Abstract
Vector-borne diseases (VBDs) are embedded within complex socio-ecological systems. While research has traditionally focused on the direct effects of VBDs on human morbidity and mortality, it is increasingly clear that their impacts are much more pervasive. VBDs are dynamically linked to feedbacks between environmental conditions, vector ecology, disease burden, and societal responses that drive transmission. As a result, VBDs have had profound influence on human history. Mechanisms include: (1) killing or debilitating large numbers of people, with demographic and population-level impacts; (2) differentially affecting populations based on prior history of disease exposure, immunity, and resistance; (3) being weaponized to promote or justify hierarchies of power, colonialism, racism, classism, and sexism; (4) catalyzing changes in ideas, institutions, infrastructure, technologies, and social practices in efforts to control disease outbreaks; and (5) changing human relationships with the land and environment. We use historical and archaeological evidence interpreted through an ecological lens to illustrate how VBDs have shaped society and culture, focusing on case studies from four pertinent VBDs: plague, malaria, yellow fever, and trypanosomiasis. By comparing across diseases, time periods, and geographies, we highlight the enormous scope and variety of mechanisms by which VBDs have influenced human history.
Keywords: disease ecology, vector-borne disease, plague, malaria, yellow fever, trypanosomiasis, mosquito, arthropod, colonialism, environment
Introduction
Vector-borne diseases (VBDs)—illnesses caused by pathogens transmitted by biting arthropods—have played a major role in human history. Today, VBDs account for more than one billion cases, one million deaths, and one-sixth of worldwide disability and illness annually (World Health Organization 2014), disproportionately impacting communities left impoverished and recovering from colonialism. While research in disease ecology predominantly focuses on the direct morbidity and mortality of VBDs, the scope of their effects is more pervasive. Understanding the full arc of VBD impacts on human history requires knowledge of how those diseases and their vectors interact with the environment, and placing those dynamics within societal and cultural contexts. Here, we synthesize the extent and mechanisms of this influence, emphasizing connections between disease ecology and the societal and environmental settings of historical places and times.
VBDs have affected human history via multiple socio-ecological mechanisms: (1) killing or debilitating large numbers of people, with demographic and population-level impacts; (2) differentially affecting populations based on prior history of disease exposure, immunity, and resistance; (3) being weaponized to promote or justify hierarchies of power, colonialism, racism, classism, and sexism; (4) catalyzing changes in ideas, institutions, infrastructure, technologies, and social practices in efforts to control disease outbreaks; and (5) changing human relationships with the land and environment. Because VBDs are intimately linked to human-modified environments and social structures through both their direct and indirect effects, we cannot understand their full impact without considering feedbacks within underlying socio-ecological systems (Fig. 1).
Figure 1. Socio-ecological feedbacks of vector-borne diseases (VBDs) throughout human history.
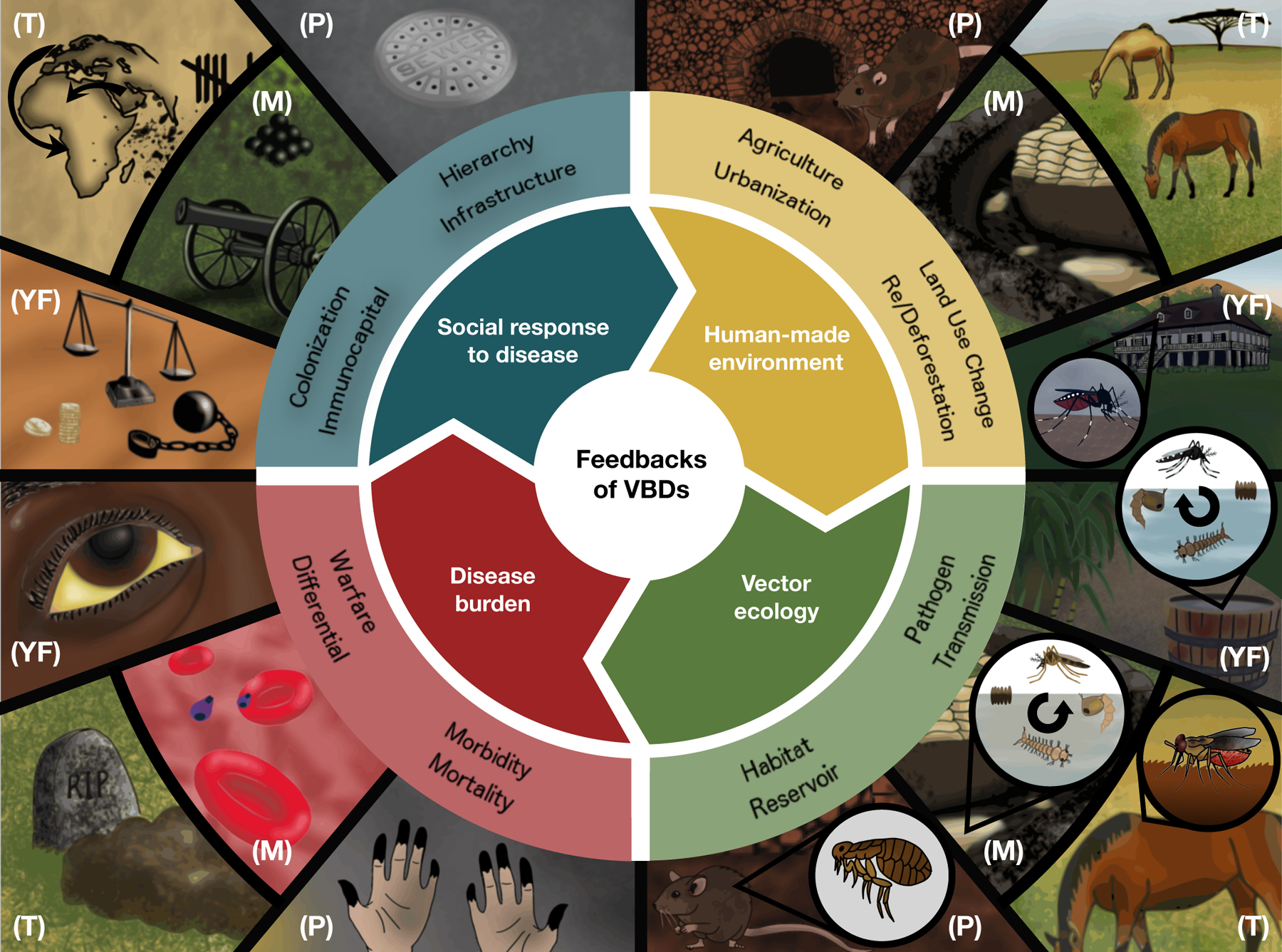
Humans have altered natural environments (yellow) in ways that led to outbreaks of diseases (red) such as plague (P), malaria (M), yellow fever (YF), and trypanosomiasis (T) via mechanisms explained by the corresponding vector ecologies (green). In response to these diseases, human societies have improved technologies, institutions, and infrastructure for human well-being, but also inflicted additional pain and suffering by weaponizing diseases in warfare, and perpetuating hierarchies of power, colonialism, racism, classism, and sexism (blue). Some of these social responses fed back into anthropogenic environmental changes (yellow).
The influence of VBDs on society and the environment requires recognizing the prominent role that racism (i.e., a system of advantage based on race) has played in many aspects of VBD ecology and social responses to disease. Of Roberts and Rizzo’s psychological factors that contribute to American racism (Roberts & Rizzo 2020), three have been particularly important in the global context of VBDs. Hierarchy perpetuates the notion that high-status groups are more valuable than low-status groups. Power codifies racism through the establishment of social norms, allocation of resources, and formation of policy. Segregation ensures that habitable surroundings are accessible to some but not others. By emerging from social hierarchy, power, and segregation, VBDs have been linked to reinforcing and exacerbating racism throughout history. Further, VBDs’ effects on population health are often mediated by racism, along with sexism, classism, and colonialism, across geography and time periods.
To understand the complex interplay between social and ecological mechanisms driving the impact of VBDs on humans throughout history, we synthesize historical and archaeological evidence within an ecological context (see Box 1 for a discussion of this review’s narrative style and interdisciplinarity). VBDs, ranging from malaria and dengue to leishmaniasis, onchocerciasis, and schistosomiasis, have caused widespread illness and disability, disproportionately affecting marginalized groups while influencing various realms of human society (Table 1). Here, we present case studies from four major diseases—plague, malaria, yellow fever, and trypanosomiasis—which have had profound and multimodal influence from the age of early humans to the present day, though the underlying mechanisms apply across all VBDs with high burdens (Fig. 2; Table 1). Each disease has a unique ecology determined by the habitat, breeding, and biting preferences of its vector, which interacts with human social structures and geography to shape VBD effects (Box 2). While there are some well-known consequences of VBDs on human history, such as malaria selecting for sickle cell and hemoglobin-related traits, and yellow fever and malaria contributing to the failed French attempt to build the Panama Canal, these are often framed as anomalous events. Here, we highlight lesser-known historical impacts and mechanisms to argue for the generality and pervasiveness of the socio-environmental consequences of VBDs.
Box 1. The narrative structure of this review.
The history of human vector-borne disease (VBD) combines the movement, activities, decisions, and social structures of people with the lands they inhabit and modify, and the interactions and conflicts that emerge between populations that come into contact. To understand these historical and modern influences, we draw on an interdisciplinary mix of history, archaeology, psychology, and disease ecology to argue that societal contexts are inextricable from the ecological settings that promote VBD transmission. While we emphasize socio-ecological mechanisms and feedbacks, and their ties to the ecology of VBDs, we present much of the main text in a narrative format that is common in historical scholarship but less common in ecology. We describe human events as a set of lived experiences, in which biases, beliefs, and social practices shaped outbreaks just as concretely as the physical landscapes and ecological environments in which they occurred.
Most ecological research, and other research in natural and physical sciences, is presented through the lens of empiricism—the idea of science as objective, and thus divorced from narrative. Physicist and race and gender scholar Dr. Chandra Prescod-Weinstein introduces the concept of White empiricism: “the phenomenon through which only White people (particularly White men) are read as having a fundamental capacity for objectivity and Black people (particularly Black women) are produced as an ontological other” (Prescod-Weinstein 2020). Similarly, research shows that psychologists tend to present White samples as more general and universal than samples from people of color, which reinforces the idea of White experiences as being more objective (Cheon et al. 2020). This work highlights how the concept of objectivity is applied unequally depending on race and gender, resulting in asymmetrical access to public recognition and resource allocation. In this review, we present historical narratives of VBD impacts on societies, as well as narratives reconstructed from archaeological evidence, as central to the ecology of VBDs. We attempt to represent previously under-recognized narratives, such as the exploitative story of Dr. Benjamin Rush during the 1793 yellow fever epidemic in Philadelphia, as recounted by two Black religious leaders whose accounts were widely suppressed by their contemporary publishers. This case is a prime example in which the narrative is inextricable from the socio-ecological setting of the disease outbreak and its impacts.
We highlight one additional stylistic note. There is some disagreement among scholars and fields about the proper capitalization of the racial designations “White” and “Black.” While many organizations advocate for capitalizing “Black” but not “white,” and others advocate for capitalizing both “Black” and “White” (Appiah 2020; Bauder 2020; Nguyen & Pendleton 2020), we have chosen to capitalize both “Black” and “White” in this paper, fully recognizing that other styles are also valid and meaningful. We invite readers to be cognizant of and to think critically about racial identities, prejudices, and injustices that persist today.
Table 1. Vector-borne diseases ranked in order of annual DALY burden, with primary vector, pathogen, geographical range, and categories of influence.
The top 10 vector-borne diseases as ranked by annual disability-adjusted life year (DALY) burden, with associated ecogeographical characteristics and influence.
| Disease* | Estimated Annual Disability-Adjusted Life Years (DALYs) in 2019 (Wang et al. 2020) | Primary Vector (Genera) | Primary Pathogen | Geographic Range | Examples of Key Categories of Influence |
|---|---|---|---|---|---|
| (1) Malaria | 46,437,811 | Mosquito (Anopheles) | Plasmodium protozoa | All inhabited continents | Outcomes of conflicts and mortality in warfare (Snowden 2008; Lockwood 2009; McNeill 2010); socioeconomic, racial, and gender inequities (Humphreys 2001; Sallares 2002; Heggenhougen et al. 2003); criminal justice (Campbell 2020) |
| (2) Dengue | 2,383,375 | Mosquito (Aedes) | DENV flavivirus | All inhabited continents | Mortality in warfare (Gibbons et al. 2012); tourism and economic decline (Nishikawa et al. 2016); genetic modification (Hoffmann et al. 2011) |
| (3) Schistosomiasis | 1,638,072 | Freshwater snail (Biomphalaria) | Schistosoma trematodes | Americas, Africa, Asia | Mortality and large-scale debilitation (McManus et al. 2018); disproportionate effects on marginalized groups (King 2010) |
| (4) Lymphatic filariasis | 1,628,649 | Mosquito (Anopheles, Culex, Aedes, Mansonia, Ochlerotatus) | Wuchereria and Brugia nematodes | Americas, Africa, Asia | Social stigmatization (Evans et al. 1993); mortality in warfare (Swartzwelder 1963; Leggat & Melrose 2005); reduction of economic output and increase in poverty (World Health Organization 1999; Ottesen 2000) |
| (5) Onchocerciasis | 1,230,433 | Black fly (Simulium) | Onchocerca nematode | Americas, Africa | Depopulation of riverine regions (Bradley 1976); psychosocial effects and female marriage age delay (Amazigo 1994; Wagbatsoma & Okojie 2004) |
| (6) Leishmaniasis | 696,703 | Sand fly (Phlebotomus) | Leishmania protozoa | Americas, Africa, Europe, Asia | Pre-Incan marker of religious significance (Bourget 2016); biowarfare and exacerbation of Syrian refugee crisis (Alawieh et al. 2014) |
| (7) Japanese encephalitis | 431,552 (Labeaud et al. 2011) | Mosquito (Culex) | JEV flavivirus | Asia | Changing agricultural practices through integrated vector management (van der Hoek et al. 2001; Keiser et al. 2005) |
| (8) Yellow fever | 290,137 | Mosquito (Aedes, Haemagogus, Sabethes) | YFV flavivirus | All inhabited continents | Colonialism and slavery (Berlin 2009; Bell 2010; McNeill 2010); racism and gaslighting (Jones & Allen 1794; Hogarth 2019); social hierarchy and power (Olivarius 2016) |
| (9) Chagas disease | 275,377 | Triatomine bug (Triatoma) | Trypanosoma protozoa | Americas | Settlement patterns in the Amazon (Coimbra 1988); improved safety of blood products (Dias et al. 2002); control efforts leading to greater sense of citizenship and rural community stability (Briceño-León et al. 1990) |
| (10) African trypanosomiasis | 82,615 | Tsetse fly (Glossina) | Trypanosoma protozoa | Africa | Colonization barrier and slave selection (Lambrecht 1980; Steverding 2008); migratory and pastoral practices (Lambrecht 1964; Gifford-Gonzalez 2000); obstacle to political centralization (Alsan 2015) |
Although plague is contemporarily not in the top-10 VBDs ranked by DALY burden, it did top the charts historically (i.e., Black Death, Plague of Justinian, etc). Plague was thus chosen as one of the four focal diseases highlighted in this paper.
Figure 2. Timeline of vector-borne disease impacts across history.
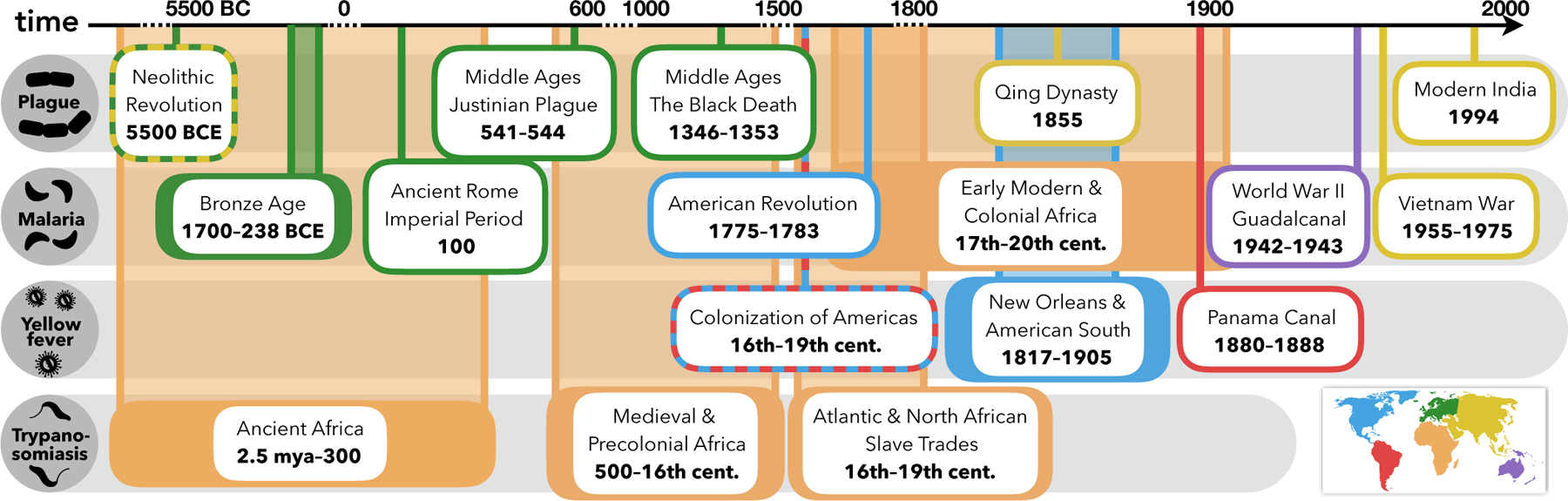
Plague, malaria, yellow fever, and trypanosomiasis have affected human history from the Paleolithic era to the modern age through a variety of mechanisms; case studies highlighted for Africa (orange), Asia (yellow), Australia (purple), Europe (green), North America (blue), and South America (red).
Box 2. Mechanisms of VBD transmission.
Transmission of VBDs to humans results from interactions among primarily arthropod vectors, pathogens, human and/or non-human hosts, and the environment. For pathogens to be transmitted, vectors must be abundant, come in contact with infected human or non-human hosts to acquire the pathogen, and bite uninfected human hosts, who either continue the chain of transmission or end the cycle as dead-end hosts (Baum 2008). Vector population size, physiology, behavior, and competence to transmit pathogens are influenced by abiotic and biotic factors, such as habitat type, climate, predation, and competition (Moore et al. 2010; Couret et al. 2014; Ferraguti et al. 2016; Mordecai et al. 2019; Shocket et al. 2020). In particular, because of the partially aquatic life cycle of mosquitoes and many other vectors, vector abundance often depends on freshwater availability and water storage practices (Poh et al. 2019).
Human behavior interacts with environmental factors to affect disease transmission. For example, human modification of the physical environment can drive vector breeding habitat availability. Some vector species (e.g., Anopheles spp. mosquitoes) thrive in agricultural contexts and breed in ditches, canals, irrigated fields, and lowland freshwater swamps, while other species (e.g., Aedes aegypti mosquitoes) breed in abandoned containers (e.g., bottles, jugs, toilets, tires) and in contaminated aquatic systems (Zahouli et al. 2017; Du et al. 2019). Dense human populations in built environments such as urban centers, army barracks, and ships can facilitate contact between vectors and human hosts (Willoughby 2017). In turn, people may respond to real or perceived disease risk in the environment by distancing themselves, emigrating, or abandoning settlements in regions with high burdens of disease (“disease avoidance”).
Finally, VBD dynamics depend on human disease susceptibility. Many pathogens induce some degree of immunity or resistance following infection, resulting in periodic epidemic cycles within populations as susceptibility waxes and wanes. When populations with differing disease histories come into contact, differential immunity to shared pathogens may cause asymmetric effects within and between populations (McNeill 2010). Together, these processes—abiotic factors, human behavior, and host susceptibility—combine to determine transmission and VBD burden in a given location (Bayoh & Lindsay 2004; Alto & Bettinardi 2013; Paaijmans et al. 2013).
Plague
Disease Ecology
Plague, caused by Yersinia pestis bacteria, is one of a few VBDs (including murine typhus) maintained in enzootic cycles with rodents and fleas (see Box 3 for disease ecology terms). While Y. pestis typically causes low mortality in rodent hosts, it occasionally causes massive die-offs, putting humans at higher exposure risk as rodent fleas seek alternative hosts (Gage & Kosoy 2005). Plague causes three forms of human disease: bubonic, septicemic, and pneumonic, the latter of which can be transmitted among people via airborne droplets. All forms have high fatality rates without antibiotic treatment. Plague is endemic to Africa, the Americas, and Asia; the disease is typically found in semi-arid forests and grasslands (Stenseth et al. 2008), but can also exist in densely-populated urban environments. While human morbidity and mortality due to plague are currently low, it has exhibited a persistent influence.
Box 3. Glossary of relevant disease ecology terms.
Acquired immunity:
Upon exposure to a pathogen, the host starts to develop immunological memory to recognize the pathogen and to activate the immune system; reliant on highly specific antibodies that can prevent reinfection or limit disease symptoms upon reinfection
Built environments:
Human-made structures and spaces in which people live, work, and recreate
Differential immunity:
State in which particular classes or groups of people are more susceptible to diseases than others
Disease avoidance:
Organisms tend to avoid infectious agents (including vectors), when feasible, since the biological benefits of remaining disease-free may outweigh the temporary costs of avoidance
Enzootic cycle:
Process by which animals, which serve as long-term reservoirs for pathogens, maintain and pass on infection to a vector; also known as a sylvatic cycle
Host:
An organism that harbors a pathogen, often with some energetic or fitness cost; in the context of this paper, hosts may include humans or other animals
Human-environment interactions:
Ways in which humans and their social systems, decision-making, and behavioral processes interact with the natural world
Infrastructure:
Basic organizational structures, facilities, and programs which are needed for the successful operation of a human society
Innate immunity:
Intrinsic resistance possessed by a host prior to exposure to a pathogen; the general, nonspecific immune response and defense mounted by the host
Land use change:
Process of human activities transforming ecological landscapes
Pathogen:
A disease-causing agent, including bacteria, viruses, fungi, protozoa, and other infectious organisms
Reservoir host:
Non-human organisms that can harbor pathogens and can contribute to pathogen spillover into human transmission cycles
Social and racial hierarchies:
Systems of social stratification that arise from the belief that certain social classes or racial groups are superior to others
Vector breeding habitat:
Areas that are suitable for vectors to reproduce; stagnant water is often an optimal habitat for mosquito vectors
Vector competence:
Ability of vectors to acquire, maintain, and transmit pathogens to hosts
Vector ecology:
Study of arthropods that transmit pathogens, the interaction between such arthropods and disease-causing organisms, the impacts of the environment on their physiology and behavior, and their contact with humans
Vector:
Organism that functions as a carrier of pathogens between organisms of a different species, including mosquitoes, ticks, fleas, and tsetse flies
In the case studies below, plague impacted human history at the nexus between settlement, mobility, and urbanization across continents. Plague has both catalyzed and set into motion the preconditions for societal upheaval. Because it is so deadly when uncontrolled, impacts of plague have often been mediated by demographic change through the sheer numbers of lives lost during epidemics.
(i). Neolithic Revolution in Eurasia
Evidence suggests that plague outbreaks emerged from the sanitation and public health shortcomings of early urban hubs, contributing to the downfall of many agricultural settlements more than 5,000 years ago (Rascovan et al. 2019). From around 5500 BCE in Eastern Europe, the Cucuteni-Trypillian culture inhabited mega-settlements of tens of thousands of people living in compact arrangements of houses with poor sanitation, high densities of animals, and accumulated food storage: ideal conditions for rodents and plague (Barrett et al. 1998). Around 3400 BCE, many buildings were abandoned and burned, residents perished or moved, and mega-settlements collapsed. Phylogenetic and genomic analyses from human remains revealed that multiple independent lineages of Y. pestis spread across Eurasia during this decline, likely through trade routes and wheeled transport (Rascovan et al. 2019). This is one of the earliest examples of the built environment promoting a major VBD epidemic, which in turn wiped out the human settlement and the population density needed to sustain transmission (Table 2).
Table 2. Historical time periods, associated ecological characteristics, and VBDs.
The unique ecological and human social context of time periods throughout history have set the stage for specific vector-borne diseases to emerge.
| Years | Overview | Ecological Characteristics | Prominent VBDs | |
|---|---|---|---|---|
| Paleolithic Era | 2.6 million years ago → 10,000 BCE | Nomadic and semi-nomadic hunter-gatherer society; foraging for wild plants or pursuing wild animals | Hypothesized early spillover of parasites from primates to humans; diseases present in animal reservoirs and human populations; incomplete immunity makes previously infected people susceptible to future infection | Malaria |
| Neolithic Revolution | 10,000 BCE → 4,000 BCE | Transition from traditional hunter-gatherer lifestyles to settlement-based agricultural lifestyles | VBD transmission rates surge due to increased contact between humans and animals in domestic settings | Malaria |
| Age of Empires | 4,000 BCE → 400 CE | Complex societies and prominent empires in many geographic areas, such as in Mesopotamia, Mediterranean, Africa, and Asia | Mercantile international trade and war scale up societal contact, leading to VBD spread among distinct populations | Trypanosomiasis,Malaria |
| Middle Ages | 400 CE → 1400s CE | Formation of new kingdoms and changes in power structure; large-scale deurbanization | Population decline followed by growth in Europe; large-scale movements of tribes and agricultural/land use modification lead to new human-environment interactions | Plague, Trypanosomiasis |
| Atlantic Empires | 1400s CE → 1750s CE | Old World interacts with New World via colonialism and exploration | Contact between different populations facilitates exchange of crops, livestock, and diseases | Yellow Fever, Malaria |
| Industrial Revolution | 1750s CE → 1900s CE | Transition to intensive manufacturing processes; shift from agriculture to industry | Worldwide population growth; highly urbanized environments increase close proximity transmission of VBDs | Yellow Fever, Malaria |
| Modern Context | 1900s CE → Present | Advent of novel technologies; increasingly globalized world | Tourism, global travel, and economic industries affected by VBDs; political uprising; poverty traps | Dengue, Zika, Yellow Fever, Malaria |
(ii). Middle Ages in Europe
The Plague of Justinian (541–544 CE), one of the world’s deadliest pandemics, was part of an emerging pattern of trade and urbanization fueling plague pandemics severe enough to upend empires and economic systems. As large settlements and transcontinental empires emerged in Medieval Europe, the Plague of Justinian facilitated changing agricultural practices and socio-politics around Constantinople, the capital of the Byzantine Empire. The outbreak was initiated by plague-infected rat fleas found aboard merchant grain ships from Egypt (Haensch et al. 2010). Farmers infected with plague were unable to tend their crops, inflating grain prices, decreasing tax revenues, and causing famine (Sabbatani et al. 2012). Human mobility between ports spread the plague, weakening the Byzantine Empire and ushering in the invasion of the Kingdom of the Lombards (Evans 2005).
The Black Death in the mid-1300s CE, the most well-known plague pandemic, caused large-scale demographic changes that helped topple the European feudal system by altering the distribution of power among social classes and in turn facilitating forest regrowth. The pandemic killed tens of millions of people—an estimated 30% of Europe’s population (McEvedy 1988; Raoult et al. 2013). Even before the plague arrived in medieval Europe, population growth and rising demand for labor threatened the feudal system (Moore 2002). The subsequent labor shortage following the Black Death increased serf wages and power (Clark 2016), expanding economic freedom for the surviving serfs and making feudalism unprofitable (Gelman 1979; Blockmans 1980). This demographic and economic transition led to large-scale land abandonment, reduced agricultural activity and grazing, prompted woodland regrowth, and altered human-environment interactions (Williams 2000; Yeloff & Van Geel 2007). Arable land decreased and forest area increased in Southern England from 1307–1377 (Poos 1991), while in France and Denmark pollen cover increased beginning in 1375 due to agricultural decline and reforestation (Stebich et al. 2005). The Black Death transformed not just the economy but also the ecological landscape, which in turn would reduce opportunities for plague transmission in urban areas.
The Black Death also exacerbated ethnoreligious scapegoating and reignited Jewish persecution. Across Western Europe, Jewish people were accused of causing the plague by poisoning water and food, tortured into confessing, and burned alive (Cohn 2007; Voigtländer & Voth 2012). The Black Death massacres exemplified feedbacks between VBD and social oppression within the context of European antisemitism.
(iii). Modern Plague Epidemics in Asia
Plague pandemics in Asia, which occurred alongside underlying societal unrest, also caused mass mortality. In the Yunnan Province of China during the 19th century, a plague pandemic erupted from rodent reservoirs into a society undergoing demographic change, economic shifts, and ethnic conflict. Tension culminated in the Panthay (Du Wenxiu) Rebellion (1856–1873), in which Muslim Hui miners rebelled against the Qing following decades of ethnic and class disputes. Plague killed many Imperial soldiers and was disseminated via refugee and troop displacement, resulting in the depopulation of Yunnan Province (Peckham 2016). Villages were deserted following epidemics of plague and other infectious diseases (Benedict 1988); in a single county, 70–80% of the population perished, likely due to plague (Rocher 1879; Benedict 1988). The outbreak, which spiraled into the Third Plague Pandemic, had huge death tolls across Asia.
Although plague epidemics have become rare since the discovery of antibiotics, one recent outlier illustrates the continued influence of feedbacks among urbanization, mobility, plague, and social change that can occur within inequitable societies. The Indian city of Surat, formerly a key port for the British East India Company, was reshaped by the legacy of exploitative colonial mercantilism, mismanagement, and a caste hierarchy promoted by the British administration (Wisner et al. 2004; Barnes 2014). Surat’s resulting poverty, corruption, influx of migrant laborers, and segregation among classes led to unsanitary conditions and under-developed infrastructure (Jacobsen 1996; Dutt et al. 2006). These conditions prompted a plague outbreak of over one thousand cases in Surat in 1994, with deaths primarily occurring among lower castes and socioeconomic groups (Barnes 2014), similar to the previous Third Plague Pandemic (Klein 1988). Over half a million people (one-fourth of the population) emigrated from Surat within two days, leading to mass shutdown of businesses (Dutt et al. 2006). In response, the city bolstered its infrastructure, sanitation, and food hygiene standards (Chatterjee 2015), ultimately becoming a nation-wide model for sanitation and reducing potential for plague transmission.
Malaria
Disease Ecology
Like many of humanity’s highest-burden VBDs (Table 1), malaria is mosquito-borne. Human malaria is caused by protozoan Plasmodium parasites (primarily P. falciparum, P. malariae, P. vivax, and P. ovale) and transmitted by Anopheles mosquitoes (Dutta & Dutt 1978). Infection with malaria manifests as recurring fever and flu-like symptoms, but severe cases can progress to organ dysfunction, anemia, and death (Bartoloni and Zammarchi 2012). P. malariae existed hundreds of thousands of years before human origins and was the only species infecting pre-agrarian hunter-gatherer populations of Eurasia and Africa (Carter & Mendis 2002). The other Plasmodium species emerged from African non-human primates into humans and migrated out of Africa with humans: P. vivax spread to Arabia and Eurasia between 30,000 and 10,000 years ago; P. ovale spread to tropical areas like New Guinea approximately 4,000 years ago (Carter & Mendis 2002); P. falciparum emerged around the same time in Africa, and over several millennia expanded into Europe, Asia, and eventually to the Americas during the transatlantic slave trade (Rodrigues et al. 2018). Unlike the other VBDs described here, the life cycle of the predominant human malaria parasites occurs almost exclusively within humans and mosquitoes (although spillover of human malaria into primates and primate malaria into humans does occur) (Faust & Dobson 2015; Grigg & Snounou 2017).
Human movement, malaria control, and climate drive malaria dynamics, with moderate temperatures required for parasite transmission and sufficient rainfall needed for larval mosquito habitat (Thomson et al. 2006; Béguin et al. 2011; Mordecai et al. 2013; Yamana & Eltahir 2013). Malaria historically occurred in environments ranging from rural settlements in Africa to irrigated plantations in North America to the Amazon’s forest fringes. Although historically distributed throughout temperate and tropical zones in 140 countries, control efforts have restricted malaria to 88 countries in the tropics and subtropics, often centered on agricultural areas undergoing land conversion (Martens et al. 1995; Hay et al. 2004; Zahouli et al. 2017). Today, over 90% of the 228 million global malaria cases and 405,000 deaths are concentrated in sub-Saharan Africa, according to a 2018 estimate (World Health Organization 2019, 2020). Genetic mutations associated with malaria resistance, such as sickle cell and Duffy-negative alleles, remain widespread in human populations historically exposed to high malaria burdens, especially in Africa and Southeast Asia (Allison 2009).
In the case studies below, malaria and its associated ecological conditions influenced agriculture, settlement, societal inequities, human mobility, outcomes of war and conflict, racial policies, and criminal justice. Malaria has had significant effects throughout the course of human history on five continents, through all five of our socio-ecological mechanisms.
(i). Ancient Rome to Modern Italy
Malaria transmission has been associated with, and has shaped, human agricultural and settlement activities throughout history. During the Roman Empire, malaria depressed the agrarian economy and affected demography across the Italian Peninsula, starting in the Imperial period (ca. 100 CE) if not earlier. Archaeological and historical evidence includes biomarkers of malaria detected within Roman-era human skeletal remains from Apulia, Umbria, and Campania; a Late Antique child cemetery linked to a malaria epidemic; and Roman author Cicero’s letters to his friend Atticus detailing quartan fevers between 50–49 BCE (Sallares 2002; Soren 2003; Marciniak et al. 2016). Malaria in Roman Italy likely resulted from the interplay of pan-Mediterranean trade, which promoted the spread of the parasite and its vector from the South and East, and villa estate agriculture, which increased the availability of suitable vector breeding habitat (Sallares et al. 2004; Yasuoka & Levins 2007; Harper 2017). Hotspots of malaria probably occurred near coastal marshes, low-lying flood plains, and within the city of Rome (Di Luca et al. 2009).
Malaria likely reinforced class and gender inequities in Roman Italy, as it often does today (Heggenhougen et al. 2003; Shah 2010; WHO 2018). Many enslaved and poor men were forced to live and work in low-lying agricultural fields and unsanitary housing during peak malaria season, exposing them to vector bites (Joshel 2010). They lacked access to the high-altitude (non-malarious) rural estates to which wealthy Romans retreated in the summer and fall (Sallares 2002). Women in the ancient Roman world were mostly confined indoors and away from swampy countryside environments (Knapp 2011); malaria’s capacity to cause miscarriage and fetal abnormalities and its increased severity in pregnant women and children (Saito et al. 2020) may have contributed to the enforcement of this oppressive social structure.
Malaria-suitable habitat, land use, settlement patterns, and cultural practices were tightly intertwined in early civilizations. In the Bronze Age, the Nuragic civilization (1700–238 BCE) on the island of Sardinia adapted their housing and agricultural practices to malaria (Brown 1986; Setzer 2010). While most pastoralist cultures reside in lowlands near the most productive grazing grounds, Sardinians established summer settlements in high-elevation areas and grazed lowlands only during winter months with lower malaria risk (Brown 1981, 1986). Like the Romans, Sardinians restricted women (especially pregnant women) to the home and away from the more malarious countryside (Brown 1986).
The ecology of malaria in Italy was tied to land use, inequitable economic systems, and human movement that promoted transmission. The malaria burden increased after the unification of Italy in 1861, as deforestation and erosion in the Apennines mountains flooded rivers and created coastal lagoons that increased mosquito breeding habitat (Snowden 2008). In turn, this high burden of malaria discouraged investment in agriculture, left vast tracts of land minimally cultivated, and maintained high mosquito populations via accumulated water. Agriculture in the formerly-Roman South was still dominated by villa estate agriculture, which required an influx of hundreds of thousands of migrant laborers to harvest wheat during peak malaria season (Snowden 2008). These impoverished workers slept in open huts and had poor nutrition, increasing their exposure and susceptibility to malaria. Southern Italy’s high malaria burden was a major factor contributing to the Italian diaspora, in which millions emigrated to the Western hemisphere in the late 19th and early 20th centuries (Snowden 2008).
Recognition that social and ecological systems together perpetuated malaria prompted the establishment of new social infrastructure in Italy. By 1900, Italy’s malariologists knew that malaria was mosquito-borne and could be treated with quinine. Recognizing that the most at-risk population was impoverished, illiterate, and untrusting of physicians, they viewed malaria as a social disease and aimed to empower the rural poor and earn their trust (Snowden 2008). Rural schools and health centers were developed to teach the population about mosquitoes and distribute quinine, drastically reducing malaria incidence, illiteracy, and overall morbidity and mortality (Snowden 2008). By World War I, the geographic area affected by malaria had contracted and deaths had been reduced by almost 90% (Snowden 2008). The response to malaria led to solutions that broadly enhanced health and wellbeing.
(ii). American Revolution, Civil War, and Reconstruction
The convergence of favorable ecological conditions and differential histories of exposure and resistance to disease allowed malaria to play a decisive role in the American Revolution (1775–1783). The swampy Chesapeake and Carolina Lowcountry and irrigated plantations created ideal environments for Anopheles mosquitoes (McCandless 2007). While many Continental Army soldiers and local militiamen had acquired malaria resistance through repeated exposure living in the South, most British troops were unexposed (McNeill 2010). After besieging Charleston early in 1780, more than half of British general Charles Cornwallis’s troops became incapacitated with malaria infection (and typhoid) and were unable to fight (Humphreys 2001; McNeill 2010). This ultimately led to the British defeat and surrender at Yorktown, tipping the scales of the Revolutionary War in favor of the Americans (McNeill 2010).
In contrast to its asymmetric effects on the American Revolution, malaria infection, resulting from a failure to understand the habitat and climatic drivers of malaria ecology, negatively impacted both sides of the American Civil War (1861–1865). Malaria was responsible for a significant portion of the disease-related casualties and burden, forcing campaigns to be abandoned and prolonging the war (Bell 2010; Lockwood 2012). In 1862, Union General Winfield Scott encouraged delaying the campaign to take Vicksburg until the advent of cold weather in November, which would reduce fevers in latitudes below Memphis (Lockwood 2009). When his advice was ignored and the campaign began that summer, malaria decimated Union regiments, and the campaign failed. Confederate troops attempted to recapture Baton Rouge in August 1862 following the Union retreat from Vicksburg, but lost two-thirds of their troops en route, largely to malaria, allowing Union troops to narrowly escape defeat (Steiner 1968). Malaria thus postponed campaigns, prolonged the war, and increased the death toll (Sartin 1993).
Following the American Civil War and into the 20th century, White landowners weaponized malaria to justify racial hierarchy, maintain social power, and segregate themselves from at-risk environments, reinforcing exploitation of Black people (see Box 1 for a note on capitalization of racial groups). Black sharecroppers on rice and sugarcane plantations inhabited porous housing, were overworked and malnourished, and were not allowed access to adequate medical care (Humphreys 2001). White landowners imposed environments where Black workers were constantly exposed to mosquito bites and suffered a high burden of malaria and other diseases. Racists asserted that the higher burden of disease in Black people was due to biological and moral weaknesses associated with race, and used such arguments to justify Black sharecroppers’ living conditions and to legitimize Jim Crow laws (Hoffman 1896; Humphreys 2001). In this way, malaria interacted with landowner-imposed living conditions and the status quo hierarchy to entrench the social position of Black people in the American South. By contrast, malaria was less of a burden in Louisiana and Arkansas plantations, where Black farmhands lived in towns instead of “bedraggled huts” and conditions, though still terrible, were better for reducing malaria exposure (Barber 1946; Humphreys 2001).
(iii). Early Modern and Colonial Africa
While the vast majority of the modern malaria burden remains in Africa, the disease has shaped African societies throughout history by impeding colonization, being weaponized to punish criminals, shaping settlement and trade patterns, and reinforcing colonial oppression.
In 17th–19th century Madagascar, malaria and its ecological conditions precluded early European settlement and were employed as a criminal justice policy. The French attempted to establish a garrison in southeast Madagascar in 1643, and then again in the northeast in 1807. Both attempts failed due to decimation of troops and settlers from malaria (Ellis 1838). Meanwhile, the Merina Kingdom wielded the ecology of malaria as a punishment tool by banishing high-ranking criminals to malaria-endemic lowland regions of the island with notoriously unfavorable outcomes (Ellis 1838; Campbell 2020). The ecological context of malaria exhibited powerful influence on Malagasy societal systems.
In 17th–19th century Ethiopia, malaria’s lowland ecology shaped human geography by restricting settlement locations and travel, which in turn defined human disease avoidance behavior. Around 1614, King Susenyos I inhabited his palace on the shore of Lake Tana and the Blue Nile River (McCann 2015). Within years, the emperor relocated his lakeside capital to the higher-elevation site of Gondar, likely fleeing from the frequent malaria epidemics near the water and thereby initiating a new period of Ethiopian political history (McCann 2015). In the mid-1800s, travelers through the Mareb River Valley documented the seasonal practice of local traders and farmers avoiding the Abyssinian deep valleys where the climate was highly suitable for malaria (Burton 1924). Further, inhabitants only traveled to neighboring provinces during the dry months in order to avoid malaria (Baker 1883). Malaria dynamics both altered, and were altered by, human behavior.
In 20th century West Africa, malaria catalyzed the establishment of racial segregation policies. In Sierra Leone during the early 1900s, the British colonial government implemented health segregation policies in which Europeans retreated to an uphill residential zone outside of Freetown at nighttime to avoid malaria, while African locals—considered already infected—remained in the city (Spitzer 1968). This segregation policy, while formulated from an underlying knowledge of nocturnal Anopheles biting behavior, was guised under purely medical pretenses and contributed to pervasive racial biases in the colony (Frenkel & Western 1988).
(iv). Global 20th Century Military Conflicts
Combatants in both World Wars weaponized the ecology of European malaria, in some cases intentionally, to inflict maximum military and civilian damage, leaving behind a devastated socio-ecological landscape that reversed decades of control efforts. In Southern Europe, trenches and artillery craters became mosquito breeding habitats, and troop movement caused outbreaks in areas where the disease had been previously eradicated (Snowden 2008). After World War I, it took six years to reduce malaria back to pre-war levels. In World War II, Nazis used malaria as a barrier to Allied troops’ advance and as biological warfare against Italian civilians (Snowden 2008). The Nazis flooded nearly 100,000 acres of former marshland near Rome with seawater, promoting habitat for the most competent local malaria vector, An. labranchiae (Snowden 2008). In the worst-affected places, An. labranchiae became the dominant mosquito species, rising from 30% to 100% of all mosquitoes and causing over 90% of civilians to become infected with malaria (Snowden 2008). Despite this staggering human cost, malaria had little effect on the outcome of either war due to its nearly equal effect on both sides of the conflicts.
Malaria also played a substantial role in modern conflicts in Asia, lengthening the Pacific Theater of World War II and the Vietnam War by causing significant mortality on both sides of these conflicts. Malaria in WWII contributed to the largest number of military and civilian casualties for any war in human history, debilitating Allied and Axis Powers. U.S. Army General Douglas MacArthur summarized the impact: “This will be a long war if for every division I have facing the enemy I must count on a second division in hospital with malaria and a third division convalescing from this debilitating disease” (MacLeod 1999). Malaria in the Pacific forced the surrender of U.S. troops in Bataan, Philippines and the evacuation of Japanese troops from Guadalcanal (Joy 1999). Malaria also prolonged the Vietnam War (1955–1975): U.S. troops reported over 24,000 cases and nearly 400,000 sick-days due to the disease (Beadle & Hoffman 1993). Malaria prevalence among Viet Cong troops was 50–75%, and soldiers may have raided plantations and dispensaries for drugs to treat symptoms (Bruce-Chwatt 1985).
The massive death toll and weaponization of malaria during large-scale global conflicts sparked major public health efforts to manage the socio-ecology of disease transmission, as governments recognized it as a national security threat. The Malaria Control in War Areas (MCWA) program was established during World War II to manage malaria around military bases in the Southern U.S. and minimize lost productivity. MCWA trained local and state health department officials on control techniques. This led to the creation in 1946 of what became the Centers for Disease Control and Prevention (CDC), whose initial mission of preventing malaria spread across the nation soon expanded to encompass all disease prevention and surveillance (Parascandola 1996).
Yellow Fever
Disease Ecology
Like malaria, yellow fever is transmitted by mosquitoes. The primary vector of yellow fever, Aedes aegypti, also transmits other flaviviruses like dengue, Zika, and Japanese encephalitis, as well as alphaviruses like chikungunya and Ross River. Yellow fever is an acute disease caused by an RNA virus (Barnett 2007), with symptoms including hemorrhaging, jaundice, vomiting, muscle pain, and often death (McGuinness et al. 2017). The disease is endemic in tropical regions of the Americas and Africa and is maintained in a sylvatic cycle of transmission between non-human primates and tree-hole breeding mosquitoes (Barrett & Monath 2003). Spillover can result in urban outbreaks of yellow fever with transmission primarily between humans and Ae. aegypti mosquitoes (Miller et al. 1989). In Africa, a savannah cycle occurs with mixed transmission between mosquitoes, humans, and non-human primates (Barrett & Monath 2003). Historically, Ae. aegypti thrived in plantations that provided storage containers for mosquito breeding and fewer insectivorous birds compared to forests (Fig. 1) (McNeill 2010).
Children are more likely than adults to survive yellow fever infection, which confers long-term immunity. Differential immunity between groups varying in previous exposure and immunity played a central role in determining the outcomes of conflict, particularly before the discovery of the mosquito transmission cycle and a highly effective vaccine in the early 20th century (McNeill 2010). Related to true differential immunity is immunocapital: socially-acknowledged and often socially-constructed differences in disease susceptibility that reinforced the power and privilege of White men, particularly in the American South (Olivarius 2019).
In the case studies below, yellow fever impacted human history by creating and perpetuating societal hierarchies, differentially affecting conflict, catalyzing infrastructure development, being weaponized to entrench racism, and stifling economies. Primarily Euro-American examples are referenced due to the availability of evidence, although the disease almost certainly had a significant influence in other continents.
(i). Empire and Colonization in the Americas
The institutionalization of Black slavery—one of the most exploitative social and economic systems in history—was driven by profiteering and rationalized by racism that intersected with yellow fever. In Barbados, English settlers arriving in 1627 initially relied on indigenous captives and White indentured servant labor (Gragg et al. 2003; Beckles 2016), until a 1642 treaty allowed access to Portuguese slave dungeons on the African coast (Great Britain & Chalmers 1790). Slave vessels arriving with Ae. aegypti mosquitoes contributed to a major yellow fever epidemic by 1647 (Cray 2015). The system of chattel slavery, in which slaveowners generated profits from enslaved people until their deaths, was made even more profitable by the fact that African people died of yellow fever at about half the rate of other people in Barbados, likely due to previous exposure and immunity. Slavery and its unprecedented exploitation soon became ingrained as the island’s primary labor system and rendered Barbados the first full-fledged slave society and richest American colony in the British Empire (Berlin 2009; Beckles 2016). The Barbados Slave Code of 1661 provided the legal framework that extended slavery to other British colonies including Jamaica, Virginia, and South Carolina (Thomas 1930; Nicholson 1994; Dunn 2012; Beckles 2016). The introduction of yellow fever-infected Ae. aegypti mosquitoes into the plantation ecology of the Americas was thus intimately linked to the establishment of slavery.
Throughout colonial conflicts in the Americas, yellow fever ecology coupled with differential immunity created advantages for those inhabiting and defending colonies against invaders (McNeill 2010). For example, differential immunity aided Haitians in their fight for independence from France in the turn of the 19th century. The plantation economy of the French colony of Saint-Domingue (now Haiti) had created ecologically suitable environments for yellow fever transmission (Perry 2008). Toussaint Louverture, a freedman governing for the French Republic, led enslaved people in revolts between 1791 and 1804; he drew French troops into guerrilla battles away from coastal enclaves and resupply ships, where they were vulnerable to yellow fever (Bell 2009). Yellow fever killed many of the nearly 33,000 immunologically naïve troops Emperor Napoleon Bonaparte deployed to Haiti from 1801–1803 (Bollet & Jay 2004). Subsequently, Haiti gained independence in 1804, becoming the first successful uprising that led to the establishment of a new country ruled by formerly enslaved people (Maingot 1996). The victory was followed by the massacre of the remaining White colonizers (Girard 2011), leading to a “terrified consciousness” among plantation owners in the Americas and heightened cruelty against enslaved people in an attempt to suppress revolts (Maingot 1996; Michael Byrd & Clayton 2000). Realizing major defeat in the Americas and fearing further losses, Napoleon sold the Louisiana territory to the United States and withdrew from the continent.
(ii). Industrial Revolution in North America
The crowded urban environments of early America, ripe for yellow fever outbreaks, became the catalyst for public health and infrastructure development that effectively reduced transmission, despite the mosquito transmission cycle remaining unknown. In 1793, Philadelphia had high population density and few safeguards to handle a yellow fever outbreak that claimed over 5,000 lives (Foster et al. 1998). Seeking a solution to dirty well water, which citizens believed caused yellow fever (Levine 2010), the Philadelphia government in 1800 commissioned its first municipal water system to provide potable drinking water, ended unhygienic water collection, and began constructing storm sewers and sewage pipes (Donaldson 1987). The epidemic also prompted the creation of the Board of Health—an early foundation for the modern U.S. healthcare system—which implemented sanitary and housing inspections, enforced vaccination of children against diseases like smallpox, and chlorinated the water supply (Higgins 2016).
Concurrently, false claims of immunity were weaponized during the 1793 Philadelphia yellow fever epidemic to exploit Black labor and entrench social hierarchies. Based on medical treatises that falsely stated that Black people were more resistant to yellow fever, Dr. Benjamin Rush, a prominent White physician, mobilized leaders in the Black community to urge Black people to volunteer to assist the sick (Jones & Allen 1794; Hogarth 2019). Many free Black people, believing Dr. Rush’s claims that they were immune and wanting to prove their worthiness as citizens, nursed sick White people, removed corpses, and connected orphaned children to care, while nearly one-third of White residents fled the city (Jones & Allen 1794; Hogarth 2019). As hundreds of Black people died performing these services, White doctors perpetuated the myth of Black immunity by downplaying deaths of Black volunteers (Jones & Allen 1794). Despite risking their lives for little to no compensation, Black people were publicly accused of profiteering and plundering sick White people during the outbreak—a profound example of racist beliefs and practices combined with medical gaslighting (Jones & Allen 1794; Hogarth 2019).
Social constructions around purported yellow fever immunity reinforced social hierarchies in 19th century New Orleans. During this period, roughly half of all individuals who contracted yellow fever died. As a result, the concept of immunocapital took hold, in which White men with demonstrable immunity were deemed worthy of investment and granted expanded economic, political, and social power (Olivarius 2019). Meanwhile, doctors incorrectly reported lower death rates and falsely alleged that Black people were more “naturally resistant” to yellow fever than White people (Olivarius 2016), which racists perverted to argue that Black people had a duty to be enslaved into strenuous labor, impeding their upward social mobility. Immunity therefore translated into immunocapital for White, but not Black, individuals.
The environmental suitability of the Southern U.S. for yellow fever interacted with social and economic responses to stifle Southern economic development from the antebellum period into the 20th century (1840–1905). Yellow fever was a substantial burden on economic development in the American South (Humphreys 1999). A large proportion of the South’s foreign commerce consisted of agricultural products (e.g., fruit, coffee) grown in tropical regions with high yellow fever rates (Sterns 1900). Policy debates pitted the protective public health effects of ship quarantines against the negative economic effects of halting trade. Fluctuating public anxiety and the resulting quarantine restrictions rendered business investment in the South risky, limiting investment, port productivity, and the distribution of goods into rural regions (Humphreys 1999). Further, an 1878 yellow fever epidemic caused mass emigration from urban centers like Memphis, Tennessee, halting economic activity almost entirely (Evans 2012). Yellow fever therefore profoundly affected the history of the Americas, from the colonial period to the advent of modern medicine, swaying wars, reinforcing racism, stifling economies, and prompting new technologies and infrastructure.
Trypanosomiasis
Disease Ecology
African trypanosomiasis is distinct from plague, malaria, yellow fever, and all of the highest-burden VBDs (Table 1) because the ecology of its tsetse fly vector (Glossina palpalis and G. morsitans) geographically restricts the parasitic disease caused by three subspecies of Trypanosoma brucei. The tsetse fly’s relatively low reproduction rate and breeding site preference for loose soil prevented it from migrating out of Africa—unlike many disease-vectoring mosquitoes—resulting in historical impacts constrained to Africa (Alsan 2015). Additionally, although African sleeping sickness affects humans (caused by T. b. rhodesiense in East Africa and T. b. gambiense in coastal West Africa and drainages of the Congo and Niger Rivers), some of its primary historical effects have been mediated by “nagana” (animal trypanosomiasis caused by T. b. brucei), which increases livestock mortality and lowers productivity. In humans, symptoms include fever and joint pain, which can progress to behavioral changes, poor coordination, and death. The trypanosomes responsible for nagana and African sleeping sickness evolved around 380 million years ago; mammals were infected as early as 35 million years ago when the tsetse fly vector evolved. Due to this long shared evolutionary history, many native African wildlife are trypanotolerant (i.e., can be infected without showing signs of disease) and serve as reservoir hosts that can infect domesticated cattle (Lambrecht 1985; Steverding 2008).
In the case studies below, trypanosomiasis acted as a barrier to political centralization, impeded colonization attempts, altered migratory patterns, reinforced cycles of political violence, and was weaponized by enslavers.
(i). Medieval and Precolonial Africa
The effects of tsetse-transmitted trypanosomes on agriculture and society shaped the precolonial history of Africa, causing settlement patterns, agricultural practices, and socioeconomic systems to mirror the geography of the disease. Because nagana limited the use of domesticated animals for draft power in tsetse-suitable land, groups who inhabited these regions were less likely to use plows and harnesses (Alsan 2015), which precluded intensive farming, large agricultural surpluses, and long-range transportation of goods over land (Nash 1969; Diamond 1999). Together, these factors influenced human settlement structure, altered labor specialization, and decreased fiscal capacity in tsetse-endemic regions of Africa (Alsan 2015). Without surplus crops and a tax base to support a ruling class, populations in tsetse-suitable areas with higher burdens of trypanosomiasis were less likely to centralize politically, develop economically, and urbanize (Connor 1994; Gennaioli & Rainer 2007; Michalopoulos & Papaioannou 2013; Alsan 2015).
The unique ecology of trypanosomiasis may have impeded the expansion of early Arabic colonizers in Africa by making travel, cultivation, and settlement difficult within the tsetse fly-belt. Documents from 1373–1374 reveal that King Mari Djata II of the Mali Empire was overtaken by “illat an-nawm,” or sleeping sickness (Lambrecht 1964). Trypanosomiasis also determined the geographic range of the Great Zimbabwe civilization (1000–1400 CE). Located on a plateau between the Limpopo and Zambezi rivers, the settlement was described as a “peninsula in a sea of tsetse” (Connah 1987), which archaeologists have suggested climatically aligns with the boundaries of the pastoral civilization (Garlake 1978; Rogers & Randolph 1988).
African trypanosomiasis altered the routes of migrating pastoralists throughout the 1500s CE, affecting societal practices before European colonization. In the Rift Valley, the main route of travel was on either side of the valley along the high ridge (Lambrecht 1964), likely because it was tsetse-free and preferred by livestock-owning pastoralists. Resting places and watering-holes along migration routes became permanent settlements and marketplaces. Similarly, migratory patterns of pastoral groups in South Africa and the Sahel edge were heavily affected by seasonal shifts of tsetse fly-belts (Fuller 1923; Dicke 1932; Ingold 1987). Archaeological evidence highlights stalled diffusion of domestic animals as compared to ceramics, probably due to trypanosomiasis (Gifford-Gonzalez 2000). The survival advantage of wild game over domesticated animals might have encouraged hunting and gathering over food production reliant on animal husbandry.
(ii). Early Modern and Colonial Africa
Trypanosomiasis repelled early European imperialism and promoted political violence throughout Africa by causing widespread mortality of horses and camels. In the 15th and 16th centuries, Portuguese explorers launched expeditions into East Africa’s interior. Trypanosomiasis, in conjunction with existing barriers to colonization—including deaths of horses and camels from disease, difficulty navigating the landscape, and vulnerability of colonizers away from the coasts—halted further Portuguese incursion into the continental interior (Lambrecht 1964).
Equine mortality from trypanosomiasis reinforced exploitative cycles of economic trade and political violence, limiting conquest and expansion. Within the Malian and Jolof empires of North Africa and the western Sahara, trypanosomiasis prompted crossbreeding of disease-resistant horses (Webb 1993). These new breeds of horses were directly exchanged for enslaved people along the desert edge and were used in state warfare in which prisoners of war were sold into the Atlantic and North African slave trades (Webb 1993). In Northern Nigeria, the effects of trypanosomiasis on the Nupe Kingdom and Oyo Empire’s cavalry limited southward expansion and maintained the autonomy of the rival Kingdom of Dahomey (Law 1977).
The socio-ecological disruptions in tsetse-suitable areas caused by colonization and the slave trade perpetuated the burden of African trypanosomiasis. While signs of human trypanosomiasis—enlarged glands and nodules on the back of the neck—were used to select people for enslavement for centuries by Arabic colonizers, British physician Thomas Winterbottom’s medical reports in 1803 prompted European slave traders to use these signs to avoid selecting slaves deemed unlikely to survive the Atlantic crossing (Steverding 2008). Slave dealers simultaneously destroyed communities and spread human trypanosomiasis by disintegrating large settlements into smaller, dispersed populations (Fage & Oliver 1970). Additionally, caravans of enslaved people infected with trypanosomiasis were led through coastal markets, introducing the disease to naïve areas (Lambrecht 1964).
Discussion
Ongoing Societal Impacts of VBDs and Other Infectious Diseases
Socio-ecological feedbacks are neither confined to the past nor to VBDs. The association between VBD and settlement, warfare, and conflict continues to recur in the modern world. While malaria likely altered settlement patterns historically throughout the Americas, particularly in the Amazon basin (Sawyer 1993), recent evidence from 21st century Brazil suggests that malaria transmission is coupled with deforestation and reduces rates of forest clearing where incidence is highest (MacDonald & Mordecai 2019). In sub-Saharan Africa, where the modern burden of malaria remains concentrated despite decades of control efforts, zones of violence and armed conflict propagate malaria by impeding safe water provision, decreasing usage of protective measures against mosquitoes (e.g., bed nets), hindering medical supply delivery, and displacing inhabitants to marginal environments of high malaria endemicity and plentiful vector breeding habitats (Toole & Waldman 1997; Gayer et al. 2007; Fürst et al. 2009).
The expanding burden of viruses transmitted by Ae. aegypti (the primary yellow fever vector), including dengue, chikungunya, and Zika, illustrate how socio-ecological conditions for disease outbreaks thrive on social inequities and poverty. Dengue fever primarily affects those living without reliable access to clean piped water, and window or door screens (Stewart-Ibarra et al. 2013). The burden of dengue has grown dramatically in the last three decades with the rise of unplanned urbanization in Latin America and Southeast Asia (Lopez-Gatell et al. 2015). Moreover, the 2016 Zika pandemic caused thousands of babies to be born with Zika congenital syndrome, many to women who lacked access to contraception, reproductive counseling, or adequate housing and sanitation (Marteleto et al. 2017; Borges et al. 2018; Freitas et al. 2019). Zika initially led to policy changes that widely expanded reproductive rights and contraceptive access (e.g., Z-CAN in Puerto Rico) (Lathrop et al. 2018; Romero et al. 2018). Efforts to control Ae. aegypti have prompted biotechnology developments in genetic modification and Wolbachia infection of mosquitoes to prevent virus transmission (Hoffmann et al. 2011). Yet these technological approaches often overlook underlying household-level vulnerabilities: accumulation of unwanted plastics that become mosquito breeding habitats, and occupation of housing permeable to mosquitoes that transmit disease (Stewart-Ibarra et al. 2013; Krystosik et al. 2020). Breaking the socio-ecological feedbacks of inequity that promote arbovirus transmission will be critical for controlling these re-emerging diseases.
Although we have focused on vector-borne diseases, the themes of hierarchy, power, and segregation apply broadly to almost all human infectious diseases. Influenza risk and adverse outcomes in the U.S. are higher for those of lower socioeconomic status due to decreased access to primary care and under-reporting in surveillance systems (Scarpino et al. 2020). In Africa, the AIDS epidemic is linked to and perpetuates poverty, causes income losses for poorer households by incapacitating primary earners, stigmatizes women and subordinates their status, and undermines social institutions and political stability (Whiteside 2002; International Monetary Fund 2004; Price-Smith 2004; Rankin et al. 2005). Tuberculosis has become a major problem in prisons both in America (Baussano et al. 2010), where young Black people are disproportionately incarcerated (Wildeman & Wang 2017), and around the world. COVID-19 has perpetuated the inequitable burden of disease along racial and socioeconomic lines: a disproportionately high number of cases and deaths have occurred in people of color (Adhikari et al. 2020), and racial disparities exist throughout the U.S., primarily driven by occupation, ability to work from home, housing density, and pre-existing health and healthcare access disparities (Tai et al. 2020; Webb Hooper et al. 2020).
Synthesis
VBDs have impacted human history, society, and culture in multiple ways: by killing or sickening large numbers of people, often differentially across populations coming into contact and conflict; through development of new infrastructure, technology, and modified human relationships with land; and by magnifying racism, sexism, colonialism, and classism. Throughout history, people in power have used VBDs to perpetuate hierarchies by promoting factions between racial or ethnic groups based on falsely perceived differences in susceptibility to disease; these fabricated differences were then used to justify and expand exploitation and segregation (Olivarius 2019; Roberts & Rizzo 2020). VBD impacts also depend on unique ecologies that favor disease transmission and vector survival: crowded urban environments with poor sanitation for plague, lowland swamps and agricultural lands for malaria, sugar plantations and urbanizing environments for yellow fever, and a climatically suitable belt within sub-Saharan Africa containing animal reservoirs for trypanosomiasis.
The effects of VBDs not only mirror their diverse range of ecologies but also respond to and alter the societal contexts in which they occur. Historically, plague felled societies from Neolithic Eurasia to Medieval Europe to regions of modern China and India; trypanosomiasis and malaria impeded settlement, political centralization, and agriculture in Africa and South America; malaria and yellow fever swayed military conflicts and were weaponized to promote racism, classism, and sexism worldwide. While these case studies differ in their precise socio-ecological mechanisms, they are linked by a common thread: the incidence and impacts of VBDs are inextricable from their environmental and societal contexts. This applies just as tangibly to other VBD threats that persist today. For example, schistosomiasis is associated with dammed waterways and unsafe water access (Sokolow et al. 2017), highlighting the tension between global development projects and VBD; leishmaniasis has been exacerbated by the Syrian refugee crisis and the unsafe living conditions it has created (Alawieh et al. 2014), exemplifying the intersection between modern civil war and VBD; Chagas disease is linked with anthropogenic change and Amazonian deforestation (Steverding 2014), typifying the relationship between landscape disturbance, settlement, and VBD. In each case, the built and natural environments are intertwined with human behavior and societal circumstances in a feedback cycle that sustains major disease burdens (Table 1; Fig. 1).
The influence of VBDs is not confined to the past. VBD impacts are current, ongoing, and shaped by our actions today. It is imperative that research in disease ecology explicitly recognize and combat the deep-rooted structural racism, classism, and sexism that continue to perpetuate environmental and health inequities, afflict primarily those living in poverty and people of color, and promote vector-borne disease transmission. Equity must be brought to the center of ecology and global health in order to make meaningful progress for all of humanity.
Acknowledgements
We are grateful to the Stanford Introductory Seminars Program for supporting the course that developed this work, Bio 2N: “Ecology and Evolution of Infectious Disease in a Changing World,” and for supporting this publication. We thank Caroline Glidden for her insightful comments. EAM and MSS were funded by the National Science Foundation (NSF; EEID grants: DEB-1518681 to EAM and MSS, DEB-2011147 to EAM). EAM and DGK were funded by the National Institutes of Health (National Institute of General Medical Sciences R35 MIRA grant: R35GM133439). EAM was also funded by the Helman Scholarship, the Terman Fellowship, and the King Center for Global Development. NB was supported by the NSF (CNH grant: DEB-1716698) and the Huck Institutes of the Life Sciences at Penn State University. MLC was supported by the Illich-Sadowsky Fellowship through the Stanford Interdisciplinary Graduate Fellowship program. NN was supported by the Stanford Data Science Scholars program. JMC and GADL were supported by the Environmental Venture Program from the Stanford Woods Institute for the Environment. GADL was also supported by a grant from the Stanford Institute for Innovation in Developing Economies, Global Development and Poverty (GDP) Initiative. LIC was supported by the Stanford Graduate Fellowship. JNC was supported by the NSF Graduate Research Fellowship (Grant No. 1650114). OCW was supported by the NSF Graduate Research Fellowship (Grant No. 1650042). DGP was supported by the Ric Weiland Graduate Fellowship in the Humanities and Sciences.
Data Accessibility Statement:
This manuscript does not include any original data.
References
- Adhikari S, Pantaleo NP, Feldman JM, Ogedegbe O, Thorpe L & Troxel AB (2020). Assessment of Community-Level Disparities in Coronavirus Disease 2019 (COVID-19) Infections and Deaths in Large US Metropolitan Areas. JAMA Netw Open, 3, e2016938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawieh A, Musharrafieh U, Jaber A, Berry A, Ghosn N & Bizri AR (2014). Revisiting leishmaniasis in the time of war: the Syrian conflict and the Lebanese outbreak. Int. J. Infect. Dis, 29, 115–119. [DOI] [PubMed] [Google Scholar]
- Allison AC (2009). Genetic control of resistance to human malaria. Curr. Opin. Immunol, 21, 499–505. [DOI] [PubMed] [Google Scholar]
- Alsan M (2015). The Effect of the TseTse Fly on African Development. Am. Econ. Rev, 105, 382–410. [Google Scholar]
- Alto BW & Bettinardi D (2013). Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am. J. Trop. Med. Hyg, 88, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amazigo UO (1994). Detrimental effects of onchocerciasis on marriage age and breast-feeding. Trop. Geogr. Med, 46, 322–325. [PubMed] [Google Scholar]
- Appiah KA (2020). The Case for Capitalizing the “B” in Black. The Atlantic.
- Baker SW (1883). The Nile Tributaries of Abyssinia, and the Sword Hunters of the Hamran Arabs.
- Barber MA (1946). A malariologist in many lands, by Marshall A. Barber, with a foreword by Paul F. Russell. Lawrence, Kan., University of Kansas press. [Google Scholar]
- Barnes KB (2014). Social vulnerability and pneumonic plague: revisiting the 1994 outbreak in Surat, India. Environ. Hazards, 13, 161–180. [Google Scholar]
- Barnett ED (2007). Yellow fever: epidemiology and prevention. Clin. Infect. Dis, 44, 850–856. [DOI] [PubMed] [Google Scholar]
- Barrett ADT & Monath TP (2003). Epidemiology and ecology of yellow fever virus. In: Advances in Virus Research (eds. Chambers TJ & Monath TP). Academic Press, pp. 291–315. [DOI] [PubMed] [Google Scholar]
- Barrett R, Kuzawa CW, McDade T & Armelagos GJ (1998). EMERGING AND RE-EMERGING INFECTIOUS DISEASES: The Third Epidemiologic Transition. Annu. Rev. Anthropol, 27, 247–271. [Google Scholar]
- Bauder D (2020). AP says it will capitalize Black but not white. Associated Press. Available at: https://apnews.com/article/7e36c00c5af0436abc09e051261fff1f. Last accessed 21 October 2020. [Google Scholar]
- Baum SG (2008). Zoonoses-with friends like this, who needs enemies? Trans. Am. Clin. Climatol. Assoc, 119, 39–51; discussion 51–2. [PMC free article] [PubMed] [Google Scholar]
- Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U & Scano F (2010). Tuberculosis incidence in prisons: a systematic review. PLoS Med, 7, e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoh MN & Lindsay SW (2004). Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med. Vet. Entomol, 18, 174–179. [DOI] [PubMed] [Google Scholar]
- Beadle C & Hoffman SL (1993). History of Malaria in the United States Naval Forces at War: World War I Through the Vietnam Conflict. Clin. Infect. Dis, 16, 320–329. [DOI] [PubMed] [Google Scholar]
- Beckles H (2016). The First Black Slave Society: Britain’s “barbarity Time” in Barbados, 1636–1876. University of the West Indies Press. [Google Scholar]
- Béguin A, Hales S, Rocklöv J, Åström C, Louis VR & Sauerborn R (2011). The opposing effects of climate change and socio-economic development on the global distribution of malaria. Glob. Environ. Change, 21, 1209–1214. [Google Scholar]
- Bell AM (2010). Mosquito Soldiers: Malaria, Yellow Fever, and the Course of the American Civil War. Louisiana State University Press. [Google Scholar]
- Bell M (2009). Toussaint Louverture.
- Benedict C (1988). Bubonic plague in nineteenth-century China. Mod. China, 14, 107–155. [DOI] [PubMed] [Google Scholar]
- Berlin I (2009). Generations of Captivity: A History of African-American Slaves. Harvard University Press. [Google Scholar]
- Blockmans W (1980). The social and economic effects of plague in the Low Countries : 1349–1500. Rev. Belge Philol. Hist, 58, 833–863. [Google Scholar]
- Bollet AJ & Jay AB (2004). Plagues & Poxes: The Impact of Human History on Epidemic Disease. Demos Medical Publishing. [Google Scholar]
- Borges ALV, Moreau C, Burke A, Dos Santos OA & Chofakian CB (2018). Women’s reproductive health knowledge, attitudes and practices in relation to the Zika virus outbreak in northeast Brazil. PLoS One, 13, e0190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourget S (2016). Sacrifice, Violence, and Ideology Among the Moche: The Rise of Social Complexity in Ancient Peru (William and Bettye Nowlin Series in Art, History, and Culture of the Western Hemisphere ()). University of Texas Press. [Google Scholar]
- Bradley AK (1976). Effects of onchocerciasis on settlement in the Middle Hawal Valley, Nigeria. Trans. R. Soc. Trop. Med. Hyg, 70, 225–229. [DOI] [PubMed] [Google Scholar]
- Briceño-León R, Gonzales S & Phelan M (1990). Housing and Health: Psychosocial and Situational Effects in a Rural Disease Control Program. J. Soc. Issues, 46, 109–118. [Google Scholar]
- Brown PJ (1981). Part III: Cultural adaptations to endemic malaria in Sardinia. Med. Anthropol, 5, 313–339. [Google Scholar]
- Brown PJ (1986). Cultural and genetic adaptations to malaria: Problems of comparison. Hum. Ecol, 14, 311–332. [Google Scholar]
- Bruce-Chwatt LJ (1985). John Hull Grundy lecture. Mosquitoes, malaria and war; then and now. J. R. Army Med. Corps, 131, 85–99. [DOI] [PubMed] [Google Scholar]
- Burton SRF (1924). First Footsteps in East Africa. Dent. [Google Scholar]
- Campbell G (2020). Malaria in Precolonial Malagasy History. In: Disease Dispersion and Impact in the Indian Ocean World (eds. Campbell G & Knoll E-M). Springer International Publishing, Cham, pp. 129–167. [Google Scholar]
- Carter R & Mendis KN (2002). Evolutionary and historical aspects of the burden of malaria. Clin. Microbiol. Rev, 15, 564–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P (2015). How an Indian City Emerged From a Plague and Became a Public Health Leader. CityLab. Available at: http://www.citylab.com/housing/2015/06/how-surat-became-indias-public-health-leaderand-stayed-that-way/395003/. Last accessed 23 December 2019. [Google Scholar]
- Cheon BK, Melani I & Hong Y-Y (2020). How USA-Centric Is Psychology? An Archival Study of Implicit Assumptions of Generalizability of Findings to Human Nature Based on Origins of Study Samples. Soc. Psychol. Personal. Sci, 11, 928–937. [Google Scholar]
- Clark G (2016). MICROBES AND MARKETS: WAS THE BLACK DEATH AN ECONOMIC REVOLUTION? Journal of Demographic Economics, 82, 139–165. [Google Scholar]
- Cohn SK (2007). The Black Death and the Burning of Jews *. Past Present, 196, 3–36. [Google Scholar]
- Coimbra CEA Jr. (1988). Human Settlements, Demographic Pattern, and Epidemiology in Lowland Amazonia: The Case of Chagas’s Disease. Am. Anthropol, 90, 82–97. [Google Scholar]
- Connah G (1987). African Civilizations: precolonial cities and states in tropical Africa. Cambridge University Press. [Google Scholar]
- Connor RJ (1994). The impact of nagana. Onderstepoort J. Vet. Res, 61, 379–383. [PubMed] [Google Scholar]
- Couret J, Dotson E & Benedict MQ (2014). Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS One, 9, e87468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray A (2015). From Paradise to Plantation: Environmental Change in 17th Century Barbados. Salem State University. [Google Scholar]
- Diamond JM (1999). Guns, Germs, and Steel: The Fates of Human Societies 1st edition. W. W. Norton & Company. [Google Scholar]
- Dias JCP, Silveira AC & Schofield CJ (2002). The impact of Chagas disease control in Latin America: a review. Mem. Inst. Oswaldo Cruz, 97, 603–612. [DOI] [PubMed] [Google Scholar]
- Dicke BH (1932). The Tsetse-fly’s Influence on South African History. S. Afr. J. Sci, 29. [Google Scholar]
- Di Luca M, Boccolini D, Severini F, Toma L, Barbieri FM, Massa A, et al. (2009). A 2-year entomological study of potential malaria vectors in central Italy. Vector Borne Zoonotic Dis, 9, 703–711. [DOI] [PubMed] [Google Scholar]
- Donaldson GA (1987). Bringing Water to the Crescent City: Benjamin Latrobe and the New Orleans Waterworks System. Louisiana History: The Journal of the Louisiana Historical Association, 28, 381–396. [PubMed] [Google Scholar]
- Dunn RS (2012). Sugar and Slaves: The Rise of the Planter Class in the English West Indies, 1624–1713. UNC Press Books. [Google Scholar]
- Du S, Liu Y, Liu J, Zhao J, Champagne C, Tong L, et al. (2019). Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments. Nat. Commun, 10, 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta HM & Dutt AK (1978). Malarial ecology: a global perspective. Soc. Sci. Med, 12, 69–84. [PubMed] [Google Scholar]
- Dutt AK, Akhtar R & McVeigh M (2006). Surat plague of 1994 re-examined. Southeast Asian J. Trop. Med. Public Health, 37, 755–760. [PubMed] [Google Scholar]
- Ellis W (1838). History of Madagascar: Comprising Also the Progress of the Christian Mission Established in 1818. Fisher. [Google Scholar]
- Evans BB (2012). The Yellow Fever Epidemic of 1878 and Public Health Reform in Memphis
- Evans DB, Gelband H & Vlassoff C (1993). Social and economic factors and the control of lymphatic filariasis: a review. Acta Trop, 53, 1–26. [DOI] [PubMed] [Google Scholar]
- Evans JAS (2005). The Emperor Justinian and the Byzantine Empire. Greenwood Publishing Group. [Google Scholar]
- Fage JD & Oliver RA (1970). Papers in African Prehistory. CUP Archive. [Google Scholar]
- Faust C & Dobson AP (2015). Primate malarias: Diversity, distribution and insights for zoonotic Plasmodium. One Health, 1, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti M, Martínez-de la Puente J, Roiz D, Ruiz S, Soriguer R & Figuerola J (2016). Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep, 6, 29002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KR, Jenkins MF & Toogood AC (1998). The Philadelphia Yellow Fever Epidemic of 1793. Sci. Am, 279, 88–93. [DOI] [PubMed] [Google Scholar]
- Freitas P. de S.S., Soares GB, Mocelin HJS, Lacerda LCX, do Prado TN, Sales CMM, et al. (2019). [Congenital Zika syndrome: sociodemographic profile of mothersSíndrome congénito por el virus del Zika: perfil sociodemográfico de las madres]. Rev. Panam. Salud Publica, 43, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel S & Western J (1988). Pretext or prophylaxis? Racial segregation and malarial mosquitos in a British tropical colony: Sierra Leone. Ann. Assoc. Am. Geogr, 78, 211–228. [DOI] [PubMed] [Google Scholar]
- Fuller C (1923). Tsetse in the Transvaal and Surrounding Territories; an Historical Review. Tsetse in the Transvaal and Surrounding Territories; an Historical Review.
- Fürst T, Raso G, Acka CA, Tschannen AB, N’Goran EK & Utzinger J (2009). Dynamics of socioeconomic risk factors for neglected tropical diseases and malaria in an armed conflict. PLoS Negl. Trop. Dis, 3, e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage KL & Kosoy MY (2005). Natural history of plague: perspectives from more than a century of research. Annu. Rev. Entomol, 50, 505–528. [DOI] [PubMed] [Google Scholar]
- Garlake PS (1978). Pastoralism and Zimbabwe. J. Afr. Hist, 19, 479–493. [Google Scholar]
- Gayer M, Legros D, Formenty P & Connolly MA (2007). Conflict and emerging infectious diseases. Emerg. Infect. Dis, 13, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman JR (1979). The English Economy Following The Black Death, 51. [Google Scholar]
- Gennaioli N & Rainer I (2007). The modern impact of precolonial centralization in Africa. J. Econ. Growth, 12, 185–234. [Google Scholar]
- Gibbons RV, Streitz M, Babina T & Fried JR (2012). Dengue and US military operations from the Spanish-American War through today. Emerg. Infect. Dis, 18, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford-Gonzalez D (2000). Animal disease challenges to the emergence of pastoralism in sub-Saharan Africa. African Archaeological Review.
- Girard PR (2011). The Slaves Who Defeated Napoleon: Toussaint Louverture and the Haitian War of Independence, 1801–1804. University of Alabama Press. [Google Scholar]
- Gragg LD, Larry (Professor of History Gragg, University of Missouri-Rolla) & of History Larry Gragg. (2003). Englishmen Transplanted: The English Colonization of Barbados, 1627–1660. Oxford University Press. [Google Scholar]
- Great Britain & Chalmers G (1790). A Collection of Treaties Between Great Britain and Other Powers. J. Stockdale. [Google Scholar]
- Grigg MJ & Snounou G (2017). Plasmodium simium: a Brazilian focus of anthropozoonotic vivax malaria? Lancet Glob Health. [DOI] [PubMed]
- Haensch S, Bianucci R, Signoli M, Rajerison M, Schultz M, Kacki S, et al. (2010). Distinct clones of Yersinia pestis caused the black death. PLoS Pathog, 6, e1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper K (2017). The Fate of Rome: Climate, Disease, and the End of an Empire. Princeton University Press. [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Noor AM & Snow RW (2004). The global distribution and population at risk of malaria: past, present, and future. Lancet Infect. Dis, 4, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggenhougen HK, Hackethal V & Vivek P (2003). The behavioural and social aspects of malaria and its control. UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR), 3. [Google Scholar]
- Higgins J (2016). Public Health | Encyclopedia of Greater Philadelphia. Available at: https://philadelphiaencyclopedia.org/archive/public-health/. Last accessed.
- van der Hoek W, Sakthivadivel R, Renshaw M, Silver JB, Birley MH & Konradsen F (2001). Alternate Wet/dry Irrigation in Rice Cultivation: A Practical Way to Save Water and Control Malaria and Japanese Encephalitis? IWMI. [Google Scholar]
- Hoffman FL (1896). Race Traits and Tendencies of the American Negro. American Economic Association. [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature, 476, 454–457. [DOI] [PubMed] [Google Scholar]
- Hogarth R (2019). A Contemporary Black Perspective on the 1793 Yellow Fever Epidemic in Philadelphia. Am. J. Public Health, 109, 1337–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys M (1999). Yellow Fever and the South. JHU Press. [Google Scholar]
- Humphreys M (2001). Malaria: Poverty, Race, and Public Health in the United States. JHU Press. [Google Scholar]
- Ingold T (1987). The appropriation of nature: essays on human ecology and social relations.
- International Monetary Fund. (2004). The Macroeconomics of HIV/AIDS. International Monetary Fund. [Google Scholar]
- Jacobsen D (1996). Social systems. In: India, a country study, Library of Congress.
- Jones A & Allen R (1794). A narrative of the proceedings of the black people, during the late awful calamity in Philadelphia, in the year 1793: and a refutation of some censures thrown upon them in some late publications. Philadelphia: Printed for the authors, by William W. Woodward, Philadelphia. [Google Scholar]
- Joshel SR (2010). Slavery in the Roman World (Cambridge Introduction to Roman Civilization). Cambridge University Press. [Google Scholar]
- Joy RJ (1999). Malaria in American troops in the South and Southwest Pacific in World War II. Med. Hist, 43, 192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Maltese MF, Erlanger TE, Bos R, Tanner M, Singer BH, et al. (2005). Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop, 95, 40–57. [DOI] [PubMed] [Google Scholar]
- King CH (2010). Parasites and poverty: the case of schistosomiasis. Acta Trop, 113, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein I (1988). Plague, policy and popular unrest in British India. Mod. Asian Stud, 22, 723–755. [DOI] [PubMed] [Google Scholar]
- Knapp R (2011). Invisible Romans 1st Edition. Harvard University Press. [Google Scholar]
- Krystosik A, Njoroge G, Odhiambo L, Forsyth JE, Mutuku F & LaBeaud AD (2020). Solid Wastes Provide Breeding Sites, Burrows, and Food for Biological Disease Vectors, and Urban Zoonotic Reservoirs: A Call to Action for Solutions-Based Research. Frontiers in Public Health, 7, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht FL (1964). Aspects of Evolution and Ecology of Tsetse Flies and Trypanosomiasis in Prehistoric African Environment. J. Afr. Hist, 5, 1–24. [Google Scholar]
- Lambrecht FL (1980). Palaeoecology of Tsetse flies and sleeping sickness in Africa. Proc. Am. Philos. Soc, 124, 367–385. [PubMed] [Google Scholar]
- Lambrecht FL (1985). Trypanosomes and Hominid Evolution. Bioscience, 35, 640–646. [Google Scholar]
- Lathrop E, Romero L, Hurst S, Bracero N, Zapata LB, Frey MT, et al. (2018). The Zika Contraception Access Network: a feasibility programme to increase access to contraception in Puerto Rico during the 2016–17 Zika virus outbreak. Lancet Public Health, 3, e91–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R (1977). The Oyo Empire, c. 1600-c. 1836: a West African imperialism in the era of the Atlantic slave trade. Clarendon press. [Google Scholar]
- Leggat PA & Melrose W (2005). Lymphatic filariasis: disease outbreaks in military deployments from World War II. Mil. Med, 170, 585–589. [DOI] [PubMed] [Google Scholar]
- Lockwood JA (2009). Six-legged soldiers : using insects as weapons of war/. Oxford University Press, Oxford; [Google Scholar]
- Lockwood JA (2012). Insects as weapons of war, terror, and torture. Annu. Rev. Entomol, 57, 205–227. [DOI] [PubMed] [Google Scholar]
- Lopez-Gatell H, Hernandez-Avila M, Hernández Avila JE & Alpuche-Aranda CM (2015). Dengue in Latin America: A Persistent and Growing Public Health Challenge. In: Neglected Tropical Diseases - Latin America and the Caribbean (eds. Franco-Paredes, C. & Santos-Preciado, J.I.). Springer Vienna, Vienna, pp. 203–224. [Google Scholar]
- MacDonald AJ & Mordecai EA (2019). Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc. Natl. Acad. Sci. U. S. A, 116, 22212–22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod RM (1999). Science and the Pacific War: Science and Survival in the Pacific, 1939–1945. Springer Science & Business Media. [Google Scholar]
- Maingot AP (1996). Haiti and the Terrified Consciousness of the Caribbean. Ethnicity in the Caribbean: Essays in Honor of Harry Hoetink, 53–80.
- Marciniak S, Prowse TL, Herring DA, Klunk J, Kuch M, Duggan AT, et al. (2016). Plasmodium falciparum malaria in 1st–2nd century CE southern Italy. Curr. Biol, 26, R1220–R1222. [DOI] [PubMed] [Google Scholar]
- Marteleto LJ, Weitzman A, Coutinho RZ & Alves SV (2017). Women’s Reproductive Intentions and Behaviors during the Zika Epidemic in Brazil. Popul. Dev. Rev, 43, 199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens WJ, Niessen LW, Rotmans J, Jetten TH & McMichael AJ (1995). Potential impact of global climate change on malaria risk. Environ. Health Perspect, 103, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless P (2007). Revolutionary fever: disease and war in the Lower South, 1776–1783. Trans. Am. Clin. Climatol. Assoc, 118, 225–249. [PMC free article] [PubMed] [Google Scholar]
- McCann JC (2015). The Historical Ecology of Malaria in Ethiopia: Deposing the Spirits. Ohio University Press. [Google Scholar]
- McEvedy C (1988). The bubonic plague. Sci. Am, 258, 118–123. [DOI] [PubMed] [Google Scholar]
- McGuinness I, Beckham JD, Tyler KL & Pastula DM (2017). An Overview of Yellow Fever Virus Disease. The Neurohospitalist, 7, 157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ & Zhou X-N (2018). Schistosomiasis. Nat Rev Dis Primers, 4, 13. [DOI] [PubMed] [Google Scholar]
- McNeill JR (2010). Mosquito empires: ecology and war in the Greater Caribbean, 1620–1914. Cambridge University Press. [Google Scholar]
- Michael Byrd W & Clayton LA (2000). An American Health Dilemma: A Medical History of African Americans and the Problem of Race: Beginnings to 1900. 1 edition. Routledge. [PubMed] [Google Scholar]
- Michalopoulos S & Papaioannou E (2013). Pre-colonial Ethnic Institutions and Contemporary African Development. Econometrica, 81, 113–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Mitchell CJ & Ballinger ME (1989). Replication, tissue tropisms and transmission of yellow fever virus in Aedes albopictus. Trans. R. Soc. Trop. Med. Hyg, 83, 252–255. [DOI] [PubMed] [Google Scholar]
- Moore JW (2002). The Crisis of Feudalism: An Environmental History. Organ. Environ, 15, 301–322. [Google Scholar]
- Moore SM, Borer ET & Hosseini PR (2010). Predators indirectly control vector-borne disease: linking predator-prey and host-pathogen models. J. R. Soc. Interface, 7, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, et al. (2019). Thermal biology of mosquito-borne disease. Ecol. Lett, 22, 1690–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, de Moor E, et al. (2013). Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett, 16, 22–30. [DOI] [PubMed] [Google Scholar]
- Nash TAM (1969). Africa’s bane: the tsetse fly. Africa’s bane: the tsetse fly.
- Nguyen AT & Pendleton M (2020). Recognizing Race in Language: Why We Capitalize “Black” and “White.” Available at: https://cssp.org/2020/03/recognizing-race-in-language-why-we-capitalize-black-and-white/. Last accessed 21 October 2020.
- Nicholson BJ (1994). Legal Borrowing and the Origins of Slave Law in the British Colonies. Am. J. Leg. Hist, 38, 38–54. [Google Scholar]
- Nishikawa AM, Clark OA, Genovez V, Pinho A & Durand L (2016). ECONOMIC IMPACT OF DENGUE IN TOURISM IN BRAZIL. Value Health, 19, A216. [Google Scholar]
- Olivarius K (2016). Necropolis: Yellow Fever, Immunity, and Capitalism in the. University of Oxford. [Google Scholar]
- Olivarius K (2019). Immunity, Capital, and Power in Antebellum New Orleans. Am. Hist. Rev, 124, 425–455. [Google Scholar]
- Ottesen EA (2000). The global programme to eliminate lymphatic filariasis. Trop. Med. Int. Health, 5, 591–594. [DOI] [PubMed] [Google Scholar]
- Paaijmans KP, Cator LJ & Thomas MB (2013). Temperature-dependent pre-bloodmeal period and temperature-driven asynchrony between parasite development and mosquito biting rate reduce malaria transmission intensity. PLoS One, 8, e55777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parascandola J (1996). From MCWA to CDC--origins of the Centers for Disease Control and Prevention. Public Health Rep, 111, 549–551. [PMC free article] [PubMed] [Google Scholar]
- Peckham R (2016). Epidemics in Modern Asia. Cambridge University Press. [Google Scholar]
- Perry JM (2008). Arrogant Armies: Great Military Disasters and the Generals Behind Them. 1 edition. Wiley. [Google Scholar]
- Poh KC, Chaves LF, Reyna-Nava M, Roberts CM, Fredregill C, Bueno R, Jr, et al. (2019). The influence of weather and weather variability on mosquito abundance and infection with West Nile virus in Harris County, Texas, USA. Sci. Total Environ, 675, 260–272. [DOI] [PubMed] [Google Scholar]
- Poos LR (1991). A Rural Society after the Black Death by L. R. Poos. Available at: https://www.cambridge.org/core/books/rural-society-after-the-black-death/5367E8D5437D30A17FEC255802E2A3A7. Last accessed.
- Prescod-Weinstein C (2020). Making Black Women Scientists under White Empiricism: The Racialization of Epistemology in Physics. Signs: Journal of Women in Culture and Society, 45, 421–447. [Google Scholar]
- Price-Smith AT (2004). Downward Spiral: HIV/AIDS, State Capacity, and Political Conflict in Zimbabwe. United States Institute of Peace. [Google Scholar]
- Rankin WW, Brennan S, Schell E, Laviwa J & Rankin SH (2005). The stigma of being HIV-positive in Africa. PLoS Med, 2, e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D, Mouffok N, Bitam I, Piarroux R & Drancourt M (2013). Plague: history and contemporary analysis. J. Infect, 66, 18–26. [DOI] [PubMed] [Google Scholar]
- Rascovan N, Sjögren K-G, Kristiansen K, Nielsen R, Willerslev E, Desnues C, et al. (2019). Emergence and Spread of Basal Lineages of Yersinia pestis during the Neolithic Decline. Cell, 176, 295–305.e10. [DOI] [PubMed] [Google Scholar]
- Roberts SO & Rizzo MT (2020). The psychology of American racism. Am. Psychol [DOI] [PubMed]
- Rocher É (1879). La province chinoise du Yün-nan. E. Leroux. [Google Scholar]
- Rodrigues PT, Valdivia HO, de Oliveira TC, Alves JMP, Duarte AMRC, Cerutti-Junior C, et al. (2018). Human migration and the spread of malaria parasites to the New World. Sci. Rep, 8, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DJ & Randolph SE (1988). Tsetse Flies in Africa: Bane or Boon? Conserv. Biol, 2, 57–65. [Google Scholar]
- Romero L, Koonin LM, Zapata LB, Hurst S, Mendoza Z, Lathrop E, et al. (2018). Contraception as a Medical Countermeasure to Reduce Adverse Outcomes Associated With Zika Virus Infection in Puerto Rico: The Zika Contraception Access Network Program. Am. J. Public Health, 108, S227–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatani S, Manfredi R & Fiorino S (2012). [The Justinian plague (part one)]. Infez. Med, 20, 125–139. [PubMed] [Google Scholar]
- Saito M, Briand V, Min AM & McGready R (2020). Deleterious effects of malaria in pregnancy on the developing fetus: a review on prevention and treatment with antimalarial drugs. Lancet Child Adolesc Health, 4, 761–774. [DOI] [PubMed] [Google Scholar]
- Sallares R (2002). Malaria and Rome: A History of Malaria in Ancient Italy. 1 edition. Oxford University Press. [Google Scholar]
- Sallares R, Bouwman A & Anderung C (2004). The spread of malaria to Southern Europe in antiquity: new approaches to old problems. Med. Hist, 48, 311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartin JS (1993). Infectious Diseases During the Civil War: The Triumph of the “Third Army.” Clin. Infect. Dis, 16, 580–584. [DOI] [PubMed] [Google Scholar]
- Sawyer D (1993). Economic and social consequences of malaria in new colonization projects in Brazil. Soc. Sci. Med, 37, 1131–1136. [DOI] [PubMed] [Google Scholar]
- Scarpino SV, Scott JG, Eggo RM, Clements B, Dimitrov NB & Meyers LA (2020). Socioeconomic bias in influenza surveillance. PLoS Comput. Biol, 16, e1007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer TJ (2010). Malaria in prehistoric Sardinia (Italy): An examination of skeletal remains from the middle Bronze Age. University of South Florida. [Google Scholar]
- Shah S (2010). The Fever: How Malaria Has Ruled Humankind for 500,000 Years. Reprint edition. Sarah Crichton Books. [Google Scholar]
- Shocket M, Anderson C, Caldwell J, Childs M, Couper L, Han S, et al. (2020). Environmental drivers of vector-borne diseases. In: Population Biology of Vector-borne Diseases (ed. Drake J). Oxford University Press. [Google Scholar]
- Snowden FM (2008). The Conquest of Malaria: Italy, 1900–1962. Yale University Press. [Google Scholar]
- Sokolow SH, Jones IJ, Jocque M, La D, Cords O, Knight A, et al. (2017). Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Philos. Trans. R. Soc. Lond. B Biol. Sci, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soren D (2003). Can archaeologists excavate evidence of malaria? World Archaeol, 35, 193–209. [Google Scholar]
- Spitzer L (1968). The Mosquito and Segregation in Sierra Leone. Canadian Journal of African Studies / Revue Canadienne des Études Africaines, 2, 49–61. [Google Scholar]
- Stebich M, Brüchmann C, Kulbe T & Negendank JFW (2005). Vegetation history, human impact and climate change during the last 700 years recorded in annually laminated sediments of Lac Pavin, France. Review of Palaeobotany and Palynology.
- Steiner PE (1968). Disease in the Civil War: Natural Biological Warfare in 1861–1865. Charles C. Thomas, Springfield, Illinois. [Google Scholar]
- Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, Carniel E, et al. (2008). Plague: past, present, and future. PLoS Med, 5, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterns WP (1900). The Foreign Trade of the United States from 1820 to 1840. J. Polit. Econ, 8, 452–490. [Google Scholar]
- Steverding D (2008). The history of African trypanosomiasis. Parasit. Vectors, 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steverding D (2014). The history of Chagas disease. Parasit. Vectors, 7, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Ibarra AM, Ryan SJ, Beltrán E, Mejía R, Silva M & Muñoz A (2013). Dengue vector dynamics (Aedes aegypti) influenced by climate and social factors in Ecuador: implications for targeted control. PLoS One, 8, e78263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder JC (1963). Filariasis bancrofti. Preventative Medicine in World War, 2. [Google Scholar]
- Tai DBG, Shah A, Doubeni CA, Sia IG & Wieland ML (2020). The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin. Infect. Dis [DOI] [PMC free article] [PubMed]
- Thomas JP (1930). The Barbadians in Early South Carolina. The South Carolina Historical and Genealogical Magazine, 31, 75–92. [Google Scholar]
- Thomson MC, Doblas-Reyes FJ, Mason SJ, Hagedorn R, Connor SJ, Phindela T, et al. (2006). Malaria early warnings based on seasonal climate forecasts from multi-model ensembles. Nature, 439, 576–579. [DOI] [PubMed] [Google Scholar]
- Toole MJ & Waldman RJ (1997). The public health aspects of complex emergencies and refugee situations. Annu. Rev. Public Health, 18, 283–312. [DOI] [PubMed] [Google Scholar]
- Voigtländer N & Voth H-J (2012). Persecution perpetuated: the medieval origins of anti-Semitic violence in Nazi Germany. Q. J. Econ, 127, 1339–1392. [Google Scholar]
- Wagbatsoma VA & Okojie OH (2004). Psychosocial effects of river blindness in a rural community in Nigeria. J. R. Soc. Promot. Health, 124, 134–136. [DOI] [PubMed] [Google Scholar]
- Webb Hooper M, Nápoles AM & Pérez-Stable EJ (2020). COVID-19 and Racial/Ethnic Disparities. JAMA. [DOI] [PMC free article] [PubMed]
- Webb J (1993). The Horse and Slave Trade between the Western Sahara and Senegambia. J. Afr. Hist, 34, 221–246. [Google Scholar]
- Whiteside A (2002). Poverty and HIV/AIDS in Africa. Third World Q, 23, 313–332. [Google Scholar]
- WHO. (2018). World Malaria Report 2018. Geneva: World Health Organization, License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Wildeman C & Wang EA (2017). Mass incarceration, public health, and widening inequality in the USA. Lancet, 389, 1464–1474. [DOI] [PubMed] [Google Scholar]
- Williams M (2000). Dark ages and dark areas: global deforestation in the deep past. J. Hist. Geogr, 26, 28–46. [Google Scholar]
- Willoughby UE (2017). Yellow Fever, Race, and Ecology in Nineteenth-Century New Orleans. LSU Press. [Google Scholar]
- Wisner B, Blaikie P, Cannon T & Davis I (2004). At Risk: natural hazards, people’s vulnerability and disasters. Wiltshire: Rout ledge. [Google Scholar]
- World Health Organization. (1999). Building partnerships for lymphatic filariasis: strategic plan. World Health Organization. [Google Scholar]
- World Health Organization. (2014). A global brief on vector-borne diseases (No. WHO/DCO/WHD/2014.1). World Health Organization. [Google Scholar]
- World Health Organization. (2019). World Malaria Report 2019. License: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization. [Google Scholar]
- World Health Organization. (2020). The potential impact of health service disruptions on the burden of malaria: a modelling analysis for countries in sub-Saharan Africa. Geneva: World Health Organization. [Google Scholar]
- Yamana TK & Eltahir EAB (2013). Projected impacts of climate change on environmental suitability for malaria transmission in West Africa. Environ. Health Perspect, 121, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka J & Levins R (2007). Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am. J. Trop. Med. Hyg, 76, 450–460. [PubMed] [Google Scholar]
- Yeloff D & Van Geel B (2007). Abandonment of farmland and vegetation succession following the Eurasian plague pandemic of ad 1347--52. J. Biogeogr, 34, 575–582. [Google Scholar]
- Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y & Utzinger J (2017). Effect of land-use changes on the abundance, distribution, and host-seeking behavior of Aedes arbovirus vectors in oil palm-dominated landscapes, southeastern Côte d’Ivoire. PLoS One, 12, e0189082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript does not include any original data.


