Abstract
Background:
Hypoalbuminemia (HA) is common in HF, however, its pathophysiology and clinical implications are poorly understood. While multiple studies have been published in the past decade investigating the role of serum albumin in HF, there is still no consensus on the prognostic value of this widely available measure. The objective of this study is to assess the prognostic role of albumin in heart failure (HF) patient
Methods:
Unrestricted searches of MEDLINE, EMBASE, Cochrane databases were performed. The results were screened for relevance and eligibility criteria. Relevant data were extracted and analyzed using Comprehensive Meta-Analysis software. The Begg and Mazumdar rank correlation test was utilized to evaluate for publication bias.
Results:
A total of 48 studies examining 44,048 patients with HF were analyzed. HA was found in 32% (95% confidence interval [CI] 28.4%–37.4%) HF patients with marked heterogeneity (I2 = 98%). In 10 studies evaluating acute HF, in-hospital mortality was almost 4 times more likely in HA with an odds ratios (OR) of 3.77 (95% CI 1.96–7.23). HA was also associated with a significant increase in long-term mortality (OR: 1.5; 95% CI: 1.36–1.64) especially at 1-year post-discharge (OR: 2.44; 95% CI: 2.05–2.91; I2 = 11%). Pooled area under the curve (AUC 0.73; 95% CI 0.67–0.78) was comparable to serum brain natriuretic peptide (BNP) in predicting mortality in HF patients.
Conclusion:
Our results suggest that HA is associated with significantly higher in-hospital mortality as well as long-term mortality with a predictive accuracy comparable to that reported for serum BNP. These findings suggest that serum albumin may be useful in determining high-risk patients.
Keywords: heart failure, hypoalbuminemia, in-hospital mortality, length of hospital stay, long-term mortality, prevalence, prognosis, rehospitalization, serum albumin
1. Introduction
Heart failure (HF) is associated with increased frequency of hospitalization, morbidity, and mortality. Disease burden is on the rise. By 2030, 1 in every 33 people in the United States are projected to suffer from HF.[1] To optimize disease management through medication dose titration, cardiac resynchronization, or ventricular-assist-devices, risk-stratification of patients has become increasingly compelling. Brain natriuretic peptide (BNP) and N-terminal pro-BNP have been increasingly used for prognostication in acute decompensated heart failure (ADHF).[2] Novel prognostic markers have been recently suggested.[3] The 2017 American College of Cardiology focused update on guidelines for the management of HF hinted toward possible future role of these markers[4]; such as admission cardiac troponin level in ADHF, soluble ST2 (sST2) in chronic HF, and other myocardial fibrosis markers for prediction of hospitalization and mortality. However, many of these biomarkers require expensive assays which aren’t readily available. Albumin is synthesized in the liver and represents >50% of total serum proteins. Serum level depends on rate of albumin synthesis, distribution, degradation and excretion.[5–7] Hypoalbuminemia (HA) is linked to poor prognosis in multiple conditions, including left ventricular assist device (LVAD),[8] and acute kidney injury.[9] We found a vast amount of literature published in the past decade investigating the role of serum albumin (SA) as a prognostic indicator in patients with HF. Considering the ease and wide availability of this test, we performed a systematic review and meta-analysis to evaluate the prognostic role of SA in HF.
2. Methods
2.1. Search strategies
This review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[10] Unrestricted searches of MEDLINE, EMBASE, CINAHL, Cochrane bibliographic databases were performed using the terms “albumin” and “heart failure” from inception to January-2019. In addition, the reference lists of all selected publications were checked to retrieve relevant studies not identified by the search.
2.2. Study selection and eligibility criteria
Two reviewers (ME and BE) independently screened studies for inclusion based on pre-specified eligibility criteria: studies prospectively or retrospectively enrolled patients diagnosed with HF. Studies clearly reported the associations of SA and one or more outcomes of interest; all-cause mortality including in-hospital, long-term mortality, rehospitalization, or composite outcome of rehospitalization/mortality, providing a relevant hazard ratio (HR)/odds ratio (OR) and its 95% confidence interval (CI), or sufficient data to estimate them. Long-term mortality was defined as post-discharge mortality in patients admitted with acute HF and all-cause mortality in patients with chronic HF, articles published in languages other than English and conference abstracts were excluded.
2.3. Data extraction and quality assessment
Two reviewers (ME and BE) independently extracted the following details from each study: publication year; country; study design; number of centers involved; acute or chronic HF; type of HF (reduced vs preserved ejection fraction); HA cut-off point; HA prevalence; sample size; mean age; inclusion and exclusion criteria for each study; reported outcomes; follow-up period; HR and/or OR with corresponding 95% CIs and adjusted variables. When multiple studies analyzed data form the same clinical trial, we elected to include only the study which either directly evaluated the role of SA or the one with the largest sample size in our qualitative analysis. To evaluate the quality of evidence, studies were assessed by the method proposed by Hayden et al[11] in systematic reviews of prognostic studies. Studies which failed to include all of the following: age, sex, body mass index (BMI), liver function tests, and renal function tests in multivariate analysis were considered at high risk of confounding bias.
2.4. Statistical analyses
OR or HR was either extracted or calculated from individual papers. Though defined differently, OR and HR have been shown to be similar theoretically as well as empirically.[9,12] Hence, throughout this systematic review, we used the term OR to denote both OR and HR. Cumulative OR with 95% confidence interval (CI) was calculated using random effects model. Heterogeneity was assessed for using the I2 measure and the Cochran Q-statistic. We further stratified studies into subgroups based on type of analysis used (univariate vs multivariate) and follow up duration for each study. The Begg and Mazumdar rank correlation test was utilized to evaluate for publication bias. Statistical analyses were performed using the Comprehensive Meta-Analysis software version 3.3.070 (Biostat; Englewood, NJ). P-value <.05 was considered significant.
3. Results
3.1. Record allocation
Of the 2591 citations in the initial literature search, conference abstracts, review papers, letters to the editor, comments, and duplications were excluded. One hundred twenty five studies remained and were screened for eligibility criteria. A total of 48 studies were considered eligible and included in the analysis (Fig. 1). Six studies were included in the qualitative analysis, but not the quantitative analysis.
Figure 1.
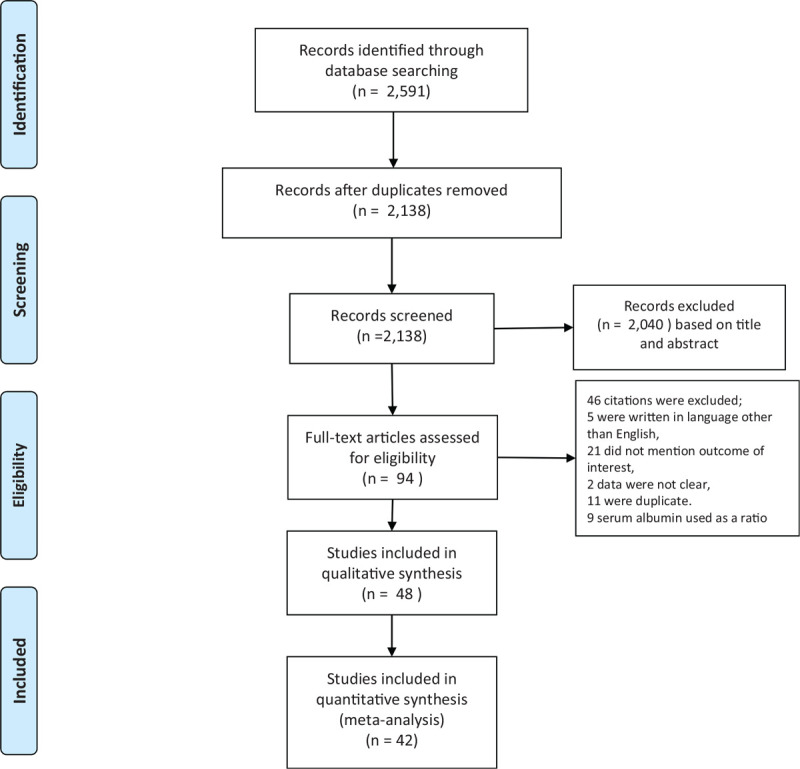
Flow diagram of record allocation.
3.2. Study characteristics
Fourty eight studies examining 44,043 HF patients were included in this review (Table 1). Study characteristics are outlined in the supplementary table.
Table 1.
Study characteristics.
| Characteristics | Number of studies | Total sample size |
| Study design, no | ||
| Prospective | 9 (19%) | 9987 (23%) |
| Retrospective | 25 (52%) | 20,060 (45%) |
| Secondary analysis of randomized controlled trials | 14 (29%) | 13,996 (32%) |
| Research country | ||
| North America | 12 (24%) | 11,348 (26%) |
| Europe | 9 (19%) | 1951 (4%) |
| Asia | 15 (31%) | 16,079 (36%) |
| Africa | 1 (2%) | 120 (0.2%) |
| Australia | 2 (4%) | 7789 (18%) |
| Inter-continent | 9 (19%) | 6756 (15%) |
| Number of hospitals | ||
| Single center | 33 (69%) | 15,293 (35%) |
| Multicenter | 15 (31%) | 28,750 (65%) |
| Sample size | ||
| >1000 | 16 (33%) | 30,379 (69%) |
| <1000 | 32 (66%) | 13,664 (31%) |
| Mean age | ||
| 50–69 | 20 (42%) | 12,476 (28%) |
| 70–80 | 16 (33%) | 16,159 (36%) |
| 80–95 | 8 (17%) | 8998 (20%) |
| NA | 4 (8%) | 6468 (15%) |
| Acute vs chronic | ||
| Acute | 42 (87%) | 34,962 (79%) |
| Chronic | 6 (13%) | 9081 (21%) |
The studies were published between 2006 and 2018, while 1 was published in 1992. Sample-size ranged from 33 to 8246. Mean-age of study populations ranged from 55 to 93 years. Percentage of male patients ranged from 13% to 100%. Nine studies were prospective, 25 retrospective, and 14 were secondary analyses of data collected in randomized controlled trials. Four[13–16] out of the 14 studies used data of population from a single trial[17] and 2 studies[18,19] used data from another trial.[20]
3.3. Quality of evidence evaluation
The overall risk of internal bias for included studies was rated as moderate. No publication bias was found in any of the analyzed outcomes using Begg and Mazumdar test. Twenty seven out of 48 of the included studies were not designed to assess the prognostic role of SA. On the other hand, 90% of the included studies used multivariate analysis to examine the prognostic role of studied parameters independently and decrease the risk of bias. The domains at low risk of bias were: population description/selection, attrition, description of statistical analysis, and outcome definition and measurement. Fourteen of the included studies used composite outcomes of rehospitalization or mortality after discharge or during follow up. In respect to prognostic factor assessment, HA was clearly defined only in 26 studies. Furthermore, techniques of albumin measurement and other laboratory parameters were not clearly described in 46% and 48% (Q3b, and Q3c), respectively. Studies which failed to include all of the following: age, sex, body mass index, liver function tests, and renal function tests in multivariate analysis were considered at high risk of confounding bias, this was the case in 50% of the included studies. Thus, the overall risk of bias in this category (Q5b) was considered to be moderate (Fig. 2).
Figure 2.
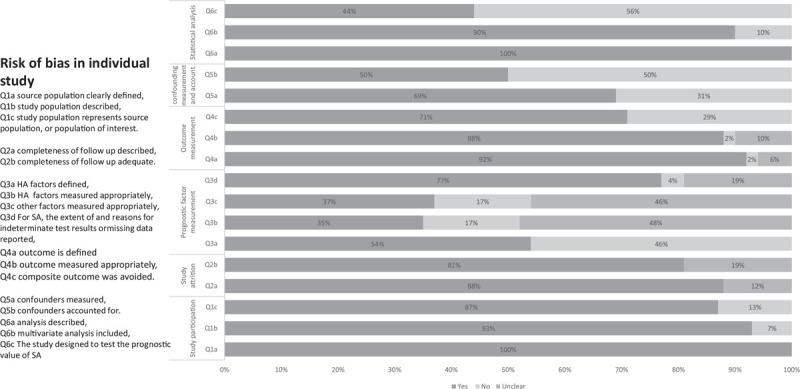
Risk of bias assessment.
3.4. Pooled prevalence of hypoalbuminemia in HF
Twenty two studies reported HA prevalence[13,21–29,29–40] ranging between 28% and 37%. Pooled prevalence was 32% (95% CI 28.4%–37.4%) with marked heterogeneity (I2 = 98%) (Fig. 3). Subgroup analysis showed that hypoalbuminemia is more prevalent in acute HF 38.5% (95% CI 34.6%–42.5%) than in chronic 19.7% (95% CI 15.2%–25.1%).
Figure 3.
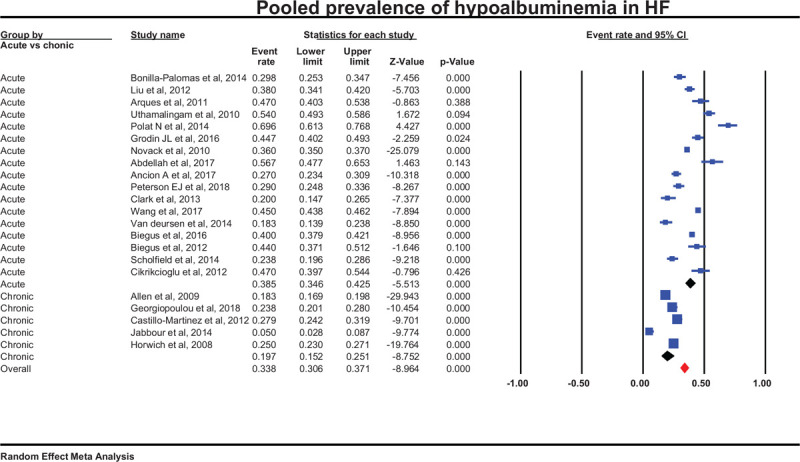
Pooled prevalence from 22 studies.
3.5. Hypoalbuminemia in HF and association to mortality
In 10 studies evaluating acute HF patients[13,21,23,30,31,34,41–44] mortality was approximately 4 times more likely in HA (OR 3.77 [95% CI 1.96–7.23] [Fig. 4A]). Further stratification by type of analysis revealed a pooled OR 5.62 (95% CI 1.86–16.95) in 5 studies performing univariate analysis compared with OR 3.05 (95% CI 1.36–6.82) in the 5 studies[13,21,30,42,43] which performed a multivariate analysis accounting for confounders such as age, sex, BMI, serum creatinine, BNP level, C-reactive peptide level, systolic blood pressure, and left ventricular ejection fraction (LVEF). OR for mortality at 3 months[26–28] was 2.12 (95% CI: 1.19–3.37), at 6 months[13,36,38,45–47] was 1.16 (95% CI: 1.03–1.36), and at 12 months[22,24,25,48–51] was 2.44 (95% CI 2.05–2.91) (Fig. 4B). HA was associated with a significant increase in long-term mortality (OR: 1.5; 95% CI: 1.36–1.64). The association was most strikingly demonstrated at 1-year follow-up (OR: 2.44; 95% CI: 2.05–2.91; I2 = 11%).
Figure 4.
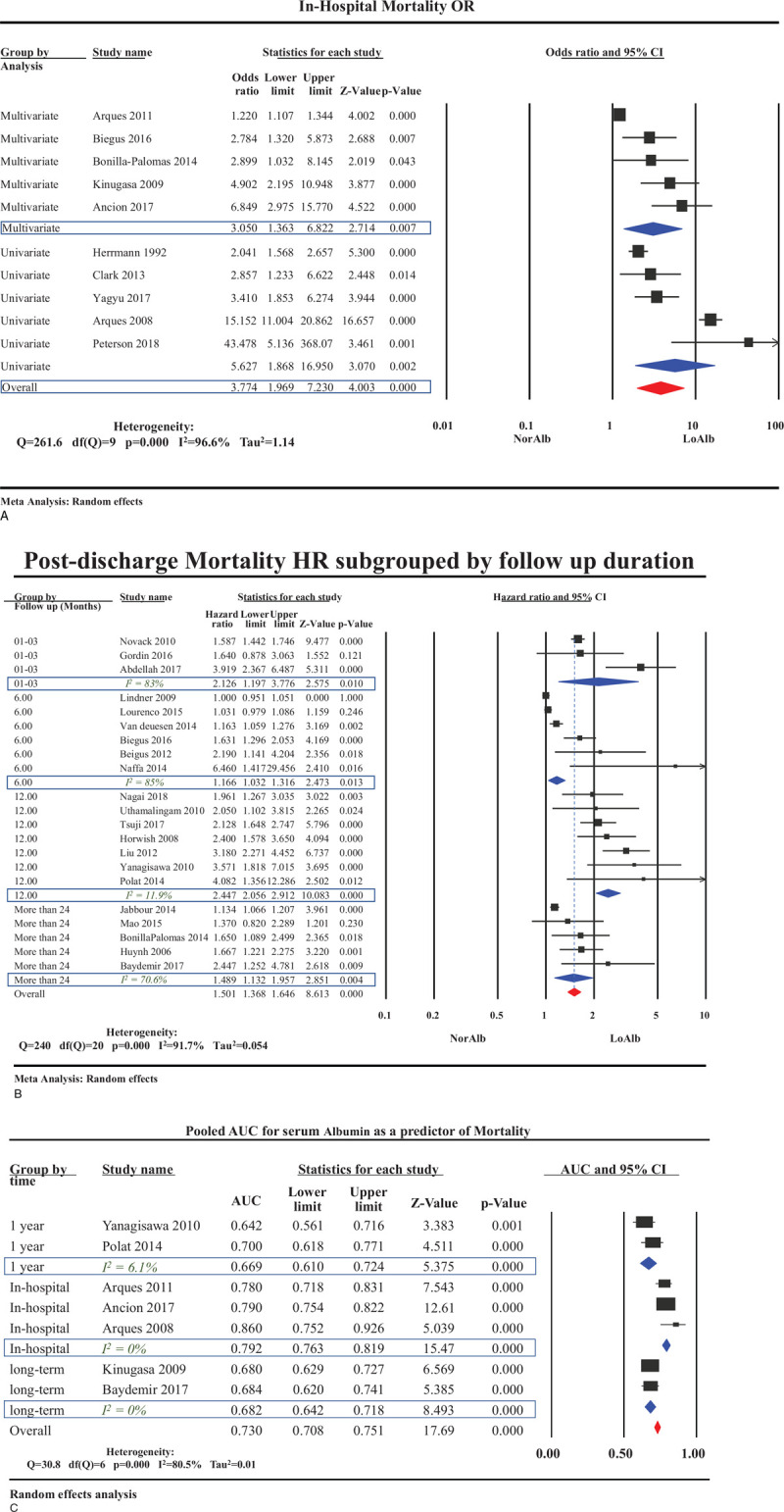
Mortality and hypoalbuminemia in patient with HF. (A) Overall odds ratio for in-hospital mortality in patient with heart failure sub-grouped according to the type of analysis conduct. (B) Long-term mortality in hypoalbuminemia group categorized by duration of follow up. (C) Pooled area under the curve for serum albumin in sub-groups based on duration of follow up. HF = heart failure.
Seven studies[23,25,30,42,43,50,52] evaluated the diagnostic accuracy of HA in predicting mortality in HF (pooled area under the curve [AUC] 0.73; 95% CI 0.67–0.78). While pooled AUC for 1-year mortality was 0.69 (95% CI 0.61–0.79) from 2 studies,[25,50] pooled AUC for in-hospital mortality was 0.79 (95% CI 0.76–0.81) from 3 studies.[23,30,42] (Fig. 4C).
3.6. Serum albumin relation to length of hospital stay (LOS) and readmission rates in heart failure patients
Six studies evaluated the relationship between HA and LOS in patients admitted with ADHF. Five studies[14,18,23,41,53] showed that HA was associated with longer hospital stay. Multivariate analysis[14,23,53] was used in 3 publications, demonstrating that SA was an independent predictor of LOS. The reporting of LOS in median (interquartile range) form hindered our ability to perform a meaningful meta-analysis of HA effect on LOS.
Four studies[26,29,52,54] reported on the association between HA and rehospitalization showing an insignificant increase in rehospitalization in the HA group (OR 1.22; 95% CI: 0.96–1.55). Four other studies[14,33,36,55] reported a significant increase in a composite outcome of rehospitalization or cardiac death in the HA group an (OR 1.17; 95% CI: 1.06–1.30) (Fig. 5A).
Figure 5.
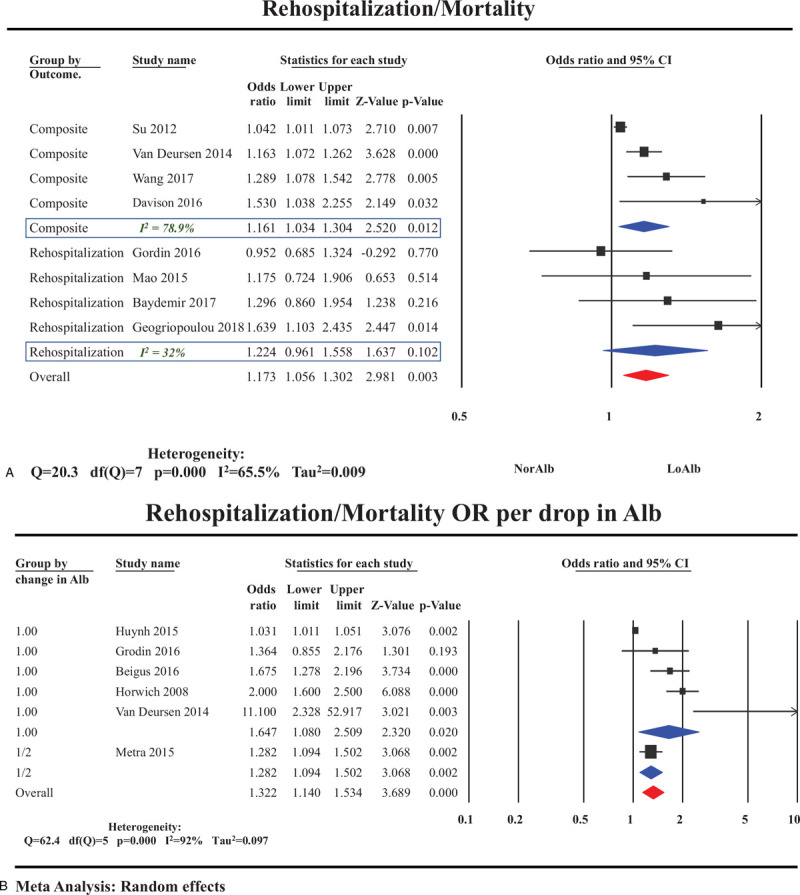
. Rehospitalization and hypoalbuminemia in patient with HF. (A) Odds ratios of rehospitalization and hypoalbuminemia sub-grouped by type of outcome (composite vs rehospitalization). (B) Odds ratios of rehospitalization/mortality composite outcome per drop in serum albumin. HF = heart failure.
3.7. Mortality is negatively correlated to SA
Five studies reported the effect of decreasing levels of SA on mortality.[13,24,26,36,56] Mortality increased as SA decreased with OR 1.64 (95% CI 1.08–2.50) for each 1 g/L decrement in SA (Fig. 5B).
4. Discussion
While multiple risk-stratification models and prognostic scores for HF have been proposed and validated, none of them investigated the role of SA.[57,58] In a recent meta-analysis, Peng et al[59] studied HA and mortality in HF, however, in our study we aimed to summarize the increasingly available evidence on the overall prognostic role of HA in HF in inpatient and outpatient mortality, LOS, and rehospitalization. Vincent et al[12] found HA to be an independent prognostic factor in all patients acutely admitted to the hospital. In this review we show this holds true for patients with acute HF. We note HA was present in one-third of HF patients and associated with increased HF mortality in the inpatient (OR 3.77, 95% CI 1.96–7.23) and outpatient (OR 2.44; 95% CI: 2.05–2.91) setting. This observation was preserved after excluding studies which only conducted univariate analysis indicating the independent effects of HA on mortality in HF. Similarly, Peng et al[59] showed in their meta-analysis that HA was associated with increased mortality in heart failure. We also found mortality to be negatively correlated to SA. Although SA is not a specific cardiac marker, admission HA had a pooled AUC of 0.79 (95% CI 0.76–0.81) in predicting in-hospital mortality which is comparable to that reported for elevated BNP on admission (AUC of 0.79).[60] This suggests SA might be a potential marker for mortality in HF.
A downward trend in SA was associated with worse prognosis in acute[13,31,61,62] and chronic HF.[32] Jabbour et al[32] followed 212 patients with chronic systolic HF for over 2 years and found that a drop in SA from baseline was associated with higher mortality compared with retained baseline SA. A study by Biegus et al[13] showed that a downward trend in SA during the first 4 days of hospitalization was associated with, increased 6-month mortality, the risk was proportional to the extent of albumin decrement. Moreover, an upward trend in SA was associated with a drop in cardiovascular and heart failure related mortality as well as all-cause mortality in ADHF.[61] The pathophysiology behind this association is not clear. However, ineffective diuresis and fluid overload might have a dilutional effect on SA and a subsequent worsening of outcome.[13] Accentuated inflammatory reaction has also been suggested.[31] These results suggest that a downward trend in SA might assist in longitudinal prognostication of HF patients over time.
SA can be affected by nutrition, inflammation, hepatorenal conditions, and volume-status of HF patients (Fig. 5). Reports have linked HA in HF to ongoing inflammation reflected by its strong correlation to C-reactive protein (CRP),[21,25,52] and serum total cholesterol[24,30] levels. Moreover, SA has been used as a marker for malnutrition and wasting syndrome in HF.[21,25] Another cause of HA can be hepatic dysfunction due to venous congestion and ischemic injury.[38] Other mechanisms include renal dysfunction,[22] protein-losing enteropathy secondary to gastrointestinal congestion[49] and hemodilution.[55] The effect of HA on HF is not completely understood. However, HA has been used as an indicator of inflammation, cachexia, and malnutrition[21,25] in patients with HF. Moreover, diuretic resistance, and pulmonary edema are more pronounced in patients with severe hypoalbuminemia.[25] This might explain the higher risk of mortality associated with HA.
Despite the findings above, no specific therapy targeting HA in HF has been studied. The only available data on rectifying HA in HF is derived from studies on LVAD outcomes. Two studies followed patients with HF who received LVAD. Longitudinal trend of SA in this population showed a progressive rise,[63,64] and even normalization of SA within 6 months.[63] Furthermore, a study found that patients with resolution of HA after LVAD had improved overall outcome.[65] This might be related to enhanced liver perfusion, decreased hepatic congestion and improved fluid status.[63] Although the co-administration of albumin and diuretics has been studied in nephrotic syndrome, and liver cirrhosis,[66] data are lacking on this treatment in HF. Future studies are needed to explore the role of this treatment strategy in HF.
4.1. Limitations
This study has the following limitations: only 9 out of the 48 studies used prospective design, half of the included studies did not adequately address relevant confounders in their multivariate analysis. Moreover, we observed significant heterogeneity between included studies. This might be related to the large variation between sample size, age, sex, and study design among the included studies. In addition, only 44% of the included papers were designed to evaluate the effect of HA on HF outcomes. Furthermore, we have used OR to denote both OR and HR, which might have introduced some bias. Despite these limitations, our findings provide valuable insights into the significance of SA in HF prognostication. Our results emphasize the need for population-based studies which longitudinally follow SA in HF patients to validate its prognostic utility.
4.2. Future direction
In Conclusion, SA is an independent predictor of mortality in HF especially in ADHF. Therefore, SA might be an additional marker for the identification of patients at high-risk of complications and mortality, who would require a higher level of monitoring, and earlier, intensive treatment. Our finding needs to be validated in controlled trial to assess whether an earlier intervention in ADHF patients with HA would change the outcome.
5. Conclusion
In summary, we found that HA in HF is associated with increased mortality rates and increased LOS. A downward trend in SA might indicate worse outcome. HA is a strong predictor of mortality in acute HF with AUC of 0.79 comparable to that reported for established factors like BNP. As such, SA level, which is a broadly available and affordable test, might provide a simple method to identify patients with increased risk of mortality.
Author contributions
Conceptualization: Mahmoud El Iskandarani, Bara El Kurdi, Timir K. Paul, Marwan M. Refaat.
Data curation: Mahmoud El Iskandarani, Bara El Kurdi, Ghulam Murtaza.
Formal analysis: Mahmoud El Iskandarani, Bara El Kurdi, Ghulam Murtaza.
Supervision: Ghulam Murtaza, Timir K. Paul, Marwan M. Refaat.
Writing – original draft: Mahmoud El Iskandarani, Bara El Kurdi, Ghulam Murtaza, Timir K. Paul.
Writing – review & editing: Timir K. Paul, Marwan M. Refaat.
Supplementary Material
Footnotes
Abbreviations: ADHF = acute decompensated heart failure, AUC = area under the curve, BNP = brain natriuretic peptide, CI = confidence interval, HA = hypoalbuminemia, HF = heart failure, HR = hazard ratio, LOS = length of hospital stay, LVAD = left ventricular assist devices, OR = odds ratio, SA = serum albumin, sST2 = soluble ST2.
How to cite this article: El Iskandarani M, El Kurdi B, Murtaza G, Paul TK, Refaat MM. Prognostic role of albumin level in heart failure: A systematic review and meta-analysis. Medicine. 2021;100:10(e24785).
Financial support: None.
Potential competing interests: None.
Institutional review board approval: Not required. The review doesn’t involve human subjects.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Santaguida PL, Don-Wauchope AC, Oremus M, et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev 2014;19:453–70. [DOI] [PubMed] [Google Scholar]
- [3].de Boer RA, Daniels LB, Maisel AS, et al. State of the Art: newer biomarkers in heart failure. Eur J Heart Fail 2015;17:559–69. [DOI] [PubMed] [Google Scholar]
- [4].Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- [5].Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432–7. [DOI] [PubMed] [Google Scholar]
- [6].De Feo P, Lucidi P. Liver protein synthesis in physiology and in disease states. Curr Opin Clin Nutr Metab Care 2002;5:47–50. [DOI] [PubMed] [Google Scholar]
- [7].Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc 2004;104:1258–64. [DOI] [PubMed] [Google Scholar]
- [8].Kato TS, Kitada S, Yang J, et al. Relation of preoperative serum albumin levels to survival in patients undergoing left ventricular assist device implantation. Am J Cardiol 2013;112:1484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med 2010;36:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- [11].Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. [DOI] [PubMed] [Google Scholar]
- [12].Vincent J-L, Dubois M-J, Navickis RJ, et al. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg 2003;237:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Biegus J, Hillege HL, Postmus D, et al. Abnormal liver function tests in acute heart failure: relationship with clinical characteristics and outcome in the PROTECT study. Eur J Heart Fail 2016;18:830–9. [DOI] [PubMed] [Google Scholar]
- [14].Davison BA, Metra M, Senger S, et al. Patient journey after admission for acute heart failure: length of stay, 30-day readmission and 90-day mortality. Eur J Heart Fail 2016;18:1041–50. [DOI] [PubMed] [Google Scholar]
- [15].O’Connor CM, Mentz RJ, Cotter G, et al. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail 2012;14:605–12. [DOI] [PubMed] [Google Scholar]
- [16].Cleland JG, Chiswell K, Teerlink JR, et al. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: a report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ Heart Fail 2014;7:76–87. [DOI] [PubMed] [Google Scholar]
- [17].Massie BM, O’Connor CM, Metra M, et al. Rolofylline, an adenosine A1−receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–28. [DOI] [PubMed] [Google Scholar]
- [18].Cotter G, Davison BA, Milo O, et al. Predictors and associations with outcomes of length of hospital stay in patients with acute heart failure: results from VERITAS. J Card Fail 2016;22:815–22. [DOI] [PubMed] [Google Scholar]
- [19].Metra M, Cotter G, El-Khorazaty J, et al. Acute heart failure in the elderly: differences in clinical characteristics, outcomes, and prognostic factors in the VERITAS Study. J Card Fail 2015;21:179–88. [DOI] [PubMed] [Google Scholar]
- [20].Teerlink JR, McMurray JJV, Bourge RC, et al. Tezosentan in patients with acute heart failure: design of the Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Study (VERITAS). Am Heart J 2005;150:46–53. [DOI] [PubMed] [Google Scholar]
- [21].Bonilla-Palomas JL, Gámez-López AL, Moreno-Conde M, et al. Hypoalbuminemia in acute heart failure patients: causes and its impact on hospital and long-term mortality. J Card Fail 2014;20:350–8. [DOI] [PubMed] [Google Scholar]
- [22].Liu M, Chan C-P, Yan BP, et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2012;14:39–44. [DOI] [PubMed] [Google Scholar]
- [23].Arques S, Roux E, Stolidi P, et al. Usefulness of serum albumin and serum total cholesterol in the prediction of hospital death in older patients with severe, acute heart failure. Arch Cardiovasc Dis 2011;104:502–8. [DOI] [PubMed] [Google Scholar]
- [24].Horwich TB, Kalantar-Zadeh K, MacLellan RW, et al. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883–9. [DOI] [PubMed] [Google Scholar]
- [25].Polat N, Aydin M, Yildiz A, et al. The prognostic significance of serum albumin in patients with acute decompensated systolic heart failure. Acta Cardiol 2014;69:648–54. [DOI] [PubMed] [Google Scholar]
- [26].Grodin JL, Lala A, Stevens SR, et al. Clinical implications of serum albumin levels in acute heart failure: insights from DOSE-AHF and ROSE-AHF. J Card Fail 2016;22:884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Novack V, Pencina M, Zahger D, et al. Routine laboratory results and thirty day and one-year mortality risk following hospitalization with acute decompensated heart failure. PLoS One 2010;5:e12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abdellah AT, Mohamed AD, Hendawi HA, et al. Clinical and laboratory characteristics of short-term mortality in Egyptian patients with acute heart failure. Egypt Heart J 2017;69:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Georgiopoulou VV, Velayati A, Burkman G, et al. Comorbidities, sociodemographic factors, and hospitalizations in outpatients with heart failure and preserved ejection fraction. Am J Cardiol 2018;121:1207–13. [DOI] [PubMed] [Google Scholar]
- [30].Ancion A, Allepaerts S, Oury C, et al. Serum albumin level and hospital mortality in acute non-ischemic heart failure. ESC Heart Fail 2017;4:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Peterson EJ, Ng TMH, Patel KA, et al. Association of admission vs. nadir serum albumin concentration with short-term treatment outcomes in patients with acute heart failure. J Int Med Res 2018;46:3665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jabbour R, Ling HZ, Norrington K, et al. Serum albumin changes and multivariate dynamic risk modelling in chronic heart failure. Int J Cardiol 2014;176:437–43. [DOI] [PubMed] [Google Scholar]
- [33].Wang N, Gallagher R, Sze D, et al. Predictors of frequent readmissions in patients with heart failure. Heart Lung Circ 2019;28:277–83. [DOI] [PubMed] [Google Scholar]
- [34].Clarke MM, Dorsch MP, Kim S, et al. Baseline albumin is associated with worsening renal function in patients with acute decompensated heart failure receiving continuous infusion loop diuretics. Pharmacotherapy 2013;33:583–8. [DOI] [PubMed] [Google Scholar]
- [35].Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 2009;11:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van Deursen VM, Edwards C, Cotter G, et al. Liver function, in-hospital, and post-discharge clinical outcome in patients with acute heart failure-results from the relaxin for the treatment of patients with acute heart failure study. J Card Fail 2014;20:407–13. [DOI] [PubMed] [Google Scholar]
- [37].Scholfield M, Schabath MB, Guglin M. Longitudinal trends, hemodynamic profiles, and prognostic value of abnormal liver function tests in patients with acute decompensated heart failure: an analysis of the ESCAPE trial. J Card Fail 2014;20:476–84. [DOI] [PubMed] [Google Scholar]
- [38].Biegus J, Zymliński R, Sokolski M, et al. Liver function tests in patients with acute heart failure. Pol Arch Med Wewn 2012;122:471–9. [DOI] [PubMed] [Google Scholar]
- [39].Castillo-Martínez L, Colín-Ramírez E, Orea-Tejeda A, et al. Cachexia assessed by bioimpedance vector analysis as a prognostic indicator in chronic stable heart failure patients. Nutrition 2012;28:886–91. [DOI] [PubMed] [Google Scholar]
- [40].Cikrikcioglu MA, Soysal P, Dikerdem D, et al. Absolute blood eosinophil count and 1-year mortality risk following hospitalization with acute heart failure. Eur J Emerg Med 2012;19:257–63. [DOI] [PubMed] [Google Scholar]
- [41].Herrmann FR, Safran C, Levkoff SE, et al. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch Intern Med 1992;152:125–30. [PubMed] [Google Scholar]
- [42].Arques S, Roux E, Sbragia P, et al. Usefulness of serum albumin concentration for in-hospital risk stratification in frail, elderly patients with acute heart failure. Insights from a prospective, monocenter study. Int J Cardiol 2008;125:265–7. [DOI] [PubMed] [Google Scholar]
- [43].Kinugasa Y, Kato M, Sugihara S, et al. A simple risk score to predict in-hospital death of elderly patients with acute decompensated heart failure--hypoalbuminemia as an additional prognostic factor. Circ J 2009;73:2276–81. [DOI] [PubMed] [Google Scholar]
- [44].Yagyu T, Kumada M, Nakagawa T. Novel risk stratification with time course assessment of in-hospital mortality in patients with acute heart failure. PLoS One 2017;12:e0187410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lindner G, Doberer E, Vychytil A, et al. Prognosis in patients with congestive heart failure and subacute renal failure treated with hemodialysis. Wien Klin Wochenschr 2009;121:391–7. [DOI] [PubMed] [Google Scholar]
- [46].Naffaa M, Makhoul BF, Tobia A, et al. Brain natriuretic peptide at discharge as a predictor of 6-month mortality in acute decompensated heart failure. Am J Emerg Med 2014;32:44–9. [DOI] [PubMed] [Google Scholar]
- [47].Lourenço P, Ribeiro A, Pintalhão M, et al. Predictors of six-month mortality in bnp-matched acute heart failure patients. Am J Cardiol 2015;116:744–8. [DOI] [PubMed] [Google Scholar]
- [48].Nagai T, Yoshikawa T, Saito Y, et al. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction- a report from the Japanese Heart Failure Syndrome With Preserved Ejection Fraction (JASPER) Registry. Circ J 2018;82:1534–45. [DOI] [PubMed] [Google Scholar]
- [49].Uthamalingam S, Kandala J, Daley M, et al. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J 2010;160:1149–55. [DOI] [PubMed] [Google Scholar]
- [50].Yanagisawa S, Miki K, Yasuda N, et al. Clinical outcomes and prognostic factor for acute heart failure in nonagenarians: impact of hypoalbuminemia on mortality. Int J Cardiol 2010;145:574–6. [DOI] [PubMed] [Google Scholar]
- [51].Tsuji K, Sakata Y, Nochioka K, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail 2017;19:1258–69. [DOI] [PubMed] [Google Scholar]
- [52].Baydemir C, Ural D, Karaüzüm K, et al. Predictors of long-term mortality and frequent re-hospitalization in patients with acute decompensated heart failure and kidney dysfunction treated with renin-angiotensin system blockers. Med Sci Monit 2017;23:3335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Davison BA, Metra M, Cotter G, et al. Worsening heart failure following admission for acute heart failure: a pooled analysis of the PROTECT and RELAX-AHF studies. JACC Heart Fail 2015;3:395–403. [DOI] [PubMed] [Google Scholar]
- [54].Mao C-T, Liu M-H, Hsu K-H, et al. Effect of multidisciplinary disease management for hospitalized heart failure under a national health insurance programme. J Cardiovasc Med (Hagerstown) 2015;16:616–24. [DOI] [PubMed] [Google Scholar]
- [55].Su W, An T, Zhou Q, et al. Serum albumin is a useful prognostic indicator and adds important information to NT-proBNP in a Chinese cohort of heart failure. Clin Biochem 2012;45:561–5. [DOI] [PubMed] [Google Scholar]
- [56].Huynh QL, Saito M, Blizzard CL, et al. Roles of nonclinical and clinical data in prediction of 30-day rehospitalization or death among heart failure patients. J Card Fail 2015;21:374–81. [DOI] [PubMed] [Google Scholar]
- [57].Fonarow GC, Adams KF, Abraham WT, et al. For the ADHERE Scientific Advisory Committee SG. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–80. [DOI] [PubMed] [Google Scholar]
- [58].Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes 2010;3:25–32. [DOI] [PubMed] [Google Scholar]
- [59].Peng W, Zhang C, Wang Z, et al. Prediction of all-cause mortality with hypoalbuminemia in patients with heart failure: a meta-analysis. Biomarkers 2019;24:631–7. [DOI] [PubMed] [Google Scholar]
- [60].Fonarow GC, Peacock WF, Phillips CO, et al. Admission B-Type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007;49:1943–50. [DOI] [PubMed] [Google Scholar]
- [61].Ambrosy AP, Vaduganathan M, Huffman MD, et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail 2012;14:302–11. [DOI] [PubMed] [Google Scholar]
- [62].Taylor P, Crouch S, Howell DA, et al. Change in physiological variables in the last two weeks of life: an observational study of hospitalized adults with heart failure. J Pain Symptom Manage 2018;55:1335–40. [DOI] [PubMed] [Google Scholar]
- [63].Guvenc TS, Güzelburc O, Ekmekci A, et al. The effect of left ventricular assist device implantation on serum albumin, total protein and body mass: a short-term, longitudinal follow-up study. Heart Lung Circ 2017;26:702–8. [DOI] [PubMed] [Google Scholar]
- [64].Deo SV, Sharma V, Altarabsheh SE, et al. Hepatic and renal function with successful long-term support on a continuous flow left ventricular assist device. Heart Lung Circ 2014;23:229–33. [DOI] [PubMed] [Google Scholar]
- [65].Gopal DJ, Hanff TC, Mazurek JA, et al. Prognostic implications of changes in albumin following left ventricular assist device implantation in patients with severe heart failure. Am J Cardiol 2017;120:2003–7. [DOI] [PubMed] [Google Scholar]
- [66].Kitsios GD, Mascari P, Ettunsi R, et al. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: a meta-analysis. J Crit Care 2014;29:253–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


