Abstract
Ileocolonoscopy is currently recognized as the gold standard for evaluating mucosal healing in patients with Crohn disease (CD). However, the ideal noninvasive marker to assess mucosal healing instead of invasive ileocolonoscopy is not available. This study aimed to determine the correlations between the mucosal healing and serological optimizing markers in CD.
This retrospective study consecutively included 62 CD patients with 137 hospitalizations between March 2014 and March 2020. On the basis of the Simple Endoscopic Score for Crohn's disease (SES-CD), the CD patients were divided into mucosal healing group (SES-CD ≤ 2) and nonmucosal healing group (SES-CD > 2). We collected the results of ileocolonoscopy examination and inflammatory markers and then serological optimizing markers, including C-reactive protein/albumin ratio (CRP/ALB), platelet/albumin ratio (PLT/ALB), neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) were calculated. The control group consisted of 50 healthy volunteers in the corresponding period.
We found that CRP/ALB, PLT/ALB, NLR, and PLR were correlated with the mucosal healing of CD, and the correlation of CRP/ALB with the mucosal healing was the highest (r = -0.64). Receiver operating characteristic (ROC) analysis showed that the area under the curve (AUC) of CRP/ALB (0.87) was higher than NLR (0.69), PLR (0.72), and PLT/ALB (0.81). In the efficacy of assessing the mucosal healing in CD, the sensitivity of CRP/ALB, NLR, PLR, and PLT/ALB were 91.1%, 83.9%, 73.2%, and 73.2%, respectively, and the specificity was 76.5%, 46.9%, 64.2%, and 75.3%, respectively.
CRP/ALB was the most appropriate marker to assess CD mucosal healing among the serological optimizing markers.
Keywords: C-reactive protein/albumin ratio, Crohn disease, mucosal healing
1. Introduction
Crohn disease (CD) is a chronic intestinal inflammatory disease characterized by transmural inflammation and involvement of the entire gastrointestinal tract. CD tends to follow a long and relapsing course with various symptoms such as abdominal pain and diarrhea.[1,2] A meta-analysis showed that achieving mucosal healing in CD was associated with improved long-term outcomes, including clinical remission, mucosal healing, and a trend toward avoiding CD-related surgery.[3] Therefore, the therapeutic target of CD has been changed from clinical remission to mucosal healing.[4,5]
The mucosal healing of CD is considered to be the absence of mucosal ulceration under the endoscopy.[6–8] In 2015, International Organization for the Study of Inflammatory Bowel Disease officially defined the Simple Endoscopic Score for Crohn 's disease (SES-CD) score 0 to 2 as mucosal healing.[9] In order to monitor the mucosal healing in CD, ileocolonoscopy need to be performed repeatedly. However, ileocolonoscopy has the disadvantages of being invasive, time-consuming, expensive, and sometimes uncomfortable for patients. Patients are usually reluctant to accept ileocolonoscopy examinations, which is not conducive to the long-term monitoring of the disease. Therefore, we hope to find an ideal noninvasive marker to replace ileocolonoscopy, and the marker should be highly sensitive, specific, and easily accepted.[10]
Numerous studies suggest that fecal calprotectin is a reliable noninvasive marker for evaluating mucosal healing in CD.[11–15] However, fecal calprotectin has not been routinely used in clinical practice in some countries and regions, mainly because of the complicated collection and processing of fecal samples and the poor compliance of some patients.[16–18] Compared with stools collection and endoscopy, some studies have shown that C-reactive protein (CRP), as a serum marker, is more suitable for long-term monitoring of CD.[6,19] However, the correlations between CRP and CD activity under endoscopy in some reports are inconsistent,[20,21] and CRP has been shown to correlate worse with mucosal inflammation compared to fecal calprotectin.[11] Thus, further research regarding serum markers correlating with mucosal healing is needed.
In recent years, some studies have found that serological optimizing markers including CRP/albumin ratio (CRP/ALB), platelet/albumin ratio (PLT/ALB), neutrophil-lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR) could be used to assess the CD clinical activity.[22–24] Nevertheless, the correlations between serological optimizing markers and mucosal healing have not been covered. Therefore, we aimed to explore the associations between serological optimizing markers and mucosal healing in CD patients, using retrospectively collected data.
2. Experimental procedures
2.1. Patients
Between March 2014 and March 2020, CD patients hospitalized in Huashan Hospital North of Fudan University were included. They were diagnosed on the basis of standard clinical, endoscopic, and histological criteria. The phenotype of CD followed the Montreal classification. Patients were randomly selected using the following exclusion criteria: concomitant infection (pulmonary infection, urinary system infection, gastrointestinal tract infection, central system infection, and other infectious diseases), low nutritional status, malignant tumors, other autoimmune diseases, regular intake of aspirin, and/or other nonsteroidal anti-inflammatory drugs, incomplete medical history. A total of 137 ileocolonoscopy procedures were performed in 62 CD patients were collected. In addition, 50 healthy subjects in the same period were enrolled as a control group. This study was approved by the ethics committee of Huashan Hospital Affiliated to Fudan University.
2.2. Data collection and collation
Data relating to age, gender, age of onset, diagnosis age, disease course, smoking history, CD-related surgery history, clinical symptoms, Montreal classification, ileocolonoscopy and radiographic examination results, and therapeutic drugs were collected from the electronic medical records. Blood samples were obtained on the day before ileocolonoscopy. Inflammatory markers such as CRP, albumin (ALB), platelet (PLT), lymphocyte (L), and neutrophil (N) were measured at the hospital clinical laboratory, and we calculated the serological optimizing markers including CRP/ALB, PLT/ALB, NLR, PLR.
2.3. The definition of mucosal healing in CD
The SES-CD has been developed to reflect intestinal mucosal inflammation and is currently the best tool for evaluating mucosal healing.[25] For calculating the SES-CD, the intestine was divided into five parts: ileum, right colon, transverse colon, left colon and rectum. The degree of involvement was determined by four parameters: ulcers, proportion of the surface covered by ulcers, proportion of the surface with any other lesions and stenosis, and each parameter was scored from 0 to 3.[11,25] Ulcers were scored in accordance with size (diameter 0.1–0.5, 0.5–2, or >2 cm); proportion of ulcerated surface in accordance with extent (<10%, 10–30%, or >30%); proportion of affected surface in accordance with extent (<50%, 50–75%, or >75%); and stenosis as single or multiple, and whether the colonoscope could be passed through the narrowed lumen,[25] as shown in Figure 1.[26] Mucosal healing is most commonly defined as the absence of mucosal ulceration in the area within reach of the ileocolonoscopy, meanwhile the guidelines also recommend SES-CD 0–2 as mucosal healing.[27] According to the pictures of ileocolonoscopy, the scores were made by 2 experienced endoscopists (more than 10 years’ working experience) without the patients’ medical histories.
Figure 1.
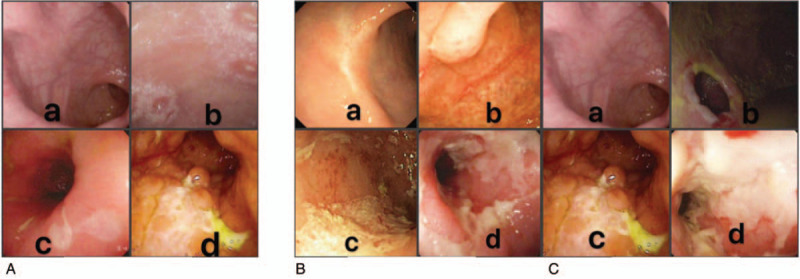
The specific scores of ulcers, proportion of the surface covered by ulcers and stenosis are shown from (A) to (C). (A) Ulcers a. 0: none; b. 1: aphtous ulcers (< 0.5 cm); c. 2: large ulcers (from 0.5 to 2 cm); d. 3: very large ulcers (> 2 cm). (B) Proportion of the surface covered by ulcers a. 0: surface involved by ulcerations 0% (none); b. 1: surface involved by ulcerations < 10%; c. 2: surface involved by ulcerations 10--30%; d. 3: surface involved by ulcerations > 30%. (C) Stenosis a. 0: none; b. 1: single, can be passed; c. 2: multiple, can be passed; d. 3: cannot be passed.
2.4. Statistical analysis
All data were analyzed by statistical software (SPSS for Mac, version 22.0, SPSS Inc., Chicago, IL). Data were expressed either as the mean plus or minus standard deviation or as the median and interquartile range for continuous variables and as percentages for categorical variables. The Mann--Whitney U-test was used to explore associations of non-parametric numerical data in 2 independent groups and the t test was used for parametric numerical data. And the χ2 tests were used to explore associations of categorical data in two independent groups. Correlations between the mucosal healing and serum markers were measured by the Spearman rank coefficient. Receiver operating characteristic (ROC) curves were plotted to compare the ability of serological optimizing markers to predict mucosal healing and for exploring their optimal cutoff values while balancing sensitivity and specificity. According to the optimal cutoff value, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy value were calculated. All P-values were 2-sided, and P < .05 was considered statistically significant.
3. Results
3.1. Clinical and laboratory characteristics of CD patients and healthy controls
According to the inclusion and exclusion criteria, a total of 137 ileocolonoscopy procedures were performed in 62 CD patients (20 women and 42 men), from whom blood samples were available for analysis. And 50 healthy control group (22 women and 28 men) were enrolled. Baseline characteristics of subjects are summarized in Table 1. Among the 62 patients, most of them had multiple ileocolonoscopies, and 24 (38.71%) underwent ileocolonoscopy once, 14 (22.58%) twice, 16 (25.81%) 3 times, 4 (6.45%) 4 times, 3 (4.84%) 5 times, and 1 (1.61%) 6 times.
Table 1.
Clinical baseline characteristics of CD patients and healthy controls.
| CD patients | Healthy controls | |
| Number of patients | 62 | 50 |
| Total number of endoscopy procedures | 137 | – |
| Sex (Female/male) | 46 (33.58%)/91 (66.42%) | 22 (44.00%)/28 (56.00%) |
| Age, yr median (IQR) | 30.50 (22.00–36.00) | 28.00 (26.00–29.00) |
| Disease duration, mo, median (IQR) | 26.00 (7.00–90.00) | – |
| Smoking status (No/Yes) | 129 (94.16%)/8 (5.84%) | 6 (12.00%)/44 (88.00%) |
| Symptoms | – | |
| Diarrhea (No/Yes) | 96 (70.07%)/41 (29.93%) | |
| Abdominal pain (No/Yes) | 81 (59.12%)/56 (40.88%) | |
| Parenteral performance (No/Yes) | 83 (60.58%)/54 (39.42%) | – |
| CD-related surgical history (No/Yes) | 90 (65.69%)/47 (34.31%) | |
| Gastroscopy (No/Yes) | 123 (89.78%)/14 (10.22%) | |
| Clinical disease activity | – | |
| Remission (CDAI ≤ 150) | 72 (52.55%) | |
| Active (CDAI > 150) | 65 (47.45%) | |
| Age at diagnosis, yr | – | |
| A1 (≤16) | 17 (12.41%) | |
| A2 (17–40) | 104 (75.91%) | |
| A3 (≥40) | 16 (11.68%) | |
| Disease location | – | |
| L1 | 30 (21.90%) | |
| L2 | 38 (27.74%) | |
| L3 | 69 (50.36%) | |
| Disease phenotype | – | |
| B1 | 65 (47.45%) | |
| B2 | 57 (41.61%) | |
| B3 | 15 (10.94%) | |
| p | 54 (39.42%) | |
| Medication∗ | – | |
| No medication | 11 (8.03%) | |
| Herbal medicine | 1 (0.73%) | |
| 5-ASA | 54 (39.42%) | |
| Corticosteroids | 13 (9.49%) | |
| Immunosuppressant | 77 (56.20%) | |
| TNF-α inhibitor | 57 (41.61%) |
IQR = interquartile range, L1 = ileal, L2 = colonic, L3 = ileocolonic, B1 = nonstricturing, nonpenetrating, B2 = structuring, B3 = penetrating, p = perianal disease, ASA = aminosalicylic acid, TNF = tumor necrosis factor.
In comparing the CD group with the control group, no significant differences were found in age, gender, and smoking history (P > .05). However, there were significant differences in the levels of serological indicators and serological optimizing markers. The levels of N, L, CRP, NLR, PLR, PLT/ALB and CRP/ALB in CD group were significantly higher than those in healthy control group, while ALB level was lower, as summarized in Table 2, and these differences were statistically significant (P < .05).
Table 2.
Biochemical characteristics in CD group and control group.
| CD group (n = 137) | Control group (n = 50) | t/x2 | P | |
| N, ∗10^9/L | 4.24 (2.94–5.56) | 3.10 (2.53–3.76) | 3.65 | <.001 |
| L, ∗10^9/L | 1.28 (0.87–1.73) | 2.05 (1.65–2.22) | 6.66 | < .001 |
| PLT, ∗10^9/L | 243.00 (198.00–328.50) | 232.00 (202.75–256.75) | 1.70 | .089 |
| CRP, mg/L | 7.81 (2.38–38.71) | 1.53 (1.30–2.27) | 7.57 | < .001 |
| ALB, g/L | 40.31 ± 6.31 | 47.40 ± 2.58 | 10.90 | < .001 |
| NLR | 3.40 (2.09–5.29) | 1.60 (1.37–1.88) | 6.92 | < .001 |
| PLR | 205.66 (138.28–309.52) | 116.29 (95.31–141.54) | 6.73 | < .001 |
| PLT/ALB | 6.17 (4.47–8.88) | 4.78 (4.21–5.55) | 3.90 | < .001 |
| CRP/ALB | 0.18 (0.54–1.06) | 0.03 (0.03–0.05) | 7.78 | < .001 |
3.2. Differences between mucosal healing group and nonmucosal healing group
According to the evaluation of digestive endoscopy experts, of the 137 ileocolonoscopy procedures, 56 cases were in the mucosal healing group and 81 cases were in the nonmucosal healing group. Table 3 presents the clinical and laboratory characteristics of CD patients in the mucosal healing group and the nonmucosal healing group. The results indicated that there were no significant differences in age, gender, body mass index (BMI), smoking history, and L level between the 2 groups (P > .05). Compared with the nonmucosal healing group, the course of disease in the mucosal healing group was longer, the hospital stay was shorter, the Crohn's disease activity index (CDAI) score was lower, the levels of N, PLT, CRP, NLR, PLR, PLT/ALB, CRP/ALB ratio were lower, and ALB level was higher (P < .05).
Table 3.
The difference between mucosal healing and mucosal activity.
| Mucosal healing (n = 56) | Nonmucosal healing (n = 81) | t/x2 | P | |
| Age, yr | 30.50 (23.00–36.50) | 31.00 (23.00–35.00) | 0.46 | .64 |
| Sex (female/male) | 16 (28.57%)/40 (71.43%) | 30 (37.04%)/51 (62.96%) | 1.03 | .30 |
| Disease duration (months) | 41.00 (20.50–116.50) | 24.00 (10.00–95.00) | 2.29 | .02 |
| BMI, kg/m2 | 19.37 (17.50–21.92) | 18.67 (17.30–20.23) | 1.35 | .18 |
| Hospital stay | 2.00 (2.00–7.00) | 7.00 (3.00–12.00) | 3.96 | < .001 |
| Smoking status (no/yes) | 5 (8.93%)/51 (91.07%) | 3 (3.70%)/78 (96.30%) | 1.28 | .20 |
| CDAI | 65.33 (42.82–109.84) | 220.34 (145.85–350.13) | 7.15 | < .001 |
| N, ∗10^9/L | 3.48 (2.56–4.73) | 4.84 (3.20–6.08) | 3.78 | < .001 |
| L, ∗10^9/L | 1.41 (0.94–1.76) | 1.24 (0.80–1.72) | 1.75 | .08 |
| CRP, mg/L | 2.38 (1.80–4.09) | 22.10 (8.13–74.60) | 7.22 | < .001 |
| ALB, g/L | 44.59 ± 4.42 | 37.35 ± 5.71 | 7.98 | < .001 |
| PLT, ∗10^9/L | 212.50 (166.25–281.50) | 262.00 (220.00–383.00) | 4.48 | < .001 |
| NLR | 2.83 (1.49–3.83) | 3.89 (2.74–6.43) | 3.62 | < .001 |
| PLR | 167.68 (111.02–210.65) | 252.08 (163.23–363.42) | 4.47 | < .001 |
| PLT/ALB | 4.67 (3.77–6.12) | 7.42 (5.57–10.46) | 6.01 | < .001 |
| CRP/ALB | 0.05 (0.04–0.10) | 0.62 (0.21–2.09) | 7.44 | < .001 |
3.3. Relationship between markers and CD mucosal healing
The correlations between biomarkers and mucosal healing in CD patients are summarized in Table 4. CDAI (Spearman's rank correlation coefficient r = -0.61), CRP level (r = -0.62), and CRP/ALB (r = -0.64) demonstrated stronger correlations with mucosal healing as compared to ALB level (r = 0.58), PLT level (r = -0.38), N level (r = -0.32), PLR (r = -0.38), NLR (r = -0.31), and PLT/ALB (r = -0.52) (all P < .05). No significant correlations were detected between mucosal healing with age, gender, BMI, smoking history, and L level (all P > .05).
Table 4.
Spearman correlations between biomarkers and mucosal healing.
| Correlation coefficient | P | |
| Sex | -0.09 | .31 |
| Age | -0.04 | .65 |
| Disease duration | 0.20 | .02 |
| BMI, kg/m2 | 0.12 | .18 |
| Smoking status | -0.11 | .20 |
| CDAI | -0.61 | < .001 |
| N, ∗10^9/L | -0.32 | < .001 |
| L, ∗10^9/L | -0.15 | .08 |
| PLT, ∗10^9/L | -0.38 | < .001 |
| CRP, mg/L | -0.62 | < .001 |
| ALB, g/L | 0.58 | < .001 |
| NLR | -0.31 | < .001 |
| PLR | -0.38 | < .001 |
| PLT/ALB | -0.52 | < .001 |
| CRP/ALB | -0.64 | < .001 |
To compare the predictive values of serum markers and serological optimizing markers for mucosal healing in CD, we analyzed ROC curves. The area under the curve (AUC) values with a 95% confidence interval (95% CI) for each biomarker for assessing mucosal healing in the entire patient cohort are presented in Table 5. The AUC of CRP/ALB for predicting mucosal healing was 0.87 (95% CI, 0.81–0.93), which was higher as compared to those of NLR, PLR, PLT/ALB (0.68, 0.72, and 0.81, respectively). Above results are further illustrated in Figure 2. On the basis of ROC analysis, the optimal cut-off value for each marker to show mucosal healing along with its sensitivity, specificity, negative predictive value, positive predictive value, and accuracy was determined, as summarized in Table 6. The optimal cut-off value of CRP/ALB in predicting mucosal healing was 0.195, and the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 91.1%, 76.5%, 72.9%, 92.5%, and 82.5% respectively. These values for CRP/ALB to predict mucosal healing were higher as compared to those of serological optimizing markers.
Table 5.
Discriminatory power of each biomarker for mucosal healing (SES-CD 0–2) by receiver operating characteristic (ROC) curves.
| Variable | AUC | 95% CI | P |
| CRP/ALB | 0.87 | 0.81–0.93 | < .001 |
| NLR | 0.68 | 0.59–0.77 | < .001 |
| PLR | 0.72 | 0.64–0.81 | < .001 |
| PLT/ALB | 0.81 | 0.74–0.88 | < .001 |
| CRP, mg/L | 0.86 | 0.80–0.93 | < .001 |
| ALB, g/L | 0.84 | 0.78–0.91 | < .001 |
| N, ∗10^9/L | 0.69 | 0.60–0.78 | < .001 |
| L, ∗10^9/L | 0.58 | 0.49–0.68 | .096 |
| PLT, ∗10^9/L | 0.73 | 0.65–0.82 | < .001 |
Figure 2.
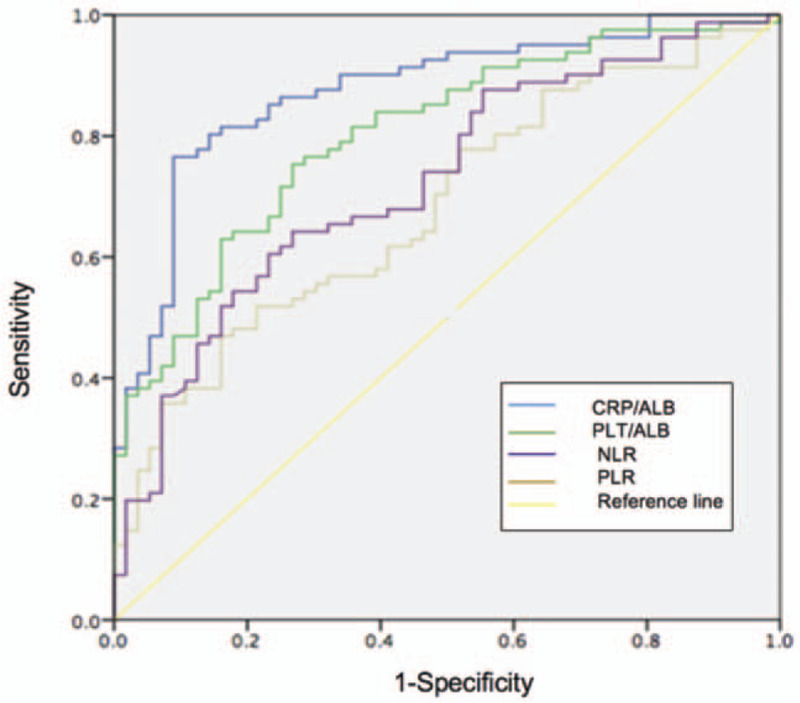
Receiver operator curves (ROCs) for serological optimizing markers to assess mucosal healing. CRP/ALB [area under the curve (AUC) = 0.87] achieved a better test performance than PLT/ALB (AUC = 0.81), NLR (AUC = 0.68), PLR (AUC = 0.72).
Table 6.
Sensitivity (SENS), specificity (SPEC), positive predictive value (PPV), and negative predictive value (NPV), as well as overall accuracy for detecting CD mucosal healing.
| Cut-off | SENS | SPEC | PPV | NPV | Accuracy | |
| CRP, mg/L | 7.82 | 0.893 | 0.765 | 0.725 | 0.912 | 0.818 |
| ALB, g/L | 39.65 | 0.893 | 0.679 | 0.658 | 0.902 | 0.766 |
| CRP/ALB | 0.195 | 0.911 | 0.765 | 0.729 | 0.925 | 0.825 |
| NLR | 4.4494 | 0.839 | 0.469 | 0.522 | 0.809 | 0.620 |
| PLR | 206.2684 | 0.732 | 0.642 | 0.586 | 0.776 | 0.679 |
| PLTALB | 5.6019 | 0.732 | 0.753 | 0.672 | 0.803 | 0.745 |
4. Discussion
CD is a chronic and recurrent inflammatory disease of the digestive system with yet unknown pathogenesis. Over the last couple of years, studies demonstrated that mucosal healing in CD was associated with lower cumulative surgery rate and better prognosis.[28,29] To monitor mucosal healing, repetitive endoscopic examinations need to be performed. Due to the limitations of repetitive endoscopic examinations, which are costly, invasive, and sometimes unpleasant for patients, considerable researches have been carried out to evaluate different markers in blood or feces regarding their correlations with mucosal healing in CD. Fecal calprotectin has been shown to correlate better with mucosal healing in CD compared with clinical activity or CRP.[11] However, some patients may be reluctant to handle fecal material.[18,30] In a recent review, Chen et al[31] summarized the applications of serum biomarkers such as CRP, serum microRNAs, and novel serum indicators such as serum free thiols, serum cathelicidin, and serum fibrinogen in monitoring the disease activity in CD. In addition, blood-based biomarkers are noninvasive, readily available, not easily contaminated, and are the most widely used. Thus, this study aimed to find another reliable marker by evaluating the correlations between mucosal healing and CRP/ALB, PLT/ALB, NLR, and PLR of CD patients.
In the current study, we found that CRP/ALB, PLT/ALB, NLR, PLR decreased in subjects with mucosal healing versus nonmucosal healing CD. CRP/ALB had a higher correlation with mucosal healing (r = -0.64, P < .01) than PLR (r = -0.384, P < .01), NLR (r = -0.310, P < .01), PLT/ALB (r = -0.515, P < .01). ROC analysis indicated that the AUC of CRP/ALB (0.87) was higher than the AUCs of NLR (0.69), of PLR (0.72), and of PLT/ALB (0.81). The AUC of CRP/ALB was the largest, which indicated that the ability of diagnosing mucosal healing was the optimum. CRP/ALB with a cut-off of ≤ 0.195 had the best overall accuracy (82.5%), sensitivity (91.1%), and specificity (76.5%) for the detection of mucosal healing in CD. In summary, this study showed that decreased CRP/ALB was more indicative of mucosal healing in CD than other serological optimizing markers.
The CRP/ALB was originally used to identify serious patients in the emergency ward.[32] Recently, the CRP/ALB had been confirmed to show out-standing prognostic value in cancers.[33] In clinical practice, we also observed that CD with a high level of CRP and low level of ALB is usually active. CRP is an important acute-phase marker, produced mainly in the liver, and can evaluate the activity and severity of CD.[30,34] In a prospective study, Weinstein-Nakar et al[35] found that the AUC of CRP in mucosal healing in CD was 0.81 (95% CI, 0.71–0.9), similar to the results of this study. A low ALB level is usually linked with chronic disease, frequently correlated with nutritional status. In addition, ALB can also be used to assess the severity of CD.[36] In our study, the specificity of ALB in predicting the mucosal healing of CD was 67.9%, similar to the research results of Kawashima et al.[14]
The CRP/ALB, integrating the effects of both inflammation and malnutrition, may be more capable of reflecting the real situation of mucosal inflammation. As a consequence of inflammation, macrophage and T-cell activation by interleukin (IL)-1 and tumor necrosis factor-α increases secretion of IL-6 with subsequent downstream stimulation of CRP synthesis.[37,38] Therefore, the CRP/ALB may be more accurate to reflect mucosal healing in CD than CRP or ALB alone, which is confirmed by our research results.
This study had some potential limitations. Firstly, the sample size included was small, which may not represent the general situation comprehensively and accurately. Secondly, ileocolonoscopy could not completely detect mucosal inflammation in the ileum 10 cm or more proximal to ileocecal valve. Considering the imbalance between diagnostic expenses on one hand and expected yield on the other hand, we did not carry out a systematic search for CD involvement of the upper gastrointestinal tract and small bowel without symptoms. Moreover, this study was a single-center retrospective study, and individual patients had probably received treatment before admission, which might interfere with the research results. Therefore, a multicenter prospective study is needed to assess correlations between serological optimizing markers and mucosal healing in CD in the future. Despite these limitations, of the serological optimizing markers, CRP/ALB is most appropriate and promising in evaluating mucosal healing in CD.
Despite the aforementioned limitations, our study confirms that CRP/ALB is the most appropriate biomarker to assesso predict CD mucosal healing among the serological optimizing markers. Therefore, CRP/ALB has the potential to replace ileocolonoscopy in the disease monitoring of CD.
Author contributions
Fu-Sheng Zhou and Nan Gao contributed equally to this work, in performing the data collection, analyzing the data, and writing the manuscript; Xu Sun, Xiao-Yun Jiang, Jia-Jie Chen, Qi-Qi Mao performed the data collection and data analysis; Liang Zhong contributed to design the research and revise manuscript. All authors reviewed the manuscript for important scientific content and approved the final version.
Conceptualization: Liang Zhong.
Data curation: Xu Sun, Qiqi Mao, Xiaoyun Jiang, Jiajie Chen.
Formal analysis: Xu Sun, Qiqi Mao, Xiaoyun Jiang, Jiajie Chen.
Project administration: Liang Zhong.
Writing – original draft: Fusheng Zhou, Nan Gao.
Writing – review & editing: Fusheng Zhou, Nan Gao, Liang Zhong.
Footnotes
Abbreviations: ALB = albumin, AUC = The area under the curve, BMI = body mass index, CD = Crohn's disease, CDAI = Crohn's disease activity index, CI = confidence interval, CRP = C-reactive protein, CRP/ALB = C-reactive protein/albumin ratio, IL= interleukin, L = lymphocyte, N = neutrophil, NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio, PLT = platelet, PLT/ALB = platelet/albumin ratio, ROC = Receiver Operating Characteristic, SES-CD = Simple Endoscopic Score for Crohn's disease.
How to cite this article: Zhou FS, Gao N, Sun X, Jiang XY, Chen JJ, Mao QQ, Zhong L. C-reactive protein/abumin ratio is a useful biomarker for predicting the mucosal healing in the Crohn disease: A retrospective study. Medicine. 2021;100:10(e24925).
FZ and NG contributed equally to this work.
The study was approved by the ethics committee of Huashan Hospital to Fudan University (number: 2020-786).
This project was supported by the Health and Family Planning Commission of Shanghai, China.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Schulze H, Esters P, Dignass A. Review article: the management of Crohn's disease and ulcerative colitis during pregnancy and lactation. Aliment Pharmacol Ther 2014;40:991–1008. [DOI] [PubMed] [Google Scholar]
- [2].Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014;146:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shah SC, Colombel JF, Sands BE, et al. Systematic review with meta analysis: mucosal healing is associated with improved long-term outcomes in Crohn's disease. Aliment Pharmacol Ther 2016;43:317–33. [DOI] [PubMed] [Google Scholar]
- [4].Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- [5].Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology 2010;138:463–8. quiz e410-461. [DOI] [PubMed] [Google Scholar]
- [6].Daperno M, Castiglione F, de Ridder L, et al. Results of the 2nd part Scientific Workshop of the ECCO. II: measures and markers of prediction to achieve, detect, and monitor intestinal healing in inflammatory bowel disease. J Crohns Colitis 2011;5:484–98. [DOI] [PubMed] [Google Scholar]
- [7].Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- [8].Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology 2012;142:1102–11. e1102. [DOI] [PubMed] [Google Scholar]
- [9].Vuitton L, Marteau P, Sandborn WJ, et al. IOIBD technical review on endoscopic indices for Crohn's disease clinical trials. Gut 2016;65:1447–55. [DOI] [PubMed] [Google Scholar]
- [10].Papp M, Lakatos PL. Serological studies in inflammatory bowel disease: how important are they? Curr Opin Gastroenterol 2014;30:359–64. [DOI] [PubMed] [Google Scholar]
- [11].Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010;105:162–9. [DOI] [PubMed] [Google Scholar]
- [12].Zubin G, Peter L. Predicting endoscopic Crohn's disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm Bowel Dis 2015;21:1386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goutorbe F, Goutte M, Minet-Quinard R, et al. Endoscopic factors influencing fecal calprotectin value in Crohn's Disease. J Crohns Colitis 2015;9:1113–9. [DOI] [PubMed] [Google Scholar]
- [14].Kawashima K, Ishihara S, Yuki T, et al. Fecal calprotectin more accurately predicts endoscopic remission of Crohn's Disease than serological biomarkers evaluated using balloon-assisted enteroscopy. Inflamm Bowel Dis 2017;23:2027–34. [DOI] [PubMed] [Google Scholar]
- [15].Pauwels RWM, de Vries AC, van der Woude CJ. Fecal calprotectin is a reliable marker of endoscopic response to vedolizumab therapy: a simple algorithm for clinical practice. J Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum 2008;51:1283–91. [DOI] [PubMed] [Google Scholar]
- [17].Marechal C, Aimone-Gastin I, Baumann C, et al. Compliance with the faecal calprotectin test in patients with inflammatory bowel disease. United European. Gastroenterol J 2017;5:702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schoepfer AM, Trummler M, Seeholzer P, et al. Accuracy of four fecal assays in the diagnosis of colitis. Dis Colon Rectum 2007;50:1697–706. [DOI] [PubMed] [Google Scholar]
- [19].Buisson A, Gonzalez F, Poullenot F, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1425–33. [DOI] [PubMed] [Google Scholar]
- [20].Sipponen T, Savilahti E, Kolho KL, et al. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis 2008;14:40–6. [DOI] [PubMed] [Google Scholar]
- [21].Meuwis MA, Vernier-Massouille G, Grimaud JC, et al. Serum calprotectin as a biomarker for Crohn's disease. J Crohns Colitis 2013;7:e678–83. [DOI] [PubMed] [Google Scholar]
- [22].Gao SQ, Huang LD, Dai RJ, et al. Neutrophil-lymphocyte ratio: a controversial marker in predicting Crohn's disease severity. Int J Clin Exp Pathol 2015;8:14779–85. [PMC free article] [PubMed] [Google Scholar]
- [23].Qin G, Tu J, Liu L, et al. Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn's disease activity. Med Sci Monit 2016;22:4393–400. [DOI] [PubMed] [Google Scholar]
- [24].Stidham RW, Guentner AS, Ruma JL, et al. Intestinal dilation and platelet: albumin ratio are predictors of surgery in stricturing small bowel Crohn's disease. Clin Gastroenterol Hepatol 2016;14:1112–9. e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- [26]. The Italian Group for the Study of Inflammatory Bowel Disease. SES-CD simple endoscopic score for Crohn's disease. Available at: https://www.igibdscores.it/it/info-sescd.html. [Google Scholar]
- [27].Koutroumpakis E, Katsanos KH. Implementation of the simple endoscopic activity. score in crohn's disease. Saudi J Gastroenterol 2016;22:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term. outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- [29].Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission. rates in short-duration Crohn's disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010;105:1574–82. [DOI] [PubMed] [Google Scholar]
- [30].Shiga H, Abe I, Onodera M, et al. Serum C-reactive protein and albumin are useful. biomarkers for tight control management of Crohn's disease in Japan. Sci Rep 2020;10:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen P, Zhou G, Lin J, et al. Serum biomarkers for inflammatory bowel disease. Front Med (Lausanne) 2020;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fairclough E, Cairns E, Hamilton J, et al. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive. protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 2009;9:30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Saito H, Kono Y, Murakami Y, et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin and neutrophil-lymphocyte ratio in gastric cancer patients. World J Surg 2018;42:1819–25. [DOI] [PubMed] [Google Scholar]
- [34].Magro F, Sousa P, Ministro P. C-reactive protein in Crohn's disease: how informative is it? Expert Rev Gastroenterol Hepatol 2014;8:393–408. [DOI] [PubMed] [Google Scholar]
- [35].Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn's disease. Clin Gastroenterol Hepatol 2018;16:1089–97. e1084. [DOI] [PubMed] [Google Scholar]
- [36].Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432–7. [DOI] [PubMed] [Google Scholar]
- [37].Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005;39: 4 Suppl 2: S143–146. [DOI] [PubMed] [Google Scholar]


