Abstract
Objective
To test the relationship between the supply of select nonpharmacologic providers (physical therapy (PT) and mental health (MH)) and use of nonpharmacologic services among older adults with a persistent musculoskeletal pain (MSP) episode.
Data sources/study setting
Claims data from a 5 percent random sample of Medicare beneficiaries enrolled fee‐for‐service (2007‐2014) and the Area Health Resource File (AHRF).
Study design
This retrospective study used generalized estimating equations to estimate the association between the county nonpharmacologic provider supply and individual service use with opioid prescriptions filled during Phase 1 (first three months of an episode) and Phase 2 (three months following Phase 1).
Data collection/extraction methods
We identified beneficiaries (>65 years) with ≥2 MSP diagnoses ≥90 days apart and no opioid prescription six months before the first pain diagnosis (N = 69 456). Beneficiaries’ county characteristics were assigned using the AHRF.
Principal findings
About 13.9 percent of beneficiaries used PT, 1.8 percent used MH services, and 10.7 percent had an opioid prescription during the first three months of a persistent MSP episode. An additional MH provider/10 000 people/county [aOR: 0.97, 95% CI: 0.96‐0.98] and PT/10 000 people/county [aOR: 0.98, 95% CI: 0.97‐1.00] was associated with lower odds of filling an opioid prescription in Phase 1. An additional MH provider/10 000 people/county [aOR: 0.97, 95% CI: 0.96‐0.98] and PT use in Phase 1 [aOR: 0.62, 95% CI: 0.58‐0.67] were associated with lower odds of filling an opioid prescription in Phase 2. The associations between the supply of providers and nonpharmacologic service use in Phase 1 and Phase 1 opioid prescriptions significantly differed by metropolitan and rural counties (P‐value: .019).
Conclusions
Limited access to nonpharmacologic services is associated with opioid prescriptions at the onset of a persistent MSP episode. Initiating PT at the onset of an episode may reduce future opioid use. Strategies for engaging beneficiaries in nonpharmacologic services should be tailored for metropolitan and rural counties.
Keywords: access to care, mental health services, musculoskeletal pain, older adults, opioid prescribing, physical therapy
What this study adds
Among older adults, the utilization rate for nonpharmacologic services (ie, physical therapy and mental health) is low during the first six months of a persistent musculoskeletal pain episode.
The supply of nonpharmacologic providers was associated with lower odds of initiating opioid prescriptions during the first three months of a persistent musculoskeletal pain episode.
Physical therapy services in the first three months of a musculoskeletal pain episode were associated with lower odds of an opioid prescription in the second three months.
Given the low utilization rate of nonpharmacologic services in this older adult population, increasing use of and access to nonpharmacologic services may reduce opioid use in older adults with persistent musculoskeletal pain.
1. INTRODUCTION
Musculoskeletal pain (MSP) affects almost two‐thirds of older adults. 1 MSP can develop into persistent pain (pain for longer than three months), which can lead to inactivity, mood disorders, isolation, and disability. 2 , 3 , 4 , 5 Persistent pain can be managed with nonpharmacologic treatments and medications, both opioids and nonopioids. 6 , 7 Clinical guidelines recommend nonpharmacologic treatments over opioids, especially for older adults. 6 , 7 , 8 Among the options for nonpharmacologic services, physical therapy (PT) and mental health (MH) services have the strongest evidence for pain relief. 7 , 8 Compared to opioids, nonpharmacologic treatments have fewer side effects and address physiological, psychological, and social needs associated with pain. 6 , 9 , 10
In 2010, nearly 17 percent of clinic visits for older adults with MSP had an opioid prescription. 11 Opioids are associated with the risk of falls, fractures, cardiac events, pneumonia, and death. 12 , 13 , 14 , 15 , 16 Although opioids may be indicated for short‐term pain relief, the number of days of the first opioid prescription increases the risk of developing long‐term opioid use. 7 , 17 , 18 , 19 Long‐term opioid treatment for persistent pain can exacerbate the risks of opioids. 20 , 21 Use of nonpharmacologic services during a pain episode, especially at the onset of an episode, may reduce opioid use. 7 , 22 , 23 , 24 , 25 , 26 , 27
Older adults with persistent MSP use opioids more frequently than nonpharmacologic treatments. 28 One reason for the differences in the use of nonpharmacologic service compared to opioids may be because of a lack of providers to deliver nonpharmacologic services. 29 Studies show significant associations between the supply of providers and opioid prescribing. 30 , 31 Increases in the number of surgeons and physicians is associated with higher county prescribing rates, after adjusting for population size. 30 , 31 At an individual level, a greater supply of physicians is associated with fewer opioid prescriptions 15 days after an acute low back pain diagnosis. 32 Disparities in opioid prescribing rates between metropolitan and rural areas may indicate differences in accessibility of pain treatments in these settings. 33 , 34 Uptake of nonpharmacologic services depends, in part, on the capacity of the health care system to deliver these services. 35
The onset of a pain episode represents a critical time period to encourage the uptake of nonpharmacologic treatments and reduce long‐term opioid use. We focus on older adults with known persistent MSP because they frequently interact with the health care system, could face more barriers to accessing nonpharmacologic pain treatments, and may initiate long‐term opioid use to manage persistent pain. 5 , 36 , 37 Identifying the drivers of early opioid use, such as access to and use of nonpharmacologic services, during a persistent MSP episode can inform strategies to promote safe pain management treatments, reduce long‐term opioid use, and prevent adverse outcomes associated with opioids.
In this retrospective observational cohort study, we estimated the association between the supply of nonpharmacologic providers (PTs and MH), use of nonpharmacologic services, and opioid prescriptions during the first six months of a new persistent MSP episode among Medicare beneficiaries >65 years. We examine two critical periods of the episode to understand the differences in treatment over time, as pain becomes persistent: Phase 1 (the first three months after the index pain diagnosis) and Phase 2 (the 3 months after Phase 1). 2
We hypothesized no association between the supply of nonpharmacologic providers and filling an opioid prescription in Phase 1 because the pain episode may be perceived to be short and opioids may only be intended for short‐term treatment. Second, we hypothesized that a greater supply of nonpharmacologic providers would be associated with lower odds of an opioid prescription in the Phase 2 because patients may require more intense treatment once pain becomes persistent. Third, we hypothesized that use of PT or MH services in Phase 1 would be associated with lower odds of filling an opioid prescription in Phase 2 because the history of pain care may influence future treatment use. Finally, to help inform policies for metropolitan and rural counties, we conducted a stratified analysis and hypothesized that the associations between the supply of nonpharmacologic providers and use of services with opioid prescriptions in either 3‐month period will differ for metropolitan and rural counties.
2. METHODS
2.1. Data sources
Data sources included the Medicare Fee‐for‐Service (FFS) claims and the Area Health Resource File (AHRF). 38 We used a 5 percent random sample of beneficiaries enrolled in Medicare FFS between January 1, 2008 and June 30, 2014. Medicare claims data from 2007 were used for the look‐back period. The Master Beneficiary Summary file contains information on demographics, enrollment, and death. Data about diagnoses, service utilization, and service dates came from claims filed for services covered by Medicare Part A (inpatient services) and Part B (outpatient services, including home health, hospice, and skilled nursing facilities). The Part D Drug Event file has claims for filled prescriptions. Referencing the beneficiaries’ county of residence and their index date from the claims data, the AHRF was used to assign county characteristics to beneficiaries. 38 The Rural‐Urban Commuting Area codes in the AHRF describe rurality for each county. 38 , 39
2.2. Study population
We identified a new episode of what would become persistent MSP using International Disease Classification Codes, 9th edition (ICD‐9) (Figure 1, Table S1). 40 Pain diagnoses are proxy measures for pain and represent when a beneficiary sought pain treatment but do not indicate when the pain first began or the severity of pain. To focus on persistent pain, beneficiaries were included if they had at least two claims for any MSP diagnosis that were ≥90 days but ≤365 days apart. 41 We excluded beneficiaries who only had claims with a pain diagnosis <90 days apart and no pain‐related claims between 90 and 365 days because these beneficiaries may stop using services if their pain is resolved or choose to no longer seek care even if pain persists. We defined a new persistent MSP episode starting with the first claim with a pain diagnosis (index date) after a year without any claims with a pain diagnosis. The same pain diagnoses were not required for the episode definition. Beneficiaries could have multiple episodes, and we selected the first episode of persistent MSP. After the index date, beneficiaries were followed for six months or until death, enrollment in Medicare Advantage, or disenrollment from Part D at which point they are censored.
FIGURE 1.
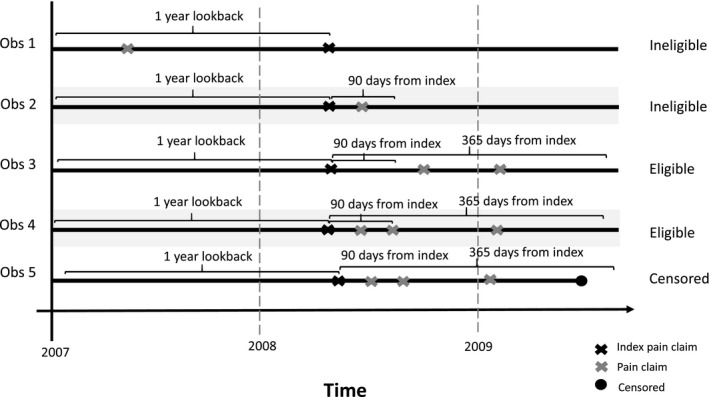
Illustration of eligible beneficiaries to identify a new episode of persistent musculoskeletal pain adapted from Gore 2012 41
Before the index date, we required continuous enrollment in Medicare FFS for one year and Part D for six months. To ensure that beneficiaries had a new pain episode and no history of opioid use, we required no claims with a pain diagnosis one year before the index date and no opioid prescriptions in the prior six months. 42 To focus on beneficiaries who enrolled in Medicare because of age, we excluded beneficiaries with Medicare for disability or end‐stage renal disease and required that beneficiaries were 66 or older. To identify beneficiaries with a new MSP episode, we excluded individuals with a trauma or surgery in the year before the index date using Current Procedural Codes (CPT) and ICD‐9 codes. 40 , 43 Beneficiaries who used hospice services or long‐term care services at any time were excluded because guidelines for opioids differ for these settings. 7
2.3. Outcomes
Because the Centers for Disease Control and Prevention (CDC) guideline states that opioids may be appropriate for short‐term use, we examine opioid prescriptions filled during the first six months of a pain episode. 7 We created binary measures for receipt of an opioid prescription in Phase 1 (first three months after the index date) and Phase 2 (three to six months after the index date). These time periods were chosen because providers and patients may try many pharmacologic and nonpharmacologic pain treatments early in a pain episode. These measures do not indicate if the prescriptions reflect guideline‐concordant care based on the days’ supply, dose, or number of prescriptions. We included opioids with an oral or transdermal formulation with the United States’ Food and Drug Administration‐approved indication to treat pain. National Drug Codes, provided by the CDC in 2017, identified opioids. 44
2.4. Primary independent variables
The primary independent variables were the supply of nonpharmacologic providers and use of nonpharmacologic services. We defined nonpharmacologic providers as providers who deliver nonpharmacologic services for pain: MH providers (psychiatrists and psychologists) and PTs. The supply of providers was a measure of local capacity to provide services. We chose MH providers based on the Health Resources and Services Administration's definition of Health Professional Shortage Areas for MH providers. 45 Psychiatrists are included because 34 percent of psychiatry visits have psychotherapy, and they prescribe 0.2 percent of opioid prescriptions. 46 , 47
We measured the supply as the number of providers per capita per county per year. We created per capita measures using county population from the AHRF multiplied by 10 000. 38 We used all available years for the annual supply measures (Psychiatrists: 2008, 2010‐2013; Psychologists: 2009, PTs: 2009). 38 We imputed missing data for the annual psychiatrist supply using linear interpolation where the existing annual supply variable was a function of the year. The interpolation was conducted separately for each county. Since one year was available for the psychologists and PT supply, data from 2009 were carried forward for the study years.
We defined binary indicators to identify beneficiaries’ use of PT and MH services in Phase 1 and/or Phase 2 of a persistent pain episode. We used CPT and Healthcare Common Procedural Coding System codes (Table S1). 48 , 49
2.5. Covariates
We adjusted for individual and county characteristics that may confound the association between the supply of providers and use of nonpharmacologic services and opioid prescription fills. Individual demographic characteristics included age, race, sex, and Medicaid dual eligibility (a proxy for income). 50 Comorbidities were measured using the Deyo‐Charlson comorbidity score which accounts for 17 clinical conditions, including cancer. 51 Based on ICD‐9 codes from claims one year before the index date, we used definitions provided by the Centers for Medicare and Medicaid's (CMS) Chronic Conditions Warehouse and the Health Cost and Utilization Project to identify depression and anxiety, which are conditions commonly comorbid with persistent pain (Table S1). 52 , 53 , 54 Substance use disorders (SUD) were not identified because claims with these diagnoses were redacted. 55 Because opioid prescriptions could be indicated for surgery and trauma, we identified opioid prescriptions, trauma, and surgery in either treatment phase. 40 , 43 , 44 We defined a categorical variable to control for the reasons for incomplete follow‐up during Phase 2, such as leaving FFS, leaving Part D, or death.
We controlled for provider supply and population demographics of a beneficiary's county. Using the same method described for the annual per capita supply of nonpharmacologic providers, we created the annual per capita supply of primary care providers (internal medicine, general practice, geriatric specialists (2008, 2010‐2013)), surgeons (2008, 2010‐2013), pain specialists (physical medicine or rehabilitation specialists: 2008, 2010‐2013), midlevel practitioners (nurse practitioners: 2009 and physician assistants: 2009), and pharmacists (2009) (Table S1). 38 The total supply of primary care providers, surgeons, and pain specialists included providers with an MD or DO training in office‐based settings, hospital staff, hospital residents, and clinical fellows. 38 We used the same methods described above for nonpharmacologic providers to account for missing data for these provider supply measures. County socioeconomic status was measured as the percent of the county population living below the Federal Poverty Level. We also controlled for the percent of the county population over 65. We assigned counties to metropolitan and rural designation based on the Rural‐Urban Commuting Area codes. 38 , 39
2.6. Statistical analysis
We calculated the means and proportions of individual and county‐level variables. T‐tests and chi‐square tests were conducted for three group comparisons in the cohort: beneficiaries with and without an opioid prescription in Phase 1, beneficiaries with and without an opioid prescription in Phase 2, and beneficiaries in metropolitan and rural counties.
Using generalized estimating equations, we estimated adjusted odds ratios (aOR) and 95% confidence intervals (CIs) for three cohorts: the full cohort, the cohort of beneficiaries in metropolitan counties, and the cohort of beneficiaries in rural counties. The dependent variables correspond to a binary variable for any opioid prescription fill, with two separate models for Phases 1 and 2. For models with an opioid prescription fill in Phase 1, we included MH provider supply, PT supply, MH use in Phase 1, and PT use in Phase 1, individual characteristics (demographics, comorbidities, trauma, and surgery), and county characteristics (provider supply as separate measures and demographics). Models for opioid prescriptions filled for Phase 2 included the same variables as Phase 1, reason for loss to follow‐up, and the following variables referencing Phase 2: nonpharmacologic service use, opioid prescriptions, trauma, and surgery. Models included year fixed effects to control for underlying time trends and minimize the potential measurement error due to the interpolation of the annual supply measures. Based on the quasi‐likelihood under the independence model criterion (QIC) goodness of fit statistic, we selected the model with the unstructured correlation.
To understand the differences in the associations for metropolitan and rural counties, we conducted subgroup analyses by stratifying the full cohort by residence in metropolitan and rural counties. We tested whether the estimates for metropolitan and rural counties were statistically significant using a type III analysis to produce a score statistic. 56
In sensitivity analyses, we explored the relationship between the supply of nonpharmacologic providers and use of nonpharmacologic services as a positive control (Tables S2 and S3). To understand the extent to which the associations for the supply of nonpharmacologic providers are independent of the use of services, we explored the association between the supply of nonpharmacologic providers and opioids prescriptions fills in Phases 1 and 2 and excluded use of services (Table S4). To assess the potential bias from measurement error due to missing years for the provider supply measures, we compared the variation between counties or states to the variation over time (Table S5). For the supply of psychiatrists, we used the AHRF data on the county supply of providers for the available years. For the supply of psychologists and PTs, we used data on the state provider supply from the Bureau of Labor Statistics’ Occupational Employment Statistics Survey. 57
We used SAS version 9.4 (SAS Institute, Inc, Cary, NC) to conduct the analyses. This study was approved by the Institutional Review Boards at the Duke University's School of Medicine and University of North Carolina‐Chapel Hill with wavier of individual consent.
3. RESULTS
We identified 197 827 beneficiaries with an episode of persistent MSP lasting at least three months without a pain diagnosis one year before the index date or an opioid prescription six months before the index pain diagnosis (Figure 2). We excluded 80 386 beneficiaries because of surgery and 1497 because of trauma within the 12 months before the index date and 5943 because of hospice. After excluding beneficiaries with fewer than 90 days of follow‐up and missing data, the final cohort included 69 456 beneficiaries. About 98 percent of the cohort (n = 68 491) was enrolled in FFS and Part D for the duration of the study period (6 months), and the average follow‐up time was 179.48 days (standard deviation: 5.11).
FIGURE 2.
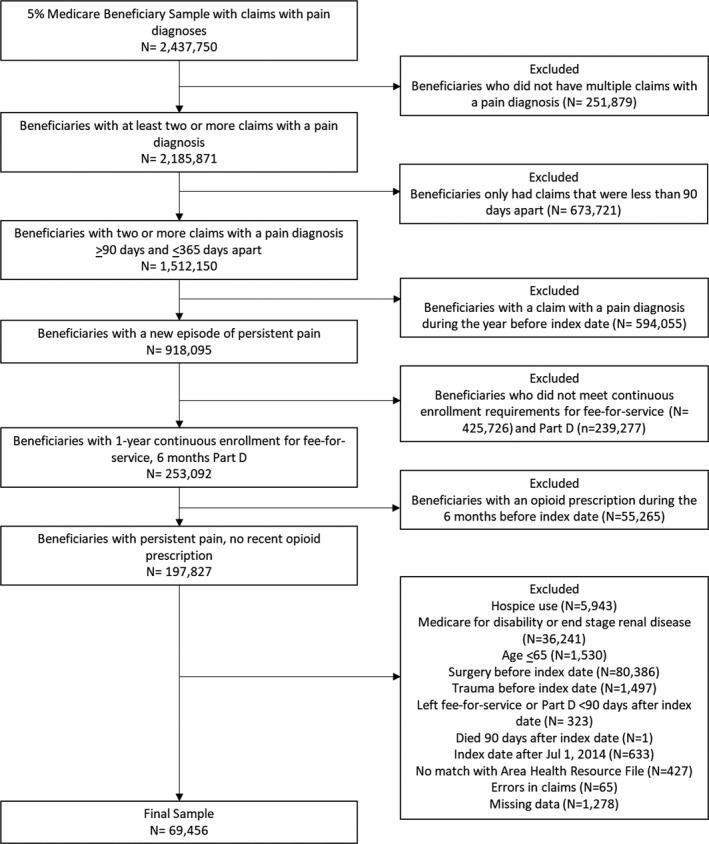
Cohort selection process
The most prevalent MSP conditions were arthritis (98.2 percent), fibromyalgia (68.3 percent), and back pain (65.7 percent) (Table 1). On average, there were 3.3 MH providers and 5.9 PTs per 10 000 people per county. Beneficiaries with at least one opioid prescription in either phase were more likely to be younger and female and were more likely to have back pain or fibromyalgia compared to beneficiaries without an opioid prescription. The county supply of MH providers and PTs was significantly lower for beneficiaries with an opioid prescription in either phase.
TABLE 1.
Characteristics of Medicare beneficiaries with persistent musculoskeletal pain by treatment phase and county type
| Entire Cohort (N = 69 456) | Phase 1 | Phase 2 | Rural (N = 12 926) | Metropolitan (N = 56 530) | P‐value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No opioid prescription (N = 60 334) | Opioid prescription (N = 9122) | P‐value | No opioid prescription (N = 62 011) | Opioid prescription (N = 7445) | P‐value | |||||
| Mean (Standard Deviation)/Number (Percent) | Mean (Standard Deviation)/Number (Percent) | Mean (Standard Deviation)/Number (Percent) | Mean (Standard Deviation)/Number (Percent) | Mean (Standard Deviation)/Number (Percent) | Mean (Standard Deviation)/Number (Percent)) | Mean (Standard Deviation)/Number (Percent) | ||||
| Outcomes | ||||||||||
| Opioid Prescription in Phase 1 | 9122 (13.1) | — | 9122 (100.0) | 6530 (10.5) | 2592 (34.8) | <.001 | 1756 (13.6) | 7366 (13.0) | .09 | |
| Opioid Prescription in Phase 2 | 7445 (10.7) | 4853 (8.0) | 2592 (28.4) | <.001 | — | 7445 (100.0) | 1453 (11.2) | 5992 (10.6) | .03 | |
| Physical Therapy in Phase 1 | 9634 (13.9) | 7297 (12.1) | 2337 (25.6) | <.001 | 8472 (13.7) | 1162 (15.6) | <.001 | 1467 (11.3) | 8167 (14.4) | <.001 |
| Physical Therapy in Phase 2 | 7409 (10.7) | 5490 (9.1) | 1919 (21.0) | <.001 | 5787 (9.3) | 1622 (21.8) | <.001 | 1101 (8.5) | 6308 (11.2) | <.001 |
| Mental Health Services in Phase 1 | 1221 (1.8) | 1065 (1.8) | 156 (1.7) | .71 | 1061 (1.7) | 160 (2.1) | .007 | 97 (0.8) | 1124 (2.0) | <.001 |
| Mental Health Services in Phase 2 | 1105 (1.6) | 973 (1.6) | 132 (1.4) | .24 | 968 (1.6) | 137 (1.8) | .07 | 88 (0.7) | 1017 (1.8) | <.001 |
| Demographics | ||||||||||
| Age | ||||||||||
| 66‐69 y | 20 159 (29.0) | 17 317 (28.7) | 2842 (31.2) | <.001 | 17 858 (28.8) | 2301 (30.9) | <.001 | 3738 (28.9) | 16 421 (29.0) | .77 |
| 70‐74 y | 16 425 (23.6) | 14 184 (23.5) | 2241 (24.6) | .03 | 14 562 (23.5) | 1863 (25.0) | .003 | 3183 (24.6) | 13 242 (23.4) | .004 |
| 75‐79 y | 12 595 (18.1) | 10 970 (18.2) | 1625 (17.8) | .40 | 11 292 (18.2) | 1303 (17.5) | .13 | 2449 (18.9) | 10 146 (17.9) | .008 |
| 80‐84 y | 9911 (14.3) | 8712 (14.4) | 1199 (13.1) | <.001 | 8964 (14.5) | 947 (12.7) | <.001 | 1738 (13.4) | 8173 (14.5) | .003 |
| >85 y | 10 366 (14.9) | 9151 (15.2) | 1215 (13.3) | <.001 | 9335 (15.1) | 1031 (13.8) | .006 | 1818 (14.1) | 8548 (15.1) | .002 |
| Sex | ||||||||||
| Female | 45625 (65.7) | 39 697 (65.8) | 5928 (65.0) | .13 | 40 822 (65.8) | 4803 (64.5) | .02 | 8470 (65.5) | 37 155 (65.7) | .67 |
| Male | 23 831 (34.3) | 20 637 (34.2) | 3 194 (35.0) | .13 | 21 189 (34.2) | 2642 (35.5) | .02 | 4456 (34.5) | 19 375 (34.3) | .67 |
| Race | ||||||||||
| White | 58 871 (84.8) | 51 094 (84.7) | 7777 (85.3) | .16 | 52 553 (84.7) | 6318 (84.9) | .8 | 11 851 (91.7) | 47 020 (83.2) | <.001 |
| Black | 4751 (6.8) | 4093 (6.8) | 658 (7.2) | .13 | 4176 (6.7) | 575 (7.7) | .001 | 727 (5.6) | 4024 (7.1) | <.001 |
| Other race | 5834 (8.4) | 5147 (8.5) | 687 (7.5) | .001 | 5282 (8.5) | 552 (7.4) | .001 | 348 (2.7) | 5486 (9.7) | <.001 |
| Medicaid dual eligible | 13 662 (19.7) | 11 690 (19.4) | 1972 (21.6) | <.001 | 11 953 (19.3) | 1709 (23.0) | <.001 | 2336 (18.1) | 11 326 (20.0) | <.001 |
| Metropolitan county | 56 530 (81.4) | 49 164 (81.5) | 7366 (80.7) | .09 | 50 538 (81.5) | 5992 (80.5) | .03 | — | 56 530 (100.0) | |
| Comorbidities | ||||||||||
| Deyo‐Charlson Comorbidity Score (mean,) | 1.1 (1.4) | 1.1 (1.4) | 1.1 (1.5) | .18 | 1.1 (1.4) | 1.1 (1.5) | .62 | 0.9 (1.3) | 1.1 (1.5) | <.001 |
| 0 | 32 774 (47.2) | 28 415 (47.1) | 4359 (47.8) | .22 | 29 300 (47.2) | 3474 (46.7) | .34 | 6813 (52.7) | 25 961 (45.9) | <.001 |
| 1 | 17 877 (25.7) | 15 532 (25.7) | 2 345 (25.7) | .94 | 15 900 (25.6) | 1 977 (26.6) | .09 | 3 300 (25.5) | 14 577 (25.8) | .55 |
| 2 | 9108 (13.1) | 7925 (13.1) | 1183 (13.0) | .66 | 8145 (13.1) | 963 (12.9) | .63 | 1447 (11.2) | 7661 (13.6) | <.001 |
| >3 | 9697 (14.0) | 8462 (14.0) | 1235 (13.5) | .21 | 8666 (14.0) | 1031 (13.8) | .77 | 1366 (10.6) | 8331 (14.7) | <.001 |
| Anxiety | 1475 (2.1) | 1274 (2.1) | 201 (2.2) | .57 | 1304 (2.1) | 171 (2.3) | .27 | 239 (1.8) | 1236 (2.2) | .02 |
| Depression | 4069 (5.9) | 3519 (5.8) | 550 (6.0) | .46 | 3624 (5.8) | 445 (6.0) | .64 | 625 (4.8) | 3444 (6.1) | <.001 |
| Pain type | ||||||||||
| Arthritis | 68 193 (98.2) | 59 259 (98.2) | 8934 (97.9) | .06 | 60 866 (98.2) | 7327 (98.4) | .11 | 12 719 (98.4) | 55 474 (98.1) | .04 |
| Back pain | 45 664 (65.7) | 39 140 (64.9) | 6524 (71.5) | <.001 | 40 196 (64.8) | 5468 (73.4) | <.001 | 8510 (65.8) | 37 154 (65.7) | .81 |
| Chronic pain | 10 743 (15.5) | 8466 (14.0) | 2277 (25.0) | <.001 | 8798 (14.2) | 1945 (26.1) | <.001 | 2076 (16.1) | 8667 (15.3) | .04 |
| Neck pain | 8201 (11.8) | 6919 (11.5) | 1282 (14.1) | <.001 | 7124 (11.5) | 1077 (14.5) | <.001 | 1326 (10.3) | 6875 (12.2) | <.001 |
| Psychogenic pain | 852 (1.2) | 731 (1.2) | 121 (1.3) | .35 | 741 (1.2) | 111 (1.5) | .03 | 126 (1.0) | 726 (1.3) | .004 |
| Sprain or strain | 19 796 (28.5) | 16 741 (27.7) | 3055 (33.5) | <.001 | 17 406 (28.1) | 2390 (32.1) | <.001 | 3647 (28.2) | 16 149 (28.6) | .42 |
| Fibromyalgia | 47 425 (68.3) | 41 043 (68.0) | 6382 (70.0) | <.001 | 42 066 (67.8) | 5359 (72.0) | <.001 | 8611 (66.6) | 38 814 (68.7) | <.001 |
| Other musculoskeletal pain | 21 269 (30.6) | 17 805 (29.5) | 3 464 (38.0) | <.001 | 18 589 (30.0) | 2680 (36.0) | <.001 | 3812 (29.5) | 17 457 (30.9) | .002 |
| County demographics | ||||||||||
| Mental Health Providers, (Median, interquartile range) a | 3.3 (1.6, 5.7) | 3.3 (1.6, 5.8) | 3.0 (1.4, 5.5) | <.001 | 3.3 (1.6, 5.8) | 2.9 (1.4, 5.4) | <.001 | 1.0 (0.3, 2.1) | 3.9 (2.1, 6.1) | <.001 |
| Physical Therapists, (Median, interquartile range) a | 5.9 (4.0, 7.6) | 6.0 (4.0, 7.7) | 5.6 (3.8, 7.4) | <.001 | 6.0 (4.0, 7.7) | 5.6 (3.8, 7.4) | <.001 | 3.6 (2.2, 5.4) | 6.4 (4.4, 7.9) | <.001 |
| Primary care providers, (Median, interquartile range) a | 6.8 (5.0, 8.9) | 6.9 (5.1, 8.9) | 6.6 (4.9, 8.5) | <.001 | 6.9 (5.1, 8.9) | 6.6 (4.8, 8.5) | <.001 | 5.0 (3.8, 6.6) | 7.2 (5.5, 9.3) | <.001 |
| Surgeons, (Median, interquartile range) a | 5.7 (3.5, 8.0) | 5.7 (3.5, 8.1) | 5.5 (3.3, 7.9) | <.001 | 5.7 (3.5, 8.1) | 5.5 (3.3, 7.9) | <.001 | 2.8 (1.1, 4.5) | 6.4 (4.2, 8.3) | <.001 |
| Pain specialist, (Median, interquartile range) a | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.5) | 0.2 (0.1, 0.4) | <.001 | 0.3 (0.1, 0.5) | 0.2 (0.1, 0.4) | <.001 | 0.0 (0.0, 0.1) | 0.3 (0.2, 0.5) | <.001 |
| Pharmacists, (Median, interquartile range ) a | 8.9 (7.2, 11.2) | 8.9 (7.2, 11.2) | 8.7 (7.2, 11.1) | <.001 | 8.9 (7.2, 11.3) | 8.7 (7.1, 11.0) | <.001 | 7.4 (5.7, 9.3) | 9.3 (7.5, 11.7) | <.001 |
| Midlevel providers, (Median, interquartile range) a | 4.9 (3.3, 7.2) | 4.9 (3.3, 7.2) | 4.9 (3.3, 7.2) | .03 | 4.9 (3.3, 7.2) | 4.8 (3.3, 7.1) | .005 | 4.1 (2.5, 6.1) | 5.1 (3.5, 7.5) | <.001 |
| Proportion of population over 65 | 0.1 (0.0) | 0.1 (0.0) | 0.1 (0.0) | .03 | 0.1 (0.0) | 0.1 (0.0) | .004 | 0.2 (0.0) | 0.1 (0.0) | <.001 |
| Proportion of population in poverty | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | <.001 | 0.1 (0.1) | 0.1 (0.1) | <.001 | 0.2 (0.1) | 0.1 (0.0) | <.001 |
Providers per 10 000 people.
About 13.1 percent of beneficiaries filled an opioid prescription in Phase 1, 13.9 percent had PT in Phase 1, and 10 percent filled a prescription or had PT in Phase 2 (Table 1). Less than 2 percent of the cohort used MH services in either phase. Beneficiaries used opioid and nonpharmacologic treatments concurrently, and the most common combination was opioid prescriptions and PT. Around 25 percent of beneficiaries who filled an opioid prescription in Phase 1 also used PT in that phase. Compared to beneficiaries in metropolitan counties, beneficiaries in rural counties were more likely to have opioid prescriptions and less likely to use PT and MH services.
In Phase 1, after controlling for individual and county characteristics, an additional nonpharmacologic provider/10 000 people/county was associated with lower odds of filling an opioid prescription [MH providers aOR: 0.97; 95% CI: 0.96‐0.98; physical therapists aOR: 0.98; 95% CI: 0.97‐1.00] (Table 2). Compared to no PT visit in Phase I, a PT visit in Phase 1 was associated with greater odds of filling an opioid prescription in Phase 1 [aOR: 2.60; 95% CI: 2.47‐2.75]. For the metropolitan and rural subgroup analysis, the directions of the associations were the same as the main analysis. For metropolitan counties, the supply of MH providers was significantly associated with lower odds of filling an opioid prescription [aOR: 0.98, 95% CI: 0.96‐0.99]. The associations for the supply of providers and use of nonpharmacologic services in Phase 1 for beneficiaries in metropolitan counties significantly differed from the associations for rural counties (Score statistic: 36.53, P‐value: .019).
TABLE 2.
Association between the supply of nonpharmacologic pain providers, use of nonpharmacologic services, and fill of an opioid prescription in Phase 1 and subgroup analysis for comparing metropolitan and rural counties
| Variable | Full cohort b | Metropolitan counties b | Rural counties b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted odds ratio | 95% Confidence interval | P‐value | Adjusted odds ratio | 95% Confidence interval | P‐value | Adjusted odds ratio | 95% Confidence interval | P‐value | |
| Mental Health Providers a | 0.97 | 0.96, 0.98 | <.0001 | 0.98 | 0.96, 0.99 | <.0001 | 0.96 | 0.93, 1.00c | .0528 |
| Physical Therapists a | 0.98 | 0.97, 1.00 c | .0142 | 0.98 | 0.97, 1.00 c | .0675 | 0.98 | 0.95, 1.00 c | .0860 |
| Used Mental Health Services in Phase 1 | 0.89 | 0.75, 1.06 | .1992 | 0.92 | 0.77, 1.11 | .3907 | 0.69 | 0.36, 1.31 | .2534 |
| Used Physical Therapy in Phase 1 | 2.60 | 2.47, 2.75 | <.0001 | 2.50 | 2.36, 2.65 | <.0001 | 3.19 | 2.80, 3.64 | <.0001 |
Providers per 10 000 people.
Adjusted for age, sex, race, Medicaid Buy‐in, Deyo‐Charlson comorbidity score, anxiety, depression, trauma or surgery in Phase 1, county‐level measures (supply of primary care providers, surgeons, pain specialists, pharmacists, midlevel providers, proportion of population over 65, proportion of population under Federal poverty level), and index year. Full cohort model adjusts for metropolitan area. Score statistic for stratified analysis: 36.53, P‐value: .019.
1.00 in the confidence interval reflects rounding.
In Phase 2, each additional MH provider/10,000 people/county was significantly associated with lower odds of filling an opioid prescription in the same phase [aOR: 0.97; 95% CI: 0.96‐0.98] (Table 3). PT use in Phase 1 was associated with lower odds of filling an opioid prescription in Phase 2 [aOR: 0.62; 95% CI: 0.58‐0.67]. Filling an opioid prescription in Phase 1 was associated with greater odds of filling an opioid prescription in Phase 2 [aOR: 4.18; 95% CI: 3.95‐4.43]. PT use in Phase 2 was associated with greater odds of filling an opioid prescription in Phase 2 [aOR: 2.75; 95% CI: 2.56‐2.96]. Associations did not differ significantly by metropolitan and rural counties (Score Statistic: 23.22, P‐value: .56).
TABLE 3.
Association between the supply of nonpharmacologic pain providers, use of nonpharmacologic services, and fill of an opioid prescription in Phase 2 of a pain episode and subgroup analysis for comparing metropolitan and rural counties
| Variable | Full Cohort b | Metropolitan Counties b | Rural Counties b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted odds ratio | 95% Confidence interval | P‐value | Adjusted odds ratio | 95% Confidence interval | P‐value | Adjusted odds ratio | 95% Confidence interval | P‐value | |
| Mental Health Providers a | 0.97 | 0.96, 0.98 | <.0001 | 0.96 | 0.95, 0.98 | <.0001 | 1.01 | 0.97, 1.04 | .7441 |
| Physical Therapists a | 1.00c | 0.98, 1.01 | .6048 | 0.99 | 0.97, 1.01 | .3531 | 1.00 c | 0.97, 1.03 | .9793 |
| Used Mental Health Services in Phase 1 | 1.20 | 0.97, 1.48 | .0992 | 1.19 | 0.95, 1.49 | .1349 | 1.32 | 0.68, 2.57 | .4167 |
| Used Physical Therapy in Phase 1 | 0.62 | 0.58, 0.67 | <.0001 | 0.62 | 0.57, 0.68 | <.0001 | 0.63 | 0.52, 0.76 | <.0001 |
| Opioid Prescription in Phase 1 | 4.18 | 3.95, 4.43 | <.0001 | 4.18 | 3.93, 4.45 | <.0001 | 4.19 | 3.68, 4.77 | <.0001 |
| Used Mental Health Services in Phase 2 | 1.00 c | 0.79, 1.25 | .9703 | 0.99 | 0.78, 1.27 | .9547 | 1.16 | 0.58, 2.31 | .6776 |
| Used Physical Therapy in Phase 2 | 2.75 | 2.56, 2.96 | <.0001 | 2.67 | 2.47, 2.89 | <.0001 | 3.17 | 2.67, 3.76 | <.0001 |
Providers per 10 000 people.
Adjusted for age, sex, race Medicaid Buy‐in, Deyo‐Charlson comorbidity score, anxiety, depression, trauma or surgery in Phase 1, trauma or surgery in Phase 2, county‐level measures (supply of primary care providers, surgeons, pain specialists, pharmacists, midlevel providers, proportion of population over 65, proportion of population under Federal poverty level), reason for censor (left fee‐for‐services or death before end of follow‐up), and index year. Full cohort model adjusts for metropolitan area. Score statistic for stratified analysis: 23.22, P‐value: .56).
1.00 in the confidence interval reflects rounding.
4. DISCUSSION
We examined the initiation rates of pain treatments in the first six months of a new episode of persistent MSP among Medicare beneficiaries over 65 years old. We found that utilization rates for opioids and PT were about 10 percent during the first six months of a pain episode. A study found that 29 percent of older adults with chronic pain reported receiving PT in the past year suggesting that PT utilization may increase as pain persists. 28 In the current study, the MH utilization rate was about 2 percent during the first six months of a pain episode, which was consistent with a previous study that found only 2 percent of older adults with chronic pain used MH services in the previous year. 58 These findings show that older adults may be more likely to use PT, but not MH, as pain persists; this suggests a focus on physical impairments and functional limitations over the psychological, emotional, and behavioral needs related to pain. 5
We found that the supply of nonpharmacologic providers has a small but significant association with initiating opioids. Although we hypothesized no association between the supply of nonpharmacologic providers and opioid prescriptions in Phase 1, the results show that the MH and PT supply was associated with lower odds of filling an opioid prescription in Phase 1. In Phase 2, only the MH supply was associated with lower odds of filling an opioid prescription. The direction of the associations for the MH supply was consistent with a study that found that the psychiatrists supply was negatively associated with county opioid prescribing rates. 30 The differences in the associations for the nonpharmacologic providers supply for each phase suggest that the local capacity to deliver services may be more important at the beginning of an episode. The small magnitude of the associations suggests that the nonpharmacologic provider supply has a limited impact on opioid initiation, and other explanations for not initiating opioid use could include fear of adverse events, lack of caregiver support, polypharmacy, and fear of misuse, addiction, or diversion. 59
We observed that PT in Phase 1 was associated with lower odds of filling an opioid prescription in Phase 2. This finding differs from a study, which found no difference in the odds of opioid use for older adults with low back pain who started PT in the first month of a new episode. 60 Several studies on younger adults with MSP had results that were similar to our study. 22 , 23 , 24 One explanation for the differences between our findings and those reported by Karvelas and colleagues could be that treatment effect could differ for populations with acute and persistent pain as well as populations with and without recent exposure to opioids. 60 We found that opioid prescriptions in Phase 1 were significantly associated with opioid prescriptions in Phase 2; more than a third of those prescribed opioids in Phase 2 had an opioid prescription filled in Phase 1. This finding is concerning because studies have found that the likelihood of long‐term opioid use was associated with a greater days’ supply for the first prescription. 18 , 19
Despite findings suggesting that the supply of MH providers was associated with lower odds of opioid prescriptions in either phase, MH service use in Phase 1 was not associated with opioid prescriptions in Phase 2. The difference in findings for the supply of MH providers and use of MH services suggests that actual use of services may not be the only path through which the supply of providers influences opioid prescribing. For example, MH providers may offer other MH treatments which could affect opioid prescribing or providers who prescribe opioids may be less willing to prescribe if other alternatives to opioids are available. The lack of association between MH service use in Phase 1 and opioid use in Phase 2 could be because the relationship between MH service use and opioid prescription fills may depend on the number of services or type of MH service. Evidence on MH services, such as cognitive behavioral therapy, suggests that addressing psychosocial factors associated with pain may be associated with improvements in pain and lower opioid use. 25 , 61
Our findings show that the relationship between the supply of nonpharmacologic providers and opioid prescriptions varies by metropolitan and rural county designations. While most associations were consistent with findings from the full cohort, there were two key differences in the associations for the nonpharmacologic provider supply. Unlike metropolitan counties, the MH provider supply was not significantly associated with the odds of opioid prescriptions in Phases 1 and 2 for rural counties. The PT supply was not significantly associated with opioid prescriptions in Phase 1 for metropolitan and rural counties, and this association differed from the findings of the full cohort. Increases in MH provider supply in metropolitan counties could reduce opioid prescriptions. Furthermore, considering that PT use in Phase 1 was associated with lower odds of opioid prescriptions in Phase 2 for both metropolitan and rural counties, future research should examine other drivers of disparities between metropolitan and rural counties, such as distance to care and patient preferences, and design targeted strategies to increase nonpharmacologic service utilization, especially PT, in metropolitan and rural counties.
Strategies that increase the number of nonpharmacologic providers may lead to reductions in opioid prescriptions in Phase 1 and indirectly reduce opioid prescriptions in Phase 2 through the use of PT. Policies that address the barriers to PT use, such as improving Medicare coverage by removing the caps on payment for PT visits, are critical for reducing opioid prescriptions. 62 Though MH visits are not associated with opioid prescriptions, encouraging MH use among beneficiaries with pain may also be important because the prevalence of MH conditions is high in populations with persistent pain. 28 , 54 , 63 Similar to a previous study on older adults with persistent pain, we found that the prevalence of depression among older adults with persistent pain was about 6 percent. 58 Similarly, among older adult, Medicare Part D beneficiaries, 3 percent had depression. 64 This finding, along with the low MH service utilization rates, could indicate that MH conditions are underdiagnosed and undertreated in this population. In addition to lack of providers, other barriers to using nonpharmacologic treatments may be poor care coordination, insufficient insurance coverage, transportation, and the knowledge, beliefs, and treatment preferences of patients and prescribers. 35 , 65 , 66 , 67 , 68
This observational study has several limitations. The associations are not causal. Unobserved characteristics (eg, pain severity, function, SUDs, interventional pain treatments, supply of substance use providers, and social workers) could bias associations. This analysis cannot be used to make inferences about whether nonpharmacologic interventions limit the transition from acute to persistent pain because claims data does not include pain severity. Temporality of nonpharmacologic service use and opioid prescriptions within each phase was not assessed. We lacked data on whether prescribed opioids were taken for the pain diagnosis identified in this study. We used administrative claims data, which only have services paid by Medicare; we lacked data on pain treatments paid for out‐of‐pocket (eg, over the counter medications, yoga, acupuncture, massage, or PT/MH services that were paid for out‐of‐pocket). We also lacked data on the opioid prescriber specialty, and psychiatrists could prescribe opioids. 46 We focused on MH services used during a persistent MSP episode, but it is possible that MH services may be used for non‐pain conditions. When this study was conducted, claims with SUD diagnoses were redacted. 55 The MH service utilization rate and the prevalence rate of MH conditions, particularly for those claims tied to SUD diagnoses, may be underestimated. One study of Medicare Part D beneficiaries estimated that the prevalence of SUDs is <1 percent. 64 The measurement error in MH service use may bias associations to the null. Though we matched county characteristics based on a beneficiary's county of residence, beneficiaries could have accessed care outside of their county, which would indicate a larger supply of providers for the beneficiary. These findings conservatively estimate the association between the provider supply and opioid prescriptions. The definition of Health Professional Shortage Areas for MH providers includes psychiatrists, psychologists, social workers, psychiatric nurse specialists, and marriage and family therapists. 45 The AHRF lacks data on the supply of social workers, psychiatrics nurses, and family therapists, so the MH provider supply may be underestimated. 38 Measurement error in the PT and MH provider supply may bias estimates toward the null. We found that the variation between counties or states for the supply measures was greater than the variation over time, which indicates that the models likely explain the geographic variation and the measurement error bias is minimal. Finally, our findings may not generalize to younger adults, adults with surgery or trauma, or adults with acute pain.
Future research should examine the association between access to nonpharmacologic services and high‐risk patterns such as the number of opioid prescriptions, high doses, and long‐term opioid use. Continuity of nonpharmacologic services and pain severity should be examined to assess the efficacy of MH and PT services on reducing pain symptoms and opioid use.
5. CONCLUSION
This study shows how the supply and use of nonpharmacologic services may influence opioid use at the onset of a persistent MSP episode. We found similar rates of opioid prescriptions and PT use but lower rates of MH service use during the first six months of an episode. PT and opioids were often used together. After adjusting for possible confounding characteristics, greater supply of nonpharmacologic providers and PT use was associated with reductions in opioid prescriptions. Promising policies that would facilitate guideline supported pain management approaches for older adults include increasing the number of nonpharmacologic providers and encouraging PT at the onset of a persistent MSP episode.
Supporting information
Supplementary Material
Table S1‐S5_v2
Karmali RN, Skinner AC, Trogdon JG, Weinberger M, George SZ, Hassmiller Lich K. The association between the supply of select nonpharmacologic providers for pain and use of nonpharmacologic pain management services and initial opioid prescribing patterns for Medicare beneficiaries with persistent musculoskeletal pain. Health Serv Res.2021;56:275–288. 10.1111/1475-6773.13561
REFERENCES
- 1. United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (BMUS). Fourth Edition, forthcoming. Rosemont, IL. http://www.boneandjointburden.org. Accessed April 28, 2018.
- 2. International Association for the Study of Pain . Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;(3):S1‐S226. [PubMed] [Google Scholar]
- 3. Molton IR, Terrill AL. Overview of persistent pain in older adults. Am Psychol. 2014;69(2):197‐207. [DOI] [PubMed] [Google Scholar]
- 4. Baumbauer KM, Young EE, Starkweather AR, et al. Managing chronic pain in special populations with emphasis on pediatric, geriatric, and drug abuser populations. Med Clin North Am. 2016;100(1):183‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. IOM (Institute of Medicine) . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, D.C.: National Academies Press; 2011. [PubMed] [Google Scholar]
- 6. American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons . Pharmacological management of persistent pain in older persons. Pain Med. 2009;10(6):1062‐1083. [DOI] [PubMed] [Google Scholar]
- 7. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain ‐ United States, 2016. MMWR Recomm Rep. 2016;65(1):1‐49. [DOI] [PubMed] [Google Scholar]
- 8. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2017;166(7):514‐530. [DOI] [PubMed] [Google Scholar]
- 9. Park J, Hughes AK. Nonpharmacological approaches to the management of chronic pain in community‐dwelling older adults: a review of empirical evidence. J Am Geriatr Soc. 2012;60(3):555‐568. [DOI] [PubMed] [Google Scholar]
- 10. Makris UE, Abrams RC, Gurland B, et al. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinman MA, Komaiko KDR, Fung KZ, et al. Use of opioids and other analgesics by older adults in the United States, 1999–2010. Pain Med. 2015;16(2):319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller M, Sturmer T, Azrael D, et al. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59(3):430‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170(22):1968‐1976. [DOI] [PubMed] [Google Scholar]
- 14. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979‐1986. [DOI] [PubMed] [Google Scholar]
- 15. Dublin S, Walker RL, Jackson ML, et al. Use of Opioids Or Benzodiazepines And Risk Of Pneumonia In Older Adults: A Population‐Based Case‐Control Study. J Am Geriatr Soc. 2011;59(10):1899‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papaleontiou M, Henderson CR Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta‐analysis. J Am Geriatr Soc. 2010;58(7):1353‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mundkur ML, Rough K, Huybrechts KF, et al. Patterns of opioid initiation at first visits for pain in United States primary care settings. Pharmacoepidemiol Drug Saf. 2017;27(5):495‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah A, Hayes CJ, Martin BC. Factors influencing long‐term opioid use among opioid naive patients: an examination of initial prescription characteristics and pain etiologies. J Pain. 2017;18(11):1374‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah AHC, Martin BC. Characteristics of initial prescription episodes and likelihood of long‐term opioid use — United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;2017(66):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Von Korff MR. Long‐term use of opioids for complex chronic pain. Best Pract Res Clin Rheumatol. 2013;27(5):663‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276‐286. [DOI] [PubMed] [Google Scholar]
- 22. Ojha HA, Wyrsta NJ, Davenport TE, et al. Timing of physical therapy initiation for nonsurgical management of musculoskeletal disorders and effects on patient outcomes: a systematic review. J Orthop Sports Phys Ther. 2016;46(2):56‐70. [DOI] [PubMed] [Google Scholar]
- 23. Fritz JM, Childs JD, Wainner RS, et al. Primary care referral of patients with low back pain to physical therapy: impact on future health care utilization and costs. Spine. 2012;37(25):2114‐2121. [DOI] [PubMed] [Google Scholar]
- 24. Childs JD, Fritz JM, Wu SS, et al. Implications of early and guideline adherent physical therapy for low back pain on utilization and costs. BMC Health Serv Res. 2015;15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non‐cancer pain. Cochrane Database Syst Rev. 2017;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanney WJ, Masaracchio M, Liu X, et al. The influence of physical therapy guideline adherence on healthcare utilization and costs among patients with low back pain: a systematic review of the literature. PLoS One. 2016;11(6):e0156799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frogner BK, Harwood K, Andrilla CHA, et al. Physical therapy as the first point of care to treat low back pain: an instrumental variables approach to estimate impact on opioid prescription, health care utilization, and costs. Health Serv Res. 2018;53(6):4629‐4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knauer SR, Freburger JK, Carey TS. Chronic low back pain among older adults: a population‐based perspective. J Aging Health. 2010;22(8):1213‐1234. [DOI] [PubMed] [Google Scholar]
- 29. Francke AL, Smit MC, de Veer AJ, et al. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta‐review. BMC Med Inform Decis Mak. 2008;8(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13(10):988‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Curtis LH, Stoddard J, Radeva JI, et al. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv Res. 2006;41: 837‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Webster BS, Cifuentes M, Verma S, et al. Geographic variation in opioid prescribing for acute, work‐related, low back pain and associated factors: a multilevel analysis. Am J Ind Med. 2009;52(2):162‐171. [DOI] [PubMed] [Google Scholar]
- 33. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prunuske JP, St. Hill CA, Hager KD, et al. Opioid prescribing patterns for non‐malignant chronic pain for rural versus non‐rural US adults: a population‐based study using 2010 NAMCS data. BMC Health Serv Res. 2014;14(1):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroenke K, Alford DP, Argoff C, et al. Challenges with implementing the Centers for Disease Control and Prevention opioid guideline: a consensus panel report. Pain Med. 2019;20(4):724‐735. [DOI] [PubMed] [Google Scholar]
- 36. Ritzwoller DP, Crounse L, Shetterly S, et al. The association of comorbidities, utilization and costs for patients identified with low back pain. BMC Musculoskelet Disord. 2006;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Axeen S. Trends in opioid use and prescribing in Medicare, 2006–2012. Health Serv Res. 2018;53(5):3309‐3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. US Department of Health and Human Services HRaSA, Bureau of Health Workforce, Data from: Area Health Resources Files (AHRF). 2015–2016. Rockville, MD.
- 39. United States Department of Agriculture Economic Research Service . Rural‐Urban Continuum Codes. 2013. https://www.ers.usda.gov/data‐products/rural‐urban‐continuum‐codes/ Accessed May 2, 2018
- 40. Watkins‐Castillo SI.ICD‐9‐CM Codes for Musculoskeletal Diseases. 2014. http://www.boneandjointburden.org/2014‐report/ik0/icd‐9‐cm‐codes‐musculoskeletal‐diseases. Accessed April 3, 2017
- 41. Gore M, Sadosky A, Stacey BR, et al. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine. 2012;37(11):E668‐E677. [DOI] [PubMed] [Google Scholar]
- 42. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Surgery Flag Software . Healthcare Cost and Utilization Project (HCUP). February 2016. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup‐us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp. Accessed December 1, 2017 [Google Scholar]
- 44. National Center for Injury Prevention and Control . CDC Compilation of Benzodiazepines, Muscle Relaxants, Stimulants, Zolpidem, and Opioid Analgesics with oral Morphine Milligram Equivalent Conversion Factors, 2017 Version. Atlanta, GA: Centers for Disease Control and Prevention; 2017. https://www.cdc.gov/drugoverdose/resources/data.html. Accessed February 26, 2018 [Google Scholar]
- 45. Health Research and Services Administration . Shortage Designation Application and Review Process. 2019. https://bhw.hrsa.gov/shortage‐designation/application‐review‐process. Accessed October 20, 2019
- 46. Guy GP Jr, Zhang K. Opioid prescribing by specialty and volume in the US. Am J Prev Med. 2018;55(5):e153‐e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mojtabai R, Olfson M. National trends in psychotherapy by office‐based psychiatrists. Arch Gen Psychiatry. 2008;65(8):962‐970. [DOI] [PubMed] [Google Scholar]
- 48. Fritz JM, Cleland JA, Brennan GP. Does adherence to the guideline recommendation for active treatments improve the quality of care for patients with acute low back pain delivered by physical therapists? Med Care. 2007;45(10):973‐980. [DOI] [PubMed] [Google Scholar]
- 49. Albrecht JS, Kiptanui Z, Tsang Y, et al. Patterns of depression treatment in Medicare beneficiaries with depression after traumatic brain injury. J Neurotrauma. 2015;32(16):1223‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Center for Medicare and Medicaid Services . Eligible Beneficiaries under Medicare and Medicaid. https://www.cms.gov/Outreach‐and‐Education/Medicare‐Learning‐Network‐MLN/MLNProducts/downloads/Medicare_Beneficiaries_Dual_Eligibles_At_a_Glance.pdf. Accessed February 26, 2018
- 51. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 52. Center for Medicare and Medicaid Services . Chronic Conditions Warehouse: Condition Categories: Other Chronic Health, Mental Health, and Potentially Disabling Conditions. https://www.ccwdata.org/web/guest/condition‐categories. Accessed October 6, 2017
- 53. HCUP Chronic Condition Indicator . Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. www.hcup‐us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed December 14, 2017 [Google Scholar]
- 54. Hooten WM. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc. 2016;91(7):955‐970. [DOI] [PubMed] [Google Scholar]
- 55. Research Data Assistance Center . Redaction of Substance Abuse Claims. https://www.resdac.org/articles/redaction‐substance‐abuse‐claims. Accessed February 09, 2020
- 56. Boos DD. On generalized score tests. Am Stat. 1992;46(4):327‐333. [Google Scholar]
- 57. Bureau of Labor Statistics . U.S. Department of Labor, Occupational Employment Statistics. https://www.bls.gov/oes/home.htm. Accessed April 27, 2020
- 58. Braden JB, Zhang L, Fan MY, et al. Mental health service use by older adults: the role of chronic pain. Am J Geriatr Psychiatry. 2008;16(2):156‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spitz A, Moore AA, Papaleontiou M, et al. Primary care providers' perspective on prescribing opioids to older adults with chronic non‐cancer pain: a qualitative study. BMC Geriatr. 2011;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karvelas DA, Rundell SD, Friedly JL, et al. Subsequent health‐care utilization associated with early physical therapy for new episodes of low back pain in older adults. Spine J. 2017;17(3):380‐389. [DOI] [PubMed] [Google Scholar]
- 61. Ehde DM, Dillworth TM, Turner JA. Cognitive‐behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153. [DOI] [PubMed] [Google Scholar]
- 62. Center for Medicare and Medicaid Services . Therapy Services. https://www.cms.gov/Medicare/Billing/TherapyServices. Accessed June 1, 2020
- 63. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1–2):127‐133. [DOI] [PubMed] [Google Scholar]
- 64. Kuo YF, Raji MA, Chen NW, et al. Trends in opioid prescriptions among part D Medicare recipients from 2007 to 2012. Am J Med. 2016;129(2):221.e21‐e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andrilla CHA, Patterson DG, Garberson LA, et al. Geographic variation in the supply of selected behavioral health providers. Am J Prev Med. 2018;54(6):S199‐S207. [DOI] [PubMed] [Google Scholar]
- 66. Zimbelman JL, Juraschek SP, Zhang X, et al. Physical therapy workforce in the United States: forecasting nationwide shortages. PM&R. 2010;2(11):1021‐1029. [DOI] [PubMed] [Google Scholar]
- 67. Park J, Hirz CE, Manotas K, et al. Nonpharmacological pain management by ethnically diverse older adults with chronic pain: barriers and facilitators. J Gerontol Soc Work. 2013;56(6):487‐508. [DOI] [PubMed] [Google Scholar]
- 68. Becker WC, Dorflinger L, Edmond SN, et al. Barriers and facilitators to use of non‐pharmacological treatments in chronic pain. BMC Fam Pract. 2017;18(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1‐S5_v2


