Abstract
Introduction:
Sleep, sedentary behavior, and physical activity are each independently associated with cardiovascular health (CVH). It is unknown how substituting time in sedentary behavior with sleep or physical activity effects overall CVH.
Methods:
Data for this analysis were taken from the Multi-Ethnic Study on Atherosclerosis (MESA) Sleep Ancillary Study. Eligible participants (n= 1718) wore Actiwatch accelerometers for 24 hours and had at least 3 days of valid accelerometry. The American Heart Association’s life simple 7 was used to represent the CVH score after excluding the physical activity component, with higher scores indicating more favorable CVH. Isotemporal substitution modeling was conducted to examine the effect of substituting 30 minutes of sedentary time for an equivalent amount of sleep, light intensity physical activity (LIPA), or moderate to vigorous physical activity (MVPA).
Results:
Substituting 30 minutes of sedentary time to sleep, LIPA, and MVPA was associated with a significantly higher CVH score [β(95%CI): 0.077(0.056), 0.039(0.033), and 0.485(0.127) respectively]. Substituting 30 minutes of sedentary time to sleep was associated with lower BMI. Substituting 30 minutes of sedentary time to LIPA was associated with higher diastolic blood pressure and total cholesterol, and lower BMI. Substituting 30 minutes of sedentary time to MVPA was associated with lower systolic and diastolic blood pressure, and lower BMI.
Conclusions:
Sleep, LIPA, and MVPA are all associated with more favorable overall CVH and several key risk factors for cardiovascular disease. These findings underscore the importance of lifestyle modifications in improving CVH.
Keywords: cardiovascular health, accelerometry, sleep, physical activity
INTRODUCTION
Sleep, sedentary behavior, and physical activity are each independently associated with cardiovascular health (CVH) (1, 2). Evidence-based guidelines recommend 7 or more hours of sleep, low levels of sustained sedentary behavior, and high levels of physical activity for optimal CVH (3–5). A significant limitation of research to date is that the relationship of these behaviors with CVH are investigated in isolation, even though a change in any given behavior will affect time spent in others.
The 24-hour activity cycle was developed as a paradigm for exploring the effects of time spent in each of four domains: sleep, sedentary behavior, light intensity physical activity (LIPA), and moderate to vigorous physical activity (MVPA) on health outcomes (6). Isotemporal substitution modeling can be used to estimate the effect of substituting a specific duration of one type of activity with an equivalent duration of another (7). For example, Buman et al. used this analytic approach to demonstrate that substituting 30 minutes per day of sedentary time to either sleep, LIPA, or MVPA is associated with improvements in various cardiovascular disease (CVD) risk biomarkers (8). Importantly, although many of these studies have utilized objectively measured physical activity information, few have used objectively measured sleep, and many have focused on single cardiometabolic risk factors on health outcomes rather than overall CVH.
To our knowledge, no study has evaluated the complex inter-relationships of accelerometer-measured sleep, sedentary behavior, LIPA, and MVPA with overall CVH. Additionally, these relationships have not been explored in an ethnically diverse cohort of middle-aged and older US adults, a population known to have high rates of sedentary behavior and a low prevalence of ideal CVH (9, 10). In light of these gaps in the literature, this study aimed to use partition and isotemporal substitution modeling to understand how relationships between these health behaviors influence CVH assessed using the American Heart Association Life’s Simple 7 (AHA LS7) score (11) in the Multi-Ethnic Study on Atherosclerosis (MESA) Sleep Ancillary Study (12, 13). We hypothesized that substituting sedentary behavior for an equivalent amount of sleep or physical activity would result in a higher overall CVH score, facilitated by increases in its component metrics.
METHODS
Study Population
MESA is an ongoing prospective cohort study designed to investigate the prevalence and progression of subclinical CVD and to identify CVD risk factors in middle-aged and older adults (45–84 y). A total of 6,814 participants free of clinical CVD from four racial/ethnic groups (38% Caucasian, 12% Chinese-American, 28% African-American, and 22% Hispanic)(12) were recruited from six field centers across the United States: Baltimore, MD; Chicago, IL; Los Angeles, CA; New York, NY; Saint Paul, MN; and Winston-Salem, NC. Six clinical exams to collect data on clinical, sociodemographic, and lifestyle factors have been completed since the inception of this cohort in 2000. At clinical exam 5 (2010–2013), 4,077 participants were invited to participate in the MESA Sleep Ancillary Study, which was designed to examine objectively measured sleep characteristics in relation to CVD risk. A total of 2,261 participants consented to participate in the Sleep Ancillary Study, of whom 2,156 wore an accelerometer for 7 days and nights on their wrist. Participants without valid accelerometer data (n=218) were not included in the analyses. Among the remaining 1,938 individuals, 214 did not have sufficient data to compute the CVH score, and an additional 6 did not have data on key covariates, resulting in a final sample of 1,718 individuals. MESA was approved by the Institutional Review Board at each site and all participants provided written informed consent.
Assessment of Sleep, Sedentary Behavior, and Physical Activity
Sleep duration, sedentary behavior, and physical activity duration and intensity were ascertained using the Actiwatch Spectrum accelerometer (Philips Respironics, Murrysville, PA) worn on each participants’ non-dominant wrist for 7 consecutive days. After removing non-wear periods, individuals without 600 daily minutes of wear time on at least 3 days were excluded from analyses. These parameters were chosen based on prior literature (14, 15). Sleep duration was estimated by scoring these data during 30-second epochs as “sleep” or “wake” by Actiware-Sleep v.5.59 analysis software (Mini Mitter Co., Inc., Bend, OR) after manually editing the sleep period using sleep diary data and event and light markers. A validated algorithm was used to calculate the activity count for each epoch (16). The average sleep duration was computed from nighttime sleep onset (sleep start time) and morning wakening (sleep end time), averaging sleep duration across all nights that the accelerometer was worn. Records were assessed by blinded, trained scorers at the Brigham and Women’s Hospital Sleep Reading Center, with high inter and intra class correlations for sleep duration metrics.
To calculate daily minutes of sedentary time, LIPA, and MVPA, non-sleep data were rescored based on 60-second epochs using published wrist-worn cut points to isolate minutes spent in sedentary behavior (<178.5 counts/min), LIPA (178.6–562.4 counts/min), and MVPA (>562.4) (17). After non-wear periods were removed, total minutes of nighttime sleep, sedentary time, LIPA, and MVPA were summed and averaged across days to produce average daily minutes of each variable.
Assessment of Cardiovascular Health Metrics
Body mass index (BMI) (kg/m2) was calculated from weight and height measurements obtained by trained personnel during MESA exam 5. A validated 128‐item food frequency questionnaire was administered to collect data on habitual dietary intake (12, 18). Seated systolic and diastolic blood pressure (SBP and DBP) were assessed from three readings after participants had rested for five minutes and the average of the last two readings was recorded. Total cholesterol (including HDL and LDL cholesterol) and blood glucose levels were measured from fasting blood samples obtained during the study visit. Smoking status (currents vs. former vs. never), blood pressure, cholesterol, and diabetes medication were assessed by self-report using a standardized questionnaire.
Operationalization of the Cardiovascular Health Score
Lifestyle behaviors, anthropometric measures, and biomarkers that constitute the AHA LS7 score assessed at exam 5, were used to compute the CVH score. Published definitions (11) for assessing CVH using the AHA LS7 were adapted based on data available in the MESA Sleep Ancillary Study and MESA exam 5, and are consistent with previous literature on the AHA LS7 score (19, 20). For the present analyses, physical activity was excluded from the CVH score given its status as an exposure variable of interest. We computed the CVH score based on meeting guidelines for smoking, diet, BMI, blood pressure, total cholesterol, and fasting glucose. Briefly, participants were assigned a score based on their level of meeting recommendations for these six CVH metrics: ideal (2 points), intermediate (1 point) or poor (0 point). Participants received a score of 2 if they met the following criteria: 1) were never smokers, 2) met 4–5 of diet guidelines (11), 3) had BMI<25 kg/m2, 4) had untreated SBP<120 mmHg and DBP<80 mmHg, 5) had untreated total cholesterol <200 mg/dl, and 6) had untreated fasting glucose <100 mg/dl. They received a score of 1 for each CVH metric if they met the following criteria: 1) were former smokers, 2) met 2–3 of diet guidelines, 3) had BMI of 25–29.9 kg/m2, 4) had SBP: 120–129 mmHg or DBP: <80 mmHg or treated to ideal level, 5) had total cholesterol 200–239mg/dl or treated to ideal level; and 6) had fasting glucose of 100–125 mg/dl or treated to normal level. Otherwise, they received a score of 0 for each CVH metric. The individual component scores were summed to create a global CVH score ranging from 0 to 12, such that higher scores represented a more favorable CVH profile. Participants with a score of 0–5, 6–9, 10–12 were considered to have poor, moderate, and high CVH, respectively (20, 21).
Statistical Analysis
Descriptive statistics were calculated for the socio-demographic, lifestyle, and cardiometabolic risk factors. Next, a series of partition models were conducted to examine the linear relationship between the average minutes of sleep, sedentary time, LIPA, MVPA, and CVH score. Due to the high correlation between sedentary time and LIPA, we ran additional sensitivity analyses. The first excluded average sedentary time while retaining average time spent in LIPA, and the second excluded time spent in LIPA while including sedentary time. All partition models controlled for sex (male vs female), age (years), marital status (married vs unmarried), education attainment (college educated vs less than college education), and race (white vs non-white).
Isotemporal substitution models were conducted to examine the effect of substituting 30 minutes of sedentary time for an equal duration of LIPA, MVPA, or sleep (7). First, a total time variable was computed by summing all time variables (i.e., total time = sedentary + LIPA + MVPA + sleep). Next, all variables were divided by 30 to facilitate examination of a 30-minute substitution. Finally, all behaviors except sedentary time, plus the total time variable (i.e., LIPA, MVPA, sleep, total time) were included with the covariates of interest. Including total time in the model constrains time, and as a result, the regression estimate for each included behavior represents the estimated mean change in levels of the outcome that is observed when that behavior is increased by 30 minutes and sedentary time is simultaneously decreased by 30 minutes. Our estimates are based on cross-sectional associations, and thus results cannot be interpreted causally or interpreted as temporal changes within individuals.
The main substitution model of interest included the CVH score as the dependent variable. We then conducted exploratory models for continuous measures of glucose, SBP, DBP, total cholesterol (as well as HDL and LDL cholesterol separately), and BMI. We also included a model stratified by sex. Models assessing diet and smoking status were not analyzed because they are not continuous (7) or binary (22) variables. All substitution models controlled for sex, age, marital status, education, and race. Additionally, models were stratified by sex but not race due to power constraints. All models also included BMI as a covariate except when CVH or BMI were the dependent variables of interest. The model examining substitution effects on glucose controlled for diabetes medication usage, both blood pressure models controlled for hypertension medication usage, and the cholesterol models accounted for the use of lipid-lowering medication. Assumptions of linearity, homoscedasticity, multicollinearity, outlying residuals, and normality of residuals were assessed for all models, and all deviations are noted in the results section. All analyses were conducted using SPSS version 25 (IBM Corp, Armonk, NY). Statistical significance was determined at p < 0.05.
RESULTS
Participant Characteristics
Study demographics are displayed by quintiles of physical activity in Table 1. In brief, the mean age of participants was 68 years, 54% were female, and on average participants were overweight (mean BMI 28.8 kg/m2). Most participants were White (39%), followed by Black (27%), Hispanic (24%), and Chinese (10%). Accelerometer mean wear time was 1329 minutes. After accounting for non-wear time (111 minutes), there was 499 minutes of sedentary time, 415 minutes of LIPA, 26 minutes of MVPA, and 388 minutes of nighttime sleep averaged over a 24-hour period, with an overall mean CVH score of 5.91. Participants in the highest physical activity quintile were generally younger, more likely to be female, and had higher CVH scores.
Table 1.
Descriptive Characteristics of the Study Population (n=1,718)
| Participant Characteristics (N=1718) | |||||
|---|---|---|---|---|---|
| Physical Activity Quintile (minutes/day) | |||||
| ≤ 319.83 | 319.84 – 403.33 | 403.34 – 472.67 | 472.68 – 555.67 | ≥ 555.68 | |
| Age (years) | 72.2 (71.2–73.2) | 70.0 (69.1 – 70.8) | 68.9 (68.0 – 69.9) | 66.7 (65.8 – 67.6) | 63.9 (63.2 – 64.7) |
| Married | 188 (57.1%) | 200 (58.3%) | 206 (59.0%) | 218 (62.8%) | 222 (63.4%) |
| College Education | 161 (48.9%) | 141 (41.1%) | 144 (41.3%) | 126 (36.3%) | 124 (35.4%) |
| Race/Ethnicity | |||||
| White | 147 (44.7%) | 145 (42.3%) | 133 (38.1%) | 125 (36.0%) | 113 (32.3%) |
| Chinese-American | 27 (8.2%) | 31 (9.0%) | 42 (12.0%) | 38 (11.0%) | 42 (12.0%) |
| Black | 100 (30.4%) | 89 (26.0%) | 98 (28.0%) | 90 (25.9%) | 91 (26.0%) |
| Hispanic | 55 (16.7%) | 78 (22.7%) | 76 (21.8) | 94 (27.1%) | 104 (29.7%) |
| Male | 186 (56.5%) | 156 (45.5%) | 156 (44.7%) | 155 (44.7%) | 131 (37.4%) |
| Diabetes Med Use | 71 (21.6%) | 48 (14.0%) | 55 (15.8%) | 51 (14.7%) | 33 (9.4%) |
| Hypertension Med Use | 216 (65.7%) | 193 (56.3%) | 187 (53.6%) | 175 (50.4%) | 143 (40.9%) |
| Lipid Lowering Med Use | 151 (45.9%) | 146 (42.6%) | 122 (35.0%) | 115 (33.1%) | 106 (30.3%) |
| Activity Parameters* | |||||
| Sedentary Time | 634.9 (623.6 – 646.3) | 563.5 (555.7 – 571.3) | 510.4 (502.7 – 518.0) | 444.7 (437.5 – 451.9) | 351.9 (344.0 – 359.9) |
| LIPA | 250.0 (244.8 – 255.2) | 350.3 (347.8 – 352.8) | 416.7 (414.2 – 419.1) | 478.7 (475.6 – 481.7) | 570.2 (563.7 – 576.6) |
| MVPA | 6.5 (5.7 – 7.3) | 12.7 (11.4 – 13.9) | 19.8 (18.2 – 21.3) | 32.7 (30.6 – 34.89) | 57.0 (52.8 – 61.2) |
| Sleep | 409.5 (399.7 – 419.2) | 403.1 (395.1 – 411.2) | 382.4 (374.1 – 390.7) | 384.9 (377.2 – 392.6) | 362.9 (355.3 – 370.4) |
| BMI (kg/m2) | 29.3 (28.6 – 29.9) | 29.6 (29.0 – 30.1) | 28.7 (28.2 – 29.3) | 28.5 (27.9 – 29.0) | 28.1 (27.5 – 28.7) |
| Systolic BP (mmHg) | 123.4 (121.2 – 125.5) | 123.1 (121.1 – 125.0) | 123.5 (121.3 – 125.7) | 122.6 (120.4 – 124.8) | 119.2 (117.3 – 121.2) |
| Diastolic BP (mmHg) | 67.4 (66.7 – 68.5) | 68.0 (67.0 – 69.0) | 68.1 (67.0 – 69.1) | 68.8 (67.7 – 69.9) | 69.0 (68.0 – 70.1) |
| Cholesterol (mg/dL) | 174.6 (170.4 – 178.8) | 184.6 (180.6 – 188.5) | 185.5 (181.8 – 189.3) | 184.4 (180.8 – 188.0) | 190.0 (186.2 – 193.9) |
| Glucose (mg/dL) | 105.2 (101.7 – 108.6) | 99.7 (97.1 – 102.3) | 101.6 (99.0 – 104.2) | 101.4 (98.4 – 104.4) | 99.2 (96.3 – 102.1) |
| CVH Score | 5.36 (5.11 – 5.60) | 5.68 (5.45 – 5.91) | 5.90 (5.66 – 6.14) | 6.15 (5.91 – 6.38) | 6.41 (6.16 – 6.65) |
Variables are presented as N(95% confidence interval) or mean (%)
Med = medication; LIPA = light intensity physical activity; MVPA = moderate to vigorous physical activity; BMI = body mass index; BP = blood pressure; CVH = cardiovascular health
activity parameters in minutes/day
Partition Models
Results from the partition models are displayed in Table 2. When all temporal variables were included, LIPA, MVPA, and sleep were each significantly and positively associated with CVH score (Model 1). These effects were maintained when sedentary behavior was removed (Model 2). There was no significant association between sedentary time and CVH score when LIPA was removed (Model 3).
Table 2.
Partition Models for Associations of Sleep, Sedentary Behavior, and Physical Activity on Cardiovascular Health
| Activity Parameter | Model 1 B(95%CI) | Model 2 B(95%CI) | Model 3 B(95%CI) |
|---|---|---|---|
| Sedentary | .001 ± .002 | Dropped | −.001 ± .002 |
| LIPA | .002 ± .002* | .001 ± .002** | Dropped |
| MVPA | .017 ± .004** | .016 ± .004** | .016 ± .004** |
| Sleep | .003 ± .002** | .003 ± .002** | .002 ± .002** |
Model 1= all temporal variables included; Model 2= all temporal variables minus sedentary behavior included; Model 3= all temporal variables minus LIPA included
LIPA = light intensity physical activity; MVPA = moderate to vigorous intensity physical activity; B = beta; CI = confidence interval
B ± 95% confidence interval represent the effect of a 1-minute increase in each activity variable on the cardiovascular health score
All models control for sex, age, marital status, education, and race
p ≤ 0.05;
p ≤ 0.01
Isotemporal Substitution Models
Results from the isotemporal substitution models are provided in Table 3 and visually represented as a forest plot in Figure 1.
Table 3.
Isotemporal Substitution Models for Associations of Sleep, Sedentary Behavior, and Physical Activity with Cardiovascular Health1–3
| Activity Parameter | CVH Score | Glucose (mg/dl)4 | Systolic BP (mmHg)5 | Diastolic BP (mmHg)5 |
Total Cholesterol (mg/dl)6 |
BMI (kg/m2) |
|---|---|---|---|---|---|---|
| Sedentary | Dropped | Dropped | Dropped | Dropped | Dropped | Dropped |
| LIPA | .039 ± .033* | −.091 ± .363 | .231 ± .290 | .147 ± .143* | .534 ± .502* | −.134 ± .080** |
| MVPA | .485 ± .127** | .279 ± 1.419 | −1.205 ± 1.133* | −.858 ± .557** | −.049 ± 1.929 | −1.179 ± .310** |
| Sleep | .077 ± .045** | −.111 ± .506 | −.182 ± .404 | −.077 ± .198 | .493 ± .694 | −.262 ± .112** |
LIPA = light intensity physical activity; MVPA = moderate to vigorous intensity physical activity; CVH = cardiovascular health; BP = blood pressure; BMI = body mass index
B ± 95% confidence interval represent the effect of substituting 30 minutes of sedentary time for LIPA, MVPA, or sleep
All models are adjusted for sex, age, marital status, education, race, and total daily time on monitor. All models except for those predicting the CVH score and BMI control for body mass index.
p ≤ 0.05;
p ≤ 0.01
Additionally adjusted for diabetes medication usage
Additionally adjusted for hypertension medication
Additionally adjusted for lipid-lowering medication
Figure 1.
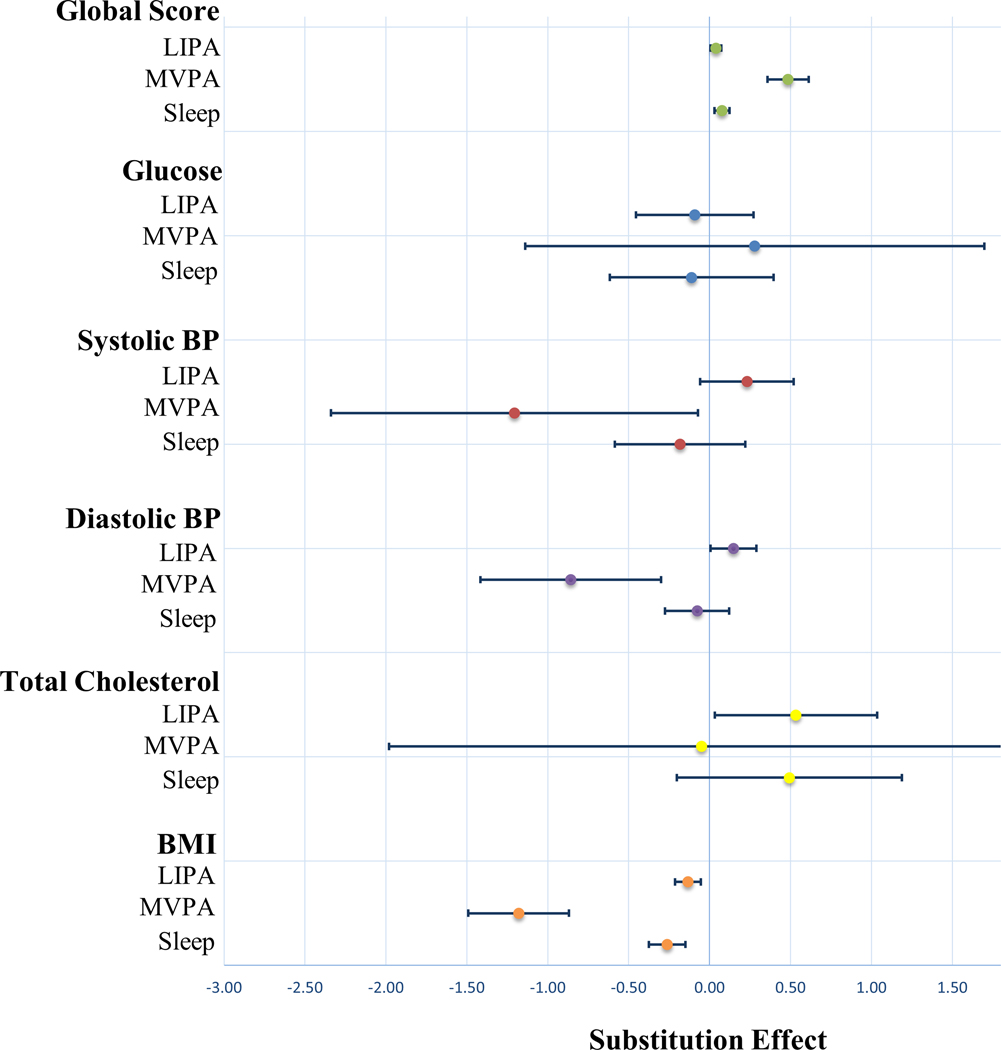
Forest Plot Depicting the Effect of Substituting 30 Minutes of Sedentary Time for LIPA, MVPA or Sleep
LIPA = light intensity physical activity; MVPA = moderate to vigorous intensity physical activity; BP = blood pressure; BMI = body mass index
*Values represent Beta and 95% Confidence Intervals
Cardiovascular Health Score
Substituting 30 minutes of sedentary time to LIPA, MVPA, and sleep was associated with a 0.039(95%CI: 0.033), 0.485(95%CI: 0.127), and 0.077(95%CI: 0.045) point increase in CVH score, respectively.
Glucose
No significant associations were observed between any substitution model and glucose.
Systolic Blood Pressure
Substituting 30 minutes of sedentary time to MVPA was associated with a 1.205 mmHg (95%CI: 1.133) decrease in SBP.
Diastolic Blood Pressure
Substituting 30 minutes of sedentary time to LIPA and MVPA was associated with a 0.147 mmHg (95%CI: 0.143) increase in DBP and 0.858 mmHg (95%CI: 0.557) decrease in DBP, respectively.
Total Cholesterol
Substituting 30 minutes of sedentary time to LIPA was associated with a 0.534 mg/dL (95%CI: 0.502) increase in total cholesterol. No significant associations were observed when substituting sedentary time for MVPA or sleep.
Body Mass Index
Substituting 30 minutes of sedentary time to LIPA, MVPA, and sleep was associated with a 0.134 kg/m2 (95%CI: 0.080), 1.179 kg/m2 (95%CI: 0.310), and 0.262 kg/m2 (95%CI: 0.112) decrease in BMI, respectively.
Stratification by Sex
In women, substituting 30 minutes of sedentary time to LIPA, MVPA, and sleep were all associated with an increase in CVH score and reduction in BMI. Substituting sedentary time to MVPA and sleep was associated with a decrease in DBP and increase in total cholesterol, respectively. No significant associations were observed between any substitution model and glucose or SBP (see Table, Supplemental Digital Content 1, Isotemporal Substitution Models for Associations of Sleep, Sedentary Behavior, and Physical Activity with Cardiovascular Health in Women).
In men, substituting 30 minutes of sedentary time to MVPA and sleep were associated with an increase in CVH score and reduction in BMI, though the relationship between these variables and LIPA was non-significant. Substituting sedentary time to LIPA was associated with an increase in DBP, while substitution to MVPA was associated with a decrease in DBP. No significant associations were observed between any substitution model and glucose, SBP, or total cholesterol (see Table, Supplemental Digital Content 2, Isotemporal Substitution Models for Associations of Sleep, Sedentary Behavior, and Physical Activity with Cardiovascular Health in Men).
Stratification of Total Cholesterol
When stratifying total cholesterol into LDL and HDL cholesterol, no significant associations were observed between any substitution model and LDL cholesterol. However, significant increases in HDL cholesterol were observed when substituting 30 minutes of sedentary time to LIPA and MVPA, but not sleep (see Table, Supplemental Digital Content 3, Isotemporal Substitution Models for Associations of Sleep, Sedentary Behavior, and Physical Activity with LDL and HLD Cholesterol). All stratified models are exploratory in nature. Interpretation of these models should be done with caution given the decreased sample size necessary to conduct these analyses.
When 14 cases with outlying residuals were censored, the effect for total cholesterol remained in the positive direction (0.434 mg/dL higher) but was no longer significant (p = .08). There were no significant substitution effects when glucose was included as a dependent variable, and effects did not meaningfully change when individuals with diabetes were removed from the analysis. There were outlying residuals (i.e., > 3 standard deviations) present in the models for systolic blood pressure, diastolic blood pressure, BMI, and glucose. Sensitivity analyses censoring outlying cases produced results that did not meaningfully differ from those presented.
DISCUSSION
Our study estimates that substituting 30 minutes of sedentary time for LIPA, MVPA, or sleep using isotemporal substitution modeling was associated with an improvement in overall CVH, assessed using metrics included in the AHA LS7 score, using data from the MESA cohort. Moreover, substituting sedentary time for LIPA, MVPA, or sleep was associated with reductions in BMI, while substituting sedentary time for MVPA was also associated with lower SBP and DBP. However, substituting sedentary time for sleep, LIPA, or MVPA was not associated with significant improvements in total cholesterol or blood glucose.
Much of the literature has focused on the benefits of MVPA on cardiovascular biomarkers and all-cause mortality (23, 24). Our results were similarly robust, showing the most favorable outcomes when substituting sedentary time for MVPA. Additionally, we uncovered an increase in the CVH score when substituting sedentary time for LIPA, which highlights the clinical and public health value of LIPA. When discussing MVPA in a clinical setting, patients often believe they must engage in more structured exercise programs which can be difficult to achieve given common barriers such as lack of time, motivation, and cost (25). Importantly, both LIPA and MVPA can be achieved during routine activities of daily living, including walking and engaging in housework (26, 27), which are more convenient than traditional exercise. Moreover, LIPA can be accumulated in large volumes with little perceived effort, offsetting the negative health effects of sedentary behavior, (28) while still achieving important health benefits including reductions in CVD, cardiovascular and all-cause mortality (26). US adults spend roughly 30% of their time in LIPA compared to 2% of their time in MVPA over a 24-hour time period, thus there is more potential public health benefit to be gained from LIPA (29).
More favorable SBP, cholesterol, and BMI were also observed when substituting sedentary time with either LIPA or MVPA, consistent with the literature on the benefits of physical activity on cardiometabolic risk factors (30–32). However, we did not find a significant benefit on blood glucose in our model. A similar study using isotemporal substitution modeling from Whitaker et al. also reported improvements in a composite cardiovascular risk score when substituting sedentary time with LIPA and MVPA, but no significant reduction in blood glucose (33). Results using isotemporal substitution have been mixed, with some studies showing no reduction in blood glucose when substituting sedentary time with physical activity (34, 35), while others have shown a significant reduction (8, 36). Differences in results may be due to the characteristics of the cohorts used in each study. Participants were generally younger and healthier, with less diabetes, in the studies that demonstrated a reduction in blood glucose with substitution, compared to the studies that demonstrated no reduction in blood glucose. Thus, the potential benefit of substituting sedentary time with physical activity on blood glucose may have been offset by the high prevalence of other cardiometabolic risk factors present in this older cohort.
We found an unexpected increase in DBP and total cholesterol when substituting sedentary time with LIPA. The relationship between physical activity and blood pressure is complex, dependent upon frequency, duration, type, and intensity of activity, which we may not have fully captured in our models. This is further acknowledged by the 2018 Physical Activity Guidelines for Americans, which states “no conclusions can be made regarding the influence of intensity on the blood pressure response to physical activity (26).” Given prior data that suggests physical activity increases HDL cholesterol (33, 37), we stratified total cholesterol into HDL and LDL cholesterol, and found that substituting sedentary time for both LIPA and MVPA resulted in increased HDL cholesterol, verifying our suspicion that the increase in total cholesterol was driven by increased HDL cholesterol.
Sleep is an important and necessary determinant of health and well-being, with evidence to suggest that both short and long sleep duration are linked to an increased risk of several cardiometabolic risk factors, cardiovascular disease, and all-cause mortality (38, 39). However, few studies have evaluated sleep in relation to sedentary behavior or physical activity. Our results are consistent with the literature on the importance of sleep in improving CVH.
A significant reduction in BMI and improvement in CVH was observed when substituting sedentary time for sleep, though we did not observe a similar benefit in SBP, DBP, total cholesterol, or blood glucose was observed. Our results are largely consistent with a recent study that used isotemporal substitution modeling in the Woman’s Health Initiative (40). Full et al. found that substituting 91 minutes of sedentary time with an equivalent amount of sleep was associated with a lower waist circumference (40). However, similar substitutions revealed no significant benefit in insulin level, HOMA-IR, cholesterol, triglycerides, or Reynolds Risk Score, a validated tool used to predict the risk of heart attack, stroke, angioplasty, coronary artery bypass surgery, or death related to heart disease in women (41). Differences in substitution time or differences in gender may explain why we observed a significant improvement in our CVH score compared to this study.
The strengths of this study include the use of objectively assessed habitual sleep and physical activity data and the rigorous assessments of the outcomes of interest. Furthermore, this represents one of the largest and earliest reports to use partition and isotemporal substitution modeling to examine the effect of substituting sedentary time with sleep, LIPA, or MVPA on overall and individual metrics of CVH in a population-based sample of middle-aged and older US adults at risk for CVD. This study is also novel because it uniquely examines physical activity and sleep concomitantly on its relationship with CVH, whereas prior studies have focused on these factors separately.
This study has limitations that we acknowledge. First, isotemporal substitution is a statistical model that does not necessarily reflect the effects of real-time substitution. Second, the MESA Sleep Ancillary Study is cross-sectional, so we were unable to examine associations of change in sleep and PA with changes in CVH over time within individuals. Third, dietary intake, which was included as a metric of CVH, was self-reported and is therefore prone to measurement error. Fourth, although we were able to adjust our analyses for several key confounders, we cannot rule out the presence of residual confounding. Fifth, the Actiwatch accelerometer was used to capture physical activity in the MESA Sleep Ancillary study, whereas the Actigraph and ActivPAL devices are more common in physical activity research. There are limited published cut points for the Actiwatch, requiring the use of cut points calibrated to a later device (Motionwatch 9, CamNtech, Cambridge, UK). However, our resulting activity data aligns well with other published accelerometer data (42), thus we believe that the objectively measured physical activity information captured from the Actiwatch proves useful insight into the potential effect of substituting low-active states (a common proxy for sedentary time) for higher active states (i.e. LIPA, MVPA) and sleep. Sixth, our sleep variable accounted for sleep duration but not measures of sleep architecture or fragmentation. There are data that increasing N3 (slow wave) sleep is associated with favorable cardiometabolic outcomes, independent of sleep duration (43). Thus, further studies assessing not only sleep duration, but also quality and character are warranted to fully understand these complex relationships. Seventh, daytime napping may have occurred, which would have been captured as sedentary behavior and not sleep time. Finally, we did not have sufficient power to fully examine differences by race/ethnicity.
In conclusion, our study suggests that LIPA, MVPA, and sleep were all associated with more favorable CVH, and that substituting 30 minutes of sedentary time with an equivalent amount of sleep, LIPA, or MVPA was associated with improvements in overall CVH and several key cardiometabolic risk factors. These findings underscore the importance of lifestyle modifications in improving CVH. Further studies targeted at elucidating the complex relationships between sleep, sedentary behavior, and physical activity are warranted to inform personalized treatment plans on an individual level for those at risk of CVD.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Polysomnography, actigraphy, and sleep questionnaire data can be accessed from the MESA section of the National Sleep Research Resource website: https://sleepdata.org/datasets/mesa.
CONFLICT OF INTEREST
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. The MESA Sleep Study was supported by NHLBI grant HL56984. S.R. was partially supported by R35HL135818.
Dr. Abdalla receives support through 18AMFDP34380732 from the American Heart Association and from the NIH/NHLBI (K23 HL141682-01A1).
The funders had no role of in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
The results of the present study do not constitute endorsement b the ACSM. The results of the study are presented clearly, honestly, and without falsification, or inappropriate data manipulation.
REFERENCES
- 1.Kwok CS, Kontopantelis E, Kuligowski G et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J Am Heart Assoc. 2018;7(15):e008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzmarzyk PT. Physical activity, sedentary behavior, and health: paradigm paralysis or paradigm shift? Diabetes. 2010;59(11):2717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piercy KL, Troiano RP, Ballard RM et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St-Onge MP, Grandner MA, Brown D et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation. 2016;134(18):e367-e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young DR, Hivert MF, Alhassan S et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation. 2016;134(13):e262–79. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberger ME, Fulton JE, Buman MP et al. The 24-Hour Activity Cycle: A New Paradigm for Physical Activity. Med Sci Sports Exerc. 2019;51(3):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buman MP, Winkler EA, Kurka JM et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol. 2014;179(3):323–34. [DOI] [PubMed] [Google Scholar]
- 9.Diaz KM, Howard VJ, Hutto B et al. Patterns of Sedentary Behavior in US Middle-Age and Older Adults: The REGARDS Study. Med Sci Sports Exerc. 2016;48(3):430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Virani SS, Callaway CW et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67-e492. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Hong Y, Labarthe D et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Wang R, Zee P et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? International Journal of Behavioral Nutrition and Physical Activity. 2011;8(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang GQ, Cui L, Mueller R et al. The National Sleep Research Resource: towards a sleep data commons. J Am Med Inform Assoc. 2018;25(10):1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakley N. Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. Bend: Mini Mitter, Cambridge Neurotechnology. 1997. [Google Scholar]
- 17.Landry GJ, Falck RS, Beets MW, Liu-Ambrose T. Measuring physical activity in older adults: calibrating cut-points for the MotionWatch 8©. Frontiers in Aging Neuroscience. 2015;7(165). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer-Davis EJ, Vitolins MZ, Carmichael SL et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9(5):314–24. [DOI] [PubMed] [Google Scholar]
- 19.Kulshreshtha A, Vaccarino V, Judd SE et al. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44(7):1909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogunmoroti O, Oni E, Michos ED et al. Life’s Simple 7 and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM. Improving the cardiovascular health of the US population. JAMA. 2012;307(12):1314–6. [DOI] [PubMed] [Google Scholar]
- 22.Mekary RA, Lucas M, Pan A et al. Isotemporal substitution analysis for physical activity, television watching, and risk of depression. Am J Epidemiol. 2013;178(3):474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loprinzi PD. Dose-response association of moderate-to-vigorous physical activity with cardiovascular biomarkers and all-cause mortality: Considerations by individual sports, exercise and recreational physical activities. Prev Med. 2015;81:73–7. [DOI] [PubMed] [Google Scholar]
- 24.Loprinzi PD, Loenneke JP, Ahmed HM, Blaha MJ. Joint effects of objectively-measured sedentary time and physical activity on all-cause mortality. Prev Med. 2016;90:47–51. [DOI] [PubMed] [Google Scholar]
- 25.Hoare E, Stavreski B, Jennings GL, Kingwell BA. Exploring Motivation and Barriers to Physical Activity among Active and Inactive Australian Adults. Sports (Basel). 2017;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Services USDoHaH. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. 2018. [Google Scholar]
- 27.Murphy MH, Donnelly P, Breslin G, Shibli S, Nevill AM. Does doing housework keep you healthy? The contribution of domestic physical activity to meeting current recommendations for health. BMC Public Health. 2013;13:966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chastin SF, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined Effects of Time Spent in Physical Activity, Sedentary Behaviors and Sleep on Obesity and Cardio-Metabolic Health Markers: A Novel Compositional Data Analysis Approach. PLoS One. 2015;10(10):e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Zhang D, Liu Y et al. Dose-Response Association Between Physical Activity and Incident Hypertension: A Systematic Review and Meta-Analysis of Cohort Studies. Hypertension. 2017;69(5):813–20. [DOI] [PubMed] [Google Scholar]
- 31.Glazer NL, Lyass A, Esliger DW et al. Sustained and shorter bouts of physical activity are related to cardiovascular health. Med Sci Sports Exerc. 2013;45(1):109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan JX, Brown BB, Hanson H, Kowaleski-Jones L, Smith KR, Zick CD. Moderate to vigorous physical activity and weight outcomes: does every minute count? Am J Health Promot. 2013;28(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitaker KM, Pettee Gabriel K, Buman MP et al. Associations of Accelerometer-Measured Sedentary Time and Physical Activity With Prospectively Assessed Cardiometabolic Risk Factors: The CARDIA Study. J Am Heart Assoc. 2019;8(1):e010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knaeps S, De Baere S, Bourgois J, Mertens E, Charlier R, Lefevre J. Substituting Sedentary Time With Light and Moderate to Vigorous Physical Activity is Associated With Better Cardiometabolic Health. J Phys Act Health. 2018;15(3):197–203. [DOI] [PubMed] [Google Scholar]
- 35.Falconer CL, Page AS, Andrews RC, Cooper AR. The Potential Impact of Displacing Sedentary Time in Adults with Type 2 Diabetes. Med Sci Sports Exerc. 2015;47(10):2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekblom-Bak E, Ekblom O, Bolam KA, Ekblom B, Bergstrom G, Borjesson M. SCAPIS Pilot Study: Sitness, Fitness and Fatness - Is Sedentary Time Substitution by Physical Activity Equally Important for Everyone’s Markers of Glucose Regulation? J Phys Act Health. 2016;13(7):697–703. [DOI] [PubMed] [Google Scholar]
- 37.Kodama S, Tanaka S, Saito K et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167(10):999–1008. [DOI] [PubMed] [Google Scholar]
- 38.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71(5):1027–36. [DOI] [PubMed] [Google Scholar]
- 39.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Full KM, Gallo LC, Malhotra A et al. Modeling the cardiometabolic benefits of sleep in older women: Exploring the 24-hour day. Sleep. 2020; 43(1):zsz205. doi: 10.1093/sleep/zsz205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–9. [DOI] [PubMed] [Google Scholar]
- 42.Hansen BH, Kolle E, Dyrstad SM, Holme I, Anderssen SA. Accelerometer-determined physical activity in adults and older people. Med Sci Sports Exerc. 2012;44(2):266–72. [DOI] [PubMed] [Google Scholar]
- 43.Javaheri S, Zhao YY, Punjabi NM, Quan SF, Gottlieb DJ, Redline S. Slow-Wave Sleep Is Associated With Incident Hypertension: The Sleep Heart Health Study. Sleep. 2018;41(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


