Abstract
Background:
Preoperative anemia is common in cardiac surgery, yet there is limited data describing the role of sex in the associations between anemia and clinical outcomes. Understanding these relationships may guide preoperative optimization efforts.
Methods:
This is an observational cohort study of adults undergoing isolated coronary artery bypass grafting or single or double valve surgery from 2008–2018 at a large tertiary medical center. Multivariable regression assessed the associations between preoperative hemoglobin concentrations and a primary outcome of postoperative acute kidney injury (AKI) and secondary outcomes of perioperative red blood cell transfusion (RBCs), reoperation, vascular complications (stroke, pulmonary embolism, myocardial infarction), and hospital length of stay (LOS). Each outcome was a single regression model, using interaction terms to assess sex-specific associations between hemoglobin and outcome.
Results:
4117 patients were included (57% male). Linear splines with sex-specific knots (13 g/dL female, 14 g/dL male) provided the best overall fit for preoperative hemoglobin and outcome relationships. In females, each 1 g/dL decrease in hemoglobin below 13 g/dL was associated with increased odds of AKI (OR 1.49, 95% CI [1.23–1.81]; p<0.001), and there was no significant association between hemoglobin per 1 g/dL above 13 g/dL and AKI (0.90 [0.56–1.45]; p=0.67). The association between hemoglobin and AKI in men did not meet statistical significance (1.10 [0.99–1.22]; p=0.076, per 1 g/dL decrease below 14 g/dL; 1.00 [0.79–1.26]; p=0.98 for hemoglobin per 1 g/dL above 14 g/dL). In females, lower preoperative hemoglobin (per 1 g/dL decrease below 13 g/dL) was associated with increased odds of RBC transfusion (2.90 [2.33–3.60]; p<0.001), reoperation (1.27 [1.11–1.45]; p<0.001) and a longer hospital LOS (multiplicative increase in geometric mean 1.05 [1.03–1.07]; p<0.001). In males, preoperative hemoglobin (per 1 g/dL decrease below 14 g/dL) was associated with increased odds of perioperative RBCs (2.56 [2.27–2.88]; p<0.001) and longer hospital LOS (multiplicative increase in geometric mean 1.02 [1.01–1.04] days; p<0.001) but not reoperation (0.94 [0.85–1.04]; p=0.256). Preoperative hemoglobin per 1 g/dL above 13 g/dL in females and 14 g/dL in males and was associated with lower odds of RBCs transfusion (0.57 [0.47–0.69]; p<0.001 and 0.74 [0.60–0.91]; p=0.005, respectively).
Conclusions:
Preoperative anemia was associated with inferior clinical outcomes after cardiac surgery. The associations between hemoglobin and outcomes were distinct for females and males, with different spline knot points identified (13 g/dL and 14 g/dL, respectively). Clinicians should consider data-driven approaches to determine preoperative hemoglobin values associated with increasing risk for adverse perioperative outcomes across sexes.
INTRODUCTION
The prevalence of preoperative anemia in cardiac surgery is high, ranging between 16%–54% (1,2). Importantly, observational studies have consistently identified preoperative anemia as a risk factor for intraoperative red blood cell transfusion and postoperative complications, including acute kidney injury (AKI), stroke, infection, and mortality (1,2). Patient sex may also influence perioperative outcomes after cardiac surgery (3–7), and female sex has been identified as an independent risk factor for preoperative anemia (8).
Notably, data assessing sex differences in the relationships between preoperative hemoglobin concentrations and clinical outcomes in cardiac surgery are limited. Understanding the role that sex may play in the associations between preoperative anemia and clinical outcomes may ultimately guide preoperative optimization prior to cardiac surgery. It is also important to consider that the typical hemoglobin cut-off values utilized to define anemia vary by sex (i.e. <12 g/dL in women, <13 g/dL in men); yet it is unclear if these thresholds, which were derived by expert consensus using epidemiological data from non-surgical patients more than 40 years ago (9), represent the optimal hemoglobin values to define a level of preoperative anemia that may be associated with inferior clinical outcomes in contemporary surgical practice. Indeed, some experts suggest targeting hemoglobin of 13 g/dL irrespective of patient sex (10).
This study investigates the associations between preoperative hemoglobin concentrations, patient sex, and postoperative clinical outcomes among a large cohort of adult cardiac surgery patients. We hypothesized that preoperative anemia would be associated with inferior postoperative clinical outcomes, and that these associations would differ by sex. In addition, this study assesses optimal hemoglobin values for defining preoperative anemia in men and women undergoing cardiac surgery by incorporating clinical outcome data.
METHODS
This was a retrospective cohort study conducted under the approval of the local Institutional Review Board with waived requirement of written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used in study design and conduct (11).
Study cohort:
The cohort included adults (≥18 years) undergoing elective cardiac surgery between May 1st, 2008 and May 1st, 2018 at a tertiary care academic medical center. Surgery types included: isolated coronary artery bypass grafting (CABG) and single or double valve surgeries (mitral, tricuspid, and aortic) with or without minor secondary procedures (i.e. ligation of left atrial appendage, patent foramen ovale closure, and Maze procedures). These common surgery types were selected a priori to improve generalizability and minimize bias related to increasingly complex surgical techniques, recognizing that outcomes may differ based upon surgical complexity. Exclusion criteria included: other cardiac procedures (i.e. off-pump CABG, robotic procedures, pulmonary valve procedures, and combined CABG and valve surgeries), prior inclusion (each patient only included once), preoperative use of extracorporeal membrane oxygenation, ventricular assist devices, and other forms of mechanical circulatory support (i.e. intra-aortic balloon pump), hospitalization prior to surgery, emergency surgery, intraoperative fresh whole blood use (recognizing that anemic patients would not be eligible for this therapy), American Society of Anesthesiologists Physical Status Classification (ASA) VI, and those with previous denial of authorization to utilize their medical records for observational research.
The study cohort was identified using the Perioperative DataMart, an institutional DataMart containing detailed perioperative clinical information (i.e. demographics, clinical characteristics, transfusion practices, laboratory information, and surgical features of each patient undergoing surgery in our institution) (12). Any information not readily available in the Perioperative DataMart were extracted using the Advanced Cohort Explorer; another institutional software containing patient-specific clinical data outside surgical environments (13). Both institutional databases undergo routine validation with accuracy of extracted data superior to that of manual extraction (14).
Exposures:
The primary exposure of interest was the preoperative hemoglobin defined as the closest hemoglobin value obtained prior to surgery, which must have been obtained within 30 days of the surgery. Covariates included as potential confounding variables were: demographic features (age, sex, body surface area), comorbidities (history of chronic kidney disease, myocardial infarction, cerebrovascular accident, diabetes mellitus, and smoking history (never versus current/former)), laboratory values (preoperative creatinine and platelet count), preoperative illness severity (New York Heart Association (NYHA) Functional Classification and left ventricular ejection fraction), and surgical features as a marker of surgical complexity including procedure type (CABG vs. valve) and intraoperative cardiopulmonary bypass (CPB) time.
Outcomes:
The primary outcome of interest was the presence of AKI, assessed as a binary outcome recognizing that any level of postoperative AKI is clinically relevant, within 7 days of intensive care unit (ICU) admission. AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria (Supplemental Table 1) (15), and AKI stages are reported for both males and females. Secondary outcomes included perioperative transfusions of allogeneic red blood cells (RBCs), reoperation (OR) within 7 days, vascular complications (i.e. composite rate of pulmonary embolism, postoperative stroke and myocardial infarction) within 7 days, hospital length of stay (LOS), and 30-day mortality.
Postoperative pulmonary embolism was diagnosed based on the presence of emboli in the pulmonary vasculature in computed tomography pulmonary angiogram scans. Stroke was diagnosed clinically according to review of all postoperative Neurology consultation notes. Myocardial infarction was defined as an elevated troponin with at least one of the following: echocardiographic evidence of new regional wall motion abnormalities deemed to be independent of post-surgical changes with congruent documentation of myocardial ischemia in a clinical note, new electrocardiographic changes consistent with ischemia with congruent documentation of myocardial ischemia in a clinical note, or angiographically-diagnosed occlusion of a native coronary artery or new coronary bypass graft (16).
Transfusion practices:
The perioperative transfusion of blood products during the study timeframe were at the discretion of the anesthesiologist. In our institution, each transfusion is conducted according to our institutional guidelines that were available at the time of the study. Indications for perioperative RBCs transfusion included: hemoglobin <7 g/dL; hemoglobin <8 g/dL with hypotension or unexplained tachycardia; hemoglobin <8 g/dL in those with chest pain, stable coronary artery disease, or heart failure symptoms; hemoglobin <10 g/dL in the presence of acute coronary syndromes; or at any hemoglobin value for patients with rapid bleeding with marked cardiovascular instability, recognizing that pre-transfusion hemoglobin values are often impractical to obtain and do not provide an accurate assessment of RBCs mass or tissue oxygenation needs in this setting.
During CPB, transfusion of RBCs was conducted with additional input from the surgeon and perfusionist according to the patients’ hemodynamic stability, hemoglobin values, and institutional guidelines. Following completion of CPB, institutionally-endorsed and validated algorithms were utilized to guide transfusion practices (17). Postoperative transfusions of blood products were at the discretion of the intensive care unit anesthesiologist in agreement with the primary surgical service in accordance with institutional transfusion guidelines (16).
Statistical Analyses:
Patient, surgical characteristics, and outcomes were summarized according to pre-operative anemia status defined according to the World Health Organization (WHO) (moderate/severe: <10 g/dL; mild: 10–12 g/dL and 10–13 g/dL for females and males respectively), and none (9). Continuous variables were summarized as median (interquartile range) and categorical variables were summarized as number (percentage). Unadjusted outcomes were compared across WHO anemia categories using Kruskal-Wallis tests for continuous outcomes and Pearson chi-square tests for categorical outcomes.
Rather than relying solely on predetermined hemoglobin thresholds to define anemia (i.e. WHO definitions, as described above), a primary goal of this investigation was to use perioperative outcome data to identify optimal preoperative hemoglobin values associated with increasing risk for adverse outcomes across sexes. To assess the associations between continuous preoperative hemoglobin concentrations and clinical outcomes, linear or logistic models were fit as appropriate with different parameterizations (functional forms) of continuous preoperative hemoglobin. Our goal was to describe the association flexibly while balancing interpretability. Models assessed were characterized by the assumptions of linear association, sex-specific linear association (allowing interaction between the linear functional form of continuous hemoglobin and sex), piecewise linear spline association with a single spline knot, sex-specific piecewise linear spline association, restricted cubic splines, and sex-specific restricted cubic splines. Piecewise linear spline parameterizations with knot points of 10, 11, 12, 13, and 14 were assessed, and sex-specific piecewise linear spline parameterizations were assessed with all 25 possible combinations of knot points for males and females. In addition, the models assuming truncation above each given knot point were assessed. Restricted cubic splines were defined with 5 knots at the 5th, 25th, 50th, 75th, and 95th percentiles. In brief, piecewise linear and restricted cubic splines allow for non-linear functional forms for the association between continuous hemoglobin and outcomes (18). A piecewise linear spline allows for estimation of an association up to the knot point and association above the knot point with the two associations connected at the knot. Similarly, a restricted cubic spline allows for a cubic association between knots and is restricted as a linear association in the tails (outside the first and last knot). For each model, the Akaike information criterion (AIC) statistic was obtained, and the final functional form was objectively selected based on the average rank of AIC statistics across all outcomes (i.e. the functional form for sex and continuous hemoglobin which reasonably fit all the outcomes). A linear spline model with sex-specific knots fit the data well, and this selected functional form was used for all regression models. Results are described per g/dL hemoglobin below the knot point and per g/dL hemoglobin above the knot point.
While selection by AIC suggests good overall fit across the outcomes by including non-linear functional forms (linear splines) and including sex-specific associations, this may not be optimal for all outcomes. For each outcome, we report p-values assessing whether there is evidence the association above vs below the knot point differs. Further, we report the model AIC values (lower AIC indicating better fit) of our selected model in comparison to a simpler model that assumes a common functional form for both males and females (sex-independent association).
Regression models were adjusted for covariates selected a priori by investigators as potential confounders, as previously described. Outcomes of AKI, receipt of perioperative RBCs, return to the OR, and a composite vascular complications were assessed with logistic regression and results reported as odds ratio per g/dL hemoglobin, with 95% confidence intervals (CI). In models for length of stay, the log transformation was applied to the outcome to improve normality of residuals and the results were transformed to report the association as the multiplicative increase in geometric mean per g/dL hemoglobin. For each outcome, the estimated regression association is plotted to illustrate the fitted model for the sex-specific association with continuous hemoglobin. First, we plot the estimated adjusted odds ratio with 95% confidence interval, comparing to a reference value of 10 g/dL. Second, we plot the marginal probability of outcome by averaging the expected outcomes over the distribution of observed covariates for a range of hemoglobin values.
A planned secondary analysis was done to assess the association between WHO anemia status and outcomes rather than employing data-driven hemoglobin thresholds. Similar to the main analyses, sex-specific associations were estimated from a single regression model for each outcome using interaction terms. P-values for the interaction of sex and anemia status assess whether there is evidence that the association differs by sex. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A two-side p-value <0.05 was used to define statistical significance.
No a priori power and sample size calculation was done. We believed 10 years of institutional data prior to a change in our electronic medical record system would provide a sufficiently sized, yet contemporary, sample for analyses. A sample size of 4000 provides 91% power to detect a clinically relevant odds ratio of 1.20 per standard deviation in hemoglobin for the outcome of AKI with 10% prevalence, based on a logistic regression model adjusted for other covariates (R-squared between other covariates and hemoglobin = 0.10) and assuming normally distributed hemoglobin.
RESULTS
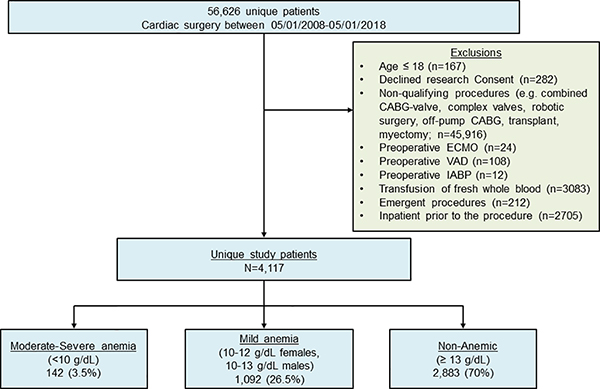
4117 unique patients met the inclusion criteria and were included (Figure 1). Baseline demographics, clinical characteristics and surgical features are provided in Table 1. The majority of patients were non-anemic prior to surgery (70.0%, n=2883) followed by those with mild (26.5%, n=1092) and moderate/severe anemia (3.5%, n=142). Anemic patients were more likely to have a history of chronic kidney disease, myocardial infarction, stroke or diabetes compared to non-anemic patients.
Figure 1. Study cohort flow diagram.
CABG – Coronary Artery Bypass Graft; ECMO – Extracorporeal Membrane Oxygenation; IABP – Intra-aortic balloon pump; VAD – ventricular assist device
Table 1 –
Patient characteristics according to preoperative anemia category*
| None (N=2883) | Mild (N=1092) | Moderate severe (N=142) | |
|---|---|---|---|
| Age, years | 68.5 (59.3, 76.3) | 72.6 (63.7, 79.2) | 69.6 (63.0, 76.6) |
| Sex | |||
| Female | 1349 (47%) | 373 (34%) | 65 (46%) |
| Male | 1534 (53%) | 719 (66%) | 77 (54%) |
| BSA, m2 | 1.9 (1.8, 2.1) | 2.0 (1.8, 2.1) | 1.9 (1.8, 2.0) |
| Ejection fraction, percent; n=2708/1032/135 | 62 (56, 66) | 61 (55, 66) | 62 (56, 65) |
| NYHA class, n=2367/922/126 | |||
| 1 | 360 (15%) | 132 (14%) | 19 (15%) |
| 2 | 1015 (43%) | 367 (40%) | 55 (44%) |
| 3 | 887 (37%) | 384 (42%) | 50 (40%) |
| 4/5 | 105 (4%) | 39 (4%) | 2 (2%) |
| Smoking history | |||
| Current | 246 (9%) | 84 (8%) | 12 (8%) |
| Former | 1083 (38%) | 484 (44%) | 70 (49%) |
| Never | 1554 (54%) | 524 (48%) | 60 (42%) |
| History of moderate/severe kidney disease | 232 (8%) | 317 (29%) | 59 (42%) |
| History of MI | 270 (9%) | 172 (16%) | 20 (14%) |
| History of CVA | 215 (7%) | 139 (13%) | 23 (16%) |
| History of DM | 613 (21%) | 391 (36%) | 59 (42%) |
| Preoperative hemoglobin, g/dL | 13.8 (13.2, 14.5) | 11.7 (11.0, 12.2) | 9.3 (8.9, 9.7) |
| Preoperative creatinine, mg/dL | 1.0 (0.8, 1.1) | 1.1 (0.9, 1.4) | 1.2 (0.9, 2.0) |
| Preoperative platelet count, x 109/L | 209 (176, 248) | 205 (166, 260) | 213 (154, 287) |
| Type of procedure | |||
| Valve | 2043 (71%) | 734 (67%) | 103 (73%) |
| CABG | 840 (29%) | 358 (33%) | 39 (27%) |
| Total Bypass Time, minutes | 78 (55, 102) | 84 (63, 111) | 83 (62, 112) |
Preoperative anemia defined as moderate/severe (<10 g/dL), mild (10 to 12 g/dL and 10 to 13 g/dL for females and males respectively). Values are median (Q1, Q3) for continuous variables and number (percent) for categorical variables. When not all data are available, numbers with complete information are presented.
BSA = body surface area; NYHA = New York Heart Association; MI = myocardial infarction; CVA = cerebrovascular accident; DM = diabetes mellitus.
In unadjusted analyses, anemic patients were more likely to experience AKI compared to non-anemic patients (18% vs. 8%; Table 2). Rates of Stage I, II, and III AKI among patients with preoperative anemia were 11%, 4%, and 3% compared to 6%, 2%, and 0% for non-anemic patients. Vascular complications were higher for patients with preoperative anemia (5% vs. 3%). Additionally, hospital LOS and readmission rates were greater in anemic versus non-anemic patients. Unadjusted outcomes by sex and anemia severity are provided in Supplemental Table 2.
Table 2 –
Hospital outcomes according to preoperative anemia status*
| Outcome | None (N=2883) | Mild (N=1092) | Moderate/severe (N=142) | P-value |
|---|---|---|---|---|
| Acute kidney injury (AKI) | 218 (7.6%) | 188 (17.2%) | 31 (21.8%) | <.001 |
| AKI stage | <.001 | |||
| None | 2665 (92.4%) | 904 (82.8%) | 111 (78.2%) | |
| 1 | 159 (5.5%) | 119 (10.9%) | 15 (10.6%) | |
| 2 | 46 (1.6%) | 44 (4.0%) | 8 (5.6%) | |
| 3 | 13 (0.5%) | 25 (2.3%) | 8 (5.6%) | |
| Any perioperative RBCs | 1166 (40%) | 849 (78%) | 138 (97%) | <.001 |
| Return to OR | 437 (15.2%) | 205 (18.8%) | 27 (19.0%) | 0.015 |
| Vascular complications† | 99 (3.4%) | 59 (5.4%) | 8 (5.6%) | 0.012 |
| ICU length of stay, days | 1.0 (0.9, 1.7) | 1.1 (0.9, 2.0) | 1.1 (1.0, 2.2) | <.001 |
| Hospital length of stay, days | 5.8 (4.9, 7.5) | 6.7 (5.7, 8.1) | 6.9 (5.7, 9.7) | <.001 |
| Hospital mortality | 4 (0.13%) | 4 (0.37%) | 0 (0%) | 0.300 |
| Hospital readmit within 30 days | 179 (6.2%) | 86 (7.9%) | 16 (11.3%) | 0.018 |
| Estimated blood loss, mL; n=2867/1087/141 | 1000 (663, 1292) | 922 (600, 1294) | 1004 (728, 1500) | 0.020 |
Values are number (percent) and median (Q1, Q3) for categorical variables and continuous variables respectively. Numbers with available estimated blood loss are presented. P-values are from Kruskal-Wallis tests for continuous outcomes and Pearson chi-square tests for categorical outcomes.
Vascular complications included myocardial infarction, pulmonary embolism, and stroke.
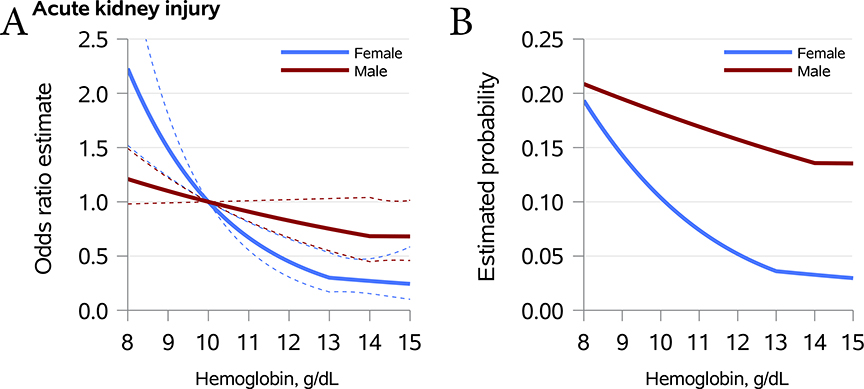
The fully adjusted associations between hemoglobin values (per 1 g/dL) and clinical outcomes by sex are provided in Table 3. Graphical representations are provided for AKI in Figure 2 and for other outcomes as supplemental digital content (Supplemental Figures 1–4). In females, the knot point for hemoglobin was set at 13 g/dL whereas in males the knot point was set at 14 g/dL based upon the model selection criteria described above. For females, each 1 g/dL decrease in the hemoglobin value below 13 g/dL was associated with higher odds of AKI (1.49 [1.23–1.81]; p<0.001). There was no statistically significant association between hemoglobin and AKI in men (1.10 [0.99–1.22]; p=0.076, for each 1 g/dL decrease in the hemoglobin value below 14 g/dL).
Table 3 -.
Associations between preoperative hemoglobin and outcomes according to sex
| Males |
Females |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hb less than 14* | Hb above 14* | Hb less than 13* | Hb above 13* | |||||
| Outcome | Estimate (95% CI) per g/dL decrease | p | Estimate (95% CI) per g/dL increase | p | Estimate (95% CI) per g/dL decrease | p | Estimate (95% CI) per g/dL increase | p |
| Acute kidney injury | 1.10 (0.99 to 1.22) | 0.076 | 1.00 (0.79 to 1.26) | 0.980 | 1.49 (1.23 to 1.81) | <.001 | 0.90 (0.56 to 1.45) | 0.668 |
| Any perioperative RBCs | 2.56 (2.27 to 2.88) | <.001 | 0.74 (0.60 to 0.91) | 0.005** | 2.90 (2.33 to 3.60) | <.001 | 0.57 (0.47 to 0.69) | <.001** |
| Return to OR | 0.94 (0.85 to 1.04) | 0.256 | 0.76 (0.60 to 0.97) | 0.030** | 1.27 (1.11 to 1.45) | <.001 | 1.21 (0.98 to 1.49) | 0.074** |
| Vascular complications | 1.04 (0.87 to 1.23) | 0.681 | 0.93 (0.61 to 1.42) | 0.734 | 1.26 (0.96 to 1.67) | 0.101 | 1.54 (1.02 to 2.32) | 0.038** |
| Hospital length of stay† | 1.02 (1.01 to 1.04) | <.001 | 0.97 (0.95 to 0.99) | 0.009 | 1.05 (1.03 to 1.07) | <.001 | 1.02 (1.00 to 1.05) | 0.097** |
Results are from multivariable piecewise linear or logistic regression models. Estimates are odds ratios unless noted and reflect the sex-specific increase in odds associated with each 1 g/dL change (i.e. increase or decrease as noted) in hemoglobin within the given range. For each outcome, a model was fit with covariates, piecewise linear splines for males with knot at 14 g/dL, and piecewise linear splines for females with knot at 13 g/dL. Estimates for males and females are estimated from the same model. Covariables included type of surgery (valve vs. CABG), age, sex, BSA, smoking history, history of moderate to severe kidney disease, MI, stroke, diabetes, preoperative creatinine, platelets, and cardio-pulmonary bypass duration.
Indicates significant evidence (p<0.05) to suggest a difference in the relationship above vs below the knot point. Tests of whether associations differed above and below the knot point for each sex were performed. For males: p=0.540, <.001, 0.030, 0.887, and 0.607 for outcomes Acute kidney injury, Any perioperative RBCs, Return to OR, Vascular complications, and Hospital length of stay, respectively. For females: p=0.323, 0.004, 0.004, 0.027, and <.001 for outcomes Acute kidney injury, Any perioperative RBCs, Return to OR, Vascular complications, and Hospital length of stay, respectively.
The current ‘full’ model with sex-specific linear splines and different sex-specific knot points was compared to a reduced model assuming no sex-specific associations and a common knot at 13g/dL using Akaike Information Criterion (where lower indicates better fit). The current model suggested better fit for AKI (AIC=2410 vs 2417), Return to OR (3599 vs 3610), Vascular Complications (1262 vs 1263), and length of stay (2062 vs 2071), but not for Any perioperative RBCS (AIC=4344 vs 4343).
Estimates reflect the sex-specific multiplicative increase in geometric mean associated with each 1 g/dL change (i.e. increase or decrease as noted) in hemoglobin within the given range.
Figure 2. Adjusted odds and expected proportion of postoperative acute kidney injury by preoperative hemoglobin stratified by sex.
Panel A – Adjusted odds ratio (95% CI) for AKI relative to a reference hemoglobin of 10 g/dL.
Panel B – Expected proportion of AKI as a marginal estimate average over the observed covariate distribution.
In females, each 1 g/dL decrease in the hemoglobin value below 13 g/dL was associated with perioperative RBC transfusion (2.90 [2.33–3.60]; p<0.001), reoperation (1.27 [1.11–1.45]; p<0.001) and a longer hospital LOS (multiplicative increase in geometric mean 1.05 [1.03–1.07]; p<0.001). Similarly, each 1 g/dL decrease in the hemoglobin value below 14 g/dL in males was associated with increased odds of perioperative RBC transfusion (OR 2.56 [95% CI 2.27–2.88]; p<0.001) and longer hospital LOS (multiplicative increase in geometric mean 1.02 [1.01–1.04] days; p<0.001) but not reoperation.
At hemoglobin values greater than 13 g/dL in females, each g/dL increase in hemoglobin was associated with increased odds of vascular complications (1.54 [1.02–2.32]; p=0.038) and decreased odds of perioperative RBC transfusions (0.57 [0.47, 0.69]; p<.001). In males with hemoglobin values greater than 14 g/dL, each g/dL increase in hemoglobin was associated with decreased odds of perioperative RBCs transfusion (0.74 [0.60–0.91]; p=0.005), reoperation (0.76 [0.60–0.97]; p=0.030) and shorter hospital length of stay (multiplicative increase in geometric mean 0.97 [0.95–0.99] days; p=0.009). When assessing whether the associations between hemoglobin and outcomes differed by sex, as assessed through linear splines with sex-specific knot points (13 g/dL females, 14 g/dL male), we compared to a reduced model with a common linear spline knot at 13 g/dL for both sexes; the sex-specific model provided superior fit for 4 out of 5 outcomes, including AKI, reoperation, vascular complications, and hospital length of stay (Table 3, footnote).
The associations between anemia status, using previously published definitions (9), and outcomes for males and females are presented in Table 4. For female patients, both mild and moderate/severe anemia were associated with increased odds of AKI (2.64 [1.63–4.27]; p<0.001, and 4.50 [2.17–9.31]; p<0.001, respectively) and perioperative RBC transfusion (7.30 [5.10–10.45)]; p<0.001, and 11.57 [4.10–32.64]; p<0.001). For male patients with mild anemia, the odds of AKI and perioperative RBCs transfusion were 1.45 (1.11–1.88) and 5.15 (4.16–6.37) times higher, respectively, when compared to non-anemic patients (p=0.006 and p<0.001, respectively). Similar associations were seen with moderate/severe preoperative anemia, though the association with AKI was not significant (p=0.073). In females, the odds of reoperation were higher in those with mild (1.53 [1.13–2.05]; p=0.005) but not moderate/severe anemia. Anemia was not associated with reoperation in males. Compared to the non-anemic reference group, females with moderate/severe but not mild anemia had a longer hospital LOS (multiplicative increase 1.28 (1.19–1.39); p<0.001). Male patients with mild and moderate/severe anemia had significantly longer hospital LOS when compared to non-anemic patients (multiplicative increase 1.08 [1.05 to 1.11] days; p<0.001, and 1.34 [1.23–1.46] days; p<0.001, respectively). The associations between anemia status and outcomes differed according to sex for AKI (p=0.032), reoperation (p=0.040), and hospital length of stay (p<0.001) but not for perioperative RBC transfusion (p=0.249) and vascular complications (p=0.851).
Table 4 -.
Associations between preoperative anemia status and outcomes according to sex
|
Males |
Females |
|||||||
|---|---|---|---|---|---|---|---|---|
| Moderate/severe§ | Mild§ | Moderate/severe§ | Mild§ | |||||
| Outcome | Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p |
| Acute kidney injury | 1.75 (0.95 to 3.23) | 0.073 | 1.45 (1.11 to 1.88) | 0.006 | 4.50 (2.17 to 9.31) | <.001 | 2.64 (1.63 to 4.27) | <.001 |
| Any perioperative RBCs‡ | - | - | 5.15 (4.16 to 6.37) | <.001 | 11.57 (4.10 to 32.64) | <.001 | 7.30 (5.10 to 10.45) | <.001 |
| Return to OR | 0.90 (0.47 to 1.70) | 0.736 | 0.94 (0.73 to 1.21) | 0.639 | 1.29 (0.69 to 2.42) | 0.431 | 1.53 (1.13 to 2.05) | 0.005 |
| Vascular complications | 1.46 (0.55 to 3.89) | 0.444 | 1.26 (0.81 to 1.95) | 0.302 | 0.90 (0.20 to 4.00) | 0.886 | 1.15 (0.60 to 2.20) | 0.672 |
| Hospital length of stay** | 1.34 (1.23 to 1.46) | <.001 | 1.08 (1.05 to 1.11) | <.001 | 1.28 (1.19 to 1.39) | <.001 | 1.04 (1.00 to 1.07) | 0.058 |
Results are from multivariable linear or logistic regression models. Estimates are odds ratios unless noted and reflect the sex-specific increase in odds associated with the given anemia category relative to patients without anemia. Moderate/severe anemia defined by hemoglobin <10 g/dL. Mild anemia defined by hemoglobin 10–12 g/dL (female) and 10–13 g/dL (males). For each outcome, a model was fit with covariates, categorical pre-operative anemia variable, and the sex by categorical pre-operative anemia interaction term. Covariables included type of surgery (valve vs. CABG), age, sex, BMI, smoking history, history of moderate to severe kidney disease, MI, stroke, diabetes, preoperative creatinine and platelet count, and cardio-pulmonary bypass duration.
To test whether the effect of WHO anemia category differed according to sex, the sex by WHO anemia category interaction term was assessed for each model. The two degree of freedom p-values associated with the interaction term were 0.032, 0.249, 0.040, 0.851, and <.001 for outcomes Acute kidney injury, Any perioperative RBCs, Return to OR, Vascular complications, and Hospital length of stay, respectively.
Effect of moderate/severe anemia is not estimable. All men classified as moderate to severely anemic required perioperative RBCs.
Estimates reflect the sex-specific multiplicative increase in geometric mean associated with the given anemia category relative to patients without anemia.
DISCUSSION
In more than 4000 patients undergoing cardiac surgery, preoperative anemia was associated with inferior clinical outcomes, including higher rates of AKI, perioperative RBC transfusion, reoperation, and longer hospital LOS. The associations between preoperative hemoglobin concentrations and most perioperative outcomes, including AKI, reoperation, vascular complications, and hospital LOS, differed between females and males, including selection of different knot points for the non-linear association (13 g/dL and 14 g/dL, respectively). Notably, the associations between preoperative hemoglobin concentrations and several clinical outcomes including AKI and reoperation seemed to be stronger for females than males. Importantly, the associations between preoperative hemoglobin and perioperative RBC transfusion were similar for both males and females, suggesting that clinicians are unlikely to consider patient sex when making transfusion decisions informed by perioperative hemoglobin concentrations.
Prior studies have shown that preoperative anemia is common and is associated with increased transfusion rates in cardiac surgery (19–21), which in turn is associated with inferior clinical outcomes including increased rates of AKI, atrial fibrillation, pulmonary complications, infection, and mortality (22,23). Additionally, there is positive a dose–response association between the number of units of RBCs transfused and postoperative morbidity (22,23). Importantly, clinical trials have shown transfusion reductions and improvement in clinical outcomes with preoperative anemia evaluation and treatment (23–30). Consistent with previous studies, preoperative anemia was highly prevalent (30%) in our cohort and was associated with inferior clinical outcomes. Importantly, female sex is a strong predictor for preoperative anemia (8). Women also experience higher rates transfusion and reoperation in cardiac surgery (31). Indeed, we found that increasing severity of preoperative anemia in females was associated with higher odds of receiving perioperative RBCs and undergoing surgical reoperation. This further highlights the importance of timely preoperative assessment of anemia and associated efforts to optimize hemoglobin recovery before elective cardiac surgery.
AKI is common after cardiac surgery, with previous studies noting rates of AKI that are approximately two to three times higher in those with preoperative anemia (2,33–35). In our study, increasing severity of preoperative anemia, utilizing both data-driven and WHO definitions for anemia, was strongly associated with AKI. In addition, the association between anemia severity and AKI seemed to be stronger for females than males, with each g/dL decrease in hemoglobin below 13 g/dL associated with an approximate 50% increase in the odds of developing postoperative AKI. This suggests that females might be more susceptible than males to anemia-mediated effects on postoperative kidney function, though further work is clearly warranted to confirm these associations and to explore the mechanisms for anemia-associated renal dysfunction. In addition to preoperative anemia, nadir intraoperative hemoglobin concentrations have been associated with clinical outcomes in cardiac surgery, and recent observational studies have noted that sex may influence these associations (36–38).
It is important to note that hemoglobin concentrations remain the most widely accepted laboratory parameter for the diagnosis of anemia even though they are not true markers of RBC mass or tissue oxygen delivery. The traditional definitions of anemia, including the widely accepted WHO criteria, are derived from the distribution of hemoglobin values in epidemiologic studies and not by the clinical and physiologic impact of those values (9,10). Therefore, data relating hemoglobin concentrations used to define anemia with clinical outcomes are lacking. Given this, our goal was to utilize robust clinical data to identify hemoglobin values best suited for defining preoperative anemia in accordance with their associations with perioperative outcomes. Importantly, the associations between hemoglobin concentrations and outcomes in both sexes were not linear, but rather followed unique and seemingly linear associations above and below values of 13 g/dL in females and 14 g/dL in males. We believe that it is important to revisit currently utilized definitions of anemia in clinical practice through data-driven approaches and to consider the role of sex in the determination of optimal hemoglobin targets prior to elective cardiac surgery. An optimal target would consider and balance the associations between hemoglobin and several important clinical outcomes. In our cohort, the data suggest little association with the primary outcome of AKI above the knot points so that the knots (13 g/dL for females and 14 g/dL for males) represent minimum targets for this outcome. Of note, some hemoglobin-outcome associations may not have clear boundaries of increasing risk. For example, higher hemoglobin concentrations were associated with reduced perioperative transfusions above and below the knot points. It is also important to recognize that the utilization of data-driven models to define the associations between continuous hemoglobin concentrations and clinical outcomes results in more precise effect estimates than the utilization of traditional anemia definitions, which rely on non-perioperative epidemiological data and hemoglobin categorization.
Our findings have important clinical implications, especially recognizing that the preoperative period offers an opportunity to identify, evaluate, and effectively treat anemia prior to elective cardiac surgery. Such treatment strategies could possibly mitigate adverse clinical outcomes, such as AKI, and thresholds for therapy initiation could incorporate hemoglobin concentrations associated with increasing risk for adverse outcomes across sexes. Indeed, there is strong evidence that preoperative anemia evaluation and management, even a single day prior to cardiac surgery, is associated with improved outcomes (40).
There are limitations of this investigation. First, despite a pre-specified statistical plan with multivariable adjustment, the possibility for residual confounding remains, which may influence observed associations between preoperative anemia and outcomes. Second, the hemoglobin concentrations best-suited to define preoperative anemia for women and men in this investigation may not be directly applicable to other practices, and each practice should consider reviewing their own clinical data to determine hemoglobin cut-offs for preoperative anemia based upon associations with clinical outcomes. Third, it is possible that the number of events of stroke, pulmonary embolism, and myocardial infarction were underdiagnosed due to the absence of routine screening, though any missed event would likely be of limited clinical significance. Moreover, postoperative AKI may occur independently of anemia-related effects and additional markers of impaired end-organ oxygen delivery should be included in future studies. Fourth, while in-hospital mortality in this population is rare (<0.2% of our sample), we model incidence of AKI prior to death or discharge as our primary outcome rather than use a composite outcome with mortality. Given this rarity, results are likely not sensitive to the analysis choice, but researchers using populations with higher mortality rates may need to consider models accounting for death. Fifth, while we adjusted our models for surgery type (valve vs. CABG), the inclusion of double valve surgeries may be associated with increased risk for adverse outcomes over single valve surgeries. However, it is worth noting that double valve surgeries are routine at our high-volume surgical center, and we adjusted for other factors that may drive any outcome differences between single and double valve surgery including cardiopulmonary bypass time, heart failure severity, and patient comorbidities. Sixth, the etiology of preoperative anemia was not diagnosed in most patients, and the vast majority of patients underwent no evaluation for iron deficiency. Future studies are necessary to assess the impact of anemia etiology on the associations between hemoglobin and outcomes. Seventh, our data reflects the experience of a large tertiary medical institution which may impact generalizability. Future investigations are warranted to assess sex-differences in the associations between preoperative anemia and postoperative clinical outcomes in high risk cardiac surgeries, such as triple valves or patients on mechanical circulatory support. Finally, we performed model/variable selection to choose the functional form for hemoglobin in our analysis, which increases the Type I error rate. This, along with multiple secondary endpoints and tests within each endpoint suggests results should be interpreted cautiously and confirmed in future study.
Conclusion:
In a large cohort of patients undergoing elective cardiac surgery, preoperative anemia was associated with inferior clinical outcomes including higher rates of AKI, perioperative RBC transfusion, and longer hospital LOS. The associations between preoperative hemoglobin concentrations and these clinical outcomes differed by sex. Ascertaining sex-specific values to identify and initiate preoperative anemia management strategies could ultimately reduce postoperative complications in adults undergoing elective cardiac surgery.
Supplementary Material
Supplemental Figure 1. Adjusted odds and expected proportion of perioperative red blood cell transfusion by preoperative hemoglobin stratified by sex
Panel A – Adjusted odds ratio (95% CI) for perioperative transfusion relative to a reference hemoglobin of 10 g/dL.
Panel B – Expected proportion of perioperative transfusion by hemoglobin. Estimates come from the adjusted regression model and represent a marginal estimate averaged over the observed distribution of covariates in our sample.
Supplemental Figure 2. Adjusted odds and expected proportion of reoperation by preoperative hemoglobin stratified by sex
Panel A – Adjusted odds ratio (95% CI) for reoperation relative to a reference hemoglobin of 10 g/dL.
Panel B – Expected proportion of reoperation by hemoglobin. Estimates come from the adjusted regression model and represent a marginal estimate averaged over the observed distribution of covariates in our sample.
Supplemental Figure 3. Adjusted odds and expected proportion of vascular complications by preoperative hemoglobin stratified by sex
Panel A – Adjusted odds ratio (95% CI) for vascular complications relative to a reference hemoglobin of 10 g/dL.
Panel B – Expected proportion of vascular complications by hemoglobin. Estimates come from the adjusted regression model and represent a marginal estimate averaged over the observed distribution of covariates in our sample.
Supplemental Figure 4. Ratio of geometric means and marginal expected hospital length of stay by preoperative hemoglobin stratified by sex
Panel A – Ratio of geometric means (95% CI) for hospital length of stay relative to a reference hemoglobin of 10 g/dL.
Panel B – Marginal hospital length of stay.
Supplemental Table 1. Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria
Supplemental Table 2. Unadjusted outcomes by patient sex and anemia severity
KEY POINTS SUMMARY.
Question:
What are the associations between preoperative hemoglobin concentrations, patient sex, and postoperative clinical outcomes in a large cohort of adult cardiac surgery patients?
Findings:
Preoperative anemia was associated with inferior clinical outcomes and the associations between hemoglobin concentrations and outcomes differed between females and males in this single center observational cohort.
Meaning:
Ascertaining sex-specific values for the identification of preoperative anemia which are driven by clinical outcome data may ultimately lead to enhanced treatment strategies to reduce postoperative complications in adults undergoing elective cardiac surgery.
Acknowledgments
Funding Statement: This study was supported by NIH R01 grant (HL121232) to Dr. Kor and by CTSA Grant Number KL2 TR002379 to Dr. Warner from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
GLOSSARY OF TERMS
- AKI
acute kidney injury
- STROBE
The Strengthening the Reporting of Observational Studies in Epidemiology
- CABG
coronary artery bypass grafting
- ECMO
extracorporeal membrane oxygenation
- VADs
ventricular assist devices
- IABP
intra-aortic balloon pump
- ASA
American Society of Anesthesiologists Physical Status Classification
- NYHA
New York Heart Association
- CPB
cardiopulmonary bypass
- ICU
intensive care unit
- KDIGO
Kidney Disease Improving Global Outcomes
- RBCs
red blood cells
- OR
operating room
- LOS
length of stay
- WHO
World Health Organization
- AIC
Akaike information criterion
- CI
confidence intervals
Footnotes
Conflict of Interest: The authors declare no competing interests
REFERENCES
- 1.Padmanabhan H, Siau K, Curtis J, et al. Preoperative Anemia and Outcomes in Cardiovascular Surgery: Systematic Review and Meta-Analysis. Ann Thorac Surg 2019;108:1840–8. [DOI] [PubMed] [Google Scholar]
- 2.Oprea AD, Del Rio JM, Cooter M, et al. Pre- and postoperative anemia, acute kidney injury, and mortality after coronary artery bypass grafting surgery: a retrospective observational study. Can J Anaesth 2018;65:46–59. [DOI] [PubMed] [Google Scholar]
- 3.Blasberg JD, Schwartz GS, Balaram SK. The role of gender in coronary surgery. Eur J Cardiothorac Surg 2011;40:715–21. [DOI] [PubMed] [Google Scholar]
- 4.Johnston A, Mesana TG, Lee DS, Eddeen AB, Sun LY. Sex Differences in Long-Term Survival After Major Cardiac Surgery: A Population-Based Cohort Study. J Am Heart Assoc 2019;8:e013260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arif R, Farag M, Gertner V, et al. Female Gender and Differences in Outcome after Isolated Coronary Artery Bypass Graft Surgery: Does Age Play a Role? PLoS One 2016;11:e0145371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ennker IC, Albert A, Pietrowski D, Bauer K, Ennker J, Florath I. Impact of gender on outcome after coronary artery bypass surgery. Asian Cardiovasc Thorac Ann 2009;17:253–8. [DOI] [PubMed] [Google Scholar]
- 7.Saxena A, Dinh D, Smith JA, Shardey G, Reid CM, Newcomb AE. Sex differences in outcomes following isolated coronary artery bypass graft surgery in Australian patients: analysis of the Australasian Society of Cardiac and Thoracic Surgeons cardiac surgery database. Eur J Cardiothorac Surg 2012;41:755–62. [DOI] [PubMed] [Google Scholar]
- 8.Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation 2007;116:471–9. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 2006;107:1747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner MA, Shore-Lesserson L, Shander A, Patel SY, Perelman SI, Guinn NR. Perioperative Anemia: Prevention, Diagnosis, and Management Throughout the Spectrum of Perioperative Care. Anesth Analg 2020;130:1364–80. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500–24. [DOI] [PubMed] [Google Scholar]
- 12.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform 2011;28:42, 4–5. [PubMed] [Google Scholar]
- 13.Chute CG, Beck SA, Fisk TB, Mohr DN. The Enterprise Data Trust at Mayo Clinic: a semantically integrated warehouse of biomedical data. J Am Med Inform Assoc 2010;17:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc 2012;87:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- 16.Will ND, Kor DJ, Frank RD, et al. Initial Postoperative Hemoglobin Values and Clinical Outcomes in Transfused Patients Undergoing Noncardiac Surgery. Anesth Analg 2019;129:819–29. [DOI] [PubMed] [Google Scholar]
- 17.Nuttall GA, Oliver WC, Ereth MH, Santrach PJ. Coagulation tests predict bleeding after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997;11:815–23. [DOI] [PubMed] [Google Scholar]
- 18.Harrel FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Second ed.: Springer, 2015. [Google Scholar]
- 19.Raphael J, Mazer CD, Subramani S, et al. Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients. Anesth Analg 2019;129:1209–21. [DOI] [PubMed] [Google Scholar]
- 20.LaPar DJ, Hawkins RB, McMurry TL, et al. Preoperative anemia versus blood transfusion: Which is the culprit for worse outcomes in cardiac surgery? J Thorac Cardiovasc Surg 2018;156:66–74.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Heymann C, Kaufner L, Sander M, et al. Does the severity of preoperative anemia or blood transfusion have a stronger impact on long-term survival after cardiac surgery? J Thorac Cardiovasc Surg 2016;152:1412–20. [DOI] [PubMed] [Google Scholar]
- 22.Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 2004;44:1453–62. [DOI] [PubMed] [Google Scholar]
- 23.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544–52. [DOI] [PubMed] [Google Scholar]
- 24.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17. [DOI] [PubMed] [Google Scholar]
- 25.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365:2453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015;372:997–1008. [DOI] [PubMed] [Google Scholar]
- 27.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. Jama 2010;304:1559–67. [DOI] [PubMed] [Google Scholar]
- 28.Shander A, Javidroozi M, Ozawa S, Hare GM. What is really dangerous: anaemia or transfusion? Br J Anaesth 2011;107 Suppl 1:i41–59. [DOI] [PubMed] [Google Scholar]
- 29.Loor G, Rajeswaran J, Li L, et al. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg 2013;146:1480–7.e6. [DOI] [PubMed] [Google Scholar]
- 30.Shehata N, Mistry N, da Costa BR, et al. Restrictive compared with liberal red cell transfusion strategies in cardiac surgery: a meta-analysis. Eur Heart J 2019;40:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranucci M, Pazzaglia A, Bianchini C, Bozzetti G, Isgro G. Body size, gender, and transfusions as determinants of outcome after coronary operations. Ann Thorac Surg 2008;85:481–6. [DOI] [PubMed] [Google Scholar]
- 32.Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost 2019;17:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karkouti K, Wijeysundera DN, Beattie WS. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 2008;117:478–84. [DOI] [PubMed] [Google Scholar]
- 34.De Santo L, Romano G, Della Corte A, et al. Preoperative anemia in patients undergoing coronary artery bypass grafting predicts acute kidney injury. J Thorac Cardiovasc Surg 2009;138:965–70. [DOI] [PubMed] [Google Scholar]
- 35.Williams ML, He X, Rankin JS, Slaughter MS, Gammie JS. Preoperative hematocrit is a powerful predictor of adverse outcomes in coronary artery bypass graft surgery: a report from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2013;96:1628–34; discussion 34. [DOI] [PubMed] [Google Scholar]
- 36.Mehta RH, Castelvecchio S, Ballotta A, Frigiola A, Bossone E, Ranucci M. Association of gender and lowest hematocrit on cardiopulmonary bypass with acute kidney injury and operative mortality in patients undergoing cardiac surgery. Ann Thorac Surg 2013;96:133–40. [DOI] [PubMed] [Google Scholar]
- 37.Brescia AA, Wu X, Paone G, et al. Effect of sex on nadir hematocrit and rates of acute kidney injury in coronary artery bypass. J Thorac Cardiovasc Surg 2019;158:1073–80.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis MC, Paugh TA, Dickinson TA, et al. Nadir Hematocrit on Bypass and Rates of Acute Kidney Injury: Does Sex Matter? Ann Thorac Surg 2015;100:1549–54; discussion 54–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy WG. The sex difference in haemoglobin levels in adults - mechanisms, causes, and consequences. Blood reviews 2014;28:41–7. [DOI] [PubMed] [Google Scholar]
- 40.Spahn DR, Schoenrath F, Spahn GH, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019;393:2201–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Adjusted odds and expected proportion of perioperative red blood cell transfusion by preoperative hemoglobin stratified by sex
Panel A – Adjusted odds ratio (95% CI) for perioperative transfusion relative to a reference hemoglobin of 10 g/dL.
Panel B – Expected proportion of perioperative transfusion by hemoglobin. Estimates come from the adjusted regression model and represent a marginal estimate averaged over the observed distribution of covariates in our sample.
Supplemental Figure 2. Adjusted odds and expected proportion of reoperation by preoperative hemoglobin stratified by sex
Panel A – Adjusted odds ratio (95% CI) for reoperation relative to a reference hemoglobin of 10 g/dL.
Panel B – Expected proportion of reoperation by hemoglobin. Estimates come from the adjusted regression model and represent a marginal estimate averaged over the observed distribution of covariates in our sample.
Supplemental Figure 3. Adjusted odds and expected proportion of vascular complications by preoperative hemoglobin stratified by sex
Panel A – Adjusted odds ratio (95% CI) for vascular complications relative to a reference hemoglobin of 10 g/dL.
Panel B – Expected proportion of vascular complications by hemoglobin. Estimates come from the adjusted regression model and represent a marginal estimate averaged over the observed distribution of covariates in our sample.
Supplemental Figure 4. Ratio of geometric means and marginal expected hospital length of stay by preoperative hemoglobin stratified by sex
Panel A – Ratio of geometric means (95% CI) for hospital length of stay relative to a reference hemoglobin of 10 g/dL.
Panel B – Marginal hospital length of stay.
Supplemental Table 1. Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria
Supplemental Table 2. Unadjusted outcomes by patient sex and anemia severity