Abstract
Background and Aims:
The long-term impact of hepatitis C virus (HCV) therapy with all-oral direct-acting antivirals (DAAs) on patient-reported outcomes (PROs) has not been well-described. We characterized changes in PROs from pre-treatment to 12 months post-treatment in a real-world cohort.
Methods:
PROP UP was a multi-center observational cohort study of 1,601 patients treated with DAAs at 11 U.S. gastroenterology/hepatology practices from 2015-2017. PROs were evaluated pre-treatment (T1) and 12 months post-treatment (T5). A minimally important change (MIC) threshold was prespecified as >5% change in PRO scores from T1 to T5. Multivariable analyses identified predictors of change.
Results:
Three-quarters of patients were 55 or older; 45% were female, 60% were white, 33% were black, nearly half had cirrhosis. The most commonly-prescribed DAA regimens were sofosbuvir-based (83%) and grazoprevir/elbasvir (11%). Study retention was greater than 95%. On average, small improvements were observed at 3 months post-treatment in all PROs and sustained at 12 months post-treatment among patients with sustained virologic response (SVR). Clinically meaningful improvements were achieved in fatigue (mean change score: −3.7 [−4.2, −3.1]), sleep (mean change score: −3.1 [−3.7, −2.5]), abdominal pain (mean change score: −2.6 [−3.3, −1.9]) and functional well-being (mean change score: −7.0 [−6.0, −8.0]). Symptom improvements were generally not sustained with no SVR (n=52). Patients with cirrhosis and MELD ≥ 12 had the greatest improvements in functional well-being (−12.9 [−17.6, −8.1]).
Conclusions:
The improvements in patient-reported outcomes reported by patients who achieved SVR following HCV DAA therapy were durable at 12 months post-treatment.
Keywords: health-related quality of life, viral hepatitis, treatment, symptoms, prospective cohort
Lay Summary:
A total of 1601 patients undergoing treatment for Hepatitis C and nearly half with cirrhosis were recruited from 11 medical practices in North America. Patients who achieved Hepatitis C cure experienced significant improvement in fatigue, sleep, stomach pain, and functional well-being that were maintained at 12 months after therapy completion. Patients reported that their medical conditions improved; those with cirrhosis achieved the greatest improvements in functional well-being.
Introduction
In addition to its effects on the liver, hepatitis C virus (HCV) causes extrahepatic manifestations and negatively impacts health-related quality of life (HRQoL) with somatic, neuropsychiatric, and gastrointestinal symptoms (1–3). Improvements in HRQoL were observed with successful HCV treatment in the era of interferon-based regimens (4). Data from studies of all oral direct-acting antivirals (DAAs), however, are largely derived from industry-sponsored trials with carefully selected patient populations (5–7) or have small sample sizes, short follow-up, or do not comprehensively evaluate a full spectrum of potential patient-reported outcomes (PROs) (8–10).
We conducted a prospective, multi-center study, PROP UP, to evaluate the impact of HCV DAA therapy on symptoms and functioning in a large real-world population. Baseline, on-treatment, and 3 month post-treatment data have been previously reported (11, 12). In this analysis we evaluate change in symptoms and functioning from baseline (T1) to 12 months (T5) following the end of HCV treatment to characterize durability of long-term benefits of cure with regards to 10 specific symptoms, total symptom burden, functional well-being, and self-reported health comorbidities.
Methods
Study Overview
PROP-UP was a multi-center, prospective, observational cohort study that from 2015 to 2017 enrolled 1,601 patients across the U.S. to evaluate experiences associated with DAA treatment for chronic HCV with details published previously (11, 12). The primary outcomes in this report include PRO scores for (a) 10 specific symptoms, (b) total symptom burden, (c) functional well-being, and (d) self-reported health conditions. Patients were prescribed one of five DAA regimens as standard of care: (sofosbuvir/ledipasvir (SOF/LED), sofosbuvir/velpatasvir (SOF/VEL), elbasvir/grazoprevir (ELB/GRZ) ombitasvir/paritaprevir/ritonavir with dasabuvir (OBV/PTV/r + DSV), and sofosbuvir/daclatasvir (SOF/DAC).
Study Settings
The University of North Carolina at Chapel Hill was the lead site. The study included eight other U.S. gastroenterology/hepatology academic medical centers and two community-based practices for a total of 11 sites. Local institutional review board approval was obtained prior to study recruitment and data collection at each site.
Inclusion/Exclusion Criteria
Eligible patients included those diagnosed with chronic HCV, English speaking, age 21 years or older, and prescribed one of the five DAA regimens. Exclusion criteria included: unable to provide informed consent, current participation in a pharmaceutical-sponsored drug trial of HCV treatment, believed to have major cognitive or mental impairment by the clinical provider, unable to read or speak English, or unwilling or unable to complete study surveys.
Recruitment, Consent, Enrollment
Patients were recruited from hepatology outpatient clinics by providers who referred eligible patients to the study. Research staff consented patients in person in the clinic or over the phone after consent forms and a recruitment letter were mailed to patients. Consented participants were officially enrolled in the study if he/she completed the baseline PRO surveys before starting DAA therapy and officially started DAA treatment. Baseline PRO surveys were required to be completed within 90 days prior to starting treatment. Patients began enrollment at University of North Carolina in November 2015 and at collaborating sites in January 2016. Recruitment ended in October 2017 and final data collection ended in July 2018.
Timing of Data Collection
In addition to baseline demographic and clinical characteristics, PROs were obtained from patients up to 90 days before starting treatment (T1); two time points during DAA treatment (T2, T3), 3 months post-treatment (T4), and 12 +/− 2 months post-treatment (T5). This analysis focused on PRO changes from T1 to T5 and durability of PRO changes from T4 to 12 months post-treatment (T5).
Minimally Important Change (MIC) in PRO Measures
At the outset of the study, we pre-defined the minimally important change (MIC) as a change in PRO mean scores of greater than 5% from baseline. The 5% MIC threshold was chosen based on: (1) guidance from our HCV Patient Engagement Group; (2) information from the PROP-UP baseline scores; and (3) review of the literature on PRO instruments(7, 13–16). The 5% MIC was reflected as a 2.5-point change for all PROMIS symptom measures, 3.0-point change for Total Memorial Symptom Assessment Scale (TMSAS), and 4.0 change for the HCV-PRO.
Overall Symptom Burden.
The Memorial Symptom Assessment Scale (MSAS) is a reliable and validated instrument that was used to capture Overall Symptom Burden(17, 18). The MSAS evaluates 32 of the most common symptoms of medical conditions. As described in our previous publications, A higher TMSAS score reflects higher symptom burden (12, 19). Change in the total symptom burden (TMSAS) score from baseline (0 to 4) was calculated and multiplied by 10 to be on similar scale as other PRO scores.
Specific Symptoms/Side Effects.
As described in previous publications, 10 short forms from the Patient-Reported Outcomes Measurement Information System® (PROMIS®) were used to measure HCV-associated symptoms (12, 19). PROMIS scores are scaled to a standardized T-score metric with a mean of 50 and standard deviation of 10 for the U.S. general population and have been validated for HCV (19). Higher scores indicate worse symptoms/side effects. The 10 PROMIS short forms used to evaluate change in symptoms over time are listed in Supplemental Table 1.
Functional Well-Being.
The HCV-PRO is a new HCV-specific survey designed to assess the well-being and functional status of HCV patients (20). HCV-PRO includes 16 items that measure physical, emotional, social functioning, productivity, intimacy, and perception of quality of life rated on a 5-point ordinal rating scale from 1= “all of the time” to 5=“none of the time”. The scale ranges from 0-100, with higher scores indicating better functioning.
Health comorbidities.
Based on medical history forms, we developed a survey of 34 common health conditions described in layperson terms. At baseline patients reported whether they (a) never had the condition; (b) had it previously; or (c) have it currently. At 12 months post-treatment (T5) participants indicated whether they believed their baseline health conditions “stayed the same,” “got worse,” or “got better.” Studies suggest a strong correlation between patient-reported chronic medical conditions and objective clinical data extracted from medical records (21).
Other Self-Report Data
Sociodemographics.
Sociodemographic information was self-reported at baseline to characterize the study sample: year of birth, sex, race, ethnicity, marital status, educational status, income level, employment status, and health insurance status.
Psychiatric and Substance Use History.
Participants self-reported responses to 10 questions related to psychiatric history and drug and alcohol use. For analytic purposes, we classified patients who reported any lifetime psychiatric hospitalizations or currently taking psychiatric medications at baseline as having “mental health issues”. Three questions from the Alcohol Use Disorder Identification Test (AUDIT)(22) evaluated frequency and quantity of alcohol consumption at baseline. Patients with alcohol use were defined as those who scored ≥ 5 on the three AUDIT items (22, 23). Two questions from the Substance Abuse Mental Illness Symptoms Screener (SAMISS) evaluated frequency of drug use in the past year, including use of nonprescription street drugs and prescription drugs(23). Patients with substance use were defined as those who reported use of non-prescription illicit street drugs or misuse of prescription medications in the year prior to enrollment.
Clinical Data from Medical Records
Laboratory, clinical and treatment variables.
HCV genotype, HCV RNA level, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total bilirubin, platelets, hemoglobin, creatinine, international normalized ratio (INR), HIV, DAA treatment regimen, treatment duration, and treatment experience were extracted from medical records, some of which were used to cross-reference with cirrhosis classification below.
Cirrhosis.
Patients were classified as having cirrhosis based on review of clinical, laboratory, imaging, histology, and transient elastography data in electronic health records. The model for End-Stage Liver Disease (MELD) was calculated among patients with cirrhosis (24); MELD ≥12 were used to indicate advanced liver disease. Adjudication of cases with inconsistent data was made by an experienced hepatologist (M.W.F.) or by site investigators/hepatologists.
Sustained virological response (SVR).
SVR status was ascertained from medical records and was defined as undetectable HCV RNA (qualitative or quantitative) at 10 or more weeks after treatment completion. In 15 patients, lack of SVR was based on quantifiable positive HCV RNA test around follow-up week 4.
Analysis Plan
The primary analysis was to evaluate mean change in PRO scores from baseline (T1) to 12 months post-treatment (T5) in patients who completed T1 and T5 surveys, had HCV RNA data available post-treatment to determine SVR status and who had achieved SVR (n=1,277). Secondarily, we described change in PROs from T4 (3 months post-treatment) to T5. Lastly, we examined changes from T1 to T5 in a small subgroup of patients who did not achieve SVR (n=52) and had complete T1 and T5 data.
To analyze change from baseline to one-year post-treatment (T5-T1) in specific symptoms and functioning in patients with documented SVR, we used a data-splitting strategy and unsupervised LASSO algorithms. Generalized linear models were used for both exploratory predictive model development (in Sample 1) and for confirmatory evaluation of models specified a priori (in Sample 2). Participants were randomly assigned to two groups: Sample 1 or Sample 2. Using Sample 1 (n=600), we evaluated a larger set of candidate predictor variables that might be associated with change in each PRO. Covariates available for selection in the Sample 1 analyses included: age, sex, race, ethnicity, cirrhosis status, MELD score, employment, education, income, alcohol use, substance use, mental health issues, HIV, DAA regimen, ribavirin (RBV) use, treatment duration, treatment experience, and number of health comorbidities. In Step 2, Sample 2 was used to validate the predictive value of the variables selected from the Sample 1 analysis. The variables in the model were considered validated if their regression coefficients were statistically significant at alpha = 0.01.
Results
The analytic cohort, including patients who completed PROs around 12 months post-treatment (11 months +/− 3 months) and had HCV RNA SVR data available (n=1329), is shown in Figure 1.
Figure 1.
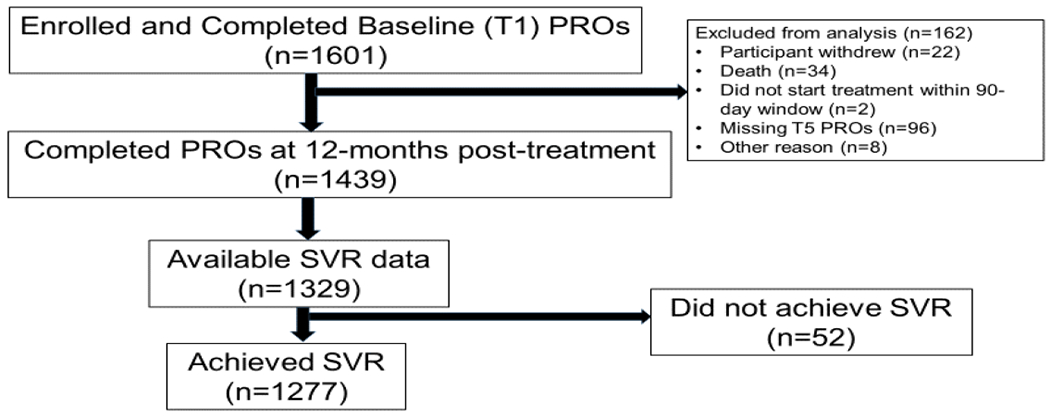
Study Cohort
Patient Characteristics
The baseline characteristics of the study sample with T5 data (n=1329) stratified by SVR are provided in Table 1. Three-quarters of patients were older than 55 years; 45% were female, 60% were white, 33% were black, 4% reported Hispanic/Latino ethnicity, 38% were working full-time, and 47% had cirrhosis. Most patients were prescribed sofosbuvir-based regimens for 12 weeks and had genotype 1 HCV. Patients had, on average five health comorbidities (range: 0-17). At baseline, 35% reported ever having mental health disturbances, 14% had baseline alcohol misuse, and 22% had reported substance use within the year prior to enrollment.
Table 1:
Baseline patient characteristics (n=1,329) stratified by SVR status
| Characteristic | Total (n=1329) nb (%) | SVR (n = 1277) | Non-SVR (n=52) |
|---|---|---|---|
| Sociodemographic Features | |||
| Age | |||
| <35 | 59 (4) | 58 (4) | 1 (2) |
| 35-55 | 292 (22) | 279 (22) | 13 (25) |
| >55 | 978 (74) | 940 (74) | 38 (73) |
| Sex | |||
| Female | 602 (45) | 582 (46) | 20 (38) |
| Male | 727 (55) | 695 (54) | 32 (62) |
| Race | |||
| Black | 801 (60) | 423 (33) | 19 (36) |
| White | 442 (33) | 769 (60) | 32 (62) |
| Other | 81 (7) | 80 (7) | 1 (2) |
| Ethnicity | |||
| Not Hispanic or Latino | 1038 (83) | 993 (82) | 45 (88) |
| Hispanic or Latino | 55 (4) | 53 (4) | 2 (4) |
| Other | 170 (13) | 166 (14) | 4 (8) |
| Education | |||
| Up to High school diploma or GED | 707 (54) | 683 (54) | 24 (49) |
| Vocational school or higher | 607 (46) | 582 (46) | 25 (51) |
| Annual Income | |||
| Under $40,000 per year | 932 (72) | 901 (72) | 31 (65) |
| $41,000 or above per year | 361 (28) | 344 (28) | 17 (35) |
| Employment status | |||
| Working full or part time | 480 (38) | 460 (37) | 20 (41) |
| Unemployed | 75 (6) | 74 (6) | 1 (2) |
| Disabled/applying | 558 (43) | 536 (44) | 22 (45) |
| Retired/homemaker/student | 170 (13) | 164 (13) | 6 (12) |
| Clinical and Treatment Features | |||
| Genotype | |||
| 1, 4, 6 | 1087 (83) | 1044 (83) | 43 (82) |
| 2 | 118 (9) | 114 (9) | 4 (8) |
| 3 | 110 (8) | 105 (8) | 5 (10) |
| Cirrhosis Status | |||
| Cirrhosis Not present | 709 (53) | 687 (54) | 22 (42) |
| Cirrhosis present | 617 (47) | 587 (46) | 30 (58) |
| MELD | |||
| 6-11 | 448 (87) | 425 (87) | 23 (85) |
| 12 or above | 68 (13) | 64 (13) | 4 (15) |
| DAA Treatment Cohorta | |||
| SOF/LED | 836 (63) | 809 (63) | 27 (51) |
| SOF/VEL | 277 (21) | 262 (21) | 15 (29) |
| GRZ/ELB | 144 (11) | 138 (11) | 6 (12) |
| OBV/PTV/r + DSV | 55 (4) | 52 (4) | 3 (6) |
| SOF/DAC | 17 (1) | 16 (1) | 1 (2) |
| Treatment Duration | |||
| 8 weeks | 132 (10) | 127 (10) | 5 (10) |
| 12 weeks | 1091 (82) | 1049 (82) | 42 (80) |
| 16 or 24 weeks | 106 (8) | 101 (8) | 5 (10) |
| Treatment Experience | |||
| Treatment naive | 1073 (81) | 1038 (81) | 35 (67) |
| Treatment experienced | 255 (19) | 238 (19) | 17 (33) |
| Ribavirin | |||
| Without Ribavirin | 1155 (87) | 1113 (87) | 42 (81) |
| With Ribavirin | 174 (13) | 164 (13) | 10 (19) |
| Medical conditions | |||
| 0-1 | 263 (20) | 255 (20) | 8 (15) |
| 2-3 | 329 (25) | 311 (24) | 18 (35) |
| ≥4 | 735 (55) | 709 (56) | 26 (50) |
| Mental Health and Substance Use Features | |||
| Lifetime Mental Health Disturbance | |||
| No | 863 (65) | 828 (65) | 35 (67) |
| Yes | 460 (35) | 443 (35) | 17 (33) |
| Alcohol Misuse | |||
| No | 1132 (86) | 1087 (86) | 45 (87) |
| Yes | 190 (14) | 183 (14) | 7 (13) |
| Substance Use in the Past Year | |||
| No | 1033 (78) | 991 (78) | 42 (81) |
| Yes | 290 (22) | 280 (22) | 10 (19) |
NOTE:
DAA: Direct-Acting Antiviral, SOF/LED: sofosbuvir/ledipasvir, SOF/VEL: sofosbuvir/velpatasvir, GRZ/ELB: grazoprevir/elbasvir, OBV/PTV/r + DSV: ombitasvir/paritaprevir/ritonavir+dasabuvir, SOF/DAC: Sofosbuvir/Daclatasvir, SVR: Sustained Virologic Response.
PRO Changes 12 Months After HCV Treatment with DAAs
PRO change scores from baseline (T1) to 12 months post-treatment (T5) by SVR status are shown in Figure 2. In 1,277 patients who achieved SVR, all PRO mean change scores improved (negative sign indicates improvement); however, not all changes met the 5% MIC threshold. Clinically significant (defined by the 5% MIC threshold) improvements at 12-months post-SVR were found for Fatigue (−3.7 [−4.2, −3.1]), Sleep Disturbance (−3.1 [−3.7, −2.5]), Abdominal Pain (−2.6 [−3.3, −1.9]) and Functional Well-being (−7.0 [−6.0, −8.0]).
Figure 2: PRO mean change scores from baseline (T1) to 12 Months Post-treatment (T5) by SVR status.
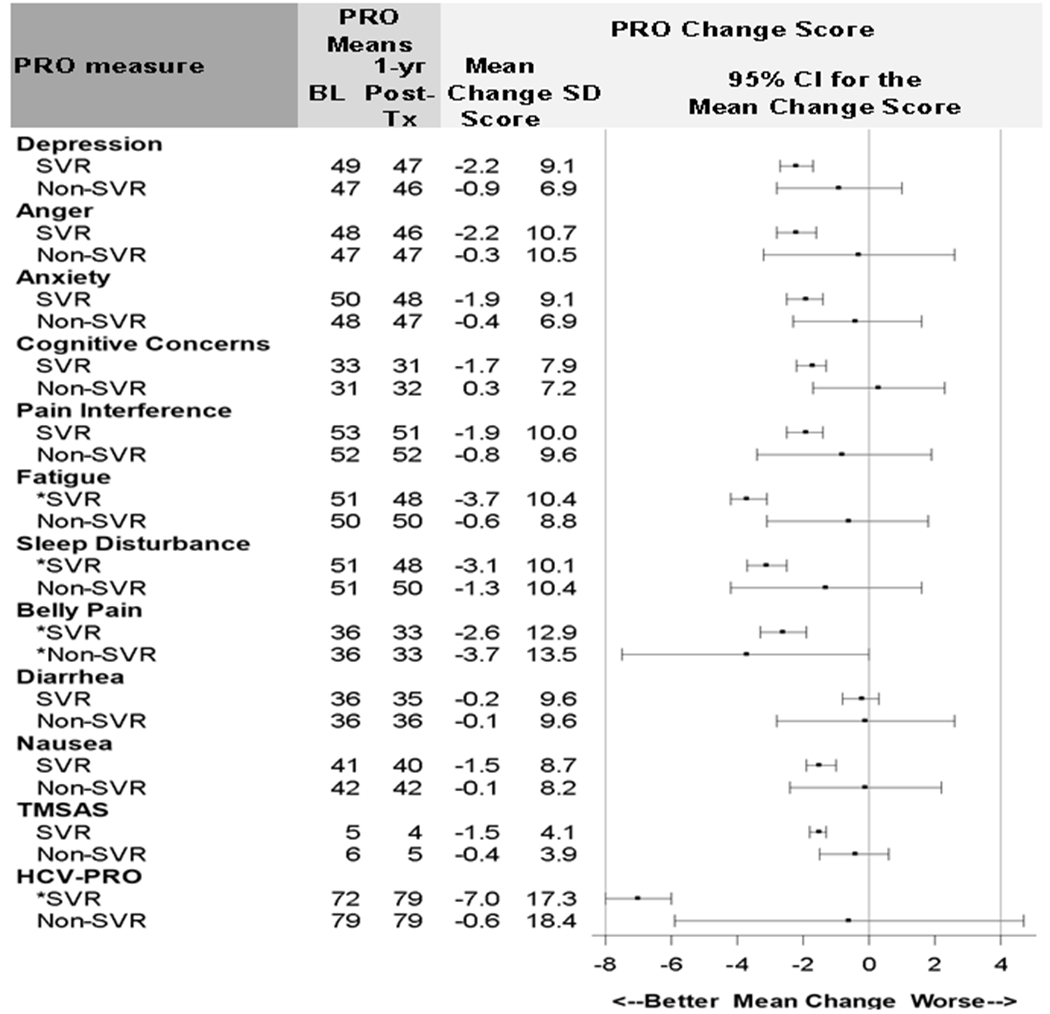
NOTE: * Mean change score greater than %5 MIC. The 5% MIC for PROMIS symptoms=2.5 points, for TMSAS=3.0, for HCV-PRO=4.0. SVR n=1,277; Non-SVR n=52. CI: confidence interval. HCV-PRO score is on 0-100 scale. HCV-PRO mean change score was reverse coded for consistency with other PROs. Negative change scores represent better outcomes. Missing values for all PRO change scores were ≤2%, except functional well-being (HCV-PRO) was missing for 6%-12% of patients.
Multivariable Models Predicting PRO Changes from Baseline (T1) to 12 months Post-Treatment (T5)
A total of 12 confirmatory regression models were fit predicting PRO changes at 12 months post-treatment in patients who achieved SVR (Supplemental Table 2). Broadly speaking, the strongest, most consistent independent predictors of symptom improvements were age, number of health comorbidities, and DAA cohort. Specifically, patients aged 35 to 55 years and those with 4+ health comorbidities had more pronounced symptoms improvements one-year after SVR compared to their counterparts (number of comorbidities stratified by age group is shown in Supplemental Table 3). Patients aged 35 to 55 years had substantial improvements in functional well-being, overall symptom burden, abdominal pain, cognitive concerns, fatigue and nausea compared with patients in other age groups. Patients reporting substance use prior to enrollment had pronounced improvements in depression at 12 months post-treatment. Patients with the highest number of comorbidities had pronounced improvements in functional well-being, overall symptom burden, nausea, as well as a trend for improved abdominal pain, cognitive concerns, and fatigue. Patients who were prescribed OBV/PTV/r + DSV had less improvement in anxiety and cognitive symptoms (as indicated by + not − coefficients) compared to patients treated with sofosbuvir-based regimens; although the sample size of patients on OBV/PTV/r+DSV was very small. Ribavirin was not selected as an independent predictor of PRO changes in multivariable models. Sociodemographic factors such as education and employment were evaluated as potential predictors of change in PRO scores, however, were not selected as significant predictors of PRO change from baseline to 12 months after SVR in the final model.(12, 25) The number of patients who did not achieve SVR (n=52) was too small to fit multivariable models for PRO change.
Durability of PRO Changes after SVR
Figure 3 shows that, among patients who achieved SVR, average PROMIS mean scores improved 1-3 points over time, overall symptom burden diminished, and functional well-being improved by 7 points. Importantly, clinically significant (>5%) improvements in fatigue, sleep disturbance and functional well-being were observed at T4 (12 weeks post treatment)(12) and were sustained at T5 (12 months post-treatment). Abdominal pain improved even more after SVR, such that clinically significant improvements were observed at 12 months post-treatment in patients who achieved cure. Among patients who did not achieve SVR (Supplemental Figure 1), a 1-2 point improvement was transiently noted during treatment, with most symptoms reverting back to baseline by T5, with the exception of abdominal pain that improved by 3 point.
Figure 3.
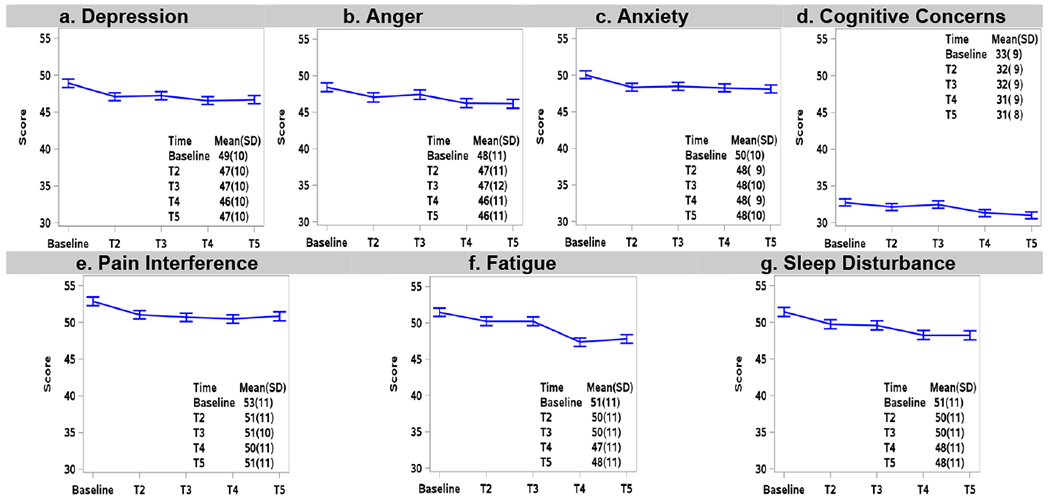
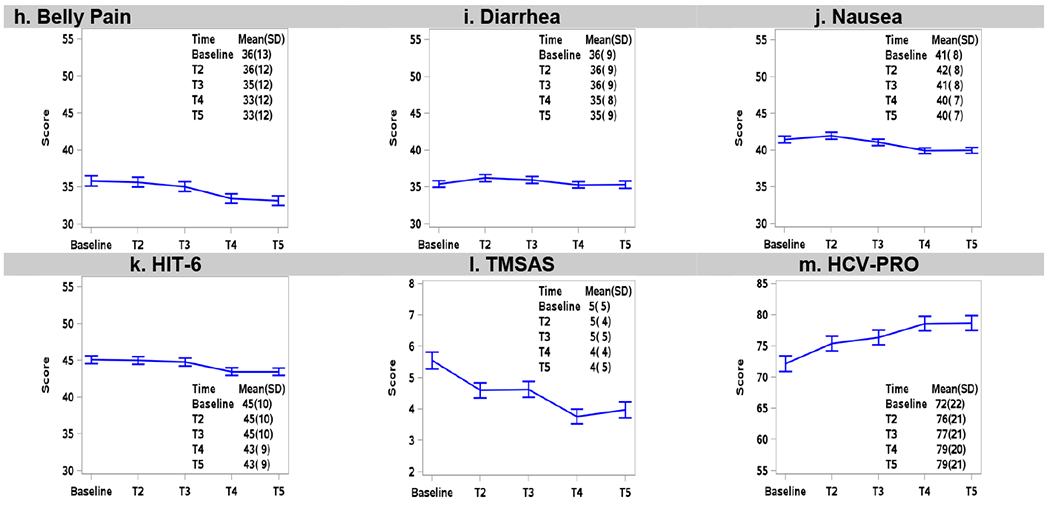
Longitudinal PRO mean scores at baseline, early-treatment (T2), late-treatment (T3), 12-weeks post-treatment (T4), and 12 months post-treatment (T5) among patients who achieved SVR (n=1277). Error bars represent 95% confidence intervals.
Change in Pre-Existing Health Comorbidities at 12 Months Post-treatment (T5) Among Patients who Achieved SVR
Patients’ self-reported experiences that their baseline conditions “got better”, “stayed the same” or “got worse” 12 months after SVR are listed in Figure 4. The majority of participants indicated that their health conditions stayed the same 12 months after achieving SVR (range per condition from 44% to 78%). Among the 15 most prevalent symptoms, nine improved in over 20% of patients with greatest improvement in diabetes (46%), cholesterol (36%) and blood pressure (32%), while five worsened in over 20% with greatest worsening in vision loss. Supplemental Figure 2 and 3 show the changes in self-reported health conditions stratified by cirrhosis status and sex, respectively.
Figure 4:
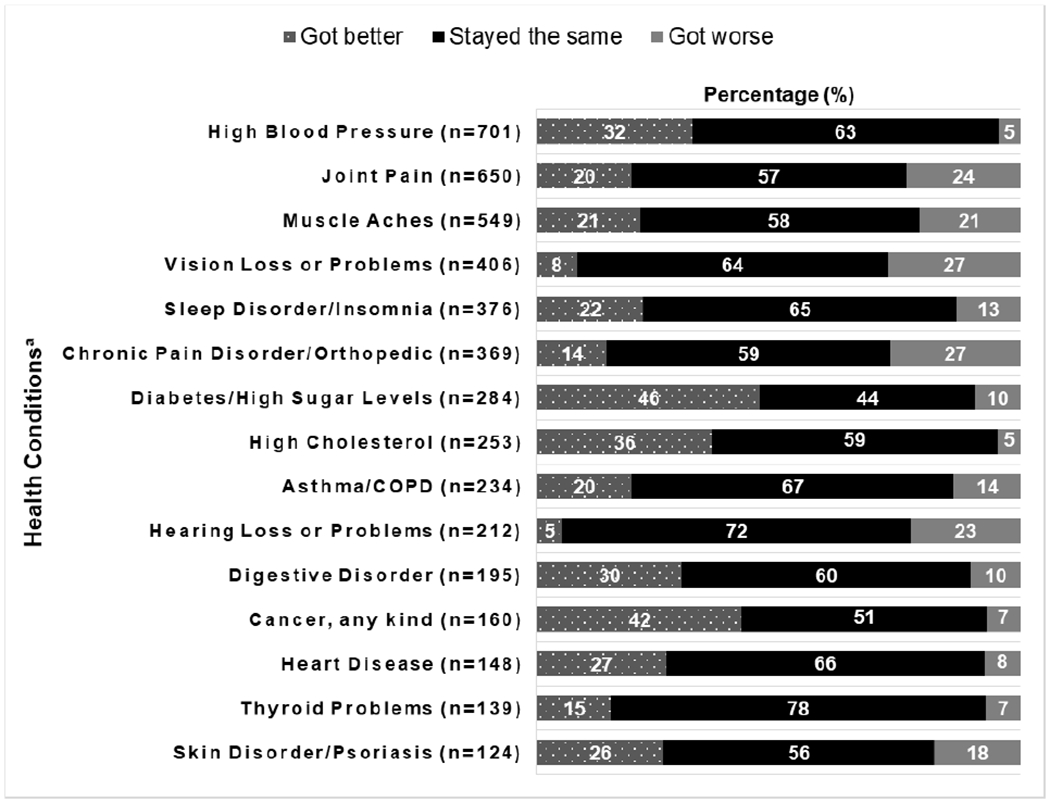
Change in pre-existing health conditions among patients who achieved SVR (n=1277)
NOTE: a Top 15 health conditions. n = number of patients who reported the health condition at baseline. The percentage (%) was computed based on the number of patients who reported a change in Pre-Existing Comorbidities at 12 Months Post-Treatment (T5).
Improvements in Symptoms and Functioning by Specific Patient Subgroups
Improvements in symptoms and functioning by patient subgroups identified from regression analysis are shown in Supplemental Table 2, Figures 5 and 6 and Supplemental Figures 4 to 8. Figure 5 demonstrates how patients aged 35 to 55 years experienced greater symptom improvements compared to patients over 55. Whereas all age groups improved on fatigue and sleep disturbance, the 35-55 age group experienced more pronounced improvements in neuropsychiatric symptoms (range: 3.5 - 4.3) and experienced twice as much improvement on the HCV-PRO (a disease-specific measure that assesses physical, emotional, social functioning, productivity, intimacy, and perception of quality of life).
Figure 5: Change in PROs 12 Months (T5) after SVR by Age (n=1277).
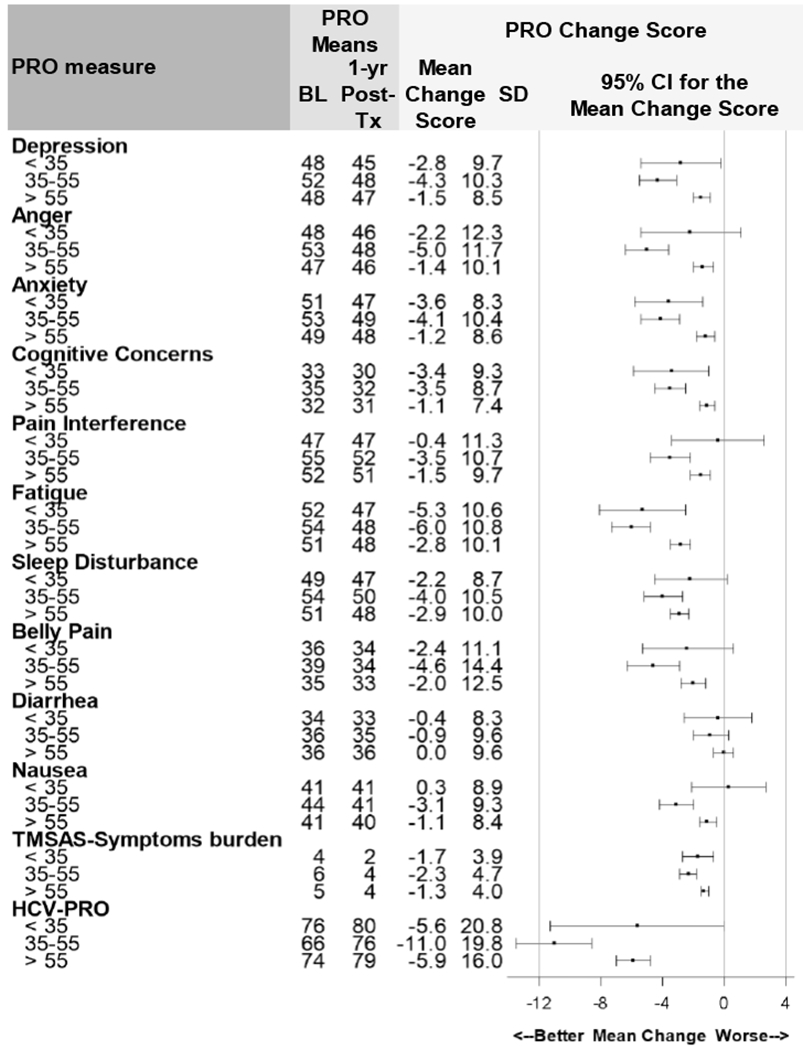
NOTE: PRO: Patient Reported Outcome. SVR: Sustained Virologic Response. BL: Baseline. 1-yr Post-Tx: 1-year Post-Treatment. SD: Standard Deviation. CI: Confidence Interval. TMSAS scores (Overall Symptom Burden) multiplied by 10. TMSAS score is on 0-40 scale. HCV-PRO score is on 0-100 scale. HCV-PRO mean change score was reverse coded for consistency with other PROs. Positive change scores represent worse PRO scores; negative change scores represent better outcomes. The 5% MIC for PROMIS symptoms=2.5 points, for TMSAS =3.0, for HCV-PRO=4.0.
Figure 6:
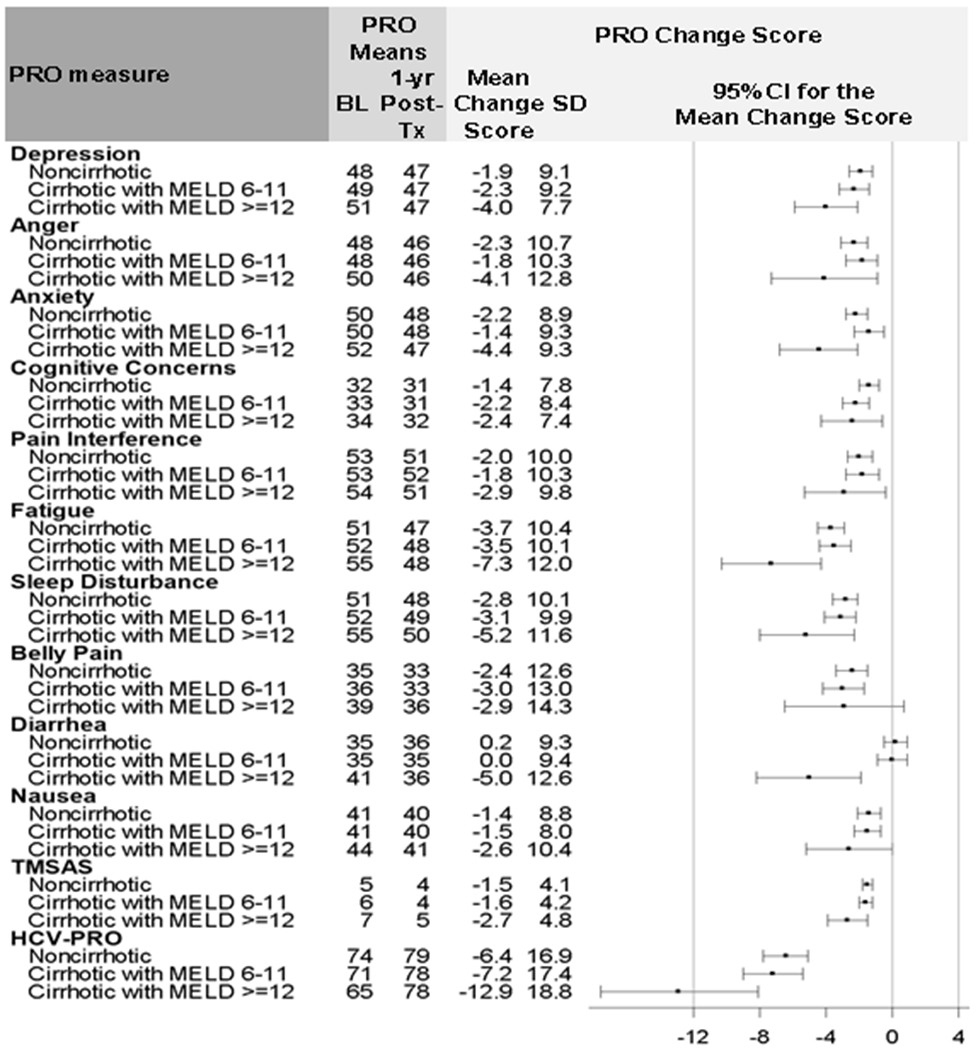
Change in PROs 12 Months post-treatment (T5) after SVR by Cirrhosis and MELD Status (n=1176)
NOTE: PRO: Patient Reported Outcome. SVR: Sustained Virologic Response. BL: Baseline. 1-yr Post-Tx: 1-year Post-Treatment. SD: Standard Deviation. CI: Confidence Interval.
Figure 6 shows the PRO change scores by cirrhosis-MELD status. Patients with and without cirrhosis had improvements in symptoms; however, those with MELD ≥ 12 had incrementally greater symptom reduction 12 months after SVR compared to patients with no cirrhosis or less advanced cirrhosis. The greatest improvements (>5%) were seen in fatigue, sleep interference, abdominal pain, and HCV-PRO in all groups. Notably, patients with cirrhosis and MELD ≥ 12 had the greatest improvements in functional well-being (−12.9 [−17.6, −8.1]).
On average, white patients experienced greater symptom and PRO improvements compared to black patients (Supplemental Figure 4). Most PRO scores improved for all DAA regimens, with clinically significant improvements in fatigue, sleep disturbance, and abdominal pain. Patients prescribed OBV/PTC/r+DSV experienced the least improvements especially for GI symptoms but the estimates were imprecise with wide confidence intervals (Supplemental Figure 5). Review of the mean change scores, suggests that all symptoms and functioning improved, regardless of treatment duration (Supplemental Figure 6). Patients on longer treatment durations (perhaps those with more advanced liver disease or prior treatment failures) showed the greatest gains in overall functioning and well-being (HCV-PRO). Patients with mental health issues experienced much more pronounced clinical benefits compared to those without mental health issues (Supplemental Figure 7) with the largest symptoms reductions observed in abdominal pain, fatigue, sleep disturbance, depression, anger, anxiety, and in overall functioning and sense of well-being. Patients with self-reported drug use (Supplemental Figure 8) experienced much more robust improvements in symptoms compared to those not using drugs at baseline.
Discussion
The PROP UP study represents the largest, most comprehensive real-world investigation of patients’ experiences during and after DAA therapy, providing novel information about HCV symptoms, possible benefits and harms during therapy, and short-term and long-term benefits of viral cure. Results in this analysis extend our prior work showing improvement in symptoms and functioning in HCV patients after achieving SVR are sustained up to 12 months after treatment.
At baseline, over 60% of our cohort had fatigue, sleep disturbance, pain, and neuropsychiatric symptoms whereas gastrointestinal symptoms were present in up to one half of patients.(19) At the end of treatment and after achieving SVR (95% in this cohort), mean PRO scores improved with clinically meaningful changes in the symptoms of fatigue, sleep disturbance, and functional well-being(12). In this follow-up at 12 months after treatment completion, we noted that all PRO scores improved, on average, from baseline with clinically meaningful improvements observed primarily for fatigue, sleep disturbance, abdominal pain and overall functional well-being, that are sustained after SVR. On average, no worsening of symptoms was noted from time of SVR to 12 months after SVR in the total cohort. These results are consistent with pooled registry data reported from patients enrolled in DAA clinical trials (26). On average, patients who did not achieve SVR experienced a return to baseline symptom levels 12 months after treatment.
We observed that certain patient subgroups: patients age 35 to 55 years, those using drugs at baseline, and those with a greater number of comorbidities derived greater symptom improvements 12 months post-treatment. It is interesting that the 35 to 55 age group had the most pronounced symptom improvements given that these patients had higher rates of mental health issues (49% vs 31%) and drug use (27% vs 20%) at baseline. This suggests that patients with psychosocial comorbidities benefit substantially from viral cure. A previous study by our group showed high SVR and DAA adherence rates among patients with drug use and mental health conditions (27); thus coupled with this study’s findings strongly suggest universal access to DAA therapy irrespective of age and psychosocial comorbidities. Older patients with HCV may not have obtained as robust PRO improvements compared to adults aged 35 to 55 given the presence of a higher number of other comorbidities that continue to cause symptoms unrelated to HCV. Patients with mental health conditions experienced improvements in PROs, which could be due to a host of factors including perhaps better linkage to care and healthcare engagement.
Although patients with and without cirrhosis derived equal benefit from viral cure, patients with cirrhosis and MELD scores over 12 experienced the greatest benefits from cure in terms of symptom reduction and improved functional well-being. These results may inform patient counseling and setting up expectations prior to treatment. Not surprisingly, DAA treatment regimen and duration did not appreciably affect long-term changes in most of the PROs at one-year post treatment as the PROs were generally affected most by the presence or absence of SVR. We did not find an independent effect of ribavirin on change in symptoms one year after SVR, however, patients prescribed OBV/PTV/r + DSV had less improvement in anxiety and cognitive symptoms after SVR. This lack of improvement may have been due to high concurrent use of ribavirin during treatment (71% of patients on OBV/PTV/r + DSV regimen versus 5-13% with other DAA regimens), however, the sample size on this regimen was only 4% making it difficult to draw firm conclusions.
Our findings are interesting and novel with regards to patient-reported changes in chronic health conditions, which have been shown to be strongly correlated with health record data (21). Near half of patients reported improvement in diabetes and about one third reported improved cholesterol and blood pressure. Although the precise reason for these results is unknown, other studies have shown improvement in short-term mortality after treatment with DAAs and SVR that were not solely explained by reduced liver-disease-related mortality (28, 29). We would postulate that patients experienced legitimate improvements in their overall health after HCV cure due to the role that HCV plays in chronic inflammation and the mounting evidence supporting extrahepatic manifestations of HCV on multiple organ systems (1, 30). Additionally, reduction in fatigue may have helped patients become more physically active. It is also possible that factors beyond HCV cure such as engagement in healthcare for other medical conditions partially explains our observed findings.
There are some study limitations that must be acknowledged. Due to the rapidly evolving treatment landscape for HCV in the last four years, some of the DAA regimens observed in PROP UP have been replaced by newer regimens making some of our findings somewhat antiquated for current clinical practice decision-making. A relatively small number of patients had advanced cirrhosis or high MELD score; therefore, our findings are not applicable in that setting. Mental health, substance use, and alcohol use were self-reported; thus, social desirability and favorable response bias could have affected responses. We did not specifically ascertain whether individuals reporting a history of substance use were involved in harm reductiontreatment, that may have improved symptoms concurrently along with HCV therapy. We are also unable to ascertain whether linkage to care and possible mental health services concurrent with HCV therapy resulted in PRO improvements in patients with mental health conditions A recent study found a significant incidence of new depressive symptoms among patients after DAA therapy while our study found a decrease in depressive symptoms post-DAA therapy.(31) The current study was conducted during the earlier era of all-oral DAA therapy from 2015-2017 and possibly could represent patients highly invested and motivated to engage in healthcare or HCV treatment. Patients were all English-speaking and were enrolled predominantly at academic centers. Thus, our findings may not generalize to the larger HCV-infected community, to patients not engaged in liver care, to people who inject drugs, Veterans, or incarcerated individuals.
Our study has a number of strengths and highlight relevant information for multiple stakeholders including patients, clinicians, and third-party payers. PROP UP is the largest investigation of PROs in a current population of patients with chronic HCV undergoing DAA therapy outside of industry-sponsored clinical trials. The sample was diverse with regards to socio-demographics, cirrhosis status, with many patients having multiple concurrent medical and psychiatric comorbidities as well as substance use issues. These subpopulations are often under-represented in registration trials. We were able to retain and collect PRO data from over 90% of the total cohort. Finally, our patient partners were engaged throughout all phases of study development to ensure that the study outcomes chosen were meaningful and important to people affected by the disease.
In conclusion, this large multi-center cohort study showed that in patients with HCV treated with DAAs, mean improvement in symptoms and functioning attained after achieving SVR was maintained up to 12 months post-treatment, particularly in fatigue, sleep disturbance, abdominal pain and overall functional well-being.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge the contributions of the following people: Alan Franciscus of www.HCVadvocate.org, Anquenette Sloan, Summer Wadsworth-Delciotto, Scott Kixmiller, Larry Huston, Finton Brown (UNC Patient Engagement Group); Virginia Sharpless, Ken Berquist, Herleesha Anderson, Courtenay Pierce, Jenn Barr, Bryonna Jackson, Jane Giang, Jama Darling, Paul Hayashi, Steven Zacks, A. Sidney Barritt IV, Scott Elliot, Dawn Harrison, Danielle Cardona (University of North Carolina); Patrick Horne (University of Florida); Kelly Borges, Danielle Ciuffetelli (University of Pennsylvania); Chrissy Ammons, Kathleen Genther, Jessica Mason (Virginia Commonwealth University); Lelani Fetrow, Vicki Shah (Rush University); Theresa Cattoor, Alisha McLendon (Saint Louis University); Mariechristi Candido, Sophia Zaragoza, Sandeep Dhaliwal, Patricia Poole, Rebecca Hluahanich, Kathleen Haight, (University of California, Davis); Andrea Gajos, Elizabeth Wu, Carrie Bergmans (University of Michigan); AnnMarie Liapakis, MD, Kristine Drozd, Carol Eggers, Hong Chau and Claudia Bertuccio (Yale University). William Harlan, Roberta Golden, Kylee Diaz (Asheville Gastroenterology Associates), William King, Megan Marles (Wilmington Gastroenterology Associates). We also extend our gratitude to the PROP UP study participants.
Conflict of interest:
Donna M. Evon receives research funding from Gilead and Merck. Michael Fried has received research funding from and served as a consultant for AbbVie, BMS, Gilead, and Merck, and TARGET PharmaSolutions. Stock in TARGET PharmaSolutions is held in an independently managed trust. Anna S. Lok has received research support from BMS, Gilead, TARGET PharmaSolutions, AbbVie (ended in 2016), and Merck (ended in 2016); and served as an advisor for Gilead (interrupted between 1/2016-12/2018). Richard K. Sterling has received research support from AbbVie, BMS, Gilead, Merck, and Roche and served as a consultant for Merck, Bayer, Salix, AbbVie, Gilead, Jansen, ViiV, Baxter, and Pfizer. Joseph K. Lim has received research support (paid to Yale University) and served as a consultant for Bristol-Myers Squibb and Gilead. Nancy Reau has received research funding (paid to Rush) from AbbVie and Intercept and has served as a consultant for Merck, AbbVie, Abbott, and Gilead. Souvik Sarkar served on a Gilead and AbbVie Advisory Board and received grant support from Gilead through UCSF (paid to UC Davis). David R. Nelson has received research grant support from AbbVie, BMS, Gilead, Janssen, and Merck and owns stock in TARGET PharmaSolutions. K. Rajender Reddy is an Ad-Hoc Advisor to Gilead, BMS, Janssen, Merck, AbbVie, Shionogi, and Dova and has received research support from Gilead, BMS, Janssen, Merck, AbbVie, Intercept, Mallinckrodt, and Conatus (paid to the University of Pennsylvania). Adrian M. Di Bisceglie has received research support from AbbVie, BMS and Gilead and has served on advisory boards for AbbVie, BMS, Gilead and Merck. He serves as Chair of the Steering Committee for TARGET HCC, a registry study funded by TARGET PharmaSolutions. Paul Stewart has served as a consultant to TARGET PharmaSolutions. Jipcy Amador served as a biostatistics intern at TARGET PharmaSolutions in 2017. Carol E. Golin, Bryce Reeve, and Marina Serper declare that they have no conflict of interests to disclose.
Financial support:
This study was funded by the Patient-Centered Outcomes Research Institute (PCORI) Award to Donna Evon (CER-1408-20660). Marina Serper is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, award #1K23DK115897. Additional support for this study (data management) came from the NIDDK-funded Center for Gastrointestinal Biology and Disease (CGIBD; PI: Sandler; P30-DK34987) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health Grant Number UL1TR002489. Additional support for Dr. Golin’s salary was partially supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K24-HD06920) and by the University of North Carolina Center for AIDS Research (CFAR) (P30 AI-50410). The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its Board of Governors or Methodology Committee, or the NIH.
Abbreviations:
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- AUDIT
Alcohol Use Disorder Identification Test
- DAA
Direct acting antiviral
- GRZ/ELB
grazoprevir/elbasvir
- HCV
Hepatitis C Virus
- HRQoL
Health-related quality of life
- MELD
Model for End Stage Liver Disease
- MIC
Minimally important change
- MSAS
Memorial Symptom Assessment Scale
- OBV/PTV/r + DSV
ombitasvir/paritaprevir/ritonavir+dasabuvir
- PROMIS
Patient-Reported Outcomes Measurement Information System
- PROs
Patient Reported Outcomes
- RBV
ribavirin
- SAMISS
Substance Abuse Mental Illness Symptoms Screener
- SOF/LED
sofosbuvir/ledipasvir
- SOF/VEL
sofosbuvir/velpatasvir
- SOF/DAC
Sofosbuvir/Daclatasvir
- SVR
sustained virologic response
Footnotes
REFERENCES
- 1.Ferri C, Ramos-Casals M, Zignego AL, Arcaini L, Roccatello D, Antonelli A, Saadoun D, Desbois AC, Sebastiani M, Casato M, Lamprecht P, Mangia A, Tzioufas AG, Younossi ZM, Cacoub P, coauthors I-E. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev. 2016;15(12):1145–60. Epub 2016/09/20. doi: 10.1016/j.autrev.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman L, Mannix S, Yuan Y, Kummer S, ĽItalien G, Revicki D. Review of patient-reported outcome measures in chronic hepatitis C. Health Qual Life Outcomes. 2012;10:92. Epub 2012/08/09. doi: 10.1186/1477-7525-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang CA, Conrad S, Garrett L, Battistutta D, Cooksley WG, Dunne MP, Macdonald GA. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J Pain Symptom Manage. 2006;31(4):335–44. Epub 2006/04/25. doi: 10.1016/j.jpainsymman.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Dusheiko G The impact of antiviral therapy for hepatitis C on the quality of life: a perspective. Liver International. 2017;37(S1):7–12. doi: 10.1111/liv.13292. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM, Stepanova M, Feld J, Zeuzem S, Jacobson I, Agarwal K, Hezode C, Nader F, Henry L, Hunt S. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: Results from ASTRAL-1 placebo-controlled trial. Journal of Hepatology. 2016;65(1):33–9. doi: 10.1016/j.jhep.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Stepanova M, Zeuzem S, Dusheiko G, Esteban R, Hezode C, Reesink HW, Weiland O, Nader F, Hunt SL. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. Journal of hepatology. 2014;61(2):228–34. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C - the impact of liver disease and new treatment regimens. Alimentary pharmacology & therapeutics. 2015;41(6):497–520. Epub 2015/01/24. doi: 10.1111/apt.13090. [DOI] [PubMed] [Google Scholar]
- 8.Juanbeltz R, Martinez-Baz I, San Miguel R, Goni-Esarte S, Cabases JM, Castilla J. Impact of successful treatment with direct-acting antiviral agents on health-related quality of life in chronic hepatitis C patients. PLoS One. 2018;13(10):e0205277. Epub 2018/10/10. doi: 10.1371/journal.pone.0205277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang ES, Kim YS, Kim KA, Lee YJ, Chung WJ, Kim IH, Lee BS, Jeong SH. Factors Associated with Health-Related Quality of Life in Korean Patients with Chronic Hepatitis C Infection Using the SF-36 and EQ-5D. Gut and liver. 2018;12(4):440–8. Epub 2018/03/29. doi: 10.5009/gnl17322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung JC, Bosh C, Wyatt B, Miller M, Harty A, Del Bello D, Knight S, Dieterich DT, Perumalswami PV, Branch AD. Hepatitis C cure improved patient-reported outcomes in patients with and without liver fibrosis in a prospective study at a large urban medical center. Journal of Viral Hepatitis.n/a(n/a). doi: 10.1111/jvh.13234. [DOI] [PubMed] [Google Scholar]
- 11.Evon DM, Golin CE, Stewart P, Fried MW, Alston S, Reeve B, Lok AS, Sterling RK, Lim JK, Reau N, Sarkar S, Nelson DR, Reddy KR, Di Bisceglie AM. Patient engagement and study design of PROP UP: A multi-site patient-centered prospective observational study of patients undergoing hepatitis C treatment. Contemp Clin Trials. 2017;57:58–68. Epub 2017/03/28. doi: 10.1016/j.cct.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evon DM, Sarkar S, Amador J, Lok AS, Sterling RK, Stewart PW, Reeve BB, Serper M, Reau N, Rajender Reddy K, Di Bisceglie AM, Nelson DR, Golin CE, Lim JK, Fried MW. Patient-reported symptoms during and after direct-acting antiviral therapies for chronic hepatitis C: The PROP UP study. Journal of Hepatology. 2019;71(3):486–97. doi: 10.1016/j.jhep.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna D, Hays RD, Shreiner AB, Melmed GY, Chang L, Khanna PP, Bolus R, Whitman C, Paz SH, Hays T, Reise SP, Spiegel B. Responsiveness to Change and Minimally Important Differences of the Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptoms Scales. Digestive diseases and sciences. 2017;62(5):1186–92. Epub 2017/03/03. doi: 10.1007/s10620-017-4499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amtmann D, Kim J, Chung H, Askew RL, Park R, Cook KF. Minimally important differences for Patient Reported Outcomes Measurement Information System pain interference for individuals with back pain. Journal of pain research. 2016;9:251–5. Epub 2016/05/14. doi: 10.2147/jpr.s93391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Annals of the rheumatic diseases. 2015;74(1):104–7. Epub 2013/10/08. doi: 10.1136/annrheumdis-2013-204053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smelt AF, Assendelft WJ, Terwee CB, Ferrari MD, Blom JW. What is a clinically relevant change on the HIT-6 questionnaire? An estimation in a primary-care population of migraine patients. Cephalalgia : an international journal of headache. 2014;34(1):29–36. Epub 2013/07/12. doi: 10.1177/0333102413497599. [DOI] [PubMed] [Google Scholar]
- 17.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326–36. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 18.Chang VT, Hwang SS, Thaler HT, Kasimis BS, Portenoy RK. Memorial symptom assessment scale. Expert review of pharmacoeconomics & outcomes research. 2004;4(2):171–8. Epub 2004/04/01. doi: 10.1586/14737167.4.2.171. [DOI] [PubMed] [Google Scholar]
- 19.Evon DM, Amador J, Stewart P, Reeve BB, Lok AS, Sterling RK, Di Bisceglie AM, Reau N, Serper M, Sarkar S, Lim JK, Golin CE, Fried MW. Psychometric properties of the PROMIS short form measures in a U.S. cohort of 961 patients with chronic hepatitis C prescribed direct acting antiviral therapy. Alimentary pharmacology & therapeutics. 2018;47(7):1001–11. Epub 2018/01/30. doi: 10.1111/apt.14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient-reported outcomes (HCV-PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(3):877–86. doi: 10.1007/s11136-013-0519-1. [DOI] [PubMed] [Google Scholar]
- 21.Ye F, Moon DH, Carpenter WR, Reeve BB, Usinger DS, Green RL, Spearman K, Sheets NC, Pearlstein KA, Lucero AR, Waddle MR, Godley PA, Chen RC. Comparison of Patient Report and Medical Records of Comorbidities: Results From a Population-Based Cohort of Patients With Prostate Cancer. JAMA oncology. 2017;3(8):1035–42. Epub 2017/02/17. doi: 10.1001/jamaoncol.2016.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders JB, Aasland OG, Babor TF, de lF Jr., Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 23.Pence BW, Gaynes BN, Whetten K, Eron JJ Jr., Ryder RW, Miller WC. Validation of a brief screening instrument for substance abuse and mental illness in HIV-positive patients. JAcquirImmuneDeficSyndr. 2005;40(4):434–44. [DOI] [PubMed] [Google Scholar]
- 24.(UNOS) UNfOS. MELD/PELD Calculator Documentation2009 July 5, 2017.
- 25.Evon DM, Stewart PW, Amador J, Serper M, Lok AS, Sterling RK, Sarkar S, Golin CE, Reeve BB, Nelson DR, Reau N, Lim JK, Reddy KR, Di Bisceglie AM, Fried MW. A comprehensive assessment of patient reported symptom burden, medical comorbidities, and functional well being in patients initiating direct acting antiviral therapy for chronic hepatitis C: Results from a large US multi-center observational study. PLoS One. 2018;13(8):e0196908. Epub 2018/08/02. doi: 10.1371/journal.pone.0196908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younossi ZM, Stepanova M, Racila A, Afendy A, Lawitz EJ, Schwabe C, Ruane PJ, Lalezari J, Reddy KR, Jacobson IM, Muir AJ, Gaggar A, Myers RP, Younossi I, Nader F. Long-term Benefits of Sustained Virologic Response for Patient-Reported Outcomes in Patients With Chronic Hepatitis C Virus Infection. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020;18(2):468–76. e11. Epub 2019/08/04. doi: 10.1016/j.cgh.2019.07.047. [DOI] [PubMed] [Google Scholar]
- 27.Serper M, Evon DM, Stewart PW, Lok AS, Amador J, Reeve BB, Golin CE, Fried MW, Reddy KR, Sterling RK, Sarkar S, Di Bisceglie AM, Lim JK, Nelson DR, Reau N. Medication Non-adherence in a Prospective, Multi-center Cohort Treated with Hepatitis C Direct-Acting Antivirals. J Gen Intern Med. 2019. Epub 2019/10/30. doi: 10.1007/s11606-019-05394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: Impact on mortality in patients without advanced liver disease. Hepatology. 2018;68(3):827–38. [DOI] [PubMed] [Google Scholar]
- 29.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69(2):487–97. [DOI] [PubMed] [Google Scholar]
- 30.Younossi ZM, Henry L, J PO, Tanaka A, Eguchi Y, Mizokami M, Lim YS, Dan YY, Yu ML, Stepanova M. Systematic review with meta-analysis: extrahepatic manifestations in chronic hepatitis C virus-infected patients in East Asia. Alimentary pharmacology & therapeutics. 2019;49(6):644–53. Epub 2019/02/15. doi: 10.1111/apt.15131. [DOI] [PubMed] [Google Scholar]
- 31.Egmond E, Marino Z, Navines R, Oriolo G, Pla A, Bartres C, Lens S, Forns X, Martin-Santos R. Incidence of depression in patients with hepatitis C treated with direct-acting antivirals. Braz J Psychiatry. 2020;42(1):72–6. Epub 2019/07/18. doi: 10.1590/1516-4446-2018-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


