Abstract
Chronic obstructive pulmonary disease (COPD) is still a constant threat to people's health. We aimed to identify the relationship between increased red cell distribution width (RDW) on admission and length of hospitalization in acute exacerbation of chronic obstructive pulmonary disease patients (AECOPD).
Patients with AECOPD were recruited and divided into 3 groups based on RDW tertiles.
Two hundred eighty six patients with AECOPD admitted to our department during January 1, 2017 and June 30, 2019 were enrolled in the study. According to the RDW tertiles (≤12.8%, 12.9% to 13.6%, >13.6%), the patients were divided into 3 groups. Length of stay was significantly related to RDW (P < .001) in AECOPD patients. Correlation analysis indicated that RDW was negatively associated with FEV1% predicted (r = −0.142, P = .016). However, RDW was positively associated with prolonged of stay (r = 0.298, P < .001) in AECOPD patients. Multivariate regression analysis discovered that RDW was independently associated with the length of hospitalization (P = .001). Receiver operating characteristic (ROC) curve showed that RDW was a good predictor of prolonged hospital stay in AECOPD patients, and the area under the curve (AUC) was 0.818 (95% CI: 0.769–0.868). The highest sensitivity to predict prolonged hospital stay was 83.8% and the specificity was 71.6% with the cut-off 13.35%.
In conclusion, prolonged hospital stay in AECOPD patients was closely associated with increased RDW. Elevated RDW may be an independent predictor for prolonged hospitalization in AECOPD patients.
Keywords: biomarkers, chronic obstructive pulmonary diseases, length of stay, prognosis, red cell distribution width
1. Introduction
Chronic obstructive pulmonary disease (COPD), which is the third leading cause of death worldwide,[1] affects more than 174 million people globally.[2,3] COPD killed 3.2 million people in 2017, a toll expected to reach 4.4 million yearly by 2040.[1,4,5] COPD is a worldwide public health challenge due to its high prevalence and related disability and mortality.[6–9] Acute exacerbation of COPD (AECOPD), particularly leading to hospitalization, play the key role in mortality and worsening disease severity, and contribute to the economic burden of COPD.[10]
As part of regular complete blood cell count report, red cell distribution width (RDW) is a parameter reflecting the heterogeneity of red blood cell volume. The increase of RDW is used to diagnosis of anemia, hematopoietic abnormality or congenital erythrocyte abnormality. In previous studies, increased RDW is reported in a variety of diseases, including COPD, pulmonary hypertension,[11,12] cerebrovascular disease,[13–15] congestive heart failure (CHF),[16] and some other diseases.[17–19]
As for COPD, previous studies have revealed that increased RDW is a useful marker to estimate clinical outcomes including: predicting COPD severity,[20,21] independent predictor of mortality,[22–24] readmission rate,[25] right heart failure,[26,27] pulmonary hypertension,[26,28] increased BODE index,[20] and other negative prognostic markers. However, as one of the prognostic factors, studies of increased RDW and length of hospitalization in AECOPD patients have not been reported.
2. Material and methods
2.1. Study design
We conducted a retrospective study on AECOPD patients hospitalized in the department of respiratory medicine of the Liyuan hospital affiliated to Tongji Medical College, Huazhong University of Science and Technology, during January 1, 2017 and June 30, 2019. The study was conducted in accordance with the Declaration of Helsinki. The research protocol was approved by the Medical Institutional Review Board Approval of Liyuan hospital. Written informed consent was obtained from each patient.
2.2. Participants
We collected 286 patients admitted for AECOPD. The inclusion criteria were as follows: All participants were adults who had been diagnosed with COPD by a physician in accordance with 2020 GOLD guidelines, which is a postbronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio <0.7. Acute exacerbation was defined as shortness of breath, expectoration of sputum, color change of sputum, cough, wheezing, chest tightness etc., with at least 2 symptoms aggravating or reappearing. Exclusion criteria included participants with severe left heart failure, pulmonary embolism, immune diseases, renal failure, mental disorders, patients with any other infections whether acute or chronic and hospitalization days >30 days or <2 days.
As is known to all, there is no published definition of prolonged length of hospital stay, we defined a length of hospitalization longer than the 75th percentile as prolonged length of hospital stay here, which is consistent with other studies.[29,30]
2.3. Data Collection
We collected each patient's age, gender, smoking history, blood gas analysis, the length of hospitalization, as well as lung function (FEV1% predicted). And the laboratory results including C-reactive protein (CRP), creatinine, hemoglobin (HB), white blood cells (WBC), PLT, neutrophils, lymphocytes, eosinophils, and RDW were recorded. At the same time, we recorded a detailed history of hypertension, diabetes, CHF, atrial fibrillation and malignancy.
Complete blood count was measured by automated hematology analyzer Sysmex XN-1000. Lung function was measured by Ledoumit automatic blood gas analyzer (Danish, ABL 800). The Ray blood gas analyzer was used to blood gas analysis (made in Danish).
2.4. Statistical analysis
The software SPSS 26.0 was used for statistical analysis. Kolmogorov–Smirnov test was used to determine whether the variables are normally distributed. Participant characteristics were compared across the RDW categories using ANOVA and Chi-Squared test, independent samples Kruskal–Wallis test was used when variances are not uniform. Pearson correlation analysis was used for correlation between RDW and other continuity parameters. Univariate and multivariate logistic regression model were used to identify the variables correlated with length of hospital stay in AECOPD patients. The predictive value of RDW for length of stay in AECOPD patients was assessed by ROC curve. P values <.05 were considered statistically significant.
3. Results
286 patients were grouped according to the RDW tertiles (≤ 12.8%, 12.9%–13.6%, >13.6%), as follows:
-
1.
102 (35.6%) in the lowest tertile,
-
2.
92 (32.2%) in the intermediate tertile,
-
3.
92 (32.2%) in the highest tertile.
The basic characteristics of AECOPD patients were presented in Table 1.
Table 1.
Baseline characteristics of patients according to tertiles of baseline RDW level.
| RDW Tertiles | ||||
| Parameters | Lowest (n = 102) | Intermediate (n = 92) | Highest (n = 92) | P |
| Age, years | 72.42 ± 10.506 | 72.36 ± 10.15 | 77.11 ± 10.22 | .002 |
| Males | 80 (78.4%) | 74 (80.4%) | 73 (79.3%) | .942 |
| Smoking (no/ ever/yes) | 57/27/18 | 43/30/19 | 52/25/15 | .681 |
| WBC × 109/L | 7.67 ± 3.50 | 8.05 ± 4.02 | 8.39 ± 4.19 | .435 |
| HB g/L | 131.75 ± 17.55 | 127.13 ± 17.77 | 113.54 ± 21.53 | <.001 |
| PLT × 109/L | 213.37 ± 68.28 | 217.21 ± 74.14 | 204.15 ± 81.45 | .475 |
| Neutrophils × 109/L | 5.64 ± 3.47 | 5.90 ± 3.94 | 6.40 ± 4.03 | .369 |
| Lymphocytes × 109/L | 1.37 ± 0.67 | 1.40 ± 0.70 | 1.16 ± 0.63 | .029 |
| Eosinophils × 109/L | 0.1349 ± 0.1337 | 0.1784 ± 0.2223 | 0.1524 ± 0.1785 | .247 |
| CRP mg/l | 10.7 (2.65, 50.7) | 9.2 (2.93, 34.20) | 34.7 (5.13, 77.93) | .149 |
| Creatinine μmmol/l | 82.99 ± 40.54 | 74.26 ± 22.14 | 81.17 ± 42.24 | .219 |
| Diabetes | 15 (14.7%) | 19 (20.7%) | 22 (23.9%) | .259 |
| Hypertension | 45 (44.1%) | 50 (54.3%) | 44 (47.8%) | .357 |
| CHF | 5 (4.9%) | 14 (15.2%) | 30 (32.6%) | <.001 |
| Atrial fibrillation | 11 (10.8%) | 6 (6.5%) | 10 (10.9%) | .509 |
| Malignant tumor | 2 (2.0%) | 6 (6.5%) | 6 (6.5%) | .231 |
| FEV1% predicted | 64.40 ± 14.09 | 64.76 ± 10.68 | 59.34 ± 12.62 | .004 |
| PaCO2 mm Hg | 39.52 ± 10.63 | 40.57 ± 9.94 | 43.55 ± 12.52 | .147 |
| PaO2 mm Hg | 84.86 ± 17.27 | 87.16 ± 20.72 | 89.21 ± 29.08 | .413 |
| pH | 7.42 ± 0.06 | 7.42 ± 0.05 | 7.41 ± 0.06 | .878 |
| length of stay | 7.41 ± 2.03 | 8.89 ± 3.56 | 10.59 ± 3.47 | <.001 |
CHF = congestive heart failure, CRP = C-reactive protein, FEV1 = forced expiratory volume in one second, HB = hemoglobin, PaCO2 = partial pressure of carbon dioxide, PaO2 = partial pressure of oxygen, PLT = platelet, RDW = red blood cell distribution width, WBC = white blood cells.
The age of patients in the lowest, intermediate, and highest tertile group was 72.42 ± 10.506, 72.36 ± 10.15 and 77.11 ± 10.22, respectively. Mean FEV1% predicted ± SD were 64.40 ± 14.09, 64.76 ± 10.68 and 59.34 ± 12.62, respectively. As tertiles increased, patients’ lung function deteriorated (P = .004). Mean white blood cell counts ± SD were 7.67 ± 3.50, 8.05 ± 4.02 and 8.39 ± 4.19, respectively. While the eosinophils were 0.1349 ± 0.1337, 0.1784 ± 0.2223 and 0.1524 ± 0.1785, respectively. HB and the increase of RDW significant correlation (P < .001). Median CRP of the 3 study group were 10.7 (2.65, 50.7), 9.20 (2.93, 34.20), and 34.7 (5.13, 77.93), respectively. In 3 groups, the length of hospital stay was 7.41 ± 2.03, 8.89 ± 3.56 and 10.59 ± 3.47, with the increase of RDW the length of hospital stay was significant extended (P < .001).
In Pearson correlation analysis, we found that there was an obvious negative correlation between RDW and HB (r = −0.470, P < .001). Similarly, RDW was positively correlated with FEV1% predicted (r = −0.142, P = .016). Nevertheless, there was a positive correlation between RDW level and length of stay (r = 0.250∗∗, P < .001) (Table 2).
Table 2.
The associations between RDW and other clinical parameters were analyzed by Pearson correlation.
| r | P | |
| age | 0.079 | .198 |
| HB | −0.470 | <.001 |
| FEV1%predicted | −0.142 | .016 |
| Length of stay (>11days) | 0.250∗ | <.001 |
Correlation is significant at the 0.01 level (2-tailed).
FEV1 = forced expiratory volume in one second, HB = hemoglobin, PLT = platelet, r = Pearson correlation coefficient, RDW = red cell distribution width.
The 75th percentile of length of stay was 11 days in 286 patients, which divided the prolonged group from the normal group. In the univariate logistic regression analysis, several factors were associated with longer hospital stay, including: age, FEV1% predicted, WBC, RDW, neutrophils, HB, acidosis (pH < 7.35), hypercapnia (PaCO2 >45 mm Hg), diabetes, and CHF (Table 3). Then, we reintegrated these factors into multivariate logistic regression analysis to determine the independent variables. Increased RDW (P = .001) and CHF (P = .030) were independent predictor for prolonged hospital stay in patients with AECOPD (Table 4).
Table 3.
Univariate regression analyses of the risk factors associated with length of stay in patients with an acute exacerbation of chronic obstructive pulmonary disease.
| Variable | Odds ratio | 95%CI | P value |
| Age | 1.005 | 1.024–1.087 | <.001 |
| FEV1% predicted | 0.971 | 0.949–0.994 | .013 |
| RDW | 1.560 | 1.266–1.923 | <.001 |
| CRP | 0.998 | 0.993–1.003 | .498 |
| Acidosis (pH < 7.35) | 2.807 | 1.060–7.431 | .038 |
| Hypercapnia (PaCO2 >45 mm Hg) | 1.648 | 0.888–3.056 | .113 |
| Hypoxemia (PaO2 <80 mm Hg) | 1.054 | 0.606–1.834 | .852 |
| Diabetes | 1.705 | 0.897–3.242 | .103 |
| Hypertension | 1.256 | 0.728–2.167 | .413 |
| CHF | 3.383 | 1.769–6.471 | <.001 |
| Atrial fibrillation | 0.908 | 0.351–2.350 | .842 |
| Malignant tumor | 0.520 | 0.114–2.384 | .400 |
| WBC | 1.054 | 0.987–1.125 | .117 |
| HB | 0.971 | 0.957–0.985 | <.001 |
| PLT | 1.001 | 0.997–1.004 | .682 |
| Neutrophils | 1.060 | 0.992–1.133 | .083 |
| Lymphocytes | 0.811 | 0.535–1.230 | .324 |
| Eosinophils | 1.890 | 0.459–7.784 | .378 |
CHF = congestive heart failure, CI = confidence interval, CRP = C-reactive protein, FEV1 = forced expiratory volume in one second, HB = hemoglobin, OR = odds ratio, PaCO2 = partial pressure of carbon dioxide, PLT = platelet, RDW = red blood cell distribution width, WBC = white blood cells.
Table 4.
Multivariate logistic regression analyses of the predictor associated with length of stay in patients with an acute exacerbation of chronic obstructive pulmonary disease.
| Variable | Odds ratio | 95%CI | P value |
| Age | 1.038 | 1.004–1.072 | .028 |
| RDW | 1.400 | 1.150–1.703 | .001 |
| WBC | 0.890 | 0.604–1.311 | .555 |
| Neutrophils | 1.207 | 0.807–1.804 | .555 |
| Eosinophils | 3.589 | 0.603–21.355 | .160 |
| FEV1% predicted | 0.985 | 0.958–1.012 | .281 |
| Acidosis (pH <7.35) | 1.482 | 0.435–5.053 | .529 |
| Hypercapnia (PaCO2 >45 mm Hg) | 1.255 | 0.576–2.737 | .567 |
| Diabetes | 1.588 | 0.774–3.261 | .207 |
| CHF | 2.236 | 1.083–4.615 | .030 |
CHF = congestive heart failure, CI = confidence interval, FEV1 = forced expiratory volume in one second, OR = odds ratio, PaCO2 = partial pressure of carbon dioxide, RDW = red blood cell distribution width, WBC = white blood cells.
ROC curve (Fig. 1) indicated that RDW was a good predictor of prolonged hospital stay in AECOPD patients, and the area under the curve (AUC) was 0.818 (95% CI: 0.769–0.868). The highest sensitivity to predict prolonged hospital stay was 83.8% and the specificity was 71.6% with the cut-off 13.35%.
Figure 1.
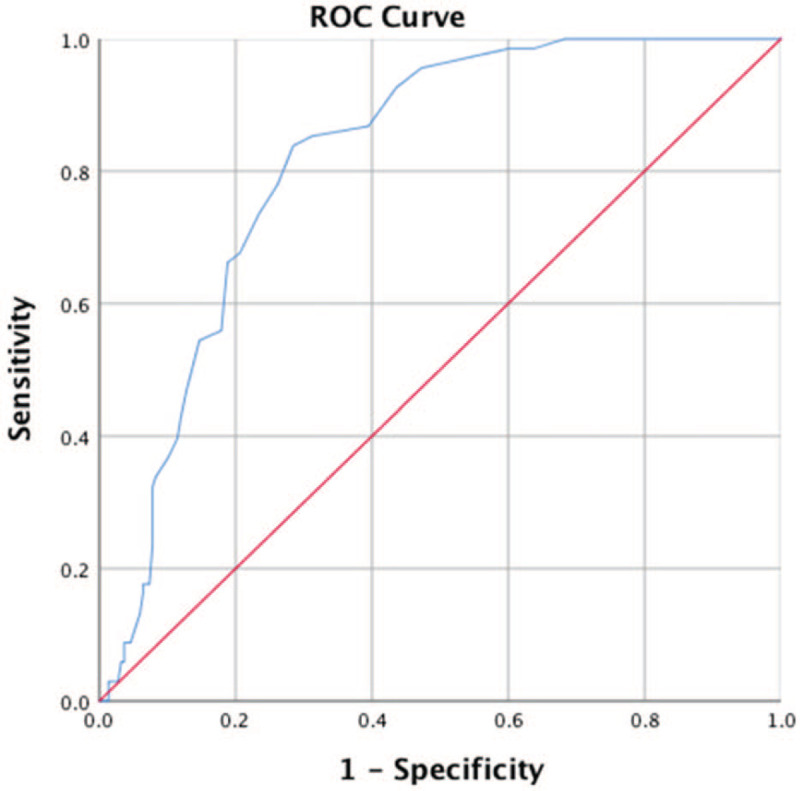
ROC curve indicated that RDW was a good predictor of prolonged hospital stay in AECOPD patients, the area under the curve (AUC) was 0.818 (95% CI: 0.769–0.868). The highest sensitivity to predict prolonged hospital stay was 83.8% and the specificity was 71.6% with the cut-off 13.35%.
4. Discussion
To our knowledge, this is the first study to report the association of increased RDW with the length of hospitalization in AECOPD. Length of hospitalization is also an important part about prognostic models for outcome prediction in COPD patients.[31] The previous study consider that patients with a prolonged length of stay had more prior admissions for AECOPD, a terrible modified Medical Research Council dyspnea scores, a higher rates of long-term home oxygen therapy, and a higher prevalence of heart failure.[32] Thus, early evaluation of whether COPD patients, hospitalized for acute exacerbations, need long-term hospitalization can effectively reduce the incidence of adverse events and the costs of treatment.[29]
RDW is a parameter that mirrors the heterogeneity of peripheral red blood cells measured, and it is an objective indicator that reveals the size difference of red blood cells.[33] Traditionally, the increase of RDW indicates anemia, hematopoietic abnormality, or congenital erythrocyte abnormality.[33] Based on recent researches, elevated RDW is connected with a variety of diseases, including cardiovascular diseases, cerebrovascular diseases, pulmonary embolism, cancer, diabetes mellitus, acute kidney failure, and other diseases. In addition, RDW is considered to be a powerful and independent dangerous factor in predicting mortality.[34] However, the possible pathophysiological mechanisms between elevated RDW and various diseases is still unclear according to the published research.[25] Most researchers believe that an elevated RDW reflects a serious imbalance of homeostasis in the red blood cell caused by impaired erythropoiesis and abnormal erythrocyte survival, due to all kinds of metabolic disturbance including shortening of telomere length, oxidative stress, inflammation, hypoproteinemia, dyslipidemia, hypertension, erythrocyte fragmentation, and changes in the function of erythropoietin.[34]
In the current study, RDW increased with age in patients with AECOPD. This was consistent with the results of a cohort study of 8089 individuals that RDW increases with age.[35] Length of hospitalization increased in the higher RDW group compared to the other groups. Our study demonstrated that elevated RDW may be an independent predictor for prolonged hospital stay. Moreover, the association between RDW and FEV1% predicted suggested that increased RDW may serve as a reference for determining the severity of disease in AECOPD patients. The result was consistent with the Tertemiz KC team's findings that the increased RDW could be used to determine the severity of the disease in patients with chronic obstructive pulmonary disease.[20] In univariate regression analysis, FEV1 was associated with LOH (P = .013), but this correlation disappeared in multivariate regression analysis (P = .281). Previous studies show that FEV1 is associated with length of hospitalization and mortality in AECOPD patients. [36,37] However, like our results, there are some other studies indicate that FEV1 is not related to length of hospitalization in AECOPD patients. [32,38,39] The discrepancy of results may be due to the limited sample capacity, and FEV1 was analyzed as a categorical variable in previous studies, whereas FEV1 was analyzed as a continuous variable in our study. Therefore, further studies are needed to confirm the relationship between FEV1 and length of hospitalization in AECOPD.
Though elevated RDW is related to prognosis in a variety of diseases, the pathophysiological mechanism of which has not been fully understood. About the association between elevated RDW and length of hospitalization, we try to provide some possible explanations.
Based on previous studies, the length of AECOPD patient's hospital stay is determined by a number of factors including age, disease severity, comorbidities, hypercapnia (PaCO2 >45 mm Hg), mechanical ventilation, hypoproteinemia, the dyspnea perception and the respiratory rate.[29,32] Previous studies have indicated that increased RDW is correlated with COPD severity,[20,21] mortality,[22–24] 60-day readmission rate,[25] combined with right heart failure,[26,27] predicting pulmonary hypertension,[26,28] and the need of mechanical ventilation[40] in COPD patients. Complications, readmitted rates, hypercapnia, severity of disease or mechanical ventilation, all lead to prolonged hospital stays in patients with COPD. The elaboration of the relationships between RDW and inflammation, oxidative stress, tissue hypoxia, disease severity, and heart failure may help understand why this marker was related to length of stay in patients with AECOPD.
This study includes some limitations. First, RDW was affected by a number of factors that we did not assess, including iron, folic and vitamin B12. Second, interventions for all AECOPD were not identical, which may lead to intervention bias. Finally, this was a single-center retrospective study with limited sample capacity.
Collectively, we indicated that elevated RDW may be an important predictor correlated with increased length of stay in AECOPD patients. As an item in the regular report of the CBC counts, although this laboratory parameter is widely available and low-cost, it has provided valuable information on the prognosis of AECOPD patients. RDW can predict the risk of adverse events and hospitalization expenses and provide high-quality discharge decisions. These findings may guide further wholesale prospective studies aimed at assessing the associated between RDW and the length of stay in AECOPD patients. And this will further enrich the prognostic indicators of clinical AECOPD patients.
5. Conclusions
Prolonged hospital stay was closely associated with increased RDW on admission in AECOPD patients. Elevated RDW may be an independent predictor for prolonged hospital stay in AECOPD patients.
Acknowledgments
The statistics guidance from Professor Ping Yin, Huazhong University of science and technology, is highly appreciated.
Author contributions
Conceptualization: Peng Hongxing, Yulan Zeng.
Data curation: Zhu Mengpei, Lei Wan.
Formal analysis: Zhang Shuling.
Visualization: Zhu Mengpei.
Writing – original draft: Zhu Mengpei.
Writing – review & editing: Zhu Mengpei, Peng Hongxing, Lei Wan, Zhang Shuling.
Footnotes
Abbreviations: AECOPD = acute exacerbation of chronic obstructive pulmonary disease, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, FEV1 = forced expiratory volume in one second, HB = hemoglobin, PaCO2 = partial pressure of carbon dioxide, PaO2 = partial pressure of oxygen, PLT = platelet, RDW = red blood cell distribution width, WBC = white blood cells.
How to cite this article: Zhu M, Peng H, Wan L, Zhang S, Zeng Y. The role of elevated red blood cell distribution width in the prognosis of AECOPD patients: a retrospective study. Medicine. 2021;100:10(e25010).
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med 2019;381:1257–66. [DOI] [PubMed] [Google Scholar]
- [2].Finks SW, Rumbak MJ, Self TH. Treating hypertension in chronic obstructive pulmonary disease. N Engl J Med 2020;382:353–63. [DOI] [PubMed] [Google Scholar]
- [3].Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the global burden of disease study, 2015. Lancet Respir, Med 2017;5:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017;389:1931–40. [DOI] [PubMed] [Google Scholar]
- [5].Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706–17. [DOI] [PubMed] [Google Scholar]
- [7].Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015;5:020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang HD, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dransfield MT, Voelker H, Bhatt SP, et al. Metoprolol for the prevention of acute exacerbations of COPD. N Engl J Med 2019;381:2304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hampole CV, Mehrotra AK, Thenappan T, et al. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol 2009;104:868–72. [DOI] [PubMed] [Google Scholar]
- [12].Smukowska-Gorynia A, Tomaszewska I, Malaczynska-Rajpold K, et al. Red blood cells distribution width as a potential prognostic biomarker in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Heart Lung Circ 2018;27:842–8. [DOI] [PubMed] [Google Scholar]
- [13].Poz D, De Falco E, Pisano C, et al. Diagnostic and prognostic relevance of red blood cell distribution width for vascular aging and cardiovascular diseases. Rejuvenation Res 2019;22:146–62. [DOI] [PubMed] [Google Scholar]
- [14].Wu T-T, Zheng Y-Y, Hou X-G, et al. Red blood cell distribution width as long-term prognostic markers in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis 2019;18:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hou H, Sun T, Li C, et al. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci Rep 2017;7:43420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roderick KV, Abelson AL, Nielsen L, et al. Evaluation of red blood cell distribution width as a prognostic indicator in cats with acquired heart disease, with and without congestive heart failure. J Feline Med Surg 2017;19:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Turcato G, Cappellari M, Follador L, et al. Red blood cell distribution width is an independent predictor of outcome in patients undergoing thrombolysis for ischemic stroke. Semin Thromb Hemost 2017;43:30–5. [DOI] [PubMed] [Google Scholar]
- [18].Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017;23:4879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang X, Wu Q, Hu T, et al. Elevated red blood cell distribution width contributes to poor prognosis in patients undergoing resection for nonmetastatic rectal cancer. Medicine (Baltimore) 2018;97:e9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tertemiz KC, Ozgen Alpaydin A, Sevinc C, et al. Could “red cell distribution width” predict COPD severity? Rev Port Pneumol (2006) 2016;22:196–201. [DOI] [PubMed] [Google Scholar]
- [21].Kalemci S, Akin F, Sarihan A, et al. The relationship between hematological parameters and the severity level of chronic obstructive lung disease. Polish Arch Intern Med 2018;128:171–7. [DOI] [PubMed] [Google Scholar]
- [22].Hu GP, Zhou YM, Wu ZL, et al. Red blood cell distribution width is an independent predictor of mortality for an acute exacerbation of COPD. Int J Tuberc Lung Dis 2019;23:817–23. [DOI] [PubMed] [Google Scholar]
- [23].Rahimirad S, Ghafari M, Ansarin K, et al. Elevated red blood cell distribution width predicts mortality in acute exacerbation of COPD. Pneumologia 2016;65:85–9. [PubMed] [Google Scholar]
- [24].Seyhan EC, Özgül MA, Tutar N, et al. Red blood cell distribution and survival in patients with chronic obstructive pulmonary disease. COPD 2013;10:416–24. [DOI] [PubMed] [Google Scholar]
- [25].Epstein D, Nasser R, Mashiach T, et al. Increased red cell distribution width: a novel predictor of adverse outcome in patients hospitalized due to acute exacerbation of chronic obstructive pulmonary disease. Respir Med 2018;136:1–7. [DOI] [PubMed] [Google Scholar]
- [26].Ozgul G, Seyhan EC, Özgül MA, et al. Red blood cell distribution width in patients with chronic obstructive pulmonary disease and healthy subjects. Arch Bronconeumol 2017;53:107–13. [DOI] [PubMed] [Google Scholar]
- [27].Sincer I, Zorlu A, Yilmaz MB, et al. Relationship between red cell distribution width and right ventricular dysfunction in patients with chronic obstructive pulmonary disease. Heart Lung 2012;41:238–43. [DOI] [PubMed] [Google Scholar]
- [28].Yang J, Liu C, Li L, et al. Red blood cell distribution width predicts pulmonary hypertension secondary to chronic obstructive pulmonary disease. Can Respir J 2019;2019:3853454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang Y, Stavem K, Dahl FA, et al. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int J Chron Obstruct Pulmon Dis 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Quintana JM, Unzurrunzaga A, Garcia-Gutierrez S, et al. Predictors of hospital length of stay in patients with exacerbations of COPD: a cohort study. J Gen Intern Med 2015;30:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bellou V, Belbasis L, Konstantinidis AK, et al. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. BMJ 2019;367:l5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Crisafulli E, Ielpo A, Barbeta E, et al. Clinical variables predicting the risk of a hospital stay for longer than 7days in patients with severe acute exacerbations of chronic obstructive pulmonary disease: a prospective study. Respir Res 2018;19:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med 1991;9: Suppl 1: 71–4. [DOI] [PubMed] [Google Scholar]
- [34].Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86–105. [DOI] [PubMed] [Google Scholar]
- [35].Hoffmann JJML, Nabbe KCAM, van den Broek NMA. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med 2015;53:2015–9. [DOI] [PubMed] [Google Scholar]
- [36].Diamantea F, Kostikas K, Bartziokas K, et al. Prediction of hospitalization stay in COPD exacerbations: the AECOPD-F score. Respir Care 2014;59:1679–86. [DOI] [PubMed] [Google Scholar]
- [37].Sprooten RTM, Rohde GGU, Lawyer G, et al. Risk stratification for short-term mortality at hospital admission for acute exacerbations of COPD. Respirology 2019;24:765–76. [DOI] [PubMed] [Google Scholar]
- [38].Ko FWS, Chan KP, Ngai J, et al. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology 2020;25:259–66. [DOI] [PubMed] [Google Scholar]
- [39].Gulati S, Zouk AN, Kalehoff JP, et al. The use of a standardized order set reduces systemic corticosteroid dose and length of stay for individuals hospitalized with acute exacerbations of COPD: a cohort study. Int J Chron Obstruct Pulmon Dis 2018;13:2271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Karampitsakos T, Dimakou K, Papaioannou O, et al. The role of increased red cell distribution width as a negative prognostic marker in patients with COPD. Pulm Pharmacol Ther 2020;60:101877. [DOI] [PubMed] [Google Scholar]


