Abstract
Background –
Type 2 diabetes (DM2) is one of the most common chronic disorders worldwide and is an important cause of cardiovascular disease. Studies investigating the risk of atrial and ventricular arrhythmias in diabetic patients taking different oral diabetes medications are sparse.
Methods –
We used IBM MarketScan® Medicare Supplemental Database to examine the risk of arrhythmias for patients on different oral diabetes medications by propensity score matching.
Results –
We found that patients on metformin monotherapy had significantly reduced risk of atrial arrhythmias, including atrial fibrillation, compared to monotherapy with DPP4 or TZD medications. Patients on metformin monotherapy had significantly reduced risk of atrial arrhythmias, ventricular arrhythmias, and bradycardia compared to monotherapy with sulfonylureas. Combination therapy with sulfonylureas and metformin had an increased risk of atrial arrhythmias compared to some other combinations.
Conclusions –
Different oral diabetes medications have significantly different long-term risk of arrhythmia. Specifically, metformin is associated with reduced risk of atrial fibrillation and ventricular arrhythmias compared to sulfonylureas.
Keywords: tachyarrhythmia, arrhythmia (heart rhythm disorders), atrial fibrillation, type 2 diabetes mellitus, ventricular fibrillation, metformin, sulfonylurea
Graphical Abstract
Introduction
Type 2 diabetes (DM2) is one of the most prevalent chronic disorders worldwide and is associated with increased mortality and disability. It affects more than 34 million people in the US with 88 million adults having pre-diabetic states 1. DM2 is associated with many serious complications, including cardiovascular disorders 2. Among the latter, atrial and ventricular arrhythmias are associated with poor clinical outcomes 3,4. The effects of antidiabetic medications on cardiovascular comorbidities have been studied extensively 5,6. American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) treatment recommendations are based on long-term macrovascular benefits observed in the United Kingdom Prospective Diabetes Study (UKPDS) and other clinical studies 7. Nevertheless, studies investigating the risk of atrial and ventricular arrhythmias in diabetic patients on different medications are sparse.
Metformin monotherapy is the recommended first-line antidiabetic treatment by the ADA and other international guidelines 8. Human data is limited, but a cohort study examined the risk of atrial fibrillation (AF) in diabetic patients on metformin and showed a reduced risk of new incidence AF, independent of co-morbidities and other medications 9. However, this study was limited to patients in Taiwan, which may not be generalizable to other populations. Moreover, the impact of different DM2 medications on the risk of other arrhythmias was not studied. There is some prior research on the impact of metformin on decreasing the risk of ischemic ventricular fibrillation (VF) in animal models 10. The current study aimed to cover this knowledge gap by studying the risk of atrial and ventricular arrhythmias in patients on metformin and other classes of antidiabetic medications.
Methods
Data source
We used IBM MarketScan® Medicare Supplemental Database, which includes adjudicated health insurance claims for more than 8 million US patients from 2010 to 2018. IBM MarketScan® Medicare Supplemental Database (MDCR) represents health services of retirees in the United States with employer-sponsored Medicare supplemental coverage through privately insured fee-for-service, point-of-service, or capitated health plans. These data include adjudicated health insurance claims (e.g. inpatient, outpatient, and outpatient pharmacy) covering a full continuum of care. Employer-provided data allowed tracking of patients across multiple plans using their unique identifiers assigned in MarketScan. The major data elements contained within this database are outpatient pharmacy dispensing claims (coded with National Drug Codes (NDC), inpatient and outpatient medical claims which provide procedure codes (CPT-4, HCPCS, ICD-9-CM or ICD-10-PCS), and diagnosis codes (ICD-9-CM or ICD-10-CM). The database underwent extensive validation 11 and was subsequently standardized to Observational Health Data Sciences and Informatics (OHDSI) Observational Medical Outcomes Partnership Common Data Model (OMOP CDM) version 5. OHDSI is an international multi-stakeholder initiative that uses common statistical approaches, data models, and standardized vocabularies to enable large-scale observational research 12.
The study used de-identified data and did not constitute human subjects research or require informed consent. The detailed analytic methods have been made available to other researchers for purposes of reproducing the results or replicating the procedure (https://github.com/aostropolets/MetforminStudy).
Study design
Our main hypothesis was that metformin prevents cardiac arrhythmias compared to sulfonylureas, thiazolidinediones (TZD), dipeptidyl peptidase 4 inhibitors (DPP4) or glucagon-like peptide-1 receptor agonists (GLP-1). We did not include patients on insulin since they are more likely to have late-stage diabetes with more complications. Our study also included several secondary hypotheses, which concerned pairwise effectiveness for preventing cardiac arrhythmias in patients on a combination therapy with metformin and each of the drug groups mentioned above.
Comparing metformin and other oral antidiabetic drugs (sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, glucagon-like peptide-1 receptor agonists)
We followed a comparative retrospective new-user cohort design. We compared new users of metformin and new users of other oral antidiabetic drugs who were observed in a database for at least a year prior. For this study, we compared metformin with other oral antidiabetic medications, including sulfonylureas, TZD, DPP4, and GLP-1 13. Patients were required to have a DM2 diagnosis prior to treatment. We excluded patients with prior atrial fibrillation, ventricular tachycardia (VT), or ventricular fibrillation (VF). We also excluded patients with previously recorded type 1 or gestational diabetes within a year prior to ensure that the patients were not misclassified.
We defined our exposures similarly to Hripcsak et al. 14 with limiting minimal required exposure to be one year (Figure 1). The cohort entry is an exposure to metformin (target group) or an exposure to other antidiabetic drugs (comparator group). We excluded patients with prior metformin exposure from the comparator group and censored patients in the comparator group if they initiated metformin treatment.
Figure 1.
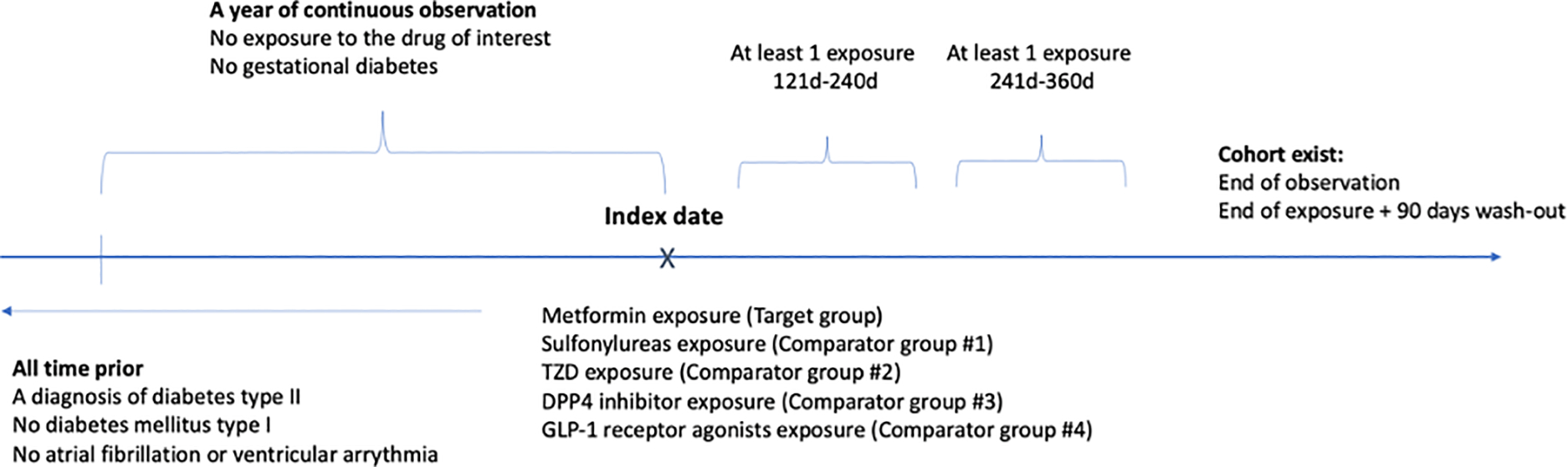
Scheme of study design, comparing antidiabetic drug monotherapies
Pairwise comparison of antidiabetic combination therapies with metformin
To examine the differences in antiarrhythmic effect among combinations of metformin with other anti-diabetic drugs, we constructed the second design comparing patients on a combination of (1) metformin and sulfonylurea, (2) metformin and DPP4 inhibitors, (3) metformin and TZD, and (4) metformin and GLP-1 receptor agonists. We followed the same rules to define our exposure (Figure 2), where an additional censoring event was initiation of another anti-diabetic drug (TZD, GLP-1 receptor agonists or DPP4 inhibitors for the target cohort; sulfonylureas, GLP-1 receptor agonists or TZD for the comparator cohort # 1; sulfonylureas, GLP-1 receptor agonists or TZD for the comparator cohort # 2; and sulfonylureas, TZD or DPP4 inhibitors for the comparator cohort # 3).
Figure 2.
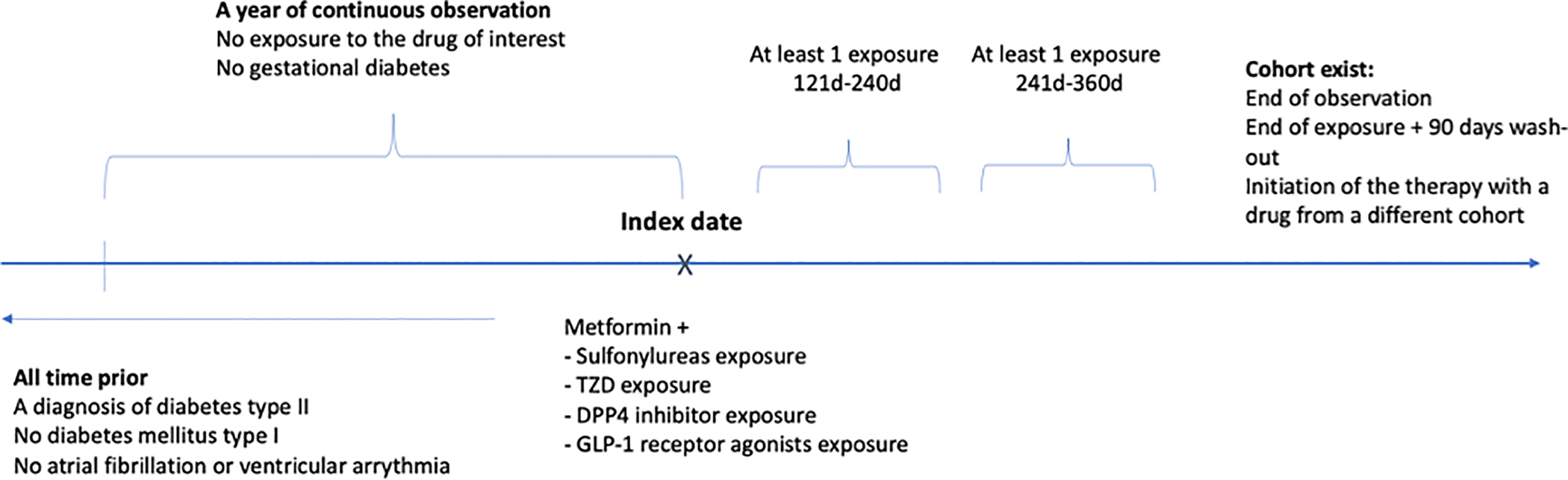
Scheme of study design, pairwise comparison of antidiabetic combo-therapies with metformin
Outcomes
We studied the occurrence of (a) atrial fibrillation (AF), (b) atrial flutter (AFL) and supraventricular arrhythmia, and (c) ventricular tachycardia (VT) or ventricular fibrillation (VF), and (d) clinically significant bradycardia. We defined the outcomes as an occurrence of diagnosis codes in patient record. The full list of codes used to define exposures and outcomes is in the supplemental materials (Supplementary Table I). We defined our time-at-risk as on-treatment analysis, which starts at the index date and continues until the medication is discontinued, an outcome occurs, or patient record ends.
Statistical analysis
To mitigate potential confounding we used propensity score matching with a ratio of up to 5 subjects from a control group to one subject in the target group. To calculate propensity scores, we used baseline covariates including demographic data, prior conditions, drug exposures, and procedures. The number of covariates varied across target and comparator drug pairs, ranging from 18,989 for a comparison of sulfonylureas and thiazolidinediones to 39,079 for a comparison of metformin and sulfonylureas. We matched patients by propensity score and used Cox proportional hazard model to estimate hazard ratios for the risk of outcomes in target and comparator cohorts 15. We characterized patients before and after propensity score matching to assess cohort balance. We also applied diagnostic tests for our propensity score models, which included examining preference score distributions and empirical equipoise using the Cyclops R package. To reduce residual study bias we additionally constructed forty-four negative controls 16. Such controls were not known to be associated with the exposure and were constructed similarly to Vashitsht et al. 17 with additional controls (e.g. rhinitis, hereditary disorders, Supplemental Table II). We used empirical null distributions to empirically calibrate hazard ratios, their confidence intervals and p-values similarly to Suchard et al. 18. We also used negative control calibration plots to evaluate potential residual bias.
In addition to following patients as long as they were on treatment, we carried out a separate analysis that is referred to in observational research as “intent-to-treat.” In this analysis, we follow the patient until an outcome occurs or until the patient record ends, regardless of whether treatment ends. We look for the secondary intent-to-treat analysis to match the primary on-treatment results, with deviation potentially pointing to missed biases.
Results
Comparing metformin to other oral antidiabetic drugs (sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, glucagon-like peptide-1 receptor agonists) as monotherapy
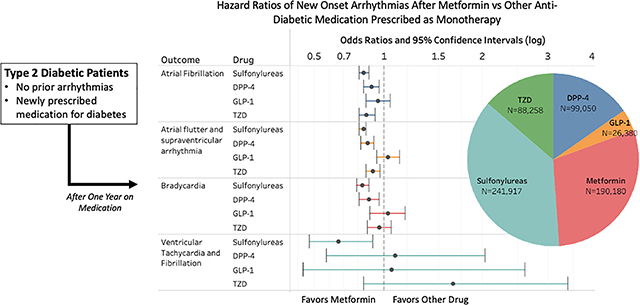
We identified 190,180 patients on metformin monotherapy, 241,917 on sulfonylureas, 99,050 on DPP4 inhibitors, 88,258 on TZD, and 26,380 on GLP-1 receptor agonists (Supplemental Table III). Patient baseline characteristics before and after propensity score matching for the target and comparators are presented in Supplemental Table IV. Supplemental Table III shows the total number of patients in the target (metformin monotherapy) and comparator groups before and after propensity score matching, as well as the mean follow-up time and the number of outcome events in each group. After matching, all of the 39,079 measured covariates achieved the standard criterion of balance with a standardized difference of the mean less than 0.1 (Supplemental Figure I). Before matching, we saw differences in sex, age, hypertension, coronary artery disease, valvular heart disorder, thyroid disorders, heart failure, asthma, chronic obstructive lung disorder, and medications like beta blockers, calcium channel blockers, and anticoagulants, but these differences were successfully addressed with matching (Supplemental Table IV).
After propensity score adjustment, we found that patients on metformin monotherapy had significantly decreased risk for all types of arrhythmia compared to those on sulfonylureas (Table 1). The largest treatment effect of metformin compared to sulfonylureas was a 34% reduction in occurrence of VT/VF (p=0.01). When compared with DPP4, metformin was associated with significantly lowered risk of AF, AFL or other supraventricular arrhythmia, and bradycardia, with a reduction in occurrence of about 10% for each (p<0.01 for all three categories). When compared with TZD, metformin was associated with significantly lowered risk of AF and AFL or supraventricular arrhythmia, with reductions in occurrence of 14% and 9% respectively (p<0.01 for both categories).
Table 1.
Estimated hazard ratios of arrhythmias among patients on metformin vs other anti-diabetic drugs. HR = hazard ratio with 95% confidence interval
| Outcome | DPP4 | GLP-1 | Sulfonylureas | TZD | ||||
|---|---|---|---|---|---|---|---|---|
| HR | P-value | HR | P-value | HR | P-value | HR | P-value | |
| Atrial Fibrillation | 0.90 (0.84– 0.96) | <0.01 | 0.95 (0.86– 1.05) | 0.34 | 0.84 (0.81– 0.88) | <0.01 | 0.86 (0.81– 0.93) | <0.01 |
| Ventricular Tachycardia and Fibrillation | 1.09 (0.58– 2.04) | 0.79 | 1.06 (0.43– 2.61) | 0.89 | 0.66 (0.47– 0.91) | 0.01 | 1.65 (0.84– 3.39) | 0.16 |
| Bradycardia | 0.88 (0.81– 0.96) | <0.01 | 1.03 (0.89– 1.18) | 0.69 | 0.83 (0.79– 0.88) | <0.01 | 0.96 (0.87– 1.06) | 0.44 |
| Atrial flutter and supraventricular arrhythmia | 0.87 (0.82– 0.92) | <0.01 | 1.03 (0.94– 1.13) | 0.59 | 0.84 (0.81– 0.86) | <0.01 | 0.91 (0.86– 0.97) | <0.01 |
Metformin was not associated with a significant difference in risk of VT/VF compared to either DPP4 or TZD. In contrast with the other types of medication, there were no significant difference in risk for any types of arrhythmia comparing metformin and GLP-1 receptor agonists. Notably, the sample size of patients on GLP-1 monotherapy was smaller than that of the other comparators, so these comparisons could be relatively underpowered to detect small differences in risk. In summary, metformin monotherapy was associated with significantly lowered risk for atrial arrhythmias, including atrial fibrillation, compared to three out of the four comparators. Metformin monotherapy also had a large reduction in ventricular arrhythmias compared to sulfonylureas. The uncalibrated and calibrated with negative contols p-values were close (Supplemental Figure II) indicating that negative controls showed little evidence of residual confounding in the study. Kaplan-Meier plots are shown in Supplementary Figure III.
Intent-to-treat analysis (Supplemental Table V – VI and Supplemental Figure IV – V) showed similar results in direction of estimates and statistical significance of hazard ratios. Eleven estimates out of sixteen did not statistically differ compared to on-treatment analysis, while five estimates confidence intervals that were different compared to on-treatment analysis.
Pairwise comparison of antidiabetic combination therapies using metformin
We then examined risk of arrhythmia on diabetic medical therapy containing metformin and another medication (combination therapy). We identified 24,772 patients who were on metformin and sulfonylurea therapy, 30,176 patients on metformin and DPP4 therapy, 26,389 patients on metformin and GLP-1 therapy and 9,793 patients on metformin and TZD therapy (Supplemental Table VII). Baseline characteristics of these patients are presented in Supplemental Table VIII.
Propensity score matching achieved balance on 25,871 measured covariates (Supplementary Figure VI). Before propensity matching, we saw differences in sex, age, hypertensive disorder, coronary heart disease, valvular heart disorder, hypothyroidism, chronic kidney disorder, chronic obstructive lung disorder, asthma, and medications like beta blockers and statins, but these differences were successfully addressed with matching (Supplemental Table VIII and Supplemental Figure VII).
After propensity score adjustment, we found no significant differences in risk of any arrhythmias when comparing combination treatment with metformin plus the second agent as DPP4, TZD or GLP-1. However, treatment with sulfonylureas plus metformin was associated with statistically higher risk of AF (OR 1.2, 95% CI 1.1–1.4, p=0.008, Table 2) and AFL or supraventricular arrhythmia (OR 1.2, 95% CI 1–1.3, p=0.012) when compared with metformin plus DPP4. Kaplan-Meier plots are presented in Supplemental Figure VIII. In summary, most combinations of oral diabetes medications did not show significant differences in risk of arrhythmia but treatment with sulfonylureas in combination with metformin is associated with higher risk for atrial arrhythmias compared to treatment with metformin and DPP4.
Table 2.
Estimated hazard ratios of arrhythmias for different combination therapy with metformin. The drug in the first column in combination with metformin is compared to the drug in the second column in combination with metformin.
| Target group | Comparator group | HR (CI 95%) | P-value |
|---|---|---|---|
| Atrial fibrillation | |||
| GLP1 | DPP4 | 1.1 (1 – 1.2) | 0.225 |
| Sulfonylureas | DPP4 | 1.2 (1.1 – 1.4) | 0.008 |
| Sulfonylureas | GLP1 | 1.1 (0.9 – 1.2) | 0.402 |
| Sulfonylureas | TZD | 1.2 (1 – 1.5) | 0.085 |
| TZD | DPP4 | 1 (0.8 – 1.2) | 0.893 |
| TZD | GLP1 | 0.9 (0.8 – 1.1) | 0.357 |
| Atrial flutter and supraventricular arrhythmia | |||
| GLP1 | DPP4 | 1 (0.9 – 1.2) | 0.533 |
| Sulfonylureas | DPP4 | 1.2 (1 – 1.3) | 0.012 |
| Sulfonylureas | GLP1 | 1.1 (1 – 1.2) | 0.153 |
| Sulfonylureas | TZD | 1.1 (0.9 – 1.3) | 0.241 |
| TZD | DPP4 | 1.1 (0.9 – 1.3) | 0.279 |
| TZD | GLP1 | 1 (0.9 – 1.2) | 0.736 |
| Bradycardia | |||
| GLP1 | DPP4 | 1 (0.9 – 1.2) | 0.795 |
| Sulfonylureas | DPP4 | 1.2 (1 – 1.4) | 0.087 |
| Sulfonylureas | GLP1 | 1.2 (1 – 1.4) | 0.131 |
| Sulfonylureas | TZD | 1.2 (0.9 – 1.6) | 0.176 |
| TZD | DPP4 | 1.2 (1 – 1.6) | 0.075 |
| TZD | GLP1 | 1.1 (0.9 – 1.4) | 0.372 |
| Ventricular Tachycardia and Ventricular Fibrillation | |||
| GLP1 | DPP4 | 2 (0.5 – 9.9) | 0.368 |
| Sulfonylureas | DPP4 | 4 (0.6 – 78.2) | 0.266 |
| Sulfonylureas | GLP1 | 0.7 (0.1 – 2.7) | 0.616 |
| TZD | GLP1 | 0.7 (0.2 – 2.8) | 0.686 |
Discussion
Metformin is recommended as the first-line anti-diabetic medication by most international guidelines. Although it has been shown to reduce cardiovascular complications in DM2 patients, there are few studies investigating risk for atrial and ventricular arrhythmias. The results of our study address this knowledge gap. This observational retrospective cohort study found that metformin was associated with significantly lowered risk of AF, AFL, and supraventricular arrhythmia compared to sulfonylureas, DPP4 inhibitors, and TZD. Metformin monotherapy also showed a significantly lowered risk for bradycardia when compared with sulfonylurea monotherapy and DPP4 monotherapy. In clinical practice, severe sinus bradycardia often co-exists with AF and it is thought that both problems can be caused by diffuse fibrosis of the atrial tissue. Medications that prevent atrial fibrosis could reduce the risk of both AF and bradycardia. In contrast, we found no significant differences in risk for arrhythmias between metformin and GLP-1 monotherapy, though the sample size for GLP-1 was smaller than the other groups. Further, this study found that sulfonylureas, as part of combination therapy with metformin, are associated with higher risk of atrial arrhythmia compared to metformin combination therapy with DPP4.
A database study of Taiwanese patients with DM2 found that metformin reduced the risk of new-onset AF 9. Notably, this protective effect was diminished within two years, underscoring the significance of our study, which examines the efficacy of metformin in combination with different second-line medications, since a second agent is often required after a few years of monotherapy. A more recent study of the Taiwanese population found that both metformin and TZD were associated with lowered risk of new-onset AF 19. In contrast with our study design, both of these studies considered metformin only as monotherapy and did not investigate the risk for other types of arrhythmias. Our study examines the impact of choice of medication on the risk of not only AF, but other types of arrhythmias. Furthermore, our study takes into account the real-world dynamics of antidiabetic treatment, wherein healthcare providers often change first-line therapy, combine different antidiabetic drugs, or proceed with a second-line treatment.
Apart from metformin, a few prior studies have examined other antidiabetic medications with regards to arrhythmic risk. One study investigated the risk of AF among DM2 patients on second-line treatment with TZD 20. The authors found that TZD was associated with fewer new diagnoses of AF compared to other second-line medications. The authors used a Danish database (perhaps not applicable the multi-ethnic population of the USA) and included patients who received both metformin and sulfonylurea as first-line treatment. A study that drew data from the DECLARE-TIMI 58 trial reported that dapagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, reduced the risk of AF/AFL in DM2 patients with multiple risk factors for atherosclerotic cardiovascular disease 21. SGLT2 inhibitors were not examined in the current study because these medications were not present in this database in sufficient numbers to allow for meaningful statistical comparisons.
An additional provocative finding is that metformin monotherapy was associated with significantly lowered risk for VT/VF when compared with sulfonylurea monotherapy. Although nonsustained VT or VF is not necessarily fatal, this risk reduction could lead to decreased mortality. Interestingly, there are prior publications from large clinical cohorts finding lower mortality with metformin compared to sulfonylureas or other anti-diabetic medications 22,23. A prior study comparing only metformin and sulfonylureas found that metformin was associated with lower risk of all-cause mortality and cardiovascular events in DM2 patients with chronic kidney disease 24. Patients with heart failure may also have a reduction in mortality on metformin 25. These prior findings, together with our work, indicate that an anti-arrhythmic effect from metformin compared to sulfonylureas could have a positive effect on mortality.
Although this is an observational study based on large database analyses, there is a substantial body of prior research on the molecular mechanisms of metformin, particularly in modulating metabolism. Increased volume of epicardial adipose tissue (EAT) in diabetic and/or obese patients has been associated with the development of severe cardiovascular disorders via paracrine signaling 26. A study of patients who underwent AF ablation found pronounced electroanatomic remodeling in regions adjacent to EAT deposits 27. A study in which rat cardiomyocytes were treated with conditioned media from EAT explants of DM2 patients showed decreased sarcomere shortening, cytosolic calcium fluxes, and SERCA2 expression (which is critical for regulating intracellular myocyte calcium) 28. It is possible that metformin influences arrhythmia risk via effects on adipose tissue, or by direct effects on the myocardium. Our group has shown that mice fed a high saturated fat diet for four weeks develop ventricular ectopy, long QT, and inducible VT/VF, which is caused by increased oxidative stress and abnormal intracellular calcium handling in cardiac myocytes 29. These findings, in accordance with epidemiologic data, suggest that metabolic dysregulation in cardiomyocytes is an important cause of arrhythmias 30.
Prior clinical studies provide convincing evidence that metformin can improve metabolic pathways during obesity or diabetes. One study found that in subcutaneous adipose tissue explanted from non-DM patients, metformin treatment increased expression and secretion of adiponectin, an insulin-sensitizing hormone released by adipocytes that reduces hepatic glucose regulation while increasing fatty acid oxidation 31,32. In vivo data from the same study showed that the blood of obese patients prescribed metformin and lifestyle changes also had higher levels of adiponectin, as well as the macrophage activation marker CD68, elucidating the possible role of metformin in anti-inflammatory pathways. In a study of DM patients transplanted with non-DM hearts, metformin decreased cardiac lipid accumulation compared to those that did not receive the drug 33.
A few animal studies have examined how modulation of lipid metabolism by metformin can prevent the development of arrhythmias. In a non-diabetic swine model of ischemia, a clinically relevant dose of metformin reduced mortality from VF, which was associated with an increase in AMPK activation and preserved myocardial ATP concentration in atrial tissue 10. A canine model of acute AF also showed an increase in AMPK activation, which prevented fatty acid deposition in the left atrial appendage by promoting the transcription of the metabolic proteins PPARα, carnitine palmitoyl transferase CPT-1, and VLCAD 34. One study found that in rats with DM2, metformin inhibits the PKC/ERK signaling pathway and prevents the downregulation of small conductance calcium-activated potassium channels (SK channels), decreased transcription of which is associated with AF 35(p3). Animal models of obesity also indicate metformin has an anti-fibrotic effect 36. Thus, there is prior literature supporting the biologic plausibility of metformin reducing the risk of arrhythmia. Previous work examining the molecular and cellular mechanisms of metformin indicates complex modulation of metabolism in different tissues (Figure 3, Central Illustration).
Figure 3.
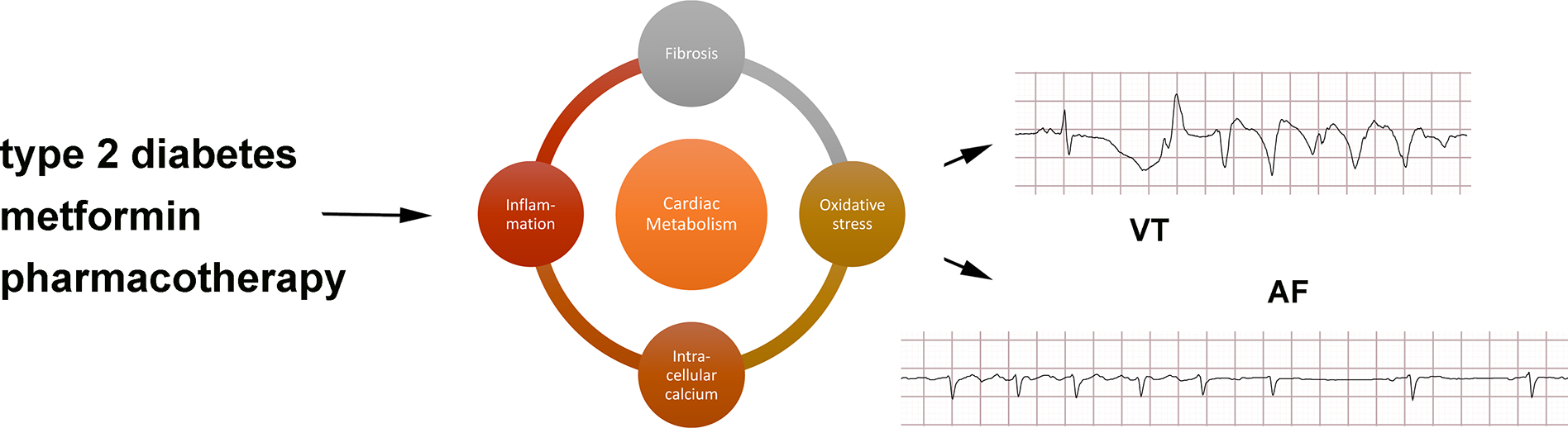
Central illustration
Our nationwide study of DM2 patients is the largest of its kind to compare metformin to other antidiabetic medications with respect to arrhythmia risk. The large number of subjects is an advantage. Our findings support the growing evidence that metformin can decrease the risk of AF and other types of arrhythmia in DM2 patients. This work does have limitations in that it is a retrospective cohort analyses using a database that is derived from claims data. Asymtomatic arrhythmias would not be captured very often by standard-of-care testing, for example. However, we have no reason to think that these diabetes medications would have an effect on symptoms from arrhythmias.
Limitations
This study has several limitations. First, administrative claims data sources lack data elements, which were not used in this study either (for example, laboratory test results). Although we adjusted for age, gender, all medications taken, diagnoses, and procedure records in the data source, we can not adjust our analysis to race or socio-economic status as the data source lacks this information. While it is possible that residual study bias may persist, we observed balance on all covariates after propensity score matching, which reduces the risk of such bias, and negative controls did not show evidence of residual bias. Second, we did not study dose-dependent effects of metformin. These results would not necessarily apply to people who do not have diabetes mellitus type II.
Conclusion
In this observational retrospective cohort study, metformin therapy was associated with a decreased risk of atrial arrhythmias in patients with diabetes mellitus type II including atrial fibrillation as compared with several other antidiabetic medications. Furthermore, there was a decreased risk of ventricular arrhythmias for patients on metformin monotherapy compared to sulfonylureas monotherapy.
Supplementary Material
What is Known?
Type 2 diabetes is a common disease with a variety of pharmacologic therapies.
Studies investigating the risk of atrial and ventricular arrhythmias in diabetic patients on different medications are sparse.
What the Study Adds?
We found that different oral diabetes medications have significantly different long-term risk of arrhythmia using a large clinical database.
Metformin is associated with reduced risk of atrial fibrillation and ventricular arrhythmias compared to sulfonylureas.
Acknowledgments
Sources of Funding: JPM is supported by NIH R01 HL136758, AO and GH are supported by NIH R01 LM006910
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- DPP4
dipeptidyl peptidase 4 inhibitors
- GLP-1
glucagon-like peptide-1 receptor agonists
- TZD
thiazolidinediones
- DM2
type 2 diabetes
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Disclosures: None
References:
- 1.National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States. Published online 2020:32.
- 2.Lin P-J, Kent DM, Winn A, Cohen JT, Neumann PJ. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care. 2015;21:e23–34. [PubMed] [Google Scholar]
- 3.Chow E, Bernjak A, Williams S, Fawdry RA, Hibbert S, Freeman J, Sheridan PJ, Heller SR. Risk of Cardiac Arrhythmias During Hypoglycemia in Patients With Type 2 Diabetes and Cardiovascular Risk. Diabetes. 2014;63:1738–1747. doi: 10.2337/db13-0468 [DOI] [PubMed] [Google Scholar]
- 4.Vidaillet H, Granada JF, Chyou P o-H, Maassen K, Ortiz M, Pulido JN, Sharma P, Smith PN, Hayes J. A population-based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113:365–370. doi: 10.1016/S0002-9343(02)01253-6 [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standards of Medical Care in Diabetes 2020. Diabetes Care. 2020;43(Supplement 1):S1–S2. doi: 10.2337/dc20-Sint [DOI] [PubMed] [Google Scholar]
- 6.Garber AJ, Handelsman Y, Grunberger G, et al. Einhorn D, Abrahamson MJ, Barzilay JI, Blonde L, Bush MA, DeFronzo RA, Garber JR, et al. CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM – 2020 EXECUTIVE SUMMARY. Endocr Pract. 2020;26:107–139. doi: 10.4158/CS-2019-0472 [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. 7. Approaches to Glycemic Treatment. Diabetes Care. 2015;38(Supplement_1):S41–S48. doi: 10.2337/dc15-S010 [DOI] [PubMed] [Google Scholar]
- 9.Chang S-H, Wu L-S, Chiou M-J, Liu Jia-Rou, Yu Kuang-Hui, Kuo Chang-Fu, Wen M-S, Chen W-J, Yeh Y-H, See L-C. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. doi: 10.1186/s12933-014-0123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L, Ye S, Scalzo RL, Reusch JEB, Greyson CR, Schwartz GG. Metformin prevents ischaemic ventricular fibrillation in metabolically normal pigs. Diabetologia. 2017;60:1550–1558. doi: 10.1007/s00125-017-4287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamson DM, Chang S, Hansen LG. Health research data for the real world: the MarketScan databases. New York: Thompson Healthcare. Published online 2008:b28. [Google Scholar]
- 12.Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, Suchard MA, Park RW, Wong ICK, Rijnbeek PR, et al. Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers. Stud Health Technol Inform. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 13.Bösenberg LH, van Zyl DG. The mechanism of action of oral antidiabetic drugs: A review of recent literature. JEMDSA. 2008;13:80–88. doi: 10.1080/22201009.2008.10872177 [DOI] [Google Scholar]
- 14.Hripcsak G, Ryan PB, Duke JD, Shah NH, Park RW, Huser V, Suchard MA, Schuemie MJ, DeFalco FJ, Perotte A, et al. Characterizing treatment pathways at scale using the OHDSI network. Proc Natl Acad Sci U S A. 2016;113:7329–7336. doi: 10.1073/pnas.1510502113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuemie MJ, Cepede MS, Suchard MA, Yang J, Tian Y, Schuler A, Ryan PB, Madigan D, Hripcsak G. How Confident Are We About Observational Findings in Health Care: A Benchmark Study. Harv Data Sci Rev. 2020;2: 10.1162/99608f92.147cc28e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D. Interpreting observational studies: why empirical calibration is needed to correct p -values. Stat Med. 2014;33:209–218. doi: 10.1002/sim.5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vashisht R, Jung K, Schuler A, Banda JM, Park RW, Jin S, Li L, Dudley JT, Johnson KW, Shervey MM, et al. Association of Hemoglobin A1c Levels With Use of Sulfonylureas, Dipeptidyl Peptidase 4 Inhibitors, and Thiazolidinediones in Patients With Type 2 Diabetes Treated With Metformin: Analysis From the Observational Health Data Sciences and Informatics Initiative. JAMA Netw Open. 2018;1:e181755. doi: 10.1001/jamanetworkopen.2018.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suchard MA, Schuemie MJ, Krumholz HM, You SC, Chen R, Pratt N, Reich CG, Duke J, Madigan D, Hripcsak G, Ryan PB. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394:1816–1826. doi: 10.1016/S0140-6736(19)32317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou Y-S, Yang F-Y, Chen H-Y, Jong G-P. Antihyperglycemic drugs use and new-onset atrial fibrillation: A population-based nested case control study. PLoS One. 2018;13:e0197245. doi: 10.1371/journal.pone.0197245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallisgaard JL, Lindhardt TB, Staerk L, Olesen JB, Torp-Pedersen C, Hansen ML, Gislason GH. Thiazolidinediones are associated with a decreased risk of atrial fibrillation compared with other antidiabetic treatment: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. 2017;3:140–146. doi: 10.1093/ehjcvp/pvw036 [DOI] [PubMed] [Google Scholar]
- 21.Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation. 2020;141:1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183 [DOI] [PubMed] [Google Scholar]
- 22.Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased Mortality Associated With the Use of Metformin Compared With Sulfonylurea Monotherapy in Type 2 Diabetes. Diabetes Care. 2002;25:2244–2248. doi: 10.2337/diacare.25.12.2244 [DOI] [PubMed] [Google Scholar]
- 23.Roussel R Metformin Use and Mortality Among Patients With Diabetes and AtherothrombosisMetformin Use With Diabetes and Atherothrombosis. Arch Intern Med. 2010;170:1892–1899. doi: 10.1001/archinternmed.2010.409 [DOI] [PubMed] [Google Scholar]
- 24.Whitlock RH, Hougen I, Komenda P, Rigatto C, Clemens KK, Tangri N. A Safety Comparison of Metformin vs Sulfonylurea Initiation in Patients With Type 2 Diabetes and Chronic Kidney Disease: A Retrospective Cohort Study. Mayo Clin Proc. 2020;95:90–100. doi: 10.1016/j.mayocp.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 25.Evans JMM, Doney ASF, AlZadjali MA, Ogston SA, Petrie JR, Morris AD, Struthers AD, Wong AKF, Lang CC. Effect of Metformin on Mortality in Patients With Heart Failure and Type 2 Diabetes Mellitus. Am J Cardiol. 2010;106:1006–1010. doi: 10.1016/j.amjcard.2010.05.031 [DOI] [PubMed] [Google Scholar]
- 26.Groves EM, Erande AS, Le C, Salcedo J, Hoang KC, Kumar S, Mohar DS, Saremi F, Im J, Agrawal Y, et al. Comparison of Epicardial Adipose Tissue Volume and Coronary Artery Disease Severity in Asymptomatic Adults With Versus Without Diabetes Mellitus. Am J Cardiol. 2014;114:686–691. doi: 10.1016/j.amjcard.2014.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, Carbone A, Teo K, Agbaedeng T, Linz D, et al. Electroanatomical Remodeling of the Atria in Obesity. JACC: Clin Electrophysiol. 2018;4:1529–1540. doi: 10.1016/j.jacep.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 28.Greulich S, Maxhera B, Vandenplas G, Herzfeld de Wiza D, Smiris K, Mueller H, Heinrichs J, Blumensatt M, Cuvelier C, Akhyari P, et al. Secretory Products From Epicardial Adipose Tissue of Patients With Type 2 Diabetes Mellitus Induce Cardiomyocyte Dysfunction. Circulation. 2012;126:2324–2334. doi: 10.1161/CIRCULATIONAHA.111.039586 [DOI] [PubMed] [Google Scholar]
- 29.Joseph LC, Avula UMR, Wan EY, Reyes MV, Lakkadi KR, Subramanyam P, Nakanishi K, Homma S, Muchir A, Pajvani UB, et al. Dietary Saturated Fat Promotes Arrhythmia by Activating NOX2 (NADPH Oxidase 2). Circ Arrhythm Electrophysiol. 2019;12:e007573. doi: 10.1161/CIRCEP.119.007573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hookana E, Junttila MJ, Puurunen V-P, et al. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. Published online July 2011. doi: 10.1016/j.hrthm.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 31.Zulian A, Cancello R, Girola A, et al. ``123414q `1AWE23Q `1 Obesity Facts. 2011;4:27–33. doi: 10.1159/000324582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100. doi: 10.1093/jmcb/mjw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marfella R, Amarelli C, Cacciatore F, Balestrieri ML, Mansueto G, D’Onofrio N, Esposito S, Mattucci I, Salerno G, De Feo M, et al. Lipid Accumulation in Hearts Transplanted From Nondiabetic Donors to Diabetic Recipients. J Am Coll Cardiol. 2020;75:1249–1262. doi: 10.1016/j.jacc.2020.01.018 [DOI] [PubMed] [Google Scholar]
- 34.Bai F, Liu Y, Tu T, Li B, Xiao Y, Ma Y, Qin F, Xie J, Zhou S, Liu Q. Metformin regulates lipid metabolism in a canine model of atrial fibrillation through AMPK/PPAR-α/VLCAD pathway. Lipids Health Dis. 2019;18:109. doi: 10.1186/s12944-019-1059-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C-H, Hua N, Fu X, Pan Y-L, Li B, Li X-D. Metformin regulates atrial SK2 and SK3 expression through inhibiting the PKC/ERK signaling pathway in type 2 diabetic rats. BMC Cardiovasc Disord. 2018;18:236. doi: 10.1186/s12872-018-0950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burlá AK, Lobato NS, Fortes ZB, Oigman W, Neves MF. Cardiac fibrosis and vascular remodeling are attenuated by metformin in obese rats. Int J Cardiol. 2013;165:483–487. doi: 10.1016/j.ijcard.2011.09.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


