Abstract
Orthodontic treatment can lead to microbial-induced gingival inflammation and aseptic periodontal inflammations. The aim of this study was to investigate the relationship between salivary pro-inflammatory cytokines levels with gingival health status and oral microbe loads among patients undergoing orthodontic treatment.
The present investigation was a cross-sectional study among a sample of 111 consecutive orthodontic patients (mean age 18.4 ± 4.4 years). Clinical examinations were conducted to assess the gingival health status employing the Modified Gingival Index, Gingival Bleeding Index, and Plaque Index. Salivary microbiological assessments of total aerobic and anaerobic bacteria count, streptococci count, and lactobacilli count were undertaken. Saliva immunological assessments included Interleukin-1Beta (IL-1β) and macrophage migration inhibitory factor (MIF) ELISA assays.
The mean ± standard deviation of salivary IL-1β was 83.52 ± 85.62 pg/ml and MIF was 4.12 ± 0.96 ng/ml. Moderate positive correlations were found between salivary IL-1β levels and total aerobic and anaerobic bacteria count, streptococci count, and lactobacilli count (r = 0.380–0.446, P < .001), and weak positive correlations between salivary MIF levels and total salivary aerobic and anaerobic bacteria counts (r = 0.249–0.306, P < .01) were observed. A positive correlation was found between salivary IL-1β levels and Bleeding Index (r = 0.216, P < .05).
The level of salivary IL-1β positively correlates with oral bacterial load among orthodontic patients; the relationship between inflammatory cytokines and oral microflora deserved further study.
Keywords: cytokines, gingivitis, microbiology, orthodontics
1. Introduction
Previous studies demonstrate that orthodontic treatment creates “new” locations for plaque retention and increases the risk for gingival inflammation. Thus, the increased potential risk for periodontal diseases is among patients undergoing orthodontic treatment is of concern for clinicians.[1,2] Traditionally, assessment or monitoring of gingival conditions has been conducted by clinical examination and expressed by various clinical parameters such as Plaque Index (PI) and Gingival Index (GI).[3] Recently attention has been focused on pro-inflammatory cytokines working as biomarkers in periodontal health assessments, this has potential in identifying early risk of inflammation and periodontal diseases.[4,5] Patients with periodontal diseases have significantly higher levels of Interleukin-1Beta (IL-1β) in the gingival crevicular fluid (GCF) and in their saliva compared with “healthy” individuals.[6,7] The result of experimental gingivitis study demonstrated a significant positive correlation between macrophage migration inhibitory factor (MIF) and plaque levels (as assessed by PI) and gingivitis (as assessed by GI).[8] Thus, evaluating the levels of pro-inflammatory cytokines may provide insight into risk of periodontal diseases and levels of destructive “activity” in itself and/or provide a more accurate assessment of periodontal health risks when combined with clinical examinations with implications for clinical practice, prevention, and management.
For those undergoing orthodontic treatment, the levels of pro-inflammatory cytokines might be affected not only by the occurrence of microbial-induced gingivitis but also by aseptic periodontal inflammations. Previous studies demonstrated that the application of orthodontic forces for teeth moving can induce aseptic inflammatory responses.[9,10] High concentrations of inflammatory cytokines such as IL-1β, Interleukin-6 (IL-6), Interleukin-8 (IL-8), and tumor necrosis factor alpha (TNFα) have been detected in the GCF in both animal models and in human clinical trials.[11–13]
The assessment of cytokines during orthodontic treatment warrants consideration in orthodontic patients in understanding their association and pathways for periodontal health. Furthermore, although the role of the pro-inflammatory cytokines on host inflammatory response to microbial challenges is well recognized, there is a dearth of information on the relationship between pro-inflammatory cytokines and oral microbial load. The aims of the present study were to investigate:
-
1.
the relationship of the salivary pro-inflammatory cytokines levels and gingival health statues,
-
2.
the relationship of the salivary pro-inflammatory cytokines levels and oral bacterial loads among patients undergoing orthodontic treatment.
2. Materials and methods
2.1. Trial registration
This study was approved by The Institution Review Board of The University of Hong Kong /Hospital Authority Hong Kong West Cluster (Reference Number: UW 10-278). ClinicalTrials.gov Identifier: NCT01637948, retrospectively registered.
2.2. Participants
All participants were provided written informed consent and a written explanation the background of the study, its objectives, and their involvement. All participants signed the written informed consent, and in the case subjects who were under 18 years old, both the patient and primary care giver were asked to sign the written informed consent.
This was a cross-sectional study conducting among a consecutive sample of 111 patients (53 males and 58 females, mean age 18.4 ± 4.4 years) who were undergoing fixed orthodontic appliance therapy at the Orthodontic Clinic of the Faculty of Dentistry, the University of Hong Kong. Patients were selected based on the following inclusion criteria: (age > 13, in good health, non-smoker, no periodontitis (periodontal disease was defined as probing depth ≥4 mm) or other dental diseases that required treatment.[14]
2.3. Clinical examinations
Periodontal health assessments were undertaken employing:
-
(i)
the Modified Gingival Index (MGI),
-
(ii)
Gingival Bleeding Index (GBI), and
-
(iii)
PI following a previous protocol[15]: assessments were carried out on Ramfjord teeth (buccal site of upper right first molar, upper left central incisor, upper left first premolar, lower left first molar, lower right central incisor, lower right first premolar.[16])
A single trained and calibrated examiner [YC] performed all clinical examinations.
2.4. Microbiological and immunological examinations
Saliva samples were obtained: No food or drink was permitted for 2 hours before collection. During the sample collection, the volunteers remained in a seated position, with their head tilted forward. The procedure was accomplished in a quiet and well-ventilated room. Saliva samples (unstimulated) were collected by means of a draining method from the participants after clinical examinations.[17] Initially, the examiner instructed each participant to rinse with distilled water once, and then collect unstimulated saliva produced during a 5-minute period with a sterile plastic bottle.[17]
Microbiological assessments: Saliva samples held for culture were packed in ice and were plated within 2 hours in the very day of collection. Serial 10-fold dilutions were made and 0.05 ml of each dilution was inoculated onto horse blood agar (HBA) plates (Oxoid columbia blood base, Oxoid Limited, UK) for aerobic culture and anaerobic culture, and Mitis-Salivarius agar (BD Difco Mitis-Salivarius Agar, BD) and Rogosa agar (BD Difco Rogosa SL Agar, BD) were used for selective culture streptococci and lactobacilli.[18,19] Inoculum was spread evenly over the agar with use of a spiral plater. The aerobic cultures and anaerobic cultures were incubated for 48 hours. Colony counts were performed on plates (Fig. 1) yielding 30 to 300 bacterial colonies per plate.[20] The number of viable bacteria per milliliter was calculated by multiplying the number of colonies by the reciprocal of the dilution factor.
Figure 1.
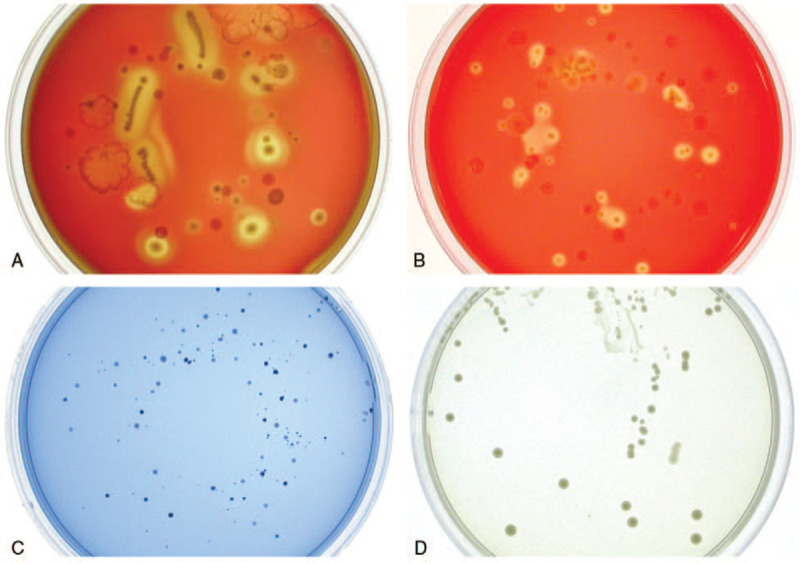
A typical human saliva bacteria cultured in different mediums and conditions: horse blood agar for total bacteria count (A: anaerobic and B: aerobic, 104 times dilution), Mitis-salivarius agar for Streptococci count (C: anaerobic, 102 times dilution), and Rogosa agar for lactobacilli count (D: anaerobic, 10 times dilution).
Immunological assay: Saliva samples held for IL-1Beta and MIF assay were collected and immediately transferred to Eppendorf tube and frozen at −70°C. Before enzyme linked immunosorbent assay (ELISA), collected saliva samples were thawed and centrifuged at 10,000 rpm for 5 minutes to precipitate bacteria and other impurities, and then the supernatant was used for assay. This assay employed the quantitative sandwich enzyme immunoassay technique. The supernatants were diluted with the reagent diluents within the ELISA kit (DuoSet ELISA Development Kit, R&D Systems, Inc.) in the ratio of 1:2 for IL-1Beta and 1:4 for MIF. Standard curves were generated as manufacture instruction: recombinant human IL-1Beta and MIF were series diluted in reagent diluents and developed for each set of samples assayed (Figs. 2 and 3). The results were reported within the linearity of the assay. The values obtained were multiplied by the dilution factor so as to obtain the actual concentration of salivary cytokines. The results were reported as concentration of cytokines in pictogram or nanogram per milliliter. All ELISA determinations were performed in duplicate.[5]
Figure 2.
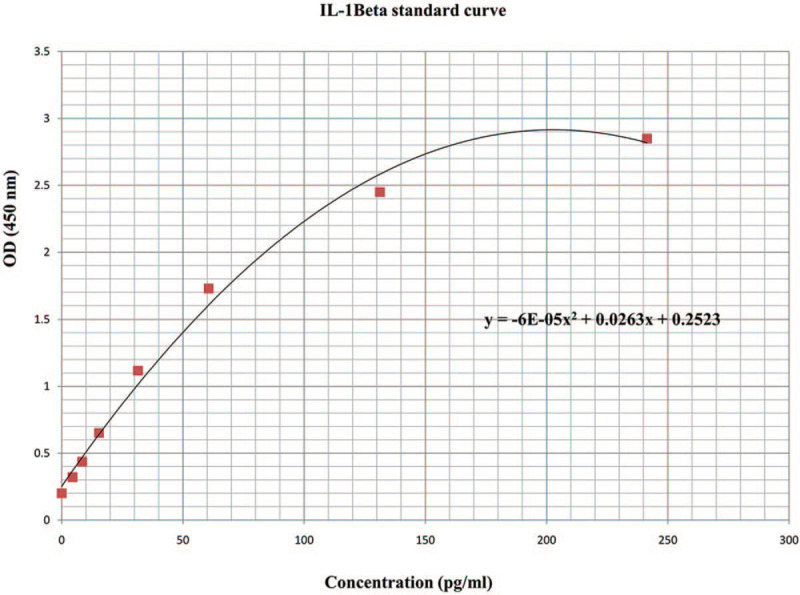
The standard curve of ELISA IL-1Beta.
Figure 3.
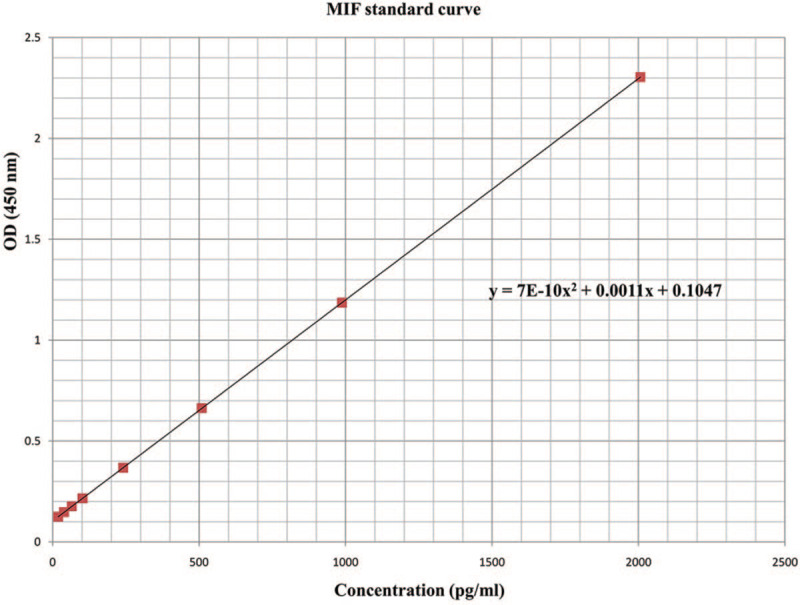
The standard curve of ELISA MIF.
2.5. Statistical analysis
Statistical analysis was performed by SPSS 17.0 (SPSS version 17.0, SPSS, Chicago, IL), Pearson correlation, and Spearman correlation test was used to assess the strength of association between salivary IL-1Beta and MIF levels and clinical and microbiological parameters, respectively. Multiple linear regressions were used to analyze the effects of the correlated parameters on salivary IL-1Beta and MIF levels. The level of significance was set at P < .05.
3. Results
Table 1 provides information on sample demographic, microbiological, and clinical parameters of all participants, the mean and standard deviation (SD) of clinical indices indicated most of the participants show a mild to moderate level of gingivitis. Detectable levels of saliva bacteria were found in all saliva samples in following culture mediums or conditions: blood agar (aerobic and anaerobic) and Mitis-Salivarius agar. In 20 saliva samples, no lactobacilli were detected in Rogosa agar culture. Detectable levels of IL-1β and MIF were found in all saliva samples.
Table 1.
Demographic, microbiological, clinical, and immunological parameters of the participants.
| Demographic, clinical, microbiological, and immunological parameters | N | Mean | SD | Minimum | Maximum |
| Age (yr) | 111 | 18.4 | 4.4 | 13.0 | 35.0 |
| Saliva volume (ml/5 min) | 111 | 3.1 | 1.6 | 0.7 | 8.9 |
| Plaque Index (PI) | 111 | 1.65 | 0.45 | 0.67 | 2.83 |
| Modified Gingival Index (MGI) | 111 | 1.64 | 0.41 | 0.67 | 2.67 |
| Bleeding Index (BI) | 111 | 0.50 | 0.38 | 0.00 | 1.67 |
| Total aerobic bacteria count (Log 10/ml) | 111 | 6.98 | 0.49 | 5.30 | 7.89 |
The correlation between participants’ salivary IL-1β and MIF levels and the microbiological and clinical parameters are shown in Table 2. Positive significant correlations were observed between salivary IL-1β levels and GBI (r = 0.216, P < .05) and between salivary IL-1β levels and salivary bacteria count in different mediums and conditions (r = 0.380–0.446, P < .001). A positive significant correlation was observed between salivary MIF levels and salivary total aerobic bacteria count and total anaerobic bacteria count (r = 0.249–0.306, P < .01). Salivary MIF levels were negatively correlated with age (r = −0.209, P < .05). There was no significant difference in salivary cytokines levels between male and female patients (P > .05).
Table 2.
The Pearson correlation of demographic, microbiological, clinical parameters and salivary IL-1β, and MIF levels.
| IL-1Beta | MIF | |||
| Demographic, clinical, and microbiological parameters | Correlation | P | Correlation | P |
| Age | −0.176 | .065 | −0.209 | .028 |
| Saliva volume (ml/5 min) | 0.089 | .352 | −0.108 | .257 |
| Plaque Index (PI) | 0.065 | .495 | 0.022 | .819 |
| Modified Gingival Index (MGI) | 0.167 | .080 | 0.082 | .390 |
| Bleeding Index (BI) | 0.216 | .023 | 0.013 | .896 |
| Total aerobic bacteria count (Log 10/ml) | 0.446 | .000 | 0.249 | .008 |
| Total anaerobic bacteria count (Log 10/ml) | 0.426 | .000 | 0.306 | .001 |
| Streptococci count (Log 10/ml) | 0.405 | .000 | 0.012 | .898 |
| Lactobacilli count (Log 10/ml) | 0.380 | .000 | 0.137 | .196 |
Multiple linear regression model investigated the relationships between the correlated clinical and microbiological factors and levels of salivary IL-1β and MIF. Since the total anaerobic bacteria count was highly correlated with total aerobic bacteria count (r = 0.85, P < .001) which suggests these 2 factors might cause multicollinearity, only the higher correlated factor: total aerobic bacteria count (r = 0.45, P < .001) was selected for determining salivary IL-1β level in the regression model. Subsequently, the relationship between total aerobic bacteria count, lactobacilli count with salivary IL-1β level according to the following equation: level of salivary IL-1β = −497.45 + 48.65 total aerobic bacteria count + 33.67 lactobacilli count. The association was positive (P < .001), the results demonstrated that the IL-1β level was directly proportional to total aerobic bacteria count and lactobacilli count (increases with bacteria count) (Table 3). Considering the total anaerobic bacteria count was highly correlated with total aerobic bacteria count which might cause multicollinearity, the relatively higher correlated factor: total anaerobic bacteria count (r = 0.31, P < .01) was selected into the salivary MIF level regression model. Subsequently, the relationship between total anaerobic bacteria count, with salivary MIF level according to the following equation: level of salivary MIF = 530.48 total aerobic bacteria count. The association was positive (P < .001), the results demonstrated that the MIF level was directly proportional to total aerobic bacteria count (increases with bacteria count) (Table 4).
Table 3.
Multiple linear regression of salivary IL-1β levels associated with independent variables (r = 0.510; r2 = 0.260).
| Unstandardized coefficients | 95% confidence interval for B | |||||
| Independent variables | B | Std. error | t | Sig. | Lower bound | Upper bound |
| Intercept | −497.45 | 126.56 | −3.93 | 0.000 | −749.04 | −245.85 |
| Bleeding Index (BI) | 20.83 | 21.10 | 0.99 | 0.326 | −21.11 | 62.77 |
| Total aerobic bacteria count (Log 10/ml) | 48.65 | 22.37 | 2.18 | 0.032 | 4.19 | 93.12 |
| Streptococci count (Log 10/ml) | 17.85 | 19.03 | 0.94 | 0.351 | −19.99 | 55.69 |
| Lactobacilli count (Log 10/ml) | 33.67 | 12.95 | 2.60 | 0.011 | 7.92 | 59.42 |
Table 4.
Multiple linear regression of salivary MIF levels associated with independent variables (r = 0.340; r2 = 0.116).
| Unstandardized coefficients | 95% confidence interval for B | |||||
| Independent variables | B | Std. error | t | Sig. | Lower bound | Upper bound |
| Intercept | 999.31 | 1384.01 | .72 | 0.472 | −1744.03 | 3742.65 |
| Age | −32.91 | 20.07 | −1.64 | 0.104 | −72.69 | 6.87 |
| Total anaerobic bacteria count (Log 10/ml) | 530.48 | 178.74 | 2.97 | 0.004 | 176.20 | 884.77 |
4. Discussion
Although pro-inflammatory cytokines work as biomarkers in periodontal health assessment in general population have been extensively investigated, the relationships between pro-inflammatory cytokines and gingival health statues among orthodontic patients remain unclear. The present cross-sectional study tries to explore the association between the salivary inflammatory mediators and microbiological and clinical features in orthodontic patients. The result shows that there was only a week positive correlation between the salivary IL-1β and BI, and no other relationship between salivary MIF and IL-1β levels and clinical gingival indices were found, which means both cytokines might not be suitable for gingival health assessment among orthodontic patients. Positive correlations were found between salivary IL-1β and MIF levels and total salivary bacteria count in the present study, which indicated that salivary MIF and IL-1β levels were mainly in response to bacterial accumulation. This finding confirms that the immunological cytokines play important roles in host inflammatory response to microbial challenges and contributes new knowledge on this research area.
In the present study, the levels of salivary IL-1β and MIF were selected to explore relationships between immunological, microbiological, and clinical parameters among orthodontic patients. IL-1β is a key regulator of the host responses to microbial infection and a major modulator of extracellular matrix catabolism and bone resorption.[21,22] It has been reported that salivary levels of IL-1β were positively correlated with BOP in periodontitis patients.[4] MIF is known to be a pro-inflammatory cytokine involved in macrophage and t-cell activation, IgE synthesis, insulin release, carbohydrate metabolism, cell growth and apoptosis, and tumor angiogenesis.[23] An experimental gingivitis study reported that the level of MIF positive correlates with plaque index and gingival index.[8] These researches indicate that both cytokines are feasible to be used to assess gingival health statues.
However, none of the clinical parameters: PI, MGI, GBI were significantly correlated with salivary MIF level, and there was only a week positive correlation between the salivary IL-1β and GBI in this study. In the multiple regression model, none of the clinical parameters significantly predicted salivary IL-1β levels. These results might due to the effect of orthodontic treatment which could affect cytokines secretion. Previous studies have shown that IL-1β, IL-6, IL-8, and TNF alpha increased in the GCF when applying orthodontic force.[9–13]Therefore, the secretion of pro-inflammatory cytokines in orthodontic patients is more complicated than that of general population, and pro-inflammatory cytokines working as biomarkers in gingival health status assessment among orthodontic patients requires further study.
The demographic parameters (age, sex, saliva flow rate) did not show a significant association with the salivary IL-1β. A negative correlation was found between salivary MIF levels and age, which suggested that salivary MIF level declines with age, which is consistent with a previous study.[8] However, in the multiple regression model, age was not significantly associated with the salivary MIF level (P > .05). It is plausible that bacteria number is a stronger factor which conceals the effect of age on salivary MIF level with the present sample size.
In this study, positive correlations were found between salivary IL-1β and MIF levels and total salivary bacteria count, and similar correlation coefficients between the total bacteria count (aerobic and anaerobic), streptococci number, and lactobacilli number with IL-1β levels which indicates that no specific bacteria combinations might be associated with gingivitis. These results are in general agreement with a previous study, which investigated a larger sample size and reported the detection of multiple pathogenic species in saliva, rather than the presence of any specific pathogens in saliva, which were associated with periodontitis.[24] Although another study reported that higher GCF levels of IL-1βcorresponded with higher proportions of orange and red complex species in periodontitis patients[25]; however, for the subjects in our study who only have gingivitis, specific pathogenic species may not have yet established.
The present study was only a pilot study try to explore the relationship of the salivary pro-inflammatory cytokines levels and gingival health statues, as well as the relationship of the salivary pro-inflammatory cytokines levels and oral bacterial loads among patients undergoing orthodontic treatment. Our findings indicate that IL-1β and MIF may be useful and appropriate biomarkers to reflect oral bacterial loads. However, since this study was a cross-sectional study with a limited sample size, it is premature to extrapolate these results to the general population. Moreover, although the analysis of salivary biomarkers offers advantages such as collection of whole saliva is easy and noninvasive, there are also some disadvantages to using whole saliva for diagnostic purposes, such as saliva contains GCF, oral bacteria, cells, and other sources that make identification of the exact site of disease activity difficult.[5] Although previous study demonstrated that cytokines play a significant role during the early stage of tooth movement but not during the linear stage,[26] as most of the participants in the present investigation were at linear stage (placed fixed appliance more than 2 months) which indicated that orthodontic treatment might not affect the patient's salivary cytokines level in the present investigation. Finally, as the present study was no control group, further study of the correlations between cytokines levels and oral microflora in general population with proper control group is warranted.
5. Conclusions
With the limitation of the present study, the level of salivary IL-1β positively correlates with oral bacterial load among orthodontic patients, the relationship between inflammatory cytokines and oral microflora deserved further study.
Author contributions
Conceptualization: Wing Kit Wong.
Investigation: Yong Chen.
Methodology: Yong Chen, Wing Kit Wong.
Project administration: Yong Chen, Wing Kit Wong.
Resources: Yong Chen, Jayampath C Seneviratne.
Software: Shuying Huang.
Supervision: Wing Kit Wong, Jayampath C Seneviratne, Colman McGrath, Urban Hagg.
Validation: Jayampath C Seneviratne.
Writing – original draft: Yong Chen.
Writing – review & editing: Wing Kit Wong, Jayampath C Seneviratne, Shuying Huang, Colman McGrath, Urban Hagg.
Footnotes
Abbreviations: GBI = Gingival Bleeding Index, GCF = gingival crevicular fluid, IL-1β = Interleukin-1Beta, IL-6: Interleukin-6, IL-8: Interleukin-8, MGI = Modified Gingival Index, MIF = macrophage migration inhibitory factor, PI = Plaque Index, TNFα = tumor necrosis factor alpha.
How to cite this article: Chen Y, Wong WK, Seneviratne JC, Huang S, McGrath C, Hagg U. Associations between salivary cytokines and periodontal and microbiological parameters in orthodontic patients. Medicine. 2021;100:10(e24924).
This manuscript was previously posted to Research square: DOI: 10.21203/rs.2.17349/v1.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Diamanti-Kipioti A, Gusberti FA, Lang NP. Clinical and microbiological effects of fixed orthodontic appliances. J Clin Periodontol 1987;14:326–33. [DOI] [PubMed] [Google Scholar]
- [2].Pandis N, Papaioannou W, Kontou E, et al. Salivary Streptococcus mutans levels in patients with conventional and self-ligating brackets. Eur J Orthod 2010;32:94–9. [DOI] [PubMed] [Google Scholar]
- [3].Hasegawa K, Furuichi Y, Shimotsu A, et al. Associations between systemic status, periodontal status, serum cytokine levels, and delivery outcomes in pregnant women with a diagnosis of threatened premature labor. J Periodontol 2003;74:1764–70. [DOI] [PubMed] [Google Scholar]
- [4].Jaedicke KM, Preshaw PM, Taylor JJ. Salivary cytokines as biomarkers of periodontal diseases. Periodontol 2000 2016;70:164–83. [DOI] [PubMed] [Google Scholar]
- [5].Miller CS, King CP, Jr, Langub MC, et al. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc 2006;137:322–9. [DOI] [PubMed] [Google Scholar]
- [6].Javed F, Al-Kheraif AA, Al Amri MD, et al. Periodontal parameters and whole salivary cytokine profiles among habitual gutka chewers and non-chewers. J Periodontol 2015;86:689–95. [DOI] [PubMed] [Google Scholar]
- [7].Gamonal J, Acevedo A, Bascones A, et al. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 2000;71:1535–45. [DOI] [PubMed] [Google Scholar]
- [8].Nonnenmacher C, Helms K, Bacher M, et al. Effect of age on gingival crevicular fluid concentrations of MIF and PGE2. J Dent Res 2009;88:639–43. [DOI] [PubMed] [Google Scholar]
- [9].Garlet T, Coelho U, Silva J, et al. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci 2007;115:355–62. [DOI] [PubMed] [Google Scholar]
- [10].Ren Y, Vissink A. Cytokines in crevicular fluid and orthodontic tooth movement. Eur J Oral Sci 2008;116:89–97. [DOI] [PubMed] [Google Scholar]
- [11].Kapoor P, Kharbanda OP, Monga N, et al. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog Orthod 2014;15:65.doi: 10.1186/s40510-014-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bletsa A, Berggreen E, Brudvik P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur J Oral Sci 2006;114:423–9. [DOI] [PubMed] [Google Scholar]
- [13].Tuncer BB, Özmeriç N, Tuncer C, et al. Levels of interleukin-8 during tooth movement. Angle Orthod 2005;75:631–6. [DOI] [PubMed] [Google Scholar]
- [14].Yoshii S, Tsuboi S, Morita I, et al. Temporal association of elevated C-reactive protein and periodontal disease in men. J Periodontol 2009;80:734–9. [DOI] [PubMed] [Google Scholar]
- [15].Tufekci E, Casagrande ZA, Lindauer SJ, et al. Effectiveness of an essential oil mouthrinse in improving oral health in orthodontic patients. Angle Orthod 2008;78:294–8. [DOI] [PubMed] [Google Scholar]
- [16].Mumghamba EGS, Pitiphat W, Matee MIN, et al. The usefulness of using Ramfjord teeth in predicting periodontal status of a Tanzanian adult population. J Clin Periodontol 2004;31:16–8. [DOI] [PubMed] [Google Scholar]
- [17].Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res 1992;71:1363–9. [DOI] [PubMed] [Google Scholar]
- [18].Vallor AC, Antonio MAD, Hawes SE, et al. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis 2001;184:1431–6. [DOI] [PubMed] [Google Scholar]
- [19].Almstahl A, Wikstrom M. Oral microflora in subjects with reduced salivary secretion. J Dent Res 1999;78:1410–6. [DOI] [PubMed] [Google Scholar]
- [20].Behnen MJ, West LA, Liewehr FR, et al. Antimicrobial activity of several calcium hydroxide preparations in root canal dentin. J Endod 2001;27:765–7. [DOI] [PubMed] [Google Scholar]
- [21].Barksby HE, Lea SR, Preshaw PM, et al. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol 2007;149:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, et al. Gingival crevicular fluid levels of interleukin-1β and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol 2004;75:1203–8. [DOI] [PubMed] [Google Scholar]
- [23].Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF) – the potential missing link. QJM: An Int J Med 2010;103:831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].D’Ercole S, Catamo G, Piccolomini R. Diagnosis in periodontology: a further aid through microbiological tests. Crit Rev Microbiol 2008;34:33–41. [DOI] [PubMed] [Google Scholar]
- [25].Teles R, Sakellari D, Teles F, et al. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol 2010;81:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ren Y, Hazemeijer H, de Haan B, et al. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. J Periodontol 2007;78:453–8. [DOI] [PubMed] [Google Scholar]


