Abstract
Background:
Procalcitonin (PCT) was used for predicting the development of acute kidney injury (AKI) in several studies recently. We aimed to investigate the accuracy of PCT for predicting AKI in this study.
Methods:
Studies that assessed the predictive performance of PCT for the development of AKI in adult patients were searched from Medline, Embase, and the Cochrane Library from inception to June 2020. We calculated the pooled sensitivities and specificities and the area under the summary receiver-operating characteristic (SROC) curves. I2 was used to test the heterogeneity and the potential heterogeneity was investigated by meta-regression.
Results:
In total, 9 of 119 studies with 4852 patients were included, 1272 were diagnosed with AKI. In the overall analysis, the area under the SROC curve was 0.82 (95% CI, 0.79–0.85) and the pooled sensitivity and specificity were 0.76 (95% confidence interval [CI], 0.64–0.85) and 0.75 (95% CI, 0.61–0.86), respectively. In the subgroup analysis among septic patients, the pooled sensitivity and specificity were 0.59 (95% CI, 0.29–0.84) and 0.53 (95% CI, 0.31–0.74), and the area under the SROC was 0.57 (95% CI, 0.53–0.62).
Conclusion:
PCT may be a potential predictor for the development of AKI.
Keywords: acute kidney injury, meta-analysis, predictor, procalcitonin
1. Introduction
Acute kidney injury (AKI) is a common clinical syndrome that occurs in 10% to 15% of overall hospitalized patients.[1] It is associated with the increased risk for chronic kidney disease and high mortality,[2–4] and can lead to considerable health care cost for both patients and institutions.[5] Though medical technology has significantly improved in the past decades, treatments for AKI have been still disappointing. Renal replacement therapy is an effective supportive treatment for AKI. However, renal replacement therapy is expensive and not universally available in medical resource-limited areas.[6] Thus, early and rapid identification of patients with a high risk of the development of AKI is an important clinical practice to improve overall outcomes. Early identification of AKI could prompt clinicians to be more cautious in using interventions that are potentially nephrotoxic to avoid additional kidney damage as far as possible.
In most scenarios, AKI is a silent condition since it does not cause pain or any specific signs or symptoms in the early stages. Assessment of the biomarkers is useful to detect AKI early. Recently, some researchers have used procalcitonin (PCT) to predict the AKI and showed promising results.[7,8] However, until now, there has been no meta-analysis investigating the predictive accuracy of PCT for the development of AKI.
2. Methods
2.1. Search strategy and selection criteria
This study was registered on PROSPERO (CRD42020192037). We reported this meta-analysis according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement.[9] Ethical approval was not necessary since all analyses were based on previously published studies. We systematically searched 3 electronic databases (MEDLINE via Pubmed, Embase via Ovid, and Cochrane library) for clinical studies that evaluated the performance of PCT for predicting AKI from the inception until June 2020. The following terms were used to search for the relevant studies: “(“procalcitonin” OR “procalcitonin” OR “PCT”) AND (“acute kidney injury” OR “acute renal injury” OR “acute kidney failure” OR “AKI”)”.
We included the studies that evaluated PCT as a predictive biomarker for predicting the development of AKI and used a standard definition for AKI in accordance with the RIFLE criteria[10] or KDIGO guideline for AKI.[11] The RIFLE definition was established in 2004 and the KDIGO guideline was developed from the RIFLE criteria. The 2 criteria both defined AKI as an increase of the serum creatinine level within particular time, or decreased urine output for hours. Besides, the included studies had to provide sufficient information to calculate numbers of true-positive (TP), false-positive (FP), false-negative (FN), and true-negative (TN) results for constructing the 2 × 2 contingency table. We only included articles written in English or Chinese. Exclusion criteria were animal experiments, case reports, reviews, editorials, correspondences, and clinical guidelines.
Two investigators independently screened titles and abstracts of all studies according to the eligibility criteria. Relevant articles considered by any investigator were retrieved for full-text review. Data including characteristics of the publications (population, setting, etc.), cut-offs for PCT concentration, and numbers of true and false positives and negatives were extracted independently by the 2 investigators.
2.2. Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist was used to assess the methodological quality of the included studies,[12] as recommended by the Cochrane Collaboration.
2.3. Statistical analysis
Univariate analysis was performed to synthesize each summary statistic including sensitivity, specificity, and diagnostic odds ratio (DOR). Sensitivity and specificity are 2 widely used unidimensional measurements to estimate diagnostic accuracy, which are negatively correlative with each other in most cases. DOR, the ratio of the odds of positivity in disease relative to the odds of positivity in the non-diseased, is an indicator with two-dimensional nature. It is calculated as following: (TP/FN)/(FP/TN) or (sensitivity/(1- sensitivity))/(specificity/(1- specificity)).[13] Receiver-operating characteristics curve is another two-dimensional measurement. In meta-analysis, the summary receiver-operating characteristics (SROC) curve was drawn by using a bivariate model to pool the sensitivity and specificity.[14] An area under the SROC curve >0.9 was regarded as excellent accuracy, >0.8 was regarded as good, >0.7 was considered as acceptable.[15] Between-study statistical heterogeneity was quantified by using the Cochrane Q test to calculate the I2 (with a scale of 0%–100%).[16] In the Cochrane Q test, the P value lower than .1 indicated a presence of heterogeneity. If I2 was larger than 50%, a substantial between-study heterogeneity was considered, and a random-effect model would be used. Meta-regression was performed to identify the source of heterogeneity. Publication bias was estimated by using the Deeks test for funnel plot asymmetry.[17] All data were analyzed by using STATA 14.0 (Stata Corp, College Station, TX).
3. Results
3.1. Literature search
Figure 1 shows the detailed selection procedure. According to the search strategy, we found a total of 119 articles. One hundred and 4 articles were excluded after reviewing the titles and abstracts. We reviewed full texts of the rest 15 studies, and further excluded 6 studies. Finally, a total of 9 studies were identified for analysis.[7,8,18–24]
Figure 1.

Flowchart of study selection.
3.2. Study characteristics and quality assessment
Table 1 lists some characteristics of the included 9 studies. In total, 4852 patients were included in the analysis, of whom 1272 were diagnosed with AKI. Most studies (6 of 9) were conducted in ICU, 2 in wards, and 1 in emergency department. Most studies (7 of 9) were in Asia. All studies provided the area under receiver operating characteristics curve values, sensitivity, and specificity except 1. The prevalence of AKI varied from11.79% to 52.71% with the overall prevalence of 26.22%. In the ICU population, the prevalence of AKI was 36.92%. The cut-off values for PCT concentration ranged from 0.065 ng/ml to 10 ng/ml. All blood samples for the measurement of PCT were collected within 24 hours of admissions.
Table 1.
Characteristics of included studies.
| Author | Year | Country | Admission category | Setting | No. of patients | Cut-off ng/ml | AKI n (%) | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
| Rajeev Jeeha[8] | 2018 | South Africa | Multidisciplinary | ICU | 201 | 10.0 | 74 (36.82%) | 46 | 38 | 89 | 28 | 62.2% | 70.1% |
| Kayeong Chun[7] | 2019 | Korea | Critically ill | ICU | 790 | 0.315 | 266 (33.67%) | 162 | 226 | 298 | 104 | 60.9% | 56.9% |
| Zhou Xiao[17] | 2018 | China | Infection | ICU | 754 | 0.40 | 405 (53.71%) | 381 | 256 | 92 | 24 | 94.2% | 26.5% |
| Alparslan Kurtul[19] | 2015 | Turkey | Acute STEMI or NSTE-ACS | Ward | 814 | 0.065 | 96 (11.79%) | 69 | 215 | 503 | 27 | 72% | 70% |
| Hua-Lan Huang[20] | 2013 | China | Acute pancreatitis | ICU | 305 | 3.30 | 52 (17.05%) | 50 | 20 | 233 | 2 | 97.2% | 92.3% |
| Xin Nie[21] | 2013 | China | Infection | Ward | 1361 | 1.575 | 199 (14.62%) | 123 | 179 | 983 | 76 | 63.82% | 87.18% |
| Hua Liu[18] | 2019 | China | Cardiac surgery | ICU | 328 | 3.425 | 105 (32.01%) | 84 | 49 | 174 | 21 | 80% | 78% |
| Hee Su Park[22] | 2019 | Korea | Sepsis | ED | 85 | 2.210 | 19 (22.35%) | 12 | 14 | 52 | 7 | 62.1% | 78.9% |
| Ruoran Wang[23] | 2020 | China | Traumatic Brain Injury | ICU | 214 | 4.695 | 55 (25.70%) | 35 | 13 | 146 | 20 | 63.6% | 91.8% |
AKI = acute kidney injury, CI = confidence interval, ED = emergency department, FN = false negative, FP = false positive, ICU = intensive care unit, NSTE-ACS = non-ST-segment elevation acute coronary syndromes, STEMI = ST-segment elevation myocardial infarction, TN = true negative, TP = true positive.
The methodological quality of the included studies assessed by QUADAS-2 is summarized in Figure 2. None of the studies fulfilled all of the items, but most of the studies fulfilled at least 4 items. The main problem was no prespecified cut-off of PCT concentration for predicting AKI. Overall, the quality of the studies was acceptable. Deeks regression test of asymmetry and the funnel plots indicated no significant publication bias (t = 0.3, P = .77), as shown in Figure 3.
Figure 2.

Quality Assessment of Diagnostic Accuracy Studies criteria for the included studies.
Figure 3.

Deeks funnel plot of publication bias.
3.3. The diagnostic accuracy of procalcitonin for AKI
In the overall meta-analysis, the pooled sensitivity and specificity were 0.76 (95% confidence interval [CI], 0.64–0.85) and 0.75 (95% CI, 0.61–0.86), respectively (Fig. 4). The area under the SROC curve was 0.82 (95% CI, 0.79–0.85) (Fig. 5A). The pooled diagnostic odds ratio was 9.63 (95% CI, 4.38–21.18) (Fig. 6A). There was significant heterogeneity in the sensitivities and specificities among the studies. We performed meta-regression to identify the source of heterogeneity. The results are listed in Figure 7. Though the included studies had varied PCT concentration cut-offs, the cut-offs had no significant association with the heterogeneity in the regression analysis. We compared studies conducted on patients from Asia and patients from non-Asian countries, patients in the intensive care unit (ICU) and patients outside the ICU, and septic patients and patients not suffering from sepsis. We also analyzed age and gender. However, there were no significant results, which suggested that the heterogeneity could not be explained by meta-regression analysis.
Figure 4.

Forest plot of the sensitivity and specificity for studies using procalcitonin to predict AKI.
Figure 5.

Summary receiver operating characteristic curves (receiver operating characteristic curve is a two-dimensional indicator including information of sensitivity and specificity, larger of area under receiver operating characteristic curve means better diagnostic performance) and the corresponding 95% confidence contours and 95% prediction contours (A plot for all included studies, B plot for studies of septic patients).
Figure 6.

Forest plot of the diagnostic odds ratio (diagnostic odds ratio if the ratio of the odds of positivity in disease relative to the odds of positivity in the non-diseased, it can be calculated as following: (TP/FN)/(FP/TN)) for the use of PCT in predicting AKI (A plot for all included studies, B plot for studies of septic patients).
Figure 7.

Forest plot of meta regression (meta regression was performed to investigate the sources of heterogeneity).
Diagnostic accuracy for AKI using PCT among septic patients was lower than that in the overall population of the enrolled studies. The pooled sensitivity and specificity of PCT for predicting AKI in septic patients were 0.59 (95% CI, 0.29–0.84) and 0.53 (95% CI, 0.31–0.74) (Fig. 8), respectively. The DOR was 1.64 (95% CI, 0.51–5.26) (Fig. 6B). In the bivariate model, the area under the SROC was 0.57 (95% CI, 0.53–0.62) (Fig. 5B), suggesting poor performance of PCT for predicting AKI in septic patients.
Figure 8.
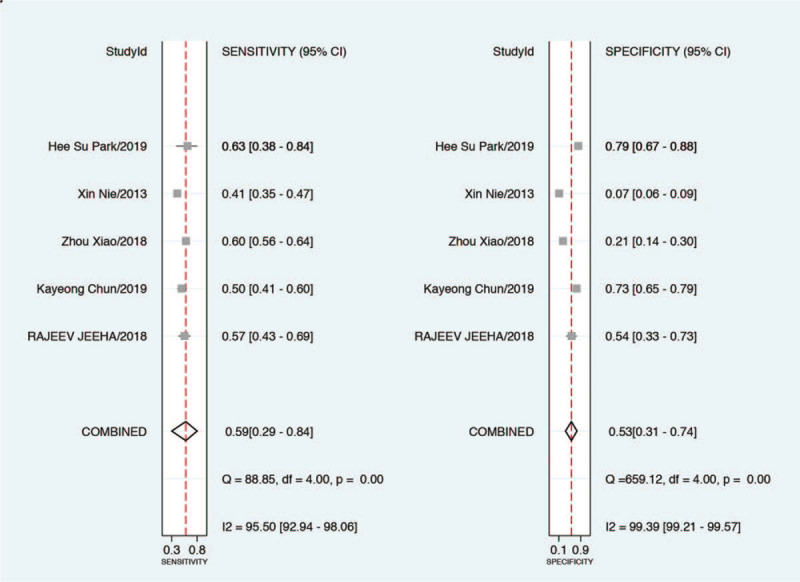
Forest plot of the sensitivity and specificity for studies using procalcitonin to predict AKI among patients with sepsis.
4. Discussion
In the current meta-analysis, we evaluated the diagnostic accuracy of PCT concentration for predicting AKI. We found that PCT had a good predictive value (sensitivity 0.76, specificity 0.75) for AKI with a pooled area under the SROC of 0.82, which suggested that PCT may be a potential biomarker for AKI. We also found that AKI was highly prevalent in the hospitalized patients. Especially in the ICU population, nearly one-third of patients were affected by AKI.
As a global health problem, many studies have suggested that AKI was highly prevalent in the hospitalized population. In a multicenter study with 528,108 patients, AKI occurred in 12.2% of hospitalized patients and the mortality of patients with AKI was higher than those without AKI.[25] A multicenter epidemiological study conducted in Southeast Asia intensive care units reported that the rate of AKI was 52.9% in critically ill patients.[26] Another multicenter study in Europe also reported a high prevalence of 57.3% in the ICU population.[27] Both the studies provided evidence that AKI severity was associated with mortality. The mortality rate of patients with stage 3 AKI appeared to be 3 to 7 times higher than those without AKI.[26,27] AKI not only increases hospital mortality, but also all-cause mortality in the year following hospital discharge.[4] In addition, AKI is an established risk factor for chronic kidney disease which can result in adverse long-term health outcomes and increased health-care cost.[4]
Because there is no effective treatment, early detection and prevention to avoid further damage are key in the management of AKI to improve the short- and long-term outcomes. Several risk prediction tools have been created to predict the development of AKI,[28,29] but most of these models were developed in specific settings and populations and lacked validation, which limit the spread of these tools to other populations and settings.[6] Serum creatinine as a classic measurement also lacked the ability to identify AKI early.[30] In the study, many patients with histological diagnosis of AKI failed to be clinically diagnosed with AKI because the rate of increase of serum creatinine was slower than the rate of increase required by the definition of AKI.
Procalcitonin, an inflammatory biomarker, may be a potential early biomarker of AKI. There is evidence that AKI was associated with activation of proinflammatory cytokines and chemokines in the patients with acute renal dysfunction.[31,32] Increased cytokines including interleukin (IL)-2, IL-6, tumor necrosis factor (TNF)-α, and interferon(IFN)-γ etc. were observed both in human and animal models with acute renal failure.[33,34] Moreover, a previous study revealed that PCT had direct cytotoxicity and induced apoptosis in mesangial cells through increasing IL-6 and TNF-α.[35] All the included studies provided conclusions that PCT was useful in predicting AKI except for the study conducted by Chun et al.[7] Nevertheless, Chun et al still found a significant association between PCT and AKI both in univariate and multivariable regression models. PCT has several advantages in predicting AKI. Firstly, it is hardly detected in healthy individuals, but it can rapidly rise in 6 hours and peak in 24 hours during inflammation.[36] In addition, PCT has been widely used as a diagnostic biomarker for sepsis in clinical practice and it is available in most institutions.[37,38]
It is notable that PCT is not specific for acute kidney injuries and can increase in other underlying conditions, especially sepsis.[39] Thus, PCT may be less accurate in predicting AKI in sepsis, which was supported by the results of the subgroup analysis of patients with sepsis in this study. The area under the SROC of PCT for predicting AKI in patients with sepsis was lower than 0.6, and the DOR was low as 1.64, which indicated poor diagnostic value. In one of the included studies,[8] Jeeha et al revealed that PCT was a significant predictor of AKI in the non-septic patients but not in septic patients. However, it is controversial on this issue. Some researchers hold that PCT had good predictive ability for AKI in septic patients.[40,41]
Interestingly, a previous meta-analysis provided the evidence that renal impairment could affect the diagnostic accuracy of PCT in diagnosing bacterial infection.[42] On one hand, an increase in PCT in renal-dysfunction patients with or without infection, was reported in previous study.[43] Furthermore, PCT levels could be further affected by renal dysfunction since PCT is partially removed via the kidney.[44]
Investigating the sources of heterogeneity among studies is an important work of meta-analysis to determine whether differences between studies explain the heterogeneity. In our study, we found wide heterogeneity among the included studies, but no potential sources of heterogeneity were explained by the meta-regression analysis. We supposed sepsis might be one of the sources of the heterogeneity since it can substantially affect the serum level of PCT. Generally, the illness severity of patients in ICUs is higher than that in wards, so whether the studies were conducted in ICUs or not may also contribute to the heterogeneity. Furthermore, the cut-offs with wide variation among the studies may be a potential contributor. In addition, we also analyzed other potential factors including variety in region, age, and gender that could contribute to heterogeneity. However, all the factors in meta-regression analysis failed to explain the heterogeneity. Multivariate analysis may be useful in detecting the source of heterogeneity, but the number of the included studies limited the use of multivariate regression model.
Our meta-analysis has several limitations. Firstly, substantial heterogeneity between the included studies could not be explained by subgroup analysis. Secondly, the included studies were mostly retrospective that may lead to inevitable bias. Moreover, we also found that the cut-off for PCT varied considerably from study to study and the best diagnostic threshold for PCT was unknown.
5. Conclusion
PCT may be a potential early biomarker of AKI, however, the result should be further validated.
Author contributions
YXF and HYH planned this study. CJ and ZHX searched literature, assessed studies, extracted data. YL and DL analyzed the data. YXF and HYH drafted the manuscript. DL contributed to its revision. All authors read the manuscript carefully and approved the final revision.
Conceptualization: Yunxia Feng, Haiyan He.
Data curation: Chao Jia, Zhihua Xu.
Formal analysis: Yuan Li.
Funding acquisition: Zhihua Xu, Dan Liao.
Investigation: Chao Jia, Zhihua Xu.
Methodology: Yunxia Feng, Yuan Li, Dan Liao.
Resources: Chao Jia, Zhihua Xu.
Writing – original draft: Yunxia Feng, Haiyan He.
Writing – review & editing: Dan Liao.
Footnotes
Abbreviations: AKI = acute kidney injury, CI = confidence interval, DOR = diagnostic odds ratio, FN = false negative, FP = false positive, ICU = intensive care unit, IFN = interferon, IL = interleukin, PCT = procalcitonin, SROC = summary receiver-operating characteristic curve, TN = true negative, TNF = tumor necrosis factor, TP = true positive, QUADAS = Quality Assessment of Diagnostic Accuracy Studies.
How to cite this article: Feng Y, He H, Jia C, Xu Z, Li Y, Liao D. Meta-analysis of procalcitonin as a predictor for acute kidney injury. Medicine. 2021;100:10(e24999).
YF and HH contributed equally to this work.
This project was supported by Research Projects of Health Commission of Mianyang City (Grants 201930 and 201938).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Al-Jaghbeer M, Dealmeida D, Bilderback A, et al. Clinical decision support for in-hospital AKI. J Am Soc Nephrol 2018;29:654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009;302:1179–85. [DOI] [PubMed] [Google Scholar]
- [4].See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 2019;95:160–72. [DOI] [PubMed] [Google Scholar]
- [5].Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet (London, England) 2019;394:1949–64. [DOI] [PubMed] [Google Scholar]
- [6].Vanmassenhove J, Kielstein J, Jorres A, et al. Management of patients at risk of acute kidney injury. Lancet (London, England) 2017;389:2139–51. [DOI] [PubMed] [Google Scholar]
- [7].Chun K, Chung W, Kim AJ, et al. Association between acute kidney injury and serum procalcitonin levels and their diagnostic usefulness in critically ill patients. Sci Rep 2019;9:4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jeeha R, Skinner DL, De Vasconcellos K, et al. Serum procalcitonin levels predict acute kidney injury in critically ill patients. Nephrology (Carlton, Vic) 2018;23:1090–5. [DOI] [PubMed] [Google Scholar]
- [9].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [10].Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–38. [Google Scholar]
- [12].Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129–35. [DOI] [PubMed] [Google Scholar]
- [14].Leeflang MMG, Deeks JJ, Gatsonis C, et al. Group obotCDTAW. Systematic reviews of diagnostic test accuracy. Ann Internal Med 2008;149:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murphy-Filkins R, Teres D, Lemeshow S, et al. Effect of changing patient mix on the performance of an intensive care unit severity-of-illness model: how to distinguish a general from a specialty intensive care unit. Crit Care Med 1996;24:1968–73. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- [18].Zhou X, Liu J, Ji X, et al. Predictive value of inflammatory markers for acute kidney injury in sepsis patients: analysis of 753 cases in 7 years. Zhonghua wei zhong bing ji jiu yi xue 2018;30:346–50. [DOI] [PubMed] [Google Scholar]
- [19].Liu H, Luo Z, Liu L, et al. Inflammatory biomarkers to predict adverse outcomes in postoperative patients with acute type A aortic dissection. Scandinavian Cardiovascular J 2020;54:37–46. [DOI] [PubMed] [Google Scholar]
- [20].Kurtul A, Murat SN, Yarlioglues M, et al. Procalcitonin as an early predictor of contrast-induced acute kidney injury in patients with acute coronary syndromes who underwent percutaneous coronary intervention. Angiology 2015;66:957–63. [DOI] [PubMed] [Google Scholar]
- [21].Huang HL, Nie X, Cai B, et al. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: a prospective study. PloS One 2013;8:e82250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nie X, Wu B, He Y, et al. Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection. Clin Chem Lab Med 2013;51:1655–61. [DOI] [PubMed] [Google Scholar]
- [23].Park HS, Kim JW, Lee KR, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury in sepsis patients in the emergency department. Clin Chim Acta 2019;495:552–5. [DOI] [PubMed] [Google Scholar]
- [24].Wang R, He M, Ou XF, et al. Serum procalcitonin level predicts acute kidney injury after traumatic brain injury. World Neurosurg 2020;141:e112-e7. [DOI] [PubMed] [Google Scholar]
- [25].Al-Jaghbeer M, Dealmeida D, Bilderback A, et al. Clinical decision support for in-hospital AKI. J Am Society Nephrol 2018;29:654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Srisawat N, Kulvichit W, Mahamitra N, et al. The epidemiology and characteristics of acute kidney injury in the Southeast Asia intensive care unit: a prospective multicentre study. Nephrol Dial Transplant 2020;35:1729–38. [DOI] [PubMed] [Google Scholar]
- [27].Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. [DOI] [PubMed] [Google Scholar]
- [28].Wilson T, Quan S, Cheema K, et al. Risk prediction models for acute kidney injury following major noncardiac surgery: systematic review. Nephrol Dial Transplant 2016;31:231–40. [DOI] [PubMed] [Google Scholar]
- [29].Koyner JL, Adhikari R, Edelson DP, et al. Development of a multicenter ward-based AKI prediction model. Clin J Am Soc Nephrol 2016;11:1935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chu R, Li C, Wang S, et al. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol 2014;9:1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chang CF, Lu TM, Yang WC, et al. Gene polymorphisms of interleukin-10 and tumor necrosis factor-alpha are associated with contrast-induced nephropathy. Am JNephrol 2013;37:110–7. [DOI] [PubMed] [Google Scholar]
- [32].Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 2002;110:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lemay S, Rabb H, Postler G, et al. Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation 2000;69:959–63. [DOI] [PubMed] [Google Scholar]
- [34].Takada M, Nadeau KC, Shaw GD, et al. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest 1997;99:2682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Araujo M, Doi SQ, Palant CE, et al. Procalcitonin induced cytotoxicity and apoptosis in mesangial cells: implications for septic renal injury. Inflammation Res 2013;62:887–94. [DOI] [PubMed] [Google Scholar]
- [36].Vijayan AL, Vanimaya, Ravindran S, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care 2017;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018;18:95–107. [DOI] [PubMed] [Google Scholar]
- [38].Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hatzistilianou M. Diagnostic and prognostic role of procalcitonin in infections. ScientificWorldJournal 2010;10:1941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shiao CC, Chueh YF, Yang L. Using procalcitonin to predict acute kidney injury in septic patients: caveat emptor? J Formos Med Assoc 2019;118:542–4. [DOI] [PubMed] [Google Scholar]
- [41].Heredia-Rodríguez M, Bustamante-Munguira J, Fierro I, et al. Procalcitonin cannot be used as a biomarker of infection in heart surgery patients with acute kidney injury. J Crit Care 2016;33:233–9. [DOI] [PubMed] [Google Scholar]
- [42].Lu XL, Xiao ZH, Yang MY, et al. Diagnostic value of serum procalcitonin in patients with chronic renal insufficiency: a systematic review and meta-analysis. Nephrol Dial Transplant 2013;28:122–9. [DOI] [PubMed] [Google Scholar]
- [43].Amour J, Birenbaum A, Langeron O, et al. Influence of renal dysfunction on the accuracy of procalcitonin for the diagnosis of postoperative infection after vascular surgery. Crit Care Med 2008;36:1147–54. [DOI] [PubMed] [Google Scholar]
- [44].Meisner M, Lohs T, Huettemann E, et al. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol 2001;18:79–87. [DOI] [PubMed] [Google Scholar]


