Supplemental digital content is available in the text.
Key Words: HAMSTRINGS, BI- AND MONOARTICULAR MUSCLES, MUSCLE VOLUME, T2
ABSTRACT
The biarticular hamstrings are lengthened more in a seated (hip-flexed) than prone (hip-extended) position.
Purpose
We investigated the effects of seated versus prone leg curl training on hamstrings muscle hypertrophy and susceptibility to eccentric exercise-induced muscle damage.
Methods
Part 1: Twenty healthy adults conducted seated leg curl training with one leg (Seated-Leg) and prone with the other (Prone-Leg), at 70% one-repetition maximum (1RM), 10 repetitions per set, 5 sets per session, 2 sessions per week for 12 wk. Magnetic resonance imaging (MRI)–measured muscle volume of the individual and whole hamstrings was assessed pre- and posttraining. Part 2: Nineteen participants from part 1 and another 12 untrained controls (Control-Leg) performed eccentric phase-only leg curl exercise at 90% 1RM, 10 repetitions per set, 3 sets for each of the seated/prone conditions with each leg. MRI-measured transverse relaxation time (T2) and 1RM of seated/prone leg curl were assessed before, 24, 48, and 72 h after exercise.
Results
Part 1: Training-induced increases in muscle volume were greater in Seated-Leg versus Prone-Leg for the whole hamstrings (+14% vs +9%) and each biarticular (+8%–24% vs +4%–19%), but not monoarticular (+10% vs +9%), hamstring muscle. Part 2: After eccentric exercise, Control-Leg had greater increases in T2 in each hamstring muscle (e.g., semitendinosus at 72 h: +52%) than Seated-Leg (+4%) and Prone-Leg (+6%). Decreases in 1RM were also greater in Control-Leg (e.g., seated/prone 1RM at 24 h: −12%/−24%) than Seated-Leg (0%/−3%) and Prone-Leg (+2%/−5%). None of the changes significantly differed between Seated-Leg and Prone-Leg at any time points.
Conclusion
Hamstrings muscle size can be more effectively increased by seated than prone leg curl training, suggesting that training at long muscle lengths promotes muscle hypertrophy, but both are similarly effective in reducing susceptibility to muscle damage.
Strengthening the hamstrings is thought to improve sprint performance (1) and reduce the risk of hamstring strain injury (2), therefore giving benefits to many athletes and sports enthusiasts. The single-joint knee flexion (“leg curl”) is one of the most common exercises to train the hamstrings because it isolates the target muscles by using a weight machine, which stabilizes the body and prevents excessive joint movement (3,4). In fact, there is considerable evidence that single-joint leg curl training can increase strength and size of the hamstrings (5–7).
The leg curl can be performed in seated and prone positions, between which there is a marked difference in the hip joint angle, therefore the muscle lengths of the hamstrings. Namely, because of the biarticular nature of three out of four hamstring muscles, they are lengthened more in the seated (hip-flexed) than prone (hip-extended) position (8–10) (Fig. 1). Some studies (11–13) reported that training-induced muscle hypertrophy was greater when trained at long versus short muscle lengths, but others (14–16) did not find a statistically significant difference. This discrepancy seems at least partly due to relatively small sample sizes (n = 8–13 per condition) (11–16) and/or short intervention periods (6–8 wk) (11–14,16) of these studies. Importantly, the previous studies all adopted isometric (11,12,14,15) or partial range of motion (13,16) training at different joint angles about the same joint to compare long versus short muscle length conditions (e.g., knee flexed vs extended positions to train the quadriceps). However, it is generally recommended that resistance training be performed with full range of motion for general fitness (4) and muscle hypertrophy (17). Thus, exploring the effect of seated versus prone leg curl training, performed with full range of motion, on hamstrings muscle hypertrophy would be useful from both basic research and applied points of view.
FIGURE 1.
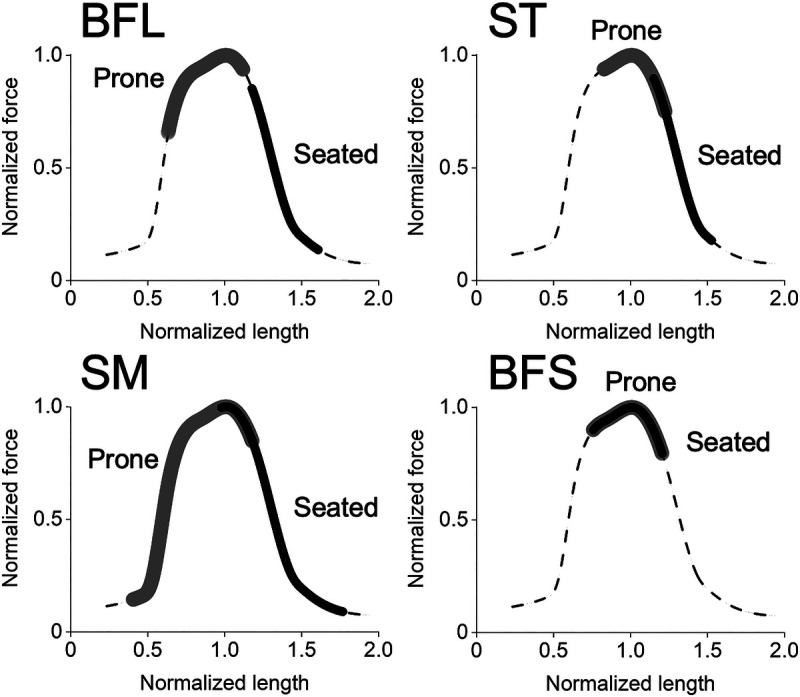
Operating ranges of each hamstring muscle on the normalized force–length curve during the seated and prone knee flexion (leg curl) exercise. These were calculated using the OpenSim Lower Limb model (8,9), with the hip joint angle at 90° and 30° for the seated and prone conditions, respectively, and the knee joint angle ranging from 0° to 90° for both conditions as conducted in this study. It is clearly seen that the three biarticular hamstrings (BFL, ST, and SM) operate at longer muscle lengths during the seated than prone condition, while there is no difference in the monoarticular BFS. Some differences within the biarticular muscles are likely due to their different musculotendinous architecture and moment arms (10).
Although it is challenging to test the protective effect of any kind of interventions against muscle strain injury that is multifactorial, some insights can be gained by examining its effect on susceptibility to exercise-induced muscle damage, which manifests as decreased muscle function and muscle swelling/edema (18). Both muscle damage and strain injury are triggered by eccentric contractions, and it is suggested that there is a link between susceptibility to damage and likelihood of strain injury (19,20). Eccentric exercise-induced muscle damage is known to be mitigated by performing priming exercise, with a greater effect conferred when the exercise had been performed at long versus short muscle lengths (21). Thus, it is envisaged that the protective effect against muscle damage would be greater for seated versus prone leg curl training, but this has never been investigated.
The purpose of this study was to examine the effects of seated versus prone leg curl training on hamstrings muscle hypertrophy and susceptibility to eccentric exercise-induced muscle damage. To this end, we designed a two-part study. Part 1 involved intervention of seated versus prone leg curl training. Subsequently in part 2, eccentric exercise was performed by those who had undergone part 1 as well as by another cohort of untrained controls to examine the effectiveness of the previous seated and prone leg curl training on preventing muscle damage. The primary measures of this study were MRI-measured muscle volume for part 1 and transverse relaxation time (T2) for part 2 to evaluate training-induced muscle hypertrophy and exercise-induced muscle edema of each hamstring muscle, respectively. Because hamstring strain injuries most commonly occur in the biceps femoris long head (BFL) and secondly in the semitendinosus (ST) muscles, often at their musculotendinous junctions (22,23), these muscles and regions were analyzed in detail. We hypothesized that 1) hypertrophic effects would be greater for the seated than prone leg curl training and 2) protective effects would also be greater for the seated than prone leg curl training.
METHODS
Study Design and Participants
This study was approved by the Ethics Committee of Ritsumeikan University (BKC-IRB-2018-087) and consisted of two parts: training intervention (part 1) and eccentric exercise (part 2) (Fig. 2). In part 1, 20 young adults conducted 12-wk unilateral leg curl training in a seated position with one leg (Seated-Leg) and in a prone position with the other leg (Prone-Leg). After the intervention, 19 participants from part 1 and another 12 young adults as untrained controls (Control-Leg) performed unilateral eccentric phase-only leg curl exercise in each of the seated and prone positions with each leg in part 2. One participant from part 1 did not attend part 2 due to an unrelated reason to this study. Participants were recruited by convenience sampling for attending both part 1 and part 2 or only part 2 depending on their schedule availability (Fig. 2), until the sample sizes for each condition/leg reached about 20. The participants were all healthy, but none had been involved in any type of systematic resistance training program in the past 12 months. Written informed consent was obtained from each participant.
FIGURE 2.
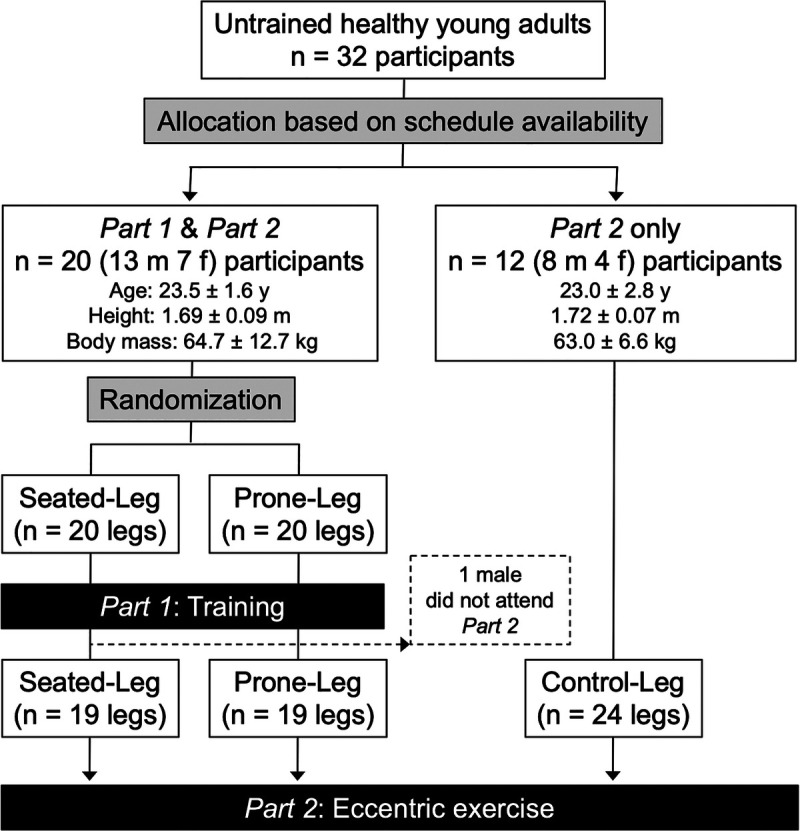
Flow diagram and demographic information of participants.
Part 1
Training program
Each leg was randomly assigned to Seated-Leg or Prone-Leg, with the dominant and nondominant legs counterbalanced by the use of a computer-generated list. Both legs were trained unilaterally with the assigned training condition, by using a modified (a backrest inserted) seated leg curl machine (Pro 2 Series, Life Fitness, Chicago, IL) with the hip joint fixed at ~90° and a prone leg curl machine (Toredo, Senoh, Japan) with the hip joint fixed at ~30° (0° = anatomical position). The knee joint range of motion was 0°–90° for both conditions, which we defined as the full range of motion to standardize it among participants and between conditions/legs. Adjustable straps were tightly fastened across the pelvis to prevent extraneous movement. Individualized machine settings (i.e., joint and seat positions) were kept the same throughout the study for each participant.
Each training session commenced with 5–10 warm-up repetitions at 50% of the load prescribed for that session (detailed below) with the assigned training condition. Participants then performed the seated or prone leg curl 10 repetitions per set for 5 sets, taking 2 s for each of the concentric (knee flexing) and eccentric (knee extending) phases without a pausing phase with the guide of a metronome (60 bpm). Two-minute rest intervals were taken in between sets. After training one leg (5 sets), the other leg was trained. The preceding leg was counterbalanced in the first training session among participants, and it was switched every session for each participant. Training was conducted twice per week, separated by ≥~48 h. Training load was gradually increased at the first, second, and third sessions from 50%, 60%, and 70% of one-repetition maximum (1RM) measured pretraining (detailed below), respectively, and 70% of 1RM was used thereafter. At least one examiner always supervised the training sessions and provided verbal encouragement and also corrected the joint positions and/or movement speed of the exercise when necessary. The examiners also assisted (spotted) the participants in executing the exercise when they could no longer repeat the repetitions up to 10 in each set. This was done in such a way that the participants could complete the task in a controlled manner with their continuous (~maximum) efforts, which accounted for 20% of all repetitions (typically in the final 1–2 sets within a session). If the participants could complete all of the prescribed protocol at the third session and thereafter without examiners’ assist, +5% of 1RM was added at the subsequent sessions.
Measurements
All participants attended three measurement sessions; two sessions before the training period (Pre-TR 1 and Pre-TR 2) separated by 2–7 d (≥~72 h) and one session after the training period (Post-TR) 2–4 d after the final training session. Participants were instructed to avoid any intensive and unfamiliar physical activities within 2 d before Pre-TR 1 and throughout the experimental period. The following variables were measured.
1RM
1RM was measured in each leg with the assigned training condition using the same machines as for the training. At Pre-TR 1, participants were familiarized with the exercise by performing 3–5 repetitions with a light load, which was gradually increased (three to five stages) with a short rest period (10–20 s) until the participants felt it somewhat heavy. Thereafter, only 1 repetition was performed at each increasing load by increments of 2.0 kg and 1.7 kg for the seated and prone leg curl, respectively, with ≥2 min rest in between trials. 1RM was defined as the maximum load lifted with the proper joint positions, which was checked by the examiner(s). At Pre-TR 2, participants performed 5, 3, and 1 warm-up repetitions at 50%, 75%, and 90% of 1RM of Pre-TR 1, respectively, with 30 s rest, and tried the same 1RM load of Pre-TR 1 after ≥2 min rest. 1RM was determined by increasing or decreasing the load thereafter. At Post-TR, the warm-up and the 1RM assessment were similarly conducted but based on the 1RM of Pre-TR 2. The training load and its increments in the training sessions described above were also based on the 1RM of Pre-TR 2. The mean within-participant coefficient of variation between the two pretraining sessions was 6.8% and 3.4% for the seated and prone 1RM, respectively.
T1-MRI
Preceding the 1RM measurement, longitudinal relaxation time (T1)–weighted cross-sectional MRI scans of the thigh were obtained for each leg using body array and spine coils (Body 18 and CP Spine Array Coil; Siemens Healthineer, Erlangen, Germany) with the following parameters: field of view, 200 × 200 mm; matrix, 512 × 512; slice thickness, 5 mm; voxel size, 0.39 × 0.39 × 5 mm; in-plane resolution, 0.39 mm; TR, 700 ms; TE, 10 ms; flip angle, 120°; number of channels, 1; gap, 5 mm; number of slices, 21 × 3 blocks (Fig. 3). Participants lay supine with their legs extended and muscles relaxed in a 3-T magnet bore (MAGNETOM Skyra, Siemens Healthineer). To obtain cross-sectional images at the same positions throughout the study within each participant, the following steps were taken: 1) by using coronal localizer images, the thigh length was measured as the distance between the greater trochanter and the distal end of the femur, 2) the center slice of a block of 21 slices (i.e., the 11th) was set at 50% of the thigh length, 3) the block was then moved proximally with the fixed 5-mm slice gap/increment until the most proximal slice was set at slightly above the ischial tuberosity (i.e., the origin for the biarticular hamstrings), and 4) 3 series of blocks were taken to cover the whole hamstrings (WH).
FIGURE 3.
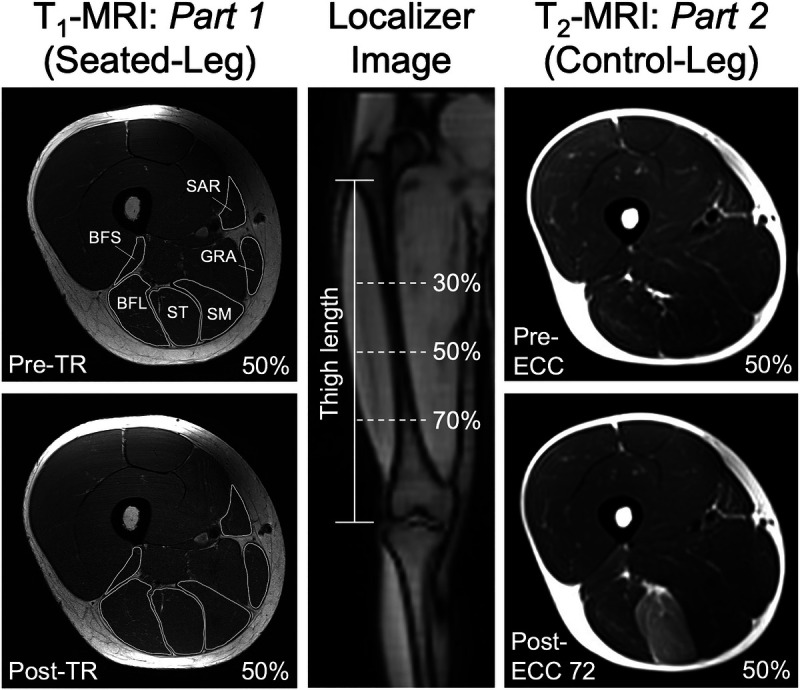
Example images for the T1-weighted MRI scans (for Seated-Leg in part 1) and T2-weighted MRI scans (for Control-Leg in part 2) at 50% of the thigh length, and a coronal localizer image. The images shown here are all for the right leg, but both legs were scanned in both part 1 and part 2.
Images were analyzed by using image analysis software (Horos, Horos Project), with the MRI data anonymized and investigators blinded to the training conditions. Anatomical cross-sectional areas (ACSA) of the individual hamstring muscles (i.e., BFL, ST, semimembranosus [SM], and biceps femoris short head [BFS]) were manually outlined in every other image from the most proximal to the most distal image in which the muscle was visible. In addition, the gracilis (GRA) and sartorius (SAR) muscles, which also work as knee flexors located in the thigh, were analyzed in the same manner as above. Because the origin for the SAR is the anterior superior iliac spine, the most proximal parts of this muscle were not fully covered in this study. Thus, the SAR was analyzed from the image at the ischial tuberosity to the most distal image in which this muscle was visible. Care was taken to exclude visible adipose and connective tissue incursions. ACSA for the skipped images and gaps was estimated based on linear interpolation between the images in which ACSA was outlined (24). The volume of individual muscle was determined by summing all ACSA for that muscle multiplied by the slice thickness. The WH volume was calculated by summing the individual muscle volumes of the four hamstring muscles. To explore the effects of seated versus prone leg curl training on muscle hypertrophy at the most commonly injured locations within the hamstrings, changes in ACSA of the BFL and ST at 30% (proximal) and 70% (distal) of the thigh length were measured using the nearest slices to these locations (named as BFLProximal, BFLDistal, etc.). The mean within-participant coefficient of variations between the two pretraining sessions for the muscle size measures were as follows: muscle volume of the WH, 1.4%; BFL, 1.6%; ST, 1.7%; SM, 1.4%; BFS, 2.1%; GRA, 1.4%; SAR, 1.5%; ACSA of the BFLProximal, 5.2%; BFLDistal, 5.2%; STProximal, 2.1%; STDistal, 4.0%.
Part 2
Eccentric exercise
Using the same leg curl machines as above, participants performed unilateral eccentric phase-only leg curl exercise at 90% of 1RM (detailed below), 10 repetitions per set, 3 sets in each of the seated and prone conditions (6 sets in total) with each leg (18). During these exercises, the examiner(s) moved (pushed/lifted) the load to the starting position (knee joint, 90°), and the participants moved the load to the finish position (knee joint, 0°) by performing eccentric contractions of the knee flexors in a controlled manner over a 2-s count with the guide of a metronome. Two-second between-repetition intervals were taken, during which the load was moved back to the starting position by the examiner (18). After completing 1 set with one leg in either the seated or the prone condition, the order of which was counterbalanced among participants, the other leg performed the same eccentric exercise (based on its 1RM) with a 1-min interval. This was repeated until each leg performed 3 sets. After a rest period of 5 min, participants then performed the eccentric exercise in the other condition. Participants familiarized themselves with the eccentric exercise using a light load (5 repetitions with ~50% of 1RM) and took at least 2 min rest before conducting the actual eccentric task.
Measurements
Before (Pre-ECC) and 24, 48, and 72 h after (Post-ECC 24–72) the eccentric exercise, 1RM and MRI-measured T2 were assessed as indices of muscle damage markers (18,25). The Pre-ECC measurement in part 2 was included in the Post-TR measurement in part 1 for those who underwent both parts. Measurements were conducted as follows.
1RM
1RM was assessed in the same manner as described in part 1, but in both seated and prone conditions with each leg in part 2. Briefly, the seated or prone leg curl 1RM was assessed in each leg after a warm-up, alternatively between legs with 1-min intervals (i.e., ≥2 min rest for the same leg). Orders of the conditions and legs were counterbalanced among participants. After assessing 1 RM in the preceding condition, participants took at least 2 min rest and proceeded to the other condition.
T2-MRI
T2-weighted MRI scans for both thighs were conducted using the same device described above, with the following parameters: field of view, 450 × 450 mm; matrix, 256 × 256; slice thickness, 4 mm; voxel size, 1.76 × 1.76 × 4 mm; in-plane resolution, 1.76 mm; TR, 2000 ms; TE, 10 increments from 10 to 100 ms; flip angle, 180°; number of channels, 1; gap, 12 mm; number of slices, 15 × 1 block (Fig. 3). The thigh length was measured as described above, and the center slice of a block of 15 slices (i.e., the 7th) was set at 50% of the thigh length. One block of T1-weighted MRI scans, centered at the 50% of the thigh length, was also taken for each thigh with the parameters described in the part 1 section, which was used as a guide to outline the ACSA of each hamstring muscle in T2 images. Images were analyzed using Osirix Lite software (Pixmeo, Geneva, Switzerland). Regions of interest for each of the four hamstrings were outlined at 50% of the thigh length (18). The BFL and the ST were additionally analyzed at 30% and 70% of the thigh length using the nearest slices to these locations (but only for two time points of Pre-ECC and Post-ECC 72 h). T2 relaxation time was calculated by least squares analysis fitting the signal intensity at each of the 10 echo times (n × 10 ms: 10 to 100 ms at 10 incremental steps) to a monoexponential decay using the following equation:
where TE is echo time, S0 is signal intensity at 0 ms, and Sn is signal intensity at TEn.
Statistical analysis
All data were analyzed using SPSS software (version 25; IBM Corp, USA) unless otherwise noted. Statistical significance was set at P < 0.05. For part 1, data from the two pretraining sessions were averaged and used for further analysis as Pre-TR values. An ANCOVA was used to compare changes in muscle size measures (volume and ACSA) between Seated-Leg and Prone-Leg, with the Pre-TR values as covariates and the Post-TR values as the dependent variables. A linear mixed-effects model was used with a subject as a random effect and a leg (training condition) as a fixed effect. Because the 1RM in part 1 was measured under the different conditions between the legs (i.e., seated 1RM or prone 1RM), the Post-TR values were z-scored using the Pre-TR mean and SD for each condition [i.e., the z-score = (individual Post-TR value – Pre-TR mean)/Pre-TR SD] and were compared between the legs by a paired t-test. For part 2, a one-way ANCOVA was used to compare changes in muscle damage indices (T2 and 1RM) between Seated-Leg, Prone-leg, and Control-Leg, with the Pre-ECC values as covariates and the values at each of the Post-ECC time points as the dependent variables. A general linear model with a least significant difference (LSD) post hoc test was used. Residuals were checked for normality and homoscedasticity by Shapiro–Wilk’s test and Levene’s test, respectively, and some data sets for T2 and 1RM in part 2 were rejected by both or either of these. However, the main statistical results were the same when these data sets were analyzed with either an ANCOVA or equivalent nonparametric test (rank-transformed values [Quade’s test]). Thus, all results are shown as those based on ANCOVA with raw values (except for z-score s in 1RM for part 1) for ease of interpretation. Finally, to improve statistical inference, mean difference from baseline on raw data with their bootstrap 95% confidence interval (CI) was calculated by using estimation statistics (26). Detailed descriptive and test statistics are provided in Supplemental Digital Contents.
RESULTS
Part 1
Muscle volume
There were significant differences in mean muscle volume change between the legs in each of the WH, BFL, ST, SM, and SAR (P ≤ 0.010), but not in the BFS (P = 0.190) and GRA (P = 0.097) (Fig. 4; see Table for details, Supplemental Digital Content 1, Muscle volume before and after leg curl training, http://links.lww.com/MSS/C152). The increases in muscle volume were greater for Seated-Leg than Prone-Leg in the WH (ANCOVA-adjusted mean change: +14.1% vs +9.3%), BFL (+14.4% vs +6.5%), ST (+23.6% vs +19.3%), and SM (+8.2% vs +3.6%). By contrast, the change in the SAR was smaller for Seated-Leg than Prone-Leg (+7.8% vs +11.8%).
FIGURE 4.
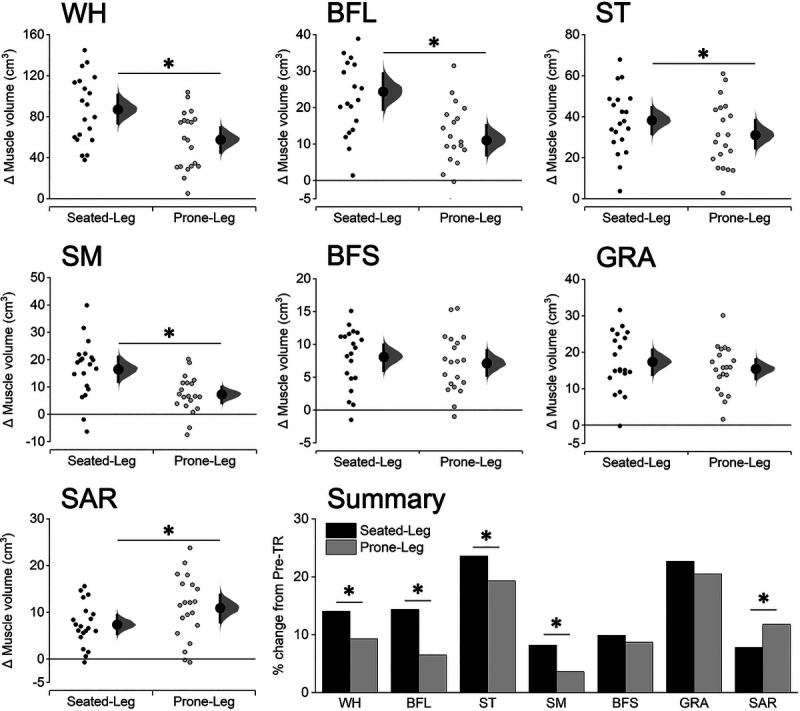
Changes in muscle volume for Seated-Leg and Prone-Leg after training. Data are plotted as individual raw change (Δ) values from baseline (small dots), with a group mean (larger dots) and its 95% CI (indicated by the ends of the vertical error bars) shown together. The CI and the bootstrap sampling distributions (5000 samples, bias-corrected and accelerated) were obtained from respective paired (pre- to posttraining) data. *Significant difference between legs at P < 0.05 based on a baseline-adjusted ANCOVA. n = 20 legs for both Seated-Leg and Prone-Leg. The bar graphs in the summary figure are based on the mean changes for each muscle.
ACSA
Changes in ACSA of the BFL and ST at the proximal and distal regions were significantly different between the legs in the BFLProximal (+20.8% vs +8.7%), BFLDistal (+10.7% vs +5.4%), and STProximal (+28.2% vs +21.1%), all showing greater increases for Seated-Leg than Prone-Leg (P = 0.002–0.039), but not in the STDistal (+21.4% vs +17.0%, P = 0.107) (Fig. 5; see Table for details, Supplemental Digital Content 2, ACSA before and after leg curl training, http://links.lww.com/MSS/C153).
FIGURE 5.
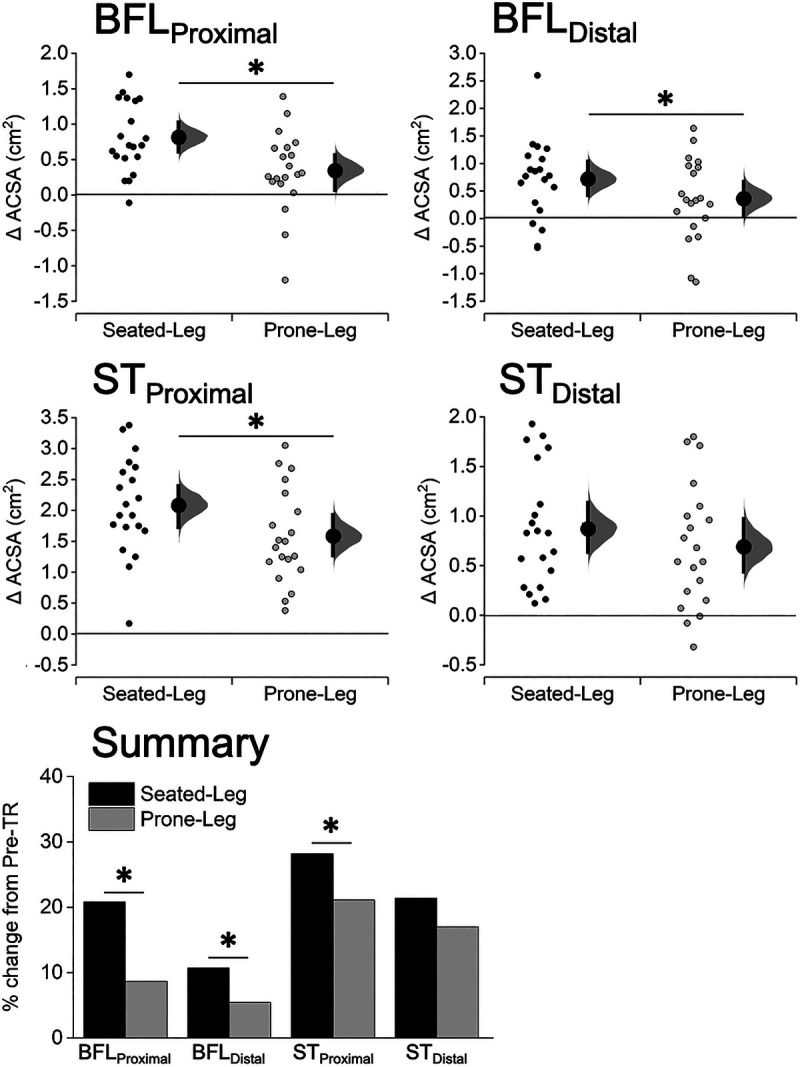
Changes in ACSA of the BFL and ST at 30% (BFLProximal, STProximal) and 70% (BFLDistal, STDistal) of the thigh length for Seated-Leg and Prone-Leg after training. Data are plotted as individual raw change (Δ) values from baseline (small dots), with a group mean (larger dots) and its 95% CI (indicated by the ends of the vertical error bars) shown together. The CI and the bootstrap sampling distributions (5000 samples, bias-corrected and accelerated) were obtained from respective paired (pre- to posttraining) data. *Significant difference between legs at P < 0.05 based on a baseline-adjusted ANCOVA. n = 20 legs for both Seated-Leg and Prone-Leg. The bar graphs in the summary figure are based on the mean changes for each muscle.
1RM
Changes in 1 RM as z-scores did not differ between Seated-Leg and Prone-Leg (0.81 ± 1.25 vs 0.76 ± 1.14, t19 = 0.442, P = 0.663). The mean ± SD values and the mean changes were as follows: Seated-Leg: Pre-TR = 40.9 ± 15.7 kg, Post-TR = 53.6 ± 19.6 kg, +31.1%; Prone-Leg: 23.9 ± 8.3 kg, 30.3 ± 9.5 kg, +26.6%.
Part 2
T2
T2 changes at the mid-thigh were more prominent at Post-ECC 72 h than Post-ECC 24–48 h for all hamstrings, so only the data for Post-ECC 72 h are shown in Figure 6, and those for Post-ECC 24–48 h are available in a supplement (see Table/Figure for details, Supplemental Digital Content 3, T2 before and after eccentric exercise, http://links.lww.com/MSS/C154). A one-way ANCOVA revealed significant differences in mean T2 change between the legs at Post-ECC 72 h in each hamstring muscle at the mid-thigh (Fig. 6). The changes in T2 at Post-ECC 72 h were greater (P ≤ 0.035) for Control-Leg than Seated-Leg and Prone-Leg in each of the BFL (+3.8% vs +0.3% vs +0.4%), ST (+56.9% vs +2.0% vs +5.7%), SM (+3.0% vs −1.8% vs −0.7%), and BFS (+5.5% vs +0.6% vs +0.1%). The T2 changes in the ST at 48 h were also greater (P ≤ 0.002) for Control-Leg than Seated-Leg and Prone-Leg (+26.9% vs +2.6% vs +4.4%), and those in the SM at 24 h were greater (P = 0.004) for Control-Leg than Seated-Leg (+2.4% vs −1.1%) but not than Prone-Leg (+0.5%, P = 0.103). There were no significant differences between Seated-Leg and Prone-Leg in all muscles at any time points (P ≥ 0.210).
FIGURE 6.
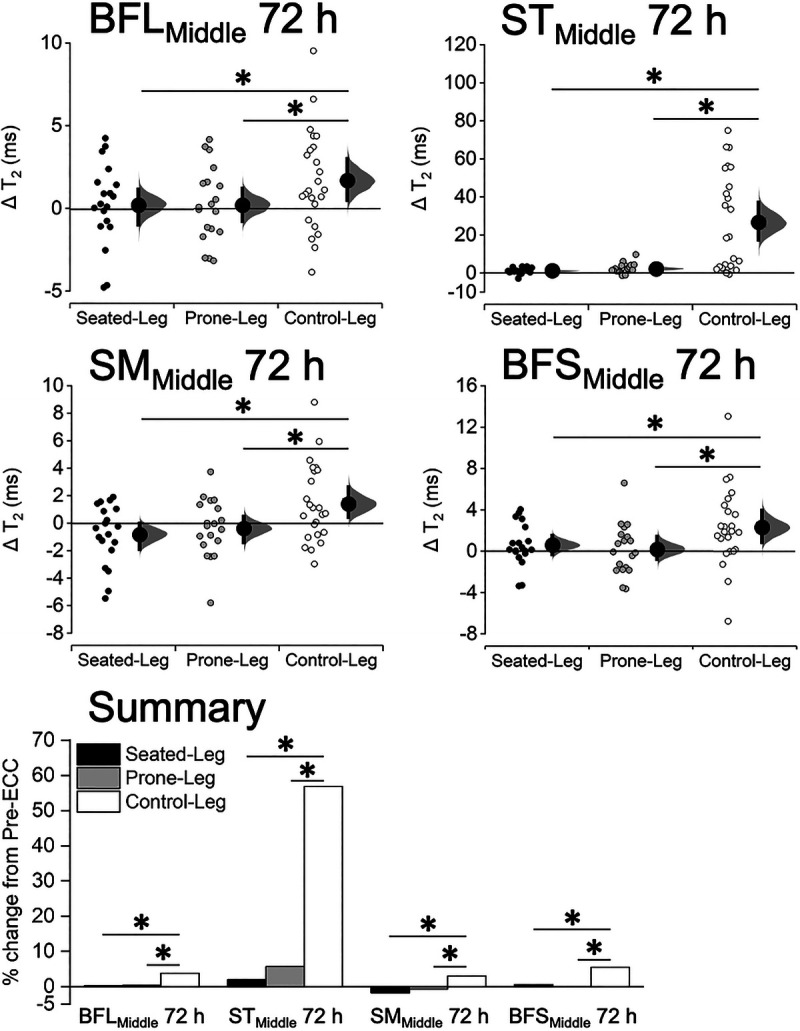
Changes in T2 of each hamstring muscle at 50% of the thigh length (BFLMiddle, STMiddle, SMMiddle, BFSMiddle) at 72 h after eccentric exercise for Seated-Leg, Prone-Leg, and Control-Leg. Data are plotted as individual raw change (Δ) values from baseline (small dots), with a group mean (larger dots) and its 95% CI (indicated by the ends of the vertical error bars) shown together. The CI and the bootstrap sampling distributions (5000 samples, bias-corrected and accelerated) were obtained from respective paired (pre- to postexercise) data. *Significant difference between legs at P < 0.05 based on a baseline-adjusted ANCOVA and an LSD post hoc test. n = 19 legs for both Seated-Leg and Prone-Leg, and 24 legs for Control-Leg. The bar graphs in the summary figure are based on the mean changes for each muscle.
At the proximal and distal regions of the BFL and ST, T2 changes at Post-ECC 72 h were significantly (P ≤ 0.046) different between the legs (Fig. 7; see Table for details, Supplemental Digital Content 4, T2 changes at the proximal/distal thigh, http://links.lww.com/MSS/C155). The changes at the BFLProximal were greater for Control-Leg than Seated-Leg (+6.5% vs +1.1%, P = 0.019) but not than Prone-Leg (+2.6%, P = 0.090). Similarly, those at the BFLDistal were significantly greater for Control-Leg than Seated-Leg (+4.8% vs +1.4%, P = 0.030) but not than Prone-Leg (+1.8%, P = 0.052). Both STProximal (+59.2% vs +4.2% vs 8.5%) and STDistal (+39.6% vs +6.1% vs 5.1%) had greater T2 changes for Control-Leg than Seated-Leg and Prone-Leg (P < 0.001). No significant differences were found between Seated-Leg and Prone-Leg (P ≥ 0.515).
FIGURE 7.
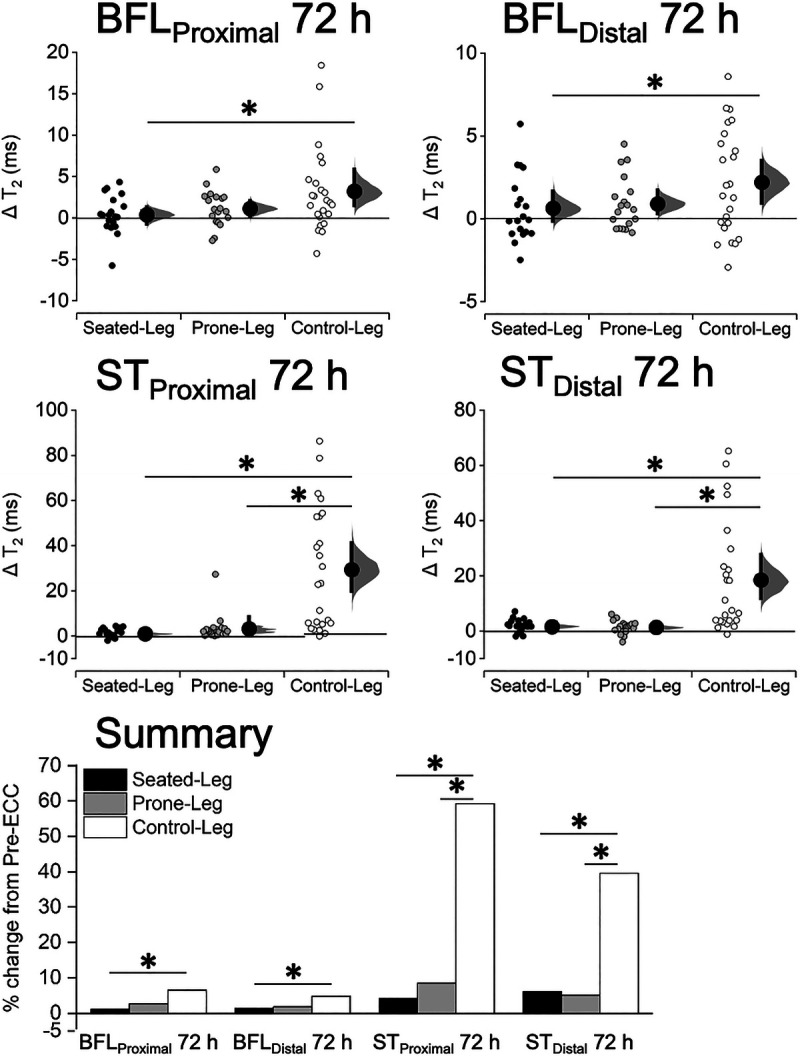
Changes in T2 of the BFL and ST at 30% (BFLProximal, STProximal) and 70% (BFLDistal, STDistal) of the thigh length at 72 h after eccentric exercise for Seated-Leg, Prone-Leg, and Control-Leg. Data are plotted as individual raw change (Δ) values from baseline (small dots), with a group mean (larger dots) and its 95% CI (indicated by the ends of the vertical error bars) shown together. The CI and the bootstrap sampling distributions (5000 samples, bias-corrected and accelerated) were obtained from respective paired (pre- to postexercise) data. *Significant difference between legs at P < 0.05 based on a baseline-adjusted ANCOVA and an LSD post hoc test. n = 19 legs for both Seated-Leg and Prone-Leg, and 24 legs for Control-Leg. The bar graphs in the summary figure are based on the mean changes for each muscle.
1RM
A one-way ANCOVA found significant (P ≤ 0.005) differences between the legs in each of the seated 1RM and prone 1RM at all Post-ECC time points (Fig. 8; see Table for details, Supplemental Digital Content 5, 1RM before and after eccentric exercise, http://links.lww.com/MSS/C156). The changes in 1RM were greater for Control-leg than Seated-Leg and Prone-Leg at all time points for both seated 1RM and prone 1RM (P ≤ 0.011), without any significant differences between Seated-Leg and Prone-Leg (P ≥ 0.351).
FIGURE 8.
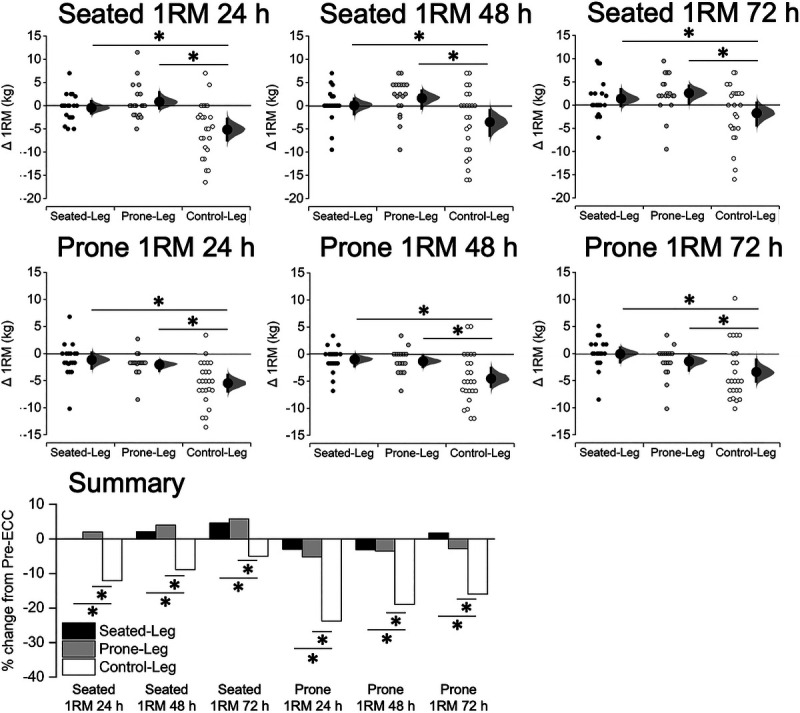
Changes in the seated and prone leg curl 1RM at 24, 48, and 72 h after eccentric exercise for Seated-Leg, Prone-Leg, and Control-Leg. Data are plotted as individual raw change (Δ) values from baseline (small dots), with a group mean (larger dots) and its 95% CI (indicated by the ends of the vertical error bars) shown together. The CI and the bootstrap sampling distributions (5000 samples, bias-corrected and accelerated) were obtained from respective paired (pre- to postexercise) data. *Significant difference between legs at P < 0.05 based on a baseline-adjusted ANCOVA and an LSD post hoc test. n = 19 legs for both Seated-Leg and Prone-Leg, and 24 legs for Control-Leg. The bar graphs in the summary figure are based on the mean changes for each 1RM measurement.
DISCUSSION
The main findings of this study were that 1) hamstrings muscle hypertrophy was clearly greater after the seated than prone leg curl training, but 2) there was no evidence for the superiority of the seated over prone leg curl training in preventing muscle damage. Thus, although our first hypothesis was supported, the second hypothesis was not. These results suggest that hamstrings muscle size can be more effectively increased by seated than prone leg curl training, while both are similarly effective in reducing susceptibility to muscle damage.
Muscle hypertrophy
The changes in the WH volume observed in this study (Seated-Leg: +14.1%, Prone-Leg: +9.3%) were in the range of the values reported in previous studies (+5.8–16.4%) (5,6) that conducted leg curl training for the same duration (12 wk), although how it was performed (i.e., seated or prone) was not specified in these studies. In either case, it seems that typical hamstrings muscle hypertrophy was induced by both training modalities conducted in this study. What this study further adds is that the training-induced change in the WH volume was ~1.5-fold greater for Seated-Leg than Prone-Leg, which is clearly attributable to the greater changes in all biarticular hamstrings, but not the monoarticular BFS, for Seated-Leg (Fig. 4). This supported our first hypothesis and indicates that only the biarticular hamstrings that were in more lengthened positions had greater hypertrophic responses after the seated leg curl training.
In part 1, the GRA and the SAR were also analyzed. Although these muscles are often overlooked in studies investigating knee flexors, the degrees of their muscle hypertrophy found in this study (Fig. 4), particularly those of the GRA, highlight their important roles as synergists in knee flexion exercise. Thus, these muscles are worth more attention and should be included as key synergists to the hamstrings in future studies. Interestingly, while the changes in muscle volume of the GRA did not statistically significantly differ between the legs (P = 0.097 in favor of Seated-Leg), that of the SAR was significantly greater for Prone-Leg than Seated-Leg (Fig. 4). Although both the GRA and the SAR are biarticular muscles that work as a knee flexor, the GRA primarily works as a hip adductor located in the medial compartment of the thigh (also assists tibial internal rotation) (27). This implies that the muscle length of the GRA is not greatly influenced by whether the hip is at a flexed or extended position (compared with the case for the biarticular hamstrings). On the other hand, the SAR works as a hip flexor located on the anterior thigh (28), indicating that the SAR is lengthened more in a prone (hip-extended) than seated (hip-flexed) position. These assumptions were supported by our follow-up simulation analysis (see Figure, Supplemental Digital Content 6, Operating ranges for the GRA/SAR, http://links.lww.com/MSS/C157). Thus, all of the six individual muscles examined here consistently demonstrated that greater hypertrophy occurred exclusively under the condition in which the muscles were trained at long muscle lengths compared with the other condition. Suggested mechanisms underpinning the greater hypertrophy include, but are not limited to, greater muscle oxygen consumption (i.e., metabolic stress) (29) and IGF-1 expression (13,30), both of which are thought to promote muscle hypertrophy (31), when exercised (13,29) or fixed (30) at long versus short muscle lengths. Although we do not have any data regarding potential mechanisms, our findings on the differences in the hypertrophic responses between the training conditions, and among muscles, should be useful for future studies in examining training-induced hypertrophy in relation to different exercise conditions.
It may be of clinical relevance that the BFL, the most commonly injured muscle within the hamstrings (22,23), had ~2.2-fold greater hypertrophy for Seated-Leg than Prone-Leg in muscle volume (Fig. 4), with similarly large (~2.0- to 2.4-fold) differences between the legs also found at its vulnerable proximal and distal regions (Fig. 5). This was also true for the second most commonly injured ST in muscle volume and at its proximal region, albeit smaller (~1.2–1.3-fold) differences between the legs. Although the difference at the distal ST was not statistically significant (P = 0.107, Fig. 5), this may be partly because the degrees of muscle hypertrophy in the ST were overall large for both legs (e.g., changes in muscle volume: ST, +23.6% vs +19.3%; BFL, +14.4% vs +6.5%). Collectively, these results suggest that 1) muscle hypertrophy of the BFL is relatively small after prone leg curl training but can be much (~2.2-fold) promoted by seated leg curl training, and 2) the ST responds (increases in size) well to both types of leg curl training, with some further (~1.2-fold) effects achieved by seated leg curl training.
Bourne et al. (32) in their recent review suggested that knee-dominant (e.g., Nordic Hamstring and prone leg curl) exercises seem to preferentially activate the ST, whereas hip-dominant (e.g., hip extension and stiff-leg deadlift) exercises appear to more heavily target the BFL and SM, based on acute T2 changes after exercise (which indicate muscle activation rather than damage [33]). In fact, Bourne et al. (34) reported that 10 wk of Nordic Hamstring training increased the muscle volume of the BFL, ST, SM, and BFS for ~6%, ~21%, ~5%, and ~15%, respectively, and hip extension training increased their volumes for ~12%, ~14%, ~8%, and ~10%, respectively (Fig. 5 in [34]). The corresponding changes after our 12-wk seated leg curl training were 14%, 24%, 8%, and 10% (×10/12 wk = 12%, 20%, 7%, and 8%), respectively. Thus, the seated leg curl appears to induce significant hypertrophy of not only the ST but also the BFL and SM, which is unachievable through either the Nordic Hamstring or the hip extension alone (34), while still inducing greater hypertrophy in the ST than the other hamstrings. Although the response of the BFS in this study seems somewhat lower compared with those of Bourne et al. (34), we do not see this as a major issue because 1) its size actually increased reasonably (+10%) and 2) the biarticular hamstrings account for a major portion of the WH in terms of size (87% in this study). Therefore, although various types of exercises may be necessary to comprehensively train the WH, performing the seated leg curl may reduce the need for substantial volumes of other hamstring exercises. Further research is warranted to substantiate these issues. The current study could be used as a foundation for such work.
Eccentric exercise-induced muscle damage
After the eccentric exercise, Control-Leg had greater changes in both T2 and 1RM compared with Seated-Leg and Prone-Leg at several time points, without significant differences between Seated-Leg and Prone-Leg at any time points (Figs. 6−8). Although some T2 changes were significantly different only between Control-Leg and Seated-Leg (Fig. 7 and Supplemental Digital Content 3, T2 changes at the mid-thigh, http://links.lww.com/MSS/C154), we do not take these as suggesting the superiority of Seated-Leg over Prone-Leg because their 95% CI of the mean changes mostly overlap with each other (and P = 0.210–0.817). Overall, the results indicate that the seated and prone leg curl training were similarly effective in reducing susceptibility to muscle damage, which refuted our second hypothesis. A previous study (21) reported that the protective effect against muscle damage was greater when priming exercise had been performed at long versus short muscle lengths. However, their priming exercise was performed only once (21), whereas the protective effect is known to be cumulative (35–37). Collectively, it appears that priming exercise at short and long muscle lengths can confer a similar protective effect against muscle damage as long as sufficient training stimulus was provided beforehand.
Within the hamstrings of Control-Leg, the ST exhibited by far the most pronounced increases in T2 among others (Figs. 6–7). The largest T2 increase in the ST is in line with previous studies, which conducted similar eccentric leg curl exercises and measured prolonged T2 changes (38–40), and also aligns with the abovementioned preferential activation/hypertrophy in the ST after knee-dominant exercises (32,34). It is not clear why the ST is prone to damage, but this may be partly attributable to its unique morphology such as being a fusiform muscle (41) and having high regional fiber length heterogeneity (42,43). What is novel in this study is that such damage in the ST based on the T2 change was substantially and similarly mitigated by both of the seated and prone leg curl training (Figs. 6−7). We also found some evidence, albeit to a weaker extent, for such protective effects in the other hamstrings including the BFL. Whether this would translate into prevention of muscle strain injury, however, is far from certain as there are more differences than similarities between muscle damage and strain injury (44). Further investigation with training intervention is warranted.
The protective effects were also evident from the 1RM changes, where Control-Leg had greater decreases than Seated-Leg and Prone-Leg over 72 h postexercise for both of the seated and prone 1RM (Fig. 8). Interestingly, the decreases in Control-Leg were apparently (~2–3-fold) larger for the prone than seated 1RM over 72 h post exercise (Fig. 8) although not statistically tested. This would be consistent with shorter working muscle lengths in the prone leg curl (Fig. 1), given that the optimum angle is transiently shifted toward longer muscle lengths with muscle damage (see Fig. 4 of [45]). Resistance training, especially eccentric training (46) or training at long muscle length (14), has been shown to induce increases in fascicle length (indicating serial sarcomere addition), which is reported to be associated with lower risk of strain injury (47). Unfortunately, we did not measure hamstring fascicle length, and it is beyond the scope of this study to discuss its potential changes and influences on the main findings of this study (i.e., muscle hypertrophy and muscle damage). Future studies should be directed toward adopting measurement techniques such as ultrasound (48), diffusion tensor imaging (49), and/or microendoscopy (50) for fascicle/sarcomere length measurements to further explore the effects of hamstring training intervention on its muscle architecture and risk of strain injuries.
Limitations
There are some limitations to this study. For part 1, although our findings on muscle hypertrophy strongly suggest that muscle length during exercise is a key determinant of resulting muscle hypertrophy, whether this is generalizable to other training modalities is yet to be examined. From an applied point of view, the approach of this study can be replicated in other biarticular muscles such as the rectus femoris as a knee extensor (with the hip joint extended vs flexed) and the gastrocnemius as a plantarflexor (with the knee joint extended vs flexed). From a viewpoint of muscle physiology, it would be necessary to take into account influences/interactions of the actual muscle lengths during exercise, associated joint moment arms, force exerted by the muscle, and neural control (excitation) to seek for the mechanisms behind greater muscle hypertrophy after training at long versus short muscle lengths. For part 2, it is unknown if the results would have been the same if we had used heavier/more strenuous protocols to induce muscle damage. We used a load of 90% 1RM with 6 sets (3 for each of the seated/prone conditions) of 10 eccentric phase-only repetitions, based on our previous study on the quadriceps (18). On the other hand, Carmona et al. (38) used a similar protocol but adopted a heavier load of 120% 1RM with 6 sets of 10 eccentric phase-only repetitions by the prone leg curl exercise and found more severe symptoms of muscle damage than those of this study. Thus, it is possible that adopting heavier/more strenuous protocols may have resulted in different findings, such as the one we secondly hypothesized. Further research is needed to better understand the influence of muscle length during resistance training on muscle hypertrophy and susceptibility to muscle damage.
CONCLUSIONS
In summary, we demonstrated that hamstring muscle hypertrophy was greater after seated than prone leg curl training, exclusively for the biarticular hamstrings that were in more lengthened positions during the seated leg curl. On the other hand, both training interventions were similarly effective in reducing susceptibility to eccentric exercise-induced muscle damage. Based on these, the seated rather than prone leg curl is recommended if training aims include increasing/maintaining muscle size of the hamstrings.
Supplementary Material
Acknowledgments
This work was supported by a research grant from Mizuno Sports Promotion Foundation (2019-5) to S. M. The authors thank the anonymous reviewers for their constructive comments that have substantially improved the quality of this study.
The authors declare that there is no conflict of interest, that no companies or manufacturers will benefit from the results of the study, and that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
Sumiaki Maeo, Email: smaeo1985@gmail.com.
Meng Huang, Email: S.Maeo@lboro.ac.uk.
Yuhang Wu, Email: s-maeo@fc.ritsumei.ac.jp.
Hikaru Sakurai, Email: gr0469ir@ed.ritsumei.ac.jp.
Yuki Kusagawa, Email: gr0417hf@ed.ritsumei.ac.jp.
Takashi Sugiyama, Email: t-sugi08@fc.ritsumei.ac.jp.
Hiroaki Kanehisa, Email: hkane@fc.ritsumei.ac.jp.
Tadao Isaka, Email: isaka@se.ritsumei.ac.jp.
REFERENCES
- 1.Morin JB Gimenez P Edouard P, et al. Sprint acceleration mechanics: the major role of hamstrings in horizontal force production. Front Physiol. 2015;6:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guex K, Millet GP. Conceptual framework for strengthening exercises to prevent hamstring strains. Sports Med. 2013;43(12):1207–15. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine . American College of Sports Medicine Position Stand: progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. [DOI] [PubMed] [Google Scholar]
- 4.Garber CE Blissmer B Deschenes MR, et al. American College of Sports Medicine . American College of Sports Medicine Position Stand: quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. [DOI] [PubMed] [Google Scholar]
- 5.Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol. 2000;81(3):174–80. [DOI] [PubMed] [Google Scholar]
- 6.Vissing K Brink M Lonbro S, et al. Muscle adaptations to plyometric vs. resistance training in untrained young men. J Strength Cond Res. 2008;22(6):1799–810. [DOI] [PubMed] [Google Scholar]
- 7.Starkey DB Pollock ML Ishida Y, et al. Effect of resistance training volume on strength and muscle thickness. Med Sci Sports Exerc. 1996;28(10):1311–20. [DOI] [PubMed] [Google Scholar]
- 8.Delp SL Anderson FC Arnold AS, et al. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54(11):1940–50. [DOI] [PubMed] [Google Scholar]
- 9.Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Ann Biomed Eng. 2010;38(2):269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold EM, Delp SL. Fibre operating lengths of human lower limb muscles during walking. Philos Trans R Soc Lond B Biol Sci. 2011;366(1570):1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alegre LM, Ferri-Morales A, Rodriguez-Casares R, Aguado X. Effects of isometric training on the knee extensor moment-angle relationship and vastus lateralis muscle architecture. Eur J Appl Physiol. 2014;114(11):2437–46. [DOI] [PubMed] [Google Scholar]
- 12.Noorkoiv M, Nosaka K, Blazevich AJ. Neuromuscular adaptations associated with knee joint angle-specific force change. Med Sci Sports Exerc. 2014;46(8):1525–37. [DOI] [PubMed] [Google Scholar]
- 13.McMahon G, Morse CI, Burden A, Winwood K, Onambele GL. Muscular adaptations and insulin-like growth factor-1 responses to resistance training are stretch-mediated. Muscle Nerve. 2014;49(1):108–19. [DOI] [PubMed] [Google Scholar]
- 14.Akagi R, Hinks A, Power GA. Differential changes in muscle architecture and neuromuscular fatigability induced by isometric resistance training at short and long muscle-tendon unit lengths. J Appl Physiol (1985). 2020;129(1):173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo K Ohgo K Takeishi R, et al. Effects of isometric training at different knee angles on the muscle-tendon complex in vivo. Scand J Med Sci Sports. 2006;16(3):159–67. [DOI] [PubMed] [Google Scholar]
- 16.Stasinaki AN Zaras N Methenitis S, et al. Triceps brachii muscle strength and architectural adaptations with resistance training exercises at short or long fascicle length. J Funct Morphol Kinesiol. 2018;3(2):28–39. [Google Scholar]
- 17.Schoenfeld BJ, Grgic J. Effects of range of motion on muscle development during resistance training interventions: a systematic review. SAGE Open Med. 2020;8:2050312120901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeo S, Saito A, Otsuka S, Shan X, Kanehisa H, Kawakami Y. Localization of muscle damage within the quadriceps femoris induced by different types of eccentric exercises. Scand J Med Sci Sports. 2018;28(1):95–106. [DOI] [PubMed] [Google Scholar]
- 19.Brockett CL, Morgan DL, Proske U. Predicting hamstring strain injury in elite athletes. Med Sci Sports Exerc. 2004;36(3):379–87. [DOI] [PubMed] [Google Scholar]
- 20.Timmins RG, Shield AJ, Williams MD, Opar DA. Is there evidence to support the use of the angle of peak torque as a marker of hamstring injury and re-injury risk? Sports Med. 2016;46(1):7–13. [DOI] [PubMed] [Google Scholar]
- 21.Nosaka K, Newton M, Sacco P, Chapman D, Lavender A. Partial protection against muscle damage by eccentric actions at short muscle lengths. Med Sci Sports Exerc. 2005;37(5):746–53. [DOI] [PubMed] [Google Scholar]
- 22.Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197–206. [DOI] [PubMed] [Google Scholar]
- 23.De Smet AA, Best TM. MR imaging of the distribution and location of acute hamstring injuries in athletes. AJR Am J Roentgenol. 2000;174(2):393–9. [DOI] [PubMed] [Google Scholar]
- 24.Maeo S, Shan X, Otsuka S, Kanehisa H, Kawakami Y. Neuromuscular adaptations to work-matched maximal eccentric versus concentric training. Med Sci Sports Exerc. 2018;50(8):1629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeo S, Ando Y, Kanehisa H, Kawakami Y. Localization of damage in the human leg muscles induced by downhill running. Sci Rep. 2017;7(1):5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat Methods. 2019;16(7):565–6. [DOI] [PubMed] [Google Scholar]
- 27.Moore KL, Agur AMR, Dalley AF. Clinically Oriented Anatomy. 8th ed. Philadelphia: Wolters Kluwer; 2018. 1153 p. [Google Scholar]
- 28.Kim J, Lee J-H. A unique case of an accessory sartorius muscle. Surg Radiol Anat. 2019;41(3):323–5. [DOI] [PubMed] [Google Scholar]
- 29.Kooistra RD, de Ruiter CJ, de Haan A. Knee angle-dependent oxygen consumption of human quadriceps muscles during maximal voluntary and electrically evoked contractions. Eur J Appl Physiol. 2008;102(2):233–42. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Alnaqeeb M, Simpson H, Goldspink G. Changes in muscle fibre type, muscle mass and IGF-I gene expression in rabbit skeletal muscle subjected to stretch. J Anat. 1997;190(Pt 4):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenfeld BJ. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013;43(3):179–94. [DOI] [PubMed] [Google Scholar]
- 32.Bourne MN Timmins RG Opar DA, et al. An evidence-based framework for strengthening exercises to prevent hamstring injury. Sports Med. 2018;48(2):251–67. [DOI] [PubMed] [Google Scholar]
- 33.Ochi E, Tsuchiya Y, Nosaka K. Differences in post-exercise T2 relaxation time changes between eccentric and concentric contractions of the elbow flexors. Eur J Appl Physiol. 2016;116(11–12):2145–54. [DOI] [PubMed] [Google Scholar]
- 34.Bourne MN Duhig SJ Timmins RG, et al. Impact of the Nordic hamstring and hip extension exercises on hamstring architecture and morphology: implications for injury prevention. Br J Sports Med. 2017;51(5):469–77. [DOI] [PubMed] [Google Scholar]
- 35.Chen TC, Chen HL, Lin MJ, Wu CJ, Nosaka K. Muscle damage responses of the elbow flexors to four maximal eccentric exercise bouts performed every 4 weeks. Eur J Appl Physiol. 2009;106(2):267–75. [DOI] [PubMed] [Google Scholar]
- 36.Maeo S, Yamamoto M, Kanehisa H. Muscular adaptations to short-term low-frequency downhill walking training. Int J Sports Med. 2015;36(2):150–6. [DOI] [PubMed] [Google Scholar]
- 37.Maeo S, Yamamoto M, Kanehisa H. Downhill walking training with and without exercise-induced muscle damage similarly increase knee extensor strength. J Sports Sci. 2016;34(21):2018–26. [DOI] [PubMed] [Google Scholar]
- 38.Carmona G Mendiguchia J Alomar X, et al. Time course and association of functional and biochemical markers in severe semitendinosus damage following intensive eccentric leg curls: differences between and within subjects. Front Physiol. 2018;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubota J, Ono T, Araki M, Torii S, Okuwaki T, Fukubayashi T. Non-uniform changes in magnetic resonance measurements of the semitendinosus muscle following intensive eccentric exercise. Eur J Appl Physiol. 2007;101(6):713–20. [DOI] [PubMed] [Google Scholar]
- 40.Mendiguchia J Garrues MA Cronin JB, et al. Nonuniform changes in MRI measurements of the thigh muscles after two hamstring strengthening exercises. J Strength Cond Res. 2013;27(3):574–81. [DOI] [PubMed] [Google Scholar]
- 41.Garrett WE, Jr., Nikolaou PK, Ribbeck BM, Glisson RR, Seaber AV. The effect of muscle architecture on the biomechanical failure properties of skeletal muscle under passive extension. Am J Sports Med. 1988;16(1):7–12. [DOI] [PubMed] [Google Scholar]
- 42.Patel TJ, Das R, Friden J, Lutz GJ, Lieber RL. Sarcomere strain and heterogeneity correlate with injury to frog skeletal muscle fiber bundles. J Appl Physiol (1985). 2004;97(5):1803–13. [DOI] [PubMed] [Google Scholar]
- 43.Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res. 2009;467(4):1074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McHugh MP, Tyler TF. Muscle strain injury vs muscle damage: two mutually exclusive clinical entities. Transl Sports Med. 2019;2(3):102–8. [Google Scholar]
- 45.Brockett CL, Morgan DL, Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Med Sci Sports Exerc. 2001;33(5):783–90. [DOI] [PubMed] [Google Scholar]
- 46.Timmins RG Ruddy JD Presland J, et al. Architectural changes of the biceps femoris long head after concentric or eccentric training. Med Sci Sports Exerc. 2016;48(3):499–508. [DOI] [PubMed] [Google Scholar]
- 47.Timmins RG, Bourne MN, Shield AJ, Williams MD, Lorenzen C, Opar DA. Short biceps femoris fascicles and eccentric knee flexor weakness increase the risk of hamstring injury in elite football (soccer): a prospective cohort study. Br J Sports Med. 2016;50(24):1524–35. [DOI] [PubMed] [Google Scholar]
- 48.Franchi MV, Fitze DP, Raiteri BJ, Hahn D, Sporri J. Ultrasound-derived biceps femoris long head fascicle length: extrapolation pitfalls. Med Sci Sports Exerc. 2020;52(1):233–43. [DOI] [PubMed] [Google Scholar]
- 49.Bolsterlee B, D’Souza A, Herbert RD. Reliability and robustness of muscle architecture measurements obtained using diffusion tensor imaging with anatomically constrained tractography. J Biomech. 2019;86:71–8. [DOI] [PubMed] [Google Scholar]
- 50.Lichtwark GA, Farris DJ, Chen X, Hodges PW, Delp SL. Microendoscopy reveals positive correlation in multiscale length changes and variable sarcomere lengths across different regions of human muscle. J Appl Physiol (1985). 2018;125(6):1812–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


