Abstract
In this review, we highlight recent discoveries regarding mechanisms contributing to nerve-cancer crosstalk and the effects of nerve-cancer crosstalk on tumor progression and dissemination. High intratumoral nerve density correlates with poor prognosis and high recurrence across multiple solid tumor types. Recent research has shown that cancer cells express neurotrophic markers such as nerve growth factor, brain-derived neurotrophic factor, and glial cell-derived neurotrophic factor and release axon guidance molecules such as Ephrin B1 to promote axonogenesis. Tumor cells recruit new neural progenitors to the tumor milieu and facilitate their maturation into adrenergic infiltrating nerves. Tumors also rewire established nerves to adrenergic phenotypes via exosome-induced neural reprogramming by p53-deficient tumors. In turn, infiltrating sympathetic nerves facilitate cancer progression. Intratumoral adrenergic nerves release noradrenaline to stimulate angiogenesis via vascular endothelial growth factor signaling and enhance the rate of tumor growth. Intratumoral parasympathetic nerves may have a dichotomous role in cancer progression and may induce Wnt-β-catenin signals that expand cancer stem cells. Importantly, infiltrating nerves not only influence the tumor cells themselves but also impact other cells of the tumor stroma. This leads to enhanced sympathetic signaling and glucocorticoid production, which influences neutrophil and macrophage differentiation, lymphocyte phenotype, and potentially lymphocyte function. Although much remains unexplored within this field, fundamental discoveries underscore the importance of nerve-cancer crosstalk to tumor progression and may provide the foundation for developing effective targets for the inhibition of tumor-induced neurogenesis and tumor progression.
Keywords: axonogenesis, neurogenesis, nerve-cancer, neural reprogramming, neurogenic, tumor microenvironment
Introduction
Mounting evidence suggests that cancer prognosis is associated with intratumoral neural infiltration. This phenomenon is most commonly observed in cancers that arise in highly innervated organs, including nearly all pancreatic cancers, 80% of head and neck cancers, 75% of prostate cancers, and 33% of colorectal cancers (1). Studies on patient tumor samples have revealed that intratumoral nerve density is associated with increased metastasis, morbidity, and mortality. Consistently, the presence of nerve fibers is an independent prognostic factor for overall survival in pancreatic ductal adenocarcinoma (PDAC) (1–4), gastric carcinoma (5,6), biliary tract tumors (7), head and neck cancer (8–10), and cervical cancer (11,12) and an indicator for recurrence risk in pancreatic cancer (13), prostate cancer (11,14), gastric cancer (15), and colorectal cancer (16–21). Tumor innervation may play an important direct role in facilitating metastasis, as tumor-associated nerves may extend into the central nervous system and cultivate pre-metastatic niches (19,22). Tumor innervation may also affect patients’ quality of life by causing pain, paresthesia, numbness, and paralysis. The purpose of this review is to help clinicians and researchers gain a deeper mechanistic understanding of nerve-cancer crosstalk. Of note, this nerve-cancer crosstalk is distinct from perineural invasion, the process by which cancer cells disseminate through lymphatic vessels within the perineural space (23,24).
Fundamental Discoveries in Nerve-Cancer Crosstalk
Despite the known impact of denervation in reducing cancer growth, investigation of nerve-cancer interaction has been slow (25–27). However, knowledge regarding nerve-cancer crosstalk has been greatly advanced by a few landmark discoveries that have elucidated three key mechanisms by which tumors regulate nerves: axonogenesis, neurogenesis, and neural reprogramming (Figure 1A).
Figure 1.
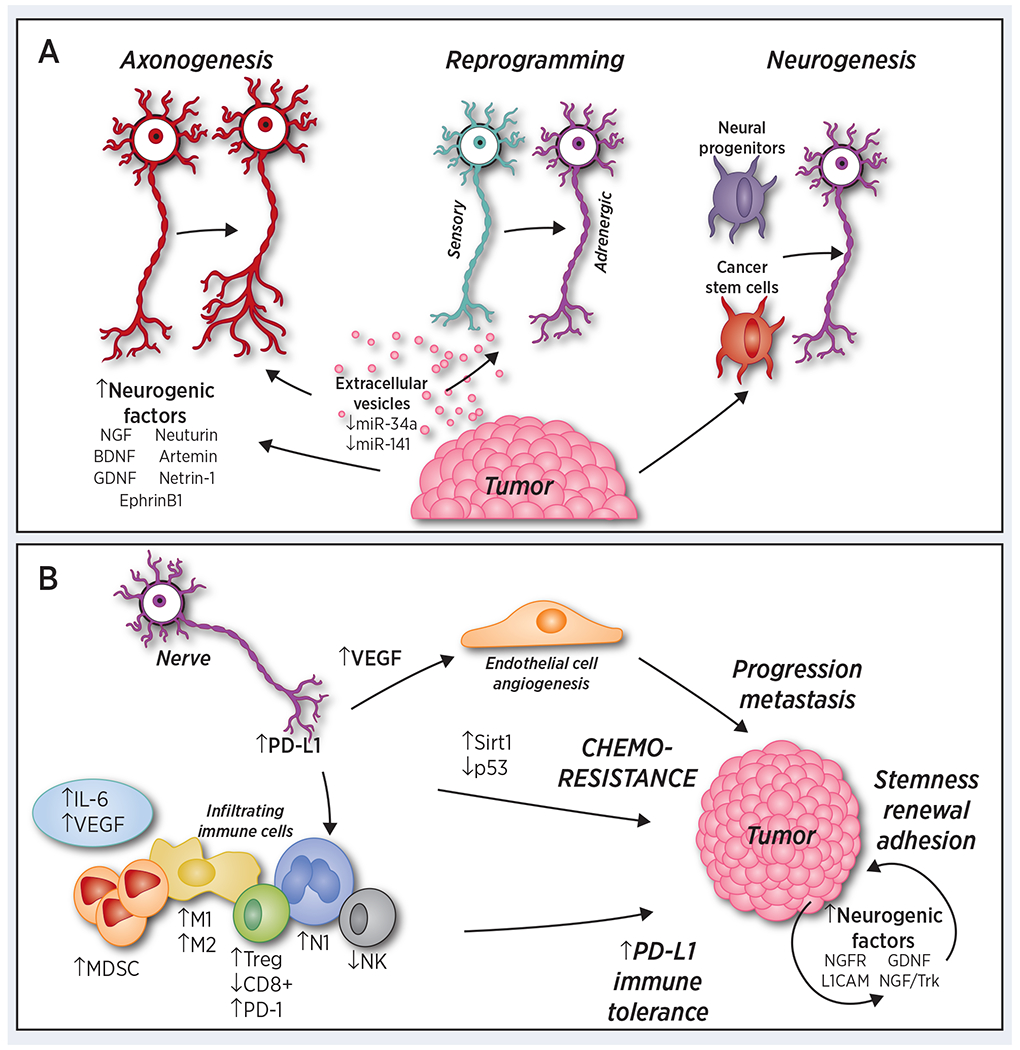
A) Cancer cells drive nerve alteration. Cancer signals to induce nerve growth and innervation through multiple mechanisms. Cancer-induced axonogenesis includes the secretion of numerous neurogenic factors, axon-guidance molecules, and extracellular vesicles containing increased levels of axonal guidance molecules. Neural reprogramming occurs through extracellular vesicles containing orchestrated levels of miR-34a and miR-121, transforming a sensory nerve into an adrenergic nerve. Finally, cancer communicate with distant organs to recruit neural progenitor cells to initiate neurogenesis, while cancer stem cells drive de novo neurogenesis. B) Sympathetic innervation promotes the tumor microenvironment and tumor growth. Sympathetic signaling induces an angiogenic switch through increased vascular endothelial growth factor (VEGF) levels and the induction of aerobic glycolysis. It also promotes the infiltration of CD11b+F4/80+, FoxP3+ Tregs, and myeloid-derived suppressor cells (MDSCs). It stimulates the secretion of interleukin (IL)-6 and decreases the numbers of CD8+ cells and natural killers (NK) cells. It participates in a tumor-type dependent M2-type/M1-type macrophage shift and N2-type/N1-type neutrophil shift. Sympathetic nerves also express protein programmed cell death-1 (PD-1) in some cancer types, potentially contributing to immune suppression. In addition, sympathetic signaling drive chemoresistance via p53-dependent Sirt1 signaling among other mechanisms, and expression of neurogenic ligands and receptors on tumor cells promote stemness and self-renewal within the tumor.
Cancer Progression Drives Axonogenesis
Following their observation that murine dorsal root ganglia form neurite outgrowths towards prostate cancer cells (28), Ayala et al. (29) investigated the symbiotic relationship between nerves and cancer. Through 2- and 3-dimensional reconstructions of entire prostates, Ayala and colleagues uncovered and confirmed cancer-related axonogenesis (the enlargement of nerves or increase in nerve density) and demonstrated an association between prostate cancer and neurogenesis (an increased number of neurons). These findings were supported by the observation that the axon-guidance molecule semaphorin 4F (S4F) is highly expressed—and may be secreted-- by DU-145 cells when co-cultured with neurons, and S4F induced neurite sprouting and increased neurite length in neurons compared to controls. Furthermore, a reduction of S4F via small interfering RNA transfection decreases neurite outgrowth. Together, these findings demonstrated, for the first time, that cancer cells produce known neurotropic molecules capable of driving axonogenesis.
Around the same time as this discovery, the revelation that chronic stress promoted angiogenesis and malignant cell growth through sympathetic β-adrenergic activation (30) caused researchers to further understand the mechanistic effects of stress on tumor growth. A closer investigation of the role of sympathetic β-adrenergic activation in tumor progression led to the landmark demonstration that tumor progression is promoted by nerve stimulation (31). In this study, surgical or pharmacological denervation of both parasympathetic and sympathetic nerves led to prostate tumor suppression in mice. Genetic deletion of sympathetic β2- and β3-adrenergic receptors in stromal cells also prevented early tumor progression. In contrast, parasympathetic stimulation contributed to later tumor progression, invasion, and metastasis through pharmacological or genetic disruption of the muscarinic 1 receptor. Additionally, clinical sympathetic and parasympathetic nerve densities were greatest in patient tumors and surrounding tissues, respectively, and were associated with poor clinical outcomes (32). This study suggests that both sympathetic and parasympathetic signaling may serve as potential therapeutic targets and that cancer-related neurogenesis may drive tumor progression.
Cancer Progression Drives Neurogenesis
In addition to studying cancer’s role in axonogenesis, Ayala et al. and Magnon et al. proposed that cancer may drive neurogenesis, the outgrowth of neural progenitors to the tumor. Mauffrey et al. (33) expounded on this hypothesis, demonstrating that neural progenitor cells expressing the neural stem cell marker doublecortin (DCX+) migrate from neurogenic regions of the brain’s subventricular zone to tumorous and metastatic niches via the bloodstream, differentiating into noradrenergic, mature neuronal phenotypes. Furthermore, DCX+ cells were observed in greater numbers in high-risk versus low-risk human prostate cancer specimens. DCX+ cell depletion also decreased incidence of neoplastic lesions and increased tumor growth in tumors with the addition of DCX+ neural progenitor cells. Additional studies to identify the mechanisms by which DCX+ progenitor cells migrate will be important next steps to confirm the conclusion of this study, cancer-related neurogenesis.
Cancer is also capable of forming de novo neurons from cancer stem cells. Lu et al. (34) showed that cancer stem cells derived from patients’ gastric and colorectal carcinomas were able to differentiate into both tyrosine hydroxylase (TH)-producing sympathetic and vesicular acetylcholine transporter-producing parasympathetic neurons. These neurons, in turn, were able reciprocally communicate with cancer cells within xenografts to facilitate tumor growth. Knockdown of the neuron-generating capacity of these stem cells by MAP2 inhibited tumor xenograft growth, thereby underscoring the importance of these de novo nerves to cancer progression.
Cancer Stimulates Neural Reprogramming
Concurrently with Mauffrey et al.’s study, Amit et al. (35) made a surprising discovery regarding cancer: reciprocal neural crosstalk. Specifically, the authors observed exosome-induced neural reprogramming--- a phenomenon typically only observed during development---by tumors and showed that cancer-derived extracellular vesicles (EVs) play a role in cancer-related axonogenesis. They discovered that genetically aberrant, p53-knockout or -mutant (p53C176F and p53A161S) oral cavity squamous cell carcinoma (OCSCC) cells release EVs that promote neuritogenesis in dorsal root ganglia. This release was dependent on Rab27A and Rab27B, GTPases both necessary for EV release (35,36–39). Analysis of micro RNA (miRNA) array revealed a decrease in the p53-deficient, cancer-derived EV-packaging of miR-34a and miR-141. The knockdown or antagonism of miR-34a in p53WT cancer cells produced similar EVs to those seen in p53-deficient cancer cells. Furthermore, Amit and colleagues were the first to report that daily intratumoral injections of p53WT OCSCC EVs can suppress noradrenergic neurogenesis. Decreased levels of miR-34a not only promoted the neuritogenesis of sensory nerves, but also induced transdifferentiation of these nerves into noradrenaline-producing adrenergic nerves, which are commonly enriched in head and neck tumors and have been shown to promote tumor growth (38). To demonstrate that neural reprogramming, rather than outgrowth of existing adrenergic nerves, drove this process, the authors performed lingual denervation prior to OCSCC implantation in mice, and found lower intratumoral tyrosine hydroxylase-positive nerve density than sham controls. In contrast, global chemical sympathectomy before OCSCC implantation in mice did not affect tumor growth. Similarly, TH+ nerve density in patients with OCSCC was associated with lower overall survival rates and thus it may potentially serve as an independent prognostic marker. Overall, these results demonstrate a novel mechanism by which cancer cells induce nerve density and initiate adrenergic neurogenesis.
Reciprocal Communication Between the Tumor, Microenvironment, and Nerves
The mechanisms that drive axonogenesis through nerve-cancer crosstalk have not yet been fully elucidated. However, evidence described in this section indicates that axonogenesis is stimulated by cancer cells’ release of neurotrophic growth factors and EVs containing altered miRNA levels. Reciprocally, nerves release neurotransmitters that stimulate cancer growth, possibly through either bona-fide or pseudo-tripartite synapses (resembling a tripartite synapse, in which a synapse consisting of two neurons is closely associated with a third, non-neural cell). Together, tumor-associated, stromal, endothelial, and immune cells are nurtured and primed for angiogenesis and inflammation (Figure 1B).
Tumor-derived EVs Induce Axonogenesis through miRNA
EVs play an important role in establishing a tumor microenvironment that can positively regulate tumor initiation and metastasis. EVs, including exosomes, which are assembled and released through a transcriptionally driven process, are released from nearly every cell type and carry complex cargos, consisting of DNA, RNA, miRNA, transfer RNA, long non-coding RNA, lipids, and proteins, across long distances (38–40). As described below, cancer-derived exosomes carry different cargo than do non-cancer-derived exosomes and signal inflammation, angiogenesis, and axonogenesis.
Exosomes extracted from human head and neck cancer samples and from murine oropharyngeal squamous cell carcinomas (OPSCC) induce increased neurite outgrowth in PC12 cells (a rat pheochromocytoma cell line) compared to exosomes derived from control plasma and tissue. In contrast, the pharmacological exosome inhibitor GW4869 and genomic knockout of Rab27A and Rab27B, genes that control the exosome secretion pathway, decrease neurite outgrowth (40). High concentrations of at least one exosome cargo, the axon-guidance molecule Ephrin B1, drive axonogenesis, also seen in cervical cancer cell lines (41). In addition, neuritogenic effects have been seen when PC12 cells treated with cancer-derived exosomes were used to recruit sensory nerves to the tumor microenvironment. These results demonstrate that cancer-derived EVs promote tumor innervation.
It is likely that additional signals packaged in exosomes drive axonogenesis. For example, cancer commonly presents with aberrant regulation of gene expression by miRNAs, which bind to mRNA to reduce its translation or increase its degradation. This binding to mRNA regulates many cellular pathways and transcription factors, such as those involved in proliferation, differentiation, and apoptosis, all of which may be corrupted in cancer. Thus, the genomic aberrations, epigenetic changes, or mutations of miRNA that affect its processing or activity may nurture a microenvironment conducive to tumorigenesis or metastasis (42–44). As such, miRNAs can be classified as oncoMIRs or tumor-suppressor miRNAs (45,46). In support of this concept, Amit et al. revealed that a combination of stimulatory miR-21 and miR-324 with scramble miRNA increased axonogenesis more than did a cocktail including inhibitory miR-34a (35). This suggests the presence of cancer-orchestrated signaling to other cells in the tumor microenvironment through specific exosome packing and release of miRNAs.
Additionally, the promotion of tumor progression through tumor-derived EVs and dysregulated miRNA has been demonstrated by a number of groups studying various cancer types. In lung cancer, low levels of miR-100-5p have been shown to increase levels of mTOR (47). In breast cancer, modified EVs and miR-23a, miR-222, miR-452, and miR-24 alter their respective targets—Sprouty2, PTEN, APC4, and p27—to confer drug resistance (48,49). Tumor-derived EVs can also provide nutrients to the tumor and mediate tumor-stem cell and tumor-progenitor cell communication (50–52). Thus, there are abundant opportunities to investigate the roles of other molecules within tumor-derived EVs in regard to nerve-cancer crosstalk.
Neurogenic Factors Promote Axonogenesis in Cancer Progression
Cancer cells increase secretion of neurogenic factors promoting axonogenesis, while nerves increase expression of the complementary receptors. Deregulation of NGF, which is responsible for the survival, differentiation, and neurite outgrowth of neurons, has been implicated in a number of cancer types that express the tyrosine-receptor kinases (Trks) TrkA, TrkB, and TrkC and the p75 neurotrophin receptor (53).
These receptors are also expressed on nerves, and p75 neurotrophin receptor has recently been reported to act as a chemoattractant for cancer cells (54). Indeed, cancer-derived NGF drives neurite growth and cancer proliferation and migration and is correlated with nerve-cancer crosstalk (1, 55–64). One notable study demonstrated an association between expanded enteric nerves and increased NGF expression in gastric cancer and the NGF/Trk signaling regulation of Dclk1+ tuft-cell coordinated crosstalk between nerves and gastric cancer (64, 65). Migration of pancreatic cancer cells toward dorsal root ganglia was reduced when NGF signaling was blocked through NGF knock down or NGF-neutralizing antibodies (66,67). Similarly, in breast cancer cells, there is a correlation between nerve fibers and NGF expression, and breast cancer cells are capable of driving axonogenesis in PC12 cells, a process partly reversed by blocking NGF (57).
The expression not only of neurogenic factors, but also their cognate receptors, influences nerve-cancer crosstalk (58). NGF’s receptor, nerve growth factor receptor (NGFR), is expressed across multiple cancer types; for example, it is expressed in luminal breast cancer in rare, basal-like cells resistant to antiestrogens (59). NGFR inhibits p53 activity within tumor cells in a negative feedback loop across multiple tumor types. This process is central to maintaining melanoma stem cells in vitro and melanoma growth in vivo (60). Through it, NGF signaling from nerves via NGFR expression on cancer stem cells may drive cancer stem cell renewal and proliferation.
Like NGFR, L1 cell adhesion molecule (L1CAM), a surface receptor central to proper cell adhesion and migration during neural development, is highly upregulated in several different tumor types. It promotes cancer cells’ motility and invasiveness, leading to cancer metastasis and chemo- and radioresistance (61,62). Recently, it has been found that L1CAM expression, in conjunction with CD133 expression, defines a cancer stem cell population in glioma and ovarian cancer. In in vivo ovarian cancer models, L1CAM expression elicited cancer stemness in several ways, e.g., through the enhancement of spherogenicity, the tumor take rate, cancer cells’ self-renewal capacity, and tumor growth (62).
Like NGF, brain-derived neurotrophic factor (BDNF) is induced by noradrenergic signaling to stimulate axonogenesis through Trk receptors (70,71) and is associated with the promotion of angiogenesis and increased tumor cell proliferation (72). Another NGF, NT-3 , is overexpressed in PDAC and its nerves, and NT-3 inhibition led to decreased growth of PDAC in a murine xenograft model (64,65,73).
Glial cell-derived neurotrophic factor (GDNF) is robustly expressed in human PDAC and is significantly correlated with neural invasion, and associated with an increase in pain levels (74–76). The cancer-promoting effects of GDNF, including neural invasion, are likely mediated through GDNF receptors including GDNF family receptors α1-3 and through RET, initiating downstream activation of the RAS/ERK, MAPK, JNK, and PI3-K/Akt signaling pathways (75,76). GDNF is also known to promote integrin expression, activate matrix metalloproteinase (MMP)-9, and increase nuclear factor κ B, which plays a significant role in nerve adhesion and invasion (77–80).
Neurturin and artemin also signal through RET and promote innervation (81). They are also highly expressed in PDAC and are associated with tumor invasiveness and nerve alteration, respectively (3,58,82,83). Artemin has been found primarily in the hypertrophic nerves of PDAC tissue samples, as determined through Western blotting and immunohistochemistry (84).
Lastly, when upregulated, the axon-guidance molecule netrin-1 acts as an oncogene in a number of cancer types (85,86) and promotes gastric cancer cell navigation along sensory dorsal root ganglia cells and sciatic nerve invasion in vitro (87). In contrast, in PDAC, restoration of pathologically decreased expression of the axon-guidance molecule, Slit2, to normal levels reduces metastasis and neural invasion (88). While these studies reveal promising therapeutic targets, further investigation will be important to translate their feasibility in clinical application.
Synapses Facilitate Nerve-cancer Crosstalk
Communication between nerves and brain cancer cells has recently been characterized as taking place via either bona-fide chemical synapses, as in glioblastomas, or via pseudo-tripartite synapses as in breast-to-brain metastases (B2BM). Gene expression of synaptic markers has been found in primary glioma cells (89). Neuron-glioma interaction via bona-fide chemical synapses have electrophysiological properties similar to those seen in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) signaling (89,90). Spontaneous excitatory postsynaptic currents from AMPA-receptor-expressing glioma cells demonstrates a functional neuroglioma synapse (90). It was also determined that these synapses do not result from a direct connection to neurons. Rather, tumor microtubules are found in glioma tissue and facilitate gap-junction signal transmission (89,91). In addition, the optogenetic stimulation of glioma cells promote glioma growth and proliferation. Furthermore, it has been demonstrated that activated glioma increases neuronal hyperexcitability in the tumor microenvironment in a reciprocal interaction (89).
Similarly, activation of glutamate ligand receptors, N-methyl-D-aspartate receptors (NMDARs), facilitates breast cancer metastasis to the brain (22). Previous reports have demonstrated tumor growth via NMDARs in neuroendocrine and ductal pancreatic cancers, and this growth is thought to result from autocrine secretion. Breast cancer transcriptional signatures have also implicated NMDARs in metastasis and NMDAR subunits have high expression levels in B2BM cell lines. It was also discovered that the extracellular glutamate in the B2BM tumor microenvironment was not sufficient to substantiate autocrine signaling. B2BM cells express adhesion molecules, such as postsynaptic density protein 95, that form pseudo-synapses between non-neuronal cells and axons and contribute to astrocytic synaptogenesis. Non-disruptive B2BM processes establish pseudo-tripartite synapses to access glutamate. The knockdown of NMDAR subunits resulted in fewer brain tumors and increased subject brain metastasis-free survival but did not affect their primary tumor burdens or lung metastases, which were rescued with the re-expression of NMDAR subunits. Together, these finding describe how tumors can co-opt neural signaling in the brain to promote tumor progression.
Sympathetic and Parasympathetic Nerves Cultivate a Microenvironment to Support Cancer
Neurotransmitters are signaling molecules that allow neurons to communicate with other neurons or cells. Because various cancers and components of their stroma express corresponding neurotransmitter receptors, the induction of a pro-tumor microenvironment can be supported by the peripheral nervous system. Autonomic nervous system responses promote tumor progression. Sympathetic stimulation is involved in the early stages of carcinogenesis and angiogenesis, while parasympathetic stimulation promotes invasion and metastasis. The sympathetic nervous system is also involved in immunomodulation and cancer-associated neuropathic pain.
Sympathetic Signals Promote Tumor Progression
Sympathetic nerve innervation has been demonstrated in human tumors and murine tumor models. Increased levels of noradrenaline have been observed in solid tumors and implicated in stress-mediated tumor progression. Furthermore, noradrenergic receptors are widely distributed in various cancer types, and modulating them has known effects on tumorigenesis and progression, described below.
Stress-induced Noradrenaline and the Angiogenic Switch
The growth of a new vascular network, angiogenesis, is marked by pro-angiogenic molecules (such as interleukin(IL)-8, tumor necrosis factor(TNF)-α, vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-α, TGF-β, angiogenin, platelet-derived growth factor, and fibroblast growth factor), the levels of which indicate tumor aggression and are important factors in prognostic outcomes (91–94). Peripheral neurons participate in vascular organization during development and wound repair and contribute to tumor angiogenesis (95–97).
The catecholamines noradrenaline and dopamine have opposing roles in angiogenesis; respectively, they stimulate and inhibit vascular networks. Noradrenaline stimulates angiogenesis by signaling increased VEGF expression in tumor-associated macrophages in primary mammary tissues and amplifying the expression of cytokines known to stimulate angiogenesis (34,98–100). Noradrenergic signaling in a β2-adrenergic and β3-adrenergic knockout in a xenogeneic orthotopic prostate cancer model in immunodeficient mice resulted in reduced tumor-associated vascular density (101). Furthermore, β2-adrenergic knockout in a spontaneous transgenic prostate cancer mouse model also exhibited decreased vascular density, migration, and branching compared to that seen in controls. Dopamine, on the other hand, downregulates the VEGF receptor-2-mediated signaling pathway, diminishing proliferation and migration in colon cancer cell lines and impairing tumor growth in mouse models of gastric cancer and ovarian cancer (98,102–106). These studies indicate that tumor innervation promotes angiogenesis and neovascularization of the tumor microenvironment.
As previously mentioned, surgical and pharmacological sympathectomy decreases prostate tumor growth and lung metastasis (33, 107). Pro-tumorigenic properties have been observed in Rv1 and LNCaP prostate cancer cell lines that express α1A-adrenergic receptors and in PC3 and DU-145 cells lines that express α1B- and α1D-adrenergic receptors. Blocking β2- and β3-adrenergic signaling was found to arrest tumor growth and angiogenesis, and it was later shown that β2-adrenergic signaling activates the angiogenic switch by inhibiting the expression of mitochondrial cytochrome c oxidase assembly factor 6, which decreases oxidative phosphorylation (aerobic glycolysis) (101). It has also been shown that noradrenergic signaling indirectly stimulates angiogenesis by stimulating M2 macrophages to secrete VEGF, which was inhibited in mice with propranolol (107). Together, these findings suggest that stress-induced sympathetic signaling drives angiogenesis and tumor progression.
Adrenergic Signaling Promotes Chemoresistance
Like dense, intratumoral sympathetic nerves, enhanced adrenergic signaling may drive primary or secondary resistance to cytotoxic chemotherapies. Eng et al. (108) noted that pancreatic cancer biology and treatment responses in their mouse colonies were heavily dependent on temperature, and that the sympathetic cold stress response enhanced noradrenaline and activated β-adrenergic receptors, driving resistance to cisplatin and paclitaxel (109). In head and neck cancer cells treated with cisplatin, the half-maximal inhibitory concentration of cisplatin was strikingly increased in oral cancer cells cocultured with neurons compared to oral cancer cells cultured alone. In addition, oral cells treated with a β2-adrenergic receptor blocker (ICI 118,551) demonstrated significantly diminished cell viability 48 hours after treatment with cisplatin (unpublished data). Similarly, blockading of β2-adrenergic receptor (ADRB2) and NGF pathways improved gemcitabine efficacy in KPC pancreatic cancer models (73). In a recent study by Chen et al. (109), signaling through upregulated β2 receptors on cervical cancer cells led to upregulation of Sirt1; inactivation of its target, p53; and downregulation of p53 target genes. As a result, these tumor cells were resistant to doxorubicin-induced p53 acetylation. Additional work needs to be done to fully determine how nerves influence cancer response and resistance to chemotherapy and other treatment modalities.
Dichotomous Role of Parasympathetic Fibers in Tumors
In addition to stimulating tumors, parasympathetic signals can also suppress tumor progression. In gastric, prostate, and breast cancers, parasympathetic signals serve as specific markers for tumors, and the corresponding receptors are expressed in gastric, pancreatic, lung, cervical, and colon cancer cells (71,110–112).
Parasympathetic Tumor Suppressors
Vagotomies performed in patients with gastric cancer and mice with pancreatic cancer have increased tumor progression (92,113). However, the activation of muscarinic cholinergic receptor 1 (Chrm1) reduces tumor incidence, perturbs cancer cell signaling pathways through the suppression of EGFR/MAPK and PI3K/AKT, and suppresses cancer stem cells (114–117). Cholinergic stimulation also prevents colorectal cancer progression by inducing trefoil factor 2 secretion by memory T cells and suppresses breast cancer by reducing PD-1 expression levels in CD4+ and CD8+ lymphocytes (118,119). Furthermore, cholinergic deprivation increases macrophage influx and TNF-α production, promoting cancer progression (107). These studies suggest a contribution of parasympathetic signaling in tumor suppression.
Parasympathetic Tumor Promoters
Conversely, cholinergic promotion of tumorigenesis and metastasis has been demonstrated in prostate and gastric cancers, respectively (32,92). Chrm1 expression in prostate cancer stromal cells is essential for metastasis (34,71). Other studies have indicated that Chrm3 mechanisms drive the pro-tumor effects of the parasympathetic nervous system. Chrm3 activation in gastric cancer induces Wnt-β-catenin signaling downstream of YAP (70,92,117). The Wnt-β-catenin signals expand cancer stem cells and induce NGF’s promotion of nerve innervation in gastric cancer (92,117). In small cell lung carcinoma, Chrm3 activates MAPK/Akt signaling (115). In colon cancer, Chrm3 activates ErbB receptors downstream of the MMP-7 adhesion molecules that promote tumor cell invasion (116). Converse to the previous section, these findings suggest parasympathetic signaling can promote tumor progression, depending on the type and stage of tumor.
Implications of Neural Signaling on Intratumoral Immune Cells
Stress, as manifested by enhanced sympathetic signaling and glucocorticoid production, contributes to many disorders, both benign and malignant. Although acute bouts of stress may enhance a CD4+ T-cell and B-cell mediated immune response, chronic repeated stress diminishes the immune response and renders important immune effectors anergic. Since many innate and adaptive immune cell types express or upregulate neural receptors including adrenergic receptors, it follows that immune cells are sensitive to neural signaling during oncogenesis.
We are now beginning to understand that the neuroregulation of inflammation plays a critical role in cancer, as inflammatory changes in nerves are observed in the early stages of some cancers (107,120). In breast cancer, CD11b+F4/80+ macrophages have been shown to infiltrate the tumor parenchyma upon pharmacologic sympathetic activation of adrenergic receptors and to contribute to a 30-fold increase in metastasis to the lymph nodes and lung. The β-adrenergic receptor blocker propranolol reversed this effect (120). CD11b+F4/80+ macrophages also secrete higher levels of GDNF in pancreatic adenocarcinoma cells compared to resting endoneurial macrophages, and a CCR2-deficient model of perineural invasion of cancer cells with reduced recruitment and activation of tumor-associated macrophages showed reduced CD11b+ F4/80+ macrophages and nerve invasion (75). Similarly, neuropathy of the sympathetic nervous system induced leukemic bone marrow infiltration in an acute myelogenous leukemia mouse model through the β2-adrenergic receptor expressed in stromal cells (121). In another study, splenic vagal denervation suppressed cytotoxic T cells and promoted carcinogenesis (119). It has also been shown that noradrenaline stimulates IL-6 production and activates macrophages and other stromal cells in the tumor microenvironment (95). Macrophages are recruited by cancer cells through cancer cell-secreted colony stimulating factor and release GDNF to promote cancer migration and nerve invasion (75). Consistently, β3-adrenergic signaling induces ovarian cancer cells to secrete brain-derived neurotrophic factor, which ultimately signals axonogenesis and plays a role in switching macrophages and neutrophils into immunocompetent M1- and N1-types and in recruiting and maintaining hemopoietic and mesenchymal stem cells (111,112).
Nerves can also interact with immune cells in an immune tolerizing fashion, allowing for tumor evasion and escape. Sensory neurons have been shown to attract myeloid-derived suppressor cells in melanoma, promoting an immune-tolerant, pro-tumor milieu (122). Within prostate cancer, regions rich in autonomic tumor-infiltrating nerves express high levels of the immune checkpoint protein programmed cell death ligand-1 (PD-L1). This inhibitory immune checkpoint ligand which binds and promotes anergy of cytotoxic CD8+ T-cells expressing the cognate receptor, PD-1. Regions high in PD-L1 expressing nerves were inversely correlated with those expressing CD8+ T-cells, and high density of PD-L1 nerves was associated with recurrence (123). Also supporting crosstalk between the autonomic nervous system and T-cell function, genetic parasympathetic stimulation of tumor decreased PD-L1 expression in tumors and PD-1 expression in T-cells, and enhanced CD8+/Treg ratios (110). In an analysis of breast cancer specimens, sympathetic nerve density was associated both with high expression of immune checkpoints and poor prognosis. Ultimately, a connection between nerve crosstalk and immune effectors may have important ramifications for the future of immune checkpoint blockade and other immunotherapies, but warrants further investigation.
Conclusions
Although advances in cancer treatment have improved patient outcomes, we still have much to learn about how tumors interact with their microenvironment. We are now beginning to realize that, in some solid tumors, infiltrating nerves and catecholaminergic signaling may play an important role in tumor initiation and progression. A deeper understanding of the mechanistic basis of cancer progression, specifically in regard to nerve-cancer crosstalk, will reveal new therapeutic targets and allow the repurposing of existing treatments, including neuromodulatory therapies to slow or stop cancer progression, or to be used in conjunction with chemo- or immunotherapies. As a greater understanding of nerve-cancer crosstalk and the neuro-immune axis emerges, new anti-neurogenic targets hold tremendous potential as novel opportunities for treating cancer (Table 1).
Table 1.
Emerging anti-neurogenic treatments
| Treatment | Mechanism | Outcome | Clinical Trial | Reference |
|---|---|---|---|---|
| Tanezumab; fulranumab | Neutralizing NGF antibody | Reduces secretion of NGF and migration of cancer cells, induction of neurites, and sympathetic nerve sprouting. Has a limited impact on neural or cognitive function. |
NCT02609828 (ongoing) | (57,124,125) |
| GW441756; carozantinib | Tyrosine kinase inhibitor | Decreases cancer cell migration and induction of neurites. Has limited cognitive effects. Previously failed to demonstrate survival benefit in men with metastatic castration-resistant prostate cancer. |
NCT01522443 (completed) NCT02219711 (ongoing) |
(57) |
| Propranolol; carvedilol | β-blocker | Reduces nerve-cancer interaction and neurotrophin secretion. Increases prostate cancer survival and reduced cancer-specific mortality. |
NCT02944201 (ongoing) NCT03152786 (ongoing) |
(66,126,127) |
| Surgical denervation | Causes gland atrophy and functional and structural deterioration of prostate epithelial cells. | (128) | ||
| Botulinum toxin (BOTOX) | Neurotoxin | Increases apoptosis in cancer cells. | NCT01520441 (withdrawn) | (129) |
| siRNA encapsulated nanoparticles | Gene silencer | Reduces neural invasion. | (4) |
Abbreviations: NGF, neuron growth factor; siRNA, small interfering RNA.
ACKNOWLEDGEMENTS
The authors would like to thank Eyal Kimhi for artistic work and the University of Texas MD Anderson Cancer Center’s Scientific Editing Services and Research Medical Library for assistance in preparing this manuscript.
Ms. Silverman’s training and projects are supported by NIH/NCI F30 CA228258, the Cullen Endowment for Higher Education, the John J. Kopchick Fellowship, and the American Legion Auxiliary Fellowship in Cancer Research. Dr. Calin is the Felix L. Haas Endowed Professor in Basic Science. Work in Dr. Calin’s laboratory is supported by National Institutes of Health (NIH/NCATS) grant UH3TR00943-01 through the NIH Common Fund, Office of Strategic Coordination (OSC), the NCI grants 1R01 CA182905-01 and 1R01CA222007-01A1, an NIGMS 1R01GM122775-01 grant, a Team DOD (CA160445P1) grant, a Chronic Lymphocytic Leukemia Moonshot Flagship project, a CLL Global Research Foundation 2019 grant, a CLL Global Research Foundation 2020 grant, donor support through Dr. Jaffer Ajani and the Estate of C. G. Johnson, Jr. Dr. Amit’s work and laboratory is supported by the MDACC Head and Neck Cancer Moonshot and NIH/NCI R37 CA242006-01A1.
Financial support: NIH/NCI F30 CA228258-01A1 (DAS), NIH UH3TR00943-01 (GAC), NCI 1R01 CA182905-01 (GAC), 1R01CA222007-01A1 (GAC), NIGMS 1R01GM122775-01 (GAC), DOD CA160445P1 (GAC), MDACC Chronic Lymphocytic Leukemia Moonshot (GAC), MDACC Head and Neck Cancer Moonshot (MA), NIH/NCI R37 CA242006-01A1 (MA)
Footnotes
Conflict of interest disclosure statement: The authors declare no conflicts of interest.
References
- 1.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. [DOI] [PubMed] [Google Scholar]
- 2.Deshmukh SD, Willmann JK, Jeffrey RB. Pathways of extrapancreatic perineural invasion by pancreatic adenocarcinoma: Evaluation with 3D volume-rendered MDCT imaging. Am J Roentgenol. 2010;194:668–74. [DOI] [PubMed] [Google Scholar]
- 3.Demir IE, Friess H, Ceyhan GO. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol. 2012;3 APR:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei Y, Tang L, Xie Y, Xianyu Y, Zhang L, Wang P, et al. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat Commun. 2017;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.España-Ferrufino A, Lino-Silva LS, Salcedo-Hernández RA. Extramural perineural invasion in pT3 and pT4 gastric carcinomas. J Pathol Transl Med. 2018;52:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng J, You Q, Gao Y, Yu Q, Zhao P, Zheng Y, et al. Prognostic value of perineural invasion in gastric cancer: A systematic review and meta-analysis. PLoS One. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oven Ustaalioglu BB, Bilici A, Seker M, Kefeli U, Aydin D, Celik S, et al. Prognostic Factors for Operated Gallbladder Cancer. J Gastrointest Cancer. 2019;50:451–7. [DOI] [PubMed] [Google Scholar]
- 8.Schmitd LB, Scanlon CS, D’Silva NJ. Perineural Invasion in Head and Neck Cancer. J Dent Res. 2018;97:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huyett P, Duvvuri U, Ferris RL, Johnson JT, Schaitkin BM, Kim S. Perineural Invasion in Parotid Gland Malignancies. Otolaryngol - Head Neck Surg. 2018;158:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cracchiolo JR, Xu B, Migliacci JC, Katabi N, Pfister DG, Lee NY, et al. Patterns of recurrence in oral tongue cancer with perineural invasion. Head Neck. 2018;40:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Zhang G, Yang Y, Cui L, Jia S, Shi Y, et al. Perineural invasion in early-stage cervical cancer and its relevance following surgery. Oncol Lett. 2018;15:6555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui L, Shi Y, Zhang GN. Perineural invasion as a prognostic factor for cervical cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;292:13–9. z [DOI] [PubMed] [Google Scholar]
- 13.Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16–22. [DOI] [PubMed] [Google Scholar]
- 14.Beard CJ, Chen MH, Cote K, Loffredo M, Renshaw AA, Hurwitz M, et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys. 2004;58:19–24. [DOI] [PubMed] [Google Scholar]
- 15.Duraker N, Şişman S, Can G. The significance of perineural invasion as a prognostic factor in patients with gastric carcinoma. Surg Today. 2003;33:95–100. [DOI] [PubMed] [Google Scholar]
- 16.Zare-Bandamiri M, Fararouei M, Zohourinia S, Daneshi N, Dianatinasab M. Risk factors predicting colorectal cancer recurrence following initial treatment: A 5-year cohort study. Asian Pacific J Cancer Prev. 2017;18:2465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, He L, Dong D, Yang C, Liang C, Chen X, et al. Individualized prediction of perineural invasion in colorectal cancer: development and validation of a radiomics prediction model. Chinese J Cancer Res. 2018;30:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.1Kinugasa T, Mizobe T, Shiraiwa S, Akagi Y, Shirouzu K. Perineural invasion is a prognostic factor and treatment indicator in patients with rectal cancer undergoing curative surgery: 2000–2011 Data from a single-center study. Anticancer Res. 2017;37:3961–8. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J Pathol. 2018;244:49–60. [DOI] [PubMed] [Google Scholar]
- 20.Jobling P, Pundavela J, Oliveira SMR, Roselli S, Walker MM, Hondermarck H. Nerve-cancer cell cross-talk: A novel promoter of tumor progression. Cancer Res. 2015;75:1777–81. [DOI] [PubMed] [Google Scholar]
- 21.Ceyhan GO, Bergmann F, Kadihasanoglu M, Erkan M, Park W, Hinz U, et al. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56:534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amit M, Na’ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nature reviews Cancer. 2016. June;16(6):399. [DOI] [PubMed] [Google Scholar]
- 24.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2009. August 1;115(15):3379–91. [DOI] [PubMed] [Google Scholar]
- 25.Batkin S, Piette LH, Wildman E. Effect of muscle denervation on growth of transplanted tumor in mice. Proc Natl Acad Sci U S A. 1970;67:1521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DE SOUSA PEREIRA A A basis for sympathectomy for cancer of the cervix uteri. Arch Surg. 1946;52:260–85. [DOI] [PubMed] [Google Scholar]
- 27.P. E. Uber das Wachstum und die VerbreitungBostariger eshwulste insbesonderedes Krebes in den Lymphbahnen der Nerven. Beitr PatholAnat. 1905;7. [Google Scholar]
- 28.Ayala GE, Wheeler TM, David Shine H, Schmelz M, Frolov A, Chakraborty S, et al. In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer. Prostate. 2001;49:213–23. [DOI] [PubMed] [Google Scholar]
- 29.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–603. [DOI] [PubMed] [Google Scholar]
- 30.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. [DOI] [PubMed] [Google Scholar]
- 31.Magnon C Role of the autonomic nervous system in tumorigenesis and metastasis. Mol Cell Oncol. 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341. [DOI] [PubMed] [Google Scholar]
- 33.Mauffrey P, Tchitchek N, Barroca V, Bemelmans A, Firlej V, Allory Y, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569:672–8. [DOI] [PubMed] [Google Scholar]
- 34.Lu R, Fan C, Shangguan W, Liu Y, Li Y, Shang Y, Yin D, Zhang S, Huang Q, Li X, Meng W. Neurons generated from carcinoma stem cells support cancer progression. Signal transduction and targeted therapy. 2017;2(1):1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578:449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda M Membrane traffic in the secretory pathway: Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 2008;2801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colombo M, Raposo G, Théry C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. [DOI] [PubMed] [Google Scholar]
- 39.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyenne V, Labouesse M, Goetz JG. The Small GTPase Ral orchestrates MVB biogenesis and exosome secretion. Small GTPases. 2018;9:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, et al. Cancer exosomes induce tumor innervation. Nat Commun. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambo S, Gröbner SN, Rausch T, Waszak SM, Schmidt C, Gorthi A, et al. The molecular landscape of ETMR at diagnosis and relapse. Nature. 2019;576:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemberg KM, Wang J, Pratilas CA. From genes to-omics: The evolving molecular landscape of malignant peripheral nerve sheath tumor. Genes (Basel). 2020;11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambo S, von Hoff K, Korshunov A, Pfister SM, Kool M. ETMR: a tumor entity in its infancy. Acta Neuropathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dragomir MP, Knutsen E, Calin GA. SnapShot: Unconventional miRNA Functions. 2018;1038–1038. e1. [DOI] [PubMed] [Google Scholar]
- 46.Anfossi S, Fu X, Nagvekar R, Calin GA. MicroRNAs, regulatory messengers inside and outside cancer cells. Adv Exp Med Biol. 2018; 87–108. [DOI] [PubMed] [Google Scholar]
- 47.Qin X, Yu S, Zhou L, Shi M, Hu Y, Xu X, et al. Cisplatin-resistant lung cancer cell–derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100–5p-dependent manner. Int J Nanomedicine. 2017;12:3721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W xian, Cai Y qin, Lv M meng, Chen L, Zhong S liang, Ma T fei, et al. Exosomes from docetaxel-resistant breast cancer cells alter chemosensitivity by delivering microRNAs. Tumor Biol. 2014;35:9649–59. [DOI] [PubMed] [Google Scholar]
- 49.Mao L, Li J, Chen W xian, Cai Y qin, Yu D dan, Zhong S liang, et al. Exosomes decrease sensitivity of breast cancer cells to adriamycin by delivering microRNAs. Tumor Biol. 2016;37:5247–56. [DOI] [PubMed] [Google Scholar]
- 50.Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peinado H Melanoma exosomes educate bone marrow progenitor cells. Nat Med. 2013;18:883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol. 2018;69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krüttgen A, Schneider I, Weis J. The dark side of the NGF family: Neurotrophins in neoplasias. Brain Pathol. 2006;16:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W, Zhao H, Zhang S, Kang E, Chen Y, Ni C, et al. Patterns of expression and function of the p75NGFR protein in pancreatic cancer cells and tumours. Eur J Surg Oncol. 2009;35:826–32. [DOI] [PubMed] [Google Scholar]
- 55.Di Donato M, Cernera G, Auricchio F, Migliaccio A, Castoria G. Cross-talk between androgen receptor and nerve growth factor receptor in prostate cancer cells: Implications for a new therapeutic approach. Cell Death Discov. 2018;4:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bapat AA, Munoz RM, Von Hoff DD, Han H. Blocking nerve growth factor signaling reduces the neural invasion potential of pancreatic cancer cells. PLoS One. 2016;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pundavela J, Roselli S, Faulkner S, Attia J, Scott RJ, Thorne RF, Forbes JF, Bradshaw RA, Walker MM, Jobling P, Hondermarck H. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Molecular oncology; 2015;8: 1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amit M, Na’ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nature reviews Cancer. 2016. June;16(6):399. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Villadsen R, Sørlie T, Fogh L, Grønlund SZ, Fridriksdottir AJ, Kuhn I, Rank F, Wielenga VT, Solvang H, Edwards PA. Tumor initiating but differentiated luminal-like breast cancer cells are highly invasive in the absence of basal-like activity. P Proc Natl Acad Sci U S A. 2012;109(16):6124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang L, Huang S, Wang J, Zhang Y, Xiong Y, Zeng SX, Lu H. Inactivating p53 is essential for nerve growth factor receptor to promote melanoma-initiating cell-stemmed tumorigenesis. Cell death & disease. 2020;11(7):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Altevogt P, Doberstein K, Fogel M. L1CAM in human cancer. International journal of cancer. 2016;138(7):1565–76. [DOI] [PubMed] [Google Scholar]
- 62.Terraneo N, Jacob F, Peitzsch C, Dubrovska A, Krudewig C, Huang YL, Heinzelmann-Schwarz V, Schibli R, Béhé M, Grünberg J. L1 cell adhesion molecule confers radioresistance to ovarian cancer and defines a new cancer stem cell population. Cancers. 2020. January;12(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ceyhan GO, Schäfer KH, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251:923–31. [DOI] [PubMed] [Google Scholar]
- 64.Miknyoczki SJ, Wan W, Chang H, Dobrzanski P, Ruggeri BA, Dionne CA, et al. The neurotrophin-trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma xenografts in nude mice. Clin Cancer Res. 2002;8:1924–31. [PubMed] [Google Scholar]
- 65.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell. 2017;31:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJP, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: Expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–27. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Z, Kleeff J, Kayed H, Wang L, Korc M, Büchler MW, et al. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol Carcinog. 2002;35:138–47. [DOI] [PubMed] [Google Scholar]
- 68.Dang C, Zhang Y, Ma Q, Shimahara Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J Gastroenterol Hepatol. 2006;21:850–8. [DOI] [PubMed] [Google Scholar]
- 69.Zhu ZW, Friess H, Wang L, Bogardus T, Korc M, Kleef J, et al. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer Res. 2001;7:105–12. [PubMed] [Google Scholar]
- 70.Schneider MB, Standop J, Ulrich A, Wittel U, Friess H, Andrén-Sandberg A, et al. Expression of nerve growth factors in pancreatic neural tissue and pancreatic cancer. J Histochem Cytochem. 2001;49:1205–10. [DOI] [PubMed] [Google Scholar]
- 71.Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell. 2018;33:75–90. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST, Poon RTP. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: Implication in hepatocellular carcinoma. Clin Cancer Res. 2011;17:3123–33. [DOI] [PubMed] [Google Scholar]
- 73.Ketterer K, Rao S, Friess H, Weiss J, Büchler MW, Korc M. Reverse Transcription-PCR Analysis of Laser-Captured Cells Points to Potential Paracrine and Autocrine Actions of Neurotrophins in Pancreatic Cancer. Clin Cancer Res. 2003;9:5127–36. [PubMed] [Google Scholar]
- 74.Zeng Q, Cheng Y, Zhu Q, Yu Z, Wu X, Huang K, et al. The relationship between overexpression of glial cell-derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J Int Med Res. 2008;36:656–64. [DOI] [PubMed] [Google Scholar]
- 75.Cavel O, Shomron O, Shabtay A, Vital J, Trejo-Leider L, Weizman N, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72:5733–43. [DOI] [PubMed] [Google Scholar]
- 76.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okada Y, Takeyama H, Sato M, Morikawa M, Sobue K, Asai K, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial- cell-line-derived neurotrophic factor (GDNF). Int J Cancer. 1999;81:67–73. [DOI] [PubMed] [Google Scholar]
- 78.Na’ara S, Amit M, Gil Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene. 2019;38:596–608. [DOI] [PubMed] [Google Scholar]
- 79.Funahashi H, Takeyama H, Sawai H, Furuta A, Sato M, Okada Y, et al. Alteration of integrin expression by glial cell line-derived neurotrophic factor (GDNF) in human pancreatic cancer cells. Pancreas. 2003;27:190–6. [DOI] [PubMed] [Google Scholar]
- 80.Funahashi H, Okada Y, Sawai H, Takahashi H, Matsuo Y, Takeyama H, et al. The role of glial cell line-derived neurotrophic factor (GDNF) and integrins for invasion and metastasis in human pancreatic cancer cells. J Surg Oncol. 2005;91:77–83. [DOI] [PubMed] [Google Scholar]
- 81.Airaksinen MS, Saarma M. The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. [DOI] [PubMed] [Google Scholar]
- 82.Wang K, Demir IE, D’Haese JG, Tieftrunk E, Kujundzic K, Schorn S, et al. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis. 2014;35:103–13. [DOI] [PubMed] [Google Scholar]
- 83.Gao L, Bo H, Wang Y, Zhang J, Zhu M. Neurotrophic factor Artemin promotes invasiveness and neurotrophic function of pancreatic Adenocarcinoma in vivo and in vitro. Pancreas. 2015;44:134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delloye-Bourgeois C, Fitamant J, Paradisi A, Cappellen D, Douc-Rasy S, Raquin MA, et al. Netrin-1 acts as a survival factor for aggressive Neuroblastoma. J Exp Med. 2009;206:833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY, et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci U S A. 2008;105:4850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin K, Wang L, Xia Y, Dang S, Zhang X, He Z, et al. Netrin-1 promotes cell neural invasion in gastric cancer via its receptor neogenin. J Cancer. 2019;10:3197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gohrig A, Detjen KM, Hilfenhaus G, Korner JL, Welzel M, Arsenic R, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74:1529–40. [DOI] [PubMed] [Google Scholar]
- 89.Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573:532–8. [DOI] [PubMed] [Google Scholar]
- 91.Folkman J Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. [DOI] [PubMed] [Google Scholar]
- 92.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McMahon G VEGF Receptor Signaling in Tumor Angiogenesis. Oncologist. 2000;5:3–10. [DOI] [PubMed] [Google Scholar]
- 95.Li S, Sun Y, Gao D. Role of the nervous system in cancer metastasis (Review). Oncol Lett. 2013;5:1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. [DOI] [PubMed] [Google Scholar]
- 97.Ekstrand AJ, Cao R, Björndahl M, Nyström S, Jönsson-Rylander AC, Hassani H, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003;100:6033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009;69:3727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, et al. Roadmap for the Emerging Field of Cancer Neuroscience. Cell. 2020;181:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Madden KS, Szpunar MJ, Brown EB. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res Treat. 2011;130:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zahalka AH, Arnal-Estapé A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–74. [DOI] [PubMed] [Google Scholar]
- 103.Kitlinska J, Tilan J. Sympathetic neurotransmitters and tumor angiogenesis link between stress and cancer progression. J Oncol. 2010;2010:14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502–10. [DOI] [PubMed] [Google Scholar]
- 105.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: Dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–56. [DOI] [PubMed] [Google Scholar]
- 106.Moreno-Smith M, Lee SJ, Chunhua L, Nagaraja AS, He G, Rupaimoole R, et al. Biologic effects of dopamine on tumor vasculature in ovarian carcinoma. Neoplasia. 2013;15:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xia Y, Wei Y, Li ZY, Cai XY, Zhang LL, Dong XR, et al. Catecholamines contribute to the neovascularization of lung cancer via tumor-associated macrophages. Brain Behav Immun. 2019;81:111–21. [DOI] [PubMed] [Google Scholar]
- 108.Eng JWL, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, Hylander BL. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β 2-adrenergic receptor activation. Nature communications; 2015. 6;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen H, Zhang W, Cheng X, Guo L, Xie S, Ma Y, Guo N, Shi M. β2-AR activation induces chemoresistance by modulating p53 acetylation through upregulating Sirt1 in cervical cancer cells. Cancer science; 2017. 108;7:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamiya A, Hayama Y, Kato S, Shimomura A, Shimomura T, Irie K, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22:1289–305. [DOI] [PubMed] [Google Scholar]
- 111.Calvani M, Bruno G, Dal Monte M, Nassini R, Fontani F, Casini A, et al. β3-Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br J Pharmacol. 2019;176:2509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calvani M, Bruno G, Dabraio A, Subbiani A, Bianchini F, Fontani F, et al. β3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Partecke LI, Käding A, Trung DN, Diedrich S, Sendler M, Weiss F, et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFα in a murine pancreatic cancer model. Oncotarget. 2017;8:22501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kodaira M, Kajimura M, Takeuchi K, Lin S, Hanai H, Kaneko E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J Gastroenterol. 1999;34:163–71. [DOI] [PubMed] [Google Scholar]
- 115.Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, et al. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–44. [DOI] [PubMed] [Google Scholar]
- 116.Belo A, Cheng K, Chahdi A, Shant J, Xie G, Khurana S, et al. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol. 2011;300:749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Konishi M, Hayakawa Y, Koike K. Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers. Biomedicines. 2019;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77:5639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dubeykovskaya Z, Si Y, Chen X, Worthley DL, Renz BW, Urbanska AM, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 2016;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer research. 2010;70:7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell Niche. Cell Stem Cell. 2014;15:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keskinov AA, Tapias V, Watkins SC, Ma Y, Shurin MR, Shurin GV. Impact of the sensory neurons on melanoma growth in vivo. PLoS One. 2016;11. [Google Scholar]
- 123.Mo RJ, Han ZD, Liang YK, Ye JH, Wu SL, Lin SX, et al. Expression of PD-L1 in tumor-associated nerves correlates with reduced CD8 + tumor-associated lymphocytes and poor prognosis in prostate cancer. Int J Cancer. 2019;144:3099–110. [DOI] [PubMed] [Google Scholar]


