Abstract
Rationale:
Herein, we report 3 hemodialysis patients with idiopathic hypereosinophilic syndrome who were successfully treated using corticosteroid therapy.
Patient concerns:
Case 1 was a 63-year-old man who was undergoing hemodialysis because of bilateral nephrectomy and developed hypereosinophilia with digestive symptoms, myocardial injury, and intradialytic hypotension. Case 2 was an 83-year-old man who was undergoing hemodialysis because of nephrosclerosis and developed hypereosinophilia with pruritus, myocardial injury, and intradialytic hypotension. Case 3 was a 59-year-old man who was undergoing hemodialysis because of diabetic nephropathy and developed hypereosinophilia with pruritus, myocardial injury, and intradialytic hypotension.
Diagnoses:
All 3 patients presented with hypereosinophilia (eosinophil count ≥1500 /μL for more than 1 month) and multiple-organ involvement (intradialytic hypotension, cardiac injury, digestive symptoms, and allergic dermatitis). A specific cause for the hypereosinophilia was not identified by systemic computed tomography, electrocardiography, echocardiography, bone marrow examination, or blood tests. Furthermore, Case 2 and 3 had not recently started taking any new drugs and drug-induced lymphocyte stimulation tests were negative in Case 1. Therefore, they were diagnosed with idiopathic hypereosinophilic syndrome.
Interventions:
All 3 patients received corticosteroid therapy with prednisolone at a dose of 40 mg/d, 30 mg/d, and 60 mg/d in Case 1, 2, and 3, respectively.
Outcomes:
Their digestive symptoms, pruritus, intradialytic hypotension, and serum troponin I concentrations were immediately improved alongside reductions in their eosinophil counts.
Lessons:
There have been few case reports of idiopathic hypereosinophilic syndrome in patients undergoing hemodialysis. We believe that recording of the clinical findings and treatments of such patients is mandatory to establish the optimal management of idiopathic hypereosinophilic syndrome.
Keywords: corticosteroid therapy, hemodialysis, idiopathic hypereosinophilic syndrome
1. Introduction
Idiopathic hypereosinophilia is a rare disease that is characterized by hypereosinophilia in the peripheral circulation and damage to multiple organs, including the lungs, heart, skin, nervous system, and gastrointestinal tract.[1] It can cause irreversible organ dysfunction, disability, or death; therefore, urgent assessment and management are necessary. Symptoms and signs of hypereosinophilic syndrome vary a great deal, depending on the affected organs.[1] In hemodialysis patients, hypereosinophilic syndrome has been reported to lead to hemodialysis intolerance, because of intradialytic hypotension or digestive symptoms.[2,3] However, there have been few case reports of idiopathic hypereosinophilic syndrome in patients undergoing hemodialysis, and its clinical features remain to be fully characterized. Here, we report 3 cases of idiopathic hypereosinophilic syndrome in patients undergoing hemodialysis that were characterized by intradialytic hypotension and cardiac injury and were successfully treated using corticosteroid therapy.
2. Case report
Table 1 summarizes the characteristics, dialysis prescriptions, treatments, and laboratory findings at the admission of the 3 patients. The treatment courses of each patient are illustrated in Figures 1–3.
Table 1.
Patient characteristics, dialysis prescription, treatment, and laboratory findings at admission.
| Case 1 | Case 2 | Case 3 | Reference range | |
| Age (yr) | 63 | 83 | 59 | |
| Sex | Male | Male | Male | |
| Cause of end-stage renal disease | Bilateral nephrectomy | Nephrosclerosis | Diabetic nephropathy | |
| Allergy | None | None | None | |
| Symptoms | Digestive symptoms, myocardial injury, and intradialytic hypotension | Pruritus, myocardial injury, and intradialytic hypotension | Pruritus, myocardial injury, and intradialytic hypotension | |
| Involved organs | Digestive tract and heart | Skin and heart | Skin and heart | |
| Dialysis prescription | ||||
| Frequency (times a week) | 3 | 3 | 3 | |
| Time (hr) | 4 | 4 | 3 | |
| Dry mass (kg) | 76.3 | 57.0 | 96.0 | |
| Blood flow (ml/min) | 200 | 200 | 200 | |
| Dialysate flow (ml/min) | 500 | 500 | 500 | |
| Dialyzer | Polysulfone dialyzer (APS-21EA; Asahi Kasei Co, Ltd) | Polysulfone dialyzer (NV-21S; Toray Medical Co, Ltd) | Polysulfone dialyzer (NV-15S; Toray Medical Co, Ltd) | |
| Anticoagulation | Heparin (600 U/h) | Low-molecular weight heparin (600 U/h) | Nafamostat (30 mg/h) | |
| Treatment | Prednisolone 40 mg/d (0.5 mg/kg/d) | Prednisolone 30 mg/d (0.5 mg/kg/d) | Prednisolone 60 mg/d (0.6 mg/kg/d) | |
| Blood test results | ||||
| White blood cell count (/μL) | 31,240 | 15,520 | 7830 | 3900–9800 |
| Band (%) | 0.4 | 0.0 | 1.0 | 0–19 |
| Segmented (%) | 13.4 | 43.5 | 55.0 | 25–72 |
| Eosinophil (%) | 79.8 | 39.5 | 28.0 | 0–7 |
| Basophil (%) | 0.4 | 0.5 | 1.0 | 0–2 |
| Lymphocyte (%) | 4.4 | 10.0 | 8.0 | 19–48 |
| Monocyte (%) | 1.0 | 6.0 | 2.0 | 3–9 |
| Eosinophil count (μL) | 24,929 | 6130 | 2192 | 0–500 |
| Hemoglobin (g/dL) | 9.9 | 10.4 | 11.3 | 12.0–17.6 |
| Platelet count (×104/μL) | 21.6 | 14.2 | 20.3 | 13.0–36.9 |
| Blood urea nitrogen (mg/dL) | 36 | 42 | 38 | 8–20 |
| Creatinine (mg/dL) | 10.96 | 10.15 | 6.25 | 0.65–1.07 |
| Aspartate aminotransferase (IU/L) | 11 | 12 | 13 | 13–30 |
| Alanine aminotransferase (IU/L) | 2 | 13 | 7 | 10–42 |
| Lactate dehydrogenase (IU/L) | 179 | 341 | 339 | 124–222 |
| Alkaline phosphatase (IU/L) | 322 | 280 | 168 | 106–322 |
| C-reactive protein (mg/dL) | 2.0 | 2.7 | 6.0 | 0–0.14 |
| Creatinine kinase (IU/L) | 98 | 51 | 30 | 59–248 |
| Creatinine kinase-MB (IU/L) | 8 | 211 | 1 | 0–25 |
| Troponin I (pg/mL) | 340.7 | 154.9 | 313.2 | <26.2 |
| IgG (mg/dL) | 818 | 1841 | 1798 | 870–1700 |
| IgA (mg/dL) | 199 | 609 | 367 | 110–410 |
| IgM (mg/dL) | 52 | 145 | 48 | 33–190 |
| IgE (mg/dL) | 29 | 1100 | 700 | <173 |
| MPO-ANCA (U/mL) | <1.0 | <1.0 | <1.0 | <1.0 |
ANCA = antineutrophil cytoplasmic antibody, Ig = immunoglobulin, MPO = myeloperoxidase.
Figure 1.
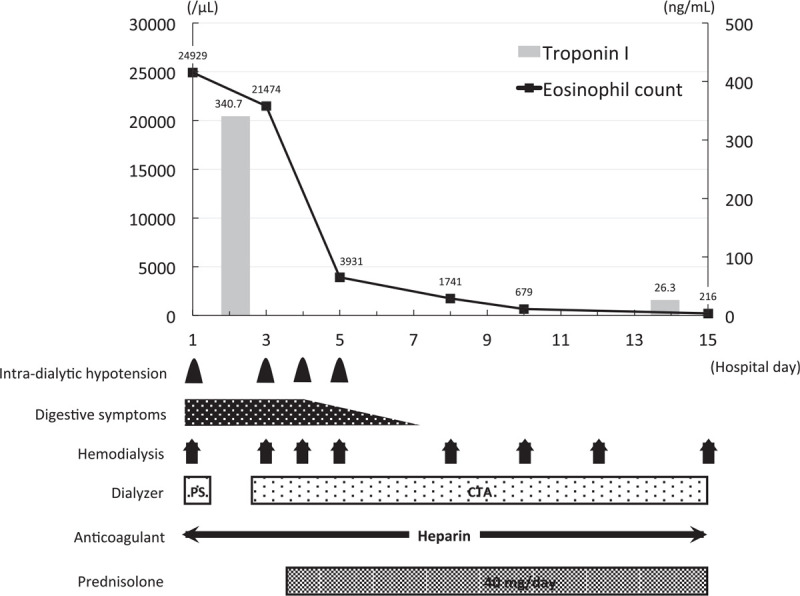
Clinical course of case 1. The x axis of the graph shows the number of days from admission and the y axis shows the eosinophil count (line) and the serum troponin I concentration (bars). The frequencies or durations of each clinical problem and treatment are shown below. CTA = cellulose triacetate, PS = polysulfone.
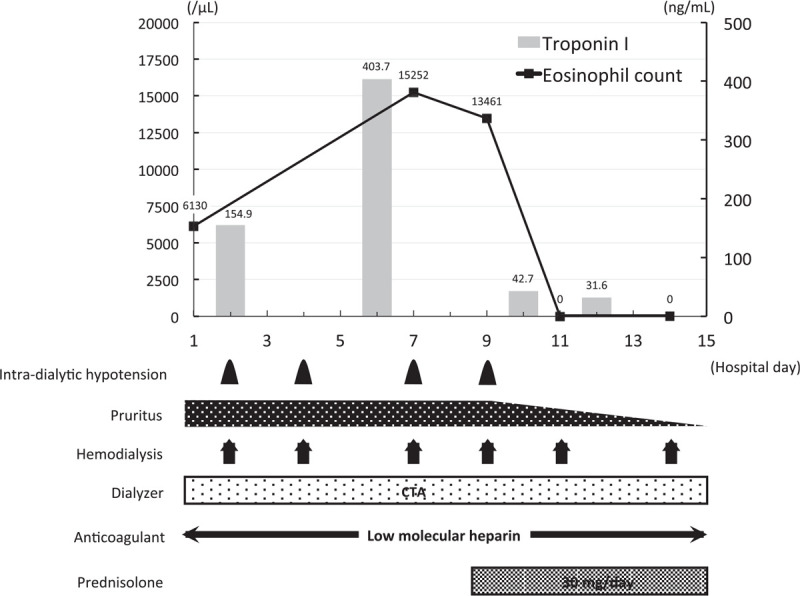
Figure 3.
Clinical course of case 3. The x axis of the graph shows the number of days from admission and the y axis shows the eosinophil count (line) and the serum troponin I concentration (bars). The frequencies or durations of each clinical problem and treatment are shown below. CTA = cellulose triacetate, PS = polysulfone.
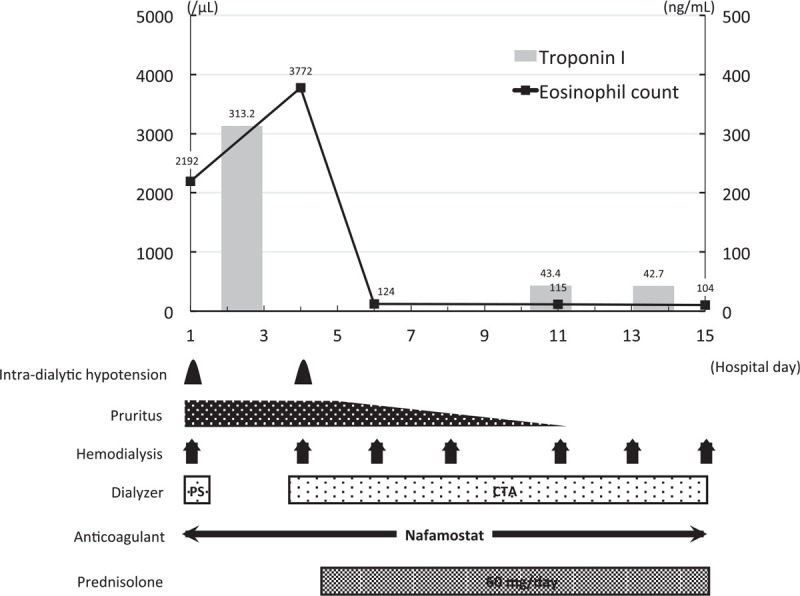
Figure 2.
Clinical course of case 2. The x axis of the graph shows the number of days from admission and the y axis shows the eosinophil count (line) and the serum troponin I concentration (bars). The frequencies or durations of each clinical problem and treatment are shown below. CTA = cellulose triacetate.
2.1. Case 1
The patient was a 63-year-old man who had been undergoing hemodialysis for ∼1 year because he had undergone bilateral nephrectomy to treat renal cell cancer. He had an 8-year history of diabetes mellitus and had recently experienced myocardial infarction. He was taking aspirin 100 mg/d, clopidogrel 75 mg/d, esomeprazole 10 mg/d, carvedilol 5 mg/d, rosuvastatin 2.5 mg/d, temocapril 2 mg/d, and a human insulin analog 10 units/d. An acetate dialysate and a dialyzer with a polysulfone dialysis membrane were being used for his hemodialysis. His medications and dialyzer membrane had not been changed for 6 months, with the exception of the aspirin, clopidogrel, and esomeprazole. One month before admission, eosinophilia (32.0%, 2659/μL) was identified, but he did not have any symptoms. One day before admission, he presented with fever, vomiting, and diarrhea and on the day of admission, he underwent hemodialysis. His systolic/diastolic blood pressure was 166/80 mmHg and his body temperature was 39.6°C. Shortly after the initiation of hemodialysis, his blood pressure dropped to 80/51 mmHg and he vomited. Therefore, the hemodialysis was stopped and he was admitted to the department. Blood tests revealed marked eosinophilia (79.8%, 24,929 /μL), high serum troponin I concentration (340.7 pg/mL), and impaired renal function (serum creatinine: 10.96 mg/dL, blood urea nitrogen: 36 mg/dL). Serological tests were negative for myeloperoxidase-anti-neutrophil cytoplasmic antibody and antiparasite antibodies. Drug-induced lymphocyte stimulation tests of aspirin, clopidogrel, and esomeprazole were negative. Thoraco-abdominal computed tomography revealed gastric and duodenal wall thickening, but there were no abnormalities in the lung fields. No abnormalities, except for those caused by the previous myocardial infarction, were noted on electrocardiography and echocardiography. Bone marrow examination revealed an eosinophil count of 42%, with no increase in blast count.
On the basis of these findings, the patient was diagnosed with idiopathic hypereosinophilic syndrome, with involvement of the digestive tract and heart. His dialyzer membrane was changed from polysulfone to cellulose triacetate, but the intradialytic hypotension and gastrointestinal symptoms did not improve. Therefore, oral prednisolone was started at a dose of 40 mg (0.5 mg/kg) per day on the 4th day of hospitalization, and his intradialytic hypotension and gastrointestinal symptoms were immediately ameliorated. On the 15th day of hospitalization, his eosinophil count and serum troponin I concentration had decreased to 216 /μL and 26.3 pg/mL, respectively, and the patient was discharged.
2.2. Case 2
The patient was an 83-year-old man who had been undergoing hemodialysis since the age of 80, because of nephrosclerosis. He had a history of undergoing surgery for sigmoid and ascending colon cancer at the ages of 77 and 80, respectively. He was taking lanthanum carbonate 1500 mg/d, febuxostat 10 mg/d, epinastine 20 mg/d, nalfurafine 5.0 μg/day, and magnesium oxide 500 mg/d. Bicarbonate dialysate and a dialyzer with a polysulfone dialysis membrane were being used for hemodialysis. His medications and dialyzer membrane had not been changed for 12 months. Three months before admission, he had presented with generalized pruritus and had eosinophilia (18.5%, 1905 /μL). One day before admission, he presented with hypotension (systolic blood pressure of 60 mmHg) and dyspnea during his hemodialysis session. Therefore, he was referred to our department for further examination and treatment. On admission, his systolic/diastolic blood pressure was 107/74 mmHg and his body temperature was 36.7°C. A generalized pruritic skin rash was observed. Blood tests showed marked eosinophilia (39.5%, 6130 /μL), high serum troponin I concentration (154.9 pg/mL), and impaired renal function (serum creatinine: 10.15 mg/dL, blood urea nitrogen: 42 mg/dL). Serological tests were negative for myeloperoxidase-anti-neutrophil cytoplasmic antibody and antiparasite antibodies. No abnormalities were detected on thoraco-abdominal computed tomography. No findings other than mild left ventricular hypertrophy were noted on electrocardiography and echocardiography. Bone marrow examination revealed an eosinophil count of 27%, with no increase in the blast count.
On the basis of these findings, the patient was diagnosed with idiopathic hypereosinophilic syndrome, with involvement of the skin and heart. All his medications were stopped and his dialyzer membrane was changed from polysulfone to cellulose triacetate. However, his intradialytic hypotension and pruritus did not improve. Therefore, oral prednisolone was started at a dose of 30 mg (0.5 mg/kg) per day on the 9th day of hospitalization, and his intradialytic hypotension and pruritus were immediately ameliorated. On the 14th day of hospitalization, his eosinophil count and serum troponin I concentration had decreased to 0/μL and 31.6 pg/mL, respectively. He was discharged on the 27th day of hospitalization.
2.3. Case 3
The patient was a 59-year-old man who had been undergoing hemodialysis for ∼1 year because of diabetic nephropathy that had developed as a complication of type II diabetes mellitus. He was taking aspirin 100 mg/d, carvedilol 1.25 mg/d, calcitriol 0.75 μg/d, calcium carbonate 1000 mg/d, esomeprazole 10 mg/d, and furosemide 160 mg/d. Acetate dialysate and a dialyzer with a polysulfone dialysis membrane were being used for hemodialysis. His medications and dialyzer membrane had not been changed for 5 months. Two months before admission, he had developed generalized pruritus and eosinophilia (22.0%, 1592 /μL). On the day of admission, he visited the hospital to undergo hemodialysis, and shortly after its initiation, his blood pressure dropped to 59/31 mmHg. Therefore, the hemodialysis was stopped. On admission, his systolic/diastolic blood pressure was 182/96 mmHg, his body temperature was 37.6°C, and a generalized pruritic skin rash was observed. Blood tests revealed marked eosinophilia (28.0%, 2192/μL), high serum troponin I concentration (313.2 pg/mL), and impaired renal function (serum creatinine: 6.25 mg/dL, blood urea nitrogen: 38 mg/dL). Serological tests were negative for myeloperoxidase-anti-neutrophil cytoplasmic antibody and antiparasite antibodies. No abnormalities were detected on thoraco-abdominal computed tomography. No findings except for mild left ventricular hypertrophy were made during electrocardiography and echocardiography. Bone marrow examination revealed an eosinophil count of 39%, with no increase in blasts.
On the basis of these findings, the patient was diagnosed with idiopathic hypereosinophilic syndrome, with involvement of the skin and heart. The dialyzer membrane was changed from polysulfone to cellulose triacetate, but his intradialytic hypotension and pruritus did not improve. Therefore, oral prednisolone was started at a dose of 60 mg (0.6 mg/kg) per day on the 5th day of hospitalization, and the intradialytic hypotension and pruritus were immediately ameliorated. On the 15th day of hospitalization, his eosinophil count and serum troponin I concentration had decreased to 104 /μL and 42.7 pg/mL, respectively and the patient was discharged on the 16th day of hospitalization.
3. Discussion
Eosinophilia (eosinophil count ≥500/μL) is a common finding in patients undergoing hemodialysis. It has been reported that the prevalence of eosinophilia in hemodialysis patients is 13% to 52%.[4,5] Dialyzer membrane materials have been considered to be the principal cause of eosinophilia in hemodialysis patients,[6,7] and in the present cases, the dialyzer membrane was changed from polysulfone to cellulose triacetate. However, this did not ameliorate the eosinophilia. Idiopathic hypereosinophilic syndrome is a systemic disease during which the eosinophil count increases in the peripheral circulation, which results in multiple-organ damage. It is diagnosed by the identification of hypereosinophilia (eosinophil count ≥1500/μL for more than 1 month) with multiple organ involvement (2 or more organs) and the exclusion of secondary causes of eosinophilia, including malignancy, eosinophilic leukemia, eosinophilic granulomatosis with polyangiitis, parasite infection, and drug reaction.[1] In the present cases, the patients presented with hypereosinophilia and multiple-organ involvement (intradialytic hypotension, cardiac injury, digestive symptoms, and allergic dermatitis). A specific cause for the hypereosinophilia was not identified using systemic computed tomography, electrocardiography, echocardiography, bone marrow examination, or blood tests. Furthermore, 2 of the patients had not recently started taking any new drugs and drug-induced lymphocyte stimulation tests were negative in the third patient. Therefore, the 3 patients were diagnosed with idiopathic hypereosinophilic syndrome.
All of these patients had intradialytic hypotension and myocardial injury. Intradialytic hypotension has previously been reported in hemodialysis patients with hypereosinophilic syndrome.[2] This is the result of the large number of eosinophils being activated by contact with the dialyzer membrane, leading to degranulation and the release of various cytokines,[8] which increase vascular permeability and dilate capillaries.[9,10] Major basic protein is also released by the eosinophils, and this is cytotoxic and induces tissue damage, including to cardiac muscle.[11] The intradialytic hypotension and serum troponin I concentrations of the present patients were ameliorated alongside reductions in their eosinophil counts. Intradialytic hypotension and myocardial injury might be common in hemodialysis patients with idiopathic hypereosinophilic syndrome, but the details of further cases should be recorded to more thoroughly characterize this idiopathic hypereosinophilic syndrome.
Corticosteroid is recommended as the first-line therapy for hypereosinophilic syndrome.[12] A previous study showed that approximately 80% of patients with hypereosinophilic syndrome had a favorable outcome following this treatment, but delayed intervention could lead to irreversible organ damage, disability, or death.[13] In the present cases, corticosteroid was promptly administered after the diagnosis of hypereosinophilic syndrome, and the patients improved, rather than experiencing irreversible organ damage or disability. Further study of additional cases of idiopathic hypereosinophilic syndrome in patients undergoing hemodialysis is required to determine the most appropriate corticosteroid dose.
In conclusion, we have described 3 patients who were undergoing hemodialysis and developed idiopathic hypereosinophilic syndrome with intradialytic hypotension and cardiac injury. They were successfully treated using a corticosteroid, and their intradialytic hypotension and serum troponin I concentrations were ameliorated alongside reductions in their eosinophil counts.
Acknowledgments
We thank Mark Cleasby, PhD, from Edanz Group for editing a draft of this manuscript.
Author contributions
Conceptualization: Junki Morino, Shohei Kaneko.
Data curation: Saori Minato, Katsunori Yanai, Hiroki Ishii.
Investigation: Momoko Matsuyama, Taisuke Kitano, Akinori Aomatsu, Haruhisa Miyazawa.
Supervision: Kiyonori Ito, Yuichiro Ueda.
Writing – original draft: Yuko Mutsuyoshi.
Writing – review & editing: Keiji Hirai, Susumu Ookawara, Yoshiyuki Morishita.
Footnotes
How to cite this article: Mutsuyoshi Y, Hirai K, Morino J, Kaneko S, Minato S, Yanai K, Ishii H, Matsuyama M, Kitano T, Aomatsu A, Miyazawa H, Ito K, Ueda Y, Ookawara S, Morishita Y. Idiopathic hypereosinophilic syndrome in hemodialysis patients: case reports. Medicine. 2021;100:10(e25164).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Declaration of Helsinki and ethical approval was obtained from the institutional review board of Saitama Medical Center, Jichi Medical University, Japan.
Informed consent was obtained from all individual participants included in the study. Written informed consent was obtained from the patient for publication of the case details and accompanying images.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012;130:607–12. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kanda E, Kida Y, Suzuki H, et al. Role of cytokines in anaphylactoid reaction with marked eosinophilia in a hemodialysis patient. Clin Exp Nephrol 2004;8:384–7. [DOI] [PubMed] [Google Scholar]
- [3].Higuchi T, Yamazaki T, Ohnishi Y, et al. A case report of hemodialysis intolerance with eosinophilia. Ther Apher Dial 2007;11:70–3. [DOI] [PubMed] [Google Scholar]
- [4].Friedberg M, Joffe P, Nielsen B, et al. Eosinophilia in hemodialysis patients. Artif Organs 1987;11:90–2. [DOI] [PubMed] [Google Scholar]
- [5].Voudiklaris S, Virvidakis K, Kalmantis T, et al. Eosinophilia in patients undergoing regular hemodialysis. Int J Artif Organs 1983;6:195–8. [PubMed] [Google Scholar]
- [6].Bodner G, Peer G, Zakuth V, et al. Dialysis-induced eosinophilia. Nephron 1982;32:63–6. [DOI] [PubMed] [Google Scholar]
- [7].Rockel A, Klinke B, Hertel J, et al. Allergy to dialysis materials. Nephrol Dial Transplant 1989;4:646–52. [PubMed] [Google Scholar]
- [8].Hertel J, Kimmel PL, Phillips TM, et al. Eosinophilia and cellular cytokine responsiveness in hemodialysis patients. J Am Soc Nephrol 1992;3:1244–52. [DOI] [PubMed] [Google Scholar]
- [9].Moqbel R, Levi-Schaffer F, Kay AB. Cytokine generation by eosinophils. J Allergy Clin Immunol 1994;94(6 Pt 2):1183–8. [DOI] [PubMed] [Google Scholar]
- [10].Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009;78:539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].deMello DE, Liapis H, Jureidini S, et al. Cardiac localization of eosinophil-granule major basic protein in acute necrotizing myocarditis. N Engl J Med 1990;323:1542–5. [DOI] [PubMed] [Google Scholar]
- [12].Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol 2014;89:325–37. [DOI] [PubMed] [Google Scholar]
- [13].Roufosse F. Management of hypereosinophilic syndromes. Immunol Allergy Clin North Am 2015;35:561–75. [DOI] [PubMed] [Google Scholar]


