed to bleed.
DESIGN
A prospective, randomised, placebo-controlled, double-blinded, international clinical trial.
SETTING
This emergency care trial was conducted in 12 Helicopter Emergency Medical Services (HEMS) and Emergency Doctors’ vehicles (NEF or NAW) and four trauma centres in Austria, Germany and Czech Republic between 2011 and 2015.
PATIENTS
A total of 53 evaluable trauma patients aged at least 18 years with major bleeding and in need of volume therapy were included, of whom 28 received fibrinogen concentrate and 25 received placebo.
INTERVENTIONS
Patients were allocated to receive either fibrinogen concentrate or placebo prehospital at the scene or during transportation to the study centre.
MAIN OUTCOME MEASURES
Primary outcome was the assessment of clot stability as reflected by maximum clot firmness in the FIBTEM assay (FIBTEM MCF) before and after administration of the study drug.
RESULTS
Median FIBTEM MCF decreased in the placebo group between baseline (before administration of study treatment) and admission to the Emergency Department, from a median of 12.5 [IQR 10.5 to 14] mm to 11 [9.5 to 13] mm (P = 0.0226), but increased in the FC Group from 13 [11 to 15] mm to 15 [13.5 to 17] mm (P = 0.0062). The median between-group difference in the change in FIBTEM MCF was 5 [3 to 7] mm (P < 0.0001). Median fibrinogen plasma concentrations in the fibrinogen concentrate Group were kept above the recommended critical threshold of 2.0 g l−1 throughout the observation period.
CONCLUSION
Early fibrinogen concentrate administration is feasible in the complex and time-sensitive environment of prehospital trauma care. It protects against early fibrinogen depletion, and promotes rapid blood clot initiation and clot stability.
TRIAL REGISTRY NUMBERS
EudraCT: 2010-022923-31 and ClinicalTrials.gov: NCT01475344.
Introduction
The presence of trauma-induced coagulopathy (TIC) in severely injured and bleeding patients on admission to the emergency department (ED) approximately doubles the mortality rate.1 Therefore, early and effective coagulation therapy is paramount to improve survival.
Fibrinogen plays a key role in haemostasis by augmenting initial platelet aggregation, limiting thrombin overload and providing the substrate for the clotting process that ensures mechanical stability of blood clots. Fibrinogen has been identified as the first coagulation factor to reach critically low levels during active bleeding.2 Hypofibrinogenaemia on admission to ED has been identified as an independent predictor of massive transfusion, morbidity and mortality in major trauma patients.3–6 Accordingly, low fibrin-based thromboelastometry (FIBTEM) amplitudes have also been shown to identify the presence of TIC and to predict massive transfusions in bleeding trauma patients.7,8
There is evidence to support early fibrinogen supplementation in trauma patients, although robust data of clinical benefits appear to be lacking.9–13 In one randomised controlled trial, early administration of fibrinogen concentrate as part of a coagulation factor concentrate-based treatment strategy decreased the transfusion of allogeneic blood products and decreased morbidity compared with standard therapy with fresh frozen plasma (FFP).9 Very early, that is prehospital, administration of fibrinogen concentrate in bleeding trauma patients has not yet been investigated. This may be attributable to the highly dynamic and complex environment of acute prehospital trauma care. We conducted a randomised, placebo-controlled, pilot trial to evaluate the feasibility of prehospital fibrinogen concentrate administration in trauma patients either bleeding or presumed to bleed.14 We hypothesised that this measure would protect against early hypofibrinogenaemia while maintaining fibrin polymerisation and clot stability. The primary objective was to assess whether early prehospital administration of fibrinogen concentrate improves the strength of fibrin-based clotting on arrival at the ED in order to avoid early TIC.
Materials and methods
The Fibrinogen in Trauma-Induced Coagulopathy (FIinTIC) trial was a prospective, international, randomised, double-blind, placebo-controlled, two-arm, parallel-group study conducted in severely injured and bleeding patients or patients presumed to bleed. The trial was performed to investigate whether early prehospital administration of fibrinogen concentrate is feasible and would promote enhanced coagulation as measured by maximum clot firmness (MCF) in the functional fibrin polymerisation (FIBTEM) assay (FIBTEM MCF) using ROTEM delta devices (Instrumentation Laboratory, Bedford, Massachusetts, USA) in all centres.
Severely injured patients aged at least 18 years with major bleeding and need for volume replacement therapy were screened and enrolled by experienced emergency physicians at the scene of trauma. Admission to one of the study trauma centres (listed in Supplemental Digital Content 2) was required to ensure adherence to the study protocol. Key exclusion criteria were isolated penetrating trauma, isolated head injury, severe haemodynamic instability refractory to therapy (vasopressor or volume), inevitable lethal course, need for cardiopulmonary resuscitation at the scene or profound hypothermia (<30°C). The full list of inclusion and exclusion criteria is given Supplemental Digital Content 3.
The study protocol was approved by the national competent authorities as well as the institutional review boards of the corresponding countries. In Austria, the Ethical Committee of the Medical University of Innsbruck provided ethical approval on 6 July 2011 (UN4310_LEK), in Germany, the Ethical Committee of the University Witten/Herdecke on 7 May 2013 (F-120/2012) and in the Czech Republic the Ethical Committee of the Regional Hospital in Liberec on 22 April 2015 (EK/78/2015).
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The trial was registered at EudraCT, number 2010-022923-31, on 3 May 2011 and at ClinicalTrials.gov, number NCT01475344, on 21 November 2011 (FIinTIC-Trial).
The randomisation schedule was stratified by site using a 1 : 1 allocation ratio. The randomisation code was generated using the random permuted blocks method with varying block size by the Department of Medical Statistics, Informatics and Health Economics, Medical University of Innsbruck. Randomisation numbers were printed onto labels, which were attached to study medication containers by the clinical pharmacy of St. Johann Hospital (Salzburg, Austria) according to Good Manufacturing Practice regulations. All study personnel involved in treating the patients, and all patients, were blinded to treatment assignment.
Study medication and procedures
Both fibrinogen concentrate (Clottafact) and placebo were manufactured by LFB Biomedicaments (Lille, France). The placebo was also designed to have the same physical form (powder) as fibrinogen concentrate. The study drug dose was 1.5 g per 30 kg of estimated bodyweight to achieve the recommended dosage of 50 mg kg−1.15 To enable the administration of the recommended dosage, the patients were stratified by the emergency physician into different body weight groups (30-kg steps). A dose of 3 g of the study drug was given in patients with bodyweight 30 to 60 kg, 4.5 g in patients with bodyweight 60 to 90 kg and 6 g in patients with bodyweight 90 to 120 kg. For all patients weighing 120 kg or more, the dose was still 6 g because blood volume increases only slightly with increasing obesity.16 Each 1.5 g of powder was reconstituted in 100 ml of solvent. The study drug was administered via a single intravenous infusion at a rate of 20 ml min−1, either at the scene of trauma or during transportation to the participating hospital.
Blood samples were taken at baseline (at the scene, prior to study drug administration (timepoint 1, T1), and on arrival at the ED (T2). If study medication was still being administered on admission to the ED, the T2 sample was taken 15 min after completion of study drug administration. Patients’ treatment after T2 was determined by standard clinical practice at the hospital to which the patient had been admitted. Blood samples could also be taken 3, 9, 24, 48 h and 7 days after ED admission (T3 to T7). These samples enabled repeated assessment of coagulation parameters. In addition, on days 5 to 9 after study drug administration, ultrasonograph examination of the lower-extremity veins was conducted for detection of venous thromboembolism not identified by routine computed tomography scans or other routine clinical examinations. Other than study drug administration, patient care was not influenced by the study protocol.
Outcome measures
The primary study endpoint was the change in FIBTEM MCF on arrival at the ED vs. baseline (T2–T1). The main clinical secondary endpoints were the need for volume and requirements for transfusions within 7 days after study inclusion. ROTEM-assessed secondary endpoints were derived from the extrinsically activated EXTEM assay, the intrinsically activated INTEM assay and the aprotinin-containing APTEM assay: clotting time (CT, time taken for a clot amplitude of 2 mm to be achieved), clot formation time (CFT, time taken for clot amplitude to increase from 2 to 20 mm) and MCF and Lysis Index at 60 min (LI60). Activated partial thromboplastin time (aPTT), prothrombin, fibrinogen concentration (Clauss method), concentrations of coagulation factor XIII (FXIII) and antithrombin, and concentration and activity of von Willebrand factor (vWF) served as additional secondary endpoints. Safety was assessed by recording the number of thromboembolic complications occurring within one week after study drug administration.
Statistical analysis
In a previous study, patients receiving fibrinogen concentrate or placebo after 30% haemodilution with hydroxyethyl starch exhibited mean ± SD FIBTEM MCF changes of 3 ± 0.6 and -1 ± 0.4 mm, respectively.17 Assuming the change in FIBTEM MCF would remain normally distributed and that the standard deviation would be higher in trauma (1 mm), a sample size of 17 per group was calculated to detect a between-group difference in mean FIBTEM MCF change of 1 mm with a power of 80% and a 5% significance level. To allow for drop-outs, we planned to randomise at least 30 patients per group. All analyses were conducted in the modified intention-to-treat (ITT) population, that is all patients receiving fibrinogen concentrate or placebo in whom posttreatment assessment of FIBTEM MCF was available.
Initial analysis of the FIBTEM MCF results (Shapiro--Wilk normality test) showed that the data were not normally distributed. A revised sample size calculation, based on the observed changes in FIBTEM MCF, implied that 20 patients per group would be sufficient to detect a 1 mm difference in FIBTEM MCF change with a power of 80%. The Wilcoxon rank sum test was used to assess between-group and within-group differences in FIBTEM MCF.
Statistical analyses were performed using R, version 3.4.1 (R Foundation, Vienna, Austria). All comparisons were two-sided, and a significance level of 5% was used. Most of the continuous secondary variables were not normally distributed. Therefore, the Wilcoxon rank sum test and Fisher's exact test were applied, and the results are presented as median [IQR]. Categorical variables are presented as frequencies (%).
To analyse the evolution of FIBTEM MCF over all seven measurement timepoints, a linear mixed-effects regression model was applied, with FIBTEM MCF as the dependent variable, study group as the fixed effect and random intercepts for patients as well as measurement timepoints. No interim analysis was planned during the study period.
Results
Between September 2011 and October 2015, a total of 67 patients were enrolled into the study. Of these, 37 received FC and 30 received placebo as defined in the protocol. After randomisation, four patients in the fibrinogen concentrate group and three patients in the placebo group were withdrawn from the study due to previously undetected fulfilment of exclusion criteria. A further seven patients (five from the fibrinogen concentrate arm and two from the placebo arm) were excluded due to missing data for the primary endpoint. Thus, the modified ITT population comprised 28 patients in the fibrinogen concentrate arm and 25 patients in the placebo arm (Fig. 1). The trial ended once the necessary number of patients was reached.
Fig. 1.
Patient disposition
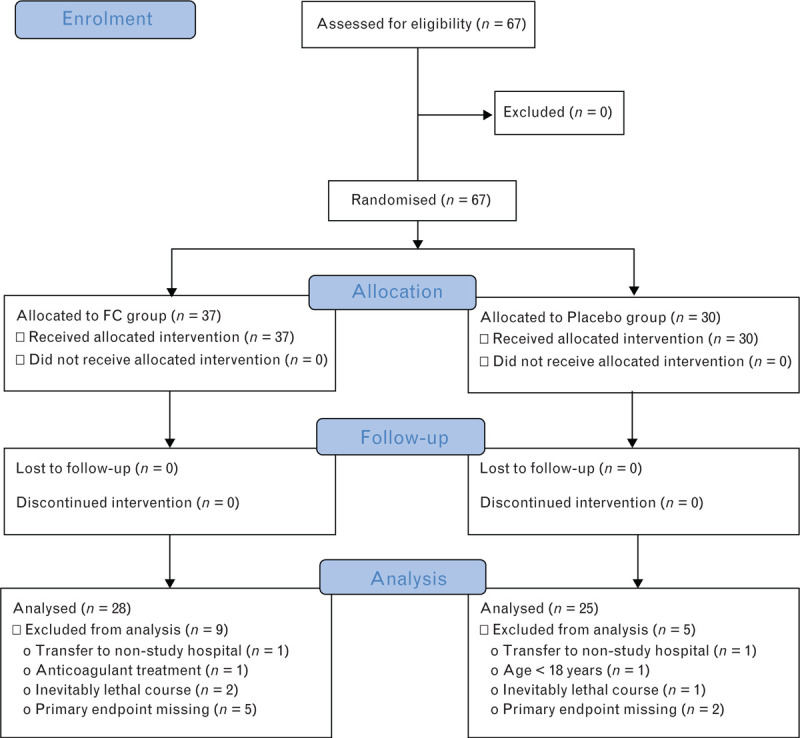
FC, fibrinogen concentrate.
The characteristics of the study patients and standard laboratory measurements at baseline are summarised in Table 1, as well as the results from the baseline functional viscoelastic testing assays. The modified ITT population had a median age of 49 years, was predominately male (83%) and had a median injury severity score (ISS) of 17 points. Almost one-third of the population exhibited concomitant traumatic brain injury. The median EXTEM CT was significantly longer in the fibrinogen concentrate group at baseline (62 vs. 50 s), and there was a trend towards higher ISS in the fibrinogen concentrate group (median 25 vs. 16 points). There was a significant difference in the prehospital administration of tranexamic acid (TXA). In the patients treated with fibrinogen concentrate, 10.7% received TXA compared with 36% in the placebo group (P = 0.0471). All other baseline characteristics were comparable in the two study arms.
Table 1.
Baseline characteristics of study population
| Placebo (n = 25) | FC (n = 28) | Estimated difference (95% CI) | P | |
| Age (years) | 54 [37 to 56] | 46 [34.5 to 58] | 4 (−6 to 13) | 0.4868 |
| Female | 4/25 (16.0%) | 5/28 (17.9%) | 0.88 (0.15 to 4.70) | 1 |
| Male | 21/25 (84.0%) | 23/28 (82.1%) | 0.88 (0.15 to 4.70) | 1 |
| BMI (kg m−2) | 26.3 [24.7 to 28.09] | 25.0 [23.4 to 27.5] | 1.28 (−0.56 to 2.94) | 0.1538 |
| ISS | 16 [16 to 34] | 25 [16 to 36] | −3 (−16 to 4) | 0.3937 |
| GCS | 15 [13 to 15] | 14.5 [6 to 15] | 0 (0 to 3) | 0.574 |
| Craniocerebral injury | 5/25 (20.0%) | 11/28 (39.3%) | 2.54 (0.65 to 11.31) | 0.1474 |
| Intubation at the scene | 5/25 (20.0%) | 11/28 (39.3%) | 2.54 (0.65 to 11.31) | 0.1474 |
| Prehospital TXA | 9/25 (36%) | 3/28 (10.7%) | 0.22 (0.03 to 1.05) | 0.0471 |
| Vital signs | ||||
| SBP (mmHg) | 90 [85 to 120] | 100 [80 to 112] | 0 (−15 to 12) | 0.9501 |
| Heart rate (beats min−1) | 100 [90 to 120] | 110 [99 to 119.5] | 0 (−15 to 10) | 0.6186 |
| Laboratory measurements | ||||
| pH | 7.34 [7.28 to 7.37] | 7.29 [7.26 to 7.36] | 0.03 (−0.02 to 0.08) | 0.2702 |
| BD (mmol l−1) | 2.5 [−0.3 to 4.2] | 3.0 [1.8 to 5.5] | −1.09 (−3.2 to 0.9) | 0.3064 |
| Lactate (mmol l−1) | 0.97 [0.72 to 1.60] | 1.28 [0.72 to 2.33] | −0.19 (−1.22 to 0.39) | 0.5453 |
| Haemoglobin (g l−1) | 137 [127 to 152] | 141 [130 to 147] | 0 (−10 to 10) | 0.9923 |
| PT quick, % | 90 [79 to 97] | 89 [68 to 96.25] | 3 (−6 to 17) | 0.4711 |
| aPTT (s) | 27 [24 to 29] | 26 [25 to 30] | −1 (−3.0 to 1.4) | 0.4496 |
| Fibrinogen (g l−1) | 235 [208 to 259] | 248 [184.75 to 272] | −6 (−38 to 35) | 0.6854 |
| Antithrombin, % | 86 [78 to 93] | 83 [73 to 94] | 4 (−3 to 11) | 0.2898 |
| FXIII, % | 118 [98 to 130] | 114 [98.25 to 130] | 0 (−11 to 16) | 0.9312 |
| vWF antigen, % | 245 [201 to 298] | 242 [194.25 to 301] | 0 (−32 to 35) | 0.8 |
| vWF ristocetin cofactor, % | 269 [215 to 301] | 259 [203.75 to 301] | 0 (−17 to 38) | 0.9255 |
| Platelet count (x109 l−1) | 239 [191 to 270] | 242 [209 to 271] | −13 (−47 to 17) | 0.3416 |
| ROTEM | ||||
| FIBMCF (mm) | 13 [10 to 15] | 13 [9 to 16.5] | 0 (−3 to 2) | 0.7817 |
| EXTEM CT (s) | 50 [46 to 61] | 62 [52 to 68.5] | −9 (−16 to −1) | 0.031 |
| EXTEM CFT (s) | 104 [90 to 118] | 96.5 [78.25 to 128.25] | 8 (−12 to 23) | 0.3449 |
| EXTEM MCF (mm) | 58 [56 to 62] | 58.5 [53 to 64.25] | 0 (−4 to 4) | 0.9218 |
| EXTEM L60, % | 94 [91.75 to 98] | 93 [91 to 96] | 1 (−2 to 3) | 0.6838 |
| INTEM CT (s) | 136 [129 to 149] | 141.5 (128.5 to 150.25] | −3.07 (−14 to 8) | 0.6558 |
| INTEM CFT (s) | 82 [67 to 96] | 86 [64.75 to 114.5] | −2.34 (−20 to 14) | 0.7148 |
| INTEM L60, % | 92 [91 to 96.25] | 94 [92 to 96] | −1 (−3 to 1) | 0.506 |
| INTEM MCF (mm) | 58 [55 to 61] | 56 [51 to 62] | 1 (−3 to 5) | 0.6169 |
| APTEM CT (s) | 56 [48.5 to 71.5] | 60 [53.5 to 72] | −4 (−12 to 5) | 0.422 |
| APTEM CFT (s) | 108.5 [95.25 to 125.5] | 96 [84 to 129.5] | 10 (−11 to 23) | 0.2654 |
| APTEM MCF (mm) | 58 [55 to 61.25] | 58 [54 to 64] | 0 (−4 to 3) | 0.8351 |
| APTEM L60, % | 93.5 [92.75 to 98] | 93 [92 to 96] | 1 (−1 to 3) | 0.4325 |
Values in Placebo and FC groups are median [IQR] or number (%).
aPTT, activated partial thromboplastin time; EP, emergency personnel; ER, emergency room; FC, fibrinogen concentrate; FXIII, factor XIII; GCS, Glasgow coma scale; ISS, injury severity score; TXA, tranexamic acid; vWF, von Willebrand factor.
The median time which elapsed between arrival of the emergency team at the trauma scene and arrival of the patient at the ED was 44 min (Table 2). During prehospital treatment, patients in the fibrinogen concentrate and placebo groups received median crystalloid volumes of 500 and 1000 ml respectively, and the corresponding colloid volumes were 750 and 500 ml (Table 3). The between-group differences in these volumes were not significant.
Table 2.
Operation times
| Placebo (n = 25) | FC (n = 28) | Estimated difference (95% CI) | P | |
| Time from accident until arrival of the emergency physician (min) | 19.5 [15 to 35.75] | 19 [14.5 to 26] | 1 (−5 to 9) | 0.6576 |
| Time from accident until start of infusion (min) | 45 [32.5 to 66] | 50 [33.5 to 61.5] | 0 (−11 to 13) | 1 |
| Time from arrival of the emergency physician until start of infusion (min) | 20 [10.5 to 34] | 24.5 [15 to 35] | −5 (−13 to 5) | 0.3434 |
| Time from accident until arrival at the emergency department (min) | 76 [57.75 to 101.75] | 73 [55 to 89.5] | 4 (−10 to 20) | 0.4673 |
| Time from start of infusion until arrival at the emergency department (min) | 26 [22 to 35] | 24 [15 to 32.75] | 3 (−4 to 10) | 0.387 |
Values in Placebo and FC groups are median [IQR]. FC, Fibrinogen concentrate.
Table 3.
Volume therapy, transfusion requirements and need for factor concentrate administration
| Placebo (n = 25) | FC (n = 28) | Estimated difference or odds ratio (95% CI) | P | |
| Until arrival to the Emergency department | ||||
| Crystalloids | ||||
| Patients | 23/25 (92.0%) | 26/28 (92.9%) | 1.13 (0.08 to 16.72) | 1 |
| Volume (ml) | 1000 [500 to 1125] | 500 [500 to 1000] | 0 (0 to 500) | 0.8218 |
| Colloids | ||||
| Patients | 15/25 (60.0%) | 10/28 (35.7%) | 0.38 (0.11 to 1.29) | 0.1017 |
| Volume (ml) | 500 [500 to 575] | 750 [500 to 1000] | 0 (−500 to 0) | 0.1689 |
| Until 24 h after accident | ||||
| Crystalloidsa | ||||
| Volume (ml) | 3100 [1500 to 5126] | 3120 [937.5 to 4905.75] | 199.24 (−1309 to 1692) | 0.7276 |
| Colloids | ||||
| Patients | 19/25 (76.0%) | 16/28 (57.1%) | 0.43 (0.11 to 1.58) | 0.245 |
| Volume (ml) | 1000 [575 to 2500] | 2475 [2000 to 5374.5] | −1385.6 (−2850 to 0) | 0.041 |
| Red blood concentrate | ||||
| Patients | 13/25 (52.0%) | 11/28 (39.3%) | 0.6 (0.17 to 2.03) | 0.4145 |
| Dose (U) | 3 [3 to 6] | 7 [4 to 9.5] | −2 (−5 to 1) | 0.2188 |
| Massive transfusionb | 2/25 (8.0%) | 3/28 (10.7%) | 1.37 (0.14 to 17.80) | 1 |
| Platelet concentrate | ||||
| Patients | 0/25 (0.0%) | 3/28 (10.7%) | ∞ (0.38 to ∞) | 0.238 |
| Fresh frozen plasma | ||||
| Patients | 4/25 (16.0%) | 4/28 (14.3%) | 0.88 (0.14 to 5.34) | 1 |
| Dose, units | 10 [8.5 to 12.5] | 17 [10.25 to 25] | −7.5 (−30 to 15) | 0.3094 |
| Fibrinogen concentrate after admission to ED | ||||
| Patients | 12/25 (48.0%) | 11/28 (39.3%) | 0.71 (0.21 to 2.39) | 0.5862 |
| Dose (g) | 4 [2 to 4.75] | 4 [4 to 5.5] | −1 (−3 to 1) | 0.2923 |
| Four-factor PCCc | ||||
| Patients | 1/25 (4.0%) | 2/28 (7.1%) | 1.83 (0.09 to 113.31) | 1 |
| Tranexamic acid after admission to ED | ||||
| Patients | 10/25 (40.0%) | 6/28 (21.4%) | 0.42 (0.10 to 1.59) | 0.2304 |
| Dose (g) | 1.5 [1 to 2] | 2 [1.25 to 2] | 0 (−1 to 1) | 0.6411 |
Values in Placebo and FC groups are median [IQR] or number (%).
ED, emergency department; PCC, prothrombin complex concentrate.
All patients received crystalloids during the first 24 h after injury.
More than 9 units in 24 h.
The patient in the Placebo group received 1200 units; the two patients in the FC group received 1000 units and 1200 units of four-factor PCC.
The change from baseline in FIBTEM MCF at T2 differed significantly between the two groups, with an increase in the fibrinogen concentrate group and a decline in the placebo group (P < 0.0001, Fig. 2a). Similarly, MCF in the EXTEM assay (EXTEM MCF) increased in response to fibrinogen concentrate treatment but decreased with placebo (P = 0.0031, Fig. 2c). EXTEM CT decreased in the fibrinogen concentrate group but increased in the placebo group (P = 0.0509; Fig. 2b). These differences were reflected by significantly higher median values for FIBTEM MCF (P = 0.0026) and EXTEM MCF (P = 0.0102) at T2 in patients receiving fibrinogen concentrate. EXTEM CT was longer at randomisation in the fibrinogen concentrate group, but similar in both groups at T2.
Fig. 2.
Values at baseline (T1) and emergency room admission (T2), and the differences between these timepoints, in selected ROTEM parameters
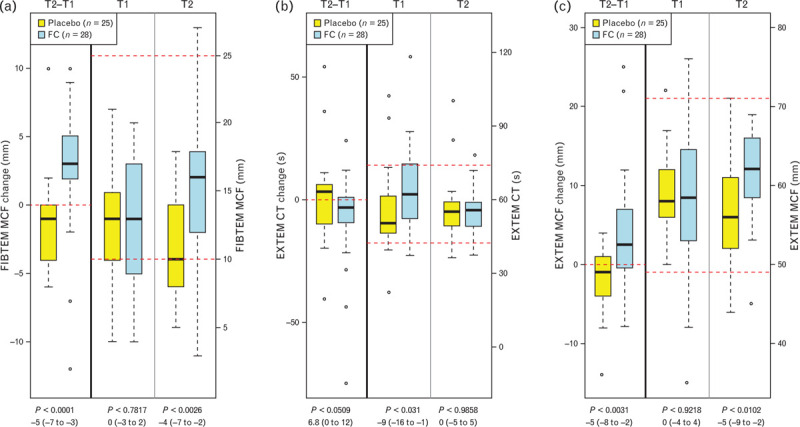
(a) FIBTEM maximum clot firmness (FIBTEM MCF); (b) EXTEM clotting time (EXTEM CT); (c) EXTEM maximum clot firmness (EXTEM MCF); FC, Factor Concentrate group. Data are presented as median [IQR] (boxes) as well as minimum and maximum range along with outliers (dots). P values are given with difference between groups and 95% CI. Horizontal dashed red lines show boundaries of the normal range for each parameter.
Figure 3 depicts the evolution of FIBTEM MCF over the entire 7-day study period. Median values for FIBTEM MCF were higher in the fibrinogen concentrate group than in the placebo group across all posttreatment timepoints, but a statistically significant between-group difference was observed only at T2. The mixed-effects model showed that, over the entire observation period (T1 to T7), fibrinogen concentrate administration increased FIBTEM MCF by 2.35 mm (95% CI 0.43 to 4.26, P = 0.0177) in comparison with placebo.
Fig. 3.
Changes in FIBTEM maximum clot firmness (FIBTEM MCF) between baseline (T1) and 7 days posttrauma (T7)
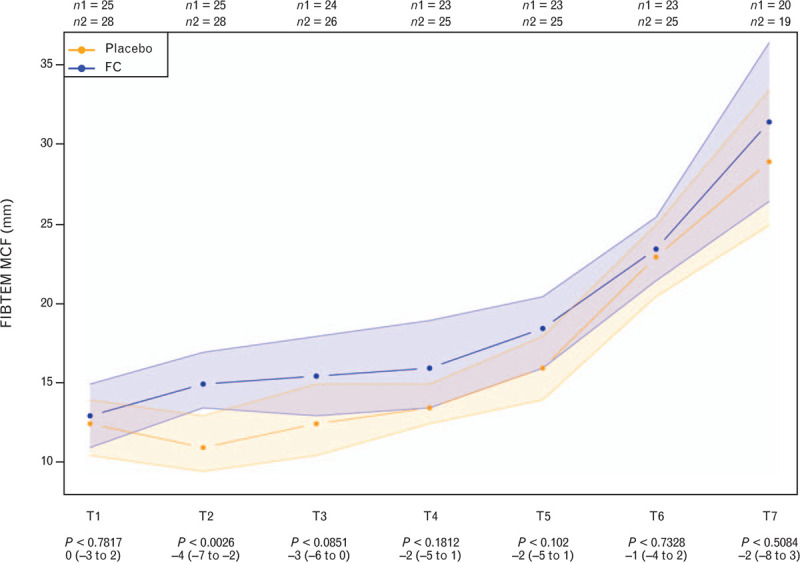
Data are presented as median [IQR] (boxes; as well as minimum and maximum plus outliers as dots). P values are given with difference between groups and 95% CI. Horizontal dashed red lines show boundaries of the normal range. FC, Factor Concentrate group; n1, Placebo group; n2, FC group.
No significant differences between the study groups were observed in 24-h volume therapy, transfusion requirements or administration of coagulation factor concentrates (Table 3). The total amount of colloid was significantly higher in patients treated with fibrinogen concentrate than in those receiving placebo (2475 vs. 1000 ml; P = 0.041). In the period from the arrival in the ED until 24 h later, TXA was given less often to patients in the fibrinogen concentrate group (21.4%) than those in the placebo group (40%), but the difference was not significant (P = 0.2304) (Table 3).
The effects of fibrinogen concentrate on further viscoelastic, coagulation and haematological parameters are shown in Supplemental Digital Content 4. In line with the FIBTEM MCF results, the median plasma fibrinogen concentration decreased below 2.0 g l−1 at T2 in the placebo group, whereas in the fibrinogen concentrate group, a modest increase in the plasma fibrinogen concentration was seen between T1 and T2 and the median fibrinogen plasma concentration did not fall below the recommended threshold of 2.0 g l−1. The between-group difference in the plasma fibrinogen concentration was significant at T2 (P = 0.0014, median [IQR] difference -83 [-134 to -42]). Results from the intrinsically activated INTEM assay and the aprotinin-containing APTEM assay reflected those of the EXTEM and the FIBTEM assays. Standard laboratory coagulation tests (prothrombin and aPTT) showed no significant between-group differences. Platelet count decreased over time in both groups until T6 but was restored almost to baseline levels at T7. Numerically higher platelet counts were observed in patients treated with fibrinogen concentrate compared with placebo from T3 onwards, but this difference did not reach statistical significance at any time. Both study groups showed a decrease over time in FXIII levels, with no statistically significant difference at any timepoint. pH was numerically lower in the fibrinogen concentrate group than the placebo group throughout the study period. Base deficit was numerically higher in the fibrinogen concentrate group at T1 and T2, but lower at T3 to T7.
In total, three (5.7%) thromboembolic complications occurred within 7 days after the administration of the study drug. Two thromboembolic events were detected in patients who had received fibrinogen concentrate and one event in patients who received placebo. The difference was not significant. There was also no significant difference between patients who received additional prehospital TXA and those who did not.
Discussion
This study indicates that early prehospital administration of fibrinogen concentrate to severely injured and bleeding patients is feasible, despite the highly complex, dynamic and time-sensitive environment. The early treatment with fibrinogen concentrate improved clot stability and avoided a significant decrease in plasma fibrinogen concentration.
The intention of early fibrinogen supplementation in trauma is to maintain clot stability in order to avoid acute trauma-associated coagulopathy, because a low fibrinogen concentration on arrival in the ED has uniformly been associated with morbidity and mortality in bleeding trauma patients.3,4,18
For practical reasons, fibrinogen concentrate used here is stable from −20°C to +50°C for more 6 months,19 which means that storage and administration is feasible even for helicopter emergency services at alpine environments and extreme temperatures. The fibrinogen concentrate was completely dissolved without producing foam and ready to use within seconds after mixing with the solvent. In contrast to our findings, Curry et al.20 were not able to administer fibrinogen concentrate within a reasonable time. We may assume that this was due to institutional shortcomings instead of procedure execution.
Our study has several limitations, including the use of a nonclinical parameter as the primary endpoint. Selection of study endpoints is one of the most challenging decisions when designing clinical trials in trauma. It is difficult to achieve measurable treatment effects on morbidity and especially mortality not only because of the heterogeneity of the underlying disease/trauma but also because of the difficulties involved in recruiting patients.21 In addition, any interventions influencing secondary outcome parameters were not controlled by the study design. Further, volume deficits have not been monitored by any preload parameter but only by blood pressure, heart rate and capillary refill time.
It may be argued that the FIBTEM MCF results of the present study are not surprising because the administration of fibrinogen concentrate is almost bound to increase the plasma fibrinogen concentration. However, we consider it important that the early prehospital administration of fibrinogen concentrate prevented a decrease in median fibrinogen plasma concentration below the critical threshold of 2.0 g l−1 that was observed in the control group. The 2019 updated guidelines15 recommend maintaining fibrinogen plasma concentration above this threshold in order to provide adequate amounts of substrate for the clotting process when needed most. As low fibrinogen plasma concentrations on arrival at the ED have been associated with both need for transfusion and outcome, the intervention may also have the potential to limit or even reduce the patient's exposure to the potentially harmful effects of subsequent allogeneic blood transfusion.4–6
Fibrinogen plasma concentrations in the fibrinogen concentrate group were kept above the recommended and critical threshold throughout the entire observation period. There were no significant between-group differences in standard laboratory coagulation test results, or transfusion of allogeneic bloods. However, these results could have been affected by the trend towards higher baseline ISS in the fibrinogen concentrate vs. the placebo group (median ISS 25 vs. 16).
In addition, it may be questioned whether the fibrinogen concentrate dosage in our study was optimal. Fibrinogen concentrate was administered without prior assessment of fibrinogen concentrations or fibrin-based clot strength, which means that some patients could have needed a higher dose while others may not have had a need for FC. It would be of interest to compare outcomes in patients treated with different prehospital fibrinogen concentrate dosing strategies. The risk of thromboembolic events irrespective of the initial fibrinogen concentrate dosing appears rather small.22 The increases of fibrinogen concentrations and FIBTEM MCF were related to a posttraumatic acute phase reaction, independently of whether patients received fibrinogen concentrate prehospital or not.
A further consideration in our study was that patients were eligible to receive any treatment considered necessary by the treating physician (e.g. coagulation factor concentrates, allogeneic blood products) after ED admission. This would have reduced the effects of study treatment on a range of outcomes. Strengths of our study include the randomised, controlled, double-blind design and the real-world setting that ensures our data are applicable to routine clinical practice. For future studies, investigation of a protocol with prehospital algorithms including coagulation factors and TXA would be of great interest and may further improve the treatment of trauma patients.
Conclusion
The FIinTIC study indicates that timely fibrinogen concentrate administration is feasible in the complex, dynamic and time-sensitive environment of acute prehospital trauma care. Early fibrinogen concentrate supplementation prevented fibrinogen plasma concentrations from decreasing to critical levels and improved blood clot stability between on-scene and ED arrival. Further studies are needed to assess whether superior clot quality on ED arrival resulting from early prehospital fibrinogen concentrate administration would translate into reduced transfusion requirement along with less morbidity and mortality in the later sequelae of trauma patients who are either bleeding or at risk to bleed.
Acknowledgements relating to this article
Assistance with the article: we thank all included patients, all physicians, paramedics and nurses from the participating hospitals. Special thanks go to Wenjun Martini, Bernard Pelletier and Wil Stevens for their valuable contribution to the study. We also thank Pamela Schech, and Dr Nicole Innerhofer for management of the FIinTIC study and Prim. Mag. Dr Günther Sumann, Dr Jürgen Simharl, Dr Pavel Sedlák, Dr Phillip Helm, Dr Sigune Kaske, Dr Michael Caspers, Dr Nadine Schäfer, and Julia Böhm, Priv.-Doz. Dr Werner Tiefenthaler, Dr Regine Korschineck, Priv.-Doz. Dr Peter Paal, Dr Hannes Dejaco, Dr Wolfgang Lederer, Dr Aurel Botz, Dr Christian Windhofer, Dr Jacob Krammer, Dr Reinhard Doppler, Dr Birgit Jaritz, Dr Gerold Muhri, Dr Dietrich Frank, Dr Jürgen Kaufmann, Dr Andreas Niederwanger, Dr Daniel Staribacher, Dr Michael Baubin, Dr Regina Unterpertinger, Dr Daniel Oberladstätter, Dr Johannes Steinmann, Dr Manuel Maurer, Dr Christian Klimmer, Dr Suzan Trübsbach, Dr Ernst Toferer, Dr Kainz Hartmann, Dr Sevak Taslakian, Dr Maximilian Schandert, Dr Jan Lejsek, Dr Lucie Langová, Dr Eva Tauchmanová and Dr Arne Driessen for including patients, treating the patients according to the protocol at the study hospitals and/or managing the FIinTIC study at the study sites. Further we thank the Department of Medical Statistics, Informatics and Health Economics at the Medical University of Innsbruck (Austria) for generating the randomisation code.
Financial support and sponsorship: the trial was funded by LFB Biomedicaments (France), the US Army/Department of Defence (Coalition Warfare Grant) and the Austrian Ministry of Defence and Sports. The study medication and an unrestricted grant were provided by LFB Biomedicaments. In addition, the sponsor of this clinical trial is the Medical University of Innsbruck (Austria). None of the funding organisations influenced or participated in study design, collection, analysis or writing.
Conflicts of interest: BS has received travel grants, honoraria for speaking or participation at meetings from CSL Behring, BBraun and Biotest. BZ has received speaker fees from CSL Behring. DF has received study funding, honoraria for consultancy and board activity from Astra Zeneca, AOP orphan, Baxter, Bayer, BBraun, Biotest, CSL Behring, Delta Select, Dade Behring, Edwards, Fresenius, Glaxo, Haemoscope, Hemogem, Lilly, LFB, Mitsubishi Pharma, NovoNordisk, Octapharm, Pfizer, Tem-Innovation. HS has received honoraria for participation in advisory board meetings for Bayer Healthcare, Böhringer Ingelheim and Tem International, and has received study grants from CSL Behring. MB has received research funding and travel grants from LFB Biomedicaments, Baxter GmbH, CSL Behring GmbH, Mitsubishi Tanabe and nonfinancial support from TEM International outside the submitted work. MM has received fees for lectures, advisory board services, travel and research support from Astra Zeneca, Bayer, Biotest, CSL Behring, IL-Werfen and LFB Biomedicaments. HS has received honoraria for participation in advisory board meetings for Bayer Healthcare, Böhringer Ingelheim and Tem International, and has received study grants from CSL Behring. PI received personal fees from Baxter GmbH, CSL Behring GmbH, Fresenius Kabi GmbH Austria, Bayer Austria GmbH, LFB, and nonfinancial support from TEM International, outside the submitted work. WJM is employee of the US Army, Department of Defence, one of the funding organisations. BT, EO, HH, HT, MT, MK, WV, IZ, CaroN, ChriN and UM declare no conflict of interest.
Presentation: none.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplemental Digital Content 1 for a full list of the study investigators.
Published online 28 October 2020
Supplemental digital content is available for this article.
References
- 1.Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury 2007; 38:298–304. [DOI] [PubMed] [Google Scholar]
- 2.Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg 1995; 81:360–365. [DOI] [PubMed] [Google Scholar]
- 3.Inaba K, Karamanos E, Lustenberger T, et al. Impact of fibrinogen levels on outcomes after acute injury in patients requiring a massive transfusion. J Am Coll Surg 2013; 216:290–297. [DOI] [PubMed] [Google Scholar]
- 4.Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 2012; 10:1342–1351. [DOI] [PubMed] [Google Scholar]
- 5.McQuilten ZK, Wood EM, Bailey M, et al. Fibrinogen is an independent predictor of mortality in major trauma patients: a five-year statewide cohort study. Injury 2017; 48:1074–1081. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura Y, Ishikura H, Kushimoto S, et al. Fibrinogen level on admission is a predictor for massive transfusion in patients with severe blunt trauma: analyses of a retrospective multicentre observational study. Injury 2017; 48:674–679. [DOI] [PubMed] [Google Scholar]
- 7.Hagemo JS, Christiaans SC, Stanworth SJ, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care 2015; 19:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veigas PV, Callum J, Rizoli S, et al. A systematic review on the rotational thrombelastometry (ROTEM() values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med 2016; 24:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Innerhofer P, Fries D, Mittermayr M, et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol 2017; 4:e258–e271. [DOI] [PubMed] [Google Scholar]
- 10.Schochl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care 2011; 15:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nascimento B, Callum J, Tien H, et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth 2016; 117:775–782. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Yamaguchi A, Sawano M, et al. Preemptive administration of fibrinogen concentrate contributes to improved prognosis in patients with severe trauma. Trauma Surg Acute Care Open 2016; 1:e000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengoli C, Franchini M, Marano G, et al. The use of fibrinogen concentrate for the management of trauma-related bleeding: a systematic review and meta-analysis. Blood Transfus 2017; 15:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maegele M, Zinser M, Schlimp C, et al. Injectable hemostatic adjuncts in trauma: fibrinogen and the FIinTIC study. J Trauma Acute Care Surg 2015; 78:S76–82. [DOI] [PubMed] [Google Scholar]
- 15.Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care 2019; 23:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg 2006; 16:773–776. [DOI] [PubMed] [Google Scholar]
- 17.Fenger-Eriksen C, Jensen TM, Kristensen BS, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost 2009; 7:795–802. [DOI] [PubMed] [Google Scholar]
- 18.Schochl H, Cotton B, Inaba K, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care 2011; 15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martini WZ, Guzman R, Dubick MA. Stability of fibrinogen concentrate in human blood samples: an in vitro study. Mil Med 2018; 183:183–188. [DOI] [PubMed] [Google Scholar]
- 20.Curry N, Foley C, Wong H, et al. Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multicentre, randomised, double blind, placebo-controlled pilot trial. Crit Care 2018; 22:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holcomb JB, Weiskopf R, Champion H, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock 2011; 35:107–113. [DOI] [PubMed] [Google Scholar]
- 22.Schlimp CJ, Ponschab M, Voelckel W, et al. Fibrinogen levels in trauma patients during the first seven days after fibrinogen concentrate therapy: a retrospective study. Scand J Trauma Resusc Emerg Med 2016; 24:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


