Abstract
Background
Pain is one of the first presenting symptoms in patients with head and neck cancer, who often develop chronic and debilitating pain as the disease progresses. Pain is also an important prognostic marker for survival. Unfortunately, patients rarely receive effective pain treatment due to our limited knowledge of the mechanisms underlying head and neck cancer pain (HNCP). Pain is often associated with neuroinflammation and particularly IL-1 signaling. The purpose of this study is to develop a novel syngeneic model of HNCP in immunocompetent mice in order to examine the contribution of IL-1 signaling.
Methods
Male C57BL/6 mice were injected with a murine model of human papillomavirus (HPV+) induced oropharyngeal squamous cell carcinoma in their right hindlimb in order to induce tumor growth. Pain sensitivity was measured via von Frey filaments. Spontaneous pain was assessed via the facial grimace scale. Interleukin (IL)-1β was measured by quantifying gene expression via qPCR and ELISA.
Results
Pain hypersensitivity and spontaneous pain develop quickly after the implantation of tumor cells, a time when tumor volume is still insignificant. Spinal and circulating IL-1β levels are significantly elevated in tumor-bearing mice. Blocking IL-1 signaling either by intrathecal administration of IL-1 receptor antagonist (IL-1ra) or by genetic deletion (Il1r1−/−) does not alleviate HNCP.
Conclusion
We established the first syngeneic model of HNCP in immunocompetent mice. Unlike inflammatory or nerve injured pain, HNCP is independent of IL-1 signaling. These findings challenge the common belief that pain results from tissue compression or IL-1 signaling in patients with head and neck cancer.
Introduction
Cancer-associated pain is one of the earliest and most commonly reported symptoms of patients with head and neck squamous cell carcinoma (HNSCC) 1,2. In fact, pain is often the motivating factor for patients to seek treatment 3,4. Importantly, head and neck cancer pain (HNCP) is associated with a poor prognosis 4,5. Patients with HNCP report both spontaneous pain and mechanical pain hypersensitivity 6,7. Despite the frequency and severity of HNCP, current treatments fail to adequately control pain 8,9.
The mechanisms responsible for the initiation and development of HNCP are not well understood. As tumors can be highly inflammatory and pain is a cardinal sign of inflammation, it is generally accepted that HNCP results from inflammation 10–12. Consistently, preclinical studies show that the proinflammatory cytokine Tumor Necrosis Factor (TNF-α) contributes to HNCP while the anti-inflammatory lipid, Resolvin D2, alleviates it 13,14. However, these studies used athymic mice which may be an important limitation given the contribution of T cells to pain modulation and cancer progression 15–17. To overcome these potential limitations, we utilize a syngeneic model of human papillomavirus-induced head and neck cancer developed as follows. Oropharyngeal cells were isolated from C57Bl/6 male mice and retrovirally transduced to stably express HPV16 viral oncogenes, E6 and E7, along with H-Ras and luciferase (mEERL cells). As such, when injected into immunocompetent mice (wildtype C57Bl/6), tumors grow with characteristics that are faithful to the human disease as a validated model of HNSCC 18,19.
Using this model, we tested the hypothesis that neuroinflammation and particularly IL-1 signaling mediates HNCP. We found an upregulation of Il1b in the spinal cord of mEERL tumor-bearing mice. Interleukin (IL)-1β is a common proinflammatory cytokine and activation of its receptor (IL-1R1) mediates chronic pain in various clinical conditions and preclinical models 20–24. Therefore, we investigated whether blocking IL-1 signaling alleviates HNCP.
Methods
Animals
C57BL/6J (WT) and Il1r1−/− (JAX#003245) male mice (10–14 weeks old) purchased from Jackson Laboratory (Bar Harbor, Maine) were housed and bred at Michigan State University (MSU). Mice were housed in a controlled temperature environment with 12-hour light-dark cycle, food and water were available ad libitum. All procedures were approved by MSU Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 86–23) and ARRIVE guidelines. Full mouse distribution is described in the attached flow chart.
Head and Neck Cancer Model
To induce HNSCC, we used a validated murine model of human papillomavirus induced (HPV+) oropharyngeal squamous cell carcinoma. This model consists of oropharyngeal epithelial cells from C57Bl/6 male mice that stably express HPV16 viral oncogenes, E6 and E7, H-Ras and luciferase (mEERL cells) 18,19. mEERL cells were grown in a T125 flask until confluent, after which cells were trypsinized and harvested, washed three times with sterile PBS, and re-suspended in 1 mL of sterile PBS to the appropriate concentration. Mice are injected subcutaneously with 20 μl solution containing either 200,000 mEERL cells or PBS (vehicle) into the right hindlimb. The injection into the leg instead of the oral cavity is justified by the 3R guideline in an effort to reduce distress, inability to feed and easier assessment of tumor growth. Although, we have previously validated the conformity of HNSCC development in the leg 18,25, the difference in the affected neuraxis: trigeminal ganglion-brainstem vs. dorsal root ganglion-spinal cord might have limitations. The day of mEERL cells injection is indicated as day 0. Tumor volume was monitored using Vernier digital calipers. Mice were terminated by CO2 exposure followed by trans-cardiac perfusion with ice-cold PBS.
Behavioral assessment of nociception
Mechanical Pain sensitivity
Mechanical sensitivity was measured using von Frey filaments (0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 1.4) starting with the application of 0.4 filament. Mice were placed on a mesh stand in plastic cages for up to 30 minutes before testing for habituation. Mice were prodded on their hind paw with a von Frey filament, A positive response was defined as shaking, clear paw withdrawal, or licking of the hind paw. The Dixon “up-and-down” method was used to quantify the mechanical force require to produce a paw withdrawal response in 50% of mice (paw withdrawal threshold), based on the statistical formula used to determine LD50s. Briefly, If there is no response, the next filament with a higher force is tested; if there is a response, the next lower force filament is tested. The 50% threshold is then calculated using the formula: 50% threshold (g) = 10(X+kd)/104, where X = the value (in log units) of the final von Frey filament, k = tabular value for the response pattern (see Appendix 1 in 26) and d = the average increment (in log units) between von Frey filaments26.
Spontaneous pain - The Mouse Grimace Scale
Mouse Grimace Scale (MGS) was used to assess spontaneous pain of mice as previously described 27. Briefly, mice were enclosed in a small cubicle. The mice were video recorded for a total of 30 minutes and 10 pictures that showed a clear view of the face were selected for scoring. We modified the MGS slightly as we measured only 4 action units of facial expressions on a scale from 0 to 2 (orbital tightening, nose bulge, cheek bulge and ear position). The whisker unit was omitted because of insufficient quality of the videos for some animals. A score of zero indicates the mouse is not experiencing spontaneous pain. Each picture was scored based on the 4 facial attributes and then averaged. Mice injected with Complete Freund’s Adjuvant (CFA, 20 μl in each hind paw, 1 mg/ml, Sigma) served as positive controls 28.
Behavioral testing was performed and scored by experimenters blinded to treatment and genotype.
Drug administration
Recombinant mouse IL-1 receptor antagonist (IL-1ra) (#480-RM-010, R&D Systems, Minneapolis, MN) was dissolved in sterile PBS and intrathecally injected (50 ng/5 μl/day) as previously reported 23,29.
Quantitative real-time polymerase chain reaction
Spinal cord and liver tissue samples were collected, snap frozen and stored at −80֯C. Total RNA was extracted using a modified version of Trizol-chloroform method to improve the quality of the isolated RNA 30 and quantified with high-performing Qubit 4 Fluorometer instrument (Qubit 4, Invitrogen, Carlsbad, CA). A total of 1 μg RNA was reverse transcribed into complementary DNA (cDNA) and quantitative real-time polymerase chain reaction was performed using a CFX96 (Biorad). Validated PrimeTime qPCR primers (Integrated DNA Technologies, Coralville, IA) were used to quantify the expression of Il1b, Il1rn, Tnf, Csf1r, Tlr4, Nfkbia, Gapdh and Acntb. Expression levels were calculated using the ΔΔCT method and normalized to Gapdh and Acntb to confirm that housekeeping gene expression is not affected by tumor growth. Data are presented as the average relative fold change normalized by both housekeeping genes independently and normalized to the control group. In addition to the spinal cord where neuroinflammation is a key player in pain regulation, we measured Il1b expression in the liver because this organ is enriched in liver-resident macrophages (Kupffer cells) which are great producers of IL-1β.
Enzyme-linked immunosorbent assay (ELISA)
Serum IL-1β was quantified by ELISA. Blood was collected by cardiac puncture. Samples were allowed to clot at room temperature for 2 hours followed by a centrifuging for 20 minutes at 2000x g following instructions provided from the Quantikine® HS ELISA kit (Cat#MHSLB00, R&D systems, Minneapolis, MN).
Statistical analysis
Differences in behavioral testing and expression levels were assessed by Student’s t-test, one-way or repeated-measure two-way ANOVA followed by Bonferroni correction for multiple tests, depending on experimental design. Significant differences are indicated in graphs as *** = P<0.001, ** = P<0.01, * = P<0.05. The number of animals required to reach a statistically significant 30% difference in group means were predicted based on a power analysis and previous publication using similar tests. For the power analysis, type I error was set at 0.05 and a type II error set at 0.80. Statistical analysis and graphical representations are performed using GraphPad Prism V8 (San Diego, CA).
COVID19 shutdown
The University shutdown due to the pandemic and health concern for students and employees drastically affected the present research and explains some missing/omitted time points in the last experiments. However, we think that it does not alter the main findings of this manuscript.
Results
Implantation of mEERL cells induces evoked- and spontaneous pain
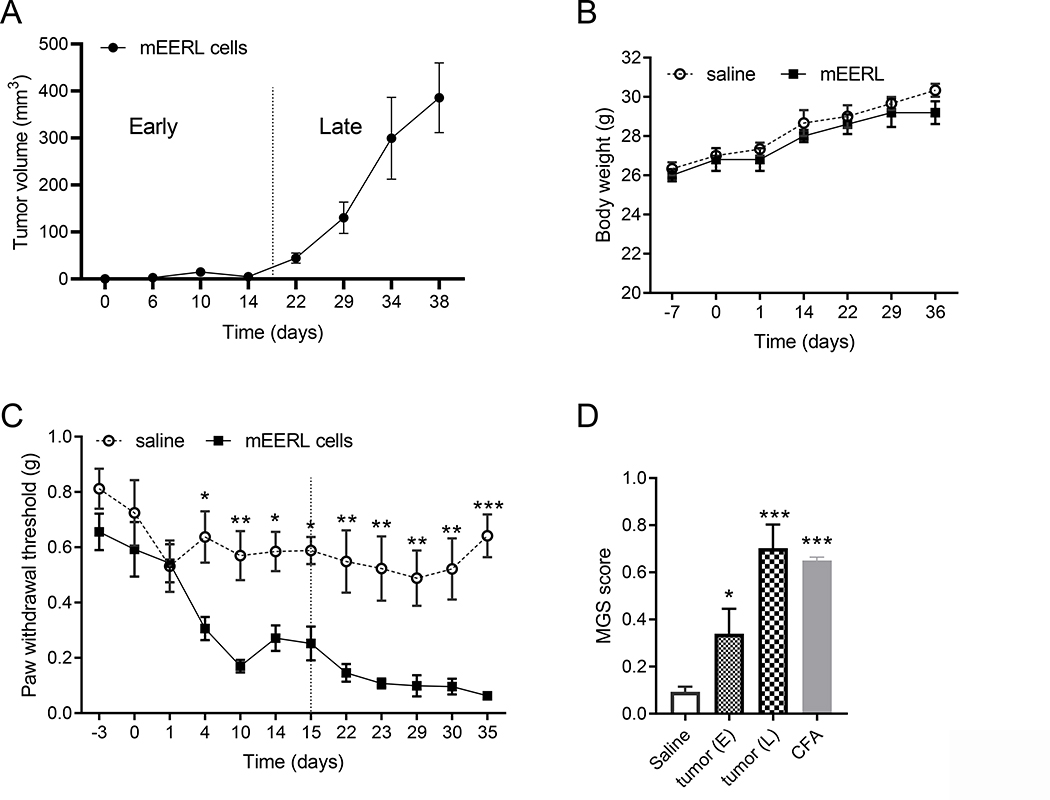
Injection of mEERL cells induces tumor growth which becomes detectable after 15 days (Figure 1A) but did not affect body weight (Figure 1B). Based on tumor size, we defined an “early” and a “late” phase of tumor growth (Figure 1A). The cut point was chosen on day 15 when the tumor reaches a measurable size. Pain hypersensitivity develops as early as 4 days after mEERL cells administration and pain threshold continues to drop as the tumor grows (Figure 1C). To determine whether mEERL cell implantation also induces the more clinically relevant non-evoked pain, we also assessed facial grimacing. Administration of mEERL cells produces a significant increase of facial grimacing in the early phase of tumor growth and induces severe facial grimacing in the late phase of disease. At 30 days post-injection, facial grimacing in mEERL tumor-bearing mice is similar to CFA-treated mice (Figure 1D and Supplementary Figure 1A). Implantation of mEERL tumors induces both evoked- and spontaneous pain.
Figure 1. mERRL cells induce pain hypersensitivity and spontaneous pain.
A) mEERL tumor growth curve. A dashed line separates “early” and “late” phase. B) Body weight is identical in healthy and tumor-bearing mice. Two-way ANOVA F(6, 42) = 0.15, p = 0.98 (no significant difference). C) Mechanical pain sensitivity is monitored with von Frey filaments. A dashed line separates “early” and “late” phase. Tumor-bearing mice show significant pain hypersensitivity 2-way ANOVA followed by Bonferroni’s multiple correction test (cancer effect F(1,12) = 111, p<0.0001, n = 7/group). D) Mouse facial grimace assessment is performed at 14 and 30 days post-injection in different groups of tumor-bearing mice (one-way ANOVA F(3,17) = 17.6, p<0.0001, n = 5–7/group) and 24 h post-CFA which serves as positive control. Graphs C and D result from 3 different experiments performed by 3 different experimenters in different animal facilities.
Injection of mEERL cells upregulates spinal Il1b and circulating IL-1β
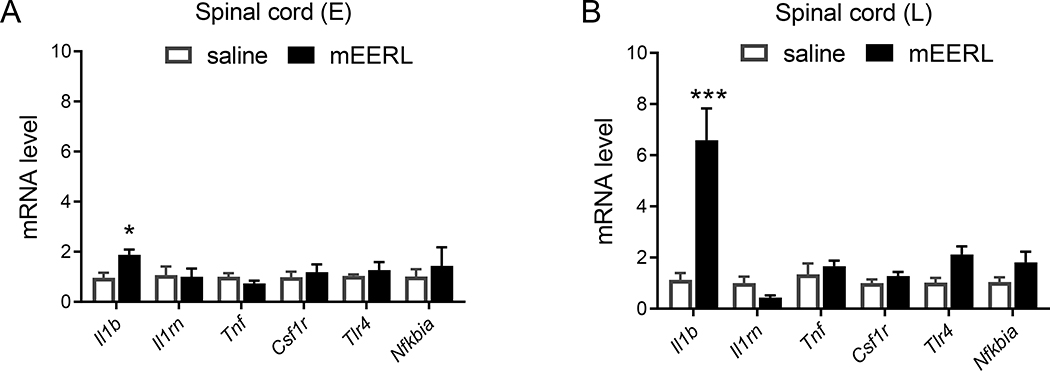
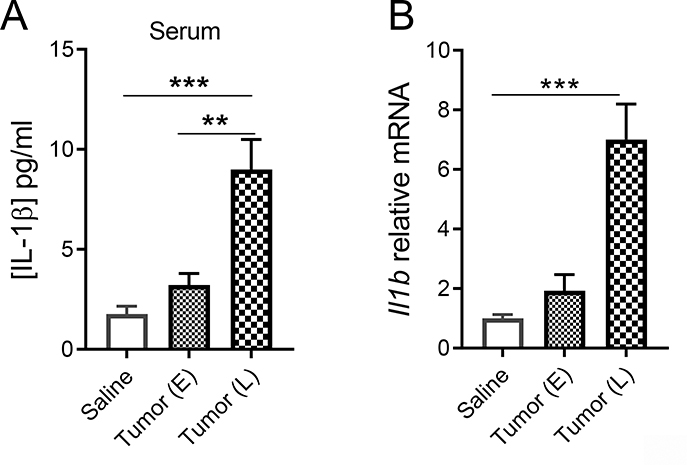
To determine whether mEERL tumor-induced pain is associated with neuroinflammation, we performed qPCR to measure the expression of cytokines and markers of neuroinflammation in the spinal cord. Il1b is the only gene upregulated in the spinal cord of HNCP mice at both the early and late stages of the disease (Figure 2). Furthermore, injection of mEERL cells also increases levels of IL-1β protein in the blood (Figure 3A) and Il1b mRNA in the liver (Figure 3B). This upregulation of IL-1β in the circulation and central nervous tissues is consistent with previous studies using this model 25,31.
Figure 2. mEERL tumor upregulates Il1b in the spinal cord.
Normalized mRNA expression levels of inflammatory markers in the spinal cord at 15 (A, E = early phase) and 30 (B, L = late phase) days after mERRL cells injection. A) Effect of mEERL cells on overall gene expression: 2-way ANOVA (injection factor) F(1, 48) = 1.69, p = 0.20. To analyze individual gene: Fisher’s correction for multiple tests saline vs mEERL Il1b p = 0.048, n = 5–6 mice /group. All other genes are not significantly affected by the mEERL tumor. B) Two-way ANOVA cancer effect F(1,66) = 21.0, p<0.0001 and gene x cancer interaction F(5, 66) = 10.7, p<0.0001, to analyze individual gene: Fisher’s correction for multiple tests saline vs mEERL Il1b p<0.0001, n = 6–7 mice /group. All other genes are not significantly affected by the mEERL tumor.
Figure 3. mEERL tumors increase systemic IL-1β.
A) Serum IL-1β assessed by ELISA at 15 (E = early) and 30 days (L = late). One-way ANOVA F(2, 12) = 15.9, p<0.0004, n = 5/group. Multiple comparisons: Saline vs E p = 0.91, Saline vs L p = 0.0005 and E vs L p = 0.0033. B) mRNA levels of Il1b in the liver at 15 and 30 days. One-way ANOVA F(2, 16) = 24.8, p<0.0001, n = 5–8/group. Multiple comparisons: Saline vs E p = 0.84, Saline vs L p<0.0001 and E vs L p = 0.0002.
Blockade of IL-1 signaling fails to affect HNCP
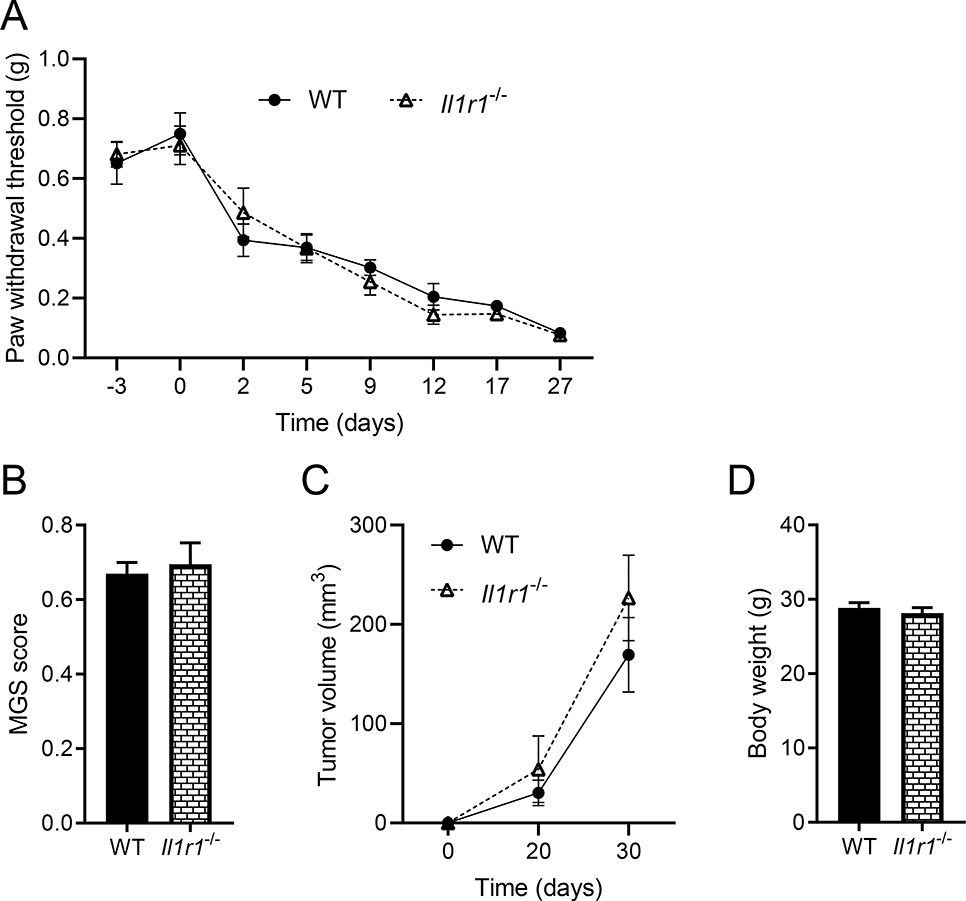
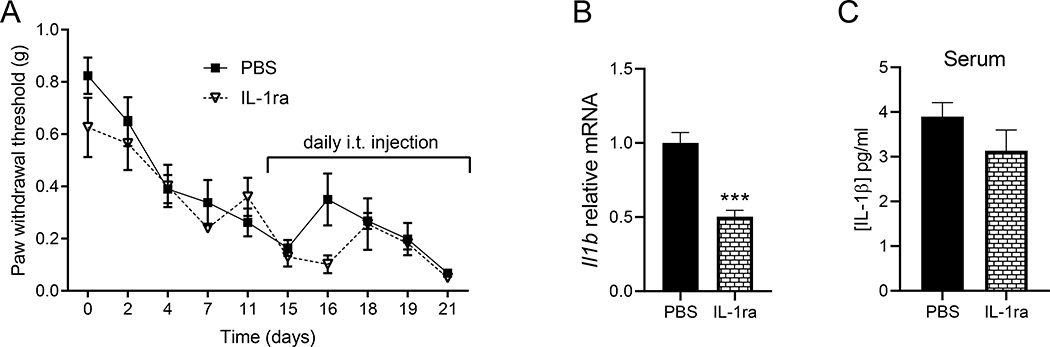
To test the effect of IL-1 signaling on HNCP, we injected mEERL cells in a different group of WT mice and Il1r1−/− mice. The lack of IL-1R1 does not alleviate pain hypersensitivity (Figure 4A) nor spontaneous pain (Figure 4B and Supplementary Figure 1B). Tumor development is also not affected by the lack of IL-1R1 (Figure 4C,D). These findings were unexpected given the contribution of IL-1 signaling in pain in inflammatory and neuropathic models 20–24. It might be possible that compensatory mechanisms take place in transgenic animals, to rule out this possibility, on days 15–21 tumor-bearing WT mice are treated with either intrathecal IL-1ra or PBS. Il-1ra has no effect on the pain threshold (Figure 5A). We confirmed the effectiveness of this antagonist as it significantly decreases Il1b expression compared to mice treated with PBS (Figure 5B). Because it is known that Il1b is upregulated in response to IL-1 receptor activation as a positive feedback mechanism 21. However, circulating IL-1β was not significantly affected (Figure 5C).
Figure 4. The lack of IL-1R1 does not affect mEERL tumor-induced pain.
A) Pain hypersensitivity measured by von Frey filaments is similar in WT (C57BL/6) and Il1r1−/− mice. Two-way ANOVA (genotype effect) F(1, 10) = 0.05, p = 0.81 (no significant difference between genotype). B) Facial grimacing in tumor-bearing mice is identical in WT (C57BL/6) and Il1r1−/− mice. C) mEERL tumor growth curve. D) Body weight at termination (n = 6 mice /group). No statistical difference between WT and Il1r1−/− mice.
Figure 5. Blocking IL-1 signaling does not alleviate HNCP.
A) Intrathecal injection of IL-1 receptor antagonist (IL-1ra) does not reduce mEERL tumor-induced pain hypersensitivity. IL-1ra was injected daily from day 15 to day 21, 2-way ANOVA (drug effect) F(1, 8) = 2.61, p = 0.15 (no significant difference between genotype), n = 5 mice/group. B) IL-1ra reduces the expression of Il1b in the spinal cord 21 days after cancer cells injection (unpaired t-test p = 0.0003, t = 6.02, df = 8). C) Serum IL-1β assessed by ELISA at 21 days (unpaired t-test p = 0.210, t = 1.36, df = 8), n = 5 mice/group.
Discussion
The main objective of this study is to establish a novel immunocompetent murine model of HNCP to study the potential effect of IL-1 signaling on HNCP. Previous studies used models with immunocompromised animals which lack CD8+ T cells that critically modulate chronic pain 15,32. Using a well-established syngeneic immunocompetent model of HNSCC, we show the rapid development of severe pain hypersensitivity and spontaneous pain in tumor-bearing mice which is consistent with clinical observations 2,6,7.
A striking finding is that both pain hypersensitivity and spontaneous pain are present before tumors reach a measurable size. Pain hypersensitivity, a very sensitive measure, is detectable 4 days after the injection of mEERL cells; such an early event suggests that a biological mechanism such as secreted factors by the tumor triggers the development of pain rather than tissue and/or nerve compression. Moreover, in this model, pain is not dependent on tumor size. To our knowledge, our study is also the first to use the MGS in a model of HNCP. In the late phase of the disease, facial grimacing induced by mEERL tumors is similar to that induced by CFA, a well-established model of severe inflammatory pain 28.
While neuroinflammation and IL-1 signaling often underlies chronic pain conditions 20–24, blocking IL-1 signaling in this model was insufficient in alleviating evoked- and spontaneous pain. Even if we targeted only IL-1 signaling, it is unlikely that neuroinflammation plays a role in this model because none of the inflammatory markers are upregulated, even at the terminal stage. Consistent with our findings, IL-1 signaling also does not contribute to cancer-induced fatigue 31 which is also in contrast with preclinical models of endotoxin/inflammation-induced fatigue 33.
The present data are in opposition to previous studies utilizing athymic murine model of HNCP in which neuroinflammation and cytokines play important roles 13,14. These discrepancies may be explained by the important role of T cells in pain modulation 15. We and others have shown that behavioral alterations such as pain and depression-like behavior in response to inflammation are critically regulated by T cells 28,32,34–36. One of the potential explanations is the production of anti-inflammatory mediators and/or endogenous opioid ligands by T cells that reverse inflammation-induced neuronal hyperexcitability 34,35.
The elevated levels of circulating and spinal IL-1β could result from multiple cellular sources; cancer cells and immune cells in response to the tumor25. While we demonstrate that IL-1 signaling does not contribute to HNCP, its role in this model remains undetermined. Even if IL-1β is the only cytokine upregulated, we cannot exclude a potential role of other proinflammatory cytokines.
Instead of neuroinflammation, the tumor might induce pain by altering the nervous system bioenergetic status37. Indeed, recent data support a strong link between mitochondrial function in sensory neurons and pain 38,39. Additionally, tumor-infiltrating nerves may also contribute to HNCP7. HNSSC are often highly innervated and intriguingly, they are mostly innervated by transient receptor potential vanilloid (TRPV)1-positive fibers 18. TRPV1 is both a marker of nociceptors and a nociception generator upon activation. The acidic tumor microenvironment may facilitate persistent activation of TRPV1 and chronic pain 40. Secreted factors by tumor cells such as neurotrophic factor may also contribute to HNCP but future studies using our immunocompetent syngeneic model will be required to address these questions 18,41.
In summary, we established a novel murine immunocompetent syngeneic model of HNCP, and we show that HNCP is not mediated by IL-1 signaling. Based on the present data, anti-inflammatory therapeutic strategies for HNCP may be limited in efficacy, and further investigation into the non-neuroinflammatory mechanisms of HNCP are necessary.
Supplementary Material
Key Points.
Question: Does IL-1 signaling contribute to HNCP?
Findings: In an immunocompetent syngeneic model, HNCP is independent of IL-1 signaling.
Meaning: Anti-inflammatory therapeutic strategies to HNCP may be limited in efficacy and it is critical to use immunocompetent model to assess the contribution of inflammation to HNCP.
Acknowledgement.
We thank Dr. Elisabeth G. Vichaya (Baylor University, Waco, TX) for expert advice on establishing the model and Sophie Laumet for technical assistance. We thank the Rita Allen Foundation Award in Pain (G.L.), College of Natural Science of Michigan State University (G.L.), and the National Institute of Health (P20GM103548) (P.D.V.) for supporting this work.
Funding: The present work is supported by the College of Natural Science of Michigan State University (G.L.), The Rita Allen Foundation Award in Pain (G.L.) and the National Institute of Health (P20GM103548) (P.D.V.).
Glossary of Terms
- cDNA
Complementary DNA
- CFA
Complete Freund’s Adjuvant
- HNCP
Head and Neck Cancer Pain
- HNSCC
Head and Neck Squamous cell carcinoma
- HPV
Human Papillomavirus
- IL1
Interleukin-1
- IL-1ra
Interleukin-1 Receptor Antagonist
- Il1r1−/−
Interleukin-1 receptor knockout
- mEERL
murine E6, E7, h-Ras, Luciferase
- MGS
Mouse Grimace Scale
- MSU
Michigan State University
- NGF
Nerve Growth Factor
- OPSCC
Oropharyngeal Squamous Cell Carcinoma
- TRPV
Transient Receptor Potential Vanilloid
- TNF-α
Tumor Necrosis Factor-α
Footnotes
Conflicts of Interests: NONE. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions.
References
- 1.van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi: 10.1093/annonc/mdm056 [DOI] [PubMed] [Google Scholar]
- 2.Bjordal K, Ahlner-Elmqvist M, Hammerlid E, et al. A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope. 2001;111(8):1440–1452. doi: 10.1097/00005537-200108000-00022 [DOI] [PubMed] [Google Scholar]
- 3.Marshall JA, Mahanna GK. Cancer in the differential diagnosis of orofacial pain. Dent Clin North Am. 1997;41(2):355–365. [PubMed] [Google Scholar]
- 4.Sato J, Yamazaki Y, Satoh A, et al. Pain may predict poor prognosis in patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(5):587–592. doi: 10.1016/j.tripleo.2010.11.033 [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, Hanna EE. Survival patterns in squamous cell carcinoma of the head and neck: Pain as an independent prognostic factor for survival. J Pain. 2014;15(10):1015–1022. doi: 10.1016/j.jpain.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connelly ST, Schmidt BL. Evaluation of pain in patients with oral squamous cell carcinoma. J Pain. 2004;5(9):505–510. doi: 10.1016/j.jpain.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 7.Salvo E, Campana WM, Scheff NN, et al. Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain. Published online July 8, 2020. doi: 10.1097/j.pain.0000000000001986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S-C, Yu W-P, Chu T-L, Hung H-C, Tsai M-C, Liao C-T. Prevalence and correlates of supportive care needs in oral cancer patients with and without anxiety during the diagnostic period. Cancer Nurs. 2010;33(4):280–289. doi: 10.1097/NCC.0b013e3181d0b5ef [DOI] [PubMed] [Google Scholar]
- 9.Lin Y-L, Lin I-C, Liou J-C. Symptom patterns of patients with head and neck cancer in a palliative care unit. J Palliat Med. 2011;14(5):556–559. doi: 10.1089/jpm.2010.0461 [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira KG, von Zeidler SV, Lamas AZ, et al. Relationship of inflammatory markers and pain in patients with head and neck cancer prior to anticancer therapy. Braz J Med Biol Res. 2014;47(7):600–604. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992 [DOI] [PubMed] [Google Scholar]
- 13.Scheff NN, Ye Y, Bhattacharya A, et al. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain. 2017;158(12):2396–2409. doi: 10.1097/j.pain.0000000000001044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Y, Scheff NN, Bernabé D, et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology. 2018;139:182–193. doi: 10.1016/j.neuropharm.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 15.Laumet G, Ma J, Robison AJ, Kumari S, Heijnen CJ, Kavelaars A. T Cells as an Emerging Target for Chronic Pain Therapy. Front Mol Neurosci. 2019;12:216. doi: 10.3389/fnmol.2019.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei Z-G, Ren X-H, Wang S-S, Liang X-H, Tang Y-L. Immunocompromised and immunocompetent mouse models for head and neck squamous cell carcinoma. Onco Targets Ther. 2016;9:545–555. doi: 10.2147/OTT.S95633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology. 2017;6(11):e1356148. doi: 10.1080/2162402X.2017.1356148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madeo M, Colbert PL, Vermeer DW, et al. Cancer exosomes induce tumor innervation. Nat Commun. 2018;9(1):4284. doi: 10.1038/s41467-018-06640-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams R, Lee DW, Elzey BD, Anderson ME, Hostager BS, Lee JH. Preclinical models of HPV+ and HPV- HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck. 2009;31(7):911–918. doi: 10.1002/hed.21040 [DOI] [PubMed] [Google Scholar]
- 20.Cohen SB, Moreland LW, Cush JJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63(9):1062–1068. doi: 10.1136/ard.2003.016014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cell Signal. 2019;53:212–223. doi: 10.1016/j.cellsig.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Laumet G, Zhou W, Dantzer R, et al. Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain Behav Immun. 2017;66:94–102. doi: 10.1016/j.bbi.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mailhot B, Christin M, Tessandier N, et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J Exp Med. 2020;217(9). doi: 10.1084/jem.20191430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vichaya EG, Vermeer DW, Christian DL, et al. Neuroimmune mechanisms of behavioral alterations in a syngeneic murine model of human papilloma virus-related head and neck cancer. Psychoneuroendocrinology. 2017;79:59–66. doi: 10.1016/j.psyneuen.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- 27.Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7(6):447–449. doi: 10.1038/nmeth.1455 [DOI] [PubMed] [Google Scholar]
- 28.Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. CD3+ T cells are critical for the resolution of comorbid inflammatory pain and depression-like behavior. Neurobiology of Pain. 2020;7:100043. doi: 10.1016/j.ynpai.2020.100043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laumet G, Bavencoffe A, Edralin JD, et al. Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain. Published online May 13, 2020. doi: 10.1097/j.pain.0000000000001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toni LS, Garcia AM, Jeffrey DA, et al. Optimization of phenol-chloroform RNA extraction. MethodsX. 2018;5:599–608. doi: 10.1016/j.mex.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossberg AJ, Vichaya EG, Christian DL, et al. Tumor-Associated Fatigue in Cancer Patients Develops Independently of IL1 Signaling. Cancer Res. 2018;78(3):695–705. doi: 10.1158/0008-5472.CAN-17-2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain. 2019;160(6):1459–1468. doi: 10.1097/j.pain.0000000000001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vichaya EG, Laumet G, Christian DL, et al. Motivational changes that develop in a mouse model of inflammation-induced depression are independent of indoleamine 2,3 dioxygenase. Neuropsychopharmacology. 2019;44(2):364–371. doi: 10.1038/s41386-018-0075-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basso L, Boué J, Mahiddine K, et al. Endogenous analgesia mediated by CD4(+) T lymphocytes is dependent on enkephalins in mice. J Neuroinflammation. 2016;13(1):132. doi: 10.1186/s12974-016-0591-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boué J, Blanpied C, Brousset P, Vergnolle N, Dietrich G. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J Immunol. 2011;186(9):5078–5084. doi: 10.4049/jimmunol.1003335 [DOI] [PubMed] [Google Scholar]
- 36.Laumet G, Edralin JD, Chiang AC-A, Dantzer R, Heijnen CJ, Kavelaars A. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology. 2018;43(13):2597–2605. doi: 10.1038/s41386-018-0154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell MI, Engelbrecht A-M. Metabolic hijacking: A survival strategy cancer cells exploit? Crit Rev Oncol Hematol. 2017;109:1–8. doi: 10.1016/j.critrevonc.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 38.Joseph EK, Levine JD. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain. 2006;121(1–2):105–114. doi: 10.1016/j.pain.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 39.Krukowski K, Ma J, Golonzhka O, et al. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 2017;158(6):1126–1137. doi: 10.1097/j.pain.0000000000000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julius D TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833 [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Demir IE, D’Haese JG, et al. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis. 2014;35(1):103–113. doi: 10.1093/carcin/bgt312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.