Abstract
This study examined the developmental trajectory of neurodevelopmental motor signs among boys and girls with attention-deficit/hyperactivity disorder (ADHD) and typically-developing (TD) children. Seventy children with ADHD and 48 TD children, aged 8–17 years, were evaluated on at least two time-points using the Physical and Neurological Assessment of Subtle Signs (PANESS). Age-related changes in subtle motor signs (overflow, dysrhythmia, speed) were modeled using linear mixed-effects models to compare the developmental trajectories among four subgroups (ADHD girls and boys and TD girls and boys). Across visits, both boys and girls with ADHD showed greater overflow, dysrhythmia, and slower speed on repetitive motor tasks compared to TD peers; whereas, only girls with ADHD were slower on sequential motor tasks than TD girls. Developmental trajectory analyses revealed a greater reduction in overflow with age among boys with ADHD than TD boys; whereas, trajectories did not differ among girls with and without ADHD, or among boys and girls with ADHD. For dysrhythmia and speed, there were no trajectory differences between the subgroups, with all groups showing similar reductions with age. Children with ADHD show developmental trajectories of subtle motor signs that are consistent with those of TD children, with one clear exception: Boys with ADHD show more significant reductions in overflow from childhood to adolescence than do their TD peers. Our findings affirm the presence of subtle motor signs in children with ADHD and suggest that some of these signs, particularly motor overflow in boys, resolve through adolescence while dysrhythmia and slow speed, may persist.
Keywords: ADHD, motor, development, overflow, PANESS
Background
Attention-deficit/hyperactivity disorder (ADHD) is a highly prevalent neurodevelopmental disorder characterized by excessive inattentive, hyperactive, and impulsive behavior. ADHD is typically chronic, persisting into adolescence, and often adulthood (Sibley et al., 2012; Willoughby, 2003). Children with ADHD also show abnormalities in motor control with approximately 50% of children with ADHD presenting with comorbid developmental coordination disorder (DCD; Cole et al., 2008; Goulardins et al. 2015; Sweeney et al., 2018). ADHD is also associated with anomalous motor physiology, with studies showing reduced transcranial magnetic stimulation (TMS)-evoked short interval cortical inhibition in the motor cortex in children with ADHD (Gilbert et al., 2011; Gilbert et al., 2019). These motor deficits parallel core impairment in higher-order behavioral control and might thereby serve as markers for inefficiency in neighboring parallel neural networks responsible for executive control (Mostofsky et al., 2003). Compared to cognitive/executive functions, motor behaviors develop earlier, are more overt, and are easily measured (Fjørtoft et al., 2013). Impaired motor control in ADHD is associated with difficulties in performing activities of daily living such as handwriting (Borella et al., 2011) and tool use (Scharoun et al., 2013). Thus, motor behaviors represent an ideal quantitative biomarker to study the risk for poor outcomes in later adolescence and adulthood in ADHD. As such, characterizing developmental trajectories of motor function may yield early predictive clinical biomarkers and facilitate the design of personalized early interventions. However, there is a paucity of longitudinal studies examining developmental trajectories of motor function from childhood through adolescence in ADHD.
Typical developmental changes in motor control include improvements in speed and reduced subtle signs of dysrhythmia and overflow (Martins et al., 2008). Dysrhythmia refers to improper timing or rhythm during controlled movements, and overflow refers to the co-movement of body parts not needed to efficiently execute a task (Gidley Larson et al., 2007). Motor overflow is age-appropriate in young children, and its decrease with age is attributed to motor system maturation (myelination) and improved inhibitory competence (Arányi & Rösler, 2002). The persistence of overflow into adolescence may represent abnormal or delayed motor development(Gidley Larson et al., 2007) and have significant implications for a range of functional outcomes involving skilled behaviors, including handwriting and activities of daily living (Fuentes et al., 2009). A large longitudinal study in typically-developing children (11–19 years) showed that subtle motor signs decrease rapidly in adolescence with girls showing a greater reduction with increasing age than boys (Martins et al., 2008).
The presence of subtle motor dysfunction in young children with ADHD is well-established, (Cole et al., 2008; Mostofsky et al., 2003; Sweeney et al., 2018) but whether motor deficits persist into late adolescence is not known. In a cross-sectional sample, compared to typically-developing (TD) controls, children with ADHD, aged 7–15 years showed greater overflow and dysrhythmia and reduced speed on repetitive and sequential timed tasks (Cole et al., 2008). While TD controls (both boys and girls) and girls with ADHD showed a steady reduction in overflow and dysrhythmia and an increase in speed with increasing age, boys with ADHD had little improvement with age (Cole et al., 2008). Additionally, across both diagnostic groups and regardless of age, girls were faster with fewer subtle motor signs (Cole et al., 2008). This sex difference is attributed to earlier brain maturation in girls.
Despite cumulative cross-sectional studies demonstrating subtle motor deficits in ADHD (Cole et al., 2008; Gaddis et al., 2015; MacNeil et al., 2011; Mostofsky et al., 2003), there have been no longitudinal studies examining subtle motor signs and factors affecting the developmental trajectory of motor function from childhood to adolescence. In a longitudinal study of preschool children with ADHD, motor performance rapidly improved from ages 4–7 years in children with and without ADHD, with greater improvement in finger tapping speed in the ADHD group (Sweeney et al., 2018). Preschool children with ADHD showed slower speed on repetitive tasks than TD controls, but there were no group differences in overflow and speed on sequential tasks (Sweeney et al., 2018). This suggests that impairments in overflow and sequenced movements are present in older (e.g., 7–15-year-olds) (Cole et al., 2008) but not younger (e.g., 4–7-year-olds) children with ADHD (Sweeney et al., 2018). Thus, there appears to be differential sensitivity of basic versus complex elements of motor control in preschool versus school-aged children with ADHD. However, this longitudinal study did not examine the effect of sex on motor function (Sweeney et al., 2018). Moreover, most studies of motor function in the ADHD population have predominantly examined school-age boys (Cole et al., 2008; Rosch et al., 2013). Hence, there is limited research examining age- and sex-related developmental changes in motor function across boys and girls with ADHD, and no studies to date examined developmental trajectories into adolescence using longitudinal methods allowing for estimation of within-person change.
Given these gaps in knowledge, this longitudinal study assessed the developmental trajectory of subtle motor signs in boys and girls with and without ADHD from childhood to adolescence. First, we hypothesized that, regardless of age, children with ADHD would show subtle motor deficits compared to the age-matched TD group. Second, we hypothesized that subtle signs would reduce with age, and specifically, that speed would improve with age across all participants, with girls showing greater improvements than boys. Lastly, we hypothesized that the developmental trajectory of improvement over time would be different for the ADHD group.
Methods
Seventy children with ADHD (25 girls) and 48 TD (19 girls) participants were included. All participants had their first visit between ages 8–12 years (at visit 1, ADHD mean age=9.81, SD=1.35; TD mean age=10.26, SD=1.21), with all participants confirmed as being pre-pubertal (Tanner stage 1 or 2) at that time. Participants were recruited from local schools and community centers using flyers and word-of-mouth. For inclusion in the ADHD group, children had to meet full DSM-IV or -5 criteria for ADHD based on the following criteria: (1) an ADHD diagnosis according to the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997) including the presence of 6/9 symptoms of inattention, hyperactivity or both and cross-situational impairment, and (2) T-score of 65 or higher on the Conners 3 Inattentive Type T-score or Conners 3 Hyperactive/Impulsive Type T-score (Conners, 1997). At visit 1, children with ADHD were allowed to meet criteria for comorbid psychiatric diagnoses on either the Diagnostic Interview for Children and Adolescents (DICA-IV; Reich, 2000) or K-SADS including oppositional defiant disorder (ODD; n=25), anxiety disorders (n=1), and depression (n=0; Table 1). There was no difference between girls and boys with ADHD in the comorbid diagnosis of ODD (p=.11). At visit 1, the Developmental Coordination Disorder Questionnaire (DCDQ; Wilson et al., 2000) was administered to all participants and three girls with ADHD and 11 boys with ADHD met criteria for DCD based on clinical cut-off scores (Total < 48), while none of the participants in the TD control group met criteria for DCD (Table 1). Master’s level clinicians conducted the diagnostic interview and integrated information from rating scales to confirm a diagnosis of ADHD under the supervision of licensed clinical psychologists (KSR, KES) or a neurologist (SHM) with extensive experience diagnosing ADHD in children and adolescents. Participants were eligible for inclusion as a TD control if they (1) did not have a history of neurodevelopmental or mental health disorders and did not meet criteria for these disorders on the DICA-IV or K-SADS, and (2) were below clinical cut-offs (T < 60) on the Conners rating scales listed above. Across diagnostic groups, participants were excluded if they had a history of seizures, head injury, or other neurodevelopmental disorders (other than ADHD).
Table 1.
Demographic data of study participants. Values indicate means and (standard deviation) unless otherwise indicated.
| ADHD | TD | ADHD vs TD (p values) | ||||
|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Boys | Girls | |
| Participants with 2 visits (n; %) | 45 (64%) | 25 (36%) | 29 (60%) | 19 (40%) | .67a | |
| Participants with 3 visits (n) | 12 | 3 | 9 | 4 | ||
| Age (years) at visit 1 | 10 (1.44) | 9.47 (1.11) | 10.51 (1.25) | 9.88 (1.06) | .12 | .22 |
| IQ at visit 1 | 110.67 (10.47) | 107.80 (14.07) | 115.90 (13.01) | 114.95 (7.87) | .05 | .06 |
| ADHD Boys vs. Girls | ||||||
| ADHD Subtype (n, IA:HI:C) | 13:1:31 | 5:0:20 | - | - | .64 | |
| Comorbid ODD (n) | 13 | 12 | 0 | 0 | .11 | |
| Comorbid depression (n) | 0 | 0 | 0 | 0 | - | |
| Comorbid anxiety (n) | 0 | 1 | 0 | 0 | - | |
| Comorbid DCD (n) | 11 | 3 | 0 | 0 | .05 | |
| Stimulant medication use (n) | 35 | 18 | 0 | 0 | .19 | |
| Non-stimulant medication use*(n) | 2 | 0 | 0 | 0 | .31 | |
| ADHD participants no longer meeting ADHD criteria at follow-up (n) | 8 | 4 | - | - | .98 | |
| ADHD vs TD (p values) | ||||||
| Boys | Girls | |||||
| Total timed overflow | 13.29 (4.96) | 10.36 (4.35) | 6.45 (3.64) | 7.11 (5.71) | <.0001 | .038 |
| Total dysrhythmia | 6.93 (2.82) | 6.80 (2.78) | 5.38 (2.48) | 4.53 (2.70) | .018 | .009 |
| Total time Repetitive tasks | 33.25 (5.39) | 36.85 (7.45) | 31.52 (5.15) | 32.25 (6.40) | .17 | .037 |
| Total time Sequential tasks | 45.75 (11.13) | 48.40 (13.44) | 45.11 (8.04) | 42.21 (7.89) | .79 | .08 |
Group comparisons using independent samples t-tests. ADHD Subtype IA = Predominantly Inattentive Presentation, HI = Hyperactive/Impulsive Presentation, CO = Combined Presentation. ODD = Oppositional Defiant Disorder, DCD = Developmental Coordination Disorder.
Both participants were taking Strattera on the day of testing.
Chi-square test examining diagnostic group differences on sex.
Scores from the Physical and Neurological Assessment of Subtle Signs (PANESS) at visit 1 (ages 8 – 12 years).
All participants had their second visit and a subset (15 ADHD, 13 TD) returned for a third visit between 10–17 years (Figure 1). Time between visits ranged from 1.3–9.6 years (mean=3.66 years, mode=2.1 years; Supplementary figure 1). All participants were right-handed and had a Full-Scale IQ above 80 (range: 83–152) using the Wechsler Intelligence Scales for Children (WISC) current at the time of testing. Participants were also screened for a reading disorder with the Wechsler Individual Achievement Test, Second (WIAT-II, n=100), or Third Edition (WIAT- III, n=18) and were excluded for standard scores below 85. Study approval was granted by the Johns Hopkins university Institutional Review Board. Parents of all participants provided written consent, and all participants provided assent. During each visit, participants completed a neuropsychological assessment battery, including the Revised Physical and Neurological Assessment of Subtle Signs (PANESS; Denckla, 1985).
Figure 1.
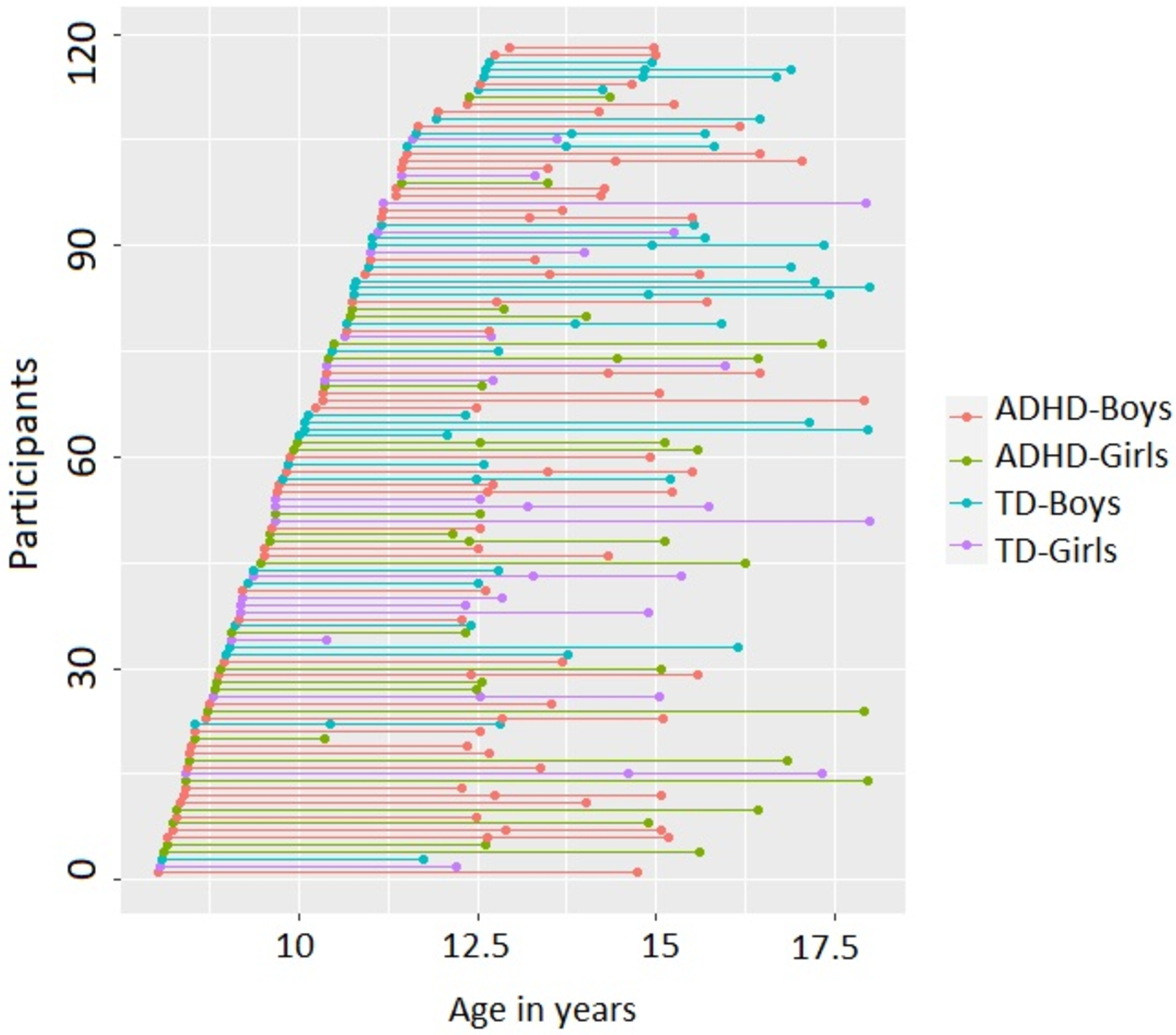
Age and sex distribution for children with ADHD and TD controls for the study visits. Each dot represents a study visit. All participants had two study visits and a subset had three study visits.
Motor assessment.
The PANESS examines subtle signs of motor impairment, including overflow and dysrhythmia during gait, balance, and timed activities (Denckla, 1985); detailed administration and scoring procedures have been previously published (Gidley Larson et al., 2007). The PANESS has adequate test-retest and inter-rater reliability (kappa ≥ 0.5; intraclass coefficient ≥ 0.7), internal consistency (Cronbach’s alpha=0.74), and sensitivity to age-related changes (Cole et al., 2008; Stephens et al., 2018; Vitiello et al., 1989).
Speed of timed movements was measured as time in seconds to complete 20 movements for six activities, including three repetitive (foot-tapping, hand-patting, and finger-tapping) and three sequential tasks (heel-toe tapping, hand pronation-supination, and finger sequencing) performed bilaterally. Total timed overflow includes the total number of movements (proximal, mirror, or orofacial) during the timed tasks. Proximal overflow indicates extraneous movement on the same side involving larger muscle groups, even if in a different limb (e.g., lifting at elbow rather than wrist during hand-patting). Mirror overflow refers to extraneous movement on the same limb of the opposite side, while orofacial overflow denotes any extraneous orofacial movement during the tasks. Overflow scores were not age-corrected. Total dysrhythmia refers to the total number of timed tasks in which the child failed to maintain a steady rhythm for the task duration. Participants received a score of 1 each if overflow or dysrhythmia was present and 0 if absent. Overflow and dysrhythmia were scored for each of the six timed tasks for the right and left extremities (Range: 0–12). On all measures, lower scores indicate better performance. The study variables include total timed overflow, dysrhythmia, and total time on a) repetitive and b) sequential tasks.
Statistical analysis.
The PANESS variables were normally-distributed and free of outliers. Linear regression models were used to examine the effect of diagnosis and sex on motor function (regardless of age), and the two-way interaction of diagnosis and sex was included to test for differences between subgroups. To investigate how motor function changes with age, as well as the heterogeneity in the association across diagnosis and sex groups, linear mixed-effects models with a three-way interaction, i.e., diagnosis by sex by age, were employed. Diagnosis (0 - TD, 1 - ADHD) and sex (0 - boys, 1 - girls) were binary dummy-coded variables. For the age variable, the minimum age (8.02 years) was subtracted across all participants. The three-way interaction of diagnosis, sex, and age were used to derive age coefficients for the four subgroups, i.e., TD-Boys, TD-Girls, ADHD-Boys, and ADHD-Girls. Developmental trajectory comparisons were performed to examine differences between the subgroups, including TD-Boys vs. ADHD-Boys, TD-Girls vs. ADHD-Girls, TD-Girls vs. TD-Boys, and ADHD-Girls vs. ADHD-Boys. This method allows for the inclusion of multiple time points per participant while accounting for the unbalanced data structure of irregular time intervals between the PANESS assessments. Examination of model fit indicated that the linear model provided the best model fit. Model parameters were estimated for each PANESS outcome separately. Modeling and visualization were performed in R(R Core Team, 2014) using linear mixed model package lme4 (Bates et al., 2014). Bonferroni-corrected alpha for the four PANESS outcomes was .01 (.05/4).
Results
Participant characteristics.
Participant demographics are listed in Table 1. At visit 1, for boys and girls, there was no group difference in age; however, TD boys and girls had significantly higher full-scale IQ than ADHD boys and girls respectively (Table 1). Diagnostic groups did not differ in the distribution of boys and girls (p-values >.05). Group differences on the PANESS at visit 1 are presented in Table 1. There was no group difference on time between visits (p=.61). The models examining the effect of diagnosis and sex (regardless of age) and the models examining developmental trajectories, along with their main and interaction effects are included in Supplementary Tables 1–4. The subgroup results reported in supplementary tables 1 and 2 were derived from the regression models reported in supplementary tables 3 and 4.
Total timed overflow.
Linear regression models testing for effects of diagnosis, sex, and their interaction (regardless of age) revealed increased overflow among girls (β=3.04, p=.007) and boys (β=5.2, p<.001) with ADHD compared to same-sex TD children (Supplementary Table 1, Figure 2A). Additionally, the difference between boys and girls with ADHD trended towards significance (β=−1.74, p=.06) with boys with ADHD showing greater overflow than girls with ADHD.
Figure 2.
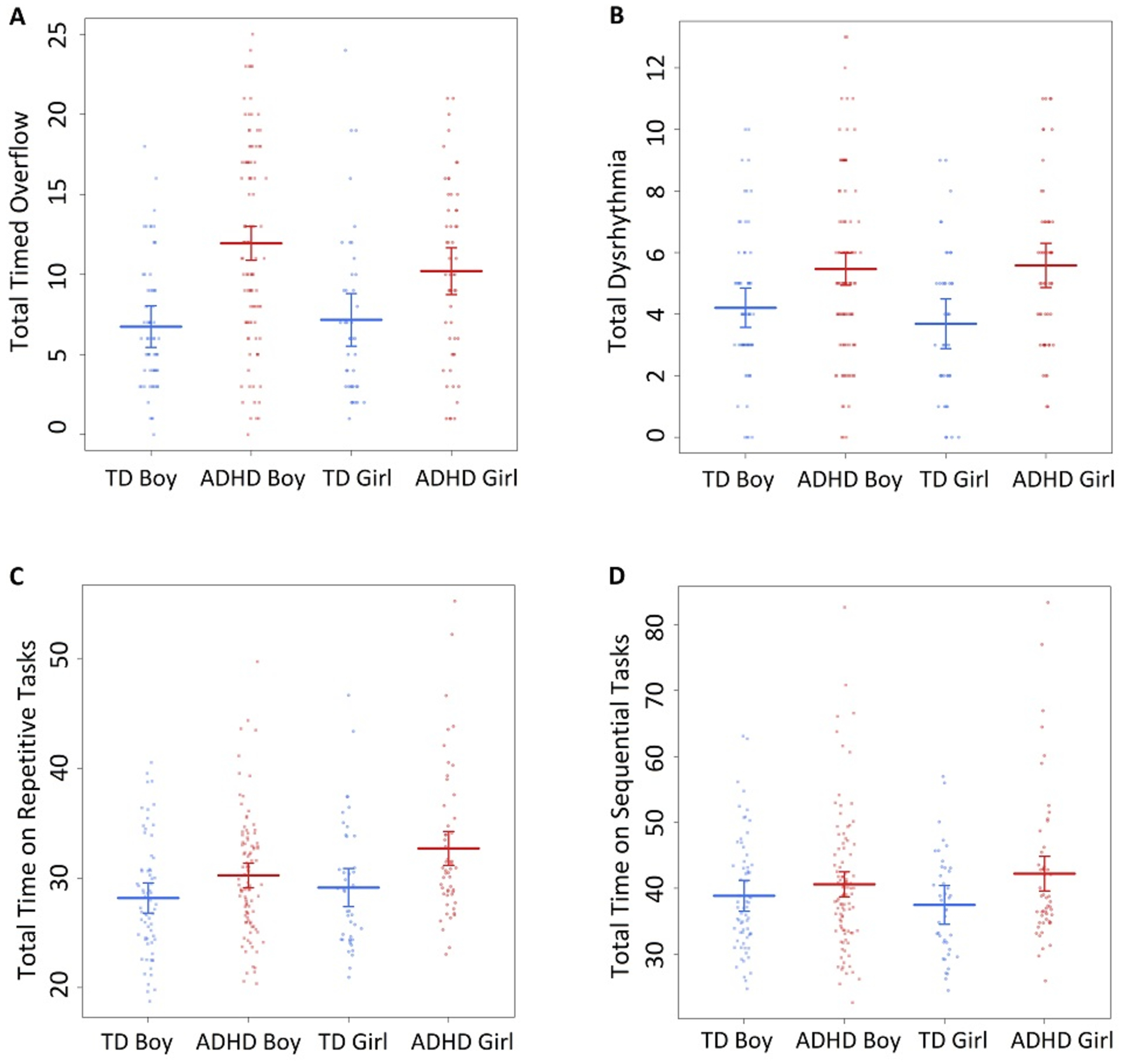
Scatter plot of PANESS outcomes by subgroup. The Y-axis depicts PANESS scores on A. Total timed overflow. B. Total Dysrhythmia. C. Total Time on Repetitive Tasks, and D. Total Time on Sequential tasks.
Trajectory analyses revealed a significant reduction in overflow with age in all four subgroups (Supplementary Table 2, Figure 3A–B), with evidence of differential improvement in ADHD boys (β=−1.34) compared to TD boys (β=−0.45, p<0.001). There was a significant diagnosis by age interaction effect (β=−0.89, p<.0001) while the diagnosis by sex interaction (β=−5.36, p=.018) and the 3-way interaction between diagnosis, sex, and age (β=0.74, p=.055) approached significance (Supplementary Table 4). As seen in Figure 3A, the trajectories for TD and ADHD boys converge after age 15 years (indicated by overlapping confidence intervals). The separate models with boys only and increasing age revealed an effect of diagnosis (p<0.01) from ages 8–14 years, with no significant difference from ages 15–17 years. In contrast, the developmental trajectories for ADHD and TD girls did not differ (Supplementary Table 2, Figure 3B).
Figure 3.
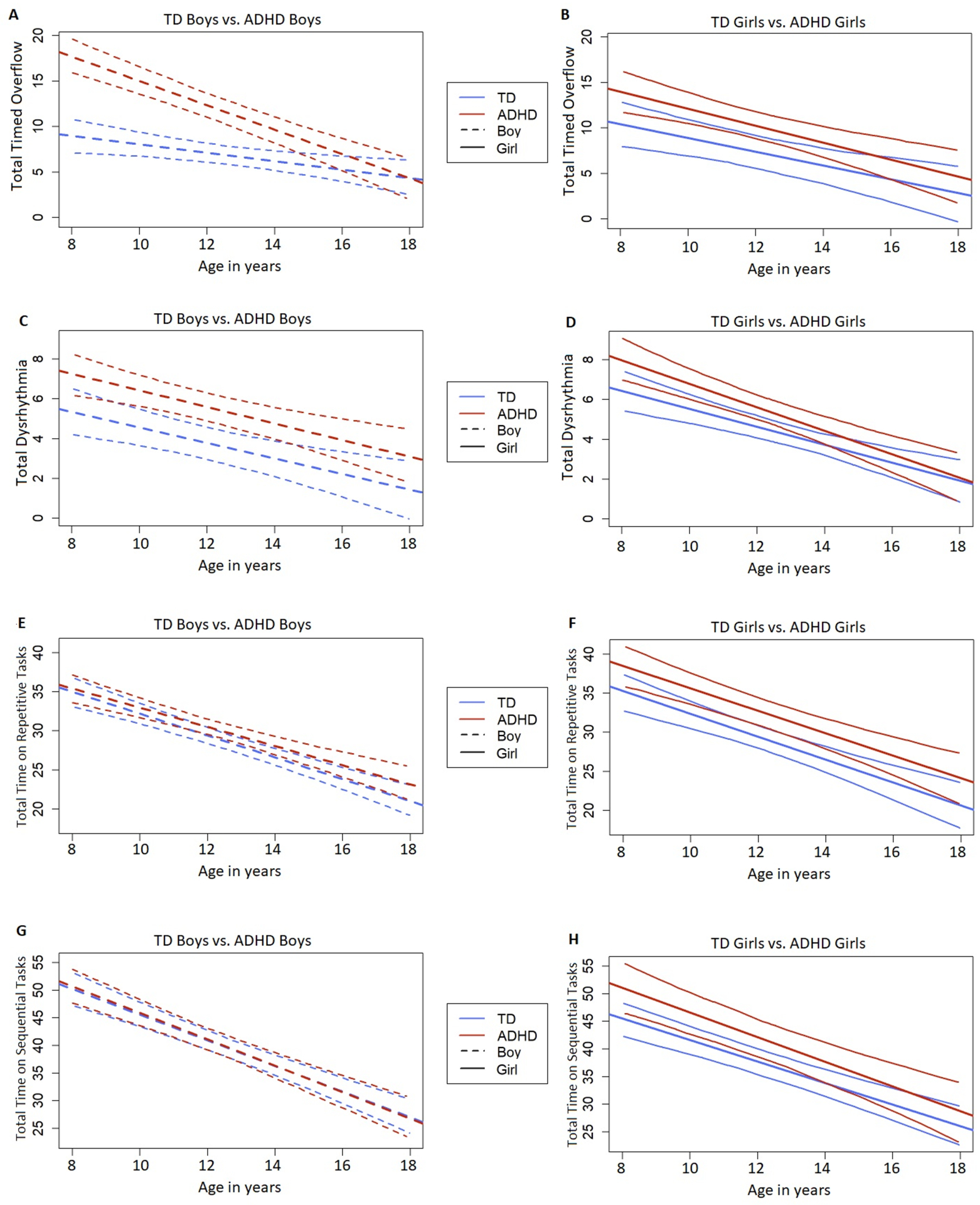
Trajectory plots depicting the model-fitted longitudinal change with the best fit line and 95% confidence intervals for TD boys versus ADHD boys (A, C, E, and G) and TD girls versus ADHD girls (B, D, F, and H). The X-axis depicts age in years. Plots A and B depict Total Timed Overflow scores. Plot C and D depict Total Dysrhythmia scores. Plots E and F depict Total Time on Repetitive Tasks and plots G and H depict Total Time on Sequential Tasks.
No sex differences in developmental trajectories were identified within either diagnostic group (Supplementary Table 2, Figure 3A–B).
Dysrhythmia.
Regardless of age, both boys (β=1.21, p=0.004) and girls (β=1.89, p<0.001) with ADHD showed significantly greater dysrhythmia than TD boys and girls respectively (Supplementary Table 1, Figure 2B). There was no difference in dysrhythmia between boys and girls with ADHD. Trajectory analyses revealed a significant reduction in dysrhythmia with age in all subgroups, with no differences in developmental trajectories between the subgroups (Supplementary Table 2, Figure 3C–D).
Speed/Total Time.
Regardless of age, girls (β=3.57, p<0.001) and boys (β=1.99, p=0.030; not significant after Bonferroni correction) with ADHD showed slower speeds on repetitive tasks than TD girls and boys, respectively (Supplementary Table 1, Figure 2C). Additionally, girls with ADHD were slower on repetitive tasks than boys with ADHD (β=2.54, p=.009).
However, only girls with ADHD (β=4.73, p=.019; not significant after Bonferroni correction) showed slower speeds on sequential tasks than TD girls, with no diagnostic group difference in boys (β=1.61, p=.30). There was no difference between boys and girls with ADHD in speed on sequential tasks (β=1.74, p=.290). Trajectory analyses revealed faster speed on repetitive and sequential tasks with age, with no differences in developmental trajectories between the subgroups (Supplementary Table 2, Figure 3E–H).
Discussion
This study examined the longitudinal trajectory of subtle motor signs, including overflow, dysrhythmia, and speed using the PANESS, in children and adolescents with and without ADHD. Consistent with our first hypothesis, we found that across the entire childhood-adolescent age range, both boys and girls with ADHD showed greater subtle motor signs than TD peers. Further, regardless of age, boys and girls within the TD and ADHD groups showed comparable motor performance, providing evidence against sex differences in motor function across this age range; although there was a trend for boys with ADHD showing greater motor overflow than girls with ADHD. Consistent with our second hypothesis, children with and without ADHD showed improved motor function from childhood into adolescence. We found mixed evidence in support of our last hypothesis, that the developmental trajectory of improvement over time would be different for the ADHD group. Specifically, this is the first study to show that boys with ADHD show a greater reduction of overflow with age compared to TD boys. In contrast, the developmental trajectory of overflow did not differ among girls with and without ADHD. Developmental trajectories of dysrhythmia and speed also did not differ among girls and boys with and without ADHD.
Models examining the impact of diagnosis and sex, regardless of age, indicated that boys and girls with ADHD showed greater overflow, dysrhythmia, and slower speeds on repetitive timed tasks than TD boys and girls respectively. Additionally, girls with ADHD were slower on sequential tasks than TD girls. Our findings of atypical motor function in children and adolescents with ADHD are consistent with prior studies, some of which have also suggested ADHD-related sex differences. Specifically, increased overflow and dysrhythmia, and reduced speed has been reported in school-aged (7–15 years) children with ADHD compared to TD peers,(Cole et al., 2008; Mostofsky et al., 2003) with some evidence that this is specific to boys with ADHD (Cole et al., 2008; MacNeil et al., 2011). Although overflow was not significantly different between girls and boys with ADHD when averaged across age, a closer examination of the trajectories suggests that boys with ADHD show the greatest amount of overflow in the 8–12-year-old age range. Given that boys with ADHD also show the greatest improvement into adolescence, the overall mean comparison (regardless of age) was not significantly different between girls and boys with ADHD, although it was trending (p=.06). Therefore, these results remain consistent with prior research in children and extend these findings into late adolescence where ADHD-related sex differences in overflow are no longer present. A study using parent-report measures of motor coordination in 5–19 year-olds also showed similar motor performance between boys and girls with ADHD through adolescence (Fliers et al., 2008). Our findings suggest that girls with ADHD, in particular, performed timed tasks more slowly compared to TD girls. Collectively, these results provide evidence for some distinctions in motor deficits in girls and boys with ADHD.
Regarding developmental trajectories of motor function, our findings reveal that among youth with and without ADHD, overflow and dysrhythmia decrease with age, and speed increases from childhood through adolescence. These findings are consistent with a longitudinal study (11–19 year-olds) and a cross-sectional study (7–14 year-old) of TD children, which revealed similar reductions in overflow and dysrhythmia with age among girls and boys (Gidley Larson et al., 2007; Martins et al., 2008). Continued improvements in motor performance throughout adolescence have been attributed to the protracted development of the cerebellum, which undergoes dramatic changes into adolescence (Tiemeier et al., 2010), and the continued maturation of white matter, including the corpus callosum, through adolescence and into adulthood (Gidley Larson et al., 2007). As these neural structures underlying motor function mature, inhibitory signaling increases, resulting in reduced overflow and dysrhythmia. Additional longitudinal studies examining trajectories of motor function along with brain structure and function would help elucidate the neural contributions to these developmental changes.
The literature on sex-related differences in motor performance in TD children and adolescents is mixed (Largo et al., 2003; Quatman-Yates et al., 2012). A systematic review examining motor function in neurotypical children (8–22 years) showed that while several studies reported significant differences between males and females, some studies reported no sex differences (Quatman-Yates et al., 2012). A cross-sectional study of 5–18 year-olds showed that boys were faster than girls on simple motor functions, while girls were faster on complex sequential movements and showed better motor coordination than boys (Largo et al., 2003). A longitudinal study in 11–19-year-old TD children showed that girls showed greater improvement in subtle motor signs with age than boys, with younger boys lagging behind girls but catching up with them by late adolescence (Martins et al., 2008). Similarly, an early cross-sectional study with the PANESS found that girls may be faster than boys early in development (e.g., ages 5–7 years; Denckla, 1973) yet these sex differences in subtle signs disappear by later childhood (Denckla, 1974; Gidley Larson et al., 2007). In the current study, TD girls and boys showed comparable motor function and similar developmental trajectories suggesting that sex differences in subtle motor signs as assessed by the PANESS may be specific to early childhood.
Our examination of developmental trajectories of motor function revealed one distinct ADHD-associated difference: That boys with ADHD showed greater reductions in overflow from childhood into adolescence than TD boys. Developmental trajectories of other subtle motor signs, dysrhythmia, and speed, appeared generally similar between children with and without ADHD. Further, there were no significant differences in developmental trajectories between girls and boys with ADHD. This implies that, while boys with ADHD show particularly high degrees of overflow during childhood, they also show the greatest improvement across adolescent development. Limited comparisons can be made with the existing literature due to the lack of longitudinal studies of motor development in ADHD. However, the cross-sectional study discussed above, which examined age-related changes across ages 8–14 years, found the opposite pattern, that girls with ADHD showed a greater reduction in overflow with age than did boys with ADHD. These divergent findings may be due to differences in study methodology and ages assessed. In addition, a cross-sectional study using parent-report measures of motor coordination in 5–19 year-olds also showed similar developmental trajectories in the ADHD and TD children and also between boys and girls with ADHD through adolescence (Fliers et al., 2008).
Our overflow findings are generally consistent with the delayed maturation hypothesis ADHD, such that differences in motor overflow are no longer observed in late adolescence due to a developmental lag rather than persistent differences (Rubia, 2007). This may be due to delayed developmental myelination of white matter tracts, which is thought to relate to the negligible overflow observed in TD children beyond early childhood (Hoy et al., 2004). Abnormal overflow in ADHD has been linked to reduced activation in the contralateral primary motor, bilateral premotor, and supplementary motor cortices (Gaddis et al., 2015). This reduced activation reflects delayed maturation and insufficient recruitment of inhibitory networks involved in active suppression of homologous motor circuitry unnecessary for task execution (Gaddis et al., 2015). Studies have also shown reduced volume in these regions and atypical white matter microstructure in boys with ADHD in childhood (Dirlikov et al., 2015; Jacobson et al., 2015). Longitudinal neuroimaging studies are needed to understand whether sex differences in neuroanatomical development and atypical development in ADHD, relate to the observed developmental trajectories of motor deficits.
Contrary to the findings for overflow, there were no diagnostic group differences in the developmental trajectories of dysrhythmia or speed, suggesting a similar improvement in these motor functions with age across children with and without ADHD. Sweeney et al. showed that speed on repetitive tasks was a more sensitive marker of anomalous motor development than overflow and sequencing speed in preschool (4–7 years) children with and without ADHD (Sweeney et al., 2018). Our findings of overflow as compared to speed being a more sensitive marker of anomalous motor development in later childhood and adolescence implies that the neural areas supporting these motor functions undergo differential changes over time. Slower speeds on repetitive tasks appear to remain more sensitive to motor deficits in ADHD in childhood and adolescence as compared to speed on sequential tasks. Compared to same-sex TD peers, both boys and girls with ADHD showed slower speeds on repetitive tasks while only girls with ADHD showed slower speeds on sequential tasks. The neural substrates supporting dysrhythmia and motor speed appear to differ from those involved in the inhibition of overflow. Dysrhythmia is attributed to impairments in the cerebellar-premotor networks(Del Olmo et al., 2007) while speed of movements depends on a network of cortical areas including the primary motor and supplementary motor complex and subcortical areas including striatal (putamen) circuits (Barber et al., 2012). Additionally, motor overflow in ADHD has been associated with impaired response inhibition, a core deficit in ADHD (Mostofsky et al., 2003). Thus, impairments in inhibitory control may underlie motor deficits in ADHD. Further research is required to examine how cognition and executive function impact motor performance in children with ADHD.
The current study included a large longitudinal sample of boys and girls with and without ADHD. Possible limitations of our study include the wide range and smaller number of adolescents compared to younger participants and the small number of girls with ADHD. Since this study focused on examining the developmental trajectory of childhood ADHD, children with ADHD who did not meet the full diagnostic criteria at a follow-up visit were included in the analyses. Further research studying age and sex effects, as well as differences across ADHD subtypes in a larger cohort of adolescents and young adults, is warranted. Additionally, research investigating the impact of environmental effects in the apparent resolution of some ADHD-associated motor deficits in late adolescence is required. Future research may also include longitudinal analyses of brain regions involved in motor control in boys and girls with ADHD through childhood and adolescence.
Conclusion
This study expands on our previous research by examining the developmental trajectory of motor function in a large longitudinal sample of boys and girls with ADHD from childhood through adolescence. Across childhood/early adolescence, both boys and girls with ADHD show greater overflow, dysrhythmia, and slower speed on repetitive tasks than same-sex peers, while only girls with ADHD showed slower speeds on sequential tasks than TD girls. Boys with ADHD show a particularly steep decline in motor overflow through adolescence compared with their TD peers; in contrast, developmental trajectories of dysrhythmia and motor speed are similar in youth with and without ADHD. These findings bolster prior studies revealing that ADHD is associated with prominent signs of atypical motor development during childhood. The findings also build on the extant literature, with developmental trajectories revealing, for the first time, that some ADHD-associated motor deficits, particularly motor overflow, appear to resolve by late adolescence, while others, particularly dysrhythmia and slow speed, may persist. Additional research examining how overflow, dysrhythmia, and speed may serve as differential biomarkers for ADHD, reflecting distinct underlying neurologic processes, is thereby warranted.
Supplementary Material
Funding statement:
This research was supported by the National Institutes of Health (SS: R01HD090266; SM: RO1MH078160 and RO1MH085328; KR: K23MH101322 and R03MH119457; KS: K23MH107734) and the Brain and Behavior Foundation (NARSAD Young Investigator Award to KS).
Footnotes
Disclosure statement: The authors report no conflict of interest.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Arányi Z, & Rösler KM (2002). Effort-induced mirror movements. Experimental Brain Research, 145(1), 76–82. 10.1007/s00221-002-1101-1 [DOI] [PubMed] [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, & Mostofsky SH (2012). Motor “Dexterity”?: Evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cerebral Cortex, 22(1), 51–59. 10.1093/cercor/bhr062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2014). Fitting linear mixed-effects models using lme4. ArXiv:1406.5823 [Stat] http://arxiv.org/abs/1406.5823 [Google Scholar]
- Borella E, Chicherio C, Re AM, Sensini V, & Cornoldi C (2011). Increased intraindividual variability is a marker of ADHD but also of dyslexia: A study on handwriting. Brain and Cognition, 77(1), 33–39. 10.1016/j.bandc.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JCG, Denckla MB, & Mahone EM (2008). Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology, 71(19), 1514. 10.1212/01.wnl.0000334275.57734.5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C (1997). Conners’ Rating Scales: Revised. North Tonawanda, NJ: Multi-Health Systems Inc. [Google Scholar]
- Del Olmo MF, Cheeran B, Koch G, & Rothwell JC (2007). Role of the cerebellum in externally paced rhythmic finger movements. Journal of Neurophysiology, 98(1), 145–152. 10.1152/jn.01088.2006 [DOI] [PubMed] [Google Scholar]
- Denckla MB (1985). Revised neurological examination and subtle signs. Psychopharmacology Bulletin, 21, 773–779. [PubMed] [Google Scholar]
- Denckla MB (1973). Development of speed in repetitive and successive finger-movements in normal children. Developmental Medicine & Child Neurology, 15(5), 635–645. 10.1111/j.1469-8749.1973.tb05174.x [DOI] [PubMed] [Google Scholar]
- Denckla MB (1974). Development of motor co-ordination in normal children. Developmental Medicine & Child Neurology, 16(6), 729–741. 10.1111/j.1469-8749.1974.tb03393.x [DOI] [PubMed] [Google Scholar]
- Dirlikov B, Shiels Rosch K, Crocetti D, Denckla MB, Mahone EM, & Mostofsky SH (2015). Distinct frontal lobe morphology in girls and boys with ADHD. NeuroImage: Clinical, 7, 222–229. 10.1016/j.nicl.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjørtoft T, Grunewaldt KH, Løhaugen GCC, Mørkved S, Skranes J, & Evensen KAI (2013). Assessment of motor behaviour in high-risk-infants at 3months predicts motor and cognitive outcomes in 10years old children. Early Human Development, 89(10), 787–793. 10.1016/j.earlhumdev.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Fliers E, Rommelse N, Vermeulen SHHM, Altink M, Buschgens CJM, Faraone SV, Sergeant JA, Franke B, & Buitelaar JK (2008). Motor coordination problems in children and adolescents with ADHD rated by parents and teachers: Effects of age and gender. Journal of Neural Transmission, 115(2), 211–220. 10.1007/s00702-007-0827-0 [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, & Bastian AJ (2009). Children with autism show specific handwriting impairments. Neurology, 73(19), 1532–1537. 10.1212/WNL.0b013e3181c0d48c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddis A, Rosch KS, Dirlikov B, Crocetti D, MacNeil L, Barber AD, Muschelli J, Caffo B, Pekar JJ, & Mostofsky SH (2015). Motor overflow in children with attention-deficit/hyperactivity disorder is associated with decreased extent of neural activation in the motor cortex. Psychiatry Research: Neuroimaging, 233(3), 488–495. 10.1016/j.pscychresns.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB, & Mahone EM (2007). Effects of gender and age on motor exam in typically developing children. Developmental Neuropsychology, 32(1), 543–562. 10.1080/87565640701361013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, MacNeil LK, & Mostofsky SH (2011). Motor cortex inhibition: A marker of ADHD behavior and motor development in children. Neurology, 76(7), 615–621. 10.1212/WNL.0b013e31820c2ebd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Huddleston DA, Wu SW, Pedapati EV, Horn PS, Hirabayashi K, Crocetti D, Wassermann EM, & Mostofsky SH (2019). Motor cortex inhibition and modulation in children with ADHD. Neurology, 93(6), e599–e610. 10.1212/WNL.0000000000007899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulardins JB, Rigoli D, Licari M, Piek JP, Hasue RH, Oosterlaan J, & Oliveira JA (2015). Attention deficit hyperactivity disorder and developmental coordination disorder: Two separate disorders or do they share a common etiology. Behavioural Brain Research, 292, 484–492. 10.1016/j.bbr.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, & Georgiou-Karistianis N (2004). Investigating the cortical origins of motor overflow. Brain Research Reviews, 46(3), 315–327. 10.1016/j.brainresrev.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Jacobson LA, Peterson DJ, Rosch KS, Crocetti D, Mori S, & Mostofsky SH (2015). Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 54(11), 938–946. 10.1016/j.jaac.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Largo RH, Fischer JE, & Rousson V (2003). Neuromotor development from kindergarten age to adolescence: Developmental course and variability. Swiss Medical Weekly, 133(13–14), 193–193. 10.5167/uzh-34720 [DOI] [PubMed] [Google Scholar]
- MacNeil LK, Xavier P, Garvey MA, Gilbert DL, Ranta ME, Denckla MB, & Mostofsky SH (2011). Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology, 76(7), 622. 10.1212/WNL.0b013e31820c3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I, Lauterbach M, Slade P, Luís H, DeRouen T, Martin M, Caldas A, Leitão J, Rosenbaum G, & Townes B (2008). A longitudinal study of neurological soft signs from late childhood into early adulthood. Developmental Medicine & Child Neurology, 50(8), 602–607. 10.1111/j.1469-8749.2008.03043.x [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, & Denckla MB (2003). Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and Motor Skills, 97(3_suppl), 1315–1331. 10.2466/pms.2003.97.3f.1315 [DOI] [PubMed] [Google Scholar]
- Quatman-Yates CC, Quatman CE, Meszaros AJ, Paterno MV, & Hewett TE (2012). A systematic review of sensorimotor function during adolescence: A developmental stage of increased motor awkwardness? British Journal of Sports Medicine, 46(9), 649–655. 10.1136/bjsm.2010.079616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/ [Google Scholar]
- Reich W (2000). Diagnostic interview for children and adolescents (DICA). Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 59–66. 10.1097/00004583-200001000-00017 [DOI] [PubMed] [Google Scholar]
- Rosch KS, Dirlikov B, & Mostofsky SH (2013). Increased intrasubject variability in boys with ADHD across tests of motor and cognitive control. Journal of Abnormal Child Psychology, 41(3), 485–495. 10.1007/s10802-012-9690-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K (2007). Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proceedings of the National Academy of Sciences, 104(50), 19663–19664. 10.1073/pnas.0710329105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharoun SM, Bryden PJ, Otipkova Z, Musalek M, & Lejcarova A (2013). Motor skills in Czech children with attention-deficit/hyperactivity disorder and their neurotypical counterparts. Research in Developmental Disabilities, 34(11), 4142–4153. 10.1016/j.ridd.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Garefino AC, Kuriyan AB, Babinski DE, & Karch KM (2012). Diagnosing ADHD in adolescence. Journal of Consulting and Clinical Psychology, 80(1), 139–150. 10.1037/a0026577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Denckla MB, McCambridge T, Slomine BS, Mahone EM, & Suskauer SJ (2018). Preliminary use of the physical and neurological examination of subtle signs for detecting subtle motor signs in adolescents with sport-related concussion. American Journal of Physical Medicine & Rehabilitation, 97(6), 456. 10.1097/PHM.0000000000000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney KL, Ryan M, Schneider H, Ferenc L, Denckla MB, & Mahone EM (2018). Developmental trajectory of motor deficits in preschool children with ADHD. Developmental Neuropsychology, 43(5), 419–429. 10.1080/87565641.2018.1466888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, & Giedd JN (2010). Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. NeuroImage, 49(1), 63–70. 10.1016/j.neuroimage.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Ricciuti AJ, Stoff DM, Behar D, & Denckla MB (1989). Reliability of subtle (soft) neurological signs in children. Journal of the American Academy of Child & Adolescent Psychiatry, 28(5), 749–753. 10.1097/00004583-198909000-00017 [DOI] [PubMed] [Google Scholar]
- Willoughby MT (2003). Developmental course of ADHD symptomatology during the transition from childhood to adolescence: A review with recommendations. Journal of Child Psychology and Psychiatry, 44(1), 88–106. 10.1111/1469-7610.t01-1-00104 [DOI] [PubMed] [Google Scholar]
- Wilson BN, Kaplan BJ, Crawford SG, Campbell A, & Dewey D (2000). Reliability and validity of a parent questionnaire on childhood motor skills. American Journal of Occupational Therapy, 54(5), 484–493. 10.5014/ajot.54.5.484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


