Abstract
Background
Histopathology of first-trimester abortion products may be useful in document an intrauterine pregnancy, identifying an important pathology affecting the mother or the embryo and diagnosing conditions that are likely to recur in future pregnancies or that explain the adverse fetal outcome. Relevant information provided by histology is essential to determine the cause and to guide the patients with early pregnancy failure.
Aims
Histopathological classification proposal in first-trimester miscarriage.
Methods
Published pathological criteria in first-trimester abortion specimens were collected, standardized and focused into a comprehensive diagnosis. The idea was to create a comprehensive classification related to major pathophysiological processes. Thus, the histological criteria were grouped into 7 categories: i. Changes suggesting aneuploidy (CSA) or metabolic storage disease; ii. Embryo anomaly (EA); iii. Multifactorial (MF) causes; iv. Maternal causes (MC); v. Gestational trophoblastic disease, such as hydatidiform mole (HM) and non neoplastic lesions and neoplasms; vi. Ectopic pregnancy; vii. Other. So, a 6-years retrospective study of first-trimester spontaneous miscarriage were reviewed. Two groups were created: i. Study group include specimens with pathological diagnosis; ii. Control group incorporate specimens with pathological diagnosis and additional genetic study in order to validate pathological criteria.
Results
Pathological criteria concordance between inter-observers was generally good, with an excellent correlation in EA and HM categories. Despite greater inter-observer disagreement in the CSA and MC categories the correlation with the genetic results was very positive.
Conclusion
A standardized, reproducible and biologically comprehensive histopathological classification may improve fetal follow-up and couple's management.
Keywords: First-trimester miscarriage, Histopathology, Chorionic villi morphology, Embryo pathology, Classification proposal system, Clinical relevance
First-trimester miscarriage; Histopathology; Chorionic villi morphology; Embryo pathology; Classification proposal system; Clinical relevance
1. Introduction
Spontaneous abortion (SA) is one of the most common first-trimester complications affecting over 15% of pregnant women in the childbearing age and can rise up to 45% [1, 2, 3, 4]. A precise incidence of first-trimester spontaneous abortion (FTSA) is not well-established [1, 2, 3, 4].
Regardless of the high incidence of early abortion, these samples are often poor described from a developmental perspective, which contributes to the low number of pathology examination requests.
Histopathological examination is essential to identify previously unsuspected disease [5, 6, 7, 8, 9, 10]. Also, the success of pathological examination is partially dependent on the skill and experience of the examiner [5]. As in general surgical pathology it is wise to follow a routine protocol [5, 6, 7, 8, 9, 10, 11, 12]. First-trimester specimens differ greatly in their composition [5, 11]. Histopathology is an integral and a routine component of the management of patients with sporadic and recurrent early pregnancy failure [5, 6, 7, 8, 9].
Useful information depends on the examiner's experience in evaluating these types of samples and interpreting the pathophysiological processes underlying the changes identified in the different components. So, first-trimester specimens histology is crucial to: (1) Document an intrauterine pregnancy; (2) Identification of previously unsuspected disease affecting the mother or the embryo that require immediate attention (e.g., unusual infections, hydatidiform mole; changes suggesting aneuploidy or metabolic storage disease); (3) Identification of conditions that are likely to recur in future pregnancies or that explain an adverse outcome (e.g., chronic histiocytic intervillositis, maternal malperfusion, or hereditary settings); (4) Conditions that can guide management of future pregnancies (e.g., hydatidiform mole, extravillous trophoblastic lesions) [8, 9, 10, 11, 12, 13]. Also, histology of spontaneous or recurrent and surgically or medically evacuated abortion specimens, is beneficial in protecting obstetrician and gynecologist from medico-legal litigation.
Etiology of SA is a complex and heterogeneous process correlated with gestational age (GA) [12, 13, 14, 15, 16]. The most common causes of early or very early spontaneous abortion are aneuploidies or chromosomal aberrations often resulting in abnormal embryo and solitary or multiple malformations indicative of specific syndromes or associations [12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. Embryo anomaly (EA), maternal causes (MC), multifactorial (MF) or environmental contexts and teratogens are often adverse causes of miscarriage. These interfere with normal embryogenesis resulting in distinct embryonic abnormalities related to timing that the errors occurrence [6, 7, 8, 14, 18, 20, 21, 22, 27, 28, 29]. Gestational trophoblastic diseases (GTD) comprise a complex and challenging group of lesions that are classified into several groups [16, 30, 31, 32, 33, 34]. Molar pregnancies, e.g., hydatidiform moles (HM) are an abnormal placenta with variable degrees of trophoblastic hyperplasia and villous hydrops [33]. Morphologically and genetically are classified as complete hydatidiform mole [CHM, very early complete hydatidiform mole (VECHM)], partial hydatidiform mole (PHM) and invasive type [30, 31, 32, 33, 34, 35, 36, 37]. The incidence of HM is heterogeneous between countries and regions [16, 18, 31, 33, 37]. Non neoplastic gestational trophoblastic lesions (e.g., exaggerated placental site and placental site nodule), and gestational trophoblast neoplasms (e.g., choriocarcinoma, placental site trophoblastic tumor and epithelioid trophoblastic tumor) are a heterogeneous group of tumors consisting of an extravillous trophoblastic cell proliferation [30, 31, 32, 33, 34, 35, 36, 37]. Exaggerated placental site typify part of the spectrum of the normal implantation-site changes. Placental site nodule is a benign, well circumscribed nodule consisting of trophoblastic cell proliferation. Choriocarcinoma is a malignant trophoblastic tumor consisting of a trimorphic proliferation of extravillous trophoblastic cells, syncytiotrophoblast and cytotrophoblast, in the absence of chorionic villi [31, 37, 39, 40]. Placental site trophoblastic tumor is a trophoblastic neoplasia consisting of neoplastic implantation site-type extravillous trophoblast, while the epithelioid trophoblastic tumor consist of neoplastic chorionic-type extravillous trophoblast [31, 37, 39, 40, 41].
In a muldisciplinary practice, a biologically comprehensive, histopathological-based classification of first-trimester specimens allows a guide treatment decision and management in sporadic and recurrent early miscarriage [5, 7, 8, 9, 10, 11, 12, 29]. Also, histological criteria could improve a selection-case to genetic or molecular additional tests [5, 12, 13, 29]. Genetic correlation (based on control group) make available evidence-based histological criteria for future investigation in this area.
2. Objective
A biologically comprehensive and histologically-based classification in first-trimester miscarriage.
3. Materials and methods
A 6-years (2013–2018) retrospective crossy-study of 3,228 first-trimester specimens pathology reports.
Pathological diagnosis was based on routine hematoxylin and eosine (H&E) stained slides of paraffin-embedded material examination. Additional complementary study (e.g., immunohistochemistry) had been performed when applicable. Guidelines to first-trimester specimens examination were used [5, 9, 10, 12, 28]. The full text of pathological reports were screened to identify those potentially fulfilling the category criteria (Figure 1). Study sample comprise first-trimester abortion <12th week of GA and was categorized into two groups: – 1. Study Group: pathological reports with isolated diagnosis. – 2. Control Group: pathological reports with diagnosis and concurrent genetic study (n = 223) for validation of histological criteria. Inclusion criteria: i. histopathological diagnosis classification into one of the defined categories; ii. inter-observer agreement. Exclusion criteria: i. degenerative changes associated with retention; ii. miscarriage relating to a medical or social termination of pregnancy; iii. twin pregnancy.
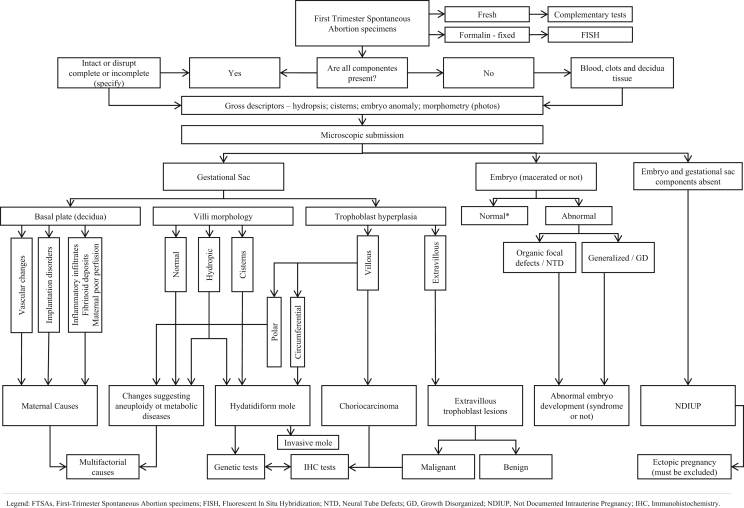
Figure 1.
Algorithm Diagnosis in First-Trimester Spontaneous Abortion specimens. FTSAs, First-Trimester Spontaneous Abortion specimens; FISH, Fluorescent In Situ Hybridization; NTD, Neural Tube Defects; GD, Growth Disorganized; NDIUP, Not Documented Intrauterine Pregnancy; IHC, Immunohistochemistry.
Grossly, first-trimester specimens may consist of blood clot admixed with minimal decidua tissue and fragmented villous tissue and embryonic parts, a complete (or not) gestational sac (GS) and an embryo; or something in between. So, gross examination allowed adequate evaluation of maternal (decidua), placental and embryonic components, and submission for microscopy. Gestational age dated from the first day of the last menstrual period was the only available information. Difficult interpretation criteria were revised by two experienced embryofetal pathologists and an inter-observer consensus was achieved together with a senior embryofetal Pathologist. When needed original H&E slides were used for the review.
In the interdisciplinary discussion we used the international guidelines for the first-trimester specimens examination in order to evaluate histopathological criteria that allow an algorithmic approach to classification (see Figure 1). Additional recommendation in simplified comment mode, could summarize an explanation in difficult cases.
The sum of the pathological features fell preferentially in a single diagnostic category integrating all the findings. So, a classification system was constructed, based on pathophysiological and histological criteria (see Figure 1).
Table 1, summarizes the cases distribution according to the 7 histopathological categories. Table 2, summarizes the main characteristics of the different specimen components concerning histopathological criteria at each category: 1. Changes suggesting aneuploidy, concerns a gestational sac and chorionic villi dysmorphic features for the GA and rare specific conditions (e.g., metabolic storage disease); 2. Embryo anomaly category encodes 5 embryo phenotypes-based on the embryonic developmental defects; 3. Maternal causes category covered microscopic features of decidual arteriopathy and impaired trophoblast invasion like as a feature related in antiphospholipid antibody (aPL)-associated loss. Also, unusual infections, and rare specific conditions such as chronic histiocytic intervillositis (CHI) and fibrin deposition were categorized under this category; 4. Multifactorial category enclosed mixed histological villous dysmorphic features and other chorion-decidual anomalies considering hindering a single unequivocal etiology; 5. Ectopic pregnancy means a pregnancy outside the uterine cavity, which can occur in different localizations. Table 3 summarizes histopathological characteristics of extravillous trophoblastic lesions.
Table 1.
Distribution of the cases according to the main seven histopathological categories given in absolute value (n) and percentage (%).
| Classification of First Trimester Abortion Specimens Lesionsa | Cases (3,228) |
|
|---|---|---|
| n | % | |
| i. Changes Suggesting Aneuploidy | 1,279 | 39.6 |
| ii. Embryo Anomaly | 953 | 29.5 |
| iii. Maternal Causes1,2 | 337 | 10.4 |
| iv. Multifactorial Causes | 317 | 9.8 |
| v. Gestational Trophoblastic Disease | ||
| Hydatidiform Mole | 122 | 3.8 |
| Extravillous Trophoblastic Lesions | 5 | 0.25 |
| Choriocarcinoma | 1 | 0.05 |
| vi. Ectopic Pregnancy | 155 | 4.8 |
| vii. Othera | 59 | 1.8 |
| Total | 3,228 | 100 |
molecular thrombophilic defects identified in 21 cases.
Cytogenetic study was performed in 23 cases.
other (e.g., undocumented intrauterine pregnancy, retention abortion components and degenerative changes associared with intrauterine retention).
Table 2.
First-trimester abortion products pathological categories.
| Histopathological Categories of First-Trimester Spontaneous Abortion Productsa | |||||||
|---|---|---|---|---|---|---|---|
| Characteristicsb | CSA | EA | MC | MF | HM |
EPc | |
| Complete | Partial | ||||||
| Embryo/Fetus | may be present | usually abnormal | may be present | may be present | rarely present | often present | may be present |
| Gestational sac histology | |||||||
|
mild to moderate | mild to moderate | rare | mild | marked 1 | mild to moderate | may be present |
|
range of villi from small to hydropic2 | range of villi from small to hydropic2 | admixture of villi, to tertiary villi |
admixture of villi from fibrotic to clubbed or round | relatively uniform; hydropic villi |
Partly normal, partly hydropic | admixture of villi from fibrotic to clubbed or round |
|
clubbed or round and scalloping | clubbed or round and scalloping | elongated to small | admixture from elongated to clubbed or round and scalloping | usually round | scalloping with inclusions | Variablec |
|
usually absent | usually absent | usually absent | usually absent | common3 | rare to mild | usually absentc |
|
rare to mild (focal) and polar | rare to mild (focal) and polar | usually absent; increase syncytial knots |
rare to mild (focal); increase syncytial knots | common; apolar,circumferential hyperplasia4 | mild to moderate (focal); apolar, circumferential hyperplasia | usually absentc |
|
none or rare | none or rare | none or rare | none or rare | common5 | minimal | none or minimal |
|
absent or rare6 | moderate | mild to moderate | mild to moderate | rare usually absent7 | common | common |
|
usually absent | usually absent | may be presentd | may be present | usually absent | usually absent | none or rare |
| Plate basal | |||||||
|
may be present | may be present | usually present | usually present | may be present | may be present | absent |
| Persistent GTD | no | no | no | no | >20%, may develop ChoCa | <5%, usually not requiring chemotherapy | none or rare |
CSA, changes suggesting of aneuploidy or metabolic storage disease; EA, embryo anomaly; MC, maternal causes; MF, multifactorial; HM, Hydatidiform Mole; EP, ectopic pregnancy; GTD, gestational trophoblastic disease; ChoCa, choriocarcinoma.
aAdapted from Benirschke and Kaufmann's, pathology of the human placenta, Baergen. Springer,2004 ISBN 0-387-2208.
bmost common presentation, but variation may occur. c molar disease may exist. d viral inclusion may be identified.
cone case with Very Early Complete Hydatidiform Mole (VECHM) included in HM category.
may be mild in VEHCM.
namely in hydropic abortion.
rare in VECHM.
moderate in VECHM.
including in VECHM.
particularly in hydropic abortion.
may be present in VECHM.
Table 3.
Histopathological classification of Extra Villous Trophoblastic Lesions.
| Extra Villous Trophoblastic Lesionsa | |||||
|---|---|---|---|---|---|
| Characteristics | PSN | EPS | PSTT | ETT | ChoCa |
| Macroscopy | |||||
|
- | - | + | + | + - |
| Histopathology | |||||
|
- | + | very rare | very rare | -1 |
|
+ | + | + | - | - |
|
- | + - | + | + | + + |
|
- | + - | + | + + | + + |
|
- | + | + | + | + |
|
- | - | - | - | + |
|
+ | + | + | + | - |
|
- | + | -2 | - | + + |
|
- | - | + + | - | + + |
|
minimal or absent | minimal or absent | + | + | + |
| History of previous Mole | - | - | 5–8% | 5–8% | 50% |
| Metastasis | none | none | occurs in 10–15% | occurs in 10–15% | potential |
| Prognosis | no sequelae | no sequelae | reserved if malignant | reserved if malignant | >90% responsive to chemo-therapy |
| Serum β-hCG | normal | appropriate for pregnancy | moderately ↑ in 80% | moderately ↑ in 80% | markedly ↑ |
PSN, placental site nodule; EPS, exaggerated placental site; PSTT, placental site trophoblastic tumor; ETT, epithelioid trophoblastic tumor; ChoCa – choriocarcinoma.; β-hCG, β-human chorionic gonadotrophin.
Adapted from Benirschke and Kaufmann's, pathology of the human placenta, Baergen. Springer,2004 ISBN 0-387-22089.
Villi are present only in placental or “in situ” ChoCa.
Multinucleate cells similar to syncytiotrophoblast may be present.
In order to validate the histopathological criteria, a control group of 223/3,169 (7%) cases with concurrent genetic study were compared which results are present in Table 4.
Table 4.
Correlation of Histopathological Categories (CSA, EA, MC, MF and HM) and Control Group (genetic results) in absolute values (n).
| Histopathological Classification of FTSAp Lesions |
Total | ||||||
|---|---|---|---|---|---|---|---|
| CSA | EA | MC1 | MF | HM | |||
| Control Group: Cytogenetic Results | Normal | 24 | 26 | 19 | 10 | 3a | 82 |
| Trisomy 2 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Trisomy 4 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Trisomy 5 | 1 | 1 | 0 | 0 | 0 | 2 | |
| Trisomy 7 | 2 | 1 | 0 | 0 | 0 | 3 | |
| Trisomy 8 | 0 | 2 | 0 | 0 | 0 | 2 | |
| Trisomy 9 | 2 | 0 | 0 | 0 | 0 | 2 | |
| Trisomy 10 | 2 | 0 | 0 | 0 | 0 | 2 | |
| Trisomy 13 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Trisomy 14 | 0 | 1 | 1 | 0 | 0 | 2 | |
| Trisomy 15 | 4 | 6 | 0 | 0 | 0 | 10 | |
| Trisomy 16 | 10 | 3 | 0 | 0 | 0 | 13 | |
| Trisomy 17 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Trisomy 18 | 4 | 2 | 0 | 1 | 0 | 7 | |
| Trisomy 20 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Trisomy 21 | 10 | 4 | 0 | 0 | 1b | 15 | |
| Trisomy 22 | 5 | 3 | 0 | 3 | 0 | 11 | |
| Monosomy X | 4 | 7 | 2 | 8 | 0 | 21 | |
| Triploidy | 4 | 7 | 0 | 2 | 8b | 21 | |
| Tetraploidy | 3 | 2 | 0 | 0 | 1b | 5 | |
| Mosaicism | 3 | 1 | 0 | 1 | 1a | 6 | |
| Structural aberration | 1 | 0 | 0 | 0 | 0 | 1 | |
| Hypertriploidy | 1 | 0 | 0 | 0 | 0 | 1 | |
| Additional material of unknown origin | 0 | 1 | 0 | 0 | 0 | 1 | |
| Monosomy 21 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Monosomy X + Trisomy 18 | 0 | 0 | 0 | 0 | 1a | 1 | |
| Robertsonian translocation | 1 | 3 | 1 | 0 | 0 | 5 | |
| Triploidy + two additional Chr. 7 and one additional Chr. 9 | 0 | 0 | 0 | 0 | 1b | 1 | |
| Triploidy + Tetrasomy 7 and 14 | 0 | 0 | 0 | 0 | 1b | 1 | |
| Trisomy 2 + Trisomy 22 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Trisomy 7 + Trisomy 18 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Trisomy X + Trisomy 22 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Total | 83 | 77 | 23 | 25 | 16 | 223 | |
FTSAp, first-trimester spontaneous abortion products; CSA, changes suggesting aneuploidy; EA, Embryo anomaly; MC, maternal causes; MF, multifactorial; HM, hydatidiform mole; PHM, partial hydatidiform mole; CHM, complete hydatidiform mole; VECHM, very-early complete hydatidiform mole; Chr. Chromosome.
molecular thrombophilic defects identified in 21/337 cases of MC category.
all CHM, 2 VECHM.
all PM.
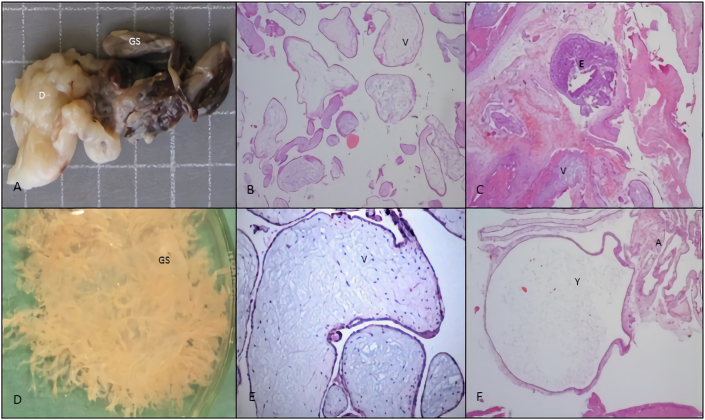
Histopathological changes suggesting aneuploidy (Figure 2) concern a gestational sac and chorionic villi dysmorphic features for the GA (e.g., enlarged scalloped, clubbed, round villi; multifocal and polar trophoblastic proliferation/hyperplasia or hypoplasia; stromal pseudo-inclusion and decreased stromal vessels) [5, 14, 30].
Figure 2.
Spontaneous abortion from 6th week GA age concerning pathological features to the category changes suggesting aneuploidy: A, macroscopic features of a shrunken GS, chorion hemorrhage and clots and 47,XX,+22 karyotype. B, hydropic avascular villi (V). H&E stain, x100. C, fibrotic villi (V), and disorganized embryo (E). H&E stain, x100. D, intact GS photographed under saline, with bulbous swelling villi. E, hydropic villi with hypoplastic trophoblast and diploid karyotype. H&E stain, x100. F, amniotic epithelium (A) and yolk sac (Y). H&E stain, x100. GA, gestational age; GS, gestational sac; H&E, hematoxylin eosin.
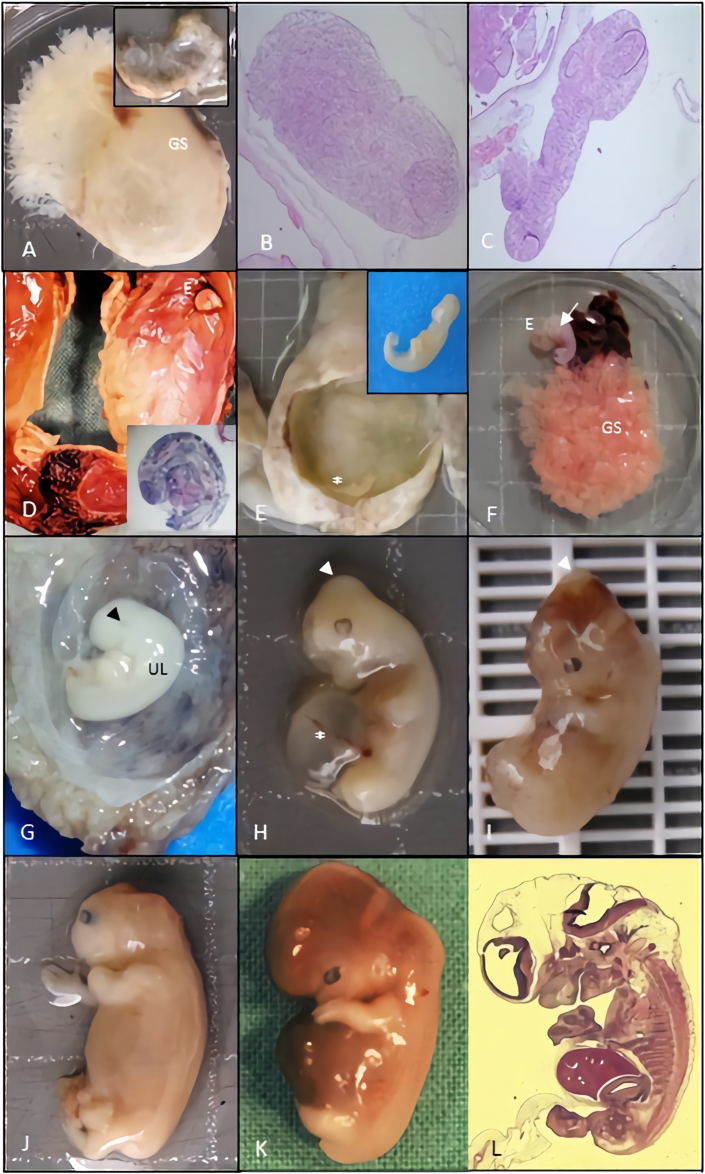
Embryo anomaly encodes 5 embryo phenotypes (Figure 3), based on the embryonic developmental defects, since individual organ malformation such as neural tube defects, to a growth disorganized (GD) embryo. So, GD1, lie of an intact GS with no evidence of embryo (Figure 3A) [5, 14]. GD2, consists of a nodular embryo moreover attached to chorionic plate (Figure 3B) [5, 14]. GD3, relates to an embryo up to 10 mm long, with caudal and cephalic poles without others recognizable external structures, moreover retinal pigment may be present (Figure 3C-G) [5, 14]. GD4, consists of an embryo with 3–17 mm long usually with a major distortion of body shape always involving head and generally with a fusion of the chin and chest (Figure 3H-I) [5, 14].
Figure 3.
Etiologic significance of macroscopic and microscopic features concerning to the embryonic development anomaly category. The following detailed pictures, comprise abortion specimens with 7–9 weeks GA dated from first day of last menstrual period. A, empty GS consistent with GD1 and normal karyotype (46,XY). B and C, histological features of a nodular embryo without any differentiation consistent with GD2 and a 47,XX,+13 and 48,XX,+2,+22 karyotype respectively; amniotic epithelium and villi at the left superior corner. H&E stain, x40. D and E, macroscopic features GS containing a GD3 embryo with 47,XX,+15 and 47,XX,+16 karyotype respectively. F, GS with hyperplastic chorion and GD4 embryo, with a major distortion of body shape (arrow) and 47,XY,+16 karyotype. G, GD4 embryo with major distortion of the body and fusion of the chin to the chest (black ar). H and I, embryo with a parietal encephalocele (white ar) and umbilical cord cyst (double ar) with a normal 46,XX, karyotype. J, embryo with acrania and diploid 46,XX karyotype. K-L, macroscopic and microscopic normal embryo with diploid karyotype. GA, gestational age; GS, gestational sac; GD, growth disorganized; H&E, hematoxylin eosin.
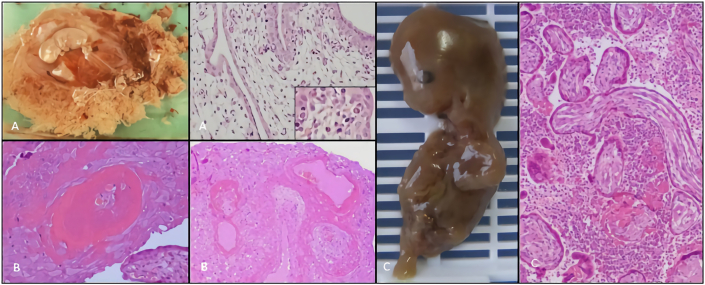
Maternal causes (Figure 4) were diagnosed hystologically as impaired trophoblastic invasion, decidual vessels pathology, decidual lymphocyte cell infiltration or fibrinoid deposition [5, 30]. Also, unusual infections (Figure 4A); massive perivillous fibrin deposition, or maternal poor perfusion (e.g., increase number of tertiary villi, fibrotic villi and decidual arteriopathy) (Figure 4B) and chronic histiocytic intervillositis (Figure 4C) were included under this category [5, 30].
Figure 4.
Etiologic significance of macroscopic and microscopic features concerning maternal cause category. The following detailed pictures, refers a 10th week's GA specimens dated of first day of last menstrual period. A, macroscopy: hydropic embryo and placental hydropsis; microscopy: marked swelling of the villi and erytroblasts with parvovirus inclusion (inset). H&E stain, x200. B, microscopy: decidual vascular changes with adjacent decidual necrosis. H&E stain, x100. C, macerated embryo with disruption of abdominal wall (iatrogenic); microscopy: massive chronic intervillitis, consisting of histiocytic and lymphocytic infiltrates in maternal space. H&E stain, x100. GA, gestational age; H&E, hematoxylin eosin.
Multifactorial category covering mixed histopathological chorionic villi pattern between maternal causes and changes suggesting aneuploidy considering hindering a single unequivocal etiology.
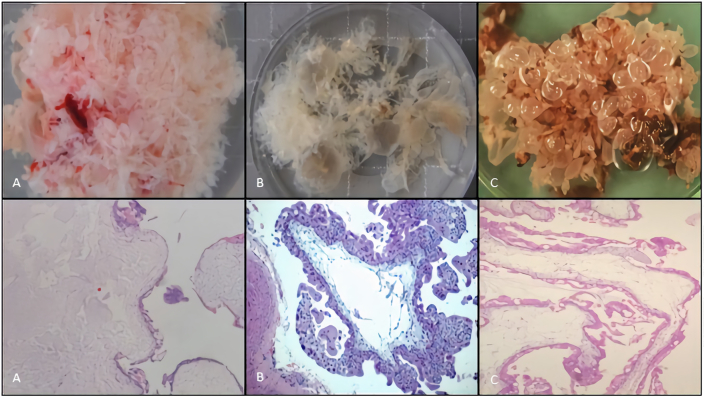
Hydatidiform moles (Figure 5) traditionally, have been subdivided into complete hydatidiform mole and partial hydatidiform mole. HM macroscopically, have abundant tissue with grossly identifiable translucent vesicles (Figure 5A-C). On microscopic examination, the villi, primarily in the terminal villi, are diffusely hydropic with a central acellular space and trophoblast hyperplasia (Figure 5A). Circumferential, trophoblast hyperplasia is universally present and is a requirement for diagnosis (Figure 5B-C) [30]. Invasive mole is a rare entity composed of trophoblastic cells and molar villi, which invade the uterus and have potential invasion of adjacent structures [17, 24, 31, 32, 33, 34, 35, 36, 37].
Figure 5.
Macroscopic and microscopic features of hydatidiform mole category. Specimens corresponding to abortions with a GA range between 6th and 10th week dated from first day of last menstrual period. A, B, C, macroscopic features of a HM photographed under saline, note bulbous swelling of villi. Microscopic features: A, hydropic villi intermixed with translucent vesicles with mild trophoblastic hyperplasia consistent with PHM and 69,XXX,+7,+14 karyotype, H&E stain, x40; B, hydropic villi with central cistern and circumferential trophoblastic hyperplasia and absence of fetally-derived tissue in a case of VECHM with a 46,X,+18 karyotype, H&E stain, x100; C, scalloping hydropic villi with central cisterns and circumferential (apolar) trophoblastic hyperplasia in a case of PHM with 69,XXY, karyotype H&E stain, x40. GA, gestational age; HM, hydatidiform mole; PHM, partial hydatidiform mole; VECHM, very early complete hydatidiform mole; H&E, hematoxylin eosin.
All the samples enrolled in the present study were unlinked and unidentified from their donors. The study was approved by the Local and Regional Ethical Committees for Medical Research. Due to the retrospective nature of the study, the Local Ethical Review Committees of the involved institutions and Minho University Medicine School, Braga, Portugal, approved the work and waived the need for written informed consent.
4. Results
The pathological criteria considered in each category allows a conclusive diagnosis in 98.2% (3,169/3,228) cases (See Table 1 and Table 2). The category “other” includes 59/3,228 (1.6%) cases of not documented intrauterine pregnancy (NDIUP) and severe degenerative changes related with retention.
Changes suggesting aneuploidy and embryo anomaly categories are the most frequent pathological diagnosis with 1,279 (39.6%) cases and 953 (29.5%) cases respectively (see Table 1). These did have a good correlation with genetic results see Table 4) with 71% (59/83) and 66.2% (51/77) respectively. Maternal causes and multifactorial categories encompass 337 (10.4%) and 317 (9.8%) cases respectively. These categories had a good correlation with cytogenetic results (see Table 4). In maternal causes category 82.6% (19/23) cases did had a normal karyotype, and molecular tests identified thrombophilic defects in 6.23% (21/337) cases also. In multifactorial category, 40% (10/25) cases had a normal karyotype. The incidence of overall HM was 3.8% (122/3,228) cases (see Table 1). HM matched to cytogenetic results, in 13.1% (16/122) cases (see Table 4). Furthermore, the classical pathological study showed PHM and CHM criteria in 12 and 4 cases respectively. Cytogenetic study documented triploidy, triploidy and tetrasomy and tetraploidy in overall PM (Table 4). One case of CHM had a 46,X,+18 karyotype and another one had a mosaicism (Table 4). Extravillous Trophoblastic lesions and choriocarcinoma occur in 0.25% and 0.05% cases respectively. Ectopic pregnancy occurred in 4.8% (155/3,228) cases, mostly (98.7%) on the Fallopian tube wall and one case on the ovarian surface, and one-other on the abdominal cavity.
5. Discussion
Spontaneous abortion has great impact on the couple who want a child. Also, first-trimester spontaneous abortion FTSA are a common and clinically significant problem. Although, there are few reports addressing the clinical value of routine histopathological examination of first-trimester miscarriage. A systematic approach to macroscopic and microscopic study allows a conclusive histopathological diagnosis in the majority of cases [5, 6, 7, 8, 9, 10, 11]. However, despite these general findings, about 2–12% of the cases in our serie remains without a specific or an obvious etiology of the miscarriage. Although, useful information from a histological study are related to: (1) confirmation of the presence of an intrauterine pregnancy; (2) identification of previously unsuspected disease process in the mother or embryo; (3) exclusion of a gestational trophoblastic disease, namely in the form of CHM or PHM; (4) identification of conditions with a high probability of recurrence in future pregnancies [14, 16, 21, 27, 28, 31, 32].
Pathological evaluation is important to the confirmation of an intrauterine pregnancy loss, validation of ultrasound features and an effective clinical follow-up toward parental orientation and additional complementary tests [14, 16, 21, 27, 28, 31, 32, 33, 34, 35, 36, 38]. Besides, histopathology could estimate the GA and the interval of retention after embryonic death [5, 7, 11]. Also, it is beneficial in protecting obstetrician and gynecologist from medico-legal litigation.
A systematic performed histopathological examination of first-trimester abortion specimens demonstrated that changes suggesting aneuploidy and embryo anomaly criteria had a good correlation with genetic tests results [5, 6, 7, 21, 26]. Structural chromosomal changes frequency is identical to others studies, in which the presence of structural changes occurs in about 4% of the cases of first-trimester abortion with abnormal karyotype [6, 7, 21, 26]. EA category, showed a high incidence of chromosomal abnormalities when matched the morphological criteria and cytogenetic results. However, the existence of EA with a diploid normal karyotype, may suggest the interference of teratogenic agents (e.g., drugs, viruses, alcohol, tobacco, hyperthermia) or other lethal disruptive events during normal embryogenesis [18, 21, 26, 27, 28].
Also, the frequency of normal karyotype in MC and MF categories supports and validates the histopathological criteria. Maternal or embryonic nongenetic factors including environmental contexts and teratogens are well-known causes associated with failure of protective mechanisms inherent to normal gestation or severe embryo malformation [17, 18, 19, 21, 26, 28, 29]. Despite that molecular tests identified thrombophilic defects in 21/337 (6.2%) cases. Immunological disorders, thrombophilia and antiphospholipid antibody syndrome, especially in aPL-associated loss have been associated with early miscarriage. Presently available data indicate that a small subgroup of women with recurrent first-trimester abortion may show evidence of CHI or massive perivillous fibrin deposition. Both conditions are diagnosed only in histology and represent a phenotype related with maternal homeostasis disturbance that may influence future reproductive management [17, 18, 20, 27]. Also, maternal age at the time of abortion shows a significant effect on the incidence of first-trimester miscarriage moreover associated with changes suggesting aneuploidy and embryo anomaly namely growth disorganized embryo [10, 12, 18, 19, 21, 26, 28].
An additional minority of first-trimester specimens will be due to HM. The incidence of HM was 3.8% (122/3,169), of which 53.3% (65/122) cases were not previously suspected. Pathological evaluation improves the diagnoses of GTD. So, histology had a major impact because these patients require surveillance for detection of persistent gestational trophoblastic disease and for the risk of recurrent mole in future pregnancy. Also, a very early complete hydatidiform mole represent a great challenge in diagnoses, as the pathologic features are more subtle and less developed [32]. Partial hydatidifom mole features are similar to the complete hydatidiform mole but are usually less striking and have an admixture of relatively normal immature villi and irregularly distended and scalloped hydropic villi. Although both forms of HM include excessive expression of paternally derived genes, but are slightly different conditions with different clinical implications. Complete hydatidiform mole presents an increased risk of developing choriocarcinoma and is generally diploid, while the PHM is mostly triploid. The higher incidence of overall HM could reflect the under pathological examination of first-trimester miscarriage and the difficult diagnosis of very early complete hydatidiform mole [16, 30, 33, 35, 36]. In HM group, a concurrent cytogenetic result validates the pathologic diagnosis in overall cases of PHM. Also, extravillous trophoblastic lesions are a complex and heterogeneous group of lesions, since benign non neoplastic lesions (e.g., placental site nodule and exaggerated placental site) to potentially malignant conditions such as placental site trophoblastic tumor and intraplacental choriocarcinoma. Histology of all the products of early miscarriage can increase the real incidence of gestational trophoblastic disease and consequently improve maternal management [32, 33, 34, 35, 36, 37, 40, 41, 42].
Ectopic pregnancy can occur in different localizations, mostly in the fallopian tube and rarely in the ovary or in the abdomen [41]. Pelvic inflammatory disease, tubal surgery, oophoritis and in vitro fertilization therapy have been implicated as risk factors, being a HM, a rare complication described [43].
6. Conclusions
A comprehensive histopathological classification of first-trimester abortion specimens is a prerequisite for the definition of reproducible embryonic and placental phenotypes. Understanding its clinical significance can serve as a gold standard for additional complementary tests and comparative effectiveness trials. Routinely used could improve the management of individual patients allowing better maternal and fetal monitoring in future pregnancies.
Declarations
Author contribution statement
Rosete Nogueira: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Pedro Luís Cardoso: Conceived and designed the experiments; Performed the experiments.
Jesús Cadillá, Graça Rodrigues and Jorge Correia Pinto: Analyzed and interpreted the data.
Marcos Gomes and Ana Azevedo: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was developed under the scope of project NORTE-01-0145-FEDER- 000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020) under the Portugal Partnership Agreement, through the European Regional Development Fund (FEDER), and by National funds, through the Foundation for Science and Technology (FCT) - project UIDB/50026/2020 and UIDP/50026/2020. CGC Genetics - Unilabs, Porto Portugal.
Data availability statement
The data that has been used is confidential.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work was developed under the scope of project NORTE-01-0145-FEDER- 000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020) under the Portugal Partnership Agreement, through the European Regional Development Fund (FEDER), and by National funds, through the Foundation for Science and Technology (FCT) - project UIDB/50026/2020 and UIDP/50026/2020.
CGC Genetics - Unilabs, Porto Portugal. The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Edmonds D.K., Lindsay K.S., Miller J.F., William E., Wood P.J. Early embryonic mortality in women. Fertil. Steril. 1982;38(4):447–453. [PubMed] [Google Scholar]
- 2.Wilcox A.J., Weinberg C.R., O’Connor J.F. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 3.Whittaker P.G., Taylor A., Lind T. Unsuspected pregnancy loss in healthy women. Lancet. 1983;21(1):1126–1127. doi: 10.1016/s0140-6736(83)92865-9. [DOI] [PubMed] [Google Scholar]
- 4.Zinaman M., Clegg E., Brown C., O’Connor J., Selevan S. Estimates of human fertility and pregnancy loss. Fertil. Steril. 1996;65(3):503–509. [PubMed] [Google Scholar]
- 5.Heatley M.K., Clark J. The value of histopathological examination of conceptual products. Br J Obs. Gynecol. 1995;102(3):256–258. doi: 10.1111/j.1471-0528.1995.tb09105.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalousek D.K. Anatomic and chromosome anomalies in specimens of early spontaneous abortions: seven-year experience. Birth Defects Orig. Artic. Ser. 1987;23(1):153–168. [PubMed] [Google Scholar]
- 7.Kalousek D.K. Clinical significance of morphologic and genetic examination of spontaneously aborted embryos. Am. J. Reprod. Immunol. 1998;39(2):108–119. doi: 10.1111/j.1600-0897.1998.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw K., Fayyad A. RCOG; 2000. The Management of Early Pregnancy Loss, RCOG Guidelines.http://www.rcog.org.uk/index.asp?PageID=515 [Google Scholar]
- 9.Jindal P., Regan L., Fourkala E.O., Rai R., Moore G., Goldin R.D., Sebire N.J. Placental pathology of recurrent spontaneous abortion: the role of histopathological examination of products of conception in routine clinical practice: a mini review. Hum. Reprod. 2007;22(2):313–316. doi: 10.1093/humrep/del128. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira R., Barroca H., Brandão O. Pathology of the first two periods of intra-uterine development. An epidemiological study of 749 cases. Pathol. Res. Pract. 1993;189:771. [Google Scholar]
- 11.Baergen R.N., editor. Manual of Benirschke and Kaufmann’s Pathology of the Human Placenta. Springer; New York: 2005. Evaluation of the first trimester products of conception; pp. 3–15. chap. 1. [Google Scholar]
- 12.Nogueira R., Sousa S., Braga A., Azevedo A., Pereira N., Carmo O., Tavares P., Pinto J. Measurements in first-trimester abortion products: a pathological study. Arch. Pathol. Lab Med. 2020;144(2):207–214. doi: 10.5858/arpa.2018-0181-OA. [DOI] [PubMed] [Google Scholar]
- 13.Brezina P. Classic and cutting-edge strategies for the management of early pregnancy loss. Obstet. Gynecol. Clin. N. Am. 2014;41(1):1–18. doi: 10.1016/j.ogc.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert-Barness E., Debich-Spicer D., editors. Embryo and Fetal Pathology - Color Atlas with Ultrasound Correlation. Cambrige University Press; New York: 2004. The human embryo and embryonic growth disorganization; pp. 1–22. [Google Scholar]
- 15.Sadler T.W. Langman's Medical Embriology. thirteenth ed. Lippincott Williams & Wilkins; Philadelphia: 2015. Birth defects and prenatal diagnosis; pp. 117–129. [Google Scholar]
- 16.Gerulath A.H., Ehlen T.G., Bessette P., Jolicoeur L., Savoie R. Gestational trophoblastic disease. J. Obstet. Gynaecol. Can. 2002;24(114):434–446. [PubMed] [Google Scholar]
- 17.Garcia-Enguidanos A., Calle M., Valero J., Luna S., Dominguez-Rojas V. Risk Factors in miscarriage: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;102(2):111–119. doi: 10.1016/s0301-2115(01)00613-3. [DOI] [PubMed] [Google Scholar]
- 18.Regan L., Rai R. Epidemiology and the medical causes of miscarriage. Bailliere's Best Pract. Res. Clin. Obstet. Gynaecol. 2000;14(5):839–854. doi: 10.1053/beog.2000.0123. [DOI] [PubMed] [Google Scholar]
- 19.Rodini E.S., Tsuribe P.M., Moreira L.M., Souza M.O., Capannacci J., Agostinho M.A. Spontaneous abortions: cytogenetic studies and risks of occurrence. Arq Ciên Saúde. 2004;11(1):37–39. [Google Scholar]
- 20.Van den Berg M., Vissenberg R., Goddijn M. Recurrent miscarriage clinics. Obstet. Gynecol. Clin. N. Am. 2014;41(1):145–155. doi: 10.1016/j.ogc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Philipp T., Kalousek D.K. Generalized abnormal embryonic development in missed abortion: embryoscopic and cytogenetic findings. Am. J. Med. Genet. 2002;111(1):41–47. doi: 10.1002/ajmg.10476. [DOI] [PubMed] [Google Scholar]
- 22.Criado B., Brandão O., Nogueira R., Seruca R., Castedo S. Citogenética de interfase de los cromosomas sexuales en material de archivo de abortos. Progresos Diagn. Prenat. 1997;9(7):376–382. [Google Scholar]
- 23.Warren J.E., Silver R.M. Genetics of pregnancy loss. Clin. Obstet. Gynecol. 2008;51(1):84–95. doi: 10.1097/GRF.0b013e318161719c. [DOI] [PubMed] [Google Scholar]
- 24.Fritz B., Hallermann C., Olert J. Cytogenetic analyses of culture failures by comparative genomic hybridisation (CGH)-Re-evaluation of chromosome aberration rates in early spontaneous abortions. Eur. J. Hum. Genet. 2001;9(7):539–547. doi: 10.1038/sj.ejhg.5200669. [DOI] [PubMed] [Google Scholar]
- 25.Choi T.Y., Lee H.M., Park W.K., Jeong S.Y., Moon H.S. Spontaneous abortion and recurrent miscarriage: a comparison of cytogenetic diagnosis in 250 cases. Obstet. Gynecol. Sci. 2014;57(6):518–525. doi: 10.5468/ogs.2014.57.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philipp T., Philipp K., Reiner A., Beer F., Kalousek D.K. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Human Reprod. 2003;18(8):1724–1732. doi: 10.1093/humrep/deg309. [DOI] [PubMed] [Google Scholar]
- 27.Middeldorp S. Thrombophilia and pregnancy complications: cause or association? J. Thromb. Haemostasis. 2007;5(1):276–282. doi: 10.1111/j.1538-7836.2007.02501.x. [DOI] [PubMed] [Google Scholar]
- 28.Freire K., Padilha P.C., Saunders C. Fatores associados ao uso de álcool e cigarro na gestação. Rev. Bras. Ginecol. Obstet. 2009;31(7):335–341. [PubMed] [Google Scholar]
- 29.Morin L., Van Den Hof M.C. Ultrasound evaluation of first trimester pregnancy complications. J. Obstet. Gynaecol. Can. 2005;27(6):581–585. doi: 10.1016/s1701-2163(16)30718-6. [DOI] [PubMed] [Google Scholar]
- 30.Baergen R.N., editor. Manual of Benirschke and Kaufmann’s Pathology of the Human Placenta. Springer; New York: 2005. Gestational trophoblastic diseases; pp. 416–461. chap. 23-25. [Google Scholar]
- 31.WHO Classification of Tumors . fifth ed. 2020. Female Genital Tumors; pp. 310–333. Chap 7. [Google Scholar]
- 32.Kohorn E.I. The new FIGO 2000 staging and risk factor scoring system for gestational trohoblastic disease: description and critical assessment. Int. J. Gynecol. Canc. 2001;11:73–77. doi: 10.1046/j.1525-1438.2001.011001073.x. [DOI] [PubMed] [Google Scholar]
- 33.Kurman Robert J., Carcangiu M.L., Herrington C Simon. International Agency for Research on Cancer; 2014. 4th Edition. WHO Classification of Tumours of Female Reproductive Organs; pp. 155–167. chap. 6. [Google Scholar]
- 34.Fukunaga M. Immunohistochemical characterization of p57KIP2 expression in early hydatidiform moles. Human Parhol. 2002;27:731–734. doi: 10.1053/hupa.2002.129421. [DOI] [PubMed] [Google Scholar]
- 35.Genest D.R. Partial Hydatidiform mole: clinicopathologic features, differential diagnosis, ploidy and molecular studies, and gold standards for diagnosis. Int. J. Gynecol. Pathol. 2001;20:315–322. doi: 10.1097/00004347-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Genest D.R., Laborde O., Berkowitz R.S. A clinicopathologic study of 153 cases of complete hydatidiform mole (1980-1990): histologic grade lacks prognostic significance. Obstet. Gynecol. 1991;78:402–409. [PubMed] [Google Scholar]
- 37.Freire de Oliveira C., Ayres de Campos D. Manual de Ginecologia. 2009;I:449–459. chap. 27. [Google Scholar]
- 38.Bifulco C., Johnson C., Hao L., Kermalli H., Bell S., Hui P. Genotypic analysis of hydatidiform mole: an accurate and practical method of diagnosis. Am. J. Surg. Pathol. 2008;32(3):445–451. doi: 10.1097/PAS.0b013e3181520034. [DOI] [PubMed] [Google Scholar]
- 39.Li H.W., Tsao S.W., Cheung A.N.Y. Current understandings of the molecular genetics of gestational trophoblastic diseases. Placenta. 2002;23:20–31. doi: 10.1053/plac.2001.0744. [DOI] [PubMed] [Google Scholar]
- 40.Caldas R.F., Oliveira P., Rodrigues C., Reis I., Scigliano H., Nogueira R., Araújo C., Ferreira S. Intraplacental choriocarcinoma: rare or underdiagnosed? Report of 2 cases diagnosed after an incomplete miscarriage and a preterm spontaneous vaginal delivery. Hindawi Case Rep. Med. 2017:4. doi: 10.1155/2017/7892980. Article ID 7892980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramilo I., Mendinhos G., Igreja F., Aleluia M.C., Nogueira R., Gomes F., Pereira J. Unusual combination of gestational trophoblastic neoplasias: case report. J. Bras. Patol. Med. Lab. 2014;50(5):375–378. [Google Scholar]
- 42.Jiao L., Ghorani E., Sebir N.J., Seckl M.J. Intraplacental choriocarcinoma: systematic review and management guidance. Gynecol. Oncol. 2016;141(3):624–631. doi: 10.1016/j.ygyno.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Bouyer J., Coste J., Fernandez H., Pouly J.L. Sites of ectopic pregnancy : a 10 year population-based study of 1800 cases. Hum. Reprod. 2002;17(12):3224–3230. doi: 10.1093/humrep/17.12.3224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.