Abstract
Background
Type 2 diabetes mellitus (T2DM) has been associated with impairment of cognitive functions. Since the majority of patients with diabetes in the Saudi population are between the ages of 40 and 69 years, it is crucial to ascertain whether the control of blood glucose level negatively correlates with the level of cognitive function scores similar to the way it correlates in those who are not controlling their blood glucose level with medications.
Aims
To assess cognitive functions in patients with T2DM and examine the effect of glycemic control on cognitive functions impairment in Saudi adults with T2DM.
Methods and material
Seventy-nine patients with T2DM underwent cognitive assessment testing using the Cambridge neuropsychological test automated battery (CANTAB), Mini-Mental State Examination (MMSE), and Fatigue severity scale. Their cognitive function scores were then correlated with their blood glucose levels, duration of diabetes, and levels of education. Poor glycemic control was defined as glycated hemoglobin levels more than 7.5. We excluded patients with depression or neurocognitive disorders as well as those over 75 years of age.
Results
Attention switching task (AST) total latency (P = 0.003), AST congruent score (P = 0.002), AST incongruent score (P = 0.003), AST block 3 (p = 0.004), and AST Block 7 (p = 0.006) were significantly higher in poorly-controlled DM. The intra-extra dimensional set shift (IED) total errors were significantly higher in poorly-controlled patients (p = 0.023). The difference in IED stages completed (p = 0.716) and spatial span (SSP) (p = 0.782) were not significant between the two groups. The mini-mental state exam (p = 0.336) and the fatigue severity scale (P = 0.167) did not show any statistical significance between good and poor control of T2DM. There was a significant positive correlation between the duration of T2DM and AST latencies for AST total latency, AST congruent score, and AST incongruent score.
Conclusions
Patients with T2DM have a statistically significant association between their cognitive functions and their glycemic control. Patients with uncontrolled T2DM showed decreased cognitive scores. Moreover, worsened cognitive scores were associated with longer disease duration.
Keywords: Cognitive functions, Cognitive impairment, CANTAB, Type 2 diabetes mellitus, Duration of diabetes, Glycosylated hemoglobin
Cognitive functions, Cognitive impairment, CANTAB, Type 2 diabetes mellitus, Duration of diabetes, Glycosylated hemoglobin.
1. Introduction
According to the American Diabetes Association, “Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Chronic hyperglycemia is associated with long-term multiple organ dysfunction including the eyes, kidneys, nerves, heart, and blood vessels” [1]. One of the most recently discovered complications of diabetes is the progressive decrease in mental ability and cognition, in particular, there is a decrease in processing speed, verbal memory, and executive functions, whereas visuospatial, attention, semantic and language functions seem to be preserved, controlling blood glucose levels, however, has shown to help delay such affects [2, 3, 4]. Moreover, one systemic review suggested that physical exercise decreases the diabetes-associated risk of dementia by 28% and risk of Alzheimer's disease by 45% [5]. However, other studies linked diabetes with alterations in different aspects of cognition and fatigability, regardless of blood sugar levels and disease control [6]. In 2014, a review article, including 86 articles, addressed the relationship between glucose regulation and cognitive function in type 2 diabetes mellitus (T2DM) patients and concluded that cognitive functions in type 2 diabetics without dementia are inversely correlated with levels of glycated hemoglobin [7]. And as the elevated adipose tissue is a contributor in the incidence of T2DM, a paper studying the relation between cognition and adiposity levels found that the amount of adipose tissue exceeding a certain degree in abundance would facilitate dementia progression and a decline in the overall mental status [8]. Furthermore, recent studies have compared the cognitive functions between type 2 diabetics and non-diabetics and found a trend in impairment in executive function, working memory, psychomotor and attentional functions in diabetic patients [3, 9]. A study assessing the severity of depressive symptoms among diabetic patients and their relation to adherence to the dietary regimen and the medications, concluded that the more severe the depressive symptoms, the less adherent the patients are to medications and dietary regime, resulting in poorer physical and mental functioning [10]. Another study suggested that the chronicity T2DM correlates with MRI evidence of cognitive impairment [9]. Interestingly, Diabetic patients were found to have higher fatigue scores using the Multidimensional Fatigue-Inventory (MFI) than non-diabetics regardless of insulin treatment [4]. Moreover, using different fatigue scoring scales, diabetic patients had overall higher fatigue scores regardless of the scoring scale used in comparison to age-matched non-diabetic subjects [11]. Researchers addressing the pathological basis of cognitive impairment in diabetic patients have faced difficulty in studying this relationship due to the presence of other important confounders, such as cardiovascular diseases and high adipose tissue [8]. A meta-analysis study of 24 trials that included a total of 26,137 subject compared with T2DM, which highlighted the impaired performance in tasks of executive function, attention/concentration, visual memory and verbal memory [12]. The previous study showed the poor glycemic control is associated with the impairment of cognitive dysfunction [13, 14].
Therefore, the present study aimed to assess cognitive functions in patients with T2DM and examine the effect of glycemic control on the severity of decline in cognitive functions in Saudi adults with T2DM.
2. Patients and methods
This is a cross sectional study. A total of 79 patients with T2DM (Male = 70 and Female = 9) were recruited from outpatient clinics at King Khalid University Hospital (KKUH) in Riyadh, Saudi Arabia. This study was conducted between February 2016 and October 2017. Ethical approval was obtained from the college of medicine and informed consent was obtained from all patients before participating in the study. Based on the criteria listed by the American Diabetes Association (ADA), the patients were diagnosed with T2DM with at least one year of duration. We included patients between 30 and 75 years of age who were metabolically stable. Patients were divided into two groups (Good control vs Poor control) according to their HbA1c levels. Patients were said to have good glycemic control if their glycosylated hemoglobin (HbA1c) value was 7.5% or less [1]. Patients had to complete the Cambridge neuropsychological test automated battery (CANTAB) assessment, fatigue severity scale and mini-mental state examination (MMSE). We excluded patients with a medical history of cognitive impairment, dementia, depression, liver dysfunction, or renal dysfunction.
2.1. Assessment tasks and procedures
2.1.1. Mini-mental state examination (MMSE)
The MMSE is one of the most widely used tools for quantitative assessment of cognitive functions. The test is composed of various questions aimed to assess orientation to time and place, registration, attention and calculation, recall, language, repetition, and visual construction with a maximum score of 30 [15]. The MMSE only requires 5–10 min to administer, making it a practical tool for research purposes.
2.1.2. Biomarker assessment
Fasting venous blood samples were collected from all patients and were analyzed for glucose, complete blood count, lipid profile, and HbA1c.
2.1.3. Designed performa
We obtained the patient's name, medical record number, age, education, job, history of diabetes, glycemic control, medication history, history of other co-morbid conditions or chronic illnesses (e.g. Liver dysfunction), presence, history of mental illnesses (depression, anxiety, etc.), and level of physical exercise and dietary habits. Hypertension was defined as a systolic blood pressure of 130 or above and/or diastolic blood pressure of 80 or above.
2.1.4. Fatigue severity scale (FSS)
A standardized questionnaire was used to assess fatigue severity by interviewing patients to assess overall fatigue level.
2.2. Cognitive functions
Neuropsychological testing was performed using CANTAB research suite software (version 6. 0.37, Cambridge cognition). The cognitive domains assessed using CANTAB include memory, attention, processing speed, visuospatial function, and executive function [16]. Selected tests in the battery required a total of 25–30 min to complete the tasks. The subject was made to sit comfortably on a seat and was asked to keep pressing the response button with the index finger of their dominant hand as per the manufacturer's instructions. The principle parameters of the CANTAB are the attention switching task (AST), which tests attention and cognitive flexibility, and the intra-extra dimensional set shift (IED) task, which uses an adaptation of the Wisconsin card sorting test. Another component of the IED is the number of stages completed in which the participant is asked to choose the correct shape on the screen in accordance to the underlying rule.
The IED test is essential for the assessment of cognitive flexibility, visual discrimination, and the frontostriatal area of the brain. This test is conducted by displaying a shape with an overlapping line on the screen, and the subject is asked to tap on that shape. Tapping on the wrong shape causes the device to produce a specific sound, giving the subject a hint that their choice was incorrect, and they need to reverse the rule and tap on another shape. Therefore, the IED is crucial for assessing rule learning and reversal.
The Spatial Span (SSP) test measures response latencies in millisecond and error scores that reflect the participant's attention switching ability and the interference of congruent and incongruent task-irrelevant information (i.e., a Stroop-like effect).
2.3. Statistical analysis
The data were analyzed using the statistical package for social sciences (SPSS) (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Categorical data were analyzed using CANTAB-core-numerical data and were summarized in the mean, standard deviation (SD), median, and range. Different groups were compared for categorical variables, using a Student's t test for normally distributed and non-parametric data and a Mann-Whitney U test for data that did not follow a normal distribution. Spearman's rank order and Pearson correlations. Univariate and multiple regression analysis were used where needed. A p-value of ≤0.05 was considered statistically significant.
3. Results
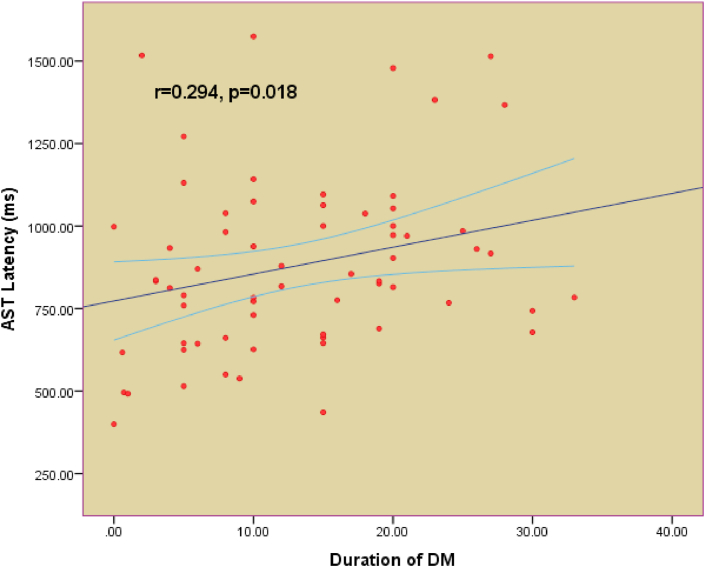
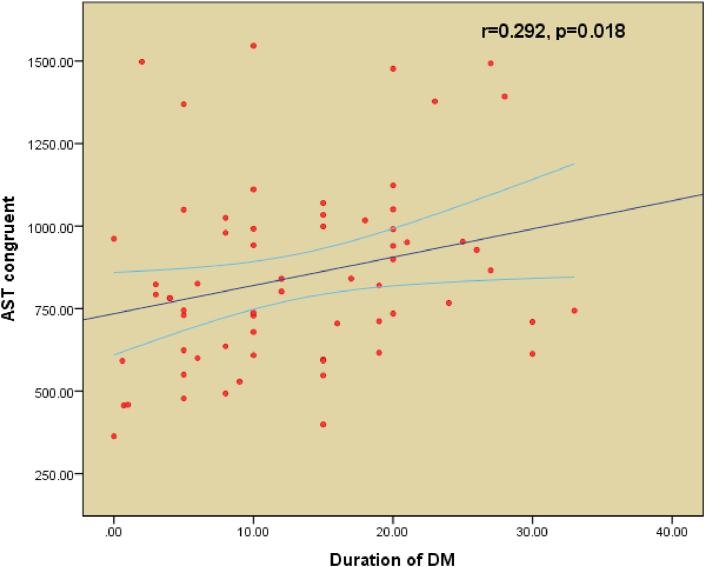
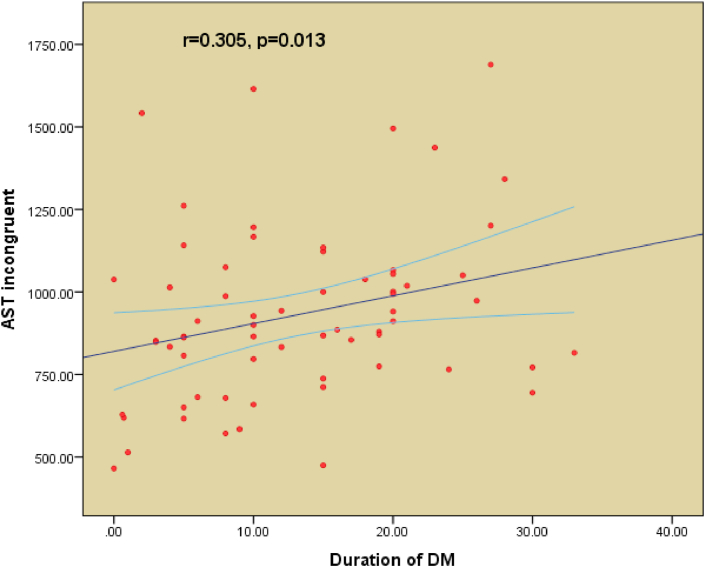
Table 1 shows the demographic characteristics of all T2DM patients. Both the good glycemic control group (n = 36) and the poor glycemic control group (n = 43) are shown in Table 2. The cutoff point for good glycemic control was a HbA1c of 7.5%. All patients were on oral hypoglycemic agents. A comparison between the demographic characteristics, clinical profiles, and biochemical profiles of good and poor glycemic control is shown in Table 2. Table 3 shows a comparison between the controlled and uncontrolled subjects in terms of cognitive function scores for AST total latency (p = 0.003), AST congruent score (p = 0.002), AST incongruent score (p = 0.003), AST block 3 (p = 0.004), and AST Block 7 (p = 0.006). The difference was insignificant for other parameters. IED total errors were significantly higher in poor-controlled patients (p = 0.023). The difference between IED stages completed (p = 0.716) and SSP (p = 0.782) were not significant between the two groups. Table 4 includes the MMSE (p = 0.336) and the fatigue severity scale (p = 0.167) parameters, which do not show any statistical significance between good and poor control of T2DM. Figures 1, 2, and 3 show the significant positive correlation between the duration of T2DM and AST latencies for AST total latency, AST congruent score, and AST incongruent score.
Table 1.
Demographic characteristics of all type 2 Diabetes Mellitus (T2DM) patients.
| Variables | All subjects | Good Glycemic Control n = 36 | Poor Glycemic Control n = 43 | P value |
|---|---|---|---|---|
| M/F | 70/9 | 31/5 | 39/4 | |
| Age (Range) | 57.25 ± 9.80 (35–75) | 55.46 ± 10.81 | 58.13 ± 9.05 | 0.249 |
| Duration of DM (years) | 13.18 ± 8.59 | 10.90 ± 9.05 | 14.82 ± 8.15 | 0.080 |
| Hypertension | 24 (30.3%) | 11 (13.9%) | 13 (16.4%) | 0.382∗ |
| Renal Dysfunction | 10 (12.6%) | 4 (5%) | 6 (7.5%) | 0.537∗ |
| Ischemic Heart Disease | 11 (13.9%) | 5 (6%) | 6 (7.5%) | 0.524∗ |
| Peripheral Vascular Disease | 14 (17.7%) | 7 (8.8%) | 7 (8.8%) | 0.495∗ |
| Retinopathy | 9 (11.3%) | 4 (5%) | 5 (6%) | 0.382∗ |
values are compared by Chi Square test.
Table 2.
Comparison of demographic characteristics and biochemical profile of study subjects between controlled and uncontrolled type 2 Diabetes Mellitus (T2DM) patients.
| Good Glycemic Control n = 36 | Poor Glycemic Control n = 43 | P value | |
|---|---|---|---|
| M/F | 31/5 | 39/4 | |
| Duration of DM (years) | 12.04 ± 8.07 | 15.02 ± 9.23 | 0.201 |
| WBC X 109/L | 6.35 ± 4.46 | 5.06 ± 3.98 | 0.320 |
| HGB g/L | 102.04 ± 65.80 | 107.10 ± 62.70 | 0.819 |
| MCV μm3 | 66.14 ± 38.21 | 57.19 ± 41.77 | 0.292 |
| AST U/L | 8.68 ± 8.61 | 9.41 ± 10.07 | 0.869 |
| CREATININE μmol/L | 73.11 ± 154.11 | 42.12 ± 44.94 | 0.441 |
| GLUCOSE mmol/L | 4.94 ± 4.244 | 5.68 ± 6.05 | 0.593 |
| Gamma GT (IU)/L | 34.23 ± 40.28 | 24.51 ± 40.19 | 0.096 |
| HbA1c % | 6.32 ± 1.75 | 9.10 ± 1.45 | 0.001 |
| CHOLESTEROL mmol/L | 2.21 ± 2.33 | 2.90 ± 2.41 | 0.213 |
| HDL mmol/L | 0.54 ± 0.56 | 0.66 ± 0.56 | 0.356 |
| LDL mmol/L | 1.30 ± 1.53 | 1.53 ± 1.46 | 0.510 |
| Triglycerides mmol/L | 0.81 ± 0.91 | 1.64 ± 2.80 | 0.045 |
| Duration of DM (in years) | 10.91 ± 9.06 | 14.83 ± 8.15 | 0.084 |
Data is expressed as Mean ± SD, Differences were studied by Student's t test.
Table 3.
Comparison of cognitive function by Cambridge Neuropsychological Test Automated Battery test variables between controlled and uncontrolled type 2 Diabetes Mellitus (T2DM) patients.
| Good Glycemic Control n = 36 | Poor Glycemic Control n = 43 | P value | |
|---|---|---|---|
| AST Total Latency | 730.53 ± 291.19 | 938.21 ± 293.76 | 0.003 |
| AST congruent | 692.55 ± 286.80 | 912.71 ± 305.14 | 0.002 |
| AST incongruent | 777.33 ± 295.77 | 990.45 ± 298.69 | 0.003 |
| AST blocks3 | 672.17 ± 279.80 | 876.92 ± 307.40 | 0.004 |
| AST7 | 836.23 ± 344.84 | 1050.91 ± 304.50 | 0.006 |
| AST Percent | 50.66 ± 34.54 | 59.51 ± 53.41 | 0.416 |
| IED Total errors | 7.41 ± 1.50 | 8.09 ± 1.06 | 0.023 |
| IED Stages completed | 4.44 ± 1.98 | 4.58 ± 1.43 | 0.716 |
| SSP | 1.81 ± 0.69 | 1.76 ± 0.77 | 0.782 |
Data is expressed as Mean ± SD, Differences were studied by Student's t test.
Table 4.
Mini-mental State Examination (MMSE) and Fatigue Severity Scale (FSS) results between controlled and uncontrolled type 2 Diabetes Mellitus T2DM patients.
| Good Glycemic Control n = 36 |
Poor Glycemic Control n = 43 |
P value | |
|---|---|---|---|
| MMSE | Mean: 26.05 ± 5.74 | Mean:26.08 ± 4.34 | .336 |
| Fatigue scale mean | Mean:33.67 ± 16.99 | Mean:38.64 ± 14.784 | .167 |
Data is expressed as Mean ± SD, Differences were studied by Student's t test.
Figure 1.
Pearson's correlation between duration of type 2 Diabetes Mellitus (T2DM) and AST Latency.
Figure 2.
Pearson's correlation between duration of type 2 Diabetes Mellitus (T2DM) and AST Congruent Latency.
Figure 3.
Pearson's correlation between duration of type 2 Diabetes Mellitus (T2DM) and AST Incongruent Latency.
4. Discussion
The results of this study suggest that patients with poorly controlled diabetes are more prone to the cognitive deterioration associated with hyperglycemia. ASTs, which are concerned with the assessment of attention and cognitive flexibility, appear to have significantly lower assessment scores in diabetic patients with poor glycemic control. Our results were similar to the study conducted by Wong which revealed that patients with T2DM have impaired attention [9]. Moreover, our current study revealed exactly what attention parameters are prone to be affected by diabetes [3]. We have found that the incongruent AST was preferentially affected, while the congruent AST showed no statistically significant difference from the control. AST incongruent is known to be negatively affected by conditions such as smoking and it could be improved by brain training games [17, 18].
We also observed that cognitive performance among diabetic patients was not affected by the presence of hypertension. This is in contrast with a previous study that concluded that control of blood pressure in diabetic patients may improve cognitive ability [11]. An important obstacle for interpreting fatigue scores in diabetic patients with a high BMI is that a high BMI, indecently of diabetes status, is known to increase mental fatigue [5, 6, 7].
Cognitive alterations, in the form of longer reaction times and impaired spatial planning, occur in diabetic patients. These impairments however, were unrelated to glycemic control, reflected by HbA1C levels, complications and duration of disease [6]. The mini-mental state exam also did not show statistical significant difference between good and poor glycemic control groups. This finding may be explained by the fact that verbal fluency is known to be the first manifestation of vascular dementia, which is a common complication of diabetes. However, verbal fluency testing is not available in the MMSE. Instead, MMSE is valuable in the detection of early stages of Alzheimer disease where memory is specifically affected. Previous literature suggesting a relationship between diabetes mellitus and fatigue have used the multidimensional fatigue inventory instead of the fatigue severity scale we used. Our finding is consistent with a previous study that showed no relationship between fatigue, glucose control (as measured by HbA1c), and diabetic complications [19].
T2DM patients have impairment in working memory, executive functions and psychomotor functions that affect their daily lives [9]. In a systematic analysis it has been reported that hyperglycemia is associated with impaired cognitive functions [20]. Patients with T2DM, regardless of their insulin treatment status have shown higher fatigue scores and cognitive impairment with significant prolongation of reaction times and defective spatial planning [21]. In a recent study by Gerstein HC et al there was a significant independent association between diabetes, small vessel vascular brain injury and cognitive impairment in patients with diabetes mellitus [22].
4.1. Limitations
Possible limitations include lack of a control group, cross sectional design, and the relatively small number of subjects. Moreover, we had a smaller female-to-male ratio in our sample. Furthermore, we could not perform nerve conduction studies to assess peripheral neuropathy. We anticipate that a larger sample size with a prospective design is needed to explore further into cognitive impairment and T2DM.
4.2. Conclusions
Patients with T2DM have a statistically significant association between their cognitive functions and their glycemic control. Patients with uncontrolled T2DM had an overall decrease in cognitive scores. Moreover, a lower attention scores were associated with longer disease duration.
4.3. Recommendations
Prospective studies at large scales are required to explore further into the areas of cognitive impairment and T2DM.
Declarations
Author contribution statement
Khaled Alkethiri, Tariq Almtroudi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Abdullah bin Jurays: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Faisal Abanumay: Performed the experiments; Analyzed and interpreted the data.
Mohammed Aldammas, Meshaal AlKhodheer: Performed the experiments; Wrote the paper.
Mohammed Iqbal: Analyzed and interpreted the data; Wrote the paper.
Syed Shahid Habib: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shahid Bashir: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Syed Shahid Habib was supported by a grant from Deanship of Scientific Research (Grant Number: RGP-1438-048) King Saud University, Riyadh, Saudi Arabia.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [published correction appears in Diabetes Care. 2010 Apr 33(4):e57] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol. Aging. 2005 Dec 1;26(1):26–30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Alfahadi A., Habib S.S., Alharbi K., Alturki D., Alshamrani F., Bashir S. Assessment of fatigue severity and neurocognitive functions in the real setting of Ramadan in patients with type 2 diabetes mellitus. Heliyon. 2020;6(5) doi: 10.1016/j.heliyon.2020.e03997. Published 2020 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umegaki H., Hayashi T., Nomura H., Yanagawa M., Nonogaki Z., Nakshima H. Cognitive dysfunction: an emerging concept of a new diabetic complication in the elderly. Geriatr. Gerontol. Int. 2013;13:28–34. doi: 10.1111/j.1447-0594.2012.00922.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamer M., Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol. Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 6.Lasselin J., Layé S., Barreau J.-B., Rivet A., Dulucq M.-J., Gin H. Fatigue and cognitive symptoms in patients with diabetes: relationship with disease phenotype and insulin treatment. Psychoneuroendocrinology. 2012 Sep;37(9):1468–1478. doi: 10.1016/j.psyneuen.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Geijselaers S.L.C., Sep S.J.S., Stehouwer C.D.A., Biessels G.J. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2014 Aug 22 doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson D.R. Adiposity and cognitive decline: underlying mechanisms. J. Alzheimers Dis. 2012;30(Suppl 2):S97–112. doi: 10.3233/JAD-2012-120487. [DOI] [PubMed] [Google Scholar]
- 9.Wong R.H.X., Scholey A., Howe P.R.C. Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus-a review with implications for future intervention studies. Curr. Diabetes Rep. 2014 Nov;14(11):547. doi: 10.1007/s11892-014-0547-4. [DOI] [PubMed] [Google Scholar]
- 10.Ciechanowski P.S., Katon W.J., Russo J.E. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch. Intern. Med. 2000 Nov 27;160(21):3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 11.Van Harten B., Oosterman J., Muslimovic D., van Loon B.-J.P., Scheltens P., Weinstein H.C. Cognitive impairment and MRI correlates in the elderly patients with type 2 diabetes mellitus. Age Ageing. 2007 Mar;36(2):164–170. doi: 10.1093/ageing/afl180. [DOI] [PubMed] [Google Scholar]
- 12.Palta P., Schneider A.L., Biessels G.J., Touradji P., Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc. 2014. March;20(3):278–291. doi: 10.1017/S1355617713001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alosco M.L., Gunstad J. The negative effects of obesity and poor glycemic control on cognitive function: a proposed model for possible mechanisms. Curr. Diabetes Rep. 2014. June;14(6):495. doi: 10.1007/s11892-014-0495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke S., Pennington K., Jones A., Bridle C., Smith M.F., Curtis F. Effects of exercise, cognitive, and dual-task interventions on cognition in type 2 diabetes mellitus: a systematic review and meta-analysis. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0232958. Published 2020 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galea M., Woodward M. Mini-mental state examination (MMSE) Aust. J. Physiother. 2005;51(3):198. doi: 10.1016/s0004-9514(05)70034-9. [DOI] [PubMed] [Google Scholar]
- 16.Cambridge Cognition . Cambridge Cognition; Cambridge: 2012. CANTABeclipse 5: Test Administration Guide. [Google Scholar]
- 17.Bashir S., Alghamd F., Alhussien A. Effect of smoking on cognitive functioning in young Saudi adults. Med. Sci. Monit. Basic Res. 2017;23:31–35. doi: 10.12659/MSMBR.902385. Published 2017 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Thaqib A., Al-Sultan F., Al-Zahrani A. Brain training games enhance cognitive function in healthy subjects. Med. Sci. Monit. Basic Res. 2018;24:63–69. doi: 10.12659/MSMBR.909022. Published 2018 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh R., Teel C., Sabus C., McGinnis P., Kluding P. Fatigue in type 2 diabetes: impact on quality of life and predictors. PloS One. 2016;11(11) doi: 10.1371/journal.pone.0165652. Published 2016 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geijselaers S.L.C., Sep S.J.S., Stehouwer C.D.A., Biessels G.J. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(1):75–89. doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- 21.Lasselin J., Layé S., Dexpert S., Aubert A., Gonzalez C., Gin H., Capuron L. Fatigue symptoms relate to systemic inflammation in patients with type 2 diabetes. Brain Behav. Immun. 2012;26(8):1211–1219. doi: 10.1016/j.bbi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Gerstein H.C., Smith E.E., Ramasundarahettige C., Desai D., Awadalla P., Broet P., Black S., Dummer T.J.B., Hicks J., Moody A., Tardif J.C., Teo K.K., Vena J., Yusuf S., Lee D.S., Friedrich M.G., Anand S.S. Diabetes, brain infarcts, cognition, and small vessels in the Canadian alliance for healthy hearts and minds study. J. Clin. Endocrinol. Metab. 2021 Jan 23;106(2):e891–e898. doi: 10.1210/clinem/dgaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.