Abstract
This study aimed to investigate the relationships between omentin-1, body composition and physical activity (PA) levels in older women.
Eighty-one older women (age = 64 ± 6years; body mass index = 24.2 ± 3.2 kg/m2; body fat percentage = 36.1 ± 5.7%) participated in this study. We divided the subjects into overweight/obesity and normal weight group. Body composition was measured by dual energy X-ray absorptiometry. Serum omentin-1 concentration was measured using enzyme linked immunosorbent assay. PA levels were obtained by using accelerometers. In addition, anthropometric and insulin resistance values were determined.
Omentin-1 level in overweight/obesity group was significantly lower than in the normal weight group (P < .01). Analysis of all subjects showed that serum omentin-1 was negatively correlated with body weight, BMI (body mass index), waist circumference (WC), WHR (waist-to-hip ratio), percentage of body fat, total body fat mass (FM), fat-free mass (FFM) (r = −.571, −0.569, −0.546, −0.382, −0.394, −0.484, −0.524, all P < .01), respectively. We also found a negative correlation between moderate-to-vigorous physical activity (MVPA) and total body FM (r = −.233, P < .05). However, no significant correlation was found between omentin-1 and sedentary behavior and MVPA (both P > .05). Moreover, the relationship between omentin-1, body composition and PA was analyzed by using multiple linear stepwise regressions. The results showed that serum omentin-1 concentration was inversely correlated with total body FM (β = −0.334, P = .004) in multiple linear stepwise regression analysis.
We found that total body FM was inversely related to serum omentin-1 concentration and PA levels, but there was no correlation between omentin-1 and PA levels. These results showed that PA may participate in the regulation of body composition, which may be also affected by serum omentin-1. However, the mechanism by which PA affects body composition may not be through omentin-1 and was more likely through other metabolic pathways.
Keywords: body composition, older women, omentin-1, physical activity
1. Introduction
Omentin-1 is secreted by adipocytes as a beneficial adipokine and plays an important role in regulating insulin resistance and lipid metabolism in obese adults.[1] Omentin-1 concentration is higher in lean subjects than obese individuals.[2] Weight loss resulting from diet and regular exercises will lead to the increase of serum omentin-1 concentration.[3,4] Omentin-1 concentration in obesity-related disease (such as insulin resistance and type 2 diabetes mellitus) populations is lower than that of normal individuals.[5,6] Aging, PA and body weight are several important factors affecting omentin-1 concentration. However, it is unclear that which factors can be used as impact predictors of circulating omentin-1 concentration.[7,8]
It is well known that aging can lead to change in body composition. At age 30 to 70 years, FFM progressively declines while fat mass (FM) increases with aging; after 70 years, both fat-free and (FM) decline.[9,10] Decreased daily PA levels and increased sedentary behaviors are the main reasons of body composition changes of elder population,[11] which is associated with an increased risk of overweight and obesity in older adults.[12] In contrast, increasing PA is positively related to a decrease in body FM.[13–15] Recent studies showed that the PA levels have been determined in older people by accelerometers successfully.[16,17] Accelerometers can provide minute-by-minute counts of PA and estimate PA volume at different intensity levels over a period of days or weeks.[17] Therefore, accelerometers are the most commonly used to evaluate objective PA levels in epidemiological research in children and non-elder adults.[18] To date, self-report is primarily used for subjective assessment methods of the daily PA levels among older adults (>65 years).[19]
Previous studies have shown that different types of exercise interventions including aerobic exercise, resistance exercise, and combined exercise can cause increase in serum omentin-1 concentration.[20–22] However, omentin-1 concentration may be affected by daily PA behaviors, though there was no consensus on the effects of the PA.[21] To our knowledge, no studies have examined the possible relationship between omentin-1 and PA levels obtained by accelerometers in elder women. Omentin-1 and daily PA behaviors both may be important factors influencing the body composition. Thus, our main aims were to examine the associations between omentin-1 concentration, accelerometer measured PA levels and body composition in older women.
2. Methods and materials
2.1. Subjects
Eighty-one older women from Beijing were enrolled in this study, including 58 women with overweight/obesity (age: 63 ± 6years, BMI: 25.7 ± 2.5 kg/m2) and 23 normal-weight women (age: 64 ± 6years, BMI: 20.6 ± 1.6 kg/m2). The candidates should be healthy postmenopausal women between 50 and 75 years. Moreover, candidates who had the habit of smoking or drinking alcohol, coffee or coke were also excluded from this study. The study was conducted in Beijing and in accordance with the ethical standards set out in the Helsinki Declaration, approved by the ethics committee for human research of China Institute of Sport Science (Ethical code: CISSIRD-201604). All subjects in this study voluntarily joined this study with informed consents.
2.2. Anthropometric and body composition measurements
Su Heng Health Scale (RGZ-120, China) was used to measure height and body weight and participants were asked to wear minimal clothing. Dual-energy X-ray absorptiometry (GE lunar prodigy) was used to determine body composition. Chest, waist and hip circumference was measured by tape (SECA, Hamburg, Germany). The SBP and (DBP) diastolic blood pressure was measured using electronic sphygmomanometer (OMRON, Kyoto, Japan). The formula of “BMI=weight (kg)/height (m) squared” was used to calculate BMI of subjects. Asian specific BMI threshold was used to define underweight (<18.5 kg/m2), normal-weight (18.5 to 23.0 kg/m2), overweight (23.0 to 27.5 kg/m2) and obese (≥ 27.5 kg/m2).[23] Those with a BMI of ≥ 23 kg/m2 were classified into overweight/obesity group, otherwise, they were classified into normal weight group. The waist-to-hip ratio (WHR) was estimated as waist circumference (cm)/hip circumference (cm). All testers in this study are professionally trained and each test project is tested by the same tester.
2.3. Physical activity levels assessment
Triaxial accelerometer was used to measure the level of physical activity (PA) (Actigraph GT3X-BT, Pensacola, FL). The monitor was worn on the right hip for 7 days except during sleeping, bathing, or swimming. The accelerometers were set to collect data in 60-seconds epochs. The data were downloaded and analyzed using ActiLife version 6.13.3. Each accelerometer file underwent the following standardized data-quality procedures to assess validity and reliability of the data.[24] We used standard methods (defined non-wear time as 60 minutes or more of consecutive zeros) to screen the wear time of accelerometer data.[25] Accelerometer values >20,000 counts/min were considered erroneous and removed from analysis. After the data-cleaning processes were employed, only days during which the accelerometer was worn for at least 600 minutes were counted as valid days of data. Only participants who had at least 4 days (including 1 weekend day) of valid accelerometer data were included in the analysis.[24] We used accelerometer cut points developed by Freedson and colleagues to convert the raw data into an estimate of physical activity intensity.[26] Sedentary behavior was characterized < 100cpm, light PA [Light-intensity physical activity (LPA)] 100 to 1951cpm, moderate PA [moderate-intensity physical activity (MPA)] 1952–5724cpm and vigorous PA (VPA) ≥ 5725cpm.[26]
2.4. Blood sampling and analysis
We asked subjects to come to the laboratory at 7:00 am. Fasting blood samples were collected between 8:00 and 9:00 am. Triglycerides and glucose were measured by GPO-PAP and hexokinase methods, respectively. Total cholesterol levels were measured by cholesterol oxidase method and high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) was measured using an automated enzymatic assay utilizing a homogeneous method (Beckman Coulter AU680, USA). Blood insulin concentration was determined using chemiluminescence immunoassay method (Architect i2000, USA). The homoeostasis model of insulin resistance (HOMA-IR) was calculated using the following equation: fasting glucose (mmol/l) × fasting insulin (mU/l)/22.5.[27] Serum omentin-1 concentration was measured by using enzyme linked immunosorbent assay (ELISA) (Apotech, Geneva, Switzerland).
2.5. Statistical analysis
All data in this study were analyzed by IBM SPSS Statistics for Windows, Version 23.0. (IBM Corp.23, Armonk, NY) and expressed as mean ± standard deviation (SD). The normal distribution was checked for all data with Kolmogorov–Smirnov test. Independent-sample t test was used to evaluate differences between overweight/obesity and normal weight group. The correlations between omentin-1 and anthropometric parameters, metabolic data, body composition as well as PA levels were determined by Pearson's correlation analysis. Multiple linear stepwise regression analysis was performed to assess the relationship between a group of independent variables [sedentary behavior, LPA, MPA, VPA, (MVPA) moderate-to-vigorous physical activity, omentin-1, glucose, insulin, total cholesterol, and triglycerides] and dependent variables (total body FM). Statistical analysis was two-sided and P < .05 represented statistical significance.
3. Results
3.1. General characteristics of the subjects
Table 1 summarizes the general characteristics of the subjects enrolled in our study. Among the 81 subjects, 58 were categorized into overweight/obesity group, while 23 were categorized into normal weight group, respectively. Overweight/obesity women had higher values than the normal weight women for weight, BMI, waist and hip circumference, WHR, percentage of body fat, total body FM, fat-free mass (FFM), fasting glucose, fasting insulin and HOMA-IR (all P < .01). Moreover, omentin-1 and HDL-C was significantly lower in the overweight/obesity group than in the normal weight group (P < .05).
Table 1.
Subject characteristics of study populations (Mean ± SD).
| Category | Subjects with overweight/obesity (n = 58) | Subjects with normal weight (n = 23) | P value |
| General information | |||
| Age (years) | 63 ± 6 | 64 ± 6 | >.05 |
| Height (cm) | 156.6 ± 5.0 | 156.1 ± 5.5 | >.05 |
| Weight (kg) | 63.1 ± 7.0 | 50.3 ± 5.1 | <.01 |
| Waist circumference (cm) | 86.3 ± 7.7 | 72.1 ± 4.8 | <.01 |
| Hip circumference (cm) | 97.2 ± 5.5 | 87.9 ± 3.9 | <.01 |
| WHR | 0.89 ± 0.07 | 0.82 ± 0.05 | <.01 |
| SBP (mm Hg) | 124 ± 14 | 114 ± 19 | .01 |
| DBP (mm Hg) | 75 ± 10 | 71 ± 9 | >.05 |
| Adipokines | |||
| Omentin-1 (ng/ml) | 12.79 ± 5.06 | 25.94 ± 9.29 | <.01 |
| Body composition | |||
| BMI (kg/m2) | 25.7 ± 2.5 | 20.6 ± 1.6 | <.01 |
| Body fat percentage (%) | 38.1 ± 4.9 | 31.1 ± 4.2 | <.01 |
| Total body FM (kg) | 23.4 ± 5.2 | 15.2 ± 3.0 | <.01 |
| Fat-free mass (kg) | 40.1 ± 4.4 | 35.1 ± 3.3 | <.01 |
| Biochemical characteristics | |||
| Fasting glucose (mmol/L) | 5.33 ± 0.99 | 4.80 ± 0.28 | <.01 |
| Fasting insulin (μIU/ml) | 8.32 ± 4.43 | 4.63 ± 2.05 | <.01 |
| HOMA-IR | 2.05 ± 1.40 | 0.99 ± 0.43 | <.01 |
| Triglycerides (mmol/L) | 1.44 ± 0.6 | 1.18 ± 0.45 | >.05 |
| Total cholesterol (mmol/L) | 4.98 ± 0.98 | 4.94 ± 0.95 | >.05 |
| HDL-C (mmol/L) | 1.29 ± 0.23 | 1.47 ± 0.39 | .013 |
| LDL-C (mmol/L) | 3.12 ± 0.77 | 2.97 ± 0.72 | >.05 |
| Physical activity | |||
| SB (min/d) | 469 ± 110 | 482 ± 88 | >.05 |
| LPA (min/d) | 247 ± 78 | 264 ± 63 | >.05 |
| MPA (min/d) | 30 ± 18 | 33 ± 23 | >.05 |
| VPA (min/d) | 0.1 ± 0.4 | 0.3 ± 0.7 | >.05 |
| MVPA (min/d) | 31 ± 19 | 34 ± 23 | >.05 |
BMI = body mass index, DBP = diastolic blood pressure, FM = fat mass, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homoeostasis model of insulin resistance, LDL-C = low-density lipoprotein cholesterol, LPA = light-intensity physical activity, MPA = moderate-intensity physical activity, MVPA = moderate-to- vigorous physical activity, SB = sedentary behavior, SBP = systolic blood pressure, VPA = vigorous-intensity physical activity.
3.2. Relationship between serum omentin-1 concentration and anthropometric and body composition data
There were negative correlations between omentin-1 concentration and body weight (r = −.571, P < .01), waist circumference (r = −.546, P < .01) and WHR (r = −.382, P < .01) in all subjects. In addition, height (r = −.506, P < .05), body weight (r = -.661, P < .01) were negatively correlated with omentin-1 in group with normal weight, but there was no correlation in group overweight/obesity (P > .05). Serum omentin-1 concentration was negatively correlated with BMI (r = −.569, P < .01), percentage of body fat (r = −.394, P < .01, Fig. 1 A), total body FM (r = −.484, P < .01, Fig. 1 B) and FFM (r = −.524, P < .01) in all subjects, respectively (Table 2). Moreover, the results of multiple linear stepwise regression analyses (Table 3) showed that serum omentin-1 concentration was negatively correlated with total body FM (β = −.334, P = .004). However, there were no significant correlations between omentin-1 and body composition (BMI, percentage of body fat, total body FM, FFM) in overweight/obesity group (all P > .05). Serum omentin-1 concentration was inversely correlated with total body FM (r = −.415, P < .05) and FFM (r = −.640, P < .01) in normal weight group.
Figure 1.
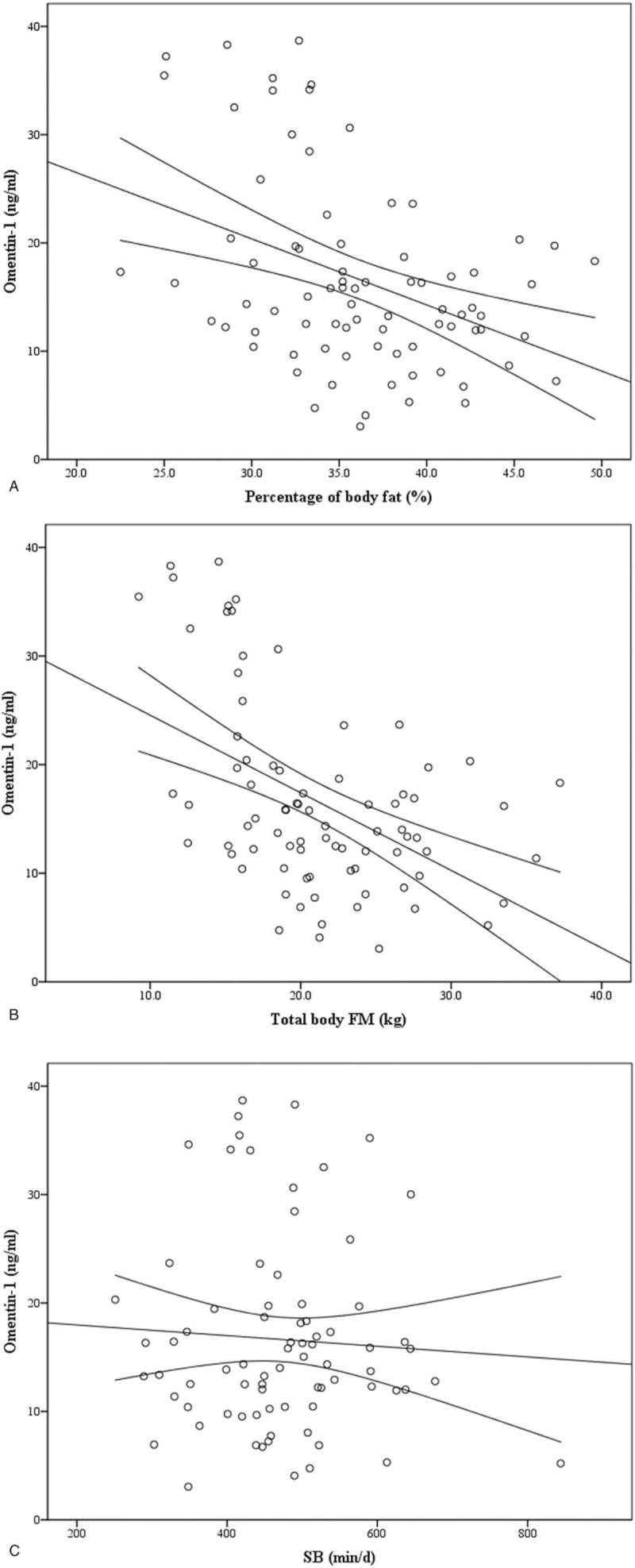
Correlation between circulating omentin-1 and a percent body fat (r = −.394, P < .01), B total body FM (r = −.484, P < .01) in all subjects. Correlation between circulating omentin-1 and C SB (r = −.057, P > .05), D MVPA (r = .166, P > .05) in all subjects. Correlation between E SB (r = −.068, P > 0.05), F MVPA (r = −.233, P < .05) and total body FM in all subjects.
Table 2.
The relationships between serum omentin-1 and body composition data in all subjects.
| Subjects with overweight/obesity (n = 58) | Subjects with normal weight (n = 23) | Total (n = 81) | |
| Height | 0.081 | −0.506∗ | −0.159 |
| Body weight | −0.023 | −0.661∗∗ | −0.571∗∗ |
| Waist circumference | −0.107 | −0.359 | −0.546∗∗ |
| WHR | −0.186 | −0.005 | −0.382∗∗ |
| BMI | −0.073 | −0.407 | −0.569∗∗ |
| Body fat percentage (%) | 0.039 | −0.131 | −0.394∗∗ |
| Total body FM | 0.010 | −0.415∗ | −0.484∗∗ |
| Fat-free mass | −0.158 | −0.640∗∗ | −0.524∗∗ |
FM = fat mass.
Values are Pearson correlation coefficient.
P < .05.
P < .01.
Table 3.
Multiple linear stepwise regression analysis for the relationship between omentin-1, fasting insulin (independent variables) and body fat mass (dependent variables) in all subjects (n = 81).
| Parameters | β-coefficient | R2 | P value |
| omentin-1 | −0.334 | 0.244 | .004 |
| Fasting insulin | 0.316 | 0.074 | .006 |
Figure 1 (Continued).
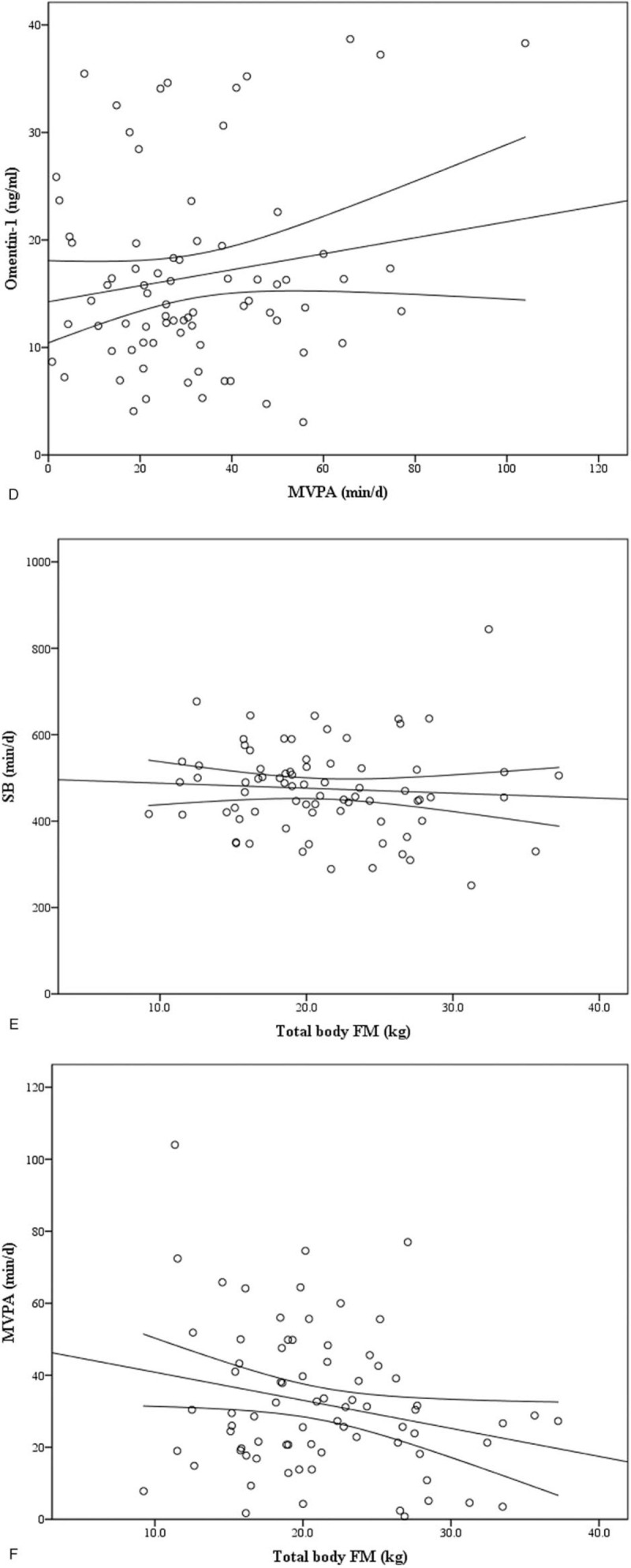
Correlation between circulating omentin-1 and a percent body fat (r = −.394, P < .01), B total body FM (r = −.484, P < .01) in all subjects. Correlation between circulating omentin-1 and C SB (r = −.057, P > .05), D MVPA (r = .166, P > .05) in all subjects. Correlation between E SB (r = −.068, P > 0.05), F MVPA (r = −.233, P < .05) and total body FM in all subjects.
3.3. Correlations of omentin-1 and metabolic factors
Serum omentin-1 concentration was negatively associated with fasting glucose (r = −.281, P < .05), insulin (r = −.500, P < .01), HOMA-IR (r = −.465, P < .01), triglycerides (r = −.321, P < .01) in all subjects. In addition, fasting insulin (r = −.403, P < .01), HOMA-IR (r = −.396, P < .01), triglycerides (r = −.263, P < .05) were negatively correlated with omentin-1 in overweight/obesity group, but there were no correlations in normal weight group. Moreover, serum omentin-1 concentration was positively correlated with HDL-C (r = .455, P < .01, Table 4) in all subjects.
Table 4.
The relationships between serum omentin-1 and metabolic data in all subjects.
| Subjects with overweight/obesity (n = 58) | Subjects with normal weight (n = 23) | Total (n = 81) | |
| Fasting glucose | −0.231 | 0.140 | −0.281∗ |
| Fasting insulin | −0.403∗∗ | −0.419∗ | −0.500∗∗ |
| HOMA-IR | −0.396∗∗ | −0.395 | −0.465∗∗ |
| Triglycerides | −0.263∗ | −0.306 | −0.321∗∗ |
| Total cholesterol | 0.124 | 0.168 | 0.087 |
| HDL-C | 0.357∗∗ | 0.398 | 0.455∗∗ |
| LDL-C | 0.069 | 0.027 | −0.024 |
HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homoeostasis model of insulin resistance, LDL-C = low-density lipoprotein cholesterol.
Values are Pearson correlation coefficient.
P < .05.
P < .01.
3.4. Correlations of omentin-1 concentration and physical activity levels
No significant statistical relationships were found between omentin-1 and SB (Fig. 1 C), LPA, MPA, VPA and MVPA (Fig. 1 D) (all P > .05, Table 5) in all subjects. There were also no significant correlations between omentin-1 concentration and all PA levels in 2 groups (all P > .05). However, MVPA was positively correlated with omentin-1 concentration in normal weight group (r = .432, P < .05).
Table 5.
The relationships between serum omentin-1 and different physical activity levels data in all subjects.
| Subjects with overweight/obesity (n = 58) | Subjects with normal weight (n = 23) | Total (n = 81) | |
| SB | −0.110 | −0.196 | −0.057 |
| LPA | −0.135 | 0.072 | 0.032 |
| MPA | −0.078 | 0.418 | 0.166 |
| VPA | −0.124 | −0.261 | −0.009 |
| MVPA | −0.068 | 0.432∗ | 0.166 |
LPA = light-intensity physical activity, MPA = moderate-intensity physical activity, MVPA = moderate-to-vigorous physical activity, SB = sedentary behavior, VPA = vigorous-intensity physical activity.
Values are Pearson correlation coefficient.
P < .05.
∗∗P < .01.
3.5. Associations between physical activity levels and body composition
Sedentary behavior was not significantly correlated with BMI, percentage of body fat, total body FM and FFM (both P > .05, Fig. 1 E) in all subjects. Moreover, MVPA was negatively correlated with total body FM (r = −.233, P < .05) in all subjects (Table 6, Fig. 1 F). However, sedentary behavior and MVPA were not correlated with BMI, percentage of body fat, body FM and FFM in overweight/obesity group and normal weight group respectively (all P > .05, Table 6). However, there was no significant correlation between total body FM and sedentary behavior, LPA, MPA, VPA, MVPA by using multiple linear stepwise regression analysis.
Table 6.
The relationships between physical activity levels and body composition data in all subjects.
| Subjects with overweight/obesity (n = 58) | Subjects with normal weight (n = 23) | Total (n = 81) | ||||||||||
| Physical activity | BMI | Body fat percentage (%) | Total body FM (kg) | Fat-free mass (kg) | BMI | Body fat percentage (%) | Total body FM (kg) | Fat-free mass (kg) | BMI | Body fat percentage (%) | Total body FM (kg) | Fat-free mass (kg) |
| SB | 0.006 | −0.034 | −0.026 | −0.119 | 0.041 | −0.229 | −0.193 | 0.174 | −0.032 | −0.087 | −0.068 | −0.086 |
| LPA | −0.047 | −0.241 | −0.178 | 0.115 | 0.257 | 0.111 | 0.235 | 0.337 | −0.074 | −0.192 | −0.150 | 0.078 |
| MPA | −0.238 | −0.275∗ | −0.275∗ | −0.069 | −0.067 | −0.134 | −0.276 | −0.324 | −0.188 | −0.229∗ | −0.247∗ | −0.152 |
| VPA | −0.090 | −0.210 | −0.184 | −0.002 | 0.111 | −0.057 | 0.083 | 0.333 | −0.160 | −0.226∗ | −0.195 | −0.018 |
| MVPA | −0.218 | −0.260 | −0.263 | −0.066 | −0.060 | −0.125 | −0.272 | −0.329 | −0.169 | −0.213 | −0.233∗ | −0.146 |
FM = fat mass, LPA = light-intensity physical activity, MPA = moderate-intensity physical activity, MVPA = moderate-to-vigorous physical activity, SB = sedentary behavior, VPA = vigorous-intensity physical activity.
Values are Pearson correlation coefficient.
P < .05.
∗∗P < .01.
4. Discussion
The present study investigated the relationship between serum omentin-1 concentration, PA levels and body composition in older women. The results of Pearson correlation analysis that was performed in all the participants indicated that serum omentin-1 concentration was negatively associated with BMI, percentage of body fat, total body FM, FFM, fasting glucose and fasting insulin. Meanwhile, MVPA was related with total body FM negatively. However, no correlations were found between PA levels and omentin-1 concentration. Above results suggested that omentin-1 and PA both were important factors affecting body composition, however, PA levels regulating body composition may not be related to omentin-1 concentration in older women.
In our results, subjects in overweight/obesity group had a lower omentin-1 concentration than normal weight group. The possible reason may be that obesity related factors (for example, insulin) downregulated omentin-1 production.[3] Omentin-1 concentration inversely correlated with BMI, percentage of body fat and FM and FFM in all subjects. Compared with normal weight adults, overweight/obesity adults with more FM also have more fat free mass,[28] which resulted in the inverse correlation between omentin-1 concentration and FFM, omentin-1 concentration and FM in all subjects. These findings are in accordance with data from several cross-sectional studies.[29,30] These studies have demonstrated that obesity related data was negatively correlated with omentin-1 concentration. Therefore, we speculated that omentin-1 was a beneficial predictor of obesity, and omentin-1 might have a positive protective effect against obesity. Moreover, previous studies have confirmed that circulating omentin-1 was closely related to glycometabolism.[31,32] Negative associations were found between serum omentin-1 and fasting glucose, insulin, HOMA-IR respectively, which was consistent with findings of Tan[32] and El-Mesallamy.[31] Tan and his colleagues[32] found that omentin mRNA expression and omentin protein production decreased while insulin increased. El-Mesallamy[31] observed that serum omentin-1 concentration negatively correlated with fasting glucose and HOMA-IR. In a word, these results indicated that omentin-1 played an important role in glucose metabolism and insulin sensitivity, and changes in serum omentin-1 concentration might affect glucose or insulin levels. In addition, a positive association between omentin-1 and HDL cholesterol was observed in this study. According to previous studies, disorder of omentin-1 may inversely affect regulation of insulin, which could influence the production of HDL-C.[33,34]
The associations of objectively measured PA levels with anthropometric, physiology and psychological variables were investigated firstly by Harris.[35] To our knowledge, associations of omentin-1 with accelerometer measured PA levels have not been examined in elder population. The effects of exercise on omentin-1 have been extensively studied, however, there have been few reports about the effects of free-living PA to omentin-1. To date, there were multiple studies indicating a weak moderate positive correlation between objectively measured various intensities of PA by accelerometers and adiponectin.[36] St-Pierre et al[37] also found similar results that there was a positive association between PA and adiponectin among healthy young women. Moreover, Sitticharoon and his colleagues[38] have confirmed that adiponectin correlated positively with omentin-1. All of these studies suggested that people with higher omentin-1 levels have greater time spent in PA or sports.[39] However, in our study, PA levels were not associated with serum omentin-1 concentration in all subjects. By contrast, multiple studies showed that longitudinal aerobic and combined exercise can lead to an increase in the serum omentin-1 concentration.[20–22] In our study, it is speculated that the intensity and duration may be an important factor to stimulate the secretion of omentin-1. The most likely reason was that older women spent a majority of their time in sedentary behavior or light-intensity activities. PA intensity did not reach the threshold for stimulating omentin-1 secretion. Another important reason is that there was no “gold standard” cut-point for older adults and the choice of the accelerometer cut-point which might influence the determination of time spent in MVPA.[40,41]
Foong and colleagues[42] found that body FM gradually decreases with increasing intensity and volume of PA. Another study[43] also showed that body FM was negatively correlated with PA levels. Our findings are consistent with these studies that have observed a negative association between MVPA and body FM in all subjects.[42,43] Physical activity, especially MVPA, stimulates higher fat oxidation and oxygen uptake, thereby increasing resting metabolic rate and total energy expenditure may be the main reasons for the negative correlation between physical activity and body composition.[44,45] Thus, these imply that the greater the amount of time spent on MVPA, the lower the body FM. Moreover, our results also found that, any of the body composition variables were not significantly correlated with sedentary time. Similar to the results of Chastin and his colleagues,[46] no significant relationship between the time spent in sedentary and body FM was found. The lack of correlation between sedentary time and body composition variables have been attributed to different lifestyles of the older women, small sample size, measurement error of sedentary time.[47]
There were several potential limitations in our study. Firstly, small sample size and the disproportionate ratio of 2 groups may limit the promotion of current research results. Future studies may investigate serum omentin-1 concentration in a larger sample size for men and women. Secondly, confounding factors such as nutritional intake that may influence omentin-1 and other metabolic variables were not investigated in this study. Thirdly, there was no consensus on the choice of the cut-point formula for the accelerometer, which could make a different PA levels when choosing a different cut-point. Fourthly, triaxial accelerometer used in this study would underestimate non-ambulatory activity, such as cycling, weight training, or swimming[48] and duration of the monitoring period of 7 days would result some errors in physical activities assessment.
5. Conclusions
Physical activity levels were inversely associated with body composition, and serum omentin-1 concentration was negatively correlated with body composition among older women. However, there was no significant association between serum omentin-1 concentration and physical activity levels. These data suggested that daily physical activity may participate in the regulation of body composition, which may be also affected by serum omentin-1. However, the relationship between physical activity levels and body composition was not mediated by serum omentin-1 concentration and was more likely due to other metabolic pathways.
Author contributions
Data curation: Shuo Li.
Formal analysis: Shuo Li, Jingjing Xue.
Funding acquisition: Ping Hong.
Investigation: Shuo Li.
Project administration: Jingjing Xue, Ping Hong.
Supervision: Jingjing Xue.
Writing – original draft: Shuo Li.
Writing – review & editing: Jingjing Xue, Ping Hong.
Footnotes
Abbreviations: BMI = body mass index, DBP = diastolic blood pressure, FFM= fat-free mass, FM = fat mass, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = Homoeostasis model of insulin resistance, LDL-C = low-density lipoprotein cholesterol, LPA = light-intensity physical activity, MPA = mderate-intensity physical activity, MVPA = moderate-to-vigorous physical activity, PA = physical activity, SB = Sedentary behavior, SBP = systolic blood pressure, VPA = Vigorous-intensity physical activity, WHR = Waist-to-hip ratio.
How to cite this article: Li S, Xue J, Hong P. Relationships between serum omentin-1 concentration, body composition and physical activity levels in older women. Medicine. 2021;100:10(e25020).
This study was supported by Ministry of Science and Technology of the People's Republic of China (Grants No.2013FY114700).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem 2004;50:1511–25. [DOI] [PubMed] [Google Scholar]
- [2].Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 2006;290:E1253–1261. [DOI] [PubMed] [Google Scholar]
- [3].Moreno-Navarrete JM, Catalán V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 2010;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang M, Tan X, Yin C, et al. Serum levels of omentin-1 are increased after weight loss and are particularly associated with increases in obese children with metabolic syndrome. Acta Paediatr 2017;106:1851–6. [DOI] [PubMed] [Google Scholar]
- [5].Teresa A, Yunuen Q, David R, et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Medical Genetics 2011;12:60–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract 2010;88:29–33. [DOI] [PubMed] [Google Scholar]
- [7].Alissa EM, Al-Salmi MM, Alama NA, et al. Role of omentin-1 and C-reactive protein in obese subjects with subclinical inflammation. J Clin Transl Endocrinol 2016;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang D, Jiang TJ, Liao L, et al. Relationships between serum omentin-1 concentration and bone mineral density, and bone biochemical markers in Chinese women. Clin Chim Acta 2013;426:64–7. [DOI] [PubMed] [Google Scholar]
- [9].Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ. Cross-sectional age differences in body composition in persons 60 + years of age. J Gerontol A Biol Sci Med Sci 1995;50:M307–16. [DOI] [PubMed] [Google Scholar]
- [10].Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83:229–39. [DOI] [PubMed] [Google Scholar]
- [11].Hughes VA, Frontera WR, Roubenoff R, et al. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 2002;76:473–81. [DOI] [PubMed] [Google Scholar]
- [12].Waleh MQ. Impacts of physical activity on the obese. Prim Care 2016;43:97–107. [DOI] [PubMed] [Google Scholar]
- [13].Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc 2001;33:S521–7. S528-529. [DOI] [PubMed] [Google Scholar]
- [14].Hornbuckle LM, Bassett DR, Thompson DL. Pedometer-determined walking and body composition variables in African-American women. Med Sci Sports Exerc 2005;37:1069–74. [PubMed] [Google Scholar]
- [15].Duncan MJ, Minatto G, Wright SL. Dose-response between pedometer assessed physical activity, functional fitness, and fatness in healthy adults aged 50-80 years. Am J Hum Biol 2016;28:890–4. [DOI] [PubMed] [Google Scholar]
- [16].Ekelund U, Tingström P, Kamwendo K, et al. The validity of the computer science and applications activity monitor for use in coronary artery disease patients during level walking. Clin Physiol Funct Imaging 2002;22:248–53. [DOI] [PubMed] [Google Scholar]
- [17].Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol 2007;100:581–9. [DOI] [PubMed] [Google Scholar]
- [18].Rowlands AV, Mirkes EM, Yates T, et al. Accelerometer-assessed physical activity in epidemiology: are monitors equivalent. Med Sci Sports Exerc 2018;50:257–65. [DOI] [PubMed] [Google Scholar]
- [19].McKee G, Kearney PM, Kenny RA. The factors associated with self-reported physical activity in older adults living in the community. Age Ageing 2015;44:586–92. [DOI] [PubMed] [Google Scholar]
- [20].AminiLari Z, Fararouei M, Amanat S, et al. The effect of 12 weeks aerobic, resistance, and combined exercises on Omentin-1 levels and lnsulin resistance among Type 2 diabetic middle-aged women. Diabetes Metab J 2017;41:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wilms B, Ernst B, Gerig R, et al. Plasma omentin-1 levels are related to exercise performance in obese women and increase upon aerobic endurance training. Exp Clin Endocrinol Diabetes 2015;123:187–92. [DOI] [PubMed] [Google Scholar]
- [22].Saremi A, Asghari M, Ghorbani A. Effects of aerobic training on serum omentin-1 and cardiometabolic risk factors in overweight and obese men. J Sports Sci 2010;28:993–8. [DOI] [PubMed] [Google Scholar]
- [23].WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- [24].Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc 2005;37:S544–554. [DOI] [PubMed] [Google Scholar]
- [25].Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the national health and nutrition examination survey 2003–2006. Prev Chronic Dis 2012;9:E113.1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–81. [DOI] [PubMed] [Google Scholar]
- [27].García-Hermoso A, Martínez-Vizcaíno V, Recio-Rodriguez JI, et al. Abdominal obesity as a mediator of the influence of physical activity on insulin resistance in Spanish adults. Prev Med 2016;82:59–64. [DOI] [PubMed] [Google Scholar]
- [28].Müller MJ, Geisler C, Blundell J, et al. The case of GWAS of obesity: does body weight control play by the rules. Int J Obes (Lond) 2018;42:1395–405. [DOI] [PubMed] [Google Scholar]
- [29].de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007;56:1655–61. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Q, Zhu L, Zheng M, et al. Changes of serum omentin-1 levels in normal subjects, type 2 diabetes and type 2 diabetes with overweight and obesity in Chinese adults. Ann Endocrinol (Paris) 2014;75:171–5. [DOI] [PubMed] [Google Scholar]
- [31].Elmesallamy HO, Elderany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabetic medicine A J British Diabetic Assoc 2011;28:1194–200. [DOI] [PubMed] [Google Scholar]
- [32].Tan BK, Adya R, Farhatullah S, et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 2008;57:801–8. [DOI] [PubMed] [Google Scholar]
- [33].Yan P, Liu D, Long M, et al. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2011;119:257–63. [DOI] [PubMed] [Google Scholar]
- [34].Rashid S, Watanabe T, Sakaue T, et al. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem 2003;36:421–9. [DOI] [PubMed] [Google Scholar]
- [35].Harris TJ, Owen CG, Victor CR, et al. What factors are associated with physical activity in older people, assessed objectively by accelerometry. Br J Sports Med 2009;43:442–50. [DOI] [PubMed] [Google Scholar]
- [36].Jürimäe J, Kums T, Jürimäe T. Plasma adiponectin concentration is associated with the average accelerometer daily steps counts in healthy elderly females. Eur J Appl Physiol 2010;109:823–8. [DOI] [PubMed] [Google Scholar]
- [37].St-Pierre DH, Faraj M, Karelis AD, et al. Lifestyle behaviours and components of energy balance as independent predictors of ghrelin and adiponectin in young non-obese women. Diabetes Metab 2006;32:131–9. [DOI] [PubMed] [Google Scholar]
- [38].Sitticharoon C, Nway NC, Chatree S, et al. Interactions between adiponectin, visfatin, and omentin in subcutaneous and visceral adipose tissues and serum, and correlations with clinical and peripheral metabolic factors. Peptides 2014;62:164–75. [DOI] [PubMed] [Google Scholar]
- [39].Dekker JM, Funahashi T, Nijpels G, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab 2008;93:1489–96. [DOI] [PubMed] [Google Scholar]
- [40].Pruitt LA, Glynn NW, King AC, et al. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. J Aging Phys Act 2008;16:416–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chronic Dis 2012;9:E26. [PMC free article] [PubMed] [Google Scholar]
- [42].Foong YC, Aitken D, Winzenberg T, et al. The association between physical activity and reduced body fat lessens with age - results from a cross-sectional study in community-dwelling older adults. Exp Gerontol 2014;55:107–12. [DOI] [PubMed] [Google Scholar]
- [43].Abbott RA, Davies PS. Habitual physical activity and physical activity intensity: their relation to body composition in 5.0-10. 5-y-old children Eur J Clin Nutr 2004;58:285–91. [DOI] [PubMed] [Google Scholar]
- [44].Yoshioka M, Doucet E, St-Pierre S, et al. Impact of high-intensity exercise on energy expenditure, lipid oxidation and body fatness. Int J Obes Relat Metab Disord 2001;25:332–9. [DOI] [PubMed] [Google Scholar]
- [45].Knab AM, Shanely RA, Corbin KD, et al. A 45-minute vigorous exercise bout increases metabolic rate for 14 hours. Med Sci Sports Exerc 2011;43:1643–8. [DOI] [PubMed] [Google Scholar]
- [46].Chastin SF, Ferriolli E, Stephens NA, et al. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing 2012;41:111–4. [DOI] [PubMed] [Google Scholar]
- [47].Scheers T, Philippaerts R, Lefevre J. Objectively-determined intensity- and domain-specific physical activity and sedentary behavior in relation to percent body fat. Clin Nutr 2013;32:999–1006. [DOI] [PubMed] [Google Scholar]
- [48].Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc 2005;37:S512–522. [DOI] [PubMed] [Google Scholar]


