Abstract
Major adverse cardiac and cerebral events (MACCE) are common complications, which prolong hospitalization and increase mortality rate in end-stage renal disease (ESRD) patients who underwent continuous ambulatory peritoneal dialysis (CAPD). Therefore, this study aimed to investigate MACCE occurrence and its potential predictive factors in those patients.
In this prospective cohort study, 196 diagnosis of ESRD patients who underwent CAPD treatment in our hospital were eligible, and their clinical data (including demographic data and biochemical indexes) were documented. Besides, their MACCE occurrence was assessed within 3-year follow-up period.
In patients, 1-, 2-, and 3-year MACCE occurrence rates were 5.1%, 11.7%, and 14.8%, respectively. Meanwhile, the mean duration of accumulating MACCE occurrence was 33.1 (95% confidence interval: 32.0–34.2) months. Furthermore, age, peritoneal dialysis duration (PDD), C-reactive protein (CRP), fasting blood glucose (FBG) and total cholesterol high correlated with increased accumulating MACCE occurrence, while high-density lipoprotein cholesterol (HDL-C) high correlated with decreased accumulating MACCE occurrence. Notably, by further multivariate Cox's proportional hazard regression analysis, age, PDD, CRP, serum uric acid, and FBG high were independent predictive factors for raised accumulating MACCE occurrence, while HDL-C high was an independent predictive factor for attenuated accumulating MACCE occurrence.
MACCE are common; besides, age, peritoneal dialysis duration, C-reactive protein, serum uric acid, fasting blood glucose, and high-density lipoprotein cholesterol serve as potential markers for indicating MACCE in ESRD patients who underwent CAPD.
Keywords: clinical features, continuous ambulatory peritoneal dialysis, end-stage renal disease, major adverse cardiac and cerebrovascular events, predictive factors
1. Introduction
End-stage renal disease (ESRD), the end stage of chronic kidney disease, is characterized by the irreversible decline in renal function with a glomerular filtration rate <15 mL/min 1.73 m2 body surface area.[1] ESRD is lethal if without supportive treatments such as hemodialysis, peritoneal dialysis, and kidney transplantation.[2] Among these treatments, kidney transplantation is the gold standard for treating ESRD, while it is limited by the availability of organ donors, appropriately skilled/trained surgeons, and financial difficulties.[3] Instead, the most patients receive maintenance dialysis therapy to prolong survival in the clinical setting.[3] Continuous ambulatory peritoneal dialysis (CAPD), the convenient and cost-effective method of dialysis, utilizes the semipermeable peritoneum as a biological dialysis membrane to exchange the electrolytes, glucose, urea, albumin, and other small molecules from the blood, which assist physiological renal function.[3,4] However, ESRD patients treated with CAPD are at high risks of experiencing additional complications such as major adverse cardiac and cerebral events (MACCE).[5,6] In ESRD patients treated with CAPD, MACCE are frequently attributed by the disruption of the equilibrium between pro-coagulation and anticoagulation activities, as well as the subsequent thrombosis, which further prolongs the hospitalization and increases the mortality rate.[7–9] Therefore, it is of clinical significance for exploring the predictive factors for MACCE in order to optimize the protection strategies against MACCE and improve prognosis in ESRD patients who underwent CAPD.
Various clinical characteristics have been unraveled with the potential as predictive factors for cardiovascular events in ESRD patients who underwent dialysis.[10–13] For instance, several demographic characteristics (e.g., age) and comorbid disease history (e.g., diabetes mellitus) are reported to predict the risk of cardiovascular events in ESRD patients who underwent dialysis.[12] In addition, several biochemical parameters (e.g., cardiac troponin T) are potential predictors for the occurrence of cardiovascular events in ESRD patient on dialysis as well.[13] While limited data is available regarding the comprehensive analysis of predictor factors for MACCE risk in ESRD patients who underwent CAPD treatment. Therefore, in the present study, we followed up 196 CAPD treated ESRD patients for 36 months, and the objective was to explore MACCE occurrence as well as its potential predictive factors in these patients, aiming to help with clinical management of MACCE and reduction of mortality rate.
2. Materials and methods
2.1. Patients
Between January 2014 and December 2016, 196 ESRD patients underwent CAPD in our hospital were consecutively recruited in this prospective cohort study. Patients were eligible for enrollment if they
-
1.
had confirmed diagnosis of ESRD,
-
2.
ambulatory peritoneal dialysis for at least 3 years, which was categorized as CAPD;
-
3.
aged more than 18 years,
-
4.
had no history of kidney cancer, kidney transplantation, or kidney surgery,
-
5.
were voluntary to participate the present study and comply with follow-up protocol.
While patients were excluded if they had malignancies, or had history of coronary artery bypass graft, percutaneous coronary intervention as well as other surgeries for valvular, aortic and peripheral arterial occlusive disease. This study was approved by the Ethics Committee of Zhongshan Hospital Xiamen University with approved number of ethic committee of M20130067, and all patients provided the written informed consents.
2.2. Flow chart
Initially, 220 ESRD patients who underwent CAPD treatment in our hospital were consecutively invited, among which 24 patients were excluded (including 16 patients who did not meet inclusion criteria or met inclusion criteria, and 8 patients who disagreed to sign informed consents) (Fig. 1). Then, the clinical data of 196 eligible patients was documented, and these eligible patients were followed up to MACCE occurrence or 36 months, among which 16 (8.2%) patients lost follow-up and 180 (91.8%) patients completed the study follow-up. For patients who lost follow-up, they were censored on the date of last visit. Finally, a total of 196 patients were included in the final analysis.
Figure 1.
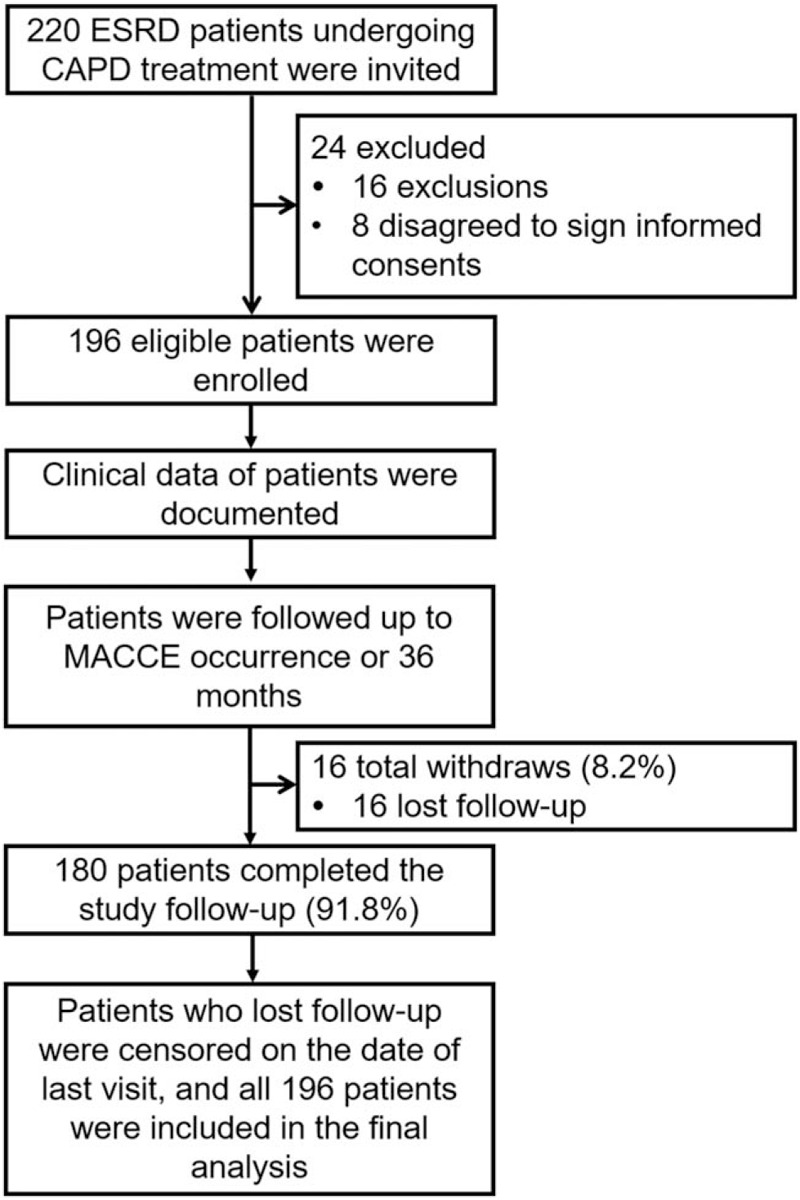
Flow chart. CAPD = continuous ambulatory peritoneal dialysis, ESRD = end-stage renal disease, MACCE = major adverse cardiac and cerebrovascular events.
2.3. Clinical data collection
Clinical data of patients at baseline were documented in Case Report Form (CRF). It covered age, gender, body mass index (BMI), current smoking status, current drinking status, peritoneal dialysis duration (PDD), Kt/V (which was calculated as clearance [K] multiplied by treatment time [t] and divided by the urea distribution volume [V]), hemoglobin (HB), white blood cell (WBC), platelet (PLT), C-reactive protein (CRP), serum creatinine (Scr), serum uric acid (SUA), calcium (Ca), phosphorus, fasting blood glucose (FBG), albumin (ALB), systolic blood pressure (SBP), diastolic blood pressure (DBP), triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
2.4. Follow-up and assessment
The follow-up for patients was performed every 1 to 3 months by clinic evaluations, or direct telephone contact. The study endpoint was the occurrence of MACCE, or the completion of 36-month follow-up. The MACCE was defined as a composite of death, acute coronary syndrome, stable angina pectoris requiring target vessel revascularization (TVR), transient ischemic attack (TIA), ischemic stroke, or hospitalization caused by cardiovascular disease or cerebrovascular disease.[14]
2.5. Statistics analysis
Statistical analysis was performed using SPSS 24.0 software (IBM, USA) and GraphPad Prism 7.01 software (GraphPad Software Inc, USA). All patients were included in the analysis, and the patients lost to follow-up were censored on the date of last visit. Continuous data were expressed as mean and standard deviation (SD), or median and interquartile range (IQR) according to characteristics of data distribution. Categorical data were expressed as number and percentage. Accumulating MACCE occurrence was displayed by Kaplan-Meier (K-M) curve, which was compared between groups by log-rank test. Factors related to accumulating MACCE occurrence were assessed by univariate and multivariate Cox's proportional hazards regression model analysis. P value < .05 was considered as statistically significant.
3. Results
3.1. Characteristics
The mean age of ESRD patients who underwent CAPD was 56.1 ± 11.4, and there were 56 (28.6%) females/140 (71.4%) males (Table 1). The mean BMI of patients was 22.5 ± 2.9 kg/m2. Furthermore, 42 (21.4%) and 16 (8.2%) patients were with current smoking and current drinking. Additionally, the median PDD of patients was 62.0 (49.3–79.0) months; the mean Kt/V was 1.9 ± 0.4. The detailed information regarding biochemical indexes was shown in Table 1.
Table 1.
Clinical characteristics of ESRD patients underwent CAPD.
| Items | Patients (N = 196) |
| Age (years), mean ± SD | 56.1 ± 11.4 |
| Gender, No. (%) | |
| Female | 56 (28.6) |
| Male | 140 (71.4) |
| BMI (kg/m2), mean ± SD | 22.5 ± 2.9 |
| Current smoking, No. (%) | 42 (21.4) |
| Current drinking, No. (%) | 16 (8.2) |
| PDD (months), median (IQR) | 62.0 (49.3–79.0) |
| Kt/V, mean ± SD | 1.9 ± 0.4 |
| HB (g/L), mean ± SD | 101.8 ± 15.0 |
| WBC (×109/L), mean ± SD | 8.1 ± 2.3 |
| PLT (×109/L), mean ± SD | 204.9 ± 53.8 |
| CRP (mg/L), median (IQR) | 4.7 (2.9–7.7) |
| Scr (μmol/L), median (IQR) | 900.4 (770.2–1103.7) |
| SUA (μmol/L), median (IQR) | 411.4 (356.0–462.8) |
| Ca (mmol/L), median (IQR) | 2.2 (2.0–2.4) |
| Phosphorus (mmol/L), mean ± SD | 1.6 ± 0.4 |
| FBG (mmol/L), median (IQR) | 5.7 (4.5–6.9) |
| ALB (g/L), mean ± SD | 38.5 ± 6.2 |
| SBP (mm Hg), median (IQR) | 139.0 (128.0–152.0) |
| DBP (mm Hg), median (IQR) | 88.0 (82.0–93.0) |
| TG (mmol/L), median (IQR) | 1.5 (1.0–2.4) |
| TC (mmol/L), median (IQR) | 4.6 (3.9–5.5) |
| LDL-C (mmol/L), mean ± SD | 2.7 ± 0.7 |
| HDL-C (mmol/L), median (IQR) | 1.0 (0.8–1.2) |
ALB = albumin, BMI = body mass index, Ca = calcium, CAPD = continuous ambulatory peritoneal dialysis, CRP = C-reactive protein, DBP = diastolic blood pressure, ESRD = end-stage renal disease, FBG = fasting blood glucose, HB = hemoglobin, HDL-C = high density lipoprotein cholesterol, IQR = interquartile range, Kt/V = calculated as clearance (K) multiplied by treatment time (t) and divided by the urea distribution volume (V), LDL-C = low density lipoprotein cholesterol, PDD = peritoneal dialysis duration, PLT = platelet, SBP = systolic pressure, Scr = serum creatinine, SD = standard deviation, SUA = serum uric acid, TC = total cholesterol, TG = triglyceride, WBC = white blood cell.
3.2. MACCE occurrence
The 1-, 2-, and 3-year MACCE occurrence rates were 10/196 (5.1%), 23/196 (11.7%), and 29/196 (14.8%) in ESRD patients who underwent CAPD, respectively (Fig. 2A). The mean time of accumulating MACCE occurrence was 33.1 months (95% confidence interval: 32.0–34.2 months) in these patients (Fig. 2B).
Figure 2.
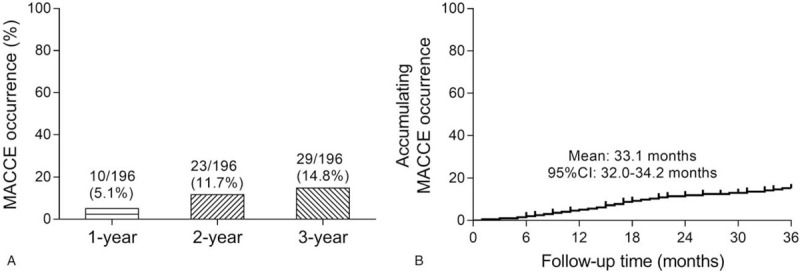
MACCE occurrence. The 1-, 2-, and 3-year MACCE occurrence rate (A), and accumulating MACCE occurrence by Kaplan–Meier curve (B) in ESRD patients who underwent CAPD. CAPD = continuous ambulatory peritoneal dialysis, ESRD = end-stage renal disease, MACCE = major adverse cardiac and cerebrovascular events.
3.3. Correlation of clinical features with accumulating MACCE occurrence
Age high (P = .042) (Fig. 3A), PDD high (P = .005) (Fig. 3B), CRP high (P = .009) (Fig. 3C), FBG high (P = .004) (Fig. 3D), and TC high (P = .034) (Fig. 3E) correlated with elevated accumulating MACCE occurrence, while HDL-C high (P = .010) (Fig. 3F) correlated with reduced accumulating MACCE occurrence in ESRD patients who underwent CAPD. Besides, no correlation of gender (P = .126) (Fig. 4A), BMI (P = .484) (Fig. 4B), current smoking (P = .314) (Fig. 4C), current drinking (P = .174) (Fig. 4D), Kt/V (P = .792) (Fig. 4E), HB (P = .530) (Fig. 4F), WBC (P = .551) (Fig. 4G), PLT (P = .315) (Fig. 4H), Scr (P = .597) (Fig. 4I), SUA (P = .165) (Fig. 4J), Ca (P = .156) (Fig. 4K), phosphorus (P = .526) (Fig. 4L), ALB (P = .167) (Fig. 4M), SBP (P = .066) (Fig. 4N), DBP (P = .164) (Fig. 4O), TG (P = .175) (Fig. 4P), or LDL-C (P = .215) (Fig. 4Q) with accumulating MACCE occurrence was observed in ESRD patients who underwent CAPD.
Figure 3.
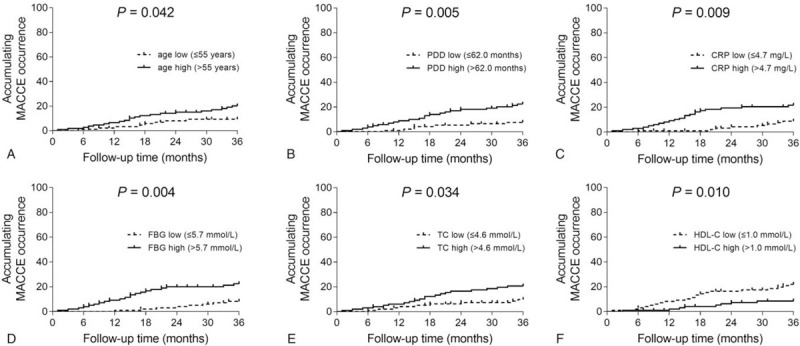
Factors correlated with accumulating MACCE occurrence. Comparisons of accumulating MACCE occurrence between patients with age low vs age high (A), patients with PDD low vs PDD high (B), patients with CRP low vs CRP high (C), patients with FBG low vs FBG high (D), patients with TC low vs TC high (E), and patients with HDL-C low vs HDL-C high (F) in ESRD patients who underwent CAPD. CAPD = continuous ambulatory peritoneal dialysis, CRP = C-reactive protein, ESRD = end-stage renal disease, FBG = fasting blood glucose, HDL-C = high-density lipoprotein cholesterol, MACCE = major adverse cardiac and cerebrovascular events, PDD = peritoneal dialysis duration, TC = total cholesterol.
Figure 4.
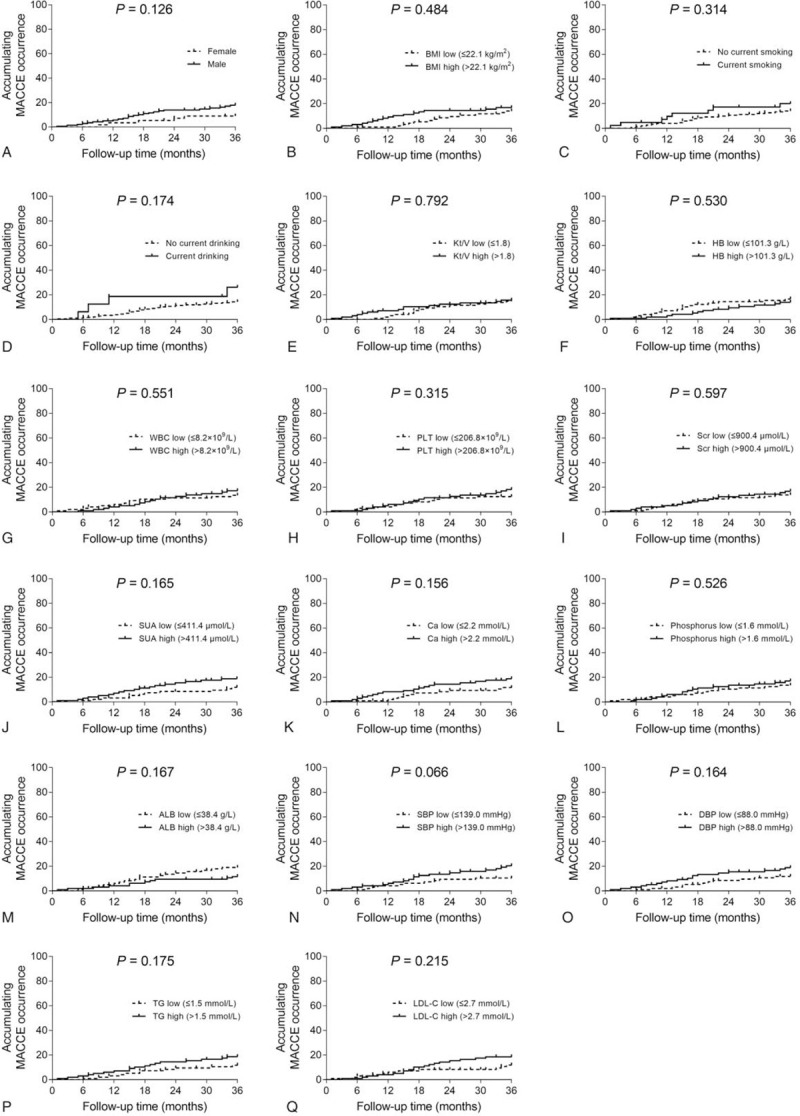
Factors not correlated with accumulating MACCE occurrence. Comparisons of accumulating MACCE occurrence between patients with female vs male (A), patients with BMI low vs BMI high (B), patients with no current smoking vs current smoking (C), patients with no current drinking vs current drinking (D), patients with Kt/V low vs Kt/V high (E), patients with HB low vs HB high (F), patients with WBC low vs WBC high (G), patients with PLT low vs PLT high (H), patients with Scr low vs Scr high (I), patients with SUA low vs SUA high (J), patients with Ca low vs Ca high (K), patients with phosphorus low vs phosphorus high (L), patients with ALB low vs ALB high (M), patients with SBP low vs SBP high (N), patients with DBP low vs DBP high (O), patients with TG low vs TG high (P), and patients with LDL-C low vs LDL-C high (Q) in ESRD patients who underwent CAPD. ALB = albumin, BMI = body mass index, Ca = calcium, CAPD = continuous ambulatory peritoneal dialysis, DBP = diastolic blood pressure, ESRD = end-stage renal disease, HB = hemoglobin, LDL-C = low density lipoprotein cholesterol, MACCE = major adverse cardiac and cerebrovascular events, PLT = platelet, Scr = serum creatinine, SBP = systolic pressure, SUA = serum uric acid, TG = triglyceride, WBC = white blood cell.
3.4. Factors predicting accumulating MACCE occurrence by Cox's proportional hazard regression
By univariate Cox's proportional hazard regression analysis, age high (P = .048, HR = 2.271), PDD high (P = .007, HR = 3.194), CRP high (P = .012, HR = 2.833), FBG high (P = .006, HR = 3.120) and TC high (P = .039, HR = 2.287) predicted elevated accumulating MACCE occurrence; HDL-C high (P = .013, HR = 0.358) predicted decreased accumulating MACCE occurrence; While gender (P = .135, HR = 2.087), BMI (P = .485, HR = 1.298), current smoking (P = .318, HR = 1.514), current drinking (P = .183, HR = 2.049), Kt/V (P = .792, HR = 1.103), HB (P = .531, HR = 0.791), WBC (P = .552, HR = 1.249), PLT (P = .318, HR = 1.457), Scr (P = .598, HR = 1.218), SUA (P = .170, HR = 1.691), Ca (P = .161, HR = 1.709), phosphorus (P = .527, HR = 1.266), ALB (P = .172, HR = 0.593), SBP (P = .072, HR = 2.022), DBP (P = .169, HR = 1.692), TG (P = .180, HR = 1.670), or LDL-C (P = .219, HR = 1.600) could not predict accumulating MACCE occurrence in ESRD patients who underwent CAPD (Table 2). Notably, by further multivariate Cox's proportional hazard regression analysis, age high (P = .017, HR = 3.378), PDD high (P = .005, HR = 3.991), CRP high (P = .022, HR = 3.041), SUA high (P = .040, HR = 2.526), and FBG high (P = .007, HR = 3.713) could independently predict higher accumulating MACCE occurrence, whereas HDL-C high (P = .007, HR = 0.235) could independently predict lower accumulating MACCE occurrence in ESRD patients who underwent CAPD (Table 3).
Table 2.
Analysis of factors related to accumulating MACCE occurrence by univariate Cox's proportional hazard regression model.
| Univariate Cox's proportional hazard regression model | ||||
| Items | P | HR | 95%CI | |
| Low | High | |||
| Age high (>55 years) | .048 | 2.271 | 1.006 | 5.127 |
| Male | .135 | 2.087 | 0.796 | 5.470 |
| BMI high (>22.1 kg/m2) | .485 | 1.298 | 0.624 | 2.698 |
| Current smoking | .318 | 1.514 | 0.671 | 3.419 |
| Current drinking | .183 | 2.049 | 0.713 | 5.888 |
| PDD high (>62.0 months) | .007 | 3.194 | 1.364 | 7.478 |
| Kt/V high (>1.8) | .792 | 1.103 | 0.532 | 2.285 |
| HB high (>101.3 g/L) | .531 | 0.791 | 0.381 | 1.645 |
| WBC high (>8.2 × 109/L) | .552 | 1.249 | 0.601 | 2.597 |
| PLT high (>206.8 × 109/L) | .318 | 1.457 | 0.696 | 3.051 |
| CRP high (>4.7 mg/L) | .012 | 2.833 | 1.255 | 6.398 |
| Scr high (>900.4 μmol/L) | .598 | 1.218 | 0.586 | 2.532 |
| SUA high (>411.4 μmol/L) | .170 | 1.691 | 0.799 | 3.581 |
| Ca high (>2.2 mmol/L) | .161 | 1.709 | 0.807 | 3.619 |
| Phosphorus high (>1.6 mmol/L) | .527 | 1.266 | 0.609 | 2.633 |
| FBG high (>5.7 mmol/L) | .006 | 3.120 | 1.381 | 7.048 |
| ALB high (>38.4 g/L) | .172 | 0.593 | 0.280 | 1.255 |
| SBP high (>139.0 mm Hg) | .072 | 2.022 | 0.940 | 4.349 |
| DBP high (>88.0 mm Hg) | .169 | 1.692 | 0.799 | 3.583 |
| TG high (>1.5 mmol/L) | .180 | 1.670 | 0.789 | 3.536 |
| TC high (>4.6 mmol/L) | .039 | 2.287 | 1.041 | 5.022 |
| LDL-C high (>2.7 mmol/L) | .219 | 1.600 | 0.756 | 3.388 |
| HDL-C high (>1.0 mmol/L) | .013 | 0.358 | 0.158 | 0.807 |
For the continuous variable in the table, “high” was classified according to the median value.
The boldface values stand for values with statistical significance.
ALB = albumin, BMI = body mass index, Ca = calcium, CI = confidence interval, CRP = C-reactive protein, DBP = diastolic blood pressure, FBG = fasting blood glucose, HB = hemoglobin, HDL-C = high density lipoprotein cholesterol, HR = hazard ratio, Kt/V = calculated as clearance (K) multiplied by treatment time (t) and divided by the urea distribution volume (V), LDL-C = low density lipoprotein cholesterol, MACCE = major adverse cardiovascular and cerebrovascular events, PDD = peritoneal dialysis duration, PLT = platelet, SBP = systolic pressure, Scr = serum creatinine, SUA = serum uric acid, TC = total cholesterol, TG = triglyceride, WBC = white blood cell.
Table 3.
Analysis of factors related to accumulating MACCE occurrence by multivariate Cox's proportional hazard regression model.
| Multivariate Cox's proportional hazard regression model | ||||
| Items | P | HR | 95%CI | |
| Low | High | |||
| Age high (>55 years) | .017 | 3.378 | 1.244 | 9.168 |
| Male | .601 | 1.358 | 0.431 | 4.275 |
| BMI high (>22.1 kg/m2) | .990 | 0.995 | 0.419 | 2.359 |
| Current smoking | .068 | 2.703 | 0.928 | 7.878 |
| Current drinking | .897 | 0.916 | 0.244 | 3.446 |
| PDD high (>62.0 months) | .005 | 3.991 | 1.518 | 10.493 |
| Kt/V high (>1.8) | .251 | 1.678 | 0.693 | 4.063 |
| HB high (>101.3 g/L) | .966 | 0.979 | 0.366 | 2.615 |
| WBC high (>8.2 × 109/L) | .325 | 0.618 | 0.237 | 1.612 |
| PLT high (>206.8 × 109/L) | .839 | 1.101 | 0.435 | 2.787 |
| CRP high (>4.7 mg/L) | .022 | 3.041 | 1.174 | 7.874 |
| Scr high (>900.4 μmol/L) | .945 | 1.034 | 0.392 | 2.726 |
| SUA high (>411.4 μmol/L) | .040 | 2.526 | 1.043 | 6.120 |
| Ca high (>2.2 mmol/L) | .191 | 1.866 | 0.733 | 4.751 |
| Phosphorus high (>1.6 mmol/L) | .524 | 1.335 | 0.549 | 3.244 |
| FBG high (>5.7 mmol/L) | .007 | 3.713 | 1.420 | 9.707 |
| ALB high (>38.4 g/L) | .729 | 0.845 | 0.327 | 2.186 |
| SBP high (>139.0 mm Hg) | .286 | 1.623 | 0.667 | 3.946 |
| DBP high (>88.0 mm Hg) | .681 | 1.223 | 0.468 | 3.201 |
| TG high (>1.5 mmol/L) | .168 | 1.907 | 0.762 | 4.772 |
| TC high (>4.6 mmol/L) | .091 | 2.842 | 0.847 | 9.534 |
| LDL-C high (>2.7 mmol/L) | .536 | 1.422 | 0.467 | 4.328 |
| HDL-C high (>1.0 mmol/L) | .007 | 0.235 | 0.081 | 0.678 |
For the continuous variable in the table, “high” was classified according to the median value. ALB = albumin, BMI = body mass index, Ca = calcium, CI = confidence interval, CRP = C-reactive protein, DBP = diastolic blood pressure, FBG = fasting blood glucose, HB = hemoglobin, HDL-C = high density lipoprotein cholesterol, HR = hazard ratio, Kt/V = calculated as clearance (K) multiplied by treatment time (t) and divided by the urea distribution volume (V), LDL-C = low density lipoprotein cholesterol, MACCE = major adverse cardiovascular and cerebrovascular events, PDD = peritoneal dialysis duration, PLT = platelet, SBP = systolic pressure, Scr = serum creatinine, SUA = serum uric acid, TC = total cholesterol, TG = triglyceride, WBC = white blood cell.
4. Discussion
In the present study, it was observed that in ESRD patients who underwent CAPD:
-
1.
the 1-, 2-, and 3-year MACCE occurrence rates were 5.1%, 11.7%, and 14.8%, respectively;
-
2.
age high, PDD high, CRP high, FBG high, and TC high were associated with increased accumulating MACCE occurrence, while HDL-C high was associated with decreased accumulating MACCE occurrence;
-
3.
age high, PDD high, CRP high, SUA high, and FBG high independently predicted raised accumulating MACCE occurrence, while HDL-C high independently predicted attenuated accumulating MACCE occurrence.
From earlier literatures, MACCE is commonly occurred in ESRD patients who underwent CAPD.[8,15] For instance, one study reveals 1-, 2-, and 3-year MACCE occurrence rates being 5.6%, 11.9%, and 15.0%, respectively in ESRD patients who underwent CAPD.[15] Another study illuminates that the 1-, 2-, and 3-year MACCE occurrence rates are 2.5%, 6.1%, and 9.1%, respectively in ESRD patients who underwent CAPD.[8] In the present study, it was revealed that the 1-, 2-, and 3-year MACCE occurrence rates were 5.1%, 11.7%, and 14.8% in ESRD patients who underwent CAPD, respectively, which were slightly different from previous studies. The Difference among study findings may result from variations in study population cohort and different follow-up duration.
Data from previous studies illustrates that factors related to cardiovascular events in ESRD patients who underwent CAPD exhibit the potential as markers for predicting cardiovascular complications.[10–13] For instance, one study reports that history of hypercholesterolemia, cardiac troponin T and history of heart failure are independent predictors for elevated occurrence of cardiovascular events in ESRD patients on chronic maintenance dialysis.[13] Another study exhibits that serum cystatin C, eGFRcysC, TC, and LDL-C predict higher risk of cardiovascular events in ESRD patients at the initiation of dialysis.[11] However, data regarding the predictive factors for MACCE is rare in ESRD patients who underwent CAPD during as long as 3 years. In the present study, we observed that age high, PDD high, CRP high, FBG high, and TC high were associated with increased accumulating MACCE occurrence, while HDL-C high was associated with decreased accumulating MACCE occurrence in ESRD patients who underwent CAPD. Of note, subsequent multivariate Cox's proportional hazard regression analysis found that age high, PDD high, CRP high, SUA high, and FBG high could independently predict raised accumulating MACCE occurrence, while HDL-C high independently predicted attenuated accumulating MACCE occurrence in ESRD patients who underwent CAPD. To explain these findings, the following reasons have been proposed:
-
1.
Older patients might have multiple comorbid conditions, such as cardiac events, cerebral dysfunction, stroke, and loss of residual renal function, thereby, age high ESRD patients who underwent CAPD were more prone to MACCE.[16]
-
2.
Longer PDD was associated with increased prevalence of dyslipidemia from exposure to a large amount of glucose in dialysate and deteriorated cardiac remodeling from overhydration with poor residual renal function and peritoneal ultrafiltration failure, thereby, resulting in a higher MACCE risk.[17–19]
-
3.
CRP elevation reflected the exaggerated inflammatory responses, which facilitated cardiovascular injury through activating complement and inducing monocyte expression of tissue factor.[20] Hence, CRP high contributed to inclined MACCE risk in ESRD patients who underwent CAPD.
-
4.
Increased FBG level might impair the vascular endothelial cells, facilitate the migration and proliferation of vascular smooth muscle cells, and mediate the activation of plasminogen activator inhibitor-1, which consequently raised the procoagulant factors and antithrombotic factors, thereby, accelerating the thrombosis formation and the MACCE occurrence in ESRD patients who underwent CAPD.[21]
-
5.
Accumulation of TC on arterial wall might result in the formation of lipid-laden foam cells and formation of atherosclerotic lesions, thereby elevating MACCE risk in ESRD patients who underwent CAPD.[22]
-
6.
HDL-C might reduce the overall level of TC via reverse cholesterol transport by its HDL particles and promote nitric oxide formation, which protected vessel walls against the development of atherosclerosis.[23] Hence, HDL-C high was associated with attenuated MACCE risk in ESRD patients who underwent CAPD.
-
7.
Although TC high correlated with higher accumulating MACCE occurrence, it could not independently predict accumulating MACCE occurrence, which was likely to explained by that TC impacted other factors (such as LDL-C and lipoprotein [a]) to indirectly result in raised accumulating MACCE occurrence in ESRD patients who underwent CAPD.[17]
-
8.
Elevated SUA level might induce the formation of uric acid crystals, which then initiated vascular inflammation, promoted platelet adhesiveness and stimulated vascular smooth cell growth, thereby, leading to a higher accumulating MACCE occurrence in ESRD patients who underwent CAPD.[24]
Some limitations should be considered when interpreting the present study. First, a total of 196 ESRD patients who underwent CAPD were included in the final analysis, while only 10/196 (5.1%), 23/196 (11.7%), and 29/196 (14.8%) cases occurred MACCE at 1, 2, and 3 years. The limited total sample size and valid events might reduce the statistic power of the analysis. Secondly, 16 ESRD patients lost follow-up, while these patients were censored on the date of last visit and included in the final analysis, which might cause potential bias. Thirdly, patients receiving long-term CAPD were at high risk of developing MACCE, thus we only recruited ESRD patients who underwent CAPD for at least 3 years. However, it might limit the generalizability of our findings. Lastly, although it was not the aim of our study, it was clinically valuable to investigate the impact of socio-economic status of patients (such as income and education) on MACCE in future studies.
To conclude, age, peritoneal dialysis duration, C-reactive protein, serum uric acid, and fasting blood glucose reflects higher accumulating MACCE risk, while high density lipoprotein cholesterol indicates lower accumulating major adverse cardiac and cerebral events risk in CAPD-treated ESRD patients. Further studies with larger samples size of CAPD-treated ESRD patients with MACCE were needed for validating our findings.
Author contributions
Conceptualization: Chunmeng Yao, Liping Zhou.
Data curation: Qinghe Huang.
Formal analysis: Qinghe Huang.
Investigation: Qinghe Huang.
Methodology: Qinghe Huang.
Resources: Chunmeng Yao, Liping Zhou.
Supervision: Chunmeng Yao, Liping Zhou.
Writing – original draft: Qinghe Huang.
Writing – review & editing: Chunmeng Yao, Liping Zhou.
Footnotes
Abbreviations: ALB = albumin, BMI = body mass index, Ca = calcium, CAPD = Continuous ambulatory peritoneal dialysis, CRF = Case Report Form, CRP = C-reactive protein, DBP = diastolic blood pressure, ESRD = End-stage renal disease, FBG = fasting blood glucose, HB = hemoglobin, HDL-C = high-density lipoprotein cholesterol, IQR = interquartile range, K-M = Kaplan–Meier, LDL-C = low density lipoprotein cholesterol, MACCE = major adverse cardiac and cerebral events, PDD = peritoneal dialysis duration, PLT = platelet, SBP = systolic blood pressure, Scr = serum creatinine, SD = standard deviation, SUA = serum uric acid, TC = total cholesterol, TG = triglyceride, TIA = transient ischemic attack, TVR = target vessel revascularization, WBC = white blood cell.
How to cite this article: Yao C, Zhou L, Huang Q. The occurrence and potential predictive factors of major adverse cardiac and cerebral events in end-stage renal disease patients on continuous ambulatory peritoneal dialysis: A prospective cohort study. Medicine. 2021;100:10(e24616).
CY and LZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- [1].Reszke R, Szepietowski JC. End-stage renal disease chronic itch and its management. Dermatol Clin 2018;36:277–92. [DOI] [PubMed] [Google Scholar]
- [2].Johannsson G, Ahlmen J. End-stage renal disease: endocrine aspects of treatment. Growth Horm IGF Res 2003;13: Suppl A: S94–101. [DOI] [PubMed] [Google Scholar]
- [3].Li H, Xie L, Yang J, et al. Symptom burden amongst patients suffering from end-stage renal disease and receiving dialysis: a literature review. Int J Nurs Sci 2018;5:427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goldstein M, Carrillo M, Ghai S. Continuous ambulatory peritoneal dialysis-a guide to imaging appearances and complications. Insights Imaging 2013;4:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khera S, Villablanca PA, Kolte D, et al. Long-term outcomes of drug-eluting stents versus bare-metal stents in end-stage renal disease patients on dialysis: a systematic review and meta-analysis. Cardiol Rev 2018;26:277–86. [DOI] [PubMed] [Google Scholar]
- [6].Kim H, Kim KH, Ahn SV, et al. Risk of major cardiovascular events among incident dialysis patients: a Korean national population-based study. Int J Cardiol 2015;198:95–101. [DOI] [PubMed] [Google Scholar]
- [7].Lee MJ, Han SH, Lee JE, et al. Endothelial dysfunction is associated with major adverse cardiovascular events in peritoneal dialysis patients. Medicine (Baltimore) 2014;93:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Y, Liu C, Wei W, et al. Predictive value of circulating coagulation related microRNAs expressions for major adverse cardiac and cerebral event risk in patients undergoing continuous ambulatory peritoneal dialysis: a cohort study. J Nephrol 2020;33:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jin DC. Major changes and improvements of dialysis therapy in Korea: review of end-stage renal disease registry. Korean J Intern Med 2015;30:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Janda K, Krzanowski M, Dumnicka P, et al. Transforming growth factor beta 1 as a risk factor for cardiovascular diseases in end-stage renal disease patients treated with peritoneal dialysis. Clin Lab 2014;60:1163–8. [DOI] [PubMed] [Google Scholar]
- [11].Shin MJ, Song SH, Kwak IS, et al. Serum cystatin C as a predictor for cardiovascular events in end-stage renal disease patients at the initiation of dialysis. Clin Exp Nephrol 2012;16:456–63. [DOI] [PubMed] [Google Scholar]
- [12].Kessler M, Zannad F, Lehert P, et al. Predictors of cardiovascular events in patients with end-stage renal disease: an analysis from the Fosinopril in dialysis study. Nephrol Dial Transplant 2007;22:3573–9. [DOI] [PubMed] [Google Scholar]
- [13].Ishii J, Nomura M, Okuma T, et al. Risk stratification using serum concentrations of cardiac troponin T in patients with end-stage renal disease on chronic maintenance dialysis. Clin Chim Acta 2001;312:69–79. [DOI] [PubMed] [Google Scholar]
- [14].Xu L, Hu X, Chen W. Fibroblast growth factor-23 correlates with advanced disease conditions and predicts high risk of major adverse cardiac and cerebral events in end-stage renal disease patients undergoing continuous ambulatory peritoneal dialysis. J Nephrol 2019;32:307–14. [DOI] [PubMed] [Google Scholar]
- [15].Liu D, Zhou S, Mao H. MicroRNA-497/fibroblast growth factor-23 axis, a predictive indictor for decreased major adverse cardiac and cerebral event risk in end-stage renal disease patients who underwent continuous ambulatory peritoneal dialysis. J Clin Lab Anal 2020;34:e23220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Power A, Brown E. Optimising treatment of end-stage renal disease in the elderly. Nephron Clin Pract 2013;124:202–8. [DOI] [PubMed] [Google Scholar]
- [17].Liu J, Rosner MH. Lipid abnormalities associated with end-stage renal disease. Semin Dial 2006;19:32–40. [DOI] [PubMed] [Google Scholar]
- [18].Konings CJ, Kooman JP, Schonck M, et al. Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int 2002;22:477–87. [PubMed] [Google Scholar]
- [19].Koc M, Toprak A, Tezcan H, et al. Uncontrolled hypertension due to volume overload contributes to higher left ventricular mass index in CAPD patients. Nephrol Dial Transplant 2002;17:1661–6. [DOI] [PubMed] [Google Scholar]
- [20].Ducloux D, Bresson-Vautrin C, Kribs M, et al. C-reactive protein and cardiovascular disease in peritoneal dialysis patients. Kidney Int 2002;62:1417–22. [DOI] [PubMed] [Google Scholar]
- [21].Wang C, Li F, Guo J, et al. Insulin resistance, blood glucose and inflammatory cytokine levels are risk factors for cardiovascular events in diabetic patients complicated with coronary heart disease. Exp Ther Med 2018;15:1515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Agabiti Rosei E, Salvetti M. Management of hypercholesterolemia, appropriateness of therapeutic approaches and new drugs in patients with high cardiovascular risk. High Blood Press Cardiovasc Prev 2016;23:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meagher EA. Addressing cardiovascular risk beyond low-density lipoprotein cholesterol: the high-density lipoprotein cholesterol story. Curr Cardiol Rep 2004;6:457–63. [DOI] [PubMed] [Google Scholar]
- [24].Jin M, Yang F, Yang I, et al. Uric acid, hyperuricemia and vascular diseases. Front Biosci (Landmark Ed) 2012;17:656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]


