Abstract
Introduction:
Prediabetes is a major risk factor for type 2 diabetes and cardiovascular diseases. While resistance exercise (RE) is recommended for individuals with prediabetes, the effects of RE on postprandial glucose metabolism in this population are poorly understood. Therefore, the purpose of this study was to elucidate how RE affects postprandial glucose kinetics, insulin sensitivity, beta cell function, and glucose oxidation during the subsequent meal in sedentary men with obesity and prediabetes.
Methods:
We studied 10 sedentary men with obesity (BMI: 33 ± 3 kg/m2) and prediabetes by using a randomized, cross-over study design. After an overnight fast, participants completed either a single bout of whole-body RE (7 exercises, 3 sets of 10-12 repetitions at 80% 1-repetition maximum each) or an equivalent period of rest. Participants subsequently completed a mixed meal test in conjunction with an intravenous [6,6-2H2]glucose infusion to determine basal and postprandial glucose rate of appearance (Ra) and disappearance (Rd) from plasma, insulin sensitivity, and the insulinogenic index (a measure of beta cell function). Skeletal muscle biopsies were obtained 90 min post-meal to evaluate pyruvate-supported and maximal mitochondrial respiration. Whole-body carbohydrate oxidation was assessed using indirect calorimetry.
Results:
RE significantly reduced the postprandial rise in glucose Ra and plasma glucose concentration. Postprandial insulin sensitivity was significantly greater after RE, while postprandial plasma insulin concentration was significantly reduced. RE had no effect on the insulinogenic index, postprandial pyruvate respiration, or carbohydrate oxidation.
Conclusion/Interpretation:
A single bout of RE has beneficial effects on postprandial glucose metabolism in men with obesity and pre-diabetes by increasing postprandial insulin sensitivity, reducing the postprandial rise in glucose Ra, and reducing postprandial plasma insulin concentration.
Keywords: Prediabetes, Postprandial, Glucose, Resistance Exercise, Insulin
Introduction
Prediabetes is a metabolic condition defined by elevated fasting (impaired fasting glucose (IFG)) and/or postprandial (impaired glucose tolerance (IGT)) plasma glucose (1). Prediabetes affects nearly 86 million adults in the United States, with most (up to 70%) ultimately progressing to type 2 diabetes (T2D) within as little as one year (2). While evidence suggests that T2D can be reversed, most cases of remission involve invasive treatment strategies, including bariatric surgery to promote weight loss, or extreme lifestyle changes (e.g. severe caloric restriction) that have poor long-term adherence (3,4). Therefore, preventing the initial progression of prediabetes to T2D would have profound health implications globally.
Previous studies have shown that lifestyle interventions combining diet and exercise can reverse prediabetes and restore normoglycemia. In 2002, the Diabetes Prevention Program Research Study demonstrated that lifestyle interventions aiming for 7% weight loss and 150 minutes per week of moderate-intensity endurance type physical activity reduced the incidence of T2D by 58%, and was significantly more effective than treatment with metformin alone in individuals with prediabetes (5). Likewise, the Finnish Diabetes Prevention Study found that a lifestyle intervention focusing on weight loss, reduced total fat intake, and physical activity (combined moderate aerobic and resistance exercise) reduced the incidence of T2D by 58% in obese men and women with impaired glucose tolerance (6). In light of these large-scale studies, the American Diabetes Association currently recommends individuals with prediabetes improve their diet and increase time spent engaged in moderate to high intensity physical activities (goal: 150 min/week of physical activity) to prevent or delay the onset of T2D (7).
The effect of resistance exercise (RE), which is a popular alternative to endurance type physical activities, on glucose metabolism and the risk of T2D is understudied and the results are equivocal (evidence grade B or C) (7). For example, in healthy, young, non-obese participants, RE reduced plasma glucose and insulin responses to an oral glucose tolerance test due to increased glucose clearance (8-10). Whereas in older, overweight subjects with prediabetes, 12 weeks of resistance exercise improved glucose tolerance, but not insulin sensitivity, during an oral glucose tolerance test (11). While these studies provide preliminary support for the benefits of resistance exercise on postprandial glucose metabolism, the use of oral glucose tolerance testing and the study of glucose concentrations alone provides limited insight into mixed meal glucose responses. It is currently unknown if resistance exercise reduces postprandial glucose appearance or increases glucose clearance (including glucose oxidation), improves beta cell function, increases insulin sensitivity, or augments hepatic insulin sensitivity following a mixed meal in individuals with prediabetes – mechanisms that would reduce postprandial hyperglycemia and reduce the risk for progression to T2D. Understanding the effects of resistance exercise on mixed-meal responses is critical to enhance exercise recommendations for these individuals, since: 1) mixed meal ingestion reflects free-living conditions (most meals consist of carbohydrates, proteins, and lipids); 2) most of the day is spent in the postprandial state; and 3) postprandial hyperglycemia is a major independent risk factor for cardiovascular disease (CVD) and other serious comorbidities (e.g. hypertension, chronic kidney disease) (12-14).
Therefore, the purpose of the present study was to determine the effects of a single bout of RE on postprandial glucose metabolism following a mixed-meal in obese, sedentary men with prediabetes. We used a stable-isotopically labeled glucose tracer infusion in conjunction with a mixed meal to measure basal and postprandial glucose rate of appearance (Ra) into and rate of disappearance (Rd) from plasma, insulin sensitivity (assessed as postprandial glucose Rd area under the curve divided by plasma insulin area under the curve), and the insulinogenic index (a measure of beta cell function). We also obtained muscle biopsies to evaluate pyruvate-supported mitochondrial respiration, and assessed postprandial whole-body substrate oxidation by using a metabolic cart. We hypothesized that a single bout of RE would reduce the glycemic response to a mixed meal by increasing glucose clearance rates, insulin sensitivity, and carbohydrate oxidation.
Methods
The participants and experimental protocol used in this study have been described in our previous publication that focused on the effects of resistance exercise on postprandial lipid metabolism (15). Here we provide additional information on the methods used to study glucose metabolism in these same participants.
Participants
Participants were recruited from the Washington University in St. Louis (WUSTL) School of Medicine Diabetes Clinic, the WUSTL Volunteers for Health, the Center for Community Based Research databases, and advertising in the St. Louis metropolitan area from 8/2015-5/2018. Potential participants completed a detailed screening evaluation including a history and physical examination, serum blood chemistries (HbA1c, a lipid panel, a comprehensive metabolic panel, and a complete blood count), and an oral glucose tolerance test [see Appendix, Supplemental Digital Content, Electronic Supplementary Materials (ESM) for further details].
Participants were included if they were male, aged 30-65 years with a BMI of 28-45 kg/m2, and met the criteria for prediabetes (HbA1c >38 but <48 mmol/mol (>5.7 but <6.5%)), or fasting plasma glucose >5.6 mmol/L but <7.0 mmol/L, or 2-hour OGTT glucose >7.8 mmol/L but <11.1 mmol/L) at 120 min (16). Only men were selected to avoid the confounding effects of sex-based differences in fasted and postprandial glucose metabolism, including a stronger predisposition for impaired glucose tolerance in men, superior postprandial glucose uptake in adipose tissue and skeletal muscle in women, increased use of carbohydrate relative to fat metabolism in the fasted and postprandial states in women; and preferential sparing of lipids during exercise recovery in women compared to men (17,18). Participants were excluded if they were diagnosed with type 2 diabetes, used insulin or other blood-sugar lowering medications, participated in regular exercise (≥2 times/week) within the previous 6 months (assessed by questionnaire during the initial phone interview), had a history of pulmonary or cardiovascular disease, coagulation disorders, anemia, or orthopedic, neurologic, metabolic or other medical condition that would prohibit them from participating in the exercise and metabolic testing protocol. Participants also completed a one-repetition maximum strength assessment, daily physical activity monitoring, and body composition measurements by using dual energy X-ray absorptiometry [DXA; see Appendix, Supplemental Digital Content, Electronic Supplementary Materials (ESM) for further details]. The study was approved by the WUSTL Human Research Protection Office (protocol #20160495) and all participants provided written informed consent prior to enrollment. All procedures were completed in accordance with the ethical principles outlined in the Declaration of Helsinki. Participant characteristics are shown in Table 1.
Table 1.
Participant Characteristics
| Measure | Mean (SD) |
|---|---|
| Age (yr) | 50 (9) |
| Height (cm) | 178 (6) |
| Weight (kg) | 105 (12) |
| BMI (kg/m2) | 33 (3) |
| Fat mass (kg) | 33 (6.4) |
| Lean mass (kg) | 67 (6.2) |
| % Body Fat | 31.6 (4) |
| HbA1c (%) | 5.7 (0.5) |
| HOMA2-%B | 139.4 (56) |
| HOMA2-%S | 46 (16) |
| Fasting glucose (mmol/L) | 5.9 (0.7) |
| 2-hr OGTT glucose (mmol/L) | 9.1 (0.5) |
| Fasting Insulin (pmol/L) | 131 (57) |
| Triglycerides (mmol/L) | 1.9 (0.27) |
| Total cholesterol (mmol/l) | 4.8 (0.83) |
| HDL-cholesterol (mmol/l) | 0.99 (0.13) |
| LDL-cholesterol (mmol/l) | 3.0 (0.75) |
| % Time Sedentary | 69 (19.2) |
| % Time Light Activity | 21 (11.4) |
| % Time Moderate Activity | 10 (9.5) |
| % Time Vigorous Activity | 0 (0) |
Values are presented as mean ± SEM. % of time spent in each activity were derived from accelerometry data (see ESM). WB = whole body. 2-hr OGTT is plasma glucose at 2-hours after a 75-gram oral glucose challenge. HOMA2 calculated using the HOMA2 calculator accessed from (http://www.dtu.oc.ac.uk) (20).
Study Protocol
Glucose Metabolism Study
Each participant completed two glucose metabolism studies (exercise and rest) in randomized order, approximately 1 week apart (Figure 1). Treatment order (rest or exercise) was assigned through simple random sampling without replacement (see Appendix, Supplemental Digital Content, Electronic Supplementary Materials). Between studies, participants were instructed to maintain their normal diet and daily activity level, which was verbally confirmed prior to beginning the second metabolism study. During the exercise study, participants completed a bout of whole-body resistance exercise (RE) 270 min before meal ingestion; for the rest study, participants rested in a chair.
Figure 1.
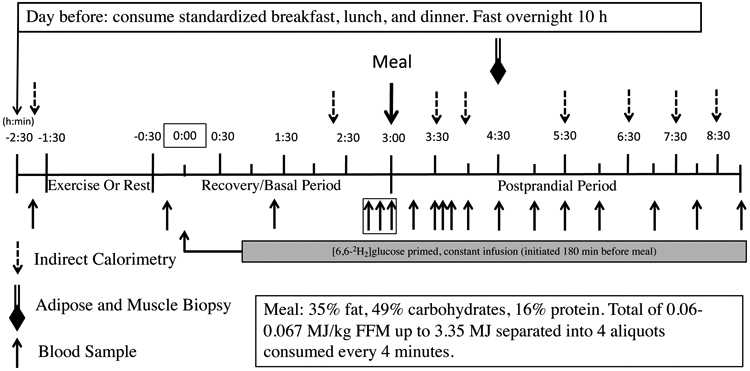
Study Protocol
The day prior to each glucose metabolism study, participants were provided standardized breakfast (2.18 MJ), lunch (3.02 MJ), afternoon snack (0.75 MJ), and dinner (3.26 MJ) from the WUSTL Clinical and Translational Research Unit (CTRU) Bionutrition Service. They consumed a liquid formula (Ensure; Ross Laboratories, Columbus, OH) containing 1.05 MJ at ~2200 h to ensure complete filling of hepatic glycogen stores. The carbohydrate, fat, and protein contents for each meal are provided in the ESM (see Appendix, Supplemental Digital Content, Electronic Supplementary Materials). Participants then fasted overnight (10 hr) except for water and reported to the CTRU at 0730 h the following morning. After admission, an intravenous catheter was inserted into the antecubital vein to collect baseline blood samples and later for tracer infusions.
During the rest study, participants rested quietly in the semi-recumbent position for 60 min from 0900 h – 1000 h. During the exercise study, participants performed 1 hr (from 0900 h – 1000 h) of RE consisting of 3 sets of 10-12 reps at 80% one-repetition maximum (1RM) of the following 7 exercises: leg press, knee extension, chest press, shoulder press, seated row, pull down, and biceps curl (see Appendix, Supplemental Digital Content, Electronic Supplementary Materials).
Following the rest or RE period for each study, a second catheter was inserted into a hand vein on the contralateral arm; the hand was warmed with a warming box (55°C) to collect arterialized blood samples. To allow time for catheter insertion, the study timer was started (study time 0) thirty minutes post-exercise or post-rest. At 0 min, a primed, constant infusion of [6,6-2H2]glucose (28 μmol/kg prime and 0.28 μmol/kg/min continuous infusion; Cambridge Isotope Laboratories, Tewksbury, MA) was initiated and continued to the end of the study. At 180-min, participants were given a liquid meal (Boost Plus) consisting of 0.08 MJ/kg fat free mass (FFM) (47.8% carbohydrate, 36.1% fat, and 16.1% protein). Blood samples were collected at −10, 80, 160, 170, 180, 196, 210, 215, 225, 240, 270, 300, 330, 360, 420, 480, and 540 minutes (Figure 1).
At 270-min (90-min post meal), biopsy samples from the vastus lateralis were obtained. We selected 90 minutes to allow adequate time for meal digestion, hormone secretion, and glucose uptake prior to probing for changes mitochondrial function, as well as to provide time for procedural set-up. Whole-body carbohydrate oxidation rate was measured at baseline (−90 min), immediately before the RE/rest period, and at 140, 210, 240, 330, 390, 450, and 510 min using indirect calorimetry as previously described (17). The time to peak carbohydrate oxidation was measured for each participant.
Sample Processing
Blood for plasma hormone and metabolite analysis was collected from an antecubital vein, immediately placed in chilled EDTA tubes, centrifuged at 2000xg for 10 minutes, and the supernatant collected and frozen at −80°C until analysis. The tracer-to-tracee ratio (TTRs) for plasma [2H2]glucose was quantified using capillary GC-MS (Agilent 6890N gas chromatograph and Agilent 5973N mass selective detector; Agilent, Palo Alto, CA), as previously described (19). Plasma insulin concentration was measured using electrochemiluminescence immunoassay and plasma glucose concentration was measured using the glucose oxidase method (Yellow Springs, OH).
Tissue Biopsies, Mitochondrial Respiration
Biopsies from the vastus lateralis were collected under local anesthesia as previously described (20,21). 6-10 mg of skeletal muscle was used for mitochondrial high-resolution respirometry analysis [Oxygraph-2k; Oroboros Instruments, Innsbruck, Austria; see Appendix, Supplemental Digital Content, Electronic Supplementary Materials, methods and ESM Table 1). Remaining muscle and adipose tissue samples were irrigated with saline, flash frozen in liquid nitrogen, and stored at −80°C until analysis. Pyruvate-supported respiration was calculated as:
(See Appendix, Supplemental Digital Content, Electronic Supplementary Materials.)
Calculations
Indices of beta cell function (HOMA-%B), and insulin sensitivity (HOMA-%S) were calculated from fasting glucose and insulin values before the mixed meal test using the Homeostatic Model Assessment 2 (HOMA2) calculator (22). Basal glucose rate of appearance (Ra) was calculated by dividing the [6,6-2H2]glucose infusion rate (μmol/min) by the plasma tracer enrichment (mean of all three measurements before the start of the mixed meal test at time 160, 170, and 180 min) (19). Non-steady state glucose kinetics during the mixed meal were assessed as previously described using plasma glucose concentration and 2H2-glucose tracer-to-tracee (TTR) values to assess the total Ra of glucose into plasma (sum of oral glucose appearance + endogenous glucose production) and total rate of glucose disappearance (Rd) (23). The postprandial [2H2]-glucose TTR for RE and rest is shown in ESM Figure 1 (see Appendix, Supplemental Digital Content, Electronic Supplementary Materials). Ra and Rd were normalized per kg of fat-free mass (FFM) from DXA.
The total area under the curve (AUC) was calculated using the trapezoid rule; incremental AUCs (iAUC) were obtained after adjusting for basal values (24). Overall postprandial insulin sensitivity was calculated as the glucose Rd AUC/insulin AUC (25). The postprandial insulinogenic index was calculated as the ratio of the change in insulin concentration to the change in glucose concentration over the first 30 minutes of the postprandial period (ΔI0-30/ΔG0-30) (26).
Statistical Analysis
Differences in glucose and insulin concentrations, carbohydrate oxidation, and glucose Ra and Rd time-courses between rest and exercise were assessed by using repeated measures ANOVA with treatment (rest/RE) and time (before/after meal) as repeated factors, and order (rest>RE or RE>rest) as a between-subjects factor. Significant interactions between treatment and time were further analyzed using paired t-tests. The Benjamini–Hochberg procedure was used to control for multiple comparisons for all repeated measures. AUCs, basal glucose Ra and Rd, insulinogenic index, insulin sensitivity, time to peak carbohydrate oxidation, and mitochondrial respirometry measures were analyzed using paired t-tests. An alpha level of 0.05 was used for significance. All analyses were completed in SPSS version 25. A conservative effect size of 1 was used to sample size calculations based on the results from Breen et al., who reported an effect size of 2 for an increase in the rate glucose disappearance after a single session of resistance exercise in healthy men (8). Therefore, a sample size of ten was required to achieve 80% power to detect differences on a paired t-test for glucose disposal (at a two-tailed alpha level of 0.05).
Results
Participant Demographics
Ten (n=10) participants were enrolled in the study. Participants were aged 39-62 years, obese, and demonstrated insulin resistance with compensatory increases in beta cell function. Participants also presented with elevated mean total triglyceride, LDL, and low HDL concentrations, and spent the majority of their day in sedentary activity (Table 1). Six participants had both IFG and IGT, two had IGT alone, one participant had IFG alone, and one participant qualified based on their HbA1c >5.7%.
Plasma Glucose and Insulin Concentrations
Compared to rest, the plasma glucose concentration was significantly elevated immediately after exercise (time −10 min in Figure 2A, p=0.005). After meal consumption, plasma glucose concentration and the glucose AUC were not different between conditions (Figure 2A, B). However, the postprandial glucose incremental AUC was significantly lower after resistance exercise than rest (180-540 min, p=0.015) indicating a reduced glycemic response to the mixed meal when normalized to pre-meal (measured at 180 min) glucose concentration (Figure 2C).
Figure 2. Plasma Glucose and Insulin Concentration.
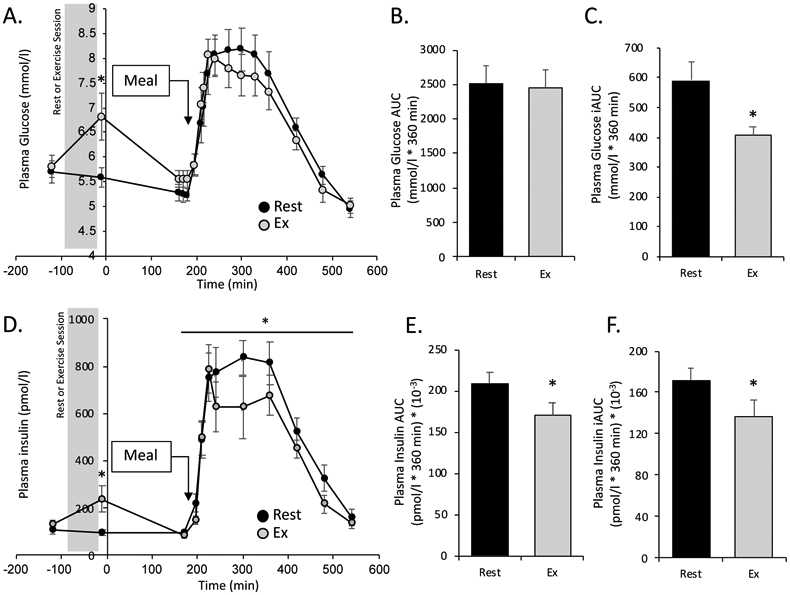
Plasma glucose and insulin concentrations measured throughout the study (mean ± SEM). Time point “0” corresponds to the start of the [6,6-2H2]glucose tracer. A. Plasma glucose (mmol/l). B & C. Postprandial total and incremental area under the curve (AUC and iAUC respectively) for plasma glucose concentration. D. Plasma insulin (pmol/l). * denotes significant elevation in plasma glucose at time -10 min in RE condition (paired t-test), and significant main effect of treatment (RE < rest) for postprandial insulin concentration (repeated-measures ANOVA, see text for p-values). E & F. Postprandial total and incremental (AUC and iAUC respectively) for plasma insulin. In B, C, E, and F * denotes significant difference between rest and exercise (paired t-test, p<0.05).
Like plasma glucose, plasma insulin was significantly elevated immediately after the resistance exercise session compared to rest (Figure 2D, −10 min, p=0.02), and returned to baseline by the end of the basal period prior to the meal. In the postprandial period, there was a main effect of time (p<0.001) and a main effect of treatment (p=0.041) for plasma insulin concentration, with total plasma insulin significantly reduced after exercise compared to rest (Figure 2B). The plasma insulin AUC and iAUC were also significantly reduced in the resistance exercise condition compared to rest (p=0.020 and p=0.038, respectively, Figure 2E, F).
Plasma Glucose Kinetics
Basal glucose Ra in plasma (an index of hepatic glucose production), was significantly greater after RE compared to rest (Table 2). In the postprandial period, there was no significant effect of RE on postprandial total glucose Ra (which represents the sum of endogenous glucose production and meal glucose appearance in plasma) or Rd (Figure 3A,D). However, both the meal-induced rise in glucose Ra and Rd (Ra iAUC and Rd iAUC) were significantly reduced in the RE condition compared to rest (p=0.002 for both measures. Figure 3C, F).
Table 2.
Basal (fasted) & Postprandial Glucose Kinetics, Total Carbohydrate Oxidation
| Rest | RE | p-value | |
|---|---|---|---|
| Basal Glucose Ra (μmol/kg FFM/min) | 13.3 (0.46) | 15.5 (0.72) | 0.02 |
| Postprandial Insulin Sensitivity (Rd AUC/Insulin AUC) (μmol/L/[pmol x Kg FFM x min]) | 0.043 (0.003) | 0.055 (0.007) | 0.02 |
| Insulinogenic Index (ΔI0-30/ΔG0-30) | 291 (46) | 312 (50) | 0.77 |
| Carbohydrate Oxidation iAUC([g/min/Kg FFM] x 360 min) | 0.18 (0.1) | 0.20 (0.1) | 0.76 |
| Time of Peak Carbohydrate Oxidation (min from start of meal) | 144 (24) | 219 (26) | 0.04 |
Values are mean ± SEM. RE = resistance exercise. Ra = glucose rate of appearance. Rd = glucose rate of disappearance. AUC = area under the curve determined using the trapezoid rule. iAUC = incremental area under the curve. FFM = fat free mass. I = insulin. G = glucose.
Figure 3. Postprandial Glucose Kinetics and Insulin Sensitivity.
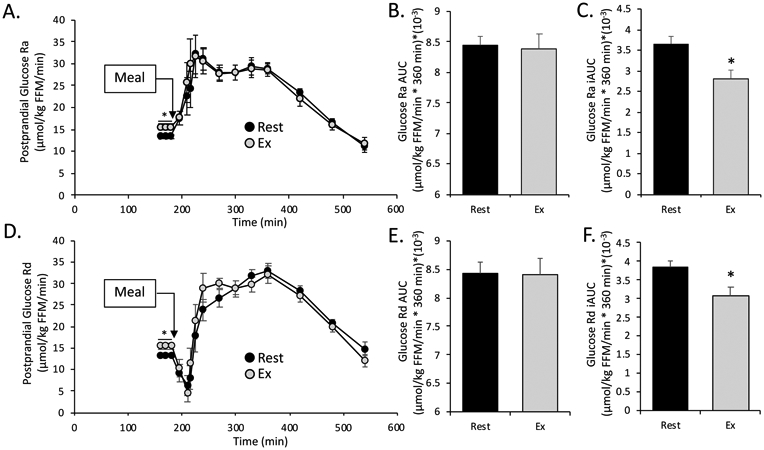
All values are mean ± SEM. A. Postprandial glucose rate of appearance (Ra). B & C. Postprandial total and incremental area under the curve (AUC and iAUC respectively) for glucose Ra. D. Postprandial glucose rate of disappearance (Rd). E & F. Postprandial total and incremental area under the curve (AUC and iAUC respectively) for glucose Rd. In all graphs * denotes significant difference between rest vs exercise (paired t-test. p<0.05).
Insulin Sensitivity and Beta Cell Function
In the postprandial period whole-body insulin sensitivity, assessed as glucose Rd AUC/insulin concentration AUC, was significantly higher after RE, indicating a greater rate of glucose clearance per unit of insulin (Table 2). The insulinogenic index, which assesses the early postprandial rise in plasma insulin in relation to the rise in plasma glucose, was not significantly different after the RE session compared to rest (Table 2).
Carbohydrate Oxidation and Mitochondrial Respiration
There was a main effect of time (p<0.001) (i.e., a meal effect) for carbohydrate oxidation rate (grams/min/Kg FFM), but no effect of RE (Figure 4A). Likewise, total postprandial carbohydrate oxidation AUC was not significantly elevated in the postprandial period after RE compared to rest (Table 2). The time-course analysis showed that the peak of carbohydrate oxidation occurred significantly later in the postprandial period after RE compared to rest (Table 2, Figure 4A). Skeletal muscle mitochondrial pyruvate-supported respiration tended to be higher (p=0.061), but the contribution of pyruvate-supported respiration contribution to maximal oxidative respiration was significantly lower (p=0.034), following RE compared to rest (Figure 4B, C).
Figure 4. Carbohydrate oxidation and mitochondrial respiration.
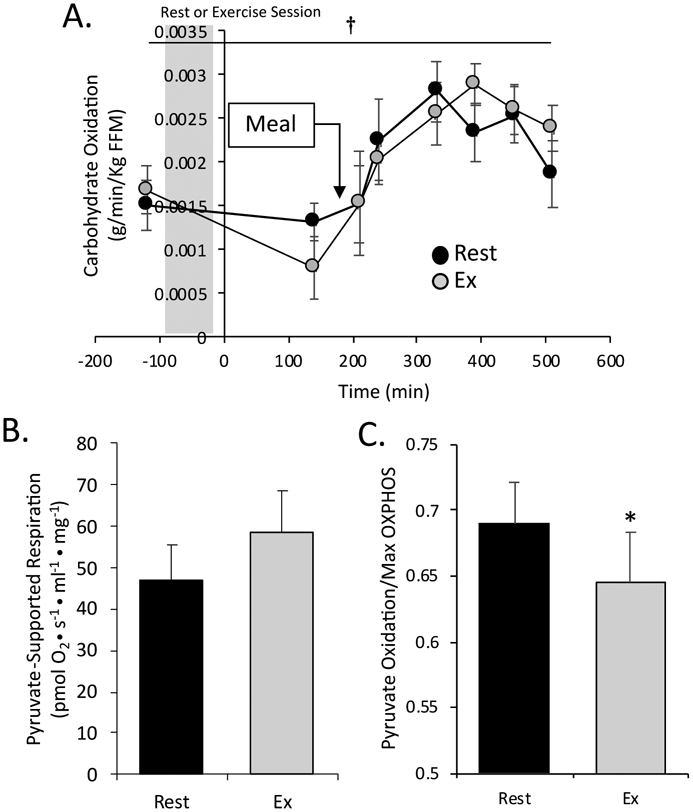
All values are presented as mean ± SEM. A. Total carbohydrate oxidation (grams/min/kg FFM) determined using indirect calorimetry. B. Pyruvate-supported mitochondrial respiration. C. Pyruvate-supported respiration normalized to maximal oxidative phosphorylation. * denotes significant difference between rest and exercise (paired t-test, p<0.05). † denotes main effect of time for carbohydrate oxidation (p<.001).
Discussion
The effects of an acute bout of resistance exercise on postprandial glucose metabolism in individuals with prediabetes are poorly understood. Previous studies have reported that post-meal resistance exercise reduces postprandial glucose excursions in healthy individuals, and in those with type 2 diabetes (27). To our knowledge, only one study has evaluated the effect of resistance exercise on post-prandial (OGTT) glucose metabolism in prediabetes, which effectively increased glucose tolerance (11). We have expanded on this initial study to show the novel finding that a single bout of resistance exercise performed 4.5 hours before a mixed meal (as opposed to an OGTT) reduced total postprandial glucose appearance (Ra iAUC), increased insulin sensitivity, and reduced the glycemic response to a mixed meal, but had no effect on glucose oxidation in obese men with prediabetes. Improvements in insulin sensitivity were complemented by reduced postprandial insulin concentration, further highlighting the beneficial effects of RE on postprandial glucoregulation.
Previous studies have found that the contribution of postprandial glycemia to plasma HbA1c increases as the degree of glycemic control decreases in healthy individuals with HbA1c between 6.0% and 7.0% (28). Therefore, in individuals with prediabetes who have only modestly impaired glycemic control, reducing the degree of postprandial glycemia could be an integral component of their treatment. We found that RE effectively minimized the rise in plasma glucose from basal values (glucose iAUC) compared to rest, which was likely due to a reduction in the rise in postprandial glucose rate of appearance above the basal rate (Ra iAUC). Since we did not utilize a meal glucose tracer, we were unable to delineate between endogenous and exogenous (meal) glucose kinetics. After meal ingestion, the liver is preferentially and immediately exposed to rising insulin concentrations through the portal vein, which serves to rapidly suppress EGP and promote glucose uptake (estimated to account for 30-40% of postprandial glucose clearance) (29). In healthy individuals, physiological postprandial insulin concentrations are sufficient to suppress EGP by ~55% (29). The preferential exposure of the liver to hyperinsulinemia may be a protective mechanism to avoid the negative consequences of increased arterial insulin concentrations (e.g. coagulation abnormalities, atherosclerosis), with skeletal muscle glucose serving as a backup system for glucose clearance (accounting for an additional 30-40% of postprandial glucose Rd) (29). The reduction in postprandial glucose Ra iAUC may be due to increased hepatic insulin sensitivity and suppression of EGP, consistent with previous studies (30). In fact, suppression of EGP may be more important for reducing postprandial glucose excursions than splanchnic glucose clearance (30, 31). The reduction in glucose Ra iAUC may have also been due to reduced glucose absorption, which was previously shown following treadmill exercise at 70% VO2max in healthy subjects (32). Overall, our data suggest that RE may reduce the glycemic response to a mixed meal in men with prediabetes, which is consistent with the findings of Andersen et al. (2007) and Lopez et al. (2014) in healthy individuals (9-10). Since individuals with type 2 diabetes demonstrate delayed suppression of endogenous postprandial glucose production and diminished glucose clearance, this could be an important means of preventing disease progression (33).
The increase in plasma glucose after intense, acute exercise is typically matched by a similar spike in plasma insulin concentration to restore normoglycemia and promote glucose uptake, which we observed in this study (34). It was previously reported that glucose concentrations return to baseline within 1-2 hours of resistance exercise cessation, which we observed in our study by the end of the 3-hour recovery period (34). However, the total glucose rate of appearance remained significantly elevated even 3 hours post-RE. The return of plasma glucose to baseline, therefore, appears to be due to a concomitant, significant increase in plasma glucose rate of disappearance measured at the end of the basal period. The elevation in basal glucose Ra may be a consequence of the lingering influence of exercise-stimulated gluconeogenic hormones (as discussed above).
Postprandial insulin concentrations were reduced after acute resistance exercise (main effect of treatment and reduced AUC and iAUC). This reduction seemed to occur more-so during the later-phase insulin response (insulin secreted over the final ~five hours of the postprandial period) to the mixed meal, as the insulin concentrations were virtually equivalent between conditions over the first 45 min of the postprandial period, and there was no effect of RE on the insulinogenic index. These results are similar to those reported by Rynders et al (2014), who found a 26% reduction in the late-phase insulin AUC during a 3-hr OGTT after bicycle ergometer exercise in prediabetic men and women (35). In the subsequent 5.5 hours in our study, the reduction in plasma insulin in the RE condition is most likely due to the increase in insulin sensitivity, which would require less insulin per unit of glucose, thereby lowering the glucose-normalized insulin response. These results are also consistent with the findings of Phillips et al., who found that a single bout resistance exercise at 65% and 85% one repetition-max significantly reduced plasma insulin concentration without changing the plasma glucose concentration response to a 100g carbohydrate meal in young, recreationally-active men, indicative of improved insulin sensitivity (36).
Multiple studies have reported that during the recovery from a single bout of RE the contribution of lipid oxidation to total energy expenditure increases, while the contribution from carbohydrate oxidation decreases in healthy subjects, which is attributed to: 1) the shunting of plasma glucose toward glycogen re-synthesis; 2) post-exercise increases in circulating free fatty acids (which can persist for 3-6 hours); and 3) declines in plasma insulin (which inhibits lipid oxidation) (37). However, while we found that acute RE significantly increased whole-body lipid oxidation (15), it did not significantly reduce carbohydrate oxidation compared to rest (eg. no difference in CHO oxidation at 140 min in Figure 4A). Our results coincide closely with those of Gaitan et al. (2019), who reported increased lipid oxidation but no change in carbohydrate oxidation in men and women with prediabetes after continuous and interval aerobic exercise training (two weeks) (38). The difference in the relative contribution of lipid vs carbohydrate to oxidative metabolism in subjects who are healthy vs. those who have prediabetes is unclear, but could be related to differences in fatty acid metabolism. As we reported previously, we did not observe increases in circulating FFA following RE, which could be due to the shunting of circulation away from adipose tissue during the higher intensity RE session, thereby limiting the ability of liberated FFA to enter the circulation to support lipid oxidation and requiring some additional glucose oxidation to support ongoing metabolism (39). We also identified a transient spike in post-exercise plasma glucose and insulin concentrations immediately post-exercise. Insulin is known to inhibit lipid oxidation, and could therefore blunt the maximal increase in lipid oxidation (though the increase is still significant vs rest) after RE, thereby requiring increased supplemental glucose oxidation (40). These findings are most likely due to the absence of sampling carbohydrate oxidation for the first 170 minutes of the recovery period, as even in healthy subjects changes in oxidation approach baseline levels by 120 minutes post-RE (41).
In contrast, after meal ingestion, the total carbohydrate oxidation rate increased in both the rest and exercise conditions (sudden rise observed from min 140 to min 240), which is consistent with previous studies showing that carbohydrate ingestion promotes a rapid shift toward carbohydrate oxidation due to the preferential oxidation of glucose over free fatty acids (42). However, we observed that peak carbohydrate oxidation was delayed in the RE condition, which may be caused by the prolonged increase postprandial lipid oxidation (15). This is supported by our observation that the percentage of maximal mitochondrial respiration from pyruvate oxidation was lower after RE, which, when combined with our previous findings of an increased lipid to glucose oxidation ratio (15), suggests that exercise increased the preference for oxidizing fatty acids even 90 minutes after meal consumption. The trend toward higher pyruvate-supported respiration after RE in this study may have been due to an elevation in total maximal mitochondrial oxidative capacity, as we reported previously (15). While the mechanism of this fatty acid respiratory preference is unclear, elevations in beta-oxidation could lead to the accumulation of acetyl-CoA and citrate, which are known to activate pyruvate dehydrogenase kinase to inhibit the pyruvate dehydrogenase complex, mitigating glycolysis and glucose oxidation (43). Further investigation is needed to elucidate the mechanisms of postprandial fuel selection preferences in skeletal muscle after RE.
Finally, although we observed improvements in postprandial insulin sensitivity, insulin concentrations, postprandial glucose rate of appearance, and glucose concentration normalized to pre-meal levels, it is appears that these benefits are waning by the end of the ~9 hour study period. Previous studies have shown that resistance exercise can lower plasma glucose concentrations for 24 hours post-RE (44), while other have reported no improvement in 24 hr glucose (45). Since we did not analyze timepoints beyond the 6-hour postprandial period , it is unclear if these benefits carry-over the subsequent meals, which could contribute to improved 24-hr glycemic control. In healthy subjects, a single bout of resistance exercise reduces plasma glucose for 14-24 hours (46, 47), suggesting that the duration of improvement is consistent with healthy populations. Overall, the effects of RE appear to dissipate by ≤24 hours, indicating the that repeated bouts (i.e. training) are required for prolonged improvement.
In conclusion, we found that a single bout of resistance exercise moderately reduced the glycemic response to a mixed meal, significantly improved insulin sensitivity, and reduced the glucose-normalized insulin response in obese, middle-aged men with prediabetes. However, there were limited effects on postprandial glucose clearance, the insulinogenic index, whole-body carbohydrate oxidation, or skeletal muscle pyruvate-supported respiration. Further investigation is needed to elucidate how resistance exercise affects exogenous (meal) vs endogenous postprandial glucose metabolism, and if additional bouts of exercise (i.e. training) produce superior outcomes for this population.
Limitations
There are several limitations to this study. First, since no oral glucose tracer was used, we could only assess total glucose Ra without resolution of exogenous oral glucose appearance from endogenous glucose production, which would be important to determine changes in postprandial hepatic insulin sensitivity. This study only enrolled adult men, thus the results cannot be generalized to obese women or children with prediabetes. Additionally, the study sample size was based on anticipated differences in glucose rate of disappearance following resistance exercise and not powered to detect differences in other variables. Despite this, we found differences in peripheral insulin sensitivity and glucose tolerance following resistance exercise. Additionally, participants were required to fast for >15 hours prior to the test meal to avoid the confounding effects of pre-exercise meals on post-exercise postprandial glucose metabolism. Fasting for this duration is associated with a switch toward increased lipid oxidation as glycogen is depleted in the liver, skeletal muscle insulin resistance (secondary to the presence of increased free fatty acid (FFA) levels, increased fat oxidation and low glucose and insulin levels), and diminished carbohydrate oxidation (48, 49). However, it was previously reported that changes in post-exercise postprandial glucose concentrations were equivalent when participants were provided with a meal vs. no meal prior to the exercise session. Therefore, while fasting could have affected some measures in this study, the magnitude of the effect is likely not large enough to significantly affect our findings.
Supplementary Material
Acknowledgements
Assistance: We would like to thank Miranda Giuffrida, and Kathryn Gratza for collection of muscle and adipose tissue biopsies, Lala Ruvinov for preparing all stable isotope tracers for infusion for this study, Sue Waller for preparing the standardized meals and the liquid test meals, Terri Pietka for assistance with mitochondrial respiration analysis, Jennifer Shew and Freida Custodio for help with sample preparation for GC/MS analysis (all Center for Human Nutrition, Washington University School of Medicine, St. Louis, MO, USA). We also thank the nursing staff at the Washington University Institute for Clinical and Translational Sciences Clinical Research Unit (Washington University School of Medicine, St. Louis, MO, USA) for their assistance with all study procedures.
Funding: This work was supported by the National Institutes of Health T32 HD007434-25 (Lang), UL1TR000448 (Powderly), and P30 DK056341 (Washington University Nutrition Obesity Research Center); the Foundation for Physical Therapy Promotion of Doctoral Studies II Scholarship (A. Bittel).
Footnotes
Disclosure Summary: The authors have no conflicts of interest or significant financial support for this work that could have influenced its outcome
Conflict of Interest: The authors declare that there is no duality of interest associated with this manuscript.
Data and Resource Sharing: The data sets generated/analyzed during this study are available from the corresponding author on reasonable request.
Declaration: The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. Results of the present study do not constitute endorsement by ACSM.
Supplemental Digital Content
Appendix: BITTEL et al. ESM.pdf
References
- 1.Bansal N Prediabetes diagnosis and treatment: A review. World Journal of Diabetes. 2015; 6(2): 296–303. doi: 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: A high-risk state for developing diabetes. Lancet. 2012; 379(9833): 2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keidar A Bariatric surgery for type 2 diabetes reversal: The risks. Diabetes Care. 2011; 34(Supplement 2): S361–S266. 10.2337/dc11-s254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jean MEL, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018; 391 (10120): 541–551 [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002; 25(12): 2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003; 26(12): 3230–6. [DOI] [PubMed] [Google Scholar]
- 7.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: A position statement of the american diabetes association. Diabetes Care. 2016. November; 39(11): 2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breen L, Philp A, Shaw CS, Jeukendrup AE, Baar K, Tipton KD. Beneficial effects of resistance exercise on glycemic control are not further improved by protein ingestion. PLoS ONE. 2011; 6(6): e20613. 10.1371/journal.pone.0020613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez IP, Kendrick KH, Smith JD. Fasting and postprandial glucose levels after a single resistance training event in Mexican-Americans. In: Proceedings from the Conference of the International Journal of Exercise Science; 2014. February 27–28: Fort Worth, TX (USA). Texas Christian University; Vol. 2, Iss. 6, Article 60. Available at: https://digitalcommons.wku.edu/ijesab/vol2/iss6/60 [Google Scholar]
- 10.Andersen E, Høstmark AT. Effect of a single bout of resistance exercise on postprandial glucose and insulin response the next day in healthy, strength-trained men. J Strength Cond Res. 2007; 21(2):487–91 [DOI] [PubMed] [Google Scholar]
- 11.Eikenberg JD, Savla J, Marinik EL, et al. Prediabetes phenotype influences improvements in glucose homeostasis with resistance training. Atkin SL, ed. PLoS ONE. 2016; 11(2): e0148009. doi: 10.1371/journal.pone.0148009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearney ML, Thyfault JP. Exercise and postprandial glycemic control in type 2 diabetes. Curr Diabetes Rev. 2016;12(3):199–210 [DOI] [PubMed] [Google Scholar]
- 13.Ikee R, Honda K, Ishioka K et al. Postprandial hyperglycemia and hyperinsulinemia associated with renal arterio-arteriolosclerosis in chronic kidney disease. Hypertens Res. 2010; 33, 499–504. [DOI] [PubMed] [Google Scholar]
- 14.Hanefeld M, Fischer S, Julius U et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow- up. Diabetologia. 1996; 39: 1577–1583 [DOI] [PubMed] [Google Scholar]
- 15.Bittel AJ, Bittel DC, Mittendorfer B, et al. A single bout of resistance exercise improves postprandial lipid metabolism in overweight/obese men with prediabetes. Diabetologia. 2020; 63(3):611–623. doi: 10.1007/s00125-019-05070-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. The BMJ. 2016; 355:i5953. doi: 10.1136/bmj.i5953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundsgaard AM, Kiens B. Gender differences in skeletal muscle substrate metabolism - molecular mechanisms and insulin sensitivity. Front Endocrinol (Lausanne). 2014; 5:195. doi: 10.3389/fendo.2014.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebeck J Editorial: Sexual Dimorphism in Glucose and Lipid Metabolism. Front Endocrinol (Lausanne). 2016; 7:166. doi: 10.3389/fendo.2016.00166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cade WT, Spencer CT, Reeds DN, et al. Substrate metabolism during basal and hyperinsulinemic conditions in adolescents and young-adults with Barth syndrome. J Inherit Metab Dis. 2013; 36:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith GI, Villareal DT, Lambert CP, Reeds DN, Mohammed BS, Mittendorfer B. Timing of the initial muscle biopsy does not affect the measured muscle protein fractional synthesis rate during basal, postabsorptive conditions. J Appl Physiol. 2009; 108(2):363–368. Doi: 10.1152/japplphysiol.00957.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabbrini E, Yoshino J, Yoshino M, et al. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015; 125(2):787–795. doi: 10.1172/JCI78425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diabetes Trials Unit. HOMA calculator. University of Oxford. Accessed from https://www.dtu.ox.ac.uk/homacalculator/ [Google Scholar]
- 23.Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122(12):4667–4674. doi: 10.1172/JCI64895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carstensen M, Thomsen C, Hermansen K. Incremental area under response curve more accurately describes the triglyceride response to an oral fat load in both healthy and type 2 diabetic subjects. Metabolism. 2003; 52(8):1034–7. [DOI] [PubMed] [Google Scholar]
- 25.Gastaldelli A, Casolaro A, Pettiti M, et al. Effect of pioglitazone on the metabolic and hormonal response to a mixed meal in type II diabetes. Clin Pharmacol Ther. 2007; 81(2):205–12 [DOI] [PubMed] [Google Scholar]
- 26.Aono D, Oka R, Kometani M, et al. Insulin secretion and risk for future diabetes in subjects with a nonpositive insulinogenic index. J Diabetes Res. 2018; 5107589. Published 2018 Mar 22. doi: 10.1155/2018/5107589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heden TD, Winn NC, Mari A, et al. Postdinner resistance exercise improves postprandial risk factors more effectively than predinner resistance exercise in patients with type 2 diabetes. J Appl Physiol. 2015; 118(5): 624–634. doi: 10.1152/japplphysiol.00917.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woerle HJ, Pimenta WP, Meyer C et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour post challenge plasma glucose and hemoglobin a1c values. Arch Intern Med. 2004. August 9–23;164(15):1627–32. [DOI] [PubMed] [Google Scholar]
- 29.Kowalski GM, Moore SM, Hamley S, Selathurai A, Bruce CR. The effect of ingested glucose dose on the suppression of endogenous glucose production in humans. Diabetes. 2017; 66(9):2400–2406 [DOI] [PubMed] [Google Scholar]
- 30.Gregory JM, Muldowney JA, Engelhardt BG, et al. Aerobic exercise training improves hepatic and muscle insulin sensitivity, but reduces splanchnic glucose uptake in obese humans with type 2 diabetes. Nutr Diabetes. 2019; 9(1):25. Published 2019 Sep 2. doi: 10.1038/s41387-019-0090-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton KS, Gibbons FK, Bracy DP, Lacy DB, Cherrington AD, Wasserman DH. Effect of prior exercise on the partitioning of an intestinal glucose load between splanchnic bed and skeletal muscle. J Clin Invest. 1996; 98(1):125–135. doi: 10.1172/JCI118756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang JA, Gisolfi CV, Lambert GP. Effect of exercise intensity on active and passive glucose absorption. Int J Sport Nutr Exerc Metab. 2006;16(5):485–93 [DOI] [PubMed] [Google Scholar]
- 33.Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010; 59(11): 2697–2707. doi: 10.2337/db10-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation. Diabetes. 2002; 51 (Suppl. 1): S271–S283 [DOI] [PubMed] [Google Scholar]
- 35.Rynders CA, Weltman JY, Jiang B, et al. Effects of exercise intensity on postprandial improvement in glucose disposal and insulin sensitivity in prediabetic adults. J Clin Endocrinol Metab. 2014;99(1):220–228. doi: 10.1210/jc.2013-2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips MD, Mitchell JB, Currie-Elolf LM, Yellott RC, Hubing KA. Influence of commonly employed resistance exercise protocols on circulating IL-6 and indices of insulin sensitivity. J Strength Cond Res. 2010. April;24(4):1091–101. doi: 10.1519/JSC.0b013e3181cc2212. [DOI] [PubMed] [Google Scholar]
- 37.Kuo CC, Fattor JA, Henderson GC, Brooks GA. Lipid oxidation in fit young adults during postexercise recovery. J Appl Physiol (1985). 2005; 99(1):349–356. doi: 10.1152/japplphysiol.00997.2004 [DOI] [PubMed] [Google Scholar]
- 38.Gaitán JM, Eichner NZM, Gilbertson NM, et al. Two Weeks of Interval Training Enhances Fat Oxidation during Exercise in Obese Adults with Prediabetes. J Sports Sci Med. 2019; 18(4):636–644. [PMC free article] [PubMed] [Google Scholar]
- 39.Melanson EL, MacLean PS, Hill JO. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc Sport Sci Rev. 2009; 37(2):93–101. doi: 10.1097/JES.0b013e31819c2f0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelley D, Reilly JP, Veneman T, Mandarino LJ. Effects of insulin on skeletal muscle glucose storage, oxidation, and glycolysis in man. Am. J. Physiol. Endocrinol. Metab 1990; 258(6 Pt 1):E923–9. doi: 10.1152/ajpendo.1990.258.6.E923. [DOI] [PubMed] [Google Scholar]
- 41.Binzen CA, Swan PD, Manore MM. Postexercise oxygen consumption and substrate use after resistance exercise in women. Med Sci Sports Exerc. 2001; 33(6):932–938. doi: 10.1097/00005768-200106000-00012) [DOI] [PubMed] [Google Scholar]
- 42.Roberts R, Bickerton AS, Fielding BA, et al. Reduced oxidation of dietary fat after a short term high-carbohydrate diet. Am. J. Clin. Nutr 2008;87(4):824–831 [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab (Lond). 2014;11(1):10. Published 2014 Feb 12. doi: 10.1186/1743-7075-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Dijk JW, Venema M, van Mechelen W, et al. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care. 2013;36(11):3448–3453. doi: 10.2337/dc12-2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georgakouli K Stamperna A, Deli CK, et al. The effects of postprandial resistance exercise on blood glucose and lipids in prediabetic, beta-thalassemia major patients. Sports. 2020; 8(5);, 57. doi: 10.3390/sports8050057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen E, Høstmark AT. Effect of a single bout of resistance exercise on postprandial glucose and insulin response the next day in healthy, strength-trained men. J Strength Cond Res. 2007;21(2):487–491. doi: 10.1519/R-20105.1 [DOI] [PubMed] [Google Scholar]
- 47.Koopman R, Manders RJ, Zorenc AH, et al. A single session of resistance exercise enhances insulin sensitivity for at least 24 h in healthy men. Eur J Appl Physiol. 2005;94(1–2):180–187. doi: 10.1007/s00421-004-1307-y [DOI] [PubMed] [Google Scholar]
- 48.Izumida Y, Yahagi N, Takeuchi Y, et al. Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization [published correction appears in Nat Commun. 2013;4:2930]. Nat Commun. 2013;4:2316. doi: 10.1038/ncomms3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoeks J, van Herpen NA, Mensink M, et al. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes. 2010;59(9):2117–2125. doi: 10.2337/db10-0519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


