Abstract
Background:
Biomedical research has recently focused on developing new models of human disease by implementing genome editing strategies in nonhuman primates (NHPs) to introduce relevant gene mutations. There is a need to establish objective semen evaluation methods to select sires for in vitro fertilization to perform germline editing in embryos.
Methods:
Sperm motility kinematic parameters were evaluated using a computer assisted semen analysis (CASA) instrument for rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fascicularis), and common marmosets (Callithrix jacchus).
Results:
Normative sperm kinematic parameters were established, revealing differences between marmosets and macaques. The impact of season on rhesus macaque sperm motility was modest, where changes in sperm motility related to season were dependent on the individual male.
Conclusions:
These data provide a baseline of normative kinematic parameters for three captive NHP species, in which implementation of CASA may serve as a tool to evaluate NHP semen quality.
Keywords: sperm, CASA, marmoset, macaque
Introduction
Nonhuman primates (NHPs) are suitable animal models for biomedical research with translation into human medicine as they reveal insight into both normal processes and abnormal pathologies [1]. NHPs allow for the study of mechanisms similar to humans in vivo as they possess a phylogenetic relationship to humans not shared with other commonly studied animal models [2]. Rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fascicularis) and common marmosets (Callithrix jacchus) share similarities with humans in their immunology, neurobiology, endocrinology, and reproductive physiology making them ideal animal models for studies in basic physiology, experimental infection, and the evaluation of vaccines and therapeutics [3–9]. They are also widely used for research studies due to the accessibility of these specimens and their tolerance of housing in research facilities [2, 9, 10]. Altogether, NHP models can provide a stepping stone from animal research to human clinical trials.
Assisted reproductive technologies, such as ovarian stimulation and in vitro fertilization (IVF) have been developed in macaques and marmosets allowing for the genetic manipulation of in vitro-produced embryos [11–15]. Advances in transgenic and genome editing technologies, such as CRISPR-Cas9, have allowed for the successful introduction of transgenes and targeted gene mutations (i.e., insertions, deletions or precise gene knock-ins) into NHP embryos [16–19]. The introduction of genetic mutations into NHP embryos provides a platform for the creation of genetically modified offspring to evaluate mutated gene function. NHP resources have greatly shifted towards deriving in vitro embryos for CRISPR-Cas9 targeting of genes associated with human disease to develop translational NHP disease models. Targeting of NHP embryos by CRISPR-Cas9 and the production of live genetically modified offspring remains inefficient. In the reports of CRISPR-Cas9 targeting in macaque embryos, 48-179 CRISPR-Cas9 microinjected embryos were transferred to 12-59 surrogates with 0.86-6.25% of the transferred embryos resulting in a live CRISPR-Cas9 edited offspring [20–23]. The creation of genetically modified offspring thus requires a large number of embryos to be transferred to produce live CRISPR-Cas9 edited offspring. Low CRISPR-Cas9 targeting rates coupled with inefficiencies of in vitro embryo production highlights the need for improved gamete selection to produce a larger pool of embryos for transfer. Moreover, evaluating the reproductive potential of the genome edited offspring will be pertinent to expanding the genetic trait within a NHP colony for studies on disease onset and the development of therapeutics.
Semen evaluation across species includes the estimation of motility (often divided into total and progressive motility), quantification of morphologically normal versus abnormal spermatozoa, and sample concentration [24, 25]. Semen quality can be evaluated using traditional methods or through automated systems such as computer assisted sperm analysis (CASA) [25]. Traditional sperm evaluation relies on the technician’s ability to assess individual samples both macro- and microscopically. Macroscopic evaluation involves visualizing samples for “normal” volume, viscosity, odor, and time to liquefaction, while microscopic evaluation includes the visualization and estimation of total motility as well as sperm morphological features [25, 26]. Despite the relative ease of traditional sperm evaluation, this assessment is highly subjective with large variability between different laboratories and technicians [27, 28].
Automated CASA systems, such as the Sperm Vision® (Minitube USA, Inc., Verona, WI, USA) or IVOS™ (Hamilton Thorne Inc, Beverly, MA, USA) systems, have been utilized to assess kinematic parameters commonly used to predict fertilization potential, such as total motility, progressive motility, individual spermatozoon velocity, and concentration [29]. These systems provide a more objective analysis for comparison between different human infertility clinics, livestock artificial insemination companies and research laboratories not previously seen due to individual evaluator subjectivity with classical semen evaluation. It has been found that there is as much as 30-60% variation between trained evaluators when assessing the same semen samples [28]. This leads to an uncomfortable level of uncertainty in the current reliability of semen evaluation results. Utilization of CASA technology to eliminate subjectivity concerns will allow for improvement in male fertility research with the goal of universal standards of normative semen parameters for NHP species.
Relatively few studies have been published that define semen parameters for NHP species. Marmoset semen has been previously evaluated to assess the effect of semen collection method on semen characteristics [30], to select sperm suspensions for AI [31], to evaluate semen separation techniques [32], to optimize cryopreservation methods [33] and to assess the maturation of epididymal sperm [34]. The maturation of cynomolgus macaque epididymal sperm has also been evaluated in parallel to marmoset [34]. Maree and van der Horst [29] reported the presence of sperm motility subpopulations in six species, including humans and rhesus macaques, which revealed that each species required individually optimized curvilinear velocity (VCL) parameters to define rapid, medium, and slow sperm. These subpopulations are thought to represent a spectrum of physiologically distinct sperm maturity states.
The objective of the present study was to establish sperm motility kinematic parameters for rhesus macaques, cynomolgus macaques, and marmosets to establish normative values for males within our colony. Macaque and marmoset semen samples collected at the Wisconsin National Primate Research Center over the course of two years were analyzed using a Hamilton-Thorne IVOS II CASA instrument. The data presented here define normative sperm kinematic parameters for each of these species, and confirms a modest impact of season on rhesus macaque sperm kinematics. This method for evaluating sperm kinematics provides a framework for future assessment of fresh or cryopreserved NHP sperm, particularly to support gamete evaluation for deriving CRISPR-Cas9 targeted embryos.
Methods
Humane Care Guidelines
All procedures were performed in accordance with the NIH guide for the Care and Use of Laboratory Animals and under the approval of the University of Wisconsin College of Letters and Sciences and Vice Chancellor Office for Research and Graduate Education Institutional Animal Care and Use Committee (protocols g005592, g005172, g005298, g005044). Animals were housed in enclosures that meet the requirements specified in the Animal Welfare Act Regulations and the Guide for the Care and Use of Laboratory Animals Guide. All animals were evaluated by veterinary staff or trained animal care staff at least twice daily for signs of pain, distress, and illness.
Animals
Nonhuman primates in this study were housed and cared for by the staff at the Wisconsin National Primate Research Center (WNPRC). Semen samples are routinely collected at the WNPRC for assisted reproduction, such as in vitro fertilization, and the present study analyzed ejaculates collected from seven marmosets, four Mauritian cynomolgus macaques, and ten rhesus macaques of Indian origin. The ages of the males evaluated were as follows: marmoset 4.63-9.21 years, cynomolgus macaques 4.67-12.42 years, and rhesus macaques 6.67-18.25 years. Body weights ranged from 0.33-0.53 kg for the marmosets, 7.92-9.54 kg for the cynomolgus macaques, and 8.48-13.93 kg for the rhesus macaques. All rhesus macaques and two of the cynomolgus macaques were individually housed, whereas two cynomolgus macaques were paired together and all marmosets were housed with a female partner.
Macaque Semen Collection
Six male rhesus macaques were pole and collar trained and placed in a restraint chair for semen collection by electroejaculation. All of the cynomolgus macaques and four rhesus macaques were not chaired for collection and rather were collected under mild sedation (0.3-0.7 mg/kg ketamine) using the same electro-ejaculator parameters for both chair trained and sedated semen collections. Electrodes were placed onto the penis for electrical stimulation (electro-ejaculator device: P-T Electronics, model no: 10130515), with a maximum level of 25 V and 25 s of stimulation until ejaculation. The stimulation regimen was repeated again within three minutes if no ejaculate was obtained with no more than three sequential attempts in a single day. The number of ejaculates analyzed for individual males were as follows: rhesus chair trained 58, 49, 44, 8, 7 and 7; rhesus sedated collections 32, 6, 1, and 1; and cynomolgus macaques 11, 5, 6, and 13. Following collection, semen was incubated at 37°C for 20-30 min to allow for liquefaction prior to analysis. The volume of each ejaculate was measured with a pipette. Semen samples were diluted with Hepes-TL medium (Caisson Labs, Inc, cat no: IVL01) supplemented with 3 mg/ml BSA to a concentration sufficient to allow for ~200-600 cells to be analyzed by CASA as described below. Sperm cell concentration was determined by diluting the sample to an appropriate concentration, loading both chambers of two Neubauer hemocytometers, and the average count across the four chambers was used to calculate the cell concentration.
Marmoset Semen Collection
Semen was collected from marmosets via penile vibratory stimulation using a Ferticare Personal Vibrator (ILTS, Inc.) [35, 36]. Males were placed into a restraint tube for the collection procedure. A 0.7 ml microcentrifuge tube was placed onto the Ferticare instrument, serving as an artificial vagina. The collection tube was positioned over the penis while the Ferticare instrument applied vibratory stimulation to the penile area. The stimulation cycle was approximately 2 min, with a maximum frequency of 90 Hz. The number of ejaculates analyzed per male was 2, 2, 1, 1, 8, 12, 3. Due to the viscosity of the coagulum, the volume of the sample was visually estimated by comparing to similar known volumes of water (10-100 μl) in the same type of 0.7 ml microcentrifuge tubes. A 3 μl aliquot of the liquified ejaculate was initially evaluated on a glass slide or via the CASA instrument to confirm the presence of sperm cells. Four volumes of Hepes-TL medium (Caisson Laboratories, Smithfield, UT; cat no: IVL01) supplemented with 3 mg/ml bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO; cat no: A8806) were layered on top of the liquified sample and coagulum. The ejaculate was then incubated at 37°C to allow for sperm swim-up. Kinematic analysis was performed after initially adding medium (denoted as 0 min), 30, 90 and 150 min post-collection. A 10 μl aliquot from the upper two-thirds of the swim-up region was used to calculate sperm concentration after 90 min of the swim-up incubation. An aliquot of the sperm cell suspension from the center of the swim-up region was diluted 1:1 in water and loaded to both chambers of two Neubauer hemocytometers. An average count across all four chambers was used to calculate the sperm cell concentration.
Computer Assisted Sperm Analysis (CASA)
An IVOS Animal-IDENT-LED Hamilton Thorne instrument was used for semen kinematic and viability evaluations. Leja 4-chamber slides ((IMV Technologies, cat no: 025170) with a depth of 20 μm were used for all CASA evaluations. A 3 μl aliquot of the semen sample was then loaded to a pre-warmed chamber of the Leja slide. Of note, the aliquot from the marmoset sample was consistently drawn from the middle of the swim-up region at 0, 30, 90 and 150 min. A total of six fields/chamber and two chambers were captured using the CASA settings for either marmosets or macaques as determined by the recommendation of a Hamilton-Thorne representative. The CASA settings are listed in Supplemental Table 1.
In addition to kinematic analysis, the CASA instrument is equipped with fluorescent illumination capable of visualizing and quantifying fluorescent staining of sperm. The Viadent stain (Hoechst 33258) supplied by Hamilton-Thorne is permeable to non-viable cells, providing a measure of sperm cell viability [37, 38]. A concentration of 10 μg/ml of Viadent stain was added to each macaque sample, and incubated for 2 min at 37°C without exposure to visible light. The stained sample (3 μl) was loaded into a Leja 4-chamber slide and six fields of two chambers were analyzed for the percentage of viable cells. Marmoset sperm cells were not analyzed for viability as to not remove additional volume from the swim-up fraction.
Statistical Analysis
Statistical analysis was performed using Prism 9 software (www.graphpad.com). The specific statistical analysis method and number of observations is specified in the legend for each table and figure.
Results
Sperm kinematics throughout swim-up incubation in the common marmoset
A swim-up method has been previously reported for preparing marmoset semen to obtain a motile sperm fraction for IVF or intracytoplasmic sperm injection (ICSI) [36, 39, 40]. In the current study, sperm motility, progressive motility and kinematic analysis of total motile sperm were assessed at 0, 30, 90 and 150 min of swim-up. An aliquot of sperm was removed from the center portion of the swim-up medium to allow for sampling of cells at earlier time points. Motility and progressive motility tended to increase between 30–150 min of incubation with ~68% motile and 59% progressive motile cells at 150 min of the swim-up incubation (Table 1). Sperm kinematic parameters were not significantly different at any time point (Supplemental Table 2). Given that there were no significant differences in motility parameters throughout the duration of swim-up, the CASA analysis at 90 min of swim-up was used for comparison to macaque sperm as there were a larger number of samples with data recorded at this time point for the WNPRC colony animals.
Table 1.
Marmoset motility and progressive motility throughout the duration of swim-up incubation.
| 0 min | 30 min | 90 min | 150 min | p-value | |
|---|---|---|---|---|---|
| motility (%) | 64.79 ± 29.05 | 60.58 ± 31.19 | 73.56 ± 22.52 | 68.64 ± 28.84 | 0.45 |
| progressive motility (%) | 49.14 ± 26.22 | 47.79 ± 26.62 | 55.71 ± 22.00 | 59.69 ± 16.92 | 0.66 |
| n | 27 | 22 | 29 | 11 |
The mean ± SD of the percent motile and progressive motile cells throughout the sperm swim-up incubation were analyzed using a non-parametric, Kruskal-Wallis test with a Dunn’s correction for multiple comparisons.
Comparison of semen and sperm kinematic parameters across nonhuman primate species
A total of 29 marmoset, 37 cynomolgus macaque and 213 rhesus macaque ejaculates were analyzed for semen and sperm kinematic parameters. Within male variation was observed in ejaculate characteristics and sperm kinematic parameters; the median as well as 25% and 75% quartile ranges for each male and parameter are listed in Table 2. When comparing across species, the mean ejaculate volumes and SD were 0.04 ± 0.01 ml (n=26) for marmosets, 0.27 ± 0.33 ml (n=37) for cynomolgus macaques and 0.43 ± 0.31 ml (n=212) for rhesus macaques (Table 3). Sperm concentration was variable within males of a species with the mean concentration for marmoset, cynomolgus macaque and rhesus macaque as follows (Table 3): 25.00 ± 17.87 SD x106 sperm cells/ml (n=21), 991.90 ± 978.50 SD x106 cells/ml (n=23) and 389.30 ± 343.10 SD x106 (n=185), respectively. The percentage of viable cells was similar between macaques with ~79% viable cells in the ejaculate (Table 3). Cell viability was not assessed in marmoset as to not remove additional sample from the swim-up region. Total motility across all three NHP species was ~72% with ~ 57% being progressive motile cells (Table 3).
Table 2.
Summary of sperm kinematic parameters and viability for individual males as analyzed by CASA. Summary data presented include the median along with the 25% and 75% quartiles in parenthesis.
| male | n | motility (%) | progressive motility (%) | viability (%) | VAP (μm/s) | VCL (μm/s) | VSL (μm/s) | STR (%) | LIN (%) | ALH (μm/s) | Area (μm) | BCF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marmoset | ||||||||||||
| A | 3 | 80.2 (97.9, 79.0) | 74.0 (66.0, 77.1) | - | 166.4 (136.4, 174.1) | 203.2 (179.0, 218.5) | 155.6 (126.2, 157.5) | 91.4 (87.6, 92.8) | 72.4 (71.7, 79.7) | 5.8 (5.6, 6.8) | 15.9 (14.3, 17.8) | 29.0 (28.7, 30.4) |
| B | 12 | 91.8 (70.7, 95.7) | 68.5 (57.1, 77.0) | - | 140.2 (128.4, 159.8) | 190.0 (177.4, 203.2) | 123.5 (117.0, 143.1) | 86.4 (85.4, 89.5) | 67.7 (64.8, 70.1) | 6.5 (6.0, 7.1) | 15.5 (14.3, 16.3) | 30.2 (27.5, 32.6) |
| C | 8 | 58.9 (42.9, 87.8) | 45.9 (27.6, 68.3) | - | 150.6 (121.3, 167.1) | 182.3 (168.6, 204.3) | 135.2 (106.7, 147.9) | 87.3 (84.7, 88.1) | 71.2 (63.5, 73.4) | 5.6 (5.0, 6.3) | 15.8 (15.5, 16.0) | 31.2 (28.0, 33.8) |
| D | 2 | 80.55 (76.4, 84.7) | 68.5 (66.6, 70.4) | - | 161.5 (160.5, 162.5) | 188.5 (187.0, 189.9) | 149.7 (149.2, 150.1) | 91.1 (90.2, 92.0) | 79.7 (78.6, 80.8) | 5.0 (4.9, 5.0) | 16.6 (16.2, 17.0) | 28.3 (27.3, 29.3) |
| E | 2 | 88.7 (84.1, 93.2) | 68.0 (63.0, 72.9) | - | 116.2 (113.5, 118.8) | 165.8 (154.8, 176.6) | 102.6 (100.0, 105.1) | 86.6 (86.4, 86.7) | 63.8 (59.3, 68.2) | 6.0 (5.6, 6.4) | 17.5 (16.4, 18.6) | 33.1 (31.3, 34.8) |
| F | 1 | 57.9 | 32.0 | - | 98.3 | 175.9 | 77.3 | 77.3 | 47.9 | 7.4 | 15.7 | 33.2 |
| G | 1 | 57.1 | 14.3 | - | 79.2 | 165.7 | 55.0 | 74.9 | 40.0 | 7.3 | 11.9 | 31.8 |
| Cynomolgus macaque | ||||||||||||
| A | 12 | 74.0 (54.8, 87.1) | 54.8 (33.5, 74.3) | 73.7 (65.7, 82.5) | 123.6 (72.5, 166.0) | 175,4 (121.1, 202.5) | 118.8 (62.5, 156.2) | 89.7 (81.4, 93.4) | 65.9 (49.6, 77.1) | 5.2 (5.0-5.9) | 20.4 (19.6, 20.9) | 37.1 (34.5, 38.4) |
| B | 5 | 67.7 (67.3, 89.6) | 60.2 (40.9, 72.5) | 75.2 (61.3, 82.2) | 138.6 (73.2, 162,3) | 188.9 (121.3, 219.4) | 130.7 (66.7, 154.1) | 93.4 (83.8, 93.8) | 65.1 (50.4, 71.4) | 4.8 (4.3-6.5) | 20,0 (19.5, 21.8) | 38.9 (35.9, 41.0) |
| C | 6 | 69.8 (62.4, 80.5) | 48.1 (41,2, 58.0) | 78.8 (77.4, 86.2) | 92.5 (79.4, 123.1) | 158.3 (137.2, 186.1) | 80.9 (64.6, 112.0) | 81.8 (77.7, 88.4) | 48.3 (43.1, 59.2) | 5.9 (5.1, 7.1) | 18.0 (14.7, 22.2) | 36.3 (34.0, 38.8) |
| D | 14 | 78.7 (73.8, 85.9) | 68 (60.2, 79.5) | 84.7 (78.4, 88.8) | 149.1 (125.0, 175.8) | 197.8 (173.4, 211.3) | 141.3 (116.1, 170.8) | 92.4 (90.2, 95.6) | 70.3 (65.9, 77.2) | 5.3 (5.2, 5.8) | 20.1 (17.6, 21.7) | 37.2 (34.9, 40.4) |
| Rhesus macaque | ||||||||||||
| A | 49 | 66.0 (52.0-89.9) | 47.8 (34.4, 58.6) | 78.0 (66.9, 84.6) | 97.9 (87.1, 119.3) | 150.1 (135.0, 184.3) | 91.2 (77.3, 106.8) | 56.6 (52.7, 60.1) | 86.2 (83.6, 88.2) | 5.8 (5.0, 6.4) | 20.2 (18.1, 22) | 35.9 (34, 37.5) |
| B | 58 | 68 .0 (52.7-78.8) | 47.4 (27.8, 64.5) | 75.7 (66.2, 86.1) | 99.2 (76.4, 135.3) | 149.1 (130.7, 183.7) | 90.1 (65.2, 124.7) | 53.4 (45.2, 62.9) | 86.1 (79.7, 90.1) | 5.5 (5.2, 6.0) | 20.6 (19.6, 21.5) | 36.8 (33.1, 39.0) |
| C | 44 | 79.5 (71.0-92.9) | 67.3 (53.6, 78.4) | 85.5 (77.6, 89.8) | 131.6 (93.7, 168.5) | 179.6 (151.5, 203.1) | 125.2 (84.1, 153.3) | 65.7 (55.3, 74.3) | 91.5 (87.3, 93.7) | 5.4 (5.0, 6.0) | 19.1 (15.3, 20.5) | 38.4 (36.1, 40.2) |
| D | 32 | 77.4 (68.6-85.4) | 68.2 (52.7, 79.7) | 83.8 (75.8, 87.3) | 145.4 (123.0, 168.5) | 193.8 (161.4, 206.5) | 139.2 (114.7, 162.8) | 71.1 (64.2, 76.7) | 93.0 (89.6, 95.3) | 5.3 (5.0, 5.6) | 19.5 (15.3, 20.5) | 37.3 (36.3, 38.6) |
| E | 8 | 60.2 (55.9, 68.1) | 43.4 (42.3, 50.6) | 79.3 (53.4, 94.3) | 114.5 (84.3, 132.2) | 163.7 (135.8, 190.3) | 104.9 (75.3, 126.1) | 59.2 (52.5, 66.7) | 87.7 (84.6, 93.0) | 5.1 (5.0, 5.6) | 23.6 (22.5, 24.5) | 40.9 (39.2, 42.1) |
| F | 7 | 74.5 (59.9, 89.5) | 56.5 (43.7, 80.4) | 94.8 (83.9, 97.4) | 108.7 (80.6, 159.8) | 152.1 (137.1, 208.8) | 100.2 (70.8, 153.0) | 61.6 (49.6, 72.2) | 87.2 (84.1, 94.4) | 5.4 (5.1, 5.7) | 21.9 (20.9, 22.0) | 38.1 (36.5, 41.5) |
| G | 7 | 63.0 (52.7, 67,8) | 55.4 (35.6, 56.5) | 79.6 (71.4, 82.7) | 122.6 (92.4, 137.8) | 187.2 (162.0, 193.1) | 116.2 (85.9, 130.0) | 63.6 (49.7, 67.2) | 90.8 (84.6, 92.0) | 5.3 (5.0, 5.5) | 16.4 (15.8, 22.4) | 39.5 (37.5, 40.4) |
| H | 6 | 78.2 (56.7, 80.3) | 59.3 (47.1, 71.3) | 81.3 (68.1, 86.0) | 136.9 (110.1, 156.4) | 190.1 (168.5, 214.7) | 130.1 (101.9, 150.5) | 65.5 (55.2, 69.9) | 91.7 (88.3, 93.6) | 5.6 (4.9, 6.5) | 21.7 (21.2, 22.5) | 41.9 (41.1, 42.8) |
| I | 1 | 47.8 | 38.7 | 76.0 | 79.9 | 141.0 | 72.5 | 51.6 | 88.3 | 5.1 | 18.9 | 39.6 |
| J | 1 | 89.6 | 84.2 | - | 138.6 | 204.9 | 130.7 | 65.1 | 93.4 | 6.1 | 20.7 | 41.7 |
Table 3.
Comparison of ejaculate characteristics across NHP species.
| species | volume (ml) | concentration (x106 cells/ml) | viability (%) | motility (%) | progressive motility (%) |
|---|---|---|---|---|---|
| marmoset | 0.04 ± 0.01 | 25.00 ± 17.87 | - | 73.56 ± 22.52 | 55.71 ± 22.00 |
| cynomolgus macaque | 0.27 ± 0.33 | 991.90 ± 978.50 | 78.94 ± 9.46 | 73.69 ± 11.20 | 60.00 ± 16.62 |
| rhesus macaque | 0.43 ± 0.31 | 389.30 ± 343.10 | 78.93 ± 11.99 | 68.38 ± 16.03 | 54.28 ± 19.44 |
The mean ± SD are displayed for each parameter. The n for each parameter are as follows: 21-29 ejaculates from 7 marmosets, 23-37 ejaculates from 4 cynomolgus macaques, and 159-213 ejaculates from 10 rhesus macaques.
Sperm kinematic parameters varied significantly across NHP species with more striking differences between marmosets and macaques. A representative image of the CASA capture field for each NHP species is shown in Figure 1. The average path velocity (VAP) was significantly lower in rhesus macaque compared to the marmoset, whereas the straight line velocity (VSL) and curvilinear velocity (VCL) were reduced in rhesus sperm compared to marmoset or cynomolgus macaques (Table 4). There were fewer differences in sperm kinematics when comparing macaque sperm with similarities in velocities, straightness (STR), linearity (LIN), amplitude of lateral head displacement (ALH), area and beat cross frequency (BCF). Marmoset sperm had significantly higher ALH, in addition to reduced area and BCF in comparison to macaques (Table 4). Overall, these data define the normative sperm kinematic parameters for each species and suggests that there are differences in sperm velocity and trajectories between marmosets and macaques.
Figure 1.
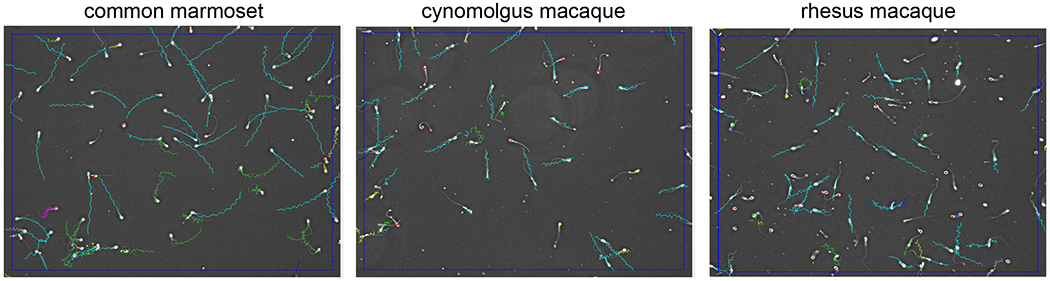
Representative fields captured by CASA for common marmoset, cynomolgus macaque and rhesus macaque sperm samples. The colored tracks annotate sperm as follows: green indicates motile sperm, turquoise indicates progressive motile sperm, red indicates static sperm, and yellow indicates a late sperm track in the capture time frame.
Table 4.
Comparison of NHP sperm kinematic parameters assessed by CASA.
| Species | n | VAP (μm/s) | VCL (μm/s) | VSL (μm/s) | STR (%, VSL/VAP) | LIN (%, VSL/VCL) | ALH (μm/s) | Area (μm) | BCF |
|---|---|---|---|---|---|---|---|---|---|
| marmoset | 29 | 138.3 ± 29.141 | 184.2 ± 27.16 | 122.9 ± 29.15 | 86.36 ± 4.281 | 67.02 ± 9.421 | 6.15 ± 0.931 | 15.85 ± 1.851 | 30.65 ± 2.861 |
| cynomolgus macaque | 37 | 130.4 ± 38.231,2 | 177.9 ± 35.52 | 121.7 ± 39.29 | 89.13 ± 6.152 | 64.46 ± 12.521,2 | 5.45 ± 0.792 | 19.83 ± 2.362 | 37.41 ± 3.192 |
| rhesus macaque | 213 | 116.5 ± 34.122 | 168.8 ± 34.86 | 107.5 ± 35.60 | 87.56 ± 6.381,2 | 59.93 ± 11.112 | 5.62 ± 0.912 | 20.30 ± 2.572 | 36.90 ± 4.662 |
| p-value | 0.0011 | 0.0347 | 0.0146 | 0.0348 | 0.0007 | 0.0021 | <0.0001 | <0.0001 |
The mean ± SD are displayed for each parameter and a non-parametric, Kruskal-Wallis test with a Dunn’s correction for multiple-comparisons was applied for each parameter. Differing superscripts within a column denote statistically significant differences between groups.
Evaluation of collection method on rhesus macaque semen quality and sperm kinematics
Rhesus macaques were either pole and collar trained and then chaired for collection (n=6), or alternatively, were collected under mild ketamine sedation (n=4). Semen collection under sedation bypasses the time and labor required to chair train an animal and may be preferred for animals that are undergoing experimental infection or that are temperamentally unsuitable for chair training. Semen quality parameters such as volume, motility, progressive motility and viability were not significantly different between collection methods, while sperm concentration approached statistical significance (Supplemental Table 3). The sperm of rhesus males collected under sedation had significantly higher velocities with a distinct trend towards straighter and more linear trajectories (Table 5). A nested t-test was performed to evaluate differences between collection methods, taking into account individual male variability as a factor. Relatively few rhesus macaques respond to electrostimulation for semen collection under sedation. A review of WNPRC records for males undergoing screening for semen collection under sedation revealed that seven of twelve males collected under sedation gave at least one positive semen sample, however, with the exception of one male, only ~33% of collection attempts for an individual male resulted in a semen sample. Hence, few males with multiple collection records were available for evaluation in this study and a more comprehensive analysis with more males should be conducted in the future.
Table 5.
Comparison of sperm kinematic parameters assessed by CASA between chair trained and sedated rhesus macaques.
| collection method | n | VAP (μm/s) | VCL (μm/s) | VSL (μm/s) | STR (%, VSL/VAP) | LIN (%, VSL/VCL) | ALH (μm/s) | Area (μm) | BCF |
|---|---|---|---|---|---|---|---|---|---|
| chair trained | 173 | 110.70 ± 33.17 | 164.40 ± 35.35 | 101.20 ± 34.52 | 86.54 ± 6.47 | 57.79 ± 10.63 | 5.65 ± 0.96 | 20.60 ± 2.46 | 36.48 ± 4.23 |
| sedated | 40 | 141.50 ± 26.12 | 187.70 ± 25.41 | 134.50 ± 26.64 | 91.95 ± 3.46 | 69.20 ± 7.97 | 5.45 ± 0.60 | 18.98 ± 2.64 | 38.74 ± 5.92 |
| p-value | 0.0477 | 0.0424 | 0.0493 | 0.0517 | 0.0568 | 0.2136 | 0.4163 | 0.1584 |
The mean ± SD are displayed for each parameter. A nested t-test was performed to compare sperm kinematic parameters between chair trained versus sedated semen collections in which the individual males were accounted for as a factor of variation.
Evaluation of season on rhesus macaque semen quality and sperm kinematics
A seasonal effect on testicular and endocrine function has been observed in male rhesus macaques [41–44]. While conceptions occur throughout the year, Dunk et al. [45] reported a peak in rhesus macaque conceptions between October to May. In the present study, the impact of season on sperm quality and kinematics was evaluated for four individual male rhesus macaques that were collected over two years. The mean and standard deviation for ejaculate volume, concentration, percentage of motile cells, percentage of progressive motile cells, and viable cells for each season are plotted for individual males in Figure 2. A total of 6–18 ejaculates were analyzed for each season for four individual males. Semen parameters for individual males varied greatly between males. A two-way ANOVA revealed that male factor was significant for all parameters (Figure 2F). An interaction was observed between males and season for volume and concentration (Figure 2F) suggesting that a seasonal impact was dependent on the male. Sperm kinematic parameters were significantly different across individual males for all parameters with the exception of no differences observed in ALH across males (Table 6). Notably, ALH was the only parameter impacted by season that was not dependent on an individual male. Altogether, there were greater differences between males, and seasonal changes in semen quality or sperm kinematics were largely dependent on the individual male.
Figure 2.
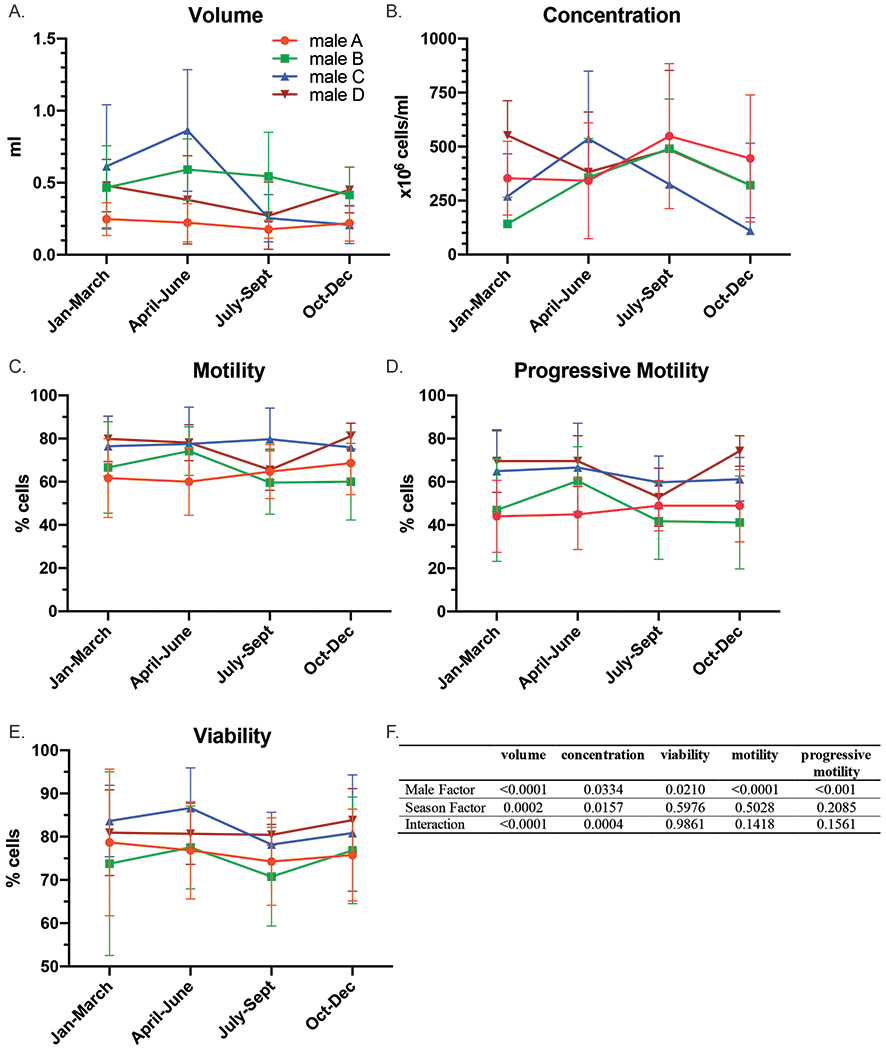
Semen characteristics for individual rhesus macaques throughout the year. The mean and standard deviation is plotted for each season and each male for the following: A) volume, B) concentration, C) motility, D) progressive motility, and E) viability. F) A two-way ANOVA was performed on the square root-transformed data for each parameter and the p-values are shown.
Table 6.
CASA kinematic parameters across seasons for four rhesus macaque males.
| VAP | VCL | VSL | STR | LIN | ALH | Area | BCF | |
|---|---|---|---|---|---|---|---|---|
| Male factor | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.175 | <0.0001 | 0.008 |
| Season factor | 0.333 | 0.156 | 0.256 | 0.358 | 0.532 | 0.026 | <0.0001 | 0.998 |
| Interaction | 0.160 | 0.269 | 0.115 | 0.124 | 0.233 | 0.709 | 0.003 | 0.113 |
The data were transformed by taking the square root of each value prior to statistical analysis. Values represented in the table are the p-values obtained from a two-way ANOVA performed for each parameter. A total of 6-18 ejaculates were analyzed per season for each male.
Discussion
Precise and accurate semen evaluation is an essential component for human infertility clinics, the livestock industry and research laboratories that heavily rely on semen samples for use in assisted reproductive technologies. Automated sperm analysis provides an attractive alternative to traditional semen evaluation, in which the technician microscopically estimates sperm motility. In the present study, a CASA instrument was used to quantify motility and sperm kinematic parameters for NHP semen samples collected at the WNPRC over the course of two years. The data presented here establish species-specific normative kinematic parameters for marmoset, cynomolgus and rhesus macaque sperm. The evaluation of marmoset sperm kinematics revealed a slight increase in progressive motility throughout the duration of the sperm swim-up incubation with non-significant changes in kinematic parameters throughout the incubation. Additionally, seasonal impact on normative kinematic parameters was explored for individual rhesus macaques. Sperm kinematics varied greatly across individual rhesus macaque males with modest seasonal changes observed that were largely dependent on the individual male. The method of analysis and normative parameters reported in this study may be used to more effectively evaluate both fresh and cryopreserved NHP semen samples.
The criteria for developing semen evaluation protocols using CASA instruments has largely been at the discretion of individual animal research laboratories. Each species’ semen has different normative parameters that dictate how CASA instruments are calibrated [27, 29]. Limited information is available to define normative kinematic parameters in a species-specific manner, and less is available when searching for those with specific CASA instruments and chambered slides. In the marmoset, past studies have been performed to define sperm kinematic parameters using the Hobson Sperm Tracker (Hobson Tracker Limited, Sheffield, United Kingdom) [30–32] and HTM-Master C (Hamilton-Thorne Research, Beverley, Massachusetts, USA) [34]. Similarly, a HTM-Master C was used to define the motility of cynomolgus macaque epididymal sperm [34]. In the rhesus macaque, sperm kinematic parameters have previously been evaluated using either a Sperm Class Analyzer (Microptic S.L., Barcelona, Spain) [29, 46] or a HTM Ceros (Hamilton-Thorne) [47] instrument. These past studies utilized a range of slide types, different numbers of analysis fields, and had variable sperm counts when investigating these parameters.
Uniform semen processing and evaluation techniques are important for obtaining both an accurate and reproducible assessment of semen following experimental manipulation, as well as for the selection of semen donors. The World Health Organization (WHO) has provided procedural guidelines for human semen analysis [24]. These guidelines describe that the CASA analysis should include the use of multiple chambers, capture of at least 200 sperm tracks per chamber, and capture of at least 6 fields per chamber [24]. Moreover, a sperm analysis slide with a chamber depth of 20 μm is recommended. Although CASA instruments are capable of estimating sperm concentrations, manual counting using hemocytometers is considered the gold standard [24, 48]. Thus, in the present study a sperm analysis protocol was developed that included manual counting of sperm with use of hemocytometers and encompassed the WHO guidelines for the number of sperm tracks to be between 200-400 sperm per chamber with six fields captured for each of two chambers analyzed.
A comparison of semen and sperm kinematic parameters revealed species-specific differences, particularly between marmosets and macaques. Notably, this is the first report assessing both marmosets and macaques using the same CASA instrument with species-specific settings. Marmoset males produced smaller ejaculate volumes with lower cell concentrations compared to macaques, however, the proportion of motile and progressive motile sperm cells were similar. When comparing sperm kinematic parameters, marmoset sperm tended to have higher sperm velocities compared to rhesus macaques but were more similar to cynomolgus males. Marmoset sperm cells also largely differed in terms of their ALH, area and BCF. Similar to a previous report assessing sperm of different species by CASA [29], these findings also highlight the differences in primate sperm kinematics and thus the need to tailor the CASA settings to each species.
Marmoset semen is often processed using a swim-up technique due to the low volume of seminal fluid and the presence of coagulum. This method reduces concentration and hinders the capture of a large proportion of sperm in some samples [49]. In the present study, evaluation of sperm kinematics throughout the duration of the sperm swim-up incubation revealed no changes in overall sperm motility or individual sperm kinematic parameters during the incubation. The volume and concentration of sperm cells were similar to previous reports for samples collected by vaginal washing and electrostimulation [30, 31, 50], but were lower in comparison to another report in which semen was collected by vibratory stimulation [35]. The percentage of progressive motile cells of ~47–59% is in agreement with a report by Cui et al. [50] in which the median progressive sperm motility was 48%. The overall motility reported for marmoset sperm ranges from ~59–75% [35], and here, the mean motility was ~73% after 90 min of sperm swim-up incubation. The mean sperm velocities and BCF in the present study were higher than those reported by Morrell et al. [31]. The differences across studies may be due to differences in sperm collection methods and the processing of sperm, particularly compared to the differences in obtaining a sample by vaginal washing.
In the present study, the middle of the fluid portion of the marmoset sperm swim-up region was analyzed to more uniformly sample sperm within the fluid fraction. The center region, however, often contained fewer sperm at earlier time points. For instance, at 30 min a mean of ~140 sperm tracks were analyzed across two chambers, whereas ~587 sperm tracks were analyzed at 90 min. An inverse relationship between sperm concentration and some sperm kinematic parameters has been previously noted [30]. Future analyses should focus on the assessment of the upper two-thirds of the sperm swim-up region or alternatively, process the sperm using a density gradient and centrifugation to obtain a more homogenous sample [32].
Sperm kinematics were similar between rhesus and cynomolgus macaques, and are consistent with previous reports of semen analysis for macaques [46, 51]. A volume of ~0.4 mls with a concentration of ~193.0×106 sperm cells/ml and motility of ~69% has been reported for rhesus macaques [46, 51], which is in agreement with our study. Likewise, the velocities are within a similar range as a previous study by Burruel et al. [47], although the absolute values were not reported, for rhesus macaque sperm analyzed using a Hamilton-Thorne Ceros CASA instrument. This study and our previous study [52] evaluating sperm cryopreservation methods are the first to report semen characteristics for Mauritian cynomolgus macaques. Yeung et al. [53] reported a range in cynomolgus macaque (animal origin not reported) sperm motility of ~40–71% with a range in mean velocities of 47–162 μm/s. Similarly, in the present study the mean motility of cynomolgus sperm was ~73% with of an average path velocity of ~130 μm/s. Overall, there were no significant differences in sperm kinematic parameters between the two species of macaques.
In comparison to marmosets or cynomolgus macaques, seasonality in breeding has been reported for rhesus macaques. In the United States, an increase in rhesus macaque conceptions have been noted between October to May [45]. Seasonal effects on testicular and endocrine function have been previously described for rhesus macaque males [1, 41–44, 54]. Although the factors which influence seasonal breeding in indoor-housed rhesus macaques are not well understood, it is thought that food availability, light, humidity, and temperature all have some effect [1]. There are few reports on the impact of season on sperm function. Testicular biopsies during the non-breeding season revealed a transformation of the seminiferous tubules from active spermatogenesis to spermatogenic arrest [41]. Levels of plasma testosterone peak to 1200 ng/100 ml during the months of October and November, whereas in summer months levels decline to as low as 200 ng/100 ml [54]. This provides a physiological explanation to the correlation between breeding season and low sperm volume/quality previously observed [54].
During the breeding season, rhesus males respond to electroejaculation stimulation to produce reliable, highly uniform ejaculates consisting of high populations of motile, structurally normal spermatozoa [41]. Comparatively, males respond less robustly to electroejaculation stimulation during the non-breeding season, producing degenerative populations of mostly nonmotile spermatozoa [41]. In the present study, the impact of season on semen quality and sperm kinematic parameters were evaluated in four rhesus macaque males. A modest seasonal effect was noted that was largely dependent on the individual male. Altogether, our results indicate that while seasonality produces more stark differences between rhesus macaque males, at the individual level there is a minimal effect on semen quality.
The challenge in interpreting results across laboratories can largely be attributable to the method of semen collection, semen processing, and the CASA instrument and slides used for conducting sperm analysis. For example, sperm kinematics have been reported for marmoset ejaculates collected by vaginal washing, electroejaculation and vibratory stimulation, but a comparison of all three methods in one report is lacking. Additionally, different laboratories have used different sperm analyzers and different slides for the analyzers. When looking at four different CASA slide types compatible with a Hamilton-Thorne instrument, including clean slide-coverslip, Makler chamber, 2-chambered Leja, and 4-chambered Leja, these slides hold different volumes and/or are comprised of chambers with differing depths, causing inconsistent results when data is compared between slide types [55]. In a report by Lenz et al. [55], the authors concluded that as long as the same slide type is consistently used in the laboratory, results remain likewise consistent and reliable. In the present study, the normative sperm kinematic parameters reported here may aid in guiding other research laboratories in using similar methods of semen collection and instrumentation for experimental evaluation of sperm parameters.
Conclusions
Overall, the CASA settings established for both marmosets and macaques under the guidance of a Hamilton-Thorne representative in this study may serve as a guide for implementation in other research laboratories using this instrument. Here, we report differences in sperm kinematics between marmosets and macaques. The variation in sperm kinematics between and within males is considerable, thus a larger cohort of males with repeated sampling within a male may be needed to fully capture changes in sperm kinematic parameters. The authors have found that the selection of males for in vitro fertilization is often contingent on the number of males collected for the experiment, and that the stark variability between males allows for facile selection based on the progressive motility and concentration of the sample. The outcomes of this study may be of utmost importance to NHP research for assessing semen characteristics following experimental manipulation as well as for selection of semen donors for assisted reproduction. Moreover, continued optimization of NHP cryopreservation protocols will rely on the ability to effectively evaluate semen samples. The improvement of effective CASA evaluation protocols would allow for potential establishment of a NHP cryobank for exchange of cryopreserved samples from proven sires or genetically engineered sires amongst laboratories worldwide.
Supplementary Material
Acknowledgements:
The authors would like to extend our thanks to the animal care staff as well as the Scientific Protocol Implementation and Veterinary Services Units at the Wisconsin National Primate Research Center for assistance with all animal procedures. Research reported in this publication was supported by the Office of the Director, National Institutes of Health under award number P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison and 1K99HD099154-01 awarded to JKS. This research was conducted at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. The authors also extend their thanks to Nicholas Keuler of the College of Agriculture and Life Sciences Statistical Consulting group for his guidance in the statistical analysis.
Footnotes
Ethics Statement: The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. All procedures were performed in accordance with the NIH guide for the Care and Use of Laboratory Animals and under the approval of the University of Wisconsin College of Letters and Sciences and Vice Chancellor Office for Research and Graduate Education Institutional Animal Care and Use Committee (protocols g005592, g005172, g005298, g005044).
Conflict of Interest Statement: The authors declare no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Harrison R, Kubisch M: Male Reproduction and Fertilization. In: The Laboratory Primate. Elsevier, 2005. [Google Scholar]
- 2.In vitro fertilization and embryo transfer in primates: Springer-Verlag; New York, 1993. [Google Scholar]
- 3.Henry Petry WL: Infection of Macaque Monkeys with Simian Immunodeficiency Virus: An Animal Model for Neuro-AIDS. Intervirology 1997; 40:112–121. [DOI] [PubMed] [Google Scholar]
- 4.DH A, DK B, RJ C, ME Y, NJ S-D: Aspects of Common Marmoset Basic Biology and Life History Important for Biomedical Research. Comparative medicine 2003; 53. [PubMed] [Google Scholar]
- 5.B L, H L, L L, RW O, Y H, M L: Current Advances in HIV Vaccine Preclinical Studies Using Macaque Models. Vaccine 2019; 37. [DOI] [PubMed] [Google Scholar]
- 6.Okano H, Hikishima K, Iriki A, Sasaki E: The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med 2012; 17:336–340. [DOI] [PubMed] [Google Scholar]
- 7.GJ C, JL B, N M, PL M, Y O, GF W: Developmental and Reproductive Toxicology Studies in Nonhuman Primates. Birth defects research Part B, Developmental and reproductive toxicology 2009; 86. [DOI] [PubMed] [Google Scholar]
- 8.DM D, MT A, EL M, CM N, TG G, TC F, DH OC: Using Macaques to Address Critical Questions in Zika Virus Research. Annual review of virology 2019; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ: Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med 2003; 53:339–350. [PubMed] [Google Scholar]
- 10.The Laboratory Primate: Elsevier, 2005. [Google Scholar]
- 11.Schmidt JK, Golos TG: In Vitro Culture of Embryos from the Common Marmoset (Callithrix jacchus). Methods Mol Biol 2019; 2006:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kropp J, Di Marzo A, Golos T: Assisted reproductive technologies in the common marmoset: an integral species for developing nonhuman primate models of human diseasesdagger. Biol Reprod 2017; 96:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki E: Creating Genetically Modified Marmosets. In: The Common Marmoset in Captivity and Biomedical Research. Marini, Wachtman , ardif T , Mansfield & Fox (eds). Academic Press; 335–353, 2019. [Google Scholar]
- 14.E C, E H: In Vitro Culture of Embryos From the Cynomolgus Macaque (Macaca Fascicularis). Methods in molecular biology (Clifton, NJ) 2019; 2006. [DOI] [PubMed] [Google Scholar]
- 15.C R, C H: In Vitro Culture of Rhesus Macaque (Macaca Mulatta) Embryos. Methods in molecular biology (Clifton, NJ) 2019; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G: Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science 2001; 291:309–312. [DOI] [PubMed] [Google Scholar]
- 17.Wolfgang MJ, Eisele SG, Browne MA, Schotzko ML, Garthwaite MA, Durning M, Ramezani A, Hawley RG, Thomson JA, Golos TG: Rhesus monkey placental transgene expression after lentiviral gene transfer into preimplantation embryos. Proc Natl Acad Sci U S A 2001; 98:10728–10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T: Generation of transgenic non-human primates with germline transmission. Nature 2009; 459:523–U550. [DOI] [PubMed] [Google Scholar]
- 19.Y K, C C, F W, Y N: CRISPR/Cas9-mediated Genome Editing in Nonhuman Primates. Disease models & mechanisms 2019; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Zheng Y, Kang Y, Yang W, Niu Y, Guo X, Tu Z, Si C, Wang H, Xing R, Pu X, Yang SH, Li S, Ji W, Li XJ: Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum Mol Genet 2015; 24:3764–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Liu Y, Tu Z, Xiao C, Yan S, Ma X, Guo X, Chen X, Yin P, Yang Z, Yang S, Jiang T, Li S, Qin C, Li XJ: CRISPR/Cas9-mediated PINK1 deletion leads to neurodegeneration in rhesus monkeys. In: Cell Res 334-336, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Sharma J, Ke Q, Landman R, Yuan J, Chen H, Hayden DS, Fisher JW 3rd, Jiang M, Menegas W, Aida T, Yan T, Zou Y, Xu D, Parmar S, Hyman JB, Fanucci-Kiss A, Meisner O, Wang D, Huang Y, Li Y, Bai Y, Ji W, Lai X, Li W, Huang L, Lu Z, Wang L, Anteraper SA, Sur M, Zhou H, Xiang AP, Desimone R, Feng G, Yang S: Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature 2019; 570:326–331. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Wan H, Feng G, Qu J, Wang J, Jing Y, Ren R, Liu Z, Zhang L, Chen Z, Wang S, Zhao Y, Wang Z, Yuan Y, Zhou Q, Li W, Liu GH, Hu B: SIRT6 deficiency results in developmental retardation in cynomolgus monkeys. Nature 2018; 560:661–665. [DOI] [PubMed] [Google Scholar]
- 24.Organization WH: WHO laboratory manual for the Examination and processing of human semen. Fifth Edition. In, 2010. [Google Scholar]
- 25.Baskaran S, Finelli R, Agarwal A, Henkel R: Diagnostic value of routine semen analysis in clinical andrology. Andrologia 2020. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson MJ, Naeem A: CASA in the medical laboratory: CASA in diagnostic andrology and assisted conception. Reproduction, Fertility and Development 2018; 30:850–859. [DOI] [PubMed] [Google Scholar]
- 27.Verstegen J, Iguer-Ouada M, Onclin K: Computer Assisted Semen Analyzers in Andrology Research and Veterinary Practice. Theriogenology 2002; 57:149–179. [DOI] [PubMed] [Google Scholar]
- 28.Amann R: Can the Fertility Potential of a seminal sample be predicted accurately? Journal of Andrology 1989; 10:89–98. [DOI] [PubMed] [Google Scholar]
- 29.Maree L, van der Horst G: Quantification and identification of sperm subpopulations using computer-aided sperm analysis and species-specific cut-off values for swimming speed. Biotech Histochem 2013; 88:181–193. [DOI] [PubMed] [Google Scholar]
- 30.Morrell JM, Kuederling I, Hodges JK: Influence of Semen Collection Method on Ejaculate Characteristics in the Common Marmoset, Callithrix jacchus. Journal of Andrology 1996; 17. [PubMed] [Google Scholar]
- 31.Morrell JM: CASA as an aid to selecting sperm suspensions for artificial insemination in Callithrix jacchus. Journal of Andrology 1997; 20:287–296. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-Lopez L, Umland N, Mondragon-Ceballos R, Nayudu PL: Comparison of the effects of Percoll and PureSperm (R) on the common marmoset (Callithrix jacchus) semen. Journal of Medical Primatology 2005; 34:86–90. [DOI] [PubMed] [Google Scholar]
- 33.Morrell JM: Cryopreservation of marmoset sperm (Callithrix jacchus). Cryo-Letters 1997; 18:45–54. [Google Scholar]
- 34.Yeung CH, Morrell JM, Cooper TG, Weinbauer GF, Hodges JK, Nieschlag E: Maturation of sperm motility in the epididymis of the common marmoset (Callithrix jacchus) and the cynomolgus monkey (Macaca fascicularis). Journal of Andrology 1996; 19:113–121. [DOI] [PubMed] [Google Scholar]
- 35.Kuederling I, Schneiders A, Sonksen J, Nayudu PL, Hodges JK: Non-invasive collection of ejaculates from the common marmoset (Callithrix jacchus) using penile vibrostimulation. American Journal of Primatology 2000; 52:149–154. [DOI] [PubMed] [Google Scholar]
- 36.Marshall VS, Browne MA, Knowles L, Golos TG, Thomson JA: Ovarian stimulation of marmoset monkeys (Callithrix jacchus) using recombinant human follicle stimulating hormone. Journal of Medical Primatology 2003; 32:57–66. [DOI] [PubMed] [Google Scholar]
- 37.Kumar P, N S, P M, P JK, S AS: Evaluating Sperm Cell Viability and Membrane Integrity | SpringerLink. In: Protocols in Semen Biology (Comparing Assays). Srivastava &M (eds). SpringerLink; 57–71, 2020. [Google Scholar]
- 38.Wessel MT, Althouse GC: Validation of an objective approach for simultaneous assessment of viability and motility of fresh and cooled equine spermatozoa. Animal Reproduction Science 2006; 94:21–22. [Google Scholar]
- 39.Gilchrist RB, Nayudu PL, Hodges JK: Maturation, fertilization, and development of marmoset monkey oocytes in vitro. Biology of Reproduction 1997; 56:238–246. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi T, Hanazawa K, Inoue T, Sato K, Sedohara A, Okahara J, Suemizu H, Yagihashi C, Yamamoto M, Eto T, Konno Y, Okano H, Suematsu M, Sasaki E: Birth of Healthy Offspring following ICSI in In Vitro-Matured Common Marmoset (Callithrix jacchus) Oocytes. Plos One 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamboni L, Conaway CH, Vanpelt L: SEASONAL-CHANGES IN PRODUCTION OF SEMEN IN FREE-RANGING RHESUS-MONKEYS. Biology of Reproduction 1974; 11:251–267. [DOI] [PubMed] [Google Scholar]
- 42.Wickings EJ, Nieschlag E: SEASONALITY IN ENDOCRINE AND EXOCRINE TESTICULAR FUNCTION OF THE ADULT RHESUS-MONKEY (MACACA-MULATTA) MAINTAINED IN A CONTROLLED LABORATORY ENVIRONMENT. International Journal of Andrology 1980; 3:87–104. [DOI] [PubMed] [Google Scholar]
- 43.Gupta G, Maikhuri J, Setty B, Dhar JD: Seasonal variations in daily sperm production rate of rhesus and bonnet monkeys. Journal of Medical Primatology 2000; 29:411–414. [DOI] [PubMed] [Google Scholar]
- 44.FW B, SR C, JD D: Seasonal Changes in the Seminiferous Epithelium of Rhesus and Bonnet Monkeys. Journal of medical primatology 2003; 32. [DOI] [PubMed] [Google Scholar]
- 45.Dunk RDP, Petto AJ, Mayer GC, Campbell BC: Seasonality of Conceptions in Captive Rhesus Macaques (Macaca mulatta). International Journal of Primatology 2015; 36:855–870. [Google Scholar]
- 46.Villiers Cd: A comparison between the semen and sperm parameters from the captive-bred Vervet monkey (Chlorocebus aethiops) and Rhesus monkey (Macaca mulatta) 2018. [DOI] [PubMed] [Google Scholar]
- 47.Burruel V, Klooster K, Barker CM, Pera RR, Meyers S: Abnormal Early Cleavage Events Predict Early Embryo Demise: Sperm Oxidative Stress and Early Abnormal Cleavage. Scientific Reports 2014; 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brito LFC, Althouse GC, Aurich C, Chenoweth PJ, Eilts BE, Love CC, Luvoni GC, Mitchell JR, Peter AT, Pugh DG, Waberski D: Andrology Laboratory review: Evaluation of sperm concentration. Theriogenology 2016; 85:1507–1527. [DOI] [PubMed] [Google Scholar]
- 49.Takabayashi S, Suzuki Y, Katoh H: Development of a modified artificial insemination technique combining penile vibration stimulation and the swim-up method in the common marmoset. Theriogenology 2015; 83:1304–1309. [DOI] [PubMed] [Google Scholar]
- 50.Cui KH, Flaherty SP, Newble CD, Guerin MV, Napier AJ, Matthews CD: COLLECTION AND ANALYSIS OF SEMEN FROM THE COMMON MARMOSET (CALLITHRIX-JACCHUS). Journal of Andrology 1991; 12:214–220. [PubMed] [Google Scholar]
- 51.Maree L, van der Horst G: Quantification and identification of sperm subpopulations using computer-aided sperm analysis and species-specific cut-off values for swimming speed. Biotechnic & Histochemistry 2013; 88:181–193. [DOI] [PubMed] [Google Scholar]
- 52.NS S, J KS, KD M, ML S, TG G, II S: Cryopreservation of Mauritian Cynomolgus Macaque (Macaca fascicularis) Sperm in Chemically Defined Medium. Journal of the American Association for Laboratory Animal Science : JAALAS 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CH Y, GF W, E N: Movement characteristics of ejaculated sperm from cynomolgus monkeys (Macaca fascicularis) analyzed by manual and automated computerized image analysis. Journal of medical primatology 1989; 18. [PubMed] [Google Scholar]
- 54.Gordon TP, Rose RM, Bernstein IS: SEASONAL RHYTHM IN PLASMA TESTOSTERONE LEVELS IN RHESUS-MONKEY (MACACA-MULATTA) - 3-YEAR-STUDY. Hormones and Behavior 1976; 7:229–243. [DOI] [PubMed] [Google Scholar]
- 55.Lenz RW, Kjelland ME, VonderHaar K, Swannack TM, Moreno JF: A comparison of bovine seminal quality assessments using different viewing chambers with a computer assisted semen analyzer. Journal of Animal Science 2011; 89:383–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


