Abstract
Context:
Outcome of acromegaly surgery is assessed by IGF-1 and glucose-suppressed GH, but whether the latter provide additional clinically relevant information when IGF-1 is normal is unclear. The role of GH suppression testing after surgery requires clarification.
Methods:
We studied 97 acromegaly patients with normal IGF-1 after surgery by measuring GH after oral glucose longitudinally, initially at ≥ 3 months after surgery and repeated one or more times ≥ 1 year later. Nadir GH was categorized as normal or abnormal relative to the 97.5th percentile of nadir GH in 100 healthy subjects, which were ≤ 0.14 μg/L (DSL IRMA) or ≤ 0.15 μg/L(IDS iSYS). Signs and symptoms scores and insulin resistance were followed longitudinally.
Results:
Of 68 patients with initial normal GH suppression 63(93%) remained in remission and of 29 with initial abnormal GH suppression, 9(31%) recurred. Recurrence was more common in patients with abnormal suppression (P<0.001). A total of 14 patients recurred, including 5 with normal GH suppression progressing to abnormal and then recurrence. Overall, serial signs and symptoms and insulin resistance assessments did not identify patients with abnormal suppression or recurrence.
Conclusion:
Risk of recurrence after surgery is increased for patients with a normal IGF-1 level, but abnormal GH suppression. We newly find, using both our and others’ cut-offs, that while normal suppression predicts long-term remission in most patients, some can progress from normal to abnormal suppression and then recurrence after many years of follow up. Nadir GH levels are of prognostic value in acromegaly patients with normal IGF-1 levels after surgery.
Clinical trials #:
Keywords: Acromegaly, GH, IGF-1
INTRODUCTION
The initiating biochemical abnormality of acromegaly is GH hypersecretion from a pituitary tumor. Traditional testing aimed to document the presence or absence of GH excess for diagnosing or excluding acromegaly, respectively. Biochemical confirmation of remission after surgery can include demonstration of normal GH suppression after oral glucose (1). A key component of the modern biochemical assessment of acromegaly is Insulin-like growth factor 1 (IGF-1) measurement. This reflects overall GH secretion, is an excellent disease marker and its normalization correlates with that of metabolic and other parameters as well as mortality rate in acromegaly (2–4). Most recent guidelines consider IGF-1 normalization to be the primary criterion for establishing remission (5) and find a less clear role for the routine use of glucose-suppressed GH levels in the monitoring of postoperative patients. Others have found immediate postoperative GH levels to be most predictive of long-term surgical outcome(6). Changing GH assay senstivity and specificity (7–16) and lack of GH assay harmonization (17,18), have resulted in debate over what nadir GH cut-off best signifies remission (19,20). The role of oral glucose GH suppression testing in the postoperative evaluation requires clarification.
We previously identified that some patients with normal IGF-1 levels after surgery do, while others do not, suppress their GH levels after oral glucose into the range of healthy subjects. The short-term follow up of 49 patients with normal IGF-1 levels, abnormal GH suppression and other evidence of higher circulating GH levels than patients with normal GH suppression, suggested that recurrence was more common in those with abnormal than normal GH suppression (21). Three other studies, however, did not find a higher recurrence rate in this group (22–24). In order to define the role of glucose-suppressed GH levels in the evaluation of patients after surgery, we undertook a long-term biochemical and clinical follow up of 97 patients who had initial biochemical remission defined by IGF-1 normalization after surgery. We aimed to determine, using state of the art, clinically available GH and IGF-1 assays, whether IGF-1 normalization alone fully informs disease status in acromegaly or if GH levels after oral glucose provide additional prognostic or clinically significant information relevant to the long-term outcome of surgically treated patients.
MATERIALS AND METHODS
Acromegaly Subjects
Subjects were selected from among those enrolled in our prospective acromegaly cohort study since 1996. The cohort study is open to all acromegaly patients including those newly diagnosed or treated with surgery or other medical therapy. Patients joined the study before surgery or at different time points after surgery or during other therapies, and were followed prospectively from the time of study enrollment. Patients participated in one or more study visits that include completion of history and signs and symptoms questionnaires, physical examination and measurement of GH(fasting and/or OGTT) and IGF-1 levels. All had preoperative confirmation of acromegaly as defined by clinical signs and symptoms, an elevated serum IGF-1 level and lack of GH suppression based on criteria appropriate for the assay used at the time of diagnosis (GH > 2 μg/L using an RIA and > 1 μg/L with a modern IRMA or chemiluminescent assay). All had transsphenoidal surgery and pathological confirmation of a pituitary tumor positive for GH on immunohistochemical (IHC) staining. For the current study, we screened the cohort for patients meeting the following criteria: 1) normalization of IGF-1 level (for at least 15 months) after surgery as the only therapy, 2) GH levels measured by 1 of 2 sensitive, specific assays (see methods) during at least 2 OGTTs, the first (initial) being perfomed ≥ 3 months after surgery and the second at least 1 year later, 3) no additional acromegaly therapy prior to surgery or during the period of observation reported in this study, 4) no diabetes mellitus at initial or follow up testing. Our study included all consecutive patients meeting all of these criteria and excluded any who did not meet 1 or more of them. Those excluded included 12 patients who were followed for < 15 months and did not have a 2nd OGTT ≥ 1 year after the 1st; one of whom developed a persistently elevated IGF-1 level at 6 months of follow up. Premenopausal acromegaly women were studied in the early follicular phase of the menstrual cycle (days 1–5). The study cohort included 40 patients from our prior report (21) who met these entry criteria. The current study includes 364.5 new person-years of follow up data on these 40 patients in addition to the 203.7 person-years of follow up data reported previously. Twenty-six patients had 2 OGTTs, 30 had 3, 15 had 4, 11 had 5, 5 had 6, 4 had 7, 4 had 8 and 2 patients had 9 OGTTs. The final study cohort consisted of 97 acromegaly patients, 54 males and 43 females.
Healthy Subjects
Forty-six healthy subjects (26 M, 20 F; median age 38 yr. (range 19–71 yr.), median BMI 23.4 kg/m2(range 18.3–34.5 kg/m2) had a 100 gm OGTT performed and GH was measured by DSL IRMA as previously reported (14,21). Fifty-four healthy subjects (34 M, 20 F; median age 39 yr.(range 20–66 yr.); median BMI 24.4 kg/m2 (range 18.3–34.5 kg/m2) had a 75 gm OGTT performed and GH was measured IDS-iSYS assay. Premenopausal healthy women were studied in the early follicular phase of the menstrual cycle (days 1–5). No females were taking oral contraceptives or estrogen therapy.
Study Procedures
At each visit, acromegaly patients had an OGTT, physical examination (including body weight and height) and completed history and signs and symptoms questionnaires. For the OGTT, after an overnight fast, while seated, blood was sampled at baseline (fasting) and then at 60, 90 and 120 minutes after drinking 75 or 100 g dextrose (Trutol 100*), which equivalently suppress GH (25). Blood was clotted at room temperature, then centrifuged and the serum was frozen at −80oC in multiple aliquots. Samples from all time points were assayed for GH in the same assay and in duplicate. Fasting blood samples were assayed for IGF-I and in 65 patients at 252 time points also for insulin and glucose levels, from which HOMA and QUICKI scores were calculated. Eighty-six patients, 56 at 3 or more visits and 30 at 2 visits, completed a total of 328 signs and symptoms questionnaires on which they rated their Headache, Perspiration, Joint Pain, Fatigue, Hand/Feet Puffiness, Snoring and Overall Health Status on a scale from 0 being best to 8 being worst (26–28). A composite score (sum of other 7 scores) was calculated for each.
Hormone Assays
Growth Hormone was measured from 1996 to 2009 with a 2 site, 22K GH specific immunoradiometric assay (IRMA)(Diagnostic Systems Laboratories (DSL)(Webster, Texas, USA) that was calibrated to World Health Organization (WHO) International Reference Standard (IRS) 88/624 and had an intra-assay coefficient of variation (CV) of 3.1% and inter-assay CV of 5.9% (9). All subsequent GH samples were assayed by a 2-site, 22K GH specific chemiluminescence immunoassay (IDS-iSYS, Immunodiagnostic Systems, Tyne & Ware, UK) calibrated to IRS 98/574 (10,29) that has an intra-assay CV of 2%−4% and inter-assay CV of 5%−7% at GH concentrations between 1.7 and 27.5 μg/L. Assay sensitivity for both assays in our laboratory is 0.05 μg/L. GH levels were measured in 44 patients by DSL IRMA or by IDS-iSYS assays at different times in their follow up, in 37 patients by DSL IRMA only and in 16 patients by IDS-iSYS only. Insulin-like growth factor-I (IGF-I) was measured by 3 different assays over time. IGF-1 was measured from 1996 to 2005 by polyclonal RIA from Nichols Institute (San Juan Capistrano, CA) calibrated to WHO 1st International Reference Reagent 1988, IGF-1 87/518 that had a intra-assay CV of 4%, and inter-assay CV of 11%(21), from 2005–2016 by chemiluminescent immunoassay IMMULITE(Siemens), calibrated to WHO IRR NIBSC code 87/518 that has an intra-assay CV of 4% and inter-assay CV of 5.9% and from 2016–2020 by chemiluminescent immunoassay (IDS-iSYS) that is calibrated to the new recombinant standard 02/254 (30) and has an intra-assay CV of 1.3%–3.7% and inter-assay CV of 3.4%–8.7%. IGF-1 levels were compared to manufacturers’ age and gender (for IDS-iSYS) specific normative ranges for each assay. Insulin was measured by IMMULITE (Siemens) and glucose by the hexokinase method.
Statistical Analysis
Acromegaly remission was defined as IGF-1 level ≤ the upper limit of the age-adjusted normal range (≤ 1X ULN). Nadir GH was defined as the lowest value at any time point before or after oral glucose administration. Acromegaly patients’ nadir GH levels were categorized as normal or abnormal relative to the upper limit of normal as defined by the 97.5th percentile of the healthy subjects’ mean nadir GH levels which was 0.14 μg/L (0.08 ± .03 μg/L (mean ± SD); range 0.05 – 0.13 μg/L)(9,14,21) with the DSL assay and 0.15 μg/L (0.07 ± 0.04 μg/L (mean ± SD); range 0.05 – 0.21 μg/L) with the IDS-iSYS assay. For our primary analysis we compared each GH nadir to its assay-specific normal range. We also assessed the comparability of GH levels measured by the DSL IRMA and IDS-iSYS assays by correlation and Bland Altman Analysis. For this, we remeasured 183 previously unassayed aliquots of GH samples that had been measured by DSL IRMA and had been stored in a −80 freezer, with the IDS-iSYS assay. GH levels measured with the two assays correlated highly (r=.947, Pearson’s) and Bland Altman showed a IDS to DSL BIAS of 0.0649, SD of the bias .255, (95% CI of agreement (−.4353 to 0.5651)). Since there was a moderate level of agreement between the assays and nadir GH levels in healthy subjects were very similar with the two assays, we conducted a secondary analysis of our data in comparison to normative data developed by others for the IDS-iSYS assay (10). For this, we used the criteria of normal GH suppression in males and pre and postmenopausal females as < 0.4 μg/L for patients with BMI < 25 kg/m2 and < 0.2 μg/L for patients with BMI ≥ 25 kg/m2. We compared the proportions of patients who recurred, males and females, types of gonadal function among groups and IGF-1 assay type used among discordant GH/IGF-1 tests by Fishers Exact Test with Bonferroni correction as needed. Mann Whitney test was used to compare continuous variables between groups and the Kruskal-Wallis test or ANOVA to test for group differences in follow up periods, BMI, age and IGF-1 levels relative to the upper limit of normal, with Bonferroni correction as needed. Receiver operating characteristic (ROC) analysis was conducted on the spectrum of initial nadir and fasting (random) GH levels to define the optimal cut point for each measure my maximizing Youden’s index and ROC AUC was calculated. Change over time in signs and symptoms scores and insulin sensitivity indices were analyzed by the generalized estimation equation (GEE) with linear link and working independence correlation structure. This analysis was planned for >20 years duration of the cohort study and when median follow up of subjects meeting inclusion criteria was > 8 years. Data were analyzed by Prism 8 for MAC and SAS 9.4 (SAS Institute, Cary, NC), and P values less than 0.05 were considered statistically significant.
RESULTS
Acromegaly Patient Characterization by Normal and Abnormal GH Suppression
Acromegaly patients were categorized based on their nadir GH level being normal (less than or equal to the healthy subjects’ upper limit) or abnormal (greater than the healthy subjects’ upper limit) on initial testing as well as being normal or abnormal on follow up based on their most recent OGTT. Table 1 shows the characteristics of the cohort overall and the acromegaly patient groups (A through E) defined by initial and patterns of change in GH suppression at last follow up or until IGF-1 became elevated (i.e. recurrence).
TABLE 1.
Demographic, anthropometric and endocrine data and duration of follow up in all 97 acromegaly patients combined and in patients divided into Groups A-F based on the pattern of initial GH suppression as either normal or abnormal and then further sub-divided based on the pattern of GH suppression on follow up testing.
| Normal GH Suppression on Initial Testing | Abnormal GH Suppression on Initial Testing | ||||||
|---|---|---|---|---|---|---|---|
| ALL PATIENTS | Normal on Follow Up [A] | Abnormal on Follow Up [B] | Abnormal → Recurrence [C] | Abnormal on Follow Up [D] | Normal on Follow Up [E] | Abnormal → Recurrence [F] | |
| Number of Acromegaly Patients | 97 | 54 | 9 | 5 | 16 | 4 | 9 |
| Gender (Male/Female) | 54M/43F | 35M/19F | 5M/4F | 2 M/3 F | 7 M/9 F | 2M/2F | 3M/6F |
| Age at Diagnosis of Acromegaly (yr.)* | 45.3 ± 11.9 | 45.9 ± 11.4 | 41.5 ±9.9 | 41.5 ± 10.9 | 48.7 ± 14 | 50.4 ± 15.6 | 38.3 ± 12.5 |
| BMI (kg/m2) | |||||||
| Mean ± SD | 28.8 ±4.5 | 30.13 ±4.6 | 26.48 ± 3.9 | 26.9 ± 3.2 | 26.66 ± 2.6 | 30.13 ± 8.1 | 27 ±4.4 |
| Median (Range) | 28.8(21.1–44.8) | 29.8(21.7–44.8) | 25.8(22.1–34.4) | 25.7(22.9–30.8) | 26.7(22.1 −31.4) | 31.1(21.6–37.7) | 27.9(21.1–34.5) |
| Years from Surgery to Initial Testing | |||||||
| Mean ± SD | 2.46 ± 3.7 | 2.25 ± 3.8 | 2.05 ± 2.8 | 1.13 ±1.6 | 3.75 ± 3.6 | 3.2 ±4.7 | 2.83 ± 3.4 |
| Median (Range) | 1.4(.25 – 24.9) | 1.34(.25–24.9) | 0.88(.25–8.34) | 0.48(0.25–4) | 2.38(.25–9.87) | 1.15(.25–10.2) | 1.9(.26–15.2) |
| Years of Follow Up Conducted | |||||||
| Mean ± SD | 9.34 ±6.4 | 9.7 ± 6.6 | 10.5 ± 6.9 | 13.6 ± 4.2 | 8.45 ± 7.4 | 5.6 ±2.1 | 8.3 ± 6.2 |
| Median (Range) | 8.1(1.52–22) | 9.23(1.52–22 ) | 9 (3–21) | 13.2(7.1–18.2) | 6.4(1.9–22) | 5.8( 3–7.6) | 6.2(3.2–21.2) |
| Nadir GH on Initial Testing | |||||||
| Mean ± SD | 0.07 ± 0.03 | 0.13 ±0.14 | 0.075 ±0.04 | 0.49 ± 0.34 | 0.18 ± 0.03 | 0.44 ± 0.46 | |
| Median (Range) | 0.06(.05-.14) | 0.05(.05-.05) | 0.06(.05-.14) | 0.36(.15–1.2) | 0.2(.15-.21) | 0.30(.15–1.65) | |
| Nadir GH on Follow Up Testing | |||||||
| Mean ± SD | 0.068 ± 0.03 | 0.29 ±0.13 | 1.37 ± 1.4 | 0.52 ± 0.42 | 0.09 ± 0.05 | 1.49 ± 1.13 | |
| Median (Range) | 0.05(.05 – .14) | 0.33(.15–.58) | 0.69(.44–2.99) | 0.4(.17–1.62) | 0.09(.05 –.14) | 1.08( 23–2.93) | |
| Random (Fasting) GH on Initial Testing | |||||||
| Mean ± SD | 0.30 ±0.43 | 0.62 ± 0.7 | 0.39 ± 0.34 | 1.73 ± 2.3 | 0.69 ± 0.33 | 0.77 ± 0.45 | |
| Median (Range) | 0.15(.05 – 2.7) | 0.29(.05 – 2) | 0.23(.ll – .97) | 1.15(0.19–8.5) | 0.77(0.21 −1) | 0.54(0.29 – 1.6) | |
Mean ± SD
On initial testing, 68 patients had normal GH suppression. In follow up, GH suppression remained normal in 54 (Table 1, Group A) and became abnormal in 14, of which 5 developed abnormal suppression and then a recurrence (Table 1, Group C). Of the 68 patients with initial normal GH suppression, 63 (93%) had a long-term persistent remission. On initial testing, 29 patients had abnormal GH suppression. In follow up, suppression normalized in 4 (Table 1, Group E), remained abnormal in 25 (Groups D, F) and 9/29 with initial abnormal suppression developed a recurrence after a median of 4.5 yr. (range 2.8–24 yr.) of follow up. Recurrence was significantly more likely in patients with initial testing showing abnormal GH suppression compared to those with normal GH suppression (P=.004). While most patients with abnormal GH supppression remained in long-term remission, recurrence was more likely in patients who had abnormal GH suppression on initial testing or developing with time, 14/43, than in those with normal GH suppression on initial testing or developing in follow up 5/72(P<.001). Recurrence remained significantly more likely in patients with abnormal suppression as measured by either the DSL IRMA assay (P<.001) or the IDS-iSYS assay (P=.006).
Overall, in follow up, 83/97(86%) patients remained in remission (normal IGF-1) and 14/97(14%) had a recurrence (elevated IGF-1). We conducted ROC analysis and determined Youden’s index for the spectrum of nadir GH and random (fasting) GH levels in our cohort. For dividing recurrent from not recurrent patients, the optimal cut point for nadir GH was 0.14 μg/L (Youden’s index = 1.437), ROC AUC=0.709 (95% CI 0.552–0.865) and for random GH the optimal cut point was 0.215 μg/L (Youden’s index= 1.410), ROC AUC=0.669 (95% CI 0.549–0.790). Positive predictive value of nadir GH < 0.14 for persistent remission was 93%. Follow up period did not differ in the recurrent, 9.4 ± 5.9 yr.(median 9.6 (2.7–21.2)), vs. not recurrent group, 9.2 ± 6.7 yr.(median 8.2 (.25–22))(P=.88). IGF-1 levels showed a trend to be higher at initial testing in the 14 patients who recurred, 64 ± 18.6 % ULN (mean ± SD)(%Upper Limit of Normal), than in those who remained in remission, 55.4 ± 22% ULN(P=.08). The proportion of patients exhibiting the abnormal GH nadir/normal IGF-1 pattern did not differ among the 3 IGF-1 assays used on initial (P=0.52) or follow up (P=0.14) testing.
For the patients that recurred, detailed OGTT GH, IGF-1 and clinical data are shown in Table 2 and the supplement to Table 2, including that for the 5 who were previously, briefly reported (21). IGF-1 levels were confirmed to be elevated in clinical laboratories as shown in Table 2. Of the 14 patients, 7 had visible tumor regrowth on MRI (including 2 previously reported) and 3 had surgery that pathologically confirmed a GH positive tumor on IHC staining. 5 patients with initial normal suppression progressed through abnormal suppression at 1–6 years of follow up and subsequently developed an elevated IGF-1 level, ie. a recurrence, at a median of 13 yr (range 2.25–18 years of follow up)(Table 2, Subjects #1–5). None had a history of multiple endocrine tumors. Clinical signs and symptoms, insulin resistance, ring size or comorbidities did not consistently worsen over time.
TABLE 2: Detailed Endocrine and Clinical Data in the 14 Acromegaly Patients who Recurred.
Growth hormone levels shown in unshaded cells were assayed by IDS-iSYS, grey shaded cells by DSL IRMA and GH and IGF-1 levels shown in patterned cells were assayed by a commercial laboratory. Patients #1–5 progressed from normal to abnormal suppression and then recurrence. Patients #6–14 progressed from abnormal suppression to recurrence. SA- Somatostatin Analog; TS- Transsphenoidal Surgery; GH +: Pathology showed a tumor + for GH on IHC; EUG - eugonadal; T-testosterone therapy; PM-postmenopausal; RM - regular menses; IM - Irregular menses; HRT - hormone replacement therapy estradiol patch; TVE - topical vaginal estrogen; NR - Nuvaring contraceptive; CTS - carpal tunnel syndrome; OA - osteoarthritis; JP - Joint Pain; HA- headache; STS - soft tissue swelling; SW- sweating; SN-snoring; F-fatigue.
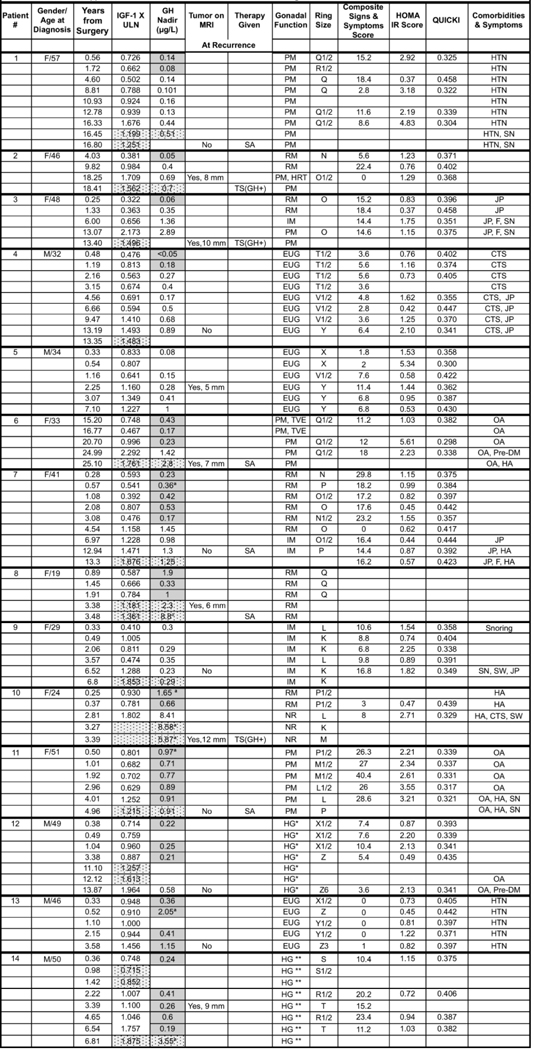 |
Testosterone levels 180–220 ng/mL range
Testosterone levels 200–360 ng/mL; IGF-1 X ULN is IGF-1 value times the upper limit of normal
denotes that GH is a fasting level.
Demographic and Anthropometric Determinants of Nadir GH
Age did not differ at initial testing in the normal, 45.3 ± 11.1 yr. (mean ± SD)(median 46.4 yr., range 18.7 – 53.4 yr.) vs. abnormal, 45.1 ± 13.9 yr. (mean ± SD)(median 46.3 yr., range 18.9 −70.6 yr.)(P=0.94), suppression groups overall or among Groups A-F (Table 1). There was a trend for more males in the normal (64% males vs. 36% females) than abnormal (males 43% vs. females 57%)(P=.07) suppression groups. Overall, the normal GH suppression group had a higher BMI at initial testing, 29.5 ± 4.62 kg/m2, compared to that in the abnormal GH suppression group, BMI 27.2 ± 4.03 kg/m2 (P=.03). BMI was higher in Group I, 30 ± 4.7 kg/m2, than in Group II, 26.6 ± 3.1 kg/m2 (P=.006) and than in Group III, 26.9 ± 3.9 kg/m2, (P=.04)(Table 3).
TABLE 3. Demographics, Gonadal Function and Signs and Symptoms Scores on Initial testing and at Follow Up in the Acromegaly Cohort.
Groups A - F (Table 1) were combined into: Group I: Normal on follow up - combines groups with GH suppression normal initially and on follow up [A] and GH suppression abnormal initially and normal on follow up[E]; Group II: Abnormal on follow up - combines groups with GH suppression normal initially that became abnormal on follow up[B] and group with abnormal GH suppression initially and on follow up [D]; Group III: Recurrence - combines group with GH suppression normal initially that became abnormal and developed a recurrence[C] and group with abnormal GH suppression initially that developed a recurrence[F]. Gonadal function is reported as number of subjects (% of group) with each type of gonadal function.
| Group I | Group II | Group III | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [A] GH suppression normal initially and on follow up | [E] GH suppression abnormal initially and became normal | [B]GH suppression normal initially and became abnormal | [D]GH suppression abnormal initially and remained abnormal | [C] GH suppression normal initially, became abnormal and recurrence developed | [F] GH suppression abnormal initially and recurrence developed | |||||||
| # Acromegaly Patients (M/F) | 35M/19F | 2M/2F | 5M/4F | 7M/9F | 2M/3F | 3M/6F | ||||||
| Age at Diagnosis/Last Follow Up | Diagnosis | Follow Up | Diagnosis | Follow Up | Diagnosis | Follow Up | Diagnosis | Follow Up | Diagnosis | Follow Up | Diagnosis | Follow Up |
| Mean ± SD | 45.9 ± 11.4 | 57.3 ± 12 | 50.4 ± 15.6 | 57.4 ± 16 | 41.5 ±9.9 | 53.9 ± 12.4 | 48.7 ± 14 | 60.6 ± 16.4 | 41.5 ± 10.9 | 54.9 ± 13.3 | 38.3 ± 12.5 | 48.7 ± 16 |
| Median (Range) | 47.4(20–72) | 56.7(33–83) | 52.6(31–65) | 60.9(36–71) | 42.9(19– 54) | 55.2(32–71) | 50.4(25–71) | 61.8(27–79) | 35.1(32–57) | 53.3(40–73) | 41.2(19–59) | 54(23–71) |
| Gonadal Function | 1 | no change | ||||||||||
| Males Eugonadal | 27 | no change | no change | 4 | 7 | no change | 2 | no change | 2 | no change | ||
| Eugonadal on testosterone | 5 | no change | 1 | no change | ||||||||
| Hypogonadal | 3 | no change | 1 | no change | no change | 1 | no change | |||||
| Females Eugonadal | 6 | no change | 1 | no change | 1 | no change | 2 | no change | ||||
| Postmenopausal (no HRT) | 8 | no change | 2 | no change | 1 | no change | 2 | no change | 1 | no change | 1 | no change |
| Hypogonadism (no HRT) | 1 | no change | 1 | 1 | 3 | no change | ||||||
| Varied | 4* | 4* | 1** | 1** | 3*** | 3*** | 2# | 2# | 3## | 3## | ||
| Significance Tests: | Men: No difference in proportion of hypogonadal men between Group 1 and II or III or between Groups II and III. Women: No difference in proportion of women who were PM, HG or with Varied gonadal function between Group 1 and II or III or between Groups II and III. |
|||||||||||
| Signs and Symptoms Scores | ||||||||||||
| Headache | 1.12 ± 1.74 | 1.02 ± 1.52 | 1.14 ±2.27 | 0.5 ±1 | 1.11 ± 1.93 | 1.9 ± 2.85 | 1.43 ± 1.77 | 1.16 ±1.91 | 1.05 ± 1.98 | 0.89 ± 1.62 | 1.24 ± 1.29 | 1.42 ± 1.83 |
| Perspiration | 1.2 ± 1.78 | 1.1 ± 1.58 | 2.2 ±2.747 | 1.2 ± 1.9 | 1.46 ± 1.92 | 2.1 ±2.03 | 1.71 ±2.03 | 1.57 ± 1.88 | 0.79 ± 0.92 | 1.67 ± 1.87 | 1.65 ± 1.47 | 1.03 ± 1.44 |
| Joint Pain | 1.87 ± 2.35 | 2.1 ± 2.29 | 2.77 ± 1.32 | 2.45 ± 1.79 | 1.97 ± 2.47 | 2.3 ± 2.55 | 1.61 ± 1.84 | 3.05 ± 1.77 | 0.89 ± 1.63 | 1.67 ± 1.41 | 2.87 ±2.52 | 2.9 ± 2.69 |
| Fatigue | 2.34 ± 2.24 | 2.31 ±2.28 | 2.8 ±2.23 | 2.1 ±2.83 | 1.53 ±2.31 | 1.8 ± 2.49 | 2.05 ± 1.89 | 1.67 ± 1.98 | 1.16 ±2.01 | 0.67 ± 1.12 | 2.95 ± 2.35 | 1.71 ± 2.05 |
| Hand and Feet Puffiness | 1.55 ± 1.52 | 1.28 ± 1.83 | 0 | 1.33 ±2.31 | 0.77 ± 2.02 | 1.5 ±2.22 | 0.85 ± 1.39 | 1.11 ± 1.22 | 0.76 ± 1.75 | 0.78 ± 1.64 | 1.7 ± 1.78 | 0.73 ± 1.27 |
| Snoring | 2.26 ± 2.33 | 1.68 ± 1.93 | 1.86 ± 1.07 | 1.85 ± 1.3 | 1.44 ± 2.03 | 1 ± 1.25 | 2.1 ±2.23 | 1.3 ± 1.99 | 0.91 ± 0.85 | 1.33 ± 2.06 | 1.97 ±2.18 | 1.3 ± 1.86 |
| Overall Health | 2.19 ± 1.77 | 2.29 ± 1.87 | 3.47 ± 1.31 | 4.4 ± 1.9 | 1.98 ± 1.83 | 2.16 ± 1.07 | 2.03 ± 1.59 | 2.61 ± 1.92 | 1.52 ± 1.36 | 1.95 ± 1.84 | 2.49 ± 1.89 | 2.67 ± 2.44 |
| Composite | 11.62 + 9.31 | 11.16 ±9.79 | 13.74 ±4.12 | 13.5 ± 7.13 | 10.02 ± 12.2 | 12.76 ± 11.16 | 11.11 ±6.67 | 12.24 ± 6.82 | 6.96 ± 6.34 | 8.95 ± 6.55 | 13.8 ± 10.68 | 11.7 ±9.37 |
| Significance Tests: | ||||||||||||
| Groups A-E | [A]: No changes | [E]: Snoring ß=0.154 (SE=0.071) p=0.030. Overall health ß=0.363 (SE=0.180) p= 0.044, Composite ß=0.508 (SE=0.175) p=0.004. | [B]: Joint pain ß-0.102 (SE=0,046) p=0.025. Fatigue ß=0.099 (SE=0.039) p=0.011. Hand/feet puffiness ß-0 097 (SE=0.032) p-0.002, Composite ß=0.523 (SE=0.181) 0.004. | [O]: Fatigue ß = −0.103 (SE=0.038) p=0.006. | [C]: Perspiration ß= −0.068 (SE-0.018| <0.001, Joint pain ß=0.146 (SE=0.061) p=0.016. | [F]: No changes | ||||||
| Groups I, II, III: Individually | I: No changes overtime | II: Perspiration: ß=0.094 (SE=0.044) p=0.033; Composite ß=0.288 (SE=0.146) p=0.049 | III: Perspiration: ß= −0.077 (SE-0.027) p=0.005 | |||||||||
| Groups I, II, II: Comparison | I compared to II or III : No difference | II compared to III: ß= 0.170 (SE=0.052) p=0.001 | ||||||||||
| HOMA IR Score | 1.89 ±1.41 | 1.83 ±3.72 | 1.16 ±0.94 | 1.44 ± 1.37 | 1.20 ±0.85 | 1.39 ± 1.71 | 1.09 ± 0.69 | 1.23 ±0.48 | 1.65 ± 1.29 | 1.86 ± 1.36 | 1.37 ± 0.85 | 1.75 ± 1.51 |
| QUICKI | 0.366 ± 0.04 | 0.37 ±0.05 | 0.39 ± 0.04 | 0.39 ± 0.07 | 0.39 ± 0.05 | 0.358 ± 0.03 | 0.39 ± 0.045 | 0.377 ± 0.02 | 0.37 ±0.04 | 0.358 ± 0.03 | 0.38 ± 0.04 | 0.372 ± 0.04 |
| Significance Tests: No significant difference in change in HOMA or QUICKI score in Groups A-F separately or in Group 1, II, II or in Group II compared to Group III. | ||||||||||||
RM - regular menses, IM-irregular menses, PM-postmenopausal, HRT - hormone replacement therapy, Data are Mean ± SD.
1) IM for 7 yr, PM 7 yr.; 2) HRT for 1 year, no HRT 9 yr.; 3 )IM 6 yr., PM 7 yr., 4) IM 18 yr, PM 4 yr.
1) HRT 6 yr. no HRT, 2 yr..
1) RM 3 yr., PM 4 yr., 2) RM 9 yr., PM 1 yr. , 3) RM 7 yr., PM 7 yr.
1) RM 17 yr., HRT 1 yr., PM 1 yr.; 2) RM 7 years, PM 6 year after that prior to recurrence
1) Vaginal estrogen 4 yr., PM 5 yr., 2) RM 2 yr., nuvaring 2 yr.,3) RM 11 yr., IM 2 yr..
Signs and Symptoms Scores and Insulin Resistance
We tested for changes in signs and symptoms scores and insulin resistance measures over time in each Group A-F, but since the number of subjects in some groups was small, we also performed analyses in 3 new groups that combined groups A-F based on initial and follow up nadir GH (Table 3). Changes in signs and symptoms scores are shown in Table 3. HOMA score at the time of initial testing did not differ in the normal, 1.338 ± .92, vs. abnormal, 1.231 ± .72 (P=0.63), suppression groups and there were no differences in change in insulin resistance or sensitivity indices, HOMA score or QUICKI, over time in Groups A-F separately or in Group I, II, II or in Group II compared to Group III (Table 3).
Gonadal Function
Groups did not differ in the proportion of hypogonadal men or in the proportions of women who were postmenopausal, pre-menopausal hypogonadal, or had varied gonadal function over the time of follow up (Table 3). Gonadal function varied in 5 women in Group 3(Tables 2,3), but this is unlikely to have contributed to their recurrences since they transitioned from normal to lower estrogen states, which would have lowered GH levels, but theirs rose. In 2 women, menopause occurred 2 years prior to their recurrences, raising the possibility that lowering of estrogen levels contributed to the rise in IGF-1 levels, unmasking their recurrences. In both, however, GH and IGF-1 rose concurrently and 1 had visible tumor regrowth.
Analysis Using IDS-iSYS Nadir Cut-Offs
We also analyzed our acromegaly patients’ data in relation to the BMI and gender adjusted normative cut offs for the IDS-iSYS assay established by others (10). We found that 9 patients were reclassified from abnormal to normal GH suppression when applying these cut-offs; 5 were reclassified on initial testing, 3 on follow up testing and 1 on both initial and follow up testing. Two patients were reclassified from each of Group E to A, B to A and D to B and 1 was reclassified from each of D to A, D to E and F to C. Interestingly, this included 1 of the 14 patients who recurred, who was reclassified on initial testing from abnormal with our cut off to normal with that of Schilbach et. al.. Using either cut off, the patient’s GH suppression was abnormal on follow up testing in transition to recurrence (Table 2, Subject #7). Using the cut offs of Schilbach et. al., recurrence was more common in those with initial abnormal (8/23) than normal (6/74) GH suppression (P=.004) and in those with abnormal suppression initially or developing on follow up (14/37) than in those with normal suppression initially or that developed in follow up (6/77)(P < .001). Of the 15 patients with normal GH suppression that progressed to abnormal suppression during the follow up, 6 recurred.
DISCUSSION
The primary findings of this long-term, prospective study are an increased risk of acromegaly recurrence in patients with abnormal GH suppression after oral glucose, either initially or developing over time, and a strong association between normal GH suppression and persistent long-term remission after surgery. These findings, importantly, reinforce our prior report of an association between disease recurrence and abnormal GH suppression. Notably, our findings support a role for postoperative OGTT testing that is contrary to guidelines that recommend reserving measurement of a nadir GH level after oral glucose for patients with a GH > 1 μg/L after surgery(5). We find that important, prognostic information can be gained from an OGTT in a patient with a normal IGF-1 level regardless of their random GH level. Our study also provides novel data that the BMI and gender-adjusted normative nadir GH cut offs developed for the IDS-iSYS GH assay(10) give similar prognostic guidance with regard to risk of progression to abnormal suppression and recurrence as our cut offs. Since this assay is in widespread clinical use in the US and Europe, our new data can guide the interpretation of nadir GH levels measured by this assay for use in long-term monitoring of surgically-treated patients.
Another important, new finding of our study is that progression from normal to abnormal GH suppression occurs in a sizable, 20% of patients with long-term follow up and this transition to abnormal suppression can be followed by a recurrence in some patients. While this study included only 5 recurrences among 14 patients who developed abnormal suppression, it differs from our prior report in which only 3.3% of those with initially normal suppression progressed to abnormal suppression and none of them recurred (21). While we and others have reported the pattern of “discordant” elevated nadir GH and normal IGF-I levels in 11 to 42% of surgically treated (9,15,31–34), as well as in somatostatin analog or radiation treated (35,36) patients, to our knowledge, progression from normal suppression, as defined by our strict criteria, to abnormal suppression and then to recurrence has not previously been reported. This study’s longer follow up likely enabled this finding. In surgical series, 7% developed a nadir GH > 1 μg/L (37) and 21% with GH > 0.3 μg/L at 3 months after surgery developed an elevated IGF-1 (31) on follow up, but details about whether these patients had true recurrences were not provided (31,37). None of our 5 patients who progressed to recurrence from normal GH suppression had evidence of predisposition to multiple endocrine tumors that might have led to a second, de novo tumor, but this is possible as is that the recurrence represents regrowth of residual cells of the original tumor. The latter suggests a much longer time frame for the development of acromegaly in some patients than previously recognized. Five of our study’s 14 recurrences occurred > 10 years after surgery. Others have reported recurrences as late as 10 or 12 yr. after surgery(38). Surgical series, which our study by design was not, report low recurrence rates of 0–2.4%, using current criteria (39–41), but potentially very long follow up is needed to identify all recurrences. We did not find better discrimination of long-term outcome with fasting than OGTT nadir GH and others have found IGF-1 to be a better predictor of long-term outcome than random GH level (42).
Contrary to our findings, three other studies did not find a higher rate of recurrence in patients with abnormal nadir GH level after surgery. Feelders et. al., performed serial OGTTs after surgery in 7 patients with nadir GH of 0.2 μg/L and 2 with nadir GH of 0.5 μg/L, but none recurred by 12 weeks follow up (22). Limitations of this study were its small size and short duration of follow up. Ronchi et. el. conducted a cross-sectional study in 40 patients in remission (defined by normal IGF-1) for a median of 14.5 yr. after surgery (23). GH was measured with a 22K specific assay calibrated to the older, human pituitary standard 80/505. The upper limit of normal nadir GH was 0.19 μg/L and 52% of patients had a nadir GH between 0.19 and 1 μg/L. The authors concluded that the pattern of abnormal GH nadir with normal IGF-1 level does not confer an increased risk of recurrence. In a subsequent study, using the same GH assay, Ronchi et. al. prospectively studied 40 surgically treated patients (17 of whom also had RT), 16 with normal nadir GH < 0.26 μg/L and 24 with abnormal nadir GH > 0.26 μg/L(24). IGF-1 was slightly higher in the latter group. Upon re-evaluation after at least 3 years, median of 6.5 yr., of follow up, GH levels did not change and none had recurred. Why our findings and those of this study differ is unclear, but our follow up was longer and, potentially, differences in GH assays and their standards played a role in our different findings.
Other data also suggest that abnormal GH suppression despite a normal IGF-1 level is a marker of mild or early GH excess along the progression to recurrence in some patients. We previously showed that some patients with abnormal GH suppression have other evidence of mild GH excess with greater mean GH on hourly sampling and GH response to arginine stimulation compared to those with normal GH suppression(21). Subtly elevated GH levels may not uniformly induce high IGF-I levels, potentially due to inter-individual differences in IGF-1 peripheral synthesis (43). Postoperative persistent thyrotrophin releasing hormone-induced growth hormone release, in particular among patients with normal GH levels, also predicted recurrence in patients with acromegaly (38,44). The fact that most patients in our study with initially normal or abnormal GH suppression continued this pattern over time after surgery also speaks to this being a true pattern for these patients as well as to the likelihood that GH neuro-secretory abnormalities, rather than mild excess, are the cause of abnormal GH suppression in most patients (45,46).
We considered other possible explanations for the abnormal GH suppression/normal IGF-1 pattern. No patients had other conditions associated with abnormal GH suppression (47–52). Nadir GH levels may be gender dependent (15,16,53,54) being higher in women, in particular those < 30 years of age, and those on estrogen (16,54). We found only a trend for more females in the abnormal than normal GH suppression group and no difference in gonadal function between GH suppression groups in males or females. The small size of some of our groups, however, may have limited these and some other analyses in this study. Age at initial testing did not differ in normal and abnormal GH suppression groups in our study, but age correlated inversely with nadir GH level in some other studies (53,54). Waist circumference correlated inversely with nadir GH level in one study (54) and higher BMI was associated with lower nadir GH in others (10,53). Schilbach et. al. found BMI-adjusted normative nadir GH cut offs to be appropriate for use with the IDS-iSYS assay(10). Interestingly, although this study found higher nadir GH levels in younger women, BMI was ultimately more important to determining normal nadir GH level cut offs, than gender or menopausal status (10). We found a lower BMI in the abnormal GH suppression group, suggesting that this may be related to the higher nadir GH levels of some acromegaly patients. However, lower BMI cannot explain abnormal suppression in those who recurred and when analyzed in relation to BMI adjusted cut-offs, abnormal GH suppression remained significantly associated with recurrence and recurrence rates were similar using BMI adjusted and unadjusted cut-offs. Timing of our postoperative OGTT is unlikely to have influenced our findings, but that which best predicts long-term outcome is debated. We chose > 3 months after surgery, in accord with other studies and guidelines (5,55), to avoid misclassifying patients during the period of early postoperative IGF-1 fluctuations. However, others found a 1-week postoperative OGTT using a GH cut off of < 1.0 μg/L to have 95% specificity and 81.7% sensitivity for persistent remission at a mean follow up of 3.8 yr. (6), which contrasts markedly with our findings. Also, our study focused on GH values for predicting recurrence rather than strict timing of assessments, since this is often not practical in clinical practice. Although IGF-1 values were expressed relative to assay-specific normal ranges and the proportion of discrepant tests did not differ among assays, our use of 3 different IGF-1 assays is a limitation of our study.
Concurrent monitoring for recurrence of clinical acromegaly as well as GH and IGF-1 levels is recommended (5,20,56), so we tested whether changes in signs and symptoms score or co-morbidities paralleled those of GH suppression pattern or biochemical recurrence. While worsening of clinical symptoms/signs seemed to accompany recurrence in some individual patients (Table 2), most groups had worsening of some signs or symptoms with concurrent improvement of others and overall, there was no consistent pattern of worsening of signs and symptoms associated with progression to abnormal GH suppression or recurrence. In 2 other smaller studies, signs and symptoms did not differ in normal vs. abnormal GH suppression groups (23,24) and in another, changes in them and IGF-1 levels during acromegaly treatment did not correlate (26). We also did not find that changes in HOMA score or QUICKI index occurred over time in any group of subjects. Even though our study, unlike others, included >3 serial signs and symptoms or insulin resistance assessments on most patients, we did not find them to be helpful, overall, in identifying patients with abnormal suppression or recurrence.
In conclusion, we find normal GH suppression after surgery to be highly predictive of persistent, long-term remission and although remission persisted in the majority of patients with abnormal GH suppression, this, either initially or developing with time, was associated with increased risk of acromegaly recurrence. We also provide novel data, using both our cut-offs and the IDS-iSYS, BMI and gender adjusted cut-offs, that some patients who progress to abnormal suppression recur after many years of follow up, suggesting a much longer time frame for acromegaly development in some patients. Our findings, therefore, show that nadir GH levels measured by highly sensitive and specific GH assays 3 or more months postoperatively provide useful prognostic information of interest to the patient and physician and have a clinically relevant role in predicting long-term outcomes of acromegaly patients after surgery.
Supplementary Material
Acknowledgments
Funding: Funded by NIH grants R01 DK 110771 and DK064720 to PUF, and in part by Columbia University’s CTSA grant No. UL1 TR000040 from NCATS/NIH.
Footnotes
Disclosure statement: PUF, JNB, CRV, SS, JD, ZJ, AGK, SC and KDP have nothing to disclose.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Hage M, Kamenicky P, Chanson P. Growth Hormone Response to Oral Glucose Load: From Normal to Pathological Conditions. Neuroendocrinology. 2019;108(3):244–255. [DOI] [PubMed] [Google Scholar]
- 2.Puder JJ, Nilavar S, Post KD, Freda PU. Relationship between Disease-related Morbidity and Biochemical Markers of Activity in Patients with Acromegaly. J Clin Endocrinol Metab. 2005;90:1972–1978. [DOI] [PubMed] [Google Scholar]
- 3.Reid TJ, Jin Z, Shen W, Reyes-Vidal CM, Fernandez JC, Bruce JN, Kostadinov J, Post KD, Freda PU. IGF-1 levels across the spectrum of normal to elevated in acromegaly: relationship to insulin sensitivity, markers of cardiovascular risk and body composition. Pituitary. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swearingen B, Barker FG 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly [see comments]. J Clin Endocrinol Metab. 1998;83(10):3419–3426. [DOI] [PubMed] [Google Scholar]
- 5.Katznelson L, Laws ER Jr., Melmed S, Molitch ME, Murad MH, Utz A, Wass JA, Endocrine S. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. [DOI] [PubMed] [Google Scholar]
- 6.Kim EH, Oh MC, Lee EJ, Kim SH. Predicting long-term remission by measuring immediate postoperative growth hormone levels and oral glucose tolerance test in acromegaly. Neurosurgery. 2012;70(5):1106–1113; discussion 1113. [DOI] [PubMed] [Google Scholar]
- 7.Reiter EO, Morris AH, MacGillivray MH, Weber D. Variable estimates of serum growth hormone concentrations by different radioassay systems. J Clin Endocrinol Metab. 1988;66(1):68–71. [DOI] [PubMed] [Google Scholar]
- 8.Celniker AC, Chen AB, Wert RM Jr., Sherman BM. Variability in the quantitation of circulating growth hormone using commercial immunoassays. J Clin Endocrinol Metab. 1989;68(2):469–476. [DOI] [PubMed] [Google Scholar]
- 9.Freda PU, Post KD, Powell JS, Wardlaw SL. Evaluation of disease status with sensitive measures of growth hormone secretion in 60 postoperative patients with acromegaly. J Clin Endocrinol Metab. 1998;83(11):3808–3816. [DOI] [PubMed] [Google Scholar]
- 10.Schilbach K, Gar C, Lechner A, Nicolay SS, Schwerdt L, Haenelt M, Dal J, Jorgensen JL, Stormann S, Schopohl J, Bidlingmaier M. Determinants of the growth hormone nadir during oral glucose tolerance test in adults. Eur J Endocrinol. 2019;181(1):55–67. [DOI] [PubMed] [Google Scholar]
- 11.Monaghan PJ, Trainer PJ. Determinants of the growth hormone nadir during oral glucose tolerance test in adults. Eur J Endocrinol. 2019;181(5):C17–C20. [DOI] [PubMed] [Google Scholar]
- 12.Hattori N, Shimatsu A, Kato Y, Koshiyama H, Ishikawa Y, Assadian H, Tanoh T, Nagao M, Imura H. Growth hormone responses to oral glucose loading measured by highly sensitive enzyme immunoassay in normal subjects and patients with glucose intolerance and acromegaly. J Clin Endocrinol Metab. 1990;70(3):771–776. [DOI] [PubMed] [Google Scholar]
- 13.Stewart PM, Smith S, Seth J, Stewart SE, Cole D, Edwards CR. Normal growth hormone response to the 75 g oral glucose tolerance test measured by immunoradiometric assay. Ann Clin Biochem. 1989;26 ( Pt 2):205–206. [DOI] [PubMed] [Google Scholar]
- 14.Freda PU, Landman RE, Sundeen RE, Post KD. Gender and age in the biochemical assessment of cure of acromegaly. Pituitary. 2001;4:163–171. [DOI] [PubMed] [Google Scholar]
- 15.Costa AC, Rossi A, Martinelli CE Jr., Machado HR, Moreira AC. Assessment of Disease Activity in Treated Acromegalic Patients Using a Sensitive GH Assay: Should We Achieve Strict Normal GH Levels for a Biochemical Cure? J Clin Endocrinol Metab. 2002;87(7):3142–3147. [DOI] [PubMed] [Google Scholar]
- 16.Chapman IM, Hartman ML, Straume M, Johnson ML, Veldhuis JD, Thorner MO. Enhanced sensitivity growth hormone (GH) chemiluminescence assay reveals lower postglucose nadir GH concentrations in men than women. J Clin Endocrinol Metab. 1994;78(6):1312–1319. [DOI] [PubMed] [Google Scholar]
- 17.Bidlingmaier M. Problems with GH assays and strategies toward standardization. Eur J Endocrinol. 2008;159 Suppl 1:S41–44. [DOI] [PubMed] [Google Scholar]
- 18.Pokrajac A, Wark G, Ellis AR, Wear J, Wieringa GE, Trainer PJ. Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin Endocrinol (Oxf). 2007;67(1):65–70. [DOI] [PubMed] [Google Scholar]
- 19.Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KK, Casanueva FF, Melmed S, Acromegaly Consensus G. Expert consensus document: A consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10(4):243–248. [DOI] [PubMed] [Google Scholar]
- 20.Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, Strasburger CJ, Luger A, Clemmons DR, Giustina A. A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freda PU, Nuruzzaman AT, Reyes CM, Sundeen RE, Post KD. Significance of “abnormal” nadir growth hormone levels after oral glucose in postoperative patients with acromegaly in remission with normal insulin-like growth factor-I levels. J Clin Endocrinol Metab. 2004;89(2):495–500. [DOI] [PubMed] [Google Scholar]
- 22.Feelders RA, Bidlingmaier M, Strasburger CJ, Janssen JA, Uitterlinden P, Hofland LJ, Lamberts SW, van der Lely AJ, de Herder WW. Postoperative evaluation of patients with acromegaly: clinical significance and timing of oral glucose tolerance testing and measurement of (free) insulin-like growth factor I, acid-labile subunit, and growth hormone-binding protein levels. J Clin Endocrinol Metab. 2005;90(12):6480–6489. [DOI] [PubMed] [Google Scholar]
- 23.Ronchi CL, Varca V, Giavoli C, Epaminonda P, Beck-Peccoz P, Spada A, Arosio M. Long-term evaluation of postoperative acromegalic patients in remission with previous and newly proposed criteria. J Clin Endocrinol Metab. 2005;90(3):1377–1382. [DOI] [PubMed] [Google Scholar]
- 24.Ronchi CL, Arosio M, Rizzo E, Lania AG, Beck-Peccoz P, Spada A. Adequacy of current postglucose GH nadir limit (< 1 microg/l) to define long-lasting remission of acromegalic disease. Clin Endocrinol (Oxf). 2007;66(4):538–542. [DOI] [PubMed] [Google Scholar]
- 25.Arafat AM, Muller L, Mohlig M, Mayr B, Kremenevskaya N, Pfeiffer AF, Buchfelder M, Schofl C. Comparison of oral glucose tolerance test (OGTT) 100 g with OGTT 75 g for evaluation of acromegalic patients and the impact of gender on test reproducibility. Clin Endocrinol (Oxf). 2011;75(5):685–691. [DOI] [PubMed] [Google Scholar]
- 26.Paisley AN, Rowles SV, Roberts ME, Webb SM, Badia X, Prieto L, Shalet SM, Trainer PJ. Treatment of acromegaly improves quality of life, measured by AcroQol. Clin Endocrinol (Oxf). 2007;67(3):358–362. [DOI] [PubMed] [Google Scholar]
- 27.Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant [see comments]. N Engl J Med. 2000;342(16):1171–1177. [DOI] [PubMed] [Google Scholar]
- 28.Bonapart IE, van Domburg R, ten Have SM, de Herder WW, Erdman RA, Janssen JA, van der Lely AJ. The ‘bio-assay’ quality of life might be a better marker of disease activity in acromegalic patients than serum total IGF-I concentrations. Eur J Endocrinol. 2005;152(2):217–224. [DOI] [PubMed] [Google Scholar]
- 29.Manolopoulou J, Alami Y, Petersenn S, Schopohl J, Wu Z, Strasburger CJ, Bidlingmaier M. Automated 22-kD growth hormone-specific assay without interference from Pegvisomant. Clin Chem. 2012;58(10):1446–1456. [DOI] [PubMed] [Google Scholar]
- 30.Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Korner A, Obermayer-Pietsch B, Hubener C, Dahlgren J, Frystyk J, Pfeiffer AF, Doering A, Bielohuby M, Wallaschofski H, Arafat AM. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712–1721. [DOI] [PubMed] [Google Scholar]
- 31.Espinosa-de-Los-Monteros AL, Sosa E, Cheng S, Ochoa R, Sandoval C, Guinto G, Mendoza V, Hernandez I, Molina M, Mercado M. Biochemical evaluation of disease activity after pituitary surgery in acromegaly: a critical analysis of patients who spontaneously change disease status. Clin Endocrinol (Oxf). 2006;64(3):245–249. [DOI] [PubMed] [Google Scholar]
- 32.Vierhapper H, Heinze G, Gessl A, Exner M, Bieglmayr C. Use of the oral glucose tolerance test to define remission in acromegaly. Metabolism. 2003;52(2):181–185. [DOI] [PubMed] [Google Scholar]
- 33.Boero L, Manavela M, Danilowicz K, Alfieri A, Ballarino MC, Chervin A, Garcia-Basavilbaso N, Glerean M, Guitelman M, Loto MG, Nahmias JA, Rogozinski AS, Servidio M, Vitale NM, Katz D, Fainstein Day P, Stalldecker G, Mallea-Gil MS. Comparison of two immunoassays in the determination of IGF-I levels and its correlation with oral glucose tolerance test (OGTT) and with clinical symptoms in acromegalic patients. Pituitary. 2012;15(4):466–471. [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulou O, Bex M, Abs R, T’Sjoen G, Velkeniers B, Maiter D. Divergence between growth hormone and insulin-like growth factor-i concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab. 2008;93(4):1324–1330. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael JD, Bonert VS, Mirocha JM, Melmed S. The utility of oral glucose tolerance testing for diagnosis and assessment of treatment outcomes in 166 patients with acromegaly. J Clin Endocrinol Metab. 2009;94(2):523–527. [DOI] [PubMed] [Google Scholar]
- 36.Sherlock M, Aragon Alonso A, Reulen RC, Ayuk J, Clayton RN, Holder G, Sheppard MC, Bates A, Stewart PM. Monitoring disease activity using GH and IGF-I in the follow-up of 501 patients with acromegaly. Clin Endocrinol (Oxf). 2009;71(1):74–81. [DOI] [PubMed] [Google Scholar]
- 37.Espinosa-de-los-Monteros AL, Mercado M, Sosa E, Lizama O, Guinto G, Lopez-Felix B, Garcia O, Hernandez I, Ovalle A, Mendoza V. Changing patterns of insulin-like growth factor-I and glucose-suppressed growth hormone levels after pituitary surgery in patients with acromegaly. J Neurosurg. 2002;97(2):287–292. [DOI] [PubMed] [Google Scholar]
- 38.Biermasz NR, Smit JW, van Dulken H, Roelfsema F. Postoperative persistent thyrotrophin releasing hormone-induced growth hormone release predicts recurrence in patients with acromegaly. Clin Endocrinol (Oxf). 2002;56(3):313–319. [DOI] [PubMed] [Google Scholar]
- 39.Losa M, Oeckler R, Schopohl J, Muller OA, Alba-Lopez J, von Werder K. Evaluation of selective transsphenoidal adenomectomy by endocrinological testing and somatomedin-C measurement in acromegaly. J Neurosurg. 1989;70(4):561–567. [DOI] [PubMed] [Google Scholar]
- 40.Kreutzer J, Vance ML, Lopes MB, Laws ER, Jr. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86(9):4072–4077. [DOI] [PubMed] [Google Scholar]
- 41.Minniti G, Jaffrain-Rea ML, Esposito V, Santoro A, Tamburrano G, Cantore G. Evolving criteria for post-operative biochemical remission of acromegaly: can we achieve a definitive cure? An audit of surgical results on a large series and a review of the literature. Endocr Relat Cancer. 2003;10(4):611–619. [DOI] [PubMed] [Google Scholar]
- 42.Cunha M, Borba LAB, Boguszewski CL. Random Gh and Igf-I levels after transsphenoidal surgery for acromegaly: relation with long-term remission. Endocrine. 2020;68(1):182–191. [DOI] [PubMed] [Google Scholar]
- 43.Elias PC, Lugao HB, Pereira MC, Machado HR, Castro M, Moreira AC. Discordant nadir GH after oral glucose and IGF-I levels on treated acromegaly: refining the biochemical markers of mild disease activity. Horm Metab Res. 2010;42(1):50–55. [DOI] [PubMed] [Google Scholar]
- 44.Arafah BM, Rosenzweig JL, Fenstermaker R, Salazar R, McBride CE, Selman W. Value of growth hormone dynamics and somatomedin C (insulin-like growth factor I) levels in predicting the long-term benefit after transsphenoidal surgery for acromegaly. J Lab Clin Med. 1987;109(3):346–354. [PubMed] [Google Scholar]
- 45.Semer M, Faria AC, Nery M, Salgado LR, Knoepfelmacher M, Wajchenberg BL, Liberman B. Growth hormone pulsatility in active and cured acromegalic subjects. J Clin Endocrinol Metab. 1995;80(12):3767–3770. [DOI] [PubMed] [Google Scholar]
- 46.Holdaway IM, Rajasoorya CR, Gamble GD, Stewart AW. Long-term treatment outcome in acromegaly. Growth Horm IGF Res. 2003;13(4):185–192. [DOI] [PubMed] [Google Scholar]
- 47.Duncan E, Wass JA. Investigation protocol: acromegaly and its investigation. Clin Endocrinol (Oxf). 1999;50(3):285–293. [DOI] [PubMed] [Google Scholar]
- 48.Chang-DeMoranville BM, Jackson IM. Diagnosis and endocrine testing in acromegaly. Endocrinol Metab Clin North Am. 1992;21(3):649–668. [PubMed] [Google Scholar]
- 49.Vinik A, Pimstone B, Buchanan-Lee B. Impairment of hyperglycemic induced growth hormone suppression in hyperthyroidism. J Clin Endocrinol Metab. 1968;28(11):1534–1538. [DOI] [PubMed] [Google Scholar]
- 50.Becker MD, Cook GC, Wright AD. Paradoxical elevation of growth hormone in active chronic hepatitis. Lancet. 1969;2(7629):1035–1039. [DOI] [PubMed] [Google Scholar]
- 51.Pieters GF, Smals AG, Kloppenborg PW. Defective suppression of growth hormone after oral glucose loading in adolescence. J Clin Endocrinol Metab. 1980;51(2):265–270. [DOI] [PubMed] [Google Scholar]
- 52.Holl RW, Bucher P, Sorgo W, Heinze E, Homoki J, Debatin KM. Suppression of growth hormone by oral glucose in the evaluation of tall stature. Horm Res. 1999;51(1):20–24. [DOI] [PubMed] [Google Scholar]
- 53.Arafat AM, Mohlig M, Weickert MO, Perschel FH, Purschwitz J, Spranger J, Strasburger CJ, Schofl C, Pfeiffer AF. Growth hormone response during oral glucose tolerance test: the impact of assay method on the estimation of reference values in patients with acromegaly and in healthy controls, and the role of gender, age, and body mass index. J Clin Endocrinol Metab. 2008;93(4):1254–1262. [DOI] [PubMed] [Google Scholar]
- 54.Colao A, Pivonello R, Auriemma RS, Grasso LF, Galdiero M, Pivonello C, Lombardi G, Savastano S. Growth hormone nadir during oral glucose load depends on waist circumference, gender and age: normative data in 231 healthy subjects. Clin Endocrinol (Oxf). 2011;74(2):234–240. [DOI] [PubMed] [Google Scholar]
- 55.Kristof RA, Neuloh G, Redel L, Klingmuller D, Schramm J. Reliability of the oral glucose tolerance test in the early postoperative assessment of acromegaly remission. J Neurosurg. 2002;97(6):1282–1286. [DOI] [PubMed] [Google Scholar]
- 56.Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, Bolanowski M, Bonert V, Bronstein MD, Casanueva FF, Clemmons D, Colao A, Ferone D, Fleseriu M, Frara S, Gadelha MR, Ghigo E, Gurnell M, Heaney AP, Ho K, Ioachimescu A, Katznelson L, Kelestimur F, Kopchick J, Krsek M, Lamberts S, Losa M, Luger A, Maffei P, Marazuela M, Mazziotti G, Mercado M, Mortini P, Neggers S, Pereira AM, Petersenn S, Puig-Domingo M, Salvatori R, Shimon I, Strasburger C, Tsagarakis S, van der Lely AJ, Wass J, Zatelli MC, Melmed S. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J Clin Endocrinol Metab. 2020;105(4). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


