Supplemental digital content is available in the text.
Key Words: Β-HYDROXYBUTYRATE, KETOSIS, ATHLETIC PERFORMANCE, LOW-CARBOHYDRATE HIGH-FAT
ABSTRACT
The consumption of a ketogenic low-carbohydrate (CHO), high-fat (LCHF) diet increases skeletal muscle fat utilization but impairs exercise economy. Whether the concomitant increase in circulating endogenous ketone bodies (KB) alters the capacity to metabolize exogenous ketone supplements such as the popular ketone monoester is unknown.
Purpose
This study aimed to determine if LCHF and ketone ester (KE) supplementation can synergistically alter exercise metabolism and improve performance.
Methods
Elite race walkers (n = 18, 15 males and 3 females; V˙O2peak, 62 ± 6 mL·min−1·kg−1) undertook a four-stage exercise economy test and real-life 10,000-m race before and after a 5-d isoenergetic high-CHO (HCHO, ~60%–65% fat; CHO, 20% fat; n = 9) or LCHF (75%–80% fat, <50 g·d−1 CHO, n = 9) diet. The LCHF group performed additional economy tests before and after diet after supplementation with 573 mg·kg−1 body mass KE (HVMN; HVMN Inc., San Francisco, CA), which was also consumed for race 2.
Results
The oxygen cost of exercise (relative V˙O2, mL·min−1·kg−1) increased across all four stages after LCHF (P < 0.005). This occurred in association with increased fat oxidation rates, with a reciprocal decrease in CHO oxidation (P < 0.001). Substrate utilization in the HCHO group remained unaltered. The consumption of KE before the LCHF diet increased circulating KB (P < 0.05), peaking at 3.2 ± 0.6 mM, but did not alter V˙O2 or RER. LCHF diet elevated resting circulating KB (0.3 ± 0.1 vs 0.1 ± 0.1 mM), but concentrations after supplementation did not differ from the earlier ketone trial. Critically, race performance was impaired by ~6% (P < 0.0001) relative to baseline in the LCHF group but was unaltered in HCHO.
Conclusion
Despite elevating endogenous KB production, an LCHF diet does not augment the metabolic responses to KE supplementation and negatively affects race performance.
During exercise, substrate utilization is influenced by several factors, including both duration and intensity (1). Prolonged exercise relies on the breakdown of endogenous fuel stores (i.e., glycogen, the storage form of carbohydrate [CHO], and intramuscular triglycerides, an endogenous store of lipids) as well as the uptake of blood-borne substrates (glucose and free fatty acids) for the production of ATP. Most Olympic middle- and long-distance events are performed at intensities greater than 75% of V˙O2max (2,3) and are therefore heavily reliant on CHO metabolism as maximal rates of lipid oxidation occur at ~60%–65% V˙O2max (4) and decline as exercise intensity increases. As a result, nutrition guidelines for endurance sport have focused heavily on matching the energy cost of the event to the body’s finite storage CHO (5), while scientists and athletes have both searched for strategies that promote “glycogen sparing” for use later in exercise. Two strategies that have received recent attention are chronic adaptation to a ketogenic low-CHO, high-fat (LCHF) diet and acute supplementation with exogenous ketones (i.e., ketone esters [KE] or ketone salts). Although these dietary strategies both rely on the provision of alternative substrates to the working skeletal muscle to delay or minimize the use of CHO, they result in the development of distinct metabolic states (6). Critically however, the interaction of the two on exercise performance has not yet been investigated.
The consumption of an LCHF diet (defined as <50 g CHO·d−1 or <5% energy intake [EI] from CHO and 75%–80% EI from fat [7,8]) is proposed to result in a host of metabolic adaptations (9). In the context of endurance performance, a key finding is that as little as 5 d of adaptation to an LCHF diet can result in rapid retooling of the skeletal muscle to increase (200%–250%) its capacity to use fat as fuel during exercise (10), as well as the relative intensity at which peak oxidation rates occur. Indeed, we have demonstrated previously that mean rates of fat oxidation were increased to ~1.4 g·min−1 when elite athletes consumed LCHF for 5–6 d (11), rates similar to those achieved by medium-term (3–4 wk) (8,12) and chronic (>12 wk) adaptation periods (13). Although hepatic ketone production also increases in the face of CHO restriction to provide substrate for the brain and other metabolically active tissues (14), it is currently unclear whether the muscle also adapts to increase the capacity to use this substrate.
Recently, Clarke and colleagues (15) developed a method for inducing acute nutritional ketosis in the absence of CHO or energy restriction, through the consumption of the synthetic ketone ester ethyl (R)-3-hydroxybutyrate and (R)-1,3-butanediol. Supplementation with 573 mg·kg−1 of this KE increased circulating ketone concentrations to ~3 mmol·L−1 after 10 min, peaking at ~6 mmol·L−1 after 20 min (16). Circulating ketone concentrations decreased to ~4 mmol·L−1 during 45 min of cycling at 40% Wmax, and to ~3 mmol·L−1 after exercise at 75% Wmax, with ketone oxidation calculated to contribute ~18% and 16%, respectively, to oxygen consumption. Critically, supplementation also resulted in a ~2% increase in the distance covered during a cycling time trial (16), suggesting that ketones could be an effective ergogenic aid. However, subsequent studies have failed to show similar improvements across a variety of performance outcomes (17–19).
There is now a growing body of evidence demonstrating that LCHF diets impair the performance of endurance exercise at high relative and absolute intensities (13), with at least part of the mechanism being an increase in the oxygen cost of exercise at the same absolute speed (9,12,13,20). In contrast, it has been suggested that ketone bodies may provide a greater Gibbs free energy (∆G) for ATP production (21) and may therefore be a more efficient metabolic fuel source. Therefore, the purpose of this study was to determine if a short-term ketogenic LCHF diet could potentiate the effects of KE supplementation on performance, and whether this supplement would offset the hallmark decrease in exercise economy seen in previous studies using this diet.
METHODS
Ethical approval
This study conformed to the standards set by the Declaration of Helsinki and was approved by the Ethics Committee of the Australian Institute of Sport (no. 20191102). After comprehensive details of the study protocol were explained to the subjects orally and in writing, all athletes provided their written informed consent.
Participants
A total of 19 athletes were initially recruited to participate in this study. After baseline testing, one athlete developed an injury and withdrew; therefore, their data were not included in the final analysis. The cohort (n = 18, 15 males and 3 females, 26.1 ± 6.7 yr, 63.9 ± 7.2 kg; V˙O2peak, 4.03 ± 0.73 L·min−1) ranged from world class athletes (e.g., Olympians, World Championship and IAAF [now World Athletics] Race Walking Team Championships medalists, and national record holders) to highly trained athletes (e.g., training partners of world class athletes). Specifically, 12 of the 18 athletes who participated in this study were selected for at least one of the major events in the previous two seasons, either the 2018 World Athletics Race Walking Team Championships or the 2019 World Athletics World Championships. Athletes were educated about the benefits and limitations of each dietary intervention and asked to nominate their preference(s) for, or non acceptance of, each intervention, as described previously (8). We were able to allocate race walkers to a preferred dietary condition while achieving suitable matching across groups based on age, body mass (BM), peak oxygen uptake (V˙O2peak), and personal best for the 10-km race walk (see Table, Supplemental Digital Content 1, Subject characteristics of elite race walkers, http://links.lww.com/MSS/C145).
Overview of study design
This study was conducted during an approximately 2.5-wk training camp (Fig. 1) that represented baseline preparation for the 2020 World Athletics race walking season. The study was broken up into three phases. Phase 1 (Baseline) served as baseline economy and performance (10,000-m race) testing (described below), all of which was performed while consuming a standardized high-CHO (HCHO) diet that was provided for all athletes. To allow athletes adequate opportunity to recover in between races, Phase 2 consisted of 7 d of regular training (see Figure, Supplemental Digital Content 2, overview of training undertaken by athletes across the study, http://links.lww.com/MSS/C146) on a habitual diet. Athletes then followed either an HCHO or an LCHF diet for 5 d, before repeating all economy and performance measures (Phase 3, Adaptation).
FIGURE 1.
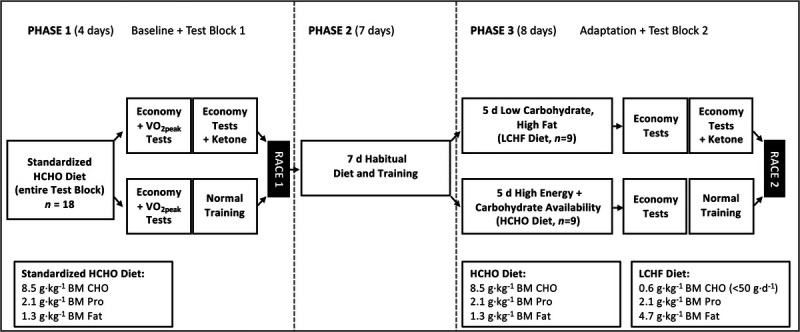
Study schematic of 2.5 wk training camp and dietary interventions for elite race walkers.
Dietary intervention
This study was undertaken with a blend of dietary control under free-living conditions with athletes accommodated in several communal houses. During the baseline (4 d total, including two testing days plus race 1) and adaptation (8 d total, including two testing days plus race 2, as above) periods of standardized food intake, each athlete was provided with an individualized daily menu and all food ingredients and fluids required to achieve this. Each period of dietary standardization involved the repetition of a 3-d rotating menu, with individualized versions constructed by a team of food service and sport dietitians to suit nutritional targets and special food needs (i.e., preferences and allergies) of each participant. Food provisions included a range of precooked/assembled meals such as commercial ready to eat meals (Dineamic Meals, Victoria, Australia), whole food ingredients, and specialty low-CHO products (Herman Brot, Queensland, Australia). Athletes were provided with kitchen scales to ensure they could portion the cooked and assembled foods as per the personalized meal plan provided. Food delivery was provided every 1–2 d to ensure that food hygiene and quality was maintained and to support meal plan compliance. Athletes were also required to provide completed daily menu checklists to the study dietary team and were encouraged to share photos of their meals.
Dietary interventions, as described previously (8,20), were used for baseline and/or adaptation periods. Both dietary treatments were designed to provide adequate energy availability, with a provision of 56 kcal (225 kJ)·kg−1 BM to account for planned training. The dietary interventions in the current study consisted of the following:
Standardized HCHO diet—constructed to provide a CHO intake of 8.5 g·kg−1 BM·d−1 with ~2.1 and 1.3 g·kg−1 BM·d−1 of protein and fat, respectively.
LCHF diet protein—matched for protein intake (2.1 g·kg−1 BM·d−1) and provided ~0.6 g·kg−1 BM·d−1 of CHO (<50 g of CHO in a single day), with the remainder of energy (4.7 g·kg−1 BM·d−1) coming from fat.
On testing days, athletes were provided with a standardized breakfast meal to be consumed 2 h before commencing exercise. Athletes consumed this before reporting to the laboratory with compliance confirmed via a time-stamped photo. On race days, the standardized meal was provided at the race site and consumed under supervision. The standardized HCHO breakfast meal provided 2 g·kg−1 BM (toast, jam, butter, and apple juice), whereas the LCHF meal was energy matched and consisted of low-CHO bread (<10 g of CHO), whole eggs, and avocado.
Incremental exercise economy and capacity tests
During both Baseline and Adaptation, athletes performed a four-stage economy test to determine submaximal walking economy and fuel utilization (8). Briefly, athletes reported to the laboratory 2 h after the intake of a standardized test meal as outlined above, and resting capillary (fingertip) blood samples were taken to assess blood lactate (Lactate Pro 2, Akray, Japan), ketones (β-hydroxybutyrate [βHB]; FreeStyle Optium Neo, Abbott Diabetes Care, Victoria, Australia), and glucose (FreeStyle Optium Neo, Abbott Diabetes Care) concentrations. Before each test, athletes performed a self-selected 10-min warm-up, which was maintained across trials. Walking economy was assessed on a motorized treadmill (Venus, h/p/cosmos, Nussdorf-Traunstein, Germany) and consisted of four submaximal stages, each lasting 4 min and increasing in speed by 1 km·h−1 each stage. Starting speeds were selected at 10–12 km·h−1 based on each individual’s capacity and sex; senior male athletes’ 20-km personal best times were compared with the 2019 World Athletics World Championship qualifying standards of 1 h 22 min 30 s, with athletes faster than this mark commencing at 12 km·h−1 and increasing to 15 km·h−1 at the final stage and the remaining male athletes commencing at 11 km·h−1 and increasing to 14 km·h−1. All female athletes commenced at 10 km·h−1 and increased to 13 km·h−1. As a result, the speeds of the second and fourth stage corresponded approximately to each individual athlete’s walking pace for the 50- and 20-km race walk events, respectively.
Each stage was followed by 1 min rest for the collection of capillary blood samples, as well as RPE (6–20 Borg scale). Heart rate (HR) was measured continuously throughout the test (Polar Heart Rate Monitor; Polar Electro, Kempele, Finland). Expired gas was collected and analyzed every 30 s via open-circuit spirometry (TrueOne 2400; Parvo Medics, Sandy, UT) with the final 60 s of gas collected accepted as steady state and rates of O2 consumption (V˙O2) and CO2 production (V˙CO2) used to calculate the RER. Before each test, gas analyzers were calibrated with commercially available gases (16% O2 and 4% CO2). To assess maximal aerobic capacity and confirm equal matching between groups, V˙O2peak was assessed at baseline. Upon completion of the final submaximal walking stage, subjects rested for 5 min before completing a ramp (speed and then gradient) test to volitional fatigue. Treadmill speed was increased by 0.5 km·h−1 every 30 s until the speed corresponding to the individual’s final submaximal stage was reached (14 or 15 km·h−1), with treadmill gradient increased by 0.5% every 30 s thereafter until exhaustion. Expired gas was collected and analyzed throughout, maximal HR recorded, and capillary blood samples collected 1 min after completion.
To assess the effects of ketone supplementation on exercise economy, athletes who had been allocated to the LCHF group returned to the laboratory the following day to repeat the submaximal economy test as outlined above. Athletes were provided with the same standardized breakfast (HCHO breakfast at baseline and LCHF breakfast after Adaptation), which was consumed 2 h before testing. Resting blood capillary samples were taken before and 25 min after the ingestion of 573 mg·kg−1 BM KE (HVMN, San Francisco CA), with the exercise protocol commencing 5 min later (30 min post-KE ingestion). All testing procedures were repeated during the Adaptation phase after the 5-d dietary intervention.
Calculation of substrate oxidation data
RER was calculated from steady-state expired gases collected over 1-min periods during the economy test and maximal aerobic capacity (V˙O2peak) protocol. Rates of CHO and fat oxidation were calculated from V˙CO2 and V˙O2 values using nonprotein RER values (22) and normalized for BM (mg·kg−1·min−1). We did not correct our calculations for the contribution of ketone oxidation to substrate use to contextualize our findings with previous work performed by our group (8,11,12) as well as other reports of substrate utilization in ultraendurance athletes who chronically consume LCHF diets (23,24). However, we acknowledge that there may be a small (but systematic) error in the use of conventional equations to calculate fat and CHO oxidation from gas exchange information (25). Substrate oxidation calculations were only performed for the tests performed without exogenous KE supplementation, as indirect calorimetry is not currently validated for the quantification of ketone oxidation.
Performance
After the completion of both baseline and adaptation phases, all athletes competed in a World Athletics–sanctioned 10,000-m race walk event held on a synthetic 400-m outdoor athletics track (Melbourne, VIC, Australia). Each race commenced at 0900 h and was conducted under World Athletics rules, which involved officiating by technical judges, invitation for participation by competitors external to the study, a feed zone allowing water intake on the outside lanes of the track in hot conditions, and electronic timing to provide official race times. Athletes arrived at the track in a fasted state and were provided with a standardized breakfast to consume ~2 h before competition. Capillary blood samples were collected in the fastest state, both 30 min and immediately before the start of the race, and as each competitor completed the race. The use of performance supplements was discussed with each participant before the first race; permission was provided when it did not interfere with the treatment diet, was documented, and was repeated for the second race. In this particular study, caffeine was the only performance supplement discussed, with three athletes consuming caffeine (1 cup black coffee) as part of their regular prerace routine. This was documented by the researchers and repeated (time consumed, preparation method, and volume) for both races.
For race 2 (adaptation), athletes allocated to the LCHF diet consumed 573 mg·kg−1 BM KE 30 min before the start of the race. Capillary bloods samples were collected in the fasted state, before KE ingestion (30 min before race start), immediately before the start of the race, and as each competitor completed the race.
Statistical analyses
The required sample size was calculated before the commencement of the study using the 10,000-m race as the primary outcome and was based on our previous work evaluating the effect of LCHF diets on performance in similar populations (12). Specific sample size estimation was calculated for performance measures using G Power software (Version 3.1, Bonn University, Bonn, Germany). Based on such data, a sample size of seven athletes per group was considered appropriate (n = 7, critical t = 2.179; expected power = 0.939; P < 0.05). To account for possible dropouts or nonadherence, we attempted to recruit 10 athletes per group (20 total) and were successful in recruiting 19. A Student’s t-test was used to determine differences between groups at baseline and changes in BM and 10,000-m race performance. A two-way repeated-measures ANOVA was used to determine differences between trials within a dietary condition for all economy test (economy stage–trial) and blood metabolite (sampling point–trial) data. If significance was detected, a Bonferroni post hoc test was applied. Significance was set at P < 0.05, where NS indicates not significant. All statistical analyses were performed using GraphPad Prism (version 8.3.1, GraphPad Software).
RESULTS
Dietary intervention
During Baseline, there were no differences between the HCHO and the LCHF groups for energy or macronutrient intake, with both groups consuming ~230 kJ·kg−1 BM·d−1. Throughout the study, protein intake was maintained at ~2 g·kg−1 BM·d−1 to maximize recovery from training. During the intervention period, the LCHF group followed a ketogenic diet, with <5% of EI (~38 g·d−1) coming from CHO and the majority (~76%, ~296 g·d−1) coming from fat. All athletes adhered to the assigned diet, with the results of the assessed actual dietary intake and mean daily intakes for baseline and adaptation phases (see Table, Supplemental Digital Content 3, actual dietary intake during baseline testing and after adaptation to a dietary intervention undertaken by elite race walkers, http://links.lww.com/MSS/C147).
Oxygen consumption and substrate utilization
For all groups, there was a main effect (P < 0.0001) of exercise intensity during the submaximal economy test, such that V˙O2 (both absolute and relative) and CHO oxidation (mg·kg−1·min−1) increased from stage 1 to stage 4, with a reciprocal decrease in fat oxidation (Fig. 2). Similarly, there was a main effect of intensity for RPE, HR, and RER, all of which increased throughout the test (Table 1, P < 0.0001). After adaptation to the LCHF diet, V˙O2 increased across all stages relative to baseline (Fig. 2A, all P < 0.005). This increase in O2 consumption was associated with a decrease in RER (Table 1, P < 0.0001), indicating a decrease in the amount of CHO oxidized (Fig. 2C, P < 0.0001) and an increased reliance on fat as a metabolic substrate. Fat oxidation rates increased across all four stages (Fig. 2E, P < 0.0001), and peaked at ~14 ± 4 mg·kg−1·min−1 (0.86 ± 0.36 g·min−1), with four athletes exceeding absolute rates of 1.0 g·min−1. The finding that RPE also increased after the LCHF diet (main effect of trial, P < 0.05) suggests that the overall decrease in exercise economy was associated with an increased metabolic and perceived cost of exercise. There was also a significant main effect of dietary intervention in the HCHO group (P < 0.05), with athletes displaying an increase in relative fat oxidation rates during stages 1 and 2 (P < 0.05), whereas V˙O2 was also increased relative to Baseline in stages 3 and 4 (P < 0.05).
FIGURE 2.
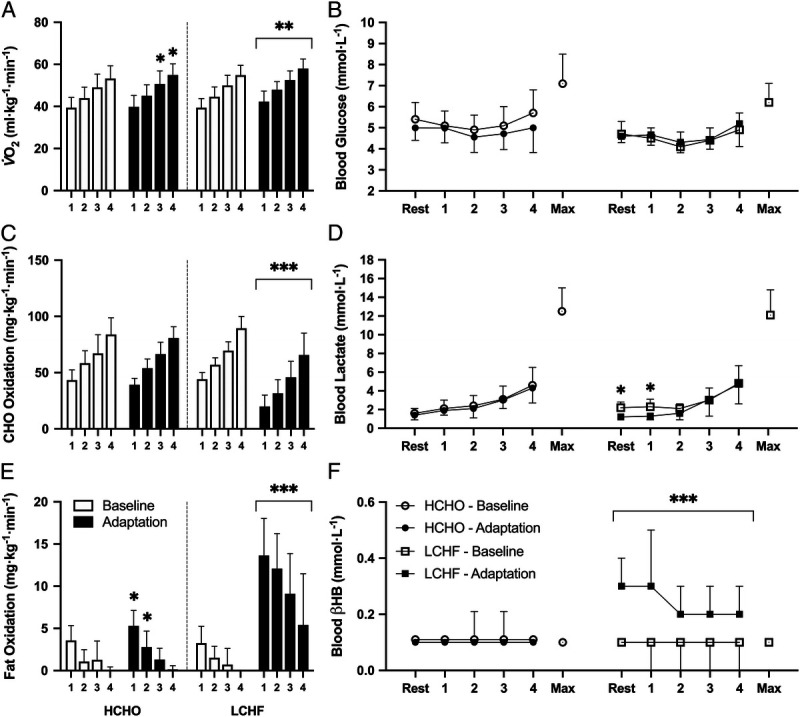
Relative oxygen uptake (A), substrate utilization (C, CHO; E, fat oxidation), and blood metabolite concentrations (B, blood glucose [mmol·L−1]; D, blood lactate [mmol·L−1]; and F, blood βHB [mmol·L−1]) during the four-stage economy test performed before and after adaptation to either an LCHF (n = 9) or an HCHO (n = 9) diet. Data are presented as mean ± SD. Significant differences within group relative to baseline are denoted by *P < 0.05, **P < 0.005, ***P < 0.0001.
TABLE 1.
Results of graded economy test and maximal aerobic capacity before and after a 7-d nutritional intervention.
| High-CHO Availability (n = 9) | LCHF (n = 9) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Max | S1 | S2 | S3 | S4 | Max | |
| BM (kg) | ||||||||||
| Baseline | 64.6 ± 7.4 | 63.1 ± 7.4 | ||||||||
| Adaptation | 64.6 ± 7.2 | 61.8 ± 7.1* | ||||||||
| RER | ||||||||||
| Baseline | 0.94 ± 0.03 | 0.99 ± 0.03 | 1.01 ± 0.03 | 1.05 ± 0.04 | 1.16 ± 0.05 | 0.95 ± 0.03 | 0.98 ± 0.03 | 1.01 ± 0.04 | 1.06 ± 0.03 | 1.19 ± 0.05 |
| Adaptation | 0.92 ± 0.02 | 0.97 ± 0.03 | 0.99 ± 0.03 | 1.02 ± 0.03 | 0.81 ± 0.05*** | 0.85 ± 0.05*** | 0.89 ± 0.05*** | 0.94 ± 0.06*** | ||
| V˙O2 (L·min−1) | ||||||||||
| Baseline | 2.56 ± 0.49 | 2.85 ± 0.53 | 3.19 ± 0.61 | 3.46 ± 0.65 | 3.98 ± 0.72 | 2.51 ± 0.52 | 2.84 ± 0.58 | 3.18 ± 0.63 | 3.48 ± 0.58 | 4.19 ± 1.06 |
| Adaptation | 2.58 ± 0.47 | 2.91 ± 0.48 | 3.28 ± 0.59* | 3.56 ± 0.64* | 2.64 ± 0.56** | 2.98 ± 0.51** | 3.26 ± 0.55 | 3.60 ± 0.59** | ||
| HR (bpm) | ||||||||||
| Baseline | 142 ± 12 | 150 ± 16 | 163 ± 12 | 172 ± 8 | 183 ± 7 | 142 ± 11 | 156 ± 13 | 169 ± 14 | 179 ± 14 | 194 ± 12 |
| Adaptation | 138 ± 8 | 153 ± 7 | 159 ± 10 | 170 ± 10 | 145 ± 12 | 156 ± 8 | 166 ± 10 | 181 ± 13 | ||
| RPE | ||||||||||
| Baseline | 11.0 ± 1.0 | 12.4 ± 1.0 | 13.6 ± 1.3 | 15.1 ± 1.6 | 10.0 ± 1.7 | 11.7 ± 1.4 | 12.9 ± 1.9 | 14.8 ± 1.5 | ||
| Adaptation | 10.3 ± 1.2 | 11.7 ± 1.1 | 12.7 ± 1.1 | 13.8 ± 1.4 | 10.9 ± 2.6 | 12.6 ± 2.4 | 14.6 ± 2.0* | 16.6 ± 2.0 | ||
Data are presented as mean ± SD. Significant differences within group relative to baseline are denoted by *P < 0.05, **P < 0.005, and ***P < 0.0001.
Exercise intensity and dietary intervention did not significantly alter circulating blood glucose responses in either treatment (Fig. 2B). Both HCHO and LCHF groups demonstrated a main effect for exercise intensity (P < 0.0001) for blood lactate, with higher concentrations at stage 4 compared with stage 1. There was also a significant (P < 0.05) effect of dietary intervention in the LCHF group; concentrations of blood lactate at rest and after the completion of stage 1 were suppressed compared with values at baseline (Fig. 2D). As measures were taken in the postprandial state after a CHO-rich meal, blood βHB concentrations were unaltered in the HCHO group regardless of exercise intensity (Fig. 2F). In contrast, there was a main effect of dietary intervention in the LCHF group (P < 0.005), as βHB concentrations were significantly elevated across all stages relative to baseline (P < 0.0001), peaking at 0.3 ± 0.2 mmol·L−1 at the end of stage 1.
Ketone supplementation economy tests
To determine the effects of KE supplementation on exercise economy, and whether an LCHF diet may augment these responses, the LCHF group performed additional economy tests before and after the dietary intervention. Consistent with the dietary effect seen in the earlier trial, the overall V˙O2 was higher in Adaptation + KE (P < 0.05), with a higher V˙O2 recorded during stages 1–3 compared with Baseline + KE (Fig. 3A, Table 2). Despite athletes adapting to the LCHF diet for 7 d, there was an increase in blood glucose across the Adaptation + KE relative to the Baseline + KE trial (Fig. 3B, P < 0.05). Critically, the consumption of the KE resulted in an increase in the concentration of βHB (P < 0.001), which remained at ~3 mmol·L−1 throughout the test, with no difference between trials (Fig. 3D). However, despite this, there were no changes in RER between the two trials. In contrast, RPE was increased (Table 2, P < 0.005), across all stages (P < 0.0001).
FIGURE 3.
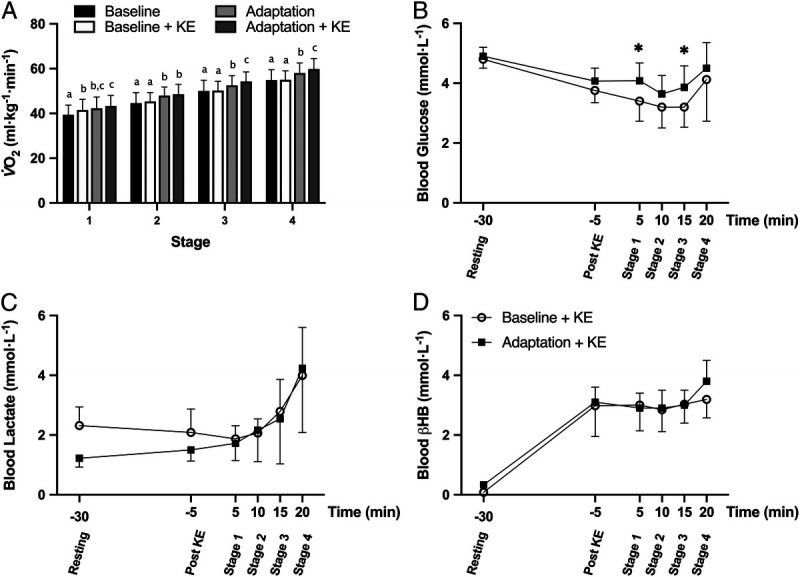
Relative oxygen uptake (A) for the four submaximal economy tests performed by the LCHF group, and blood metabolite concentrations (B, blood glucose [mmol·L−1]; C, blood lactate [mmol·L−1]; and D, blood βHB [mmol·L−1]) at rest, after supplementation with 573 mg·kg−1 ketone ester, and during the four-stage graded economy test performed before (baseline + KE) and after adaptation to an LCHF diet (adaptation + KE, n = 9). Data are presented as mean ± SD. Bars within a stage sharing a letter are not statistically different (P < 0.05). Significant differences relative to baseline + KE are denoted by *P < 0.05.
TABLE 2.
Results of ketone supplementation on graded economy test and maximal aerobic capacity before and after a 7-d nutritional intervention.
| LCHF—Ketone Supplementation Trials (n = 9) | ||||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| BM (kg) | ||||
| Baseline | 62.9 ± 7.2 | |||
| Adaptation | 61.7 ± 7.1** | |||
| RER | ||||
| Baseline | 0.93 ± 0.04 | 0.97 ± 0.04 | 1.02 ± 0.03 | 1.06 ± 0.04 |
| Adaptation | 0.78 ± 0.03 | 0.81 ± 0.03 | 1.10 ± 0.76 | 1.10 ± 0.62 |
| V˙O2 (L·min−1) | ||||
| Baseline | 2.63 ± 0.53 | 2.87 ± 0.52 | 3.17 ± 0.51 | 3.47 ± 0.58 |
| Adaptation | 2.69 ± 0.51 | 3.00 ± 0.51** | 3.36 ± 0.55*** | 3.70 ± 0.60*** |
| HR (bpm) | ||||
| Baseline | 144 ± 15 | 151 ± 14 | 163 ± 13 | 169 ± 10 |
| Adaptation | 146 ± 11 | 159 ± 11* | 169 ± 11 | 179 ± 12 |
| RPE | ||||
| Baseline | 10.0 ± 1.5 | 11.4 ± 1.3 | 13.2 ± 1.7 | 15.2 ± 1.6 |
| Adaptation | 11.9 ± 1.6*** | 13.7 ± 1.5*** | 15.4 ± 1.4*** | 17.4 ± 1.2*** |
Data are presented as mean ± SD. Significant differences within group relative to baseline denoted by *P < 0.05, **P < 0.005, and ***P < 0.0001.
10,000 m race performance
Performance of the World Athletics–sanctioned 10,000-m track race is summarized in Figure 4. Although there was no difference in the average performance of athletes in the HCHO group (Fig. 4A, P = 0.767), athletes in the LCHF group consistently performed worse compared with baseline (P < 0.0001). Specifically, performance was ~6% slower (equating to 2:34.9 ± 00:57.6 min), with all nine athletes recording slower race times. This occurred despite the athletes in the LCHF group being significantly lighter after the dietary intervention (Fig. 4B, P < 0.005). In contrast, five athletes in the HCHO group improved their performance, with two recording lifetime personal bests.
FIGURE 4.
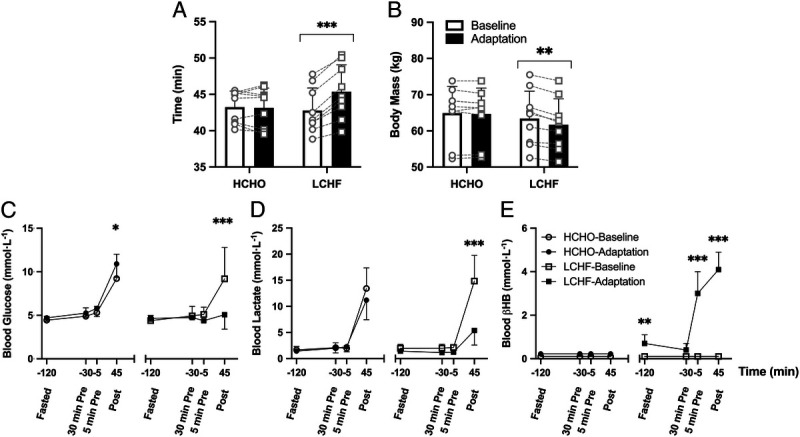
The 10,000-m race walk performance time (A), BM (B), and blood metabolite concentrations (C, blood glucose [mmol·L−1]; D, blood lactate [mmol·L−1]; and E, blood βHB [mmol·L−1]) after an overnight fast, 30 min (postprandial), and 5 min before the start and immediately after the 10,000-m race performed at baseline and after adaptation to either an HCHO (n = 9) or an LCHF (n = 9) diet. Athletes in the LCHF group supplemented with 573 mg·kg−1 ketone ester 25 min before the start of the race. Data are presented as mean ± SD. Significant differences within group relative to baseline are denoted by *P < 0.05, **P < 0.005, and ***P < 0.0001.
There was a main effect of the dietary intervention for blood glucose concentration in the HCHO group, with post hoc analysis revealing an increase immediately postrace (Fig. 4C). However, there were no differences in measured blood lactate (Fig. 4D) or βHB (Fig. 4E) compared with the race performed at Baseline. In contrast, after Adaptation in the LCHF group postrace blood glucose and lactate concentrations were decreased (P < 0.0001). Athletes in the LCHF group also displayed elevated fasting βHB concentrations compared with Baseline (0.7 ± 0.4 vs 0.1 ± 0.0 mmol·L−1, respectively), which increased to 3.0 ± 1.0 mmol·L−1 after the consumption of KE (Fig. 4E). This was maintained throughout the race, as postrace concentrations peaked at 4.1 ± 0.8 mmol·L−1. Neither group displayed any differences between races in self-reported measures of GI discomfort when measured prerace (both fasted and postprandial) or postrace (data not shown).
DISCUSSION
This study confirms, in elite athletes, that a brief (5-d) adaptation to a ketogenic LCHF diet significantly increases the capacity to use fat as a primary substrate at intensities relevant for real-world competition. However, this results in a decrease in exercise economy, with an increase in the oxygen cost of exercise at the same absolute intensity when skeletal muscle relies on fat as opposed to CHO oxidation. Supplementation with exogenous ketones has been suggested as a strategy to improve endurance performance (16), with mechanistic data suggesting it is a more efficient metabolic fuel than CHO (26). However, despite using the same dose as reported previously (16), we failed to find differences in the oxygen cost of exercise when KE was acutely supplemented under conditions of HCHO availability, nor any alterations to V˙O2 or RER when it was repeated in athletes who had adapted to the LCHF. Furthermore, LCHF adaptation plus acute KE supplementation was associated with a universal reduction in performance of a real-life 10,000-m race walking event, equivalent to a mean performance loss of 6%, whereas performance in a control HCHO group was unchanged relative to a baseline race. These findings are similar to our previous experience with short-term (5–7 d) (11) and longer-term (3.5 wk) adaptation to LCHF alone (8,12), suggesting that acute KE supplementation does not have an individual or additive effect on exercise economy or performance.
Although adaptation to a ketogenic LCHF diet has become a popular topic in sports nutrition research (5) and practice (27), there is some controversy regarding the multisystem physiological adaptations as well as the timelines required to achieved this physiological state (8,13,28). Despite contentions that periods greater than 12 wk are required for full physiological benefits to occur (23,24,29), many studies confirm that a substantial increase in capacity for fat oxidation occurs within 3–4 wk of adherence to a LCHF diet (8,12,30), with recent evidence that the underpinning physiological adaptations may occur in as little as 5–6 d (11). In the current study, we elected to use a shorter (i.e., 5 d) adaptation period as we wanted to evaluate the efficacy of combining this diet with KE supplementation in the absence of significant changes in aerobic fitness. We saw a significant increase in capacity for fat oxidation in response to the LCHF intervention in this time frame, with peak oxidation rates increasing by ~300% to a maximum of ~14 ± 4 mg·kg−1·min−1 (0.86 ± 0.36 g·min−1). We note that these values, expressed as absolute rates, are lower than we have reported previously (11). This reflects the inclusion of females and younger athletes in the subject cohort, where lower BM and absolute power outputs result in a lower associated fuel cost (12). We acknowledge that longer adherence may achieve more robust whole-body metabolic adaptations. Indeed, we have demonstrated previously that 3 wk on a ketogenic LCHF diet (<50 g·d−1 CHO) can increase fat oxidation rates to ~1.5 g·min−1 in elite male race walkers (8). However, we note that maximum values of 1.4 g·min−1 (~22 mg·kg−1·min−1) were observed in male race walkers after 5–6 d of adaptation to LCHF, especially during longer protocols of exercise (e.g., a 2-h training session) (11). Our results are therefore consistent with previous work, demonstrating drastic shifts in substrate preference across a range of exercise intensities (~65%–90% V˙O2peak) that are of relevance for race walking performance.
Nutritional ketosis is defined as circulating concentrations of ketone bodies greater than 0.5 mmol·L−1 (9). In the current study, the LCHF diet achieved fasting βHB concentrations of 0.7 ± 0.4 mmol·L−1 on the morning of the second race. In contrast, it has been suggested that to improve performance through supplementation, ketone concentrations should exceed a 2-mmol·L−1 threshold (31). Our acute KE supplementation protocol involved an intake of 573 mg·kg−1 because this is the dose previously associated with performance benefits (16). This resulted in a significant increase in circulating βHB in the LCHF group with a concentration of ~3 mmol·L−1 being measured in all KE economy trials and 5 min before the second race. Although these concentrations are lower than what has been reported previously using this dosage (16), all laboratory testing and race trials in the current study were performed in the postprandial state. This has been shown to attenuate increases in circulating ketones, perhaps as a result of delayed gastric emptying (32). However, as concentrations were >2 mmol·L−1 for all metabolic trials, we are confident that our protocol achieved the optimal scenario in which to determine if there were synergistic effects of the two interventions.
Ketone supplementation has been suggested to improve performance because of the proposed thermodynamic advantages of ketone oxidation (26,33,34). Collectively, these studies have demonstrated an increase in ATP production when 2-carbon units from d-β-hydroxybutyrate are oxidized in the citric acid cycle, in comparison with those derived from pyruvate (CHO pathway) or from fat. If this improvement in bioenergetic efficiency was translated to a whole-body level, one would expect to see a decrease in the oxygen cost of exercise (i.e., a decrease in V˙O2) at the same absolute exercise intensity. KE consumption did not alter relative V˙O2 in the two trials performed at baseline, which is supported by a growing body of literature demonstrating no change in oxygen uptake or energy expenditure after supplementation with ketone salts (35–38) or esters (16,17,39,40). In contrast, comparisons between the two trials performed after the dietary adaptation period show a small but statistically significant increase in V˙O2 during stages 3 and 4. The HCHO group also displayed an increase in the relative V˙O2 at the same absolute speed during the last two stages after adaptation, which may be indicative of fatigue. However, in the HCHO group, this occurred without any shifts in RER, signifying no change in fuel preference, and there were also no significant changes in the training volume performed when on the habitual diet (phase 2) or during adaptation (see Table, Supplemental Digital Content 1, Subject characteristics of elite race walkers, http://links.lww.com/MSS/C145), or in the 10,000-m race performance in the HCHO group.
Limitations
A major limitation of this study is the inability to accurately quantify the uptake and utilization of ketones by skeletal muscle. Currently, the use of indirect calorimetry is not validated for calculation of ketone oxidation (6), with the respiratory quotients of ketone bodies being similar to that of glucose (βHB = 0.89, acetoacetate = 1.0) (21). As a result, we cannot conclusively determine the contribution of ketones to total energy production and have instead relied on whole-body measures such as V˙O2 and circulating metabolites. In this regard, the use of tracers and/or skeletal muscle biopsies in subsequent studies is warranted. Furthermore, this will also help to determine whether an LCHF diet upregulates key molecular machinery required for ketone oxidation in humans. A perceived limitation may also be the inclusion of female athletes within the larger cohort. We have previously published data on a similar cohort, including female athletes with a more prolonged LCHF dietary intervention (12), which replicated early work performed only in men (8). We are therefore confident in our decision to include female athletes in the current study, of which there were two female athletes in the HCHO group and one in the LCHF. This was initially balanced; however, one of the female athletes developed an injury after the first race and was therefore unable to complete the adaptation trial. Finally, we note that athletes were not randomly assigned to dietary treatments; rather, they were asked to nominate their preferred diet to enhance placebo or belief effects. Although this may be a possible limitation when interpreting the data, we feel this was justified to strengthen the real-world application of results.
CONCLUSIONS
The current study sought to determine whether KE ingestion could counter the decrease in exercise economy associated with short-term adaptation to a ketogenic LCHF diet while retaining the increased capacity for ketone and fat oxidation in a cohort of well-trained race walkers. We demonstrated that supplementation with KE did not improve exercise economy before or after a 5-d ketogenic LCHF diet. Critically, race performance was impaired by ~6% in these athletes, despite increased ketone availability. In contrast, athletes who consumed an HCHO diet were able to compete unhindered, resulting in several lifetime best performances.
Supplementary Material
Acknowledgments
This study was funded by a Program Grant from the Australian Catholic University Research Fund (ACURF, grant no. 2017000034) awarded to L. M. B. J. W. is supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Postdoctoral Fellowship.
This study was conducted at the Victorian Institute for Sport and Australian Catholic University. Design of the experiments was undertaken by J. W., L. M. B., A. K. A. M., I. A. H., R. H., and A. P. S. All authors contributed to the collection, assembly, analysis, and interpretation of data. Drafting the article or revising it critically for important intellectual content was undertaken by J. W. and L. M. B. All authors edited and approved the final manuscript.
The authors do not have any conflicts to disclose. The results of the present study have been presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation and do not constitute endorsement by the American College of Sports Medicine.
Footnotes
J. W. and L. M. B. contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
LOUISE M. BURKE, Email: Louise.Burke@ausport.gov.au.
ALANNAH K. A. MCKAY, Email: 20929928@student.uwa.edu.au.
IDA A. HEIKURA, Email: ida.heikura@gmail.com.
REBECCA HALL, Email: Rebecca.Hall@acu.edu.au.
NIKITA FENSHAM, Email: nikita.fensham@myacu.edu.au.
AVISH P. SHARMA, Email: avishsharma@gmail.com.
REFERENCES
- 1.Hargreaves M, Spriet LL. Exercise metabolism: fuels for the fire. Cold Spring Harb Perspect Med. 2018;8(8):a029744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke LM, Jeukendrup AE, Jones AM, Mooses M. Contemporary nutrition strategies to optimize performance in distance runners and race walkers. Int J Sport Nutr Exerc Metab. 2019;29(2):117–29. [DOI] [PubMed] [Google Scholar]
- 3.Stellingwerff T, Bovim IM, Whitfield J. Contemporary nutrition interventions to optimize performance in middle-distance runners. Int J Sport Nutr Exerc Metab. 2019;29(2):106–16. [DOI] [PubMed] [Google Scholar]
- 4.van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536(1):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke LM, Hawley JA, Jeukendrup A, Morton JP, Stellingwerff T, Maughan RJ. Toward a common understanding of diet-exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab. 2018;28(5):451–63. [DOI] [PubMed] [Google Scholar]
- 6.Shaw DM, Merien F, Braakhuis A, Maunder E, Dulson DK. Exogenous ketone supplementation and keto-adaptation for endurance performance: disentangling the effects of two distinct metabolic states. Sports Med. 2020;50(4):641–56. [DOI] [PubMed] [Google Scholar]
- 7.Phinney SD, Bistrian BR, Wolfe RR, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism. 1983;32(8):757–68. [DOI] [PubMed] [Google Scholar]
- 8.Burke LM Ross ML Garvican-Lewis LA, et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017;595(9):2785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15(1):13–20. [DOI] [PubMed] [Google Scholar]
- 10.Burke LM, Hawley JA. Effects of short-term fat adaptation on metabolism and performance of prolonged exercise. Med Sci Sports Exerc. 2002;34(9):1492–8. [DOI] [PubMed] [Google Scholar]
- 11.Burke LM Whitfield J Heikura IA, et al. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J Physiol. 2021;599(3):771–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke LM Sharma AP Heikura IA, et al. Crisis of confidence averted: impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS One. 2020;15(6):e0234027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke LM. Ketogenic low-CHO, high-fat diet: the future of elite endurance sport? J Physiol. 2021;599(3):819–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan B, D’Agostino DP. Fueling performance: ketones enter the mix. Cell Metab. 2016;24(3):373–5. [DOI] [PubMed] [Google Scholar]
- 15.Clarke K Tchabanenko K Pawlosky R, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63(3):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox PJ Kirk T Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256–68. [DOI] [PubMed] [Google Scholar]
- 17.Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sports Exerc. 2019;51(12):2506–15. [DOI] [PubMed] [Google Scholar]
- 18.Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exerc. 2018;50(11):2330–8. [DOI] [PubMed] [Google Scholar]
- 19.Dearlove DJ, Faull OK, Rolls E, Clarke K, Cox PJ. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol. 2019;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirtschin JG Forbes SF Cato LE, et al. Organization of dietary control for nutrition-training intervention involving periodized carbohydrate availability and ketogenic low-carbohydrate high-fat diet. Int J Sport Nutr Exerc Metab. 2018;28(5):480–9. [DOI] [PubMed] [Google Scholar]
- 21.Cox PJ, Clarke K. Acute nutritional ketosis: implications for exercise performance and metabolism. Extrem Physiol Med. 2014;3(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16(1):23–9. [PubMed] [Google Scholar]
- 23.Volek JS Freidenreich DJ Saenz C, et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2016;65(3):100–10. [DOI] [PubMed] [Google Scholar]
- 24.Webster CC, Noakes TD, Chacko SK, Swart J, Kohn TA, Smith JAH. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high-fat diet. J Physiol. 2016;594(15):4389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):628–34. [DOI] [PubMed] [Google Scholar]
- 26.Murray AJ Knight NS Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30(12):4021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McSwiney FT, Doyle L, Plews DJ, Zinn C. Impact of ketogenic diet on athletes: current insights. Open Access J Sports Med. 2019;10:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindseth I. Methodological issues question the validity of observed performance impairment of a low carbohydrate, high fat diet. J Physiol. 2017;595(9):2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, Doyle L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism. 2018;81:25–34. [DOI] [PubMed] [Google Scholar]
- 30.Shaw DM, Merien F, Braakhuis A, Maunder ED, Dulson DK. Effect of a ketogenic diet on submaximal exercise capacity and efficiency in runners. Med Sci Sports Exerc. 2019;51(10):2135–46. [DOI] [PubMed] [Google Scholar]
- 31.Margolis LM, O’Fallon KS. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2020;11(2):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stubbs BJ Cox PJ Evans RD, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veech RL. Ketone ester effects on metabolism and transcription. J Lipid Res. 2014;55(10):2004–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K Kashiwaya Y Keon CA, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9(8):651–8. [DOI] [PubMed] [Google Scholar]
- 35.Evans M, Patchett E, Nally R, Kearns R, Larney M, Egan B. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur J Sport Sci. 2018;18(3):376–86. [DOI] [PubMed] [Google Scholar]
- 36.O’Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab. 2017;42(10):1031–5. [DOI] [PubMed] [Google Scholar]
- 37.Rodger S, Plews D, Laursen P, Driller M. Oral β-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise. J Sci Cycl. 2017;6(1):26–31. [Google Scholar]
- 38.Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott BE Laursen PB James LJ, et al. The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport. 2019;22(6):702–6. [DOI] [PubMed] [Google Scholar]
- 40.Shaw DM, Merien F, Braakhuis A, Plews D, Laursen P, Dulson DK. The effect of 1,3-butanediol on cycling time-trial performance. Int J Sport Nutr Exerc Metab. 2019;29(5):466–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


