Abstract
The aim of this study was to evaluate the ability of the red blood cell distribution width (RDW) to predict prognosis and treatment response in chronic myeloid leukemia (CML)-chronic phase (CP) patients treated with tyrosine kinase inhibitor (TKIs).
We retrospectively enrolled 93 newly diagnosed CML-CP patients treated with TKIs from 2009 to 2018 at the First Hospital of Lanzhou University. Patients were divided into 2 groups using an RDW of 18.65% determined by receiver operating characteristic curve analysis. We analyzed the correlation of treatment responses and the RDW compared to common scoring systems, as well as the correlation of the RDW with disease outcome, including overall survival (OS) and progression-free survival (PFS), and demographic and laboratory factors affecting outcome. Univariate analysis and Cox regression analysis were used.
The median age of patients was 40 years, and 51 patients (54.8%) were men. A high RDW could predict treatment response at 3 months (P = .03) and 6 months (P = .02). The RDW was significantly lower in patients who achieved molecular response by 3 months (P < .001) and complete cytogenetic response by 6 months (P = .001) than in those who did not respond. Patients with a high RDW (>18.65%, n = 35) had significantly worse 5-year OS (77.1% vs 96.6%; P = .008) and PFS (80.0% vs 98.3%; P = .002) than those with a low RDW (≤18.65%, n = 58). Multivariate analysis demonstrated that a high RDW was an adverse predictor of OS (P = .005, HR (hazard ratio) = 9.741) and PFS (P = .009, HR = 16.735).
The RDW is a readily available prognostic marker of outcome in patients with CML-CP and can predict treatment response to TKIs. Further larger and prospective studies are required.
Keywords: chronic myeloid leukemia, prognosis, red blood cell volume distribution width, tyrosine kinase inhibitors
1. Introduction
Chronic myeloid leukemia (CML) accounts for 15% of adult leukemias, with an estimated 8450 new cases each year.[1] Several early treatments, such as hydroxyurea and interferon-α, had unsatisfactory results.[2] However, the introduction of tyrosine kinase inhibitors (TKIs) therapy has dramatically improved survival in these patients.[3] The estimated 5-year survival rate has more than doubled since the introduction of TKIs, from 31% in the early 1990 s to around 70% in 2015.[4] The National Comprehensive Cancer Network (NCCN) recommends imatinib, dasatinib, and nilotinib as first-line therapy for newly diagnosed patients with CML in the chronic phase (CML-CP).[5] Second-generation TKIs, namely dasatinib and nilotinib, are highly effective for newly diagnosed patients who fail imatinib,[6,7] and third-generation TKIs, including ponatinib and bosutinib, have been approved for use in patients resistant to first-line therapies.[8,9] However, although TKIs therapy appears promising, 1130 people still die from CML annually.[1]
CML is a myeloproliferative neoplasm characterized by the Philadelphia chromosome and BCR-ABL (breakpoint cluster region - abl oncogene) fusion gene, which encodes the oncoprotein BCR-ABL (p210 or p190) with constitutive active tyrosine kinase activity.[10,11] TKIs treatment targeting BCR-ABL significantly improves the prognosis of CML-CP patients, but side effects, poor adherence, and economic burden decrease its utility. Further, there is no uniform and simple method to evaluate the prognosis and treatment responses of CML patients. To date, the validity of scoring systems is insufficient for predicting prognosis,[12] and there are few studies of scoring systems for predicting treatment response and clinical efficacy of TKIs.
The red cell distribution width (RDW), a measure of heterogeneity in red blood cell size,[13] is a routine parameter that can be readily assessed from the complete blood count. In addition to aiding in the diagnosis of anemia, the RDW has recently been reported to be closely related to cardiovascular disease,[14] infection,[15] and metabolic syndrome.[16] The RDW is an independent prognostic factor in numerous solid cancers, including lung cancer,[17] colon cancer,[18] breast cancer,[19] and prostate cancer,[20] as well as in several types of hematologic malignancies, such as multiple myeloma,[21] natural killer/T-cell lymphoma,[22] diffuse large B-cell lymphoma[23] and CML.[24] Iriyama et al found that patients with a higher RDW had poorer OS and PFS, although their cutoff was within the normal range, and they did not perform multivariate analyses.[24] However, the RDW calculation differs widely among most commonly used hematological analyzers.[13] Therefore, we identified the optimal cut-off value of the RDW by receiver operating curve (ROC) analysis to examine whether the RDW can predict clinical efficacy and treatment response and whether a high RDW is an independent adverse prognostic factor for newly diagnosed CML-CP patients treated with TKIs. Finally, we summarized the causes of a high RDW.
2. Methods
2.1. Patients
We enrolled 93 patients newly diagnosed with CML-CP between 2009 and 2018, and their medical records and laboratory results were collected from the Hematology Department of the First Hospital of Lanzhou University. Our study obtained consent from all patients and the approval of the ethical committee. All patients were diagnosed according to the NCCN criteria.[5] Patients were treated with any TKIs as initial therapy and were followed up for at least 3 months. Exclusion criteria were the use of interferon-α or any chemotherapy prior to or in combination with TKIs treatment and age < 18 years at diagnosis (Fig. 1). Patients who received hydroxyurea prior to TKIs treatment were included in the study. The RDW at diagnosis was obtained prior to treatment (including hydroxyurea, TKIs, and blood transfusion).
Figure 1.
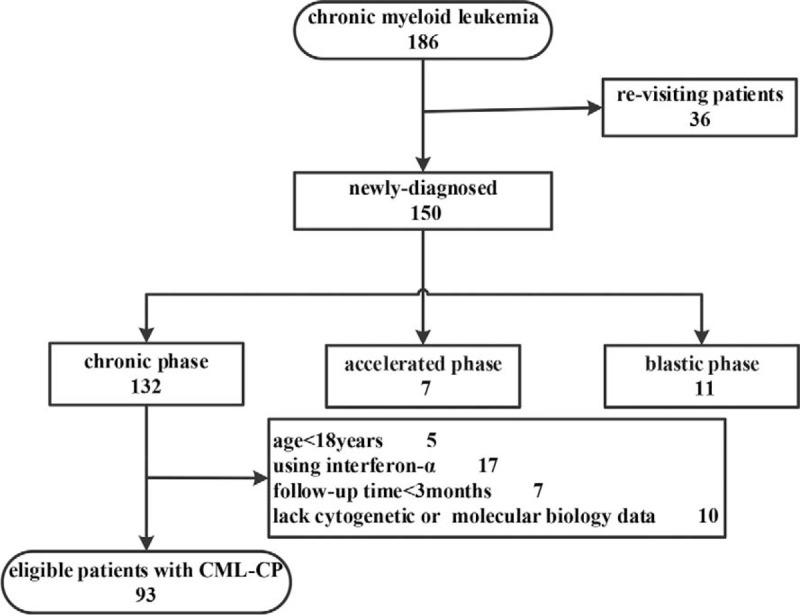
Flow chart of the screening process of this study.
2.2. Assessment of treatment responses
We defined the clinical efficacy of TKIs as first-line treatment in accordance with the NCCN 2019 recommendations.[5] BCR-ABLIS ≤10% was defined as early molecular response (EMR), Ph+ = 0 was defined as complete cytogenetic response (CCyR), and BCR-ABLIS ≤0.1% was defined as major molecular response (MMR). These treatment responses are in accordance with the European Leukemia Net (ELN) 2013 Guidelines.[25]
2.3. Statistical analysis
The statistical analyses were performed by SPSS 23 and Graphpad Prism 8. OS was defined as the period from the date of diagnosis to the date of any cause of death or the last follow-up. PFS was defined as the period from the date of diagnosis to progression to accelerated phase (AP) or blastic phase (BP) or the last follow-up. ROC curve analysis was used to determine the optimal cut-off value of the RDW for predicting OS and PFS. Continuous variables were analyzed using independent sample t-tests, and more than 2 independent samples were analyzed using one-way analysis of variance. Categorical variables were evaluated using the chi-square or Fisher exact test. For survival analysis, Kaplan–Meier curves with a log-rank test were used. Prognostic variables for OS and PFS were analyzed using the Cox proportional hazards model. P < .05 was accepted as statistically significant.
3. Results
3.1. Patients characteristics
The main baseline characteristics of the 93 patients studied are listed in Table 1. The median age was 40 years (range, 19–83 years). Fifty-one patients (54.8%) were men. ROC curve analysis indicated that the optimal cut-off value of the RDW was 18.65% for both OS and PFS (area under the curve: 0.761 and 0.784, respectively; 95% confidence interval: 0.646–0.877 and 0.661–0.907, respectively; sensitivity: 80.0% and 87.5%, respectively; and specificity: 67.5% and 67.1%, respectively; P = .007 and .008, respectively; Fig. 2). Therefore, the patients were divided into low- and high- RDW groups using a cutoff of 18.65%. There were 58 patients in the low RDW group (≤18.65%) and 35 patients in the high RDW group (>18.65%). The patients with a high RDW had higher WBC counts (P = .03), lower hemoglobin levels (P = .001), and a higher rate of splenomegaly (P = .004). However, there were no significant correlations between RDW and age, sex, basophil, eosinophil, blast, and marrow blast counts, platelet count, or lactate dehydrogenase. The initial treatment agent also did not differ between the 2 groups. With regard to scoring systems, risk stratification by the EUTOS score was associated with the RDW (P < .001), but there was no significant difference in the Sokal, Hasford, or ELTS scores between the 2 groups.
Table 1.
Patient baseline characteristics (overall and divided according to 18.65% RDW cutoff).
| Divided by RDW (%) | ||||
| Variable | Overall, n = 93 | Low RDW (≤18.65%) n = 58 | High RDW (>18.65%) n = 35 | P value |
| RDW (%), median (range) | 18 (14.6–27.7) | 17.5 (14.6–18.6) | 19.7 (17.3–27.7) | |
| Age (years), median (range) | 40 (19–83) | 45.5 (19–83) | 42 (19–73) | .281 |
| Sex, number (%) | .934 | |||
| Male | 51 (54.8) | 32 | 19 | |
| Female | 42 (45.2) | 26 | 16 | |
| WBC (×109/L), median (range) | 130.57 (5.13–566) | 116.26 (18.47–414.13) | 212.33 (5.13–566) | .025 |
| Eos (%), median (range) | 2 (0.1–6.7) | 2 (0.2–5.1) | 2 (0.1–6.7) | .708 |
| Bas (%), median (range) | 4.75 (0–20) | 5.3 (0–14) | 4.5 (0–20) | .802 |
| Hemoglobin (g/dl), median (range) | 10.0 (5.5–16.5) | 9.3 (5.5–13.1) | 8.8 (7.2–16.5) | .001 |
| MCV (fl), median (range) | 92.2 (78.3–103.2) | 92.8 (83.2–103.2) | 90.9 (78.3–102.3) | .099 |
| MCH (pg), median (range) | 29.8 (23.4–36.3) | 29.85 (24.8–36.3) | 29.8 (23.4–36.2) | .203 |
| MCHC (%), median (range) | 319 (287–367) | 321.5 (294–352) | 316 (287–367) | .911 |
| Platelet (×109/L), median (range) | 279 (4–1231) | 268 (68–1106) | 321 (4–1231) | .442 |
| PDW (%),median (range) | 15 (9.5–22.2) | 15 (11–22.2) | 14.75 (9.5–21.2) | .761 |
| LDH (U/L), median (range) | 854.6 (180.2–2072) | 876 (259.8–2072) | 818.5 (180.6–1830) | .521 |
| Blast (%), median (range) | 2 (0–8) | 0 (0–5) | 0 (0–8) | .938 |
| Marrow Blast (%), median (range) | 2 (0–9) | 2 (0–9) | 2 (0.5–8) | .686 |
| Spleen size (cm), median (range) | 6.9 (0–25) | 5.5 (0–25) | 10 (0–21) | .004 |
| Sokal score, number (%) | .229 | |||
| Low-risk | 45 (48.4) | 32 | 13 | |
| Intermediate-risk | 38 (40.9) | 21 | 17 | |
| High-risk | 10 (10.7) | 5 | 5 | |
| Hasford score, number (%) | .231 | |||
| Low-risk | 52 (55.9) | 34 | 18 | |
| Intermediate-risk | 38 (40.9) | 22 | 16 | |
| High-risk | 3 (3.2) | 2 | 1 | |
| EUTOS score, number (%) | .000 | |||
| Low-risk | 88 (94.6) | 56 | 32 | |
| High-risk | 5 (5.4) | 2 | 3 | |
| ELTS score, number (%) | .112 | |||
| Low-risk | 42 (45.2) | 31 | 11 | |
| Intermediate-risk | 36 (38.7) | 19 | 17 | |
| High-risk | 15 (16.1) | 8 | 7 | |
| Treatment, number (%) | .680 | |||
| Imatinib | 75 (80.6) | 47 | 28 | |
| Dasatinib | 8 (8.6) | 4 | 4 | |
| Nilotinib | 10 (10.8) | 7 | 3 | |
Bas = basophils, Eos = eosinophil, LDH = lactic dehydrogenase, MCH = Mean Corpuscular Hemoglobin, MCHC = Mean Corpuscular Hemoglobin Concentration, MCV = mean corpuscular volume, PDW = platelet distribution width, RDW = red blood cell volume distribution width, WBC = white blood cell.
Figure 2.
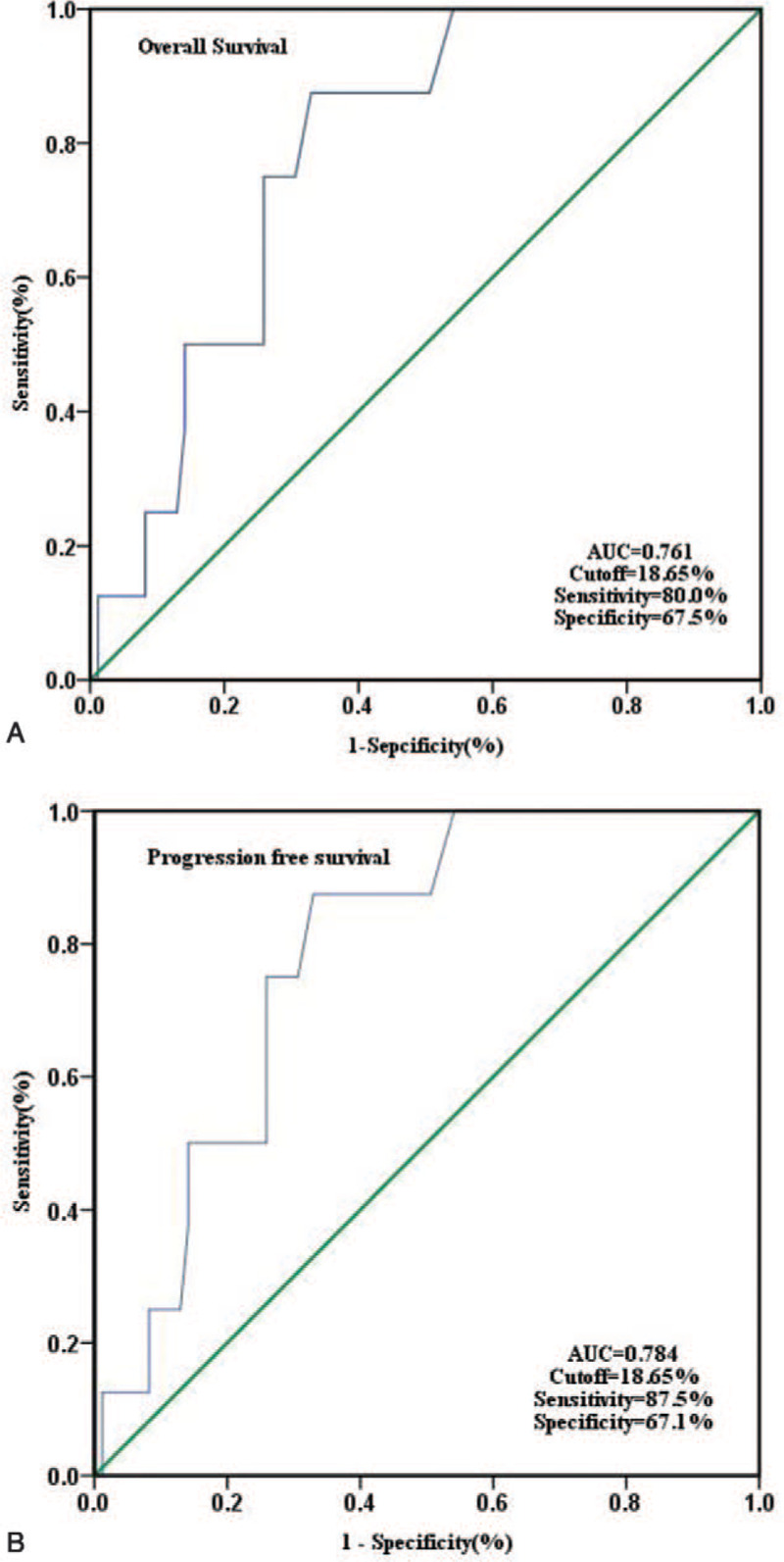
ROC analysis for determining the optimal cut-off value in predicting OS (A) and PFS (B) for RDW.
3.2. The relationship between RDW and treatment response
We examined whether the RDW could evaluate the treatment response according to ELN2013. As shown in Table 2, the high RDW group had significantly worse treatment responses at 3 months (P = .03) and 6 months (P = .02), whereas there was no significant difference in treatment responses between the 2 groups at 12 months (P = .23). As shown in Figure 3, patients with EMR at 3 months (3M-EMR) (17.65 ± 1.19% vs 20.68 ± 1.55%, P < .001, t-test, Fig. 3A) and CCyR at 6 months (6M-CCyR) (17.87 ± 1.68% vs 19.42 ± 2.10%, P < .001, t-test, Fig. 3B) had lower RDW than those who did not. The RDWs were not significantly different in patients with and without MMR at 12 months (12M-MMR) (18.31 ± 2.01% vs 18.70 ± 1.73%, P = .42, t-test, Fig. 3C). For comparison, we analyzed scoring systems (Sokal, Hasford, EUTOS, and ELTS scores) to determine their ability to evaluate the clinical efficacy of TKIs. As shown in Table 3, the Sokal score (P = .04) could predict 3M-EMR, and the ELTS score could predict 3M-EMR (P = .03) and 12M-MMR (P = .03). None of the scoring systems could predict 6M-CCyR.
Table 2.
Associations of RDW and treatment responses according to European Leukemia Net 2013 recommendations.
| Monitor time | Treatment Response | No.of patients | High-RDW | Low-RDW | P Value |
| 3months | optimal | 49 | 17 | 32 | .034 |
| warning | 12 | 8 | 4 | ||
| failure | 5 | 4 | 1 | ||
| 6months | optimal | 55 | 15 | 40 | .019 |
| warning | 12 | 7 | 5 | ||
| failure | 9 | 6 | 3 | ||
| 12months | optimal | 42 | 12 | 30 | .231 |
| warning | 9 | 5 | 4 | ||
| failure | 19 | 8 | 11 |
Figure 3.
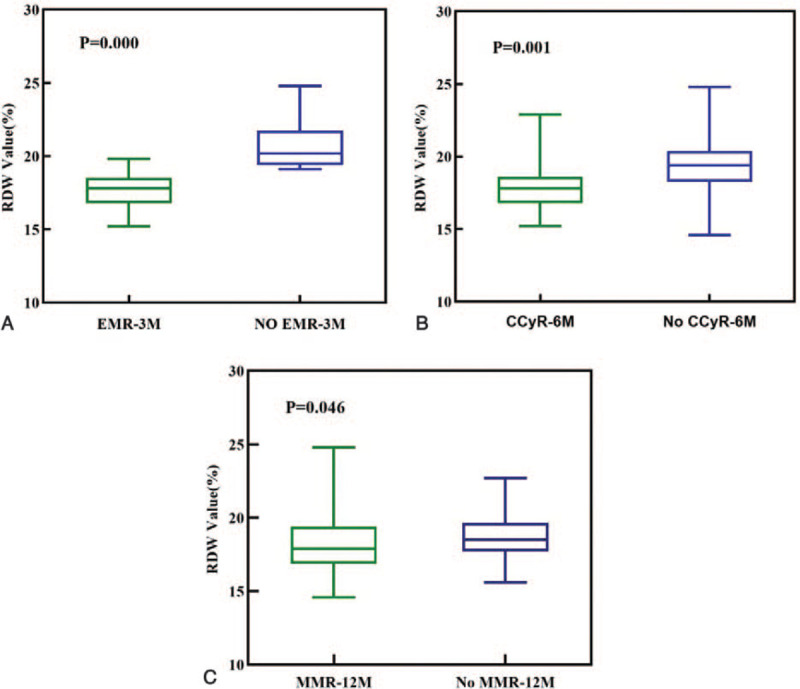
Baseline RDW value in patients with CML-CP were divided according to the clinical efficacy.(A) early molecular response by 3 months (3M-EMR), (B) complete cytogenetic response by 6 months(6M-CCyR), (C) major molecular response by 12 months (12M-MMR).
Table 3.
Evaluation of the clinical efficacy by score systems.
| Score systems | Risk stratification | 3M-EMR (n = 66) | P value | 6M-CCyR (n = 76) | P value | 12M-MMR (n = 70) | P value |
| Sokal score | Low-risk | 32/37 | .036 | 30/40 | 0.763 | 23/37 | .926 |
| Intermediate-risk | 12/20 | 20/28 | 14/23 | ||||
| High-risk | 5/9 | 5/8 | 5/10 | ||||
| Hasford score | Low-risk | 30/39 | .826 | 32/42 | 0.709 | 24/38 | .586 |
| Intermediate-risk | 17/24 | 21/31 | 17/29 | ||||
| High-risk | 2/3 | 2/3 | 1/3 | ||||
| EUTOS score | Low-risk | 45/61 | .759 | 52/75 | 0.810 | 40/66 | .674 |
| High-risk | 4/5 | 3/4 | 2/4 | ||||
| ELTS score | Low-risk | 27/32 | .028 | 28/40 | 0.815 | 25/33 | .034 |
| Intermediate-risk | 16/21 | 20/26 | 14/29 | ||||
| High-risk | 6/13 | 7/10 | 3/8 |
3.3. Impact of the RDW on clinical outcomes
The median follow-up time was 41 months (range, 3–95 months). During follow-up, 10 (10.8%) patients died, and 8 (8.60%) patients progressed to AP or BP. Patients with a high RDW had a significantly lower 5-year OS (77.1% vs 96.6%; P = .008) and PFS (80.0% vs 98.3%; P = .002) than those with a low RDW (Fig. 4). Results of the univariate analysis for the prognostic factors influencing OS and PFS are reported in Table 4. High RDW, older age, failure to achieve 3M-EMR, 6M-CCyR, and 12M-MMR, Sokal score (intermediate and high risk),and ELTS score (high risk) were significantly associated with OS and PFS. Hasford score (intermediate and high risk) was a predictor of PFS (P = .04) but not OS (P = .06). The basophil, eosinophil, blast, and marrow blast counts, hemoglobin levels, spleen size, and EUTOS score were not significantly associated with either PFS or OS. In multivariate analysis, which included all of the parameters having a P value <.2 in the univariate analysis, RDW and age at diagnosis were significant independent predictors of OS and PFS (Table 5).
Figure 4.
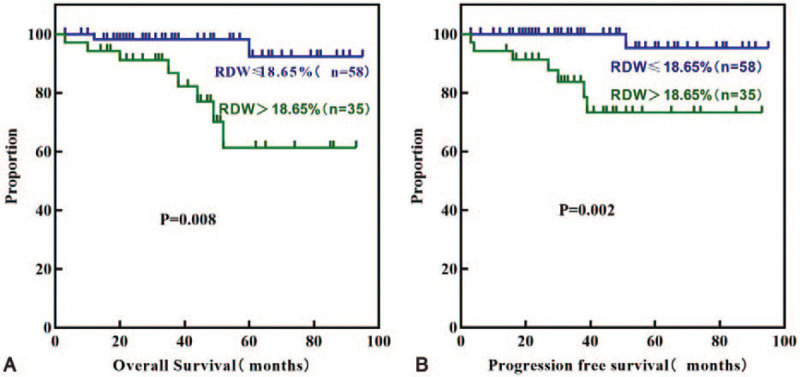
Kaplan-Meier curves for OS (A) and PFS (B) according to RDW.
Table 4.
Univariate analyses for PFS and OS.
| PFS | OS | |||||
| HR | 95%CI | P Value | HR | 95%CI | P Value | |
| RDW (>18.65%) | 12.22 | 2.896–51.59 | 0.002 | 7.303 | 1.998–26.69 | .008 |
| Age (≥50 years) | 6.103 | 1.397–26.66 | 0.011 | 4.810 | 1.283–18.03 | .011 |
| BAS (≥3%) | 0.799 | 0.116–5.516 | 0.833 | 0.628 | 0.111–3.554 | .655 |
| EOS (≥5) | – | – | 0.294 | – | – | .584 |
| Hb (<10/L) | 1.729 | 0.410–7.285 | 0.672 | 0.683 | 0.193–2.413 | .354 |
| Marrow Blast (>0%) | – | – | 0.208 | 1.551 | 0.269–8.928 | .673 |
| Blast (>0%) | 1.781 | 0.422–7.520 | 0.432 | 1.696 | 1.470–6.117 | .398 |
| Spleen size (>0cm) | 1.336 | 0.202–8.834 | 0.785 | 1.635 | 0.239–9.115 | .637 |
| Sokal socre (intermediate and high risk) | 7.605 | 1.900–30.44 | 0.025 | 4.302 | 1.244–14.87 | .044 |
| Hasford socre (intermediate and high risk) | 4.587 | 1.112–18.93 | 0.040 | 3.433 | 0.971–12.14 | .056 |
| EUTOS socre (high risk) | 2.101 | 0.123–35.90 | 0.477 | 1.669 | 0.129–21.63 | .623 |
| ELTS socre (high risk) | 21.23 | 2.097–215.0 | 0.010 | 5.592 | 1.750–41.70 | .002 |
| NO EMR-3M | 5.251 | 2.355–29.74 | 0.009 | 3.473 | 1.898–33.36 | .026 |
| NO CCyR-6M | 1.875 | 1.278–20.31 | 0.037 | 2.318 | 2.220–7.911 | .049 |
| NO MMR-12M | 1.139 | 1.010–16.96 | 0.044 | 6.509 | 2.294–7.749 | .022 |
Table 5.
Multivariate analyses for PFS and OS.
| PFS | OS | |||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| RDW (>18.65%) | 16.74 | 2.014–139.1 | .009 | 9.741 | 2.012–47.16 | .005 |
| Age (≥50 years) | 8.603 | 1.704–43.45 | .009 | 6.581 | 1.658–26.13 | .007 |
| Sokal socre (intermediate and high risk) | – | – | .669 | – | – | .911 |
| Hasford socre (intermediate and high risk) | – | – | .812 | – | – | .871 |
| ELTS socre (high risk) | – | – | .207 | – | – | .187 |
| NO EMR-3M | – | – | .385 | – | – | .421 |
| NO CCyR-6M | – | – | .673 | – | – | .545 |
| NO MMR-12M | – | – | .076 | – | – | .082 |
4. Discussion
The retrospective study showed that high RDW at diagnosis could predict poor prognosis of CML-CP and RDW was associated with treatment responses, especially at 3 months and 6 months, uniformly patients with 3M-EMR and 6M-CCyR had lower RDW. To compare the value of the RDW in evaluating the clinical efficacy of TKIs, we also evaluated 4 scoring systems. We found that the Sokal and ELTS score could predict 3M-EMR, and only the ELTS score could predict 12M-MMR. Therefore, combining the RDW with the Sokal score and the ELTS score could better predict the clinical efficacy of TKIs in CML-CP. To our knowledge, there has been few studies done.
RDW is a better predictor of prognosis than other laboratory parameters in the general population. Previous studies revealed that a higher RDW is associated with increased mortality risk[26] and is a poor prognostic factor in neoplastic diseases,[27] even in some hematological malignancies,[21–24] but the exact mechanism has not been clearly elucidated. It is thought that an increased RDW leads to a profound deregulation of erythrocyte homeostasis by affecting erythrocyte production and survival.[28] Numerous studies have reported a positive correlation between the RDW and a variety of inflammatory markers.[29] Demirkol et al found that inflammation impairs erythropoiesis and causes changes in red blood cell maturation, which contributes to an increase in the RDW.[30] Therefore, an elevated RDW might be a bridge between inflammation and tumorigenesis, thereby correlating to the poor prognosis of cancer patients.[31] We examined the association between the RDW and other clinical characteristics of CML patients and found that a higher RDW was closely related with WBC, Hb, spleen size, and EUTOS score but not with age or gender. This is consistent with the results of Iriyamas study.[24] The RDW has been shown to increase with age,[32] but we did not observe this in our findings. This may be because of the low median age of the patients included in our study.
The NCCN guidelines state that patients who achieve EMR by 3 or 6 months generally have favorable outcomes, but MMR is not a significant prognosticator of long-term outcome in patients who achieve stable CCyR.[5] We found that the RDW could predict the treatment responses at 3 and 6 months but not 12 months, and the RDW at diagnosis was significantly lower in patients who achieved 3M-EMR and 6M-CCyR. However, the RDW was not significantly different in patients who did not achieve 12M-MMR. Iriyama observed dynamic changes in the RDW before TKIs treatment and 1, 3 and 6 months after TKIs treatment. They found that the RDW was transiently elevated after 1 month but declined at 3 months and 6 months. They found no change at 12 months.[24] In our study, 60% of patients achieved 12M-MMR, which was lower than the proportions achieving 3M-EMR (74.2%) and 6M-CCyR (72.4%). If we can observe that the RDW decreases at 12 months after TKIs treatment, it suggests that RDW can also predict the treatment response at 12 months. It has been reported that individuals harboring somatic mutations in IDH1/2, TET2, or ASXL1 have higher RDW, and these somatic mutations are associated with an elevated risk of hematological disorders.[33] Importantly, mutations in IDH1/2, TET2, and ASXL1 have been detected in CML patients, and they may also contribute to progression in CML.[34] We hypothesized that somatic mutations result in an increased RDW and progression in CML. Therefore, the RDW, a marker that is easy to assess clinically, will be useful for the early identification of CML.
We further evaluated whether the RDW was an independent prognostic factor of OS and PFS. Log-rank test showed that age, RDW, clinical responses to TKIs (3M-EMR, 6M-CCyR, and 12M-MMR), the Sokal score, and the ELTS score were related to OS and PFS, although only the RDW and age were significant independent predictors in the multivariate Cox analysis. Therefore, our study results demonstrated the usefulness of the RDW as a prognostic factor. CML is a specific disease in which the CML stem cell has the potential to differentiate into erythroid lineage cells, resulting in the involvement of malignant clone-derived erythropoiesis.[35] Monika et al concluded that persistent activation of erythropoiesis through IGF-1/mTOR results in heterogeneity in red cell sizes, namely an increased RDW. Therefore, the RDW might be a marker of IGF-1/mTOR signaling, and the impact of RDW on mortality might be driven through IGF-1/mTOR signaling.[27]
There are several limitations in this analysis. First, this was a retrospective study in a single center with a small sample size. Second, nonadherence is common in CML patients and leads to treatment failure and poor outcomes. Third, we did not analyze the correlation between RDW and inflammatory markers or evaluate the RDW dynamically during TKIs treatment but only focused on the RDW at diagnosis.
5. Conclusion
Our study found that a high RDW is a readily available prognostic marker of poor outcome in patients with CML-CP. The RDW combined with the Sokal score and the ELTS score could be a good predictor of the clinical efficacy of TKIs in CML-CP patients. Our results suggest that the RDW should be given more attention in the clinic.
Author contributions
Conceptualization: Xia-Li Mao, Ya-Ming Xi.
Data curation: Li-Na Wang, Ming-Feng Jia.
Methodology: Long Zhao, Ming Li.
Writing – original draft: Xia-Li Mao, Hao Zhang.
Writing – review & editing: Zi-Jian Li.
Footnotes
Abbreviations: 12M-MMR = major molecular response at 12 months, 3M-EMR = early molecular response at 3 months, 6M-CCyR = complete cytogenetic response at 6 month, AP = accelerated phase, AUC = areas under the curve, BCR-ABL = breakpoint cluster region - abl oncogene, BP = blastic phase, CCyR = complete cytogenetic response, CI = confidence interval, CML = chronic myeloid leukemia, CP = chronic phase, ELN = European Leukemia Net, ELTS = the EUTOS long-term survival, EMR = early molecular response, EUTOS = the European Treatment and Outcome Study, HR = hazard ratio, MMR = major molecular response, NCCN = National Comprehensive Cancer Network, OS = overall survival, PFS = progression free survival, RDW = red blood cell distribution width, ROC = receiver operating curve, TKIs = Tyrosine Kinase Inhibitors, WBC = white blood cell.
How to cite this article: Mao XL, Xi YM, Li ZJ, Jia MF, Li M, Wang LN, Zhao L, Zhang H. Higher red blood cell distribution width at diagnose is a simple negative prognostic factor in chronic phase-chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: a retrospective study. Medicine. 2021;100:10(e24003).
The authors have no funding or conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Pan P, Wang L, Wang Y, et al. Systematic review and meta-analysis of -new-generation tyrosine kinase inhibitors versus imatinib for newly diagnosed chronic myeloid leukemia. Acta Haematol 2019;12:1–3. [DOI] [PubMed] [Google Scholar]
- [3].Balakumaran J, Birk T, Golemiec B, et al. Evaluating the endometabolic and bone health effects of tyrosine kinase inhibitors in chronic myeloid leukaemia: a systematic review protocol. BMJ Open 2019;9:e030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016. National Cancer Institute 2019. https://seer.cancer.gov/csr/1975_ 2016/ (accessed 3 February 2020). [Google Scholar]
- [5].Deininger MW, Shah NP, Altman JK, et al. Chronic myeloid leukemia, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020. https://www.nccn.org/professionals/physician gls/pdf/cml.pdf [DOI] [PubMed] [Google Scholar]
- [6].Mathisen MS, Kantarjian HM, Cortes J, et al. Practical issues surrounding the explosion of tyrosine kinase inhibitors for the management of chronic myeloid leukemia. Blood Rev 2014;28:179–87. [DOI] [PubMed] [Google Scholar]
- [7].Cuellar S, Vozniak M, Rhodes J, et al. BCR-ABL1 tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. J Oncol Pharm Pract 2018;24:433–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Frankfurt O, Licht JD. Ponatinib--a step forward in overcoming resistance in chronic myeloid leukemia. Clin Cancer Res 2013;19:5828–34. [DOI] [PubMed] [Google Scholar]
- [9].Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood 2012;119:3403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ben-Neriah Y, Daley GQ, Mes-Masson A-M, et al. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science 1986;233:212–4. [DOI] [PubMed] [Google Scholar]
- [11].Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1084–6. [DOI] [PubMed] [Google Scholar]
- [12].Gurrea Salas D, Glauche I, Tauer JT, et al. Can prognostic scoring systems for chronic myeloid leukemia as established in adults be applied to pediatric patients? Ann Hematol 2015;94:1363–71. [DOI] [PubMed] [Google Scholar]
- [13].Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86–105. [DOI] [PubMed] [Google Scholar]
- [14].Mozos I. Mechanisms linking red blood cell disorders and cardiovascular diseases. Biomed Res Int 2015;2015:682054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lippi G, Dipalo M, Teti L, et al. Relationship between red blood cell distribution width and prognostic biomarkers in patients admitted to the emergency department with acute infections. Eur J Intern Med 2013;24:e15–6. [DOI] [PubMed] [Google Scholar]
- [16].Sanchez-Chaparro MA, Calvo-Bonacho E, Gonzalez-Quintela A, et al. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care 2010;33:e40. [DOI] [PubMed] [Google Scholar]
- [17].Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PloS one 2013;8:e80240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ay S, Eryilmaz MA, Aksoy N, et al. Is early detection of colon cancer possible with red blood cell distribution width. Asian Pac J Cancer Prev 2015;16:753–6. [DOI] [PubMed] [Google Scholar]
- [19].Seretis C, Seretis F, Lagoudianakis E, et al. Is red cell distribution width a novel biomarker of breast cancer activity? J Clin Med Res 2013;5:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Albayrak S, Zengin K, Tanik S, et al. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev 2014;15:7781–4. [DOI] [PubMed] [Google Scholar]
- [21].Wang J, Xie X, Cheng F, et al. Evaluation of pretreatment red cell distribution width in patients with multiple myeloma. Cancer Biomarkers 2017;20:267–72. [DOI] [PubMed] [Google Scholar]
- [22].Luo H, Quan X, Song X-Y, et al. Red blood cell distribution width as a predictor of survival in nasal-type, extranodal natural killer/T-cell lymphoma. Oncotarget 2017;8:92522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou S, Fang F, Chen H, et al. Prognostic significance of the red blood cell distribution width in diffuse large B-cell lymphoma patients. Oncotarget 2017;8:40724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iriyama N, Hatta Y, Kobayashi S, et al. Higher red blood cell distribution width is an adverse prognostic factor in chronic-phase chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Anticancer Res 2015;35:5473–8. [PubMed] [Google Scholar]
- [25].Baccarani M, Deininger MW, Rosti G, et al. European leukemia net recommendations for the management of chronic myeloid leukemia: 2013. J Am Soci Hematol 2013;122:872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009;169:588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Podhorecka M, Halicka D, Szymczyk A, et al. Assessment of red blood cell distribution width as a prognostic marker in chronic lymphocytic leukemia. Oncotarget 2016;7:32846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Meng S, Ma Z, Lu C, et al. Prognostic value of elevated red blood cell distribution width in Chinese patients with multiple myeloma. Ann Clin Laboratory Sci 2017;47:282–90. [PubMed] [Google Scholar]
- [29].Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Laboratory Med 2009;133:628–32. [DOI] [PubMed] [Google Scholar]
- [30].Demirkol S, Balta S, Cakar M, et al. Red cell distribution width: A novel infl ammatory marker in clinical practice. Cardiol J 2013;20:209–19. [DOI] [PubMed] [Google Scholar]
- [31].Ai L, Mu S, Hu Y. Prognostic role of RDW in hematological malignancies: a systematic review and meta-analysis. Cancer Cell Intern 2018;18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brightwell R, Crawford G, Cale J, et al. Ageing and the haematological profiles of an Australian community. Ann Human Biol 1998;25:1–0. [DOI] [PubMed] [Google Scholar]
- [33].Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. New Eng J Med 2014;371:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Makishima H, Jankowska AM, McDevitt MA, et al. CBL, CBLB, TET2, ASXL1, and IDH1/2 mutations and additional chromosomal aberrations constitute molecular events in chronic myelogenous leukemia. Blood 2011;117:e198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dainiak N, Liu A, Dewey MC, et al. Chromosome analysis of isolated colony erythroblasts in chronic myelogenous leukaemia. Br J Haematol 1984;56:507–12. [DOI] [PubMed] [Google Scholar]


