Abstract
Objectives:
To evaluate the long-term association between a multicomponent intervention program and disability in socioeconomically vulnerable older adults
Design:
Non-randomized prospective intervention trial
Setting:
Community
Participants:
Older Koreans living alone or receiving a government assistance from a low-income program
Intervention:
A 24-week multicomponent program compromising group exercise, nutritional supplementation, management of depression, deprescribing, and home hazard reduction (n=187) versus usual care (n=196)
Measurements:
The number of dependencies in 17 basic and instrumental activities of daily living was measured every 3 months for 30 months (range: 0–17; greater values indicated worse disability). Inverse probability weighting Poisson regression was used to model the number of dependencies to adjust for confounding bias and higher dropout rates of those with greater disability.
Results:
The study population had a mean age of 76 years and 26% were men. During the 30-month follow-up, 17 died (n=8, intervention; n=9, control), 62 (n=16, intervention; n=46, control) were institutionalized or received nursing home care, and 34 (n=15, intervention; n=19, control) were lost to follow-up. After inverse probability weighting, the mean number of dependencies at baseline was 1.21 and 1.29 for the intervention group and for control group, respectively (p=0.80). The intervention group had fewer dependencies than the control group, but the difference was attenuated over time: 1.08 versus 1.60 at 6 months (p=0.04), 1.29 versus 1.87 at 12 months (p=0.03), 1.62 versus 2.17 at 18 months (p=0.06), 2.08 versus 2.51 at 24 months (p=0.18), and 2.73 versus 2.90 at 30 months (p=0.67).
Conclusions:
A 24-week multicomponent intervention was associated with a slower progression of disability, however, the diminishing association from 24 months and beyond suggests that reassessment and intervention may be necessary. Due to lack of randomization, our findings should be interpreted with caution.
Keywords: Frailty, Disability, Intervention, Public Health
INTRODUCTION
Older adults with disability experience a lower quality of life, social isolation from their community, and higher risks of adverse health outcomes, including death, nursing home admission, and prolonged hospitalization.1–4 Annual health care costs were $2,773 higher in older adults with difficulty in performing daily activities and $3,919 higher in those with inability to perform without help than in those without disability.4,5 Epidemiological studies have identified several modifiable risk factors for disability, including sarcopenia, depression, polypharmacy, and fall-related injuries.6–9 Previous research demonstrated that interventions targeting these modifiable risk factors improved frailty and physical performance.10–13 In particular, we previously reported that a 24-week multicomponent intervention consisting of group exercise, protein supplementation, management of depression, discontinuation of potentially inappropriate medications, and home hazard reduction effectively improved physical performance, frailty, sarcopenia, nutritional status, and depression severity over 12 months after the intervention.12 However, whether improvements in risk factors eventually delayed the progression of age-related disability has not been examined. Because several multicomponent intervention studies had a follow-up duration less than 12 months,14–19 it remains uncertain how long the benefit is sustained after cessation of the intervention.
In this study, we evaluated the change in disability over a 30-month period after the aforementioned 24-week multicomponent intervention program. We hypothesized that the intervention would delay the progression of disability compared with usual care in community-dwelling older adults.
METHODS
Study Design
The Aging Study of Pyeongchang Rural Area Intervention Study (ASPRA-IS) is a prospective, single-arm intervention study (ClinicalTrials.gov NCT 02554994) wherein a 24-week multicomponent intervention program was delivered in 3 geographically separate regions in Pyeongchang County, Gangwon Province, Korea.12 The intervention took place in one region at a time over a planned 6-month period (region A: August 2015-January 2016; region B: February 2016-July 2016; region C: August 2016-January 2017) (Supplementary Figure 1A). The study protocol was approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea, and written informed consent was obtained from all participants.
The current study aimed to compare disability over 30 months between individuals who received the intervention (intervention group) and otherwise eligible individuals who declined the intervention (control group). Because those who declined to participate in the intervention program were assessed as part of the observational cohort study (see details in Study Population),20 data on disability were available for both groups. All 3 regions had at least 30 months of follow-up for the assessment of disability (Supplementary Figure 1B).
Study Population
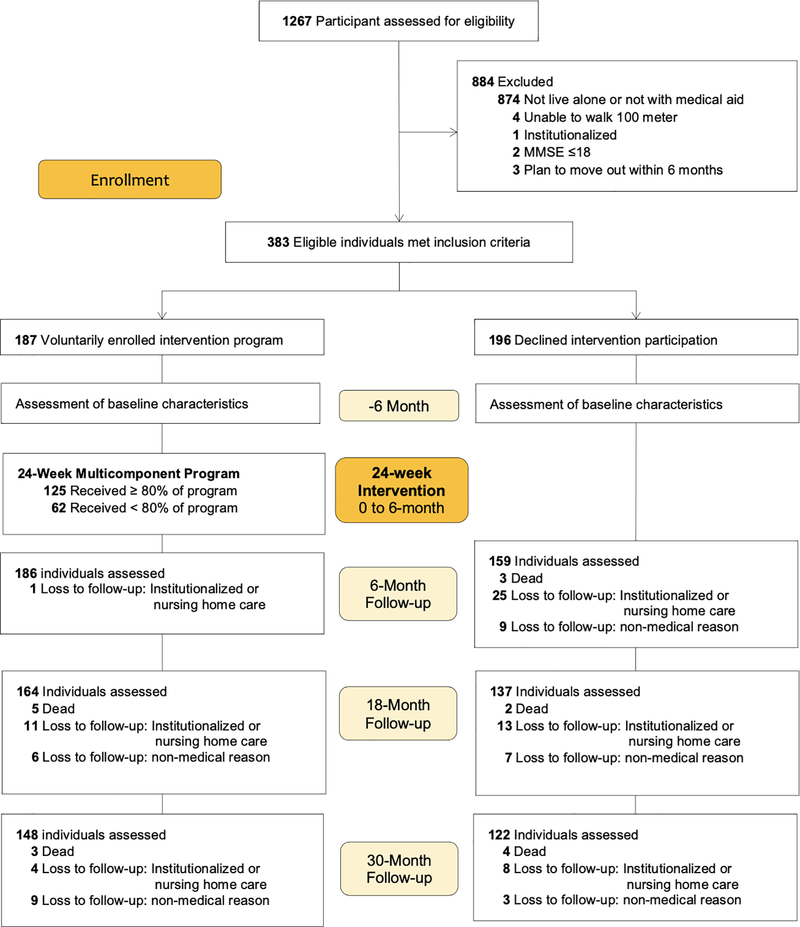
All participants were drawn from the Aging Study of Pyeongchang Rural Area (ASPRA), a population-based prospective cohort study of 1,267 community-dwelling adults aged 65 years or older who resided in Pyeongchang County. Individuals were invited to participate in the intervention program if they were living alone or receiving medical aid (government public assistance program) designated for low-income status. These eligibility criteria were determined after discussions with the local public health department in Pyeongchang County, which prioritized allocation of resources to socioeconomically vulnerable residents.20 Of the 1,267 ASPRA cohort participants, 884 were excluded owing to the following criteria: 1) not living alone or not receiving medical aid services (n=874); 2) unable to walk 100 meters unassisted (n=4); 3) institutionalized in the past 6 months (n=1); 4) cognitively impaired (Mini-Mental State Examination [MMSE] score ≤18) (n=2); and 5) planning to move out of the area within 6 months (n=3). Among the 383 eligible individuals, 187 agreed to participate in the intervention program and 196 declined. The latter group was used as the control group. The selection and follow-up status of the study population are depicted in Figure 1.
Figure 1.
Selection and Follow-up Status of Study Population
Multicomponent Intervention Program
The 24-week multicomponent intervention program included group exercise training, nutritional supplementation, depression management, discontinuation of high-risk medications, and home hazard reduction. Every participant received exercise sessions and nutritional supplementation. Participants engaged in a 60-minute group exercise session twice a week conducted by 2 licensed exercise trainers. Additionally, they were provided a written handout for home exercises. All participants also received 2 packs each of a 125mL nutritional supplement (24.5g carbohydrate, 13g protein, 5.63g essential amino acid, and 7g fat) every day, which was provided free of charge by Maeil Dairies Co., Ltd. Depression management (antidepressant medications or supportive psychotherapy) was provided by a geriatrician or psychiatrist to individuals with symptoms of severe depression, defined by the Center for Epidemiological Studies Depression scale (CES-D) score ≥21 points. A geriatrician examined individuals taking more than 5 prescription drugs monthly and reduced potentially inappropriate medications according to the Beers criteria.21 Lastly, nurses and social workers assessed the participants’ home environments using the Home Fall Prevention Checklist for older adults by the Centers for Disease Control and Prevention22 and modified the home hazards (e.g., reorganizing power cords, replacing slippers or slippery mats, and rearranging furniture to clear walking path).12 Adherence rates were 91.3% for home hazard correction, 88.5% for monthly visits for the evaluation of polypharmacy, 88.4% for monthly visits for depression management, 87.8% for the number of nutrition supplements consumed (self-report), and 83.7% for attendance in group exercise sessions.12 Of the 187 intervention participants, 125 (66.8%) completed at least 80% of the group exercise sessions and consumed 80% of the nutritional supplements.
The individuals who did not receive the intervention (control group) had monthly visits to their primary care physician for chronic disease management and preventive care. The study assessment results were not available to the primary care physicians.
Measurement of Functional Status
Self-reported functional status was measured every 3 months via telephonic interview using the validated questionnaires for activities of daily living (ADL) (7 activities: bathing, maintaining continence, dressing, eating, toileting, transferring, and washing face and hands), and instrumental activities of daily living (IADL) (10 activities: food preparation, household chores, going out for short distance, grooming, handling of finances, laundry, taking personal medication, shopping, using public transportation, and using the telephone).23 The disability score, which ranged from 0 to 17, was calculated as the total number of activities requiring assistance from another person.
Other Measurements
At baseline, trained nurses assessed clinical characteristics, including age, sex, education status, multimorbidity (5 or more of the 11 physician-diagnosed conditions: angina, arthritis, asthma, cancer excluding minor skin cancer, chronic lung disease, congestive heart failure, diabetes, heart attack, hypertension, kidney disease, and stroke), polypharmacy (taking five or more prescription drugs), and body mass index (kg/m2). Handgrip strength (kg) was assessed as the average of two measurements using a dynamometer (T.K.K. 5401 Grip-D; Takei, Tokyo, Japan) in the dominant hand. Gait speed (m/s) was calculated from a 4-meter walk at usual pace after a 1-meter acceleration. We evaluated depressive mood using the Korean version of the CES-D (range: 0–60) and defined depression as a CES-D score ≥ 21;24,25 cognitive function using the Korean MMSE;26 and malnutrition using the Mini-Nutritional Assessment Short Form (MNA-SF) (range: 0–14). Frailty was assessed according to the frailty phenotype scale (range: 0–5) based on unintentional weight loss, exhaustion, inactivity, slow gait speed, and weak grip strength27 as well as a deficit-accumulation frailty index (range: 0–1) based on 43 health deficit items (38 self-reported items and 5 performance test items).28–30
Statistical Analysis
We summarized the baseline characteristics using means and standard deviations for continuous variables and proportions for categorical variables. We conducted imputation of missing values on baseline variables (2 missing CES-D scores and 4 missing MMSE scores) and disability scores using a single multivariable imputation using chained equation.
We applied inverse probability weighting (IPW) (see details in Supplementary Methods and Supplementary Tables 1–3). To estimate inverse probability treatment weight (IPTW), we first fitted logistic regression to estimate the probability of receiving the multicomponent intervention as a function of the baseline covariates listed above (see Other Measurements). The IPTW was the inverse of the probability of receiving the treatment that the individuals actually received. To minimize bias due to differential dropout during the follow-up period (e.g., individuals with greater disability are more likely to drop out), we used Cox regression to estimate the probability of censoring at each follow-up time point as a function of baseline covariates and history of covariates and disability scores up to the time of censoring. The inverse probability censoring weight (IPCW) was calculated at each follow-up time as the inverse of the probability of non-censoring. The final weight was calculated by multiplying the IPTW and IPCW. To minimize the influence of extreme weights (>10), we used stabilized weights by multiplying the marginal probability of the treatment.31,32 We assessed covariate balance before and after IPW by calculating the standardized mean difference (SMD). An SMD <0.10 was considered an adequate balance.
After achieving covariate balance using IPW, we evaluated longitudinal changes in the disability score over 30 months using the generalized estimating equation generalized linear model with Poisson distribution and log link, and first-order autoregressive correlational structure to account for correlation of disability scores measured for an individual. The model included an indicator term for the intervention, time in months since the intervention (continuous variable and its square root term), the intervention-by-time interaction term, and offset (natural logarithm of the maximum disability score). The stabilized IPW was accounted for in the model. As a sensitivity analysis, we restricted the intervention group to those with at least 80% adherence to both group exercise and nutritional supplements and repeated the IPW analysis.
Statistical analyses were performed using Stata version 14 (mi impute command) and R version 3.6 (ipw and geeglm package). A 2-sided p-value <0.05 was considered statistically significant.
RESULTS
Characteristics of Study Population
Table 1 shows the clinical characteristics of the intervention and control groups before and after IPW. Before IPW, individuals who participated in the intervention program were older (mean [SD], 77.1 [5.1] versus 75.6 [6.2] years), more likely to be women (75.9% versus 68.4%), and less educated (4.1 [2.5] versus 4.7 [3.1] years) than those who did not. The intervention group was more likely to receive medical aid (25.7% versus 15.3%) but less likely to live alone (77.0% versus 89.3%) than the control group. Overall, the intervention group had poorer health status than the control group, as shown by the higher prevalence of multimorbidity (53.5% versus 42.3%) and polypharmacy (31.0% versus 23.5%) as well as higher levels of frailty phenotype scale scores (2.3 [1.3] versus 1.8 [1.2]) and frailty index (0.27 [0.1] versus 0.23 [0.11]).
Table 1.
Characteristics of Study Population *
| Before IPW | After IPW | |||||
|---|---|---|---|---|---|---|
| Characteristics | Intervention (n=187) | Control (n=196) | SMD | Intervention (n=179) | Control (n=199) | SMD |
| Age, years | 77.1 ± 5.1 | 75.6 ± 6.2 | 0.27 | 76.5 ± 4.9 | 76.4 ± 8.3 | 0.02 |
| Female | 142 (75.9) | 134 (68.4) | 0.17 | 132 (73.8) | 147 (74.1) | <0.01 |
| Education, years | 4.1 ± 2.5 | 4.7 ± 3.1 | 0.21 | 4.6 ± 3.2 | 4.4 ± 2.8 | 0.05 |
| Medical aid | 48 (25.7) | 30 (15.3) | 0.26 | 36 (20.3) | 41 (20.5) | <0.01 |
| Living alone | 144 (77.0) | 175 (89.3) | 0.33 | 147 (82.6) | 165 (82.8) | <0.01 |
| Location | 0.39 | 0.04 | ||||
| Region 1 | 33 (17.6) | 66 (33.7) | 39 (21.8) | 47 (23.6) | ||
| Region 2 | 88 (47.1) | 66 (33.7) | 77 (42.9) | 83 (41.8) | ||
| Region 3 | 66 (35.3) | 64 (32.7) | 63 (35.3) | 69 (34.6) | ||
| Multimorbidity | 100 (53.5) | 83 (42.3) | 0.22 | 90 (50.3) | 98 (49.1) | 0.02 |
| Polypharmacy | 58 (31.0) | 46 (23.5) | 0.17 | 54 (30.3) | 61 (30.4) | <0.01 |
| Grip strength, kg | 17.2 ± 7.0 | 20.2 ± 9.1 | 0.36 | 18.1 ± 7.5 | 18.4 ± 8.3 | 0.04 |
| Gait speed, m/s | 0.66 ± 0.25 | 0.76 ± 0.27 | 0.37 | 0.69 ± 0.25 | 0.70 ± 0.26 | 0.03 |
| CES-D (0–60), points | 9.5 ± 9.3 | 9.9 ± 9.9 | 0.04 | 9.9 ± 9.9 | 10.0 ± 9.8 | <0.01 |
| MMSE (0–30), points | 24.3 ± 4.0 | 24.9 ± 4.1 | 0.13 | 24.7 ± 3.9 | 24.4 ± 3.5 | 0.02 |
| MNA-SF (0–14), points | 11.2 ± 2.2 | 11.6 ± 2.2 | 0.16 | 11.3 ± 2.2 | 11.3 ± 2.2 | 0.02 |
| Body mass index, kg/m2 | 24.5 ± 4.0 | 24.3 ± 3.5 | 0.07 | 24.4 ± 3.8 | 24.4 ± 3.5 | 0.01 |
| Frailty phenotype scale (0–5) | 2.3 ± 1.3 | 1.8 ± 1.2 | 0.37 | 2.1 ± 1.3 | 2.1 ± 1.2 | 0.04 |
| Frailty index (0–1) | 0.27 ± 0.10 | 0.23 ± 0.11 | 0.33 | 0.25 ± 0.10 | 0.25 ± 0.11 | 0.02 |
CES-D, Center for Epidemiological Studies Depression scale; MMSE, Mini-Mental State Examination; MNA-SF, Mini-Nutritional Assessment Short Form; IPW, inverse probability weighting; SMD, standardized mean difference.
Data were presented in n (%) for categorical variables or mean ± standard deviation for continuous variables.
Inverse Probability Weighting
To reduce bias due to confounding and differential dropouts between the treatment groups, we estimated IPTW and IPCW. Predictors of receiving the intervention included geographic regions (B versus A: odds ratio [OR], 4.95 [95% confidence interval, 2.01–12.22]; and C versus A: OR, 2.92 [1.23–6.93]), older age (OR, 1.07 [1.01–1.12] per 1-year increase), lower CES-D score (OR, 0.95 [0.91–0.98] per 1-point increase), and higher frailty levels by frailty phenotype (OR, 1.58 [1.13–2.21] per 1-point increase) and frailty index (OR, 2.02 [1.03–3.96] per 0.10-increase) (Supplementary Table S1). Predictors of dropout were lower MMSE scores (hazard ratio [HR], 0.94 [95% confidence interval, 0.90–0.97] per 1-point increase) and higher disability scores (HR, 1.07 [1.02–1.12] per 1-point increase) (Supplementary Table S2). The mean weight (range) was 0.99 (0.50–5.75) for IPTW, 0.99 (0.25–3.46) for IPCW, and 0.98 (0.14–6.13) for IPW, which showed no evidence of extreme weights (Supplementary Table S3). After IPW, all baseline variables were balanced between the groups, as evidenced by an SMD < 0.1 (Table 1).
Multicomponent Intervention Program and Changes in Disability Over 30 Months
At the end of the 24-week period, the intervention group had faster mean gait speed (0.92 versus 0.65 m/s; p<0.001) and lower mean CES-D score (7.3 versus 9.6; p=0.05) than the control group (Supplementary Table S4). A similar trend was observed for the mean grip strength (22.0 versus 20.6 kg; p=0.14), but there was no difference in the mean NMA-SF scores (12.0 versus 11.9; p=0.62).
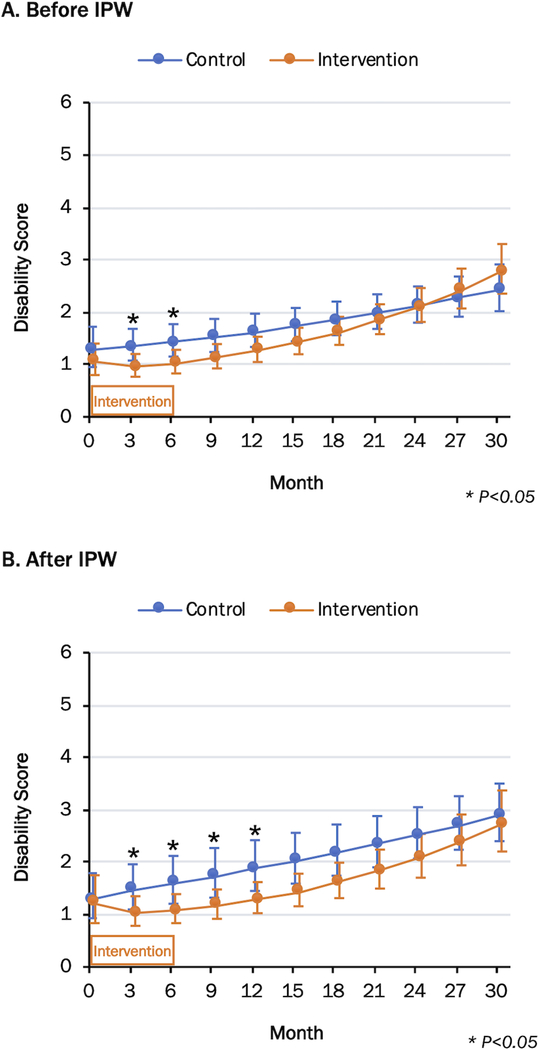
During the 30-month period, 17 participants died (n = 8 in the intervention group; n = 9 in the control group) and 62 (n = 16 in the intervention group; n = 46 in the control group) were institutionalized or received nursing home care, and 34 (n =15 intervention group; n = 19 in the control group) were lost to follow-up visits. Figure 2 and Table 2 show the least-square means of disability scores over 30 months. In general, the intervention group had an initially modest reduction in disability followed by progressive worsening, whereas the control group demonstrated a progressive increase in disability. After IPW, the mean disability scores for the intervention group versus the control group were 1.21 versus 1.29 at baseline (p=0.80), 1.08 versus 1.60 at 6 months (p=0.04), 1.29 versus 1.87 at 12 months (p=0.03), 1.62 versus 2.17 at 18 months (p=0.06), 2.08 versus 2.51 at 24 months (p=0.18), and 2.73 versus 2.90 at 30 months (p=0.67). Although the group differences in the disability scores became non-significant beyond 18 months, the intervention group had numerically lower mean scores by 0.5 points than those of the control group until 21 months. Compared with the results before IPW, the weighting procedure elevated the disability scores of the control group without affecting those of the intervention group.
Figure 2.
Change in Disability Score Over 30 Months.
Data are presented as least-square mean (95% confidence intervals shown in vertical bars) from a generalized estimating equation repeated measure Poisson regression model. IPW = inverse probability weighting
Table 2.
Multicomponent Intervention Program and Change in Disability Score Over 30 Months *
| Before IPW | After IPW | |||||
|---|---|---|---|---|---|---|
| Time (Month) | Intervention (n=187) | Control (n=196) | P value | Intervention (n=179) | Control (n=199) | P value |
| 0 | 1.06 (0.80, 1.40) | 1.28 (0.95, 1.72) | 0.37 | 1.21 (0.84, 1.75) | 1.29 (0.93, 1.79) | 0.80 |
| 3 | 0.96 (0.77, 1.20) | 1.34 (1.08, 1.68) | 0.04 | 1.03 (0.79, 1.35) | 1.47 (1.10, 1.96) | 0.08 |
| 6 | 1.03 (0.83, 1.28) | 1.43 (1.15, 1.77) | 0.04 | 1.08 (0.84, 1.39) | 1.60 (1.21, 2.12) | 0.04 |
| 9 | 1.13 (0.93, 1.39) | 1.52 (1.24, 1.87) | 0.05 | 1.17 (0.92, 1.48) | 1.73 (1.32, 2.27) | 0.03 |
| 12 | 1.27 (1.05, 1.53) | 1.62 (1.34, 1.97) | 0.08 | 1.29 (1.03, 1.62) | 1.87 (1.74, 2.72) | 0.03 |
| 15 | 1.43 (1.20, 1.70) | 1.73 (1.44, 2.08) | 0.14 | 1.44 (1.16, 1.78) | 2.02 (1.59, 2.56) | 0.04 |
| 18 | 1.62 (1.37, 1.91) | 1.85 (1.56, 2.20) | 0.27 | 1.62 (1.31, 1.99) | 2.17 (1.74, 2.72) | 0.06 |
| 21 | 1.84 (1.58, 2.15) | 1.98 (1.68, 2.34) | 0.54 | 1.83 (1.50, 2.24) | 2.34 (1.90, 2.88) | 0.09 |
| 24 | 2.11 (1.81, 2.46) | 2.12 (1.80, 2.49) | 0.98 | 2.08 (1.70, 2.54) | 2.51 (2.07, 3.05) | 0.18 |
| 27 | 2.42 (2.07, 2.83) | 2.26 (1.91, 2.68) | 0.57 | 2.38 (1.94, 2.91) | 2.70 (2.24, 3.26) | 0.36 |
| 30 | 2.79 (2.35, 3.30) | 2.42 (2.02, 2.91) | 0.27 | 2.73 (2.20, 3.37) | 2.90 (2.40, 3.50) | 0.67 |
IPW, inverse probability weighting.
Data were presented in the least-square mean (95% confidence interval) from a generalized estimating equation repeated measure Poisson regression
In a sensitivity analysis restricted to the high-adherence intervention participants (Supplementary Table S5), disability scores of this subgroup were generally lower than those of the total population. The intervention group with ≥80% adherence to both group exercise and nutritional supplements had numerically lower disability scores than those of the control group, although this difference was not statistically significant.
DISCUSSION
The ASPRA-IS previously reported that a 24-week multicomponent intervention program targeting modifiable risk factors improved physical performance, frailty, sarcopenia, nutritional risk, and depression symptoms in socioeconomically vulnerable older Koreans, and the benefit lasted up to 6 months after cessation of the intervention.12 The present study examined the change in disability over a 30-month follow-up period. Individuals who participated in the intervention program had numerically lower disability scores than those of the control group up to 21 months, and the difference decreased to less than 0.5 points from 24 months onwards. These results suggest that reassessment and intervention may be necessary after 24 months to prevent further progression of disability. This information is valuable in designing a resource-intensive public health intervention for an aging community.
Several studies have demonstrated the short-term benefits of a multicomponent intervention on physical performance and frailty.10,14,17,18,33–37 Most studies included exercise and nutritional supplementation in the intervention; however, neither the duration of the intervention nor the follow-up period exceeded 12 months.10,14,18,19,38 They showed improvement in physical performance (e.g., gait speed33 and short physical performance battery score14,17), endurance (e.g., time to complete a 400-meter walk16 and peak VO210,34), and frailty phenotype.11,14 Although these outcomes are intermediate endpoints leading to disability, the beneficial effect on intermediate endpoints may not result in a comparable benefit on disability,39,40 which is directly relevant to older adults’ quality of life and ability to live in the community.40–42
Only a small number of clinical trials have examined disability. The Utrecht primary care PROactive frailty intervention trial (U-PROFIT) examined changes in ADL before and 1 year after comprehensive geriatric assessment and the individualized care plan.43,44 The Home-Based Older People’s Exercise (HOPE) trial measured changes in ADL before and 14 weeks after home-based exercise.8 Both U-PROFIT and HOPE trial found the intervention moderately beneficial when compared with usual care.44,45 The Community Aging in Place—Advancing Better Living for Elders (CAPABLE) trial examined a 5-month multidisciplinary program for low-income older adults with disability. The CAPABLE intervention program included assessments of medical and functional status as well as home safety, followed by individualized interventions and home hazard reduction to achieve functional goals.37,46 Participants who benefited from the intervention had a 30% reduction in ADL disability scores at 5 months compared with those in the control group; however, this benefit was attenuated at 12 months.47 The CAPABLE intervention is similar to our intervention, as both interventions addressed functional, medical, and home environment needs of socioeconomically vulnerable older adults.47 While these results are promising, it remains unclear whether these benefits can be sustained beyond 1 year.
Information regarding the effectiveness of a public health intervention program and its duration is essential for public health officials to prioritize and allocate resources in the community. Previously, we demonstrated that our intervention program improved the short physical performance battery score by an average of 3.24 points at 12 months12; nevertheless, the benefit in terms of disability corresponded to a reduction in disability by approximately 0.5 activities. Such benefits, which may seem modest at an individual level, may be translated to a meaningful public health benefit by delaying the use of resource-intensive support services. If a disability score of 2 or higher was a threshold for receiving support services, our intervention could delay the use of such services by approximately 9 months (Figure 2B). Furthermore, we observed that the benefit of our 24-week multicomponent intervention was not sustained at 24 months, at which point reassessment and further interventions may be needed.
Methodologically, our study illustrates the analytical approaches that are useful to address the challenges of long-term follow-up studies of disability in older adults. During the 30-month follow-up period, more individuals in the control group did not participate in the follow-up visits than those in the intervention group. We discovered that lower cognitive function and higher disability scores were strong predictors of dropout, that is, the control group was likely over-represented by those with better cognitive function and lower disability level. Such a differential dropout causes selection bias, making the control group look healthier, and the intervention effect is attenuated. As demonstrated in Figure 2, the use of IPW (in particular, IPCW) was able to reduce such selection bias.
This study has several strengths and limitations. We assessed disability every 3 months to describe longitudinal changes in disability over 30 months. In the rural towns where our study was conducted, less than 5% of the people were immigrating or emigrating from the area and public health centers were the main source of medical care. This contributed to high adherence to the intervention program. Moreover, little improvement in grip strength, gait speed, CES-D score, and MNA-SF score in the control group makes the possibility of a temporal trend or a concurrent intervention outside our study unlikely. The main limitations of our study are related to the generalizability of our results and the possibility of a residual confounding and selection bias. Our study participants had sociodemographic characteristics that were comparable in many ways to the rural Korean population, except for a higher proportion of the participants engaged in agriculture and those with no formal education.20 Moreover, most participants were pre-frail or mildly frail, with a mean of 1.2 dependencies with daily activities. Therefore, it remains unclear whether our intervention program will provide a similar long-term benefit to older adults with severe frailty or those living in urban areas or outside Korea. We acknowledge that the IPW methods rely on several assumptions, including no unmeasured confounding and no model misspecification. Nonetheless, we were able to achieve balance in important risk factors for disability, and IPW showed a reasonable distribution, without extreme weights. In addition, a higher proportion of sicker people in the intervention group at baseline and the differential dropout of sicker people in the control group would attenuate the beneficial effect of the intervention. We believe that our intervention would have shown a larger benefit, had residual bias been entirely adjusted for.
In conclusion, our results demonstrate that a multicomponent intervention consisting of group exercise, nutritional supplementation, management of depression, deprescribing potentially inappropriate medications, and home hazard reduction was associated with a slower progression of disability; however, this beneficial association was attenuated at 24 months. Our results underscore the need for reassessment and further interventions to prevent older adults from acquiring more disability. Given our non-randomized design, future research is needed to confirm our findings.
Supplementary Material
Supplementary Figure 1. Study Design and Timeline
Supplementary Methods Estimation of Inverse Probability Weight
Supplementary Table 1. Baseline Characteristics Associated with Receiving the Intervention Program
Supplementary Table 2. Characteristics Associated with Censoring
Supplementary Table 3. Distribution of Inverse Probability Weights by Treatment Groups
Supplementary Table 4. Multicomponent Intervention Program and Change in Physical Performance, Depression Symptoms, and Nutritional Status at 24 weeks
Supplementary Table 5. Change in Disability Score for High-Adherence Subgroup
ACKNOWLEDGMENTS
We are indebted to the public health professionals and nurses of the Pyeonchang County Hospital, Public Health Center, and Community Health Posts for their administrative support and efforts in enrollment, retention, and measurements. This research was funded by a grant (2020IF0001) from the Asan Institute for Life Science, Asan Medical Center, Seoul, Republic of Korea.
SPONSOR’S ROLE
Public health professionals and nurses of Pyeongchang County Hospital were involved in data collection, but they did not have any role in the study design, analysis or interpretation of data, writing of the paper, or the decision to submit the paper for publication. Maeil Dairies Co., Ltd did not have any role in the study design, collection, analysis or interpretation of data, writing of the paper, or decision to submit the paper for publication.
Funding Sources
The Aging Study of Pyeongchang Rural Area, an intervention study, was funded by the Pyeongchang County Hospital, Pyeongchang County, Gangwon Province, Korea. This study was also supported by a grant (2020IF0001) from the Asan Institute for Life Science, Asan Medical Center, Seoul, Korea. Dr. Dae Hyun Kim is supported by grants R01AG056368, R01AG062713, R21AG060227, P30AG031679, and P30AG048785 from the National Institute on Aging.
• DHK received grants R01AG056368, R01AG062713, R21AG060227, P30AG031679, and P30AG048785 from the National Institute on Aging. He is a consultant to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturer.
Footnotes
CONFLICT OF INTERESTS
• The other authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Cournane S, Conway R, Byrne D, O’Riordan D, Silke B. Persons with disability, social deprivation and an emergency medical admission. Ir J Med Sci. 2018;187(3):593–600. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World health statistics 2019: monitoring health for the SDGs, sustainable development goals. World Health Organization. https://apps.who.int/iris/handle/10665/324835. Published 2019. Accessed May 13, 2020. [Google Scholar]
- 3.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362(13):1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang IY, Lee HY, Lee E. Geriatrics Fact Sheet in Korea 2018 From National Statistics. Ann Geriatr Med Res. 2019;23(2):50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26(2):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abellan van Kan G Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13(8):708–712. [DOI] [PubMed] [Google Scholar]
- 7.Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity Combinations and Disability in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71(6):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg A, Barber S, Young J, Forster A, Iliffe S. The Home-Based Older People’s Exercise (HOPE) trial: study protocol for a randomised controlled trial. Trials. 2011;12:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima T The Need for Actions Against Polypharmacy in Older People With Frailty. Ann Geriatr Med Res. 2018;22(3):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50(12):1921–1928. [DOI] [PubMed] [Google Scholar]
- 11.Cesari M, Vellas B, Hsu FC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70(2):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang IY, Jung HW, Park H, et al. A multicomponent frailty intervention for socioeconomically vulnerable older adults: a designed-delay study. Clin Interv Aging. 2018;13:1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, et al. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J Am Med Dir Assoc. 2016;17(5):426–433. [DOI] [PubMed] [Google Scholar]
- 15.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Van Ness PH. A prehabilitation program for the prevention of functional decline: effect on higher-level physical function. Arch Phys Med Rehabil. 2004;85(7):1043–1049. [DOI] [PubMed] [Google Scholar]
- 16.LIFE Study Investigators, Pahor M, Blair SN, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. [DOI] [PubMed] [Google Scholar]
- 17.Cameron ID, Fairhall N, Langron C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serra-Prat M, Sist X, Domenich R, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46(3):401–407. [DOI] [PubMed] [Google Scholar]
- 19.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med. 2017;376(20):1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung HW, Jang IY, Lee YS, et al. Prevalence of Frailty and Aging-Related Health Conditions in Older Koreans in Rural Communities: a Cross-Sectional Analysis of the Aging Study of Pyeongchang Rural Area. J Korean Med Sci. 2016;31(3):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Check for safety: a home fall prevention checklist for older adults. https://www.cdc.gov/steadi/pdf/check_for_safety_brochure-a.pdf. Published 2015. Accessed Jun 21, 2015.
- 23.Jang S-N, Kawachi I. Why Do Older Korean Adults Respond Differently to Activities of Daily Living and Instrumental Activities of Daily Living? A Differential Item Functioning Analysis. Ann Geriatr Med Res. 2019;23(4):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale:A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 25.Park JH, Kim KW. A review of the epidemiology of depression in Korea. J Korean Med Assoc. 2011;54(4):362–369. [Google Scholar]
- 26.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15(2):300–308. [Google Scholar]
- 27.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 28.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. [DOI] [PubMed] [Google Scholar]
- 30.Jang IY, Jung HW, Lee HY, Park H, Lee E, Kim DH. Evaluation of Clinically Meaningful Changes in Measures of Frailty. J Gerontol A Biol Sci Med Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. [DOI] [PubMed] [Google Scholar]
- 32.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 33.Gill TM, Baker DI, Gottschalk M, et al. A prehabilitation program for physically frail community-living older persons. Arch Phys Med Rehabil. 2003;84(3):394–404. [DOI] [PubMed] [Google Scholar]
- 34.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166(8):860–866. [DOI] [PubMed] [Google Scholar]
- 35.Suikkanen S, Soukkio P, Pitkala K, et al. Older persons with signs of frailty in a home-based physical exercise intervention: baseline characteristics of an RCT. Aging Clin Exp Res. 2019;31(10):1419–1427. [DOI] [PubMed] [Google Scholar]
- 36.Fairhall N, Kurrle SE, Sherrington C, et al. Effectiveness of a multifactorial intervention on preventing development of frailty in pre-frail older people: study protocol for a randomised controlled trial. BMJ Open. 2015;5(2):e007091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szanton SL, Thorpe RJ, Boyd C, et al. Community aging in place, advancing better living for elders: a bio-behavioral-environmental intervention to improve function and health-related quality of life in disabled older adults. J Am Geriatr Soc. 2011;59(12):2314–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng TP, Feng L, Nyunt MS, et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am J Med. 2015;128(11):1225–1236. [DOI] [PubMed] [Google Scholar]
- 39.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. Jama. 2014;311(23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva AG, Queiros A, Sa-Couto P, Rocha NP. Self-Reported Disability: Association With Lower Extremity Performance and Other Determinants in Older Adults Attending Primary Care. Phys Ther. 2015;95(12):1628–1637. [DOI] [PubMed] [Google Scholar]
- 41.Kim KI, Lee JH, Kim CH. Impaired health-related quality of life in elderly women is associated with multimorbidity: results from the Korean National Health and Nutrition Examination Survey. Gend Med. 2012;9(5):309–318. [DOI] [PubMed] [Google Scholar]
- 42.Choi YS, Kim MJ, Lee GY, et al. The Association between Frailty and Disability among the Elderly in Rural Areas of Korea. Int J Environ Res Public Health. 2019;16(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bleijenberg N, Drubbel I, Ten Dam VH, Numans ME, Schuurmans MJ, de Wit NJ. Proactive and integrated primary care for frail older people: design and methodological challenges of the Utrecht primary care PROactive frailty intervention trial (U-PROFIT). BMC Geriatr. 2012;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bleijenberg N, Drubbel I, Schuurmans MJ, et al. Effectiveness of a Proactive Primary Care Program on Preserving Daily Functioning of Older People: A Cluster Randomized Controlled Trial. J Am Geriatr Soc. 2016;64(9):1779–1788. [DOI] [PubMed] [Google Scholar]
- 45.Clegg A, Barber S, Young J, Iliffe S, Forster A. The Home-based Older People’s Exercise (HOPE) trial: a pilot randomised controlled trial of a home-based exercise intervention for older people with frailty. Age Ageing. 2014;43(5):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szanton SL, Wolff JW, Leff B, et al. CAPABLE trial: a randomized controlled trial of nurse, occupational therapist and handyman to reduce disability among older adults: rationale and design. Contemp Clin Trials. 2014;38(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szanton SL, Xue QL, Leff B, et al. Effect of a Biobehavioral Environmental Approach on Disability Among Low-Income Older Adults: A Randomized Clinical Trial. JAMA Intern Med. 2019;179(2):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study Design and Timeline
Supplementary Methods Estimation of Inverse Probability Weight
Supplementary Table 1. Baseline Characteristics Associated with Receiving the Intervention Program
Supplementary Table 2. Characteristics Associated with Censoring
Supplementary Table 3. Distribution of Inverse Probability Weights by Treatment Groups
Supplementary Table 4. Multicomponent Intervention Program and Change in Physical Performance, Depression Symptoms, and Nutritional Status at 24 weeks
Supplementary Table 5. Change in Disability Score for High-Adherence Subgroup