Abstract
Background:
Chronic pancreatitis (CP) does not have diagnostic or prognostic biomarkers. CP is the end stage of a progressive inflammatory syndrome that is diagnosed at late stages by morphologic features. To diagnose earlier stages of the disease, a new mechanistic definition was established based on identifying underlying pathogenic processes and biomarker evidence of disease activity and stage. Although multiple risk factors are known, the corresponding biomarkers needed to make a highly accurate diagnosis of earlier disease stages have not been established. The goal of this study is to systematically analyze the literature to identify the most likely candidates for development into biomarkers of CP.
Methods:
We conducted a systematic review of candidate analytes from easily accessible biological fluids and identified 67 studies that compared CP to nonpancreatic-disease controls. We then ranked candidate biomarkers for sensitivity and specificity by area under the receiver operator curves (AUROCs).
Results:
Five biomarkers had a large effect size (an AUROC > 0.96), whereas 30 biomarkers had a moderate effect size (an AUROC between 0.96 and 0.83) for distinguishing CP cases from controls or other diseases. However, the studies reviewed had marked variability in design, enrollment criteria, and biospecimen sample handling and collection.
Conclusions:
Several biomarkers have the potential for evaluation in prospective cohort studies and should be correlated with risk factors, clinical features, imaging studies and outcomes. The Consortium for the Study of Chronic Pancreatitis, Diabetes and Pancreas Cancer provides recommendations for avoiding design biases and heterogeneity in sample collection and handling in future studies.
Keywords: Pancreatitis, chronic pancreatitis, biomarker, PRoBE strategy, early detection
Introduction
Biomarkers are objective measures that can be indicators of normal biological or pathogenic processes or responses related to therapeutic interventions for a particular disease.1 To date, there are no reliable diagnostic, prognostic, or therapeutic biomarkers for chronic pancreatitis (CP).2 CP is characterized by chronic inflammation and progressive fibrosis of the pancreas, with loss of acinar cell mass. This leads to irreversible morphologic changes, loss of pancreatic function, and increased risk of pancreatic cancer.3–5 CP, like many chronic diseases, is defined by the consensus criteria of experts as a clinicopathologic syndrome with characteristic clinical, imaging, pathological, and functional features.6–8 Unfortunately, the detection of early-stage CP has remained elusive due to a poor understanding of the pathogenesis of CP and the nonspecific findings on endoscopic and radiologic imaging.9–11 Although detection of early-stage CP is a research challenge, it represents an opportunity for innovative CP research and discovery in the 21st century.
Current diagnostic methods for CP are highly accurate for moderate to advanced disease and include abdominal radiographic imaging,12 endoscopic procedures (EUS), and functional testing methods, including measurement of analytes in pancreas fluid after secretin or cholecystokinin stimulation.13–15 However, none of these testing methods are suitable for early-stage CP diagnosis in isolation.16 Often, a confident diagnosis of CP is not confirmed until end-stage clinical features are evident, indicating moderate to severe fibrotic changes of the pancreas gland.13, 17–19 Therefore, the lack of accurate methods for the early diagnosis of CP impedes patient evaluation and limits the development of clinical trials of potential new CP therapies, which may alter the natural course of the disease. The development of accurate diagnostic “early-stage” CP biomarkers would create the opportunity to test the effectiveness of repurposed or new antifibrotic, antioxidant, and/or anti-inflammatory drug therapies for CP.20–24 Furthermore, the development of accurate prognostic biomarkers could predict the development of end-stage CP complications, such as diabetes, exocrine insufficiency, bone disease, or pancreatic cancer, facilitating the development of strategies to block, retard, or slow disease progression. Thus, the lack of successful biomarker development in CP research has remained an elusive target for decades and represents a major research gap in our knowledge.25
The Adult Chronic Pancreatitis Working Group of the Consortium for the Study of Chronic Pancreatitis Diabetes and Pancreas Cancer (CPDPC) established a Biospecimen Working Group, which includes a Biomarker Subcommittee devoted to addressing the research gaps related to biomarker discovery and validation in CP studies.26 This paper represents our first step towards the development of accurate CP diagnostic and prognostic biomarkers. Our primary goal was to systematically review all promising candidate biomarkers of CP described in previous studies. For each biomarker identified, we evaluated the stage of biomarker development as defined by the prospective-specimen-collection, retrospective-blinded-evaluation (PRoBE) design method27–30 and the biomarkers’ quantitative diagnostic performance based on the area under the receiver operator curve (AUROC) and other metrics. Our secondary goal was to identify which candidate biomarkers merit further testing and validation using biospecimens (whole blood, urine, saliva, pancreas fluid, and/or pancreas tissue) that are being prospectively collected in the PROspective Evaluation of Chronic Pancreatitis for Epidemiologic and Translational Studies (PROCEED).27 Among the types of biomarkers evaluated were adipokines, amino acids or other intermediary metabolites, lipoproteins, chemokines, cytokines, microRNAs, extracellular matrix proteins, and glycoproteins.
Methods
Medical literature databases (PubMed and Scopus) were searched from what was available until August 2018 using multiple search strategies, including the search terms “pancreatitis,” “chronic pancreatitis,” “biomarker,” and “diagnosis.” Based on these searches, 743 articles that potentially included biomarkers of CP were identified and underwent preliminary review. Of these, 234 articles reported CP-biomarker assays and were reviewed in detail. Sixty-seven of these articles reported quantitative results of biomarker assays in a human biological fluid (whole blood, serum, plasma, buffy coat, urine, saliva, stool, or pancreas fluid) of cases with CP compared to a normal or benign-disease control group and were included in the final analysis. (Figure 1). Animal studies and data comparing CP to pancreatic ductal adenocarcinoma (PDAC) were not included. Studies assessing tissue immunohistochemistry were included only if there were also data available regarding the performance of the same biomarker in a human biological fluid. Data abstracted from these articles included the potential biomarker being studied, the assay used to measure the biomarker of interest, type of biofluid assayed, definitions of the subject groups included in the study, sample sizes, and quantitative data regarding biomarker performance (mean, standard deviation, and/or standard error of the mean, median, or other quantiles, interquartile ranges, minimum, maximum, sensitivity, specificity, AUROC, and p-values from multigroup comparison).
Figure 1.
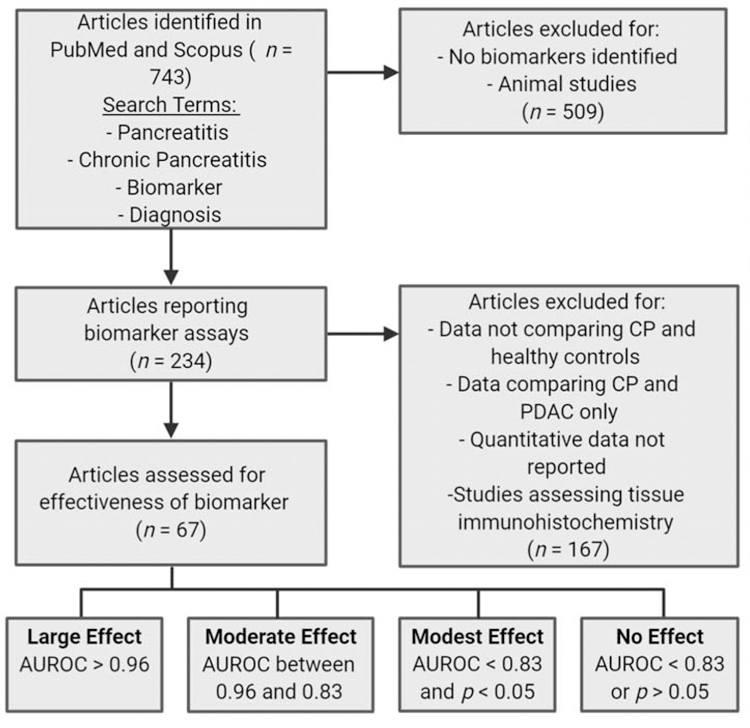
Flow diagram of literature search strategy.
The discrimination ability of a biomarker diagnostic test, that is, its ability to separate various phenotypic groups, arises from its different distribution among these groups and is of central importance to a diagnostic test evaluation. Although the AUROC (or sensitivity/specificity) is widely used as a standard measure of discrimination,31, 32 it was not reported in all the publications reviewed in this study. Even fewer studies reported the receiver operating characteristic (ROC) curve graphically. Various publications reported different summary statistics that characterize certain aspects of biomarker distributions, such as the mean, standard deviation, p-value from a test of means, Bayes factor, etc. Not all of these statistics have the same clinically relevant interpretability as the ROC curve or AUROC, which makes it difficult to compare biomarkers’ discrimination abilities across studies. In order to compare the discrimination ability of different biomarkers, we estimated the AUROC if it was feasible to do so with the published statistics in the articles that did not report AUROC. The estimation was based on a binormal model (i.e., assuming the biomarker is normally distributed in the cases and control groups), which is widely used in diagnostic medicine.31, 32 Normalization transformation was used when the published statistics suggested skewness in the data. The AUROC can be estimated from the binormal model when the mean and standard deviation are reported for the cases and controls or when the median, Q25 (25th quartile), and Q75 are reported. When the publication reported minimum and maximum values of the biomarker, we used them as Q10 and Q90 in the estimation. Because the estimated AUROCs are dependent on the binormal model assumption, our tables annotate them differently from the published AUROC results (estimated AUROCs are in bold font). We further classified the biomarker into one of four effect-size (discrimination ability) categories: large if the AUROC was greater than 0.96, moderate if the AUROC was between 0.96 and 0.83, modest if the AUROC was less than 0.83 and a significant p-value was reported, and undetermined/no effect for all others. Under a binormal model, the cutoff of 0.96 implies that the interval (Q10, Q90) of the two comparison groups does not overlap; the cutoff of 0.83 implies that the range (Q25, Q75) of the two comparison groups does not overlap (Figure 1). The level of discrimination is on a continuous spectrum, and this categorization is chosen for convenience of discussion in this paper.
Results
There was wide variation in the study populations included in the 67 articles reviewed. Most studies defined CP on the basis of unequivocal imaging and/or functional changes, but there was substantial variation in the classification systems used and in the proportion of CP cases that were attributed to alcohol. In some studies, no objective definition of CP was provided. The control groups also varied, which included healthy controls in some studies and abdominal pain patients deemed not to have pancreatic disease in others.
Overall, we analyzed the selected studies that investigated biomarker levels in human biofluids (Figure 2A) or tissues (Figure 2B) and grouped them based on their AUROCs to distinguish CP cases from healthy or benign-disease control group. We found that of the potential biomarkers analyzed from biofluids, five had a large effect size, 25 had a moderate effect size, 33 had a modest effect size, and 18 had no effect (Figure 2A). Moreover, we found that a subset of these potential biomarkers was also analyzed in tissues, for which none had a large effect size, five had a moderate effect size, and four had a modest effect size (Figure 2B). All the biomarkers analyzed from biofluids and tissues are summarized in Table 1 by analyte/biomarker category and in alphabetical order. The table provides information regarding the following: the type of biospecimen assayed, the number of control and CP cases, the AUROC reported or imputed, the p-value reported in the publication, the determined effect size, any special comments regarding the comparison, and references used for the determination. In instances where we were unable to impute AUROCs or could not make an informed judgement on the effect size of a particular potential biomarker the field in the table remains blank. Since some potential biomarkers were identified in multiple papers, we provided information on all the sample types, the range of “n”, AUROCs, and p-values provided from all the papers that mentioned each biomarker.
Figure 2.
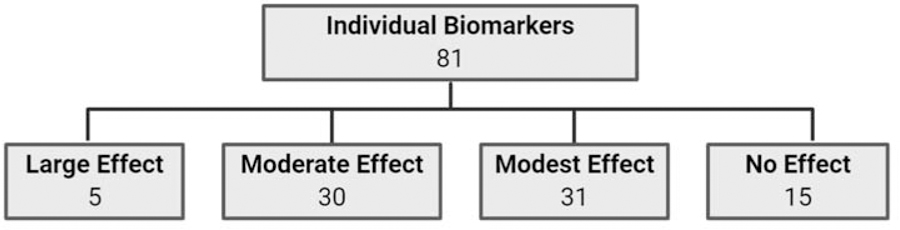
Summary of effect sizes of individual biomarkers based on AUROCs. The highest effect size is used for biomarkers with heterogeneity across studies.
Table 1.
The determined diagnostic biomarker effect size for distinguishing between control and CP cases in biological fluids. The p-values presented were reported in the original publications from various statistical tests for between-group difference. The AUROC values, shown in bold font, were imputed from published data, as described in the methods section.
| Biomarker | Biospecimen Type | n (Control) | n (CP) | AUROC | p-value | Effect Size | Comments | Ref(s) |
|---|---|---|---|---|---|---|---|---|
| Adipokines | ||||||||
| Adiponectin | Plasma, serum | 13–30 | 27–44 | 0.514–0.994 | NS–<0.0003 | None - Large | Heterogeneity across study results: two studies of serum with null results, one study with plasma showing large effect size | 45, 46, 48 |
| Leptin | Serum | 16–30 | 30–44 | 0.788–0.803 | <0.05-<0.01 | Modest | 45, 48 | |
| Neutrophil gelatinase-associated lipocalin (NGAL) | Pancreatic juice | 23 | 24 | 0.88 | <0.001 | Moderate | 63 | |
| Resistin | Serum | 16–78 | 23–81 | 0.865 | <0.001 | Moderate | May distinguish between RAP and CP | 64, 65 |
| Chemokines | ||||||||
| Chemokine ligand 5 (CCL5) | Plasma, serum | 20 | 20 | 0.92 | <0.0001 | Moderate | 66 | |
| Chemerin | Serum | 40 | 68 | 0.746 (DM), 0.787 (non-DM) | <0.01 | Modest | 67 | |
| C-reactive protein (CRP) | Serum | 28–70 | 14–45 | 0.513 –0.828 | <0.05 | Modest | 68, 69 | |
| Fractalkine | Serum | 15–116 | 78–109 | 0.898 | 0.011 to <0.0001 | Modest - Moderate | May be elevated in earlystage disease | 70, 71 |
| Fractalkine (CX3CR1) | Pancreatic tissue | 21 | 61 | 0.736 | <0.001 | Modest | Correlates with pain and degree of inflammation | 72 |
| Monocyte chemoattractant protein-1 (MCP-1) | Plasma, serum, whole blood | 15–88 | 78–142 | N/A–0.943 | NS-<0.001 | None – Moderate | Heterogeneity across study results | 64, 70, 73 |
| Platelet basic protein (PPBP) | Plasma, serum | 20 | 20 | 0.92 | <0.001 | Moderate | 66 | |
| Cytokines | ||||||||
| Cytokine array | Serum | 30 | 16 | 0.71 | - | - | 74 | |
| Endothelin-1 | Serum | 13–26 | 24–39 | 0.711–0.720 | NS | None | Correlated with tobacco use (AUROC 0.7) rather than CP | 47, 75 |
| Interleukin-1β (IL-1β) | Plasma, serum | 18–31 | 27–33 | N/A to 0.777 | NS-<0.001 | None – Modest | Heterogeneity across study results | 47, 76 |
| Interleukin-6 (IL-6) | Pancreatic juice | 3–41 | 3–39 | N/A to 0.64 | NS–0.01 | None – Modest | AUC is for study with larger n | 34, 77 |
| Interleukin-6 (IL-6) | Plasma, serum | 8–72 | 8–56 | 0.507–0.997 | NS-<0.001 | None – Large | Heterogeneity across study results: only one study shows more than a modest effect size. | 47, 50–52, 68, 78, 79 |
| Interleukin-8 (IL-8) | Serum | 45 | 49 | - | <0.05 | Modest | 79, 80 | |
| Interleukin-8 (IL-8) | Pancreatic juice | 3–41 | 3–39 | 0.82 | NS–0.011 | Modest | AUC is from study with larger n | 34, 77 |
| Interleukin-8 (IL-8) | Pancreatic tissue | 4 | 4 | - | <0.05 | Modest | Unable to estimate AUROC from data provided | 80 |
| Interleukin-10 (IL-10) | Serum | 30 | 39 | 0.614 | 0.058 | None | 81 | |
| Interleukin-18 (IL-18) | Serum | 30 | 29 | 0.764 | <0.005 | Modest | 81 | |
| Cytokine array | Serum | 30 | 16 | 0.71 | - | - | 74 | |
| Macrophage inhibitory cytokine 1 (MIC-1) | Plasma | 24–500 | 23–50 | 0.508–0.875 | NS-<0.001 | None – Moderate | Heterogeneity across study results | 63, 82 |
| Platelet-derived growth factor-AA (PDGF-AA) | Serum | 28 | 61 | 0.612 | 0.071 | None | 83 | |
| Platelet-derived growth factor-BB (PDGF-BB) | Serum | 35–40 | 40–60 | 0.732 | <0.01-<0.001 | Modest | 67, 84 | |
| Transforming growth factor-α (TGF-α) | Pancreatic juice | 3 | 3 | - | 0.176 | None | CP subjects had end-stage clinical disease | 77 |
| Transforming growth factor-α (TGF-α) | Pancreatic tissue | 5 | 12 | - | <0.01 | Modest | Unable to estimate AUROC from data provided | 85 |
| Transforming growth factor-β1 (TGF-β1) | Pancreatic juice | 41 | 39 | 0.55 | 0.4 | None | 34 | |
| Transforming growth factor-β1 (TGF-β1) | Plasma, serum | 11–116 | 10–109 | 0.513–0.913 | 0.3-<0.0001 | None –Moderate | Heterogeneity across study results | 51, 64, 69–71, 83, 84 |
| Tumor necrosis factor-α (TNF-α) | Plasma, serum | 100 | 71–100 | 0.654–0.665 | <0.014 | Modest | 69, 86 | |
| Vascular endothelial growth factor (VEGF) | Serum | 24–50 | 10–72 | 0.735 | 0.031-<0.001 | Modest | 87, 88 | |
| Extracellular Matrix Proteins | ||||||||
| Hyaluronic acid | Pancreatic juice | 20 | 20 | 0.95 | <0.01 | Moderate | 89 | |
| Hyaluronic acid | Serum | 15–78 | 78–81 | 0.688–0.733 | <0.001 | Modest | 64, 70 | |
| MAC-2 binding protein (M2BP) | Serum | 30–59 | 74–162 | 0.727-0.788 | <0.001-<0.0001 | Modest | Levels correlated with morphologic severity of CP and were highest in patients with severe disease; AUC is for mild CP group | 90, 91 |
| Glycoproteins | ||||||||
| Intercellular adhesion molecule 1 (ICAM1) | Plasma, serum | 20 | 20 | 0.92 | <0.001 | Moderate | 66 | |
| Lactotransferrin (LTF) | Plasma | 41 | 52 | 0.73–092 | 0.084-<0.0001 | None - Moderate | 66 | |
| Thrombospondin 1 (THBS1) | Plasma, serum | 20 | 20 | 0.92 | <0.001 | Moderate | 66 | |
| Tissue inhibitor of metalloproteinases 1 (TIMP-1) | Plasma, serum | 10–86 | 23–48 | 0.503 | 0.98-<0.05 | None - Modest | Heterogeneity across study results; imputed AUROC is from study with largest n | 38, 92, 93 |
| Lipoproteins | ||||||||
| Apolipoprotein 2 (APOA2) | Plasma | 41 | 52 | 0.65 | 0.199 | None | 66 | |
| High density lipoprotein-c (HDLc) | Serum | 40 | 48 | 0.647 | 0.004 | Modest | 94 | |
| Paraoxonase 1 (PON1) | Plasma | 132 | 186 | 0.586 | <0.001 | Modest | 95 | |
| Zinc-α−2-glycoprotein (AZGP1) | Plasma, serum | 20 | 20 | 0.9292 | <0.0001 | Moderate | 66 | |
| Metabolites | ||||||||
| 4-hydroxynonenal (4-HNE) | Plasma | 27 | 105 | - | <0.05 | Modest | Twofold elevation in CP; unable to impute AUROC from presented data | 96 |
| Citrate | Urine | 5 | 5 | 0.948 | 0.048 | Moderate | 43 | |
| Malondialdehyde (MDA) | Plasma | 27 | 105 | - | <0.05 | Modest | Six fold elevation in CP; unable to impute AUROC from presented data | 96 |
| Methionine | Plasma | 48 | 90 | 0.807–0.878 | <0.001 | Modest - Moderate | Mean plasma methionine levels almost two times lower in CP | 65 |
| Oxidized fatty acid 13:HODE:LA | Serum | 5 | 5 | 0.959 | 0.008 | Moderate | Values shown for “severe CP” vs. control | 33 |
| Oxidized fatty acids: 5-HETE:AA, 11-HETE:AA, 15HETE:AA, 9-HODE:LA, 9oxoODE:LA, 13-oxoODE:LA | Serum | 5 | 5 | 0.877–1 | 0.03-<0.001 | Moderate – Large | Values shown for “severe CP” vs. control | 33 |
| Phosphatidylcholine 18:2n-6 | Plasma | 108 | 96 | 0.875 | <0.001 | Moderate | Fatty acid deficiencies also occur in luminal GI diseases and PDAC | 97 |
| Phosphatidylcholines 16:1n–7, 18:1n–9, 18:1n–7, 18:3n6, 22:4n6, 22:5n–6, 22:6n–3, D9D16, D5Dn6 | Plasma | 108 | 96 | 0.586–0.750 | <0.001 | Modest | 97 | |
| Thiobarbituric acid-reactive substances (TBARS) | Serum | 28 | 57 | 0.865 | 0.001 | Moderate | 10-fold differences in median values | 83 |
| Metal Binding Proteins | ||||||||
| Core-fucosylated haptoglobin | Serum | 59 | 159 | 0.897 | 0.0001 | Moderate | 98 | |
| Matrix metalloproteinase 3 (MMP-3) | Plasma, serum | 120 | 120 | 0.56 | 0.10 | None | 99 | |
| Matrix metalloproteinase 7 (MMP-7) | Serum | 150 | 100 | 0.559 | 0.34 | None | 100 | |
| Matrix metalloproteinase 9 (MMP-9) | Plasma, serum | 100 | 71 | 0.938 | <0.001 | Moderate | 39 | |
| mRNAs | ||||||||
| miIR-106b | Plasma, exosomes | 6–46 | 11–37 | 0.578–0.713 | 0.851–0.092 | None | 35, 36 | |
| miR-10b | Plasma, exosomes | 3–46 | 3–37 | 0.551–0.591 | 1.0-< 0.001 | None - Modest | 35, 36, 101 | |
| miR-124 | Serum | 47 | 28 | 0.61 | 0.074 | None | 102 | |
| miR-148 | Pancreatic tissue | 16 | 19 | 0.92 | 0.022 | Moderate | 103 | |
| miR-155 | Plasma | 46 | 37 | 0.682 | 0.558-< 0.001 | Modest | 36 | |
| miR-181a | Whole blood, plasma, exosomes | 6–33 | 11–38 | 0.626–0.8 | 0.316 -< 0.01 | None – Modest | Best results with exosomes or cell pellet | 35, 103 |
| miR-182 | Pancreatic tissue | 16 | 19 | 0.95 | 0.005 | Moderate | 103 | |
| miR-1826 | Whole blood | 33 | 38 | 0.81 | < 0.01 | Modest | 103 | |
| miR-192 | Pancreatic tissue | 16 | 19 | 0.93 | 0.028 | Moderate | 103 | |
| miR-194 | Pancreatic tissue | 16 | 19 | 0.91 | 0.033 | Moderate | 103 | |
| miR-200a | Plasma | 11 | 31 | 0.861 | < 0.001 | Moderate | 104 | |
| miR-200c | Plasma | 11 | 31 | 0.731 | 0.004 | Modest | 104 | |
| miR-20a | Plasma, exosomes | 6 | 11 | 0.544–0.623 | 0.8–0.321 | None | 35 | |
| miR-21 | Plasma, exosomes | 6 | 11 | 0.558–0.542 | 0.828–0.719 | None | 35 | |
| miR-212 | Plasma | 46 | 37 | 0.630 | 0.578 | None | 36 | |
| miR-215 | Pancreatic tissue | 16 | 19 | 0.96 | 0.028 | Moderate | 103 | |
| miR-217 | Pancreatic tissue | 27 | 26 | - | <0.05 | Modest | Unable to estimate AUROC from data provided | 37, 105 |
| miR-30c | Plasma, exosomes | 6–38 | 48 | 0.5–0.706 | 0.999–0.126 | None | 35, 36 | |
| miR-320a | Blood, plasma | 44 | 69 | 0.83–0.848 | <0.01–0.001 | Moderate | 103, 104 | |
| miR-375 | Plasma | 11 | 31 | 0.823 | <0.001 | Modest | 104 | |
| miR-378 | Whole blood | 33 | 38 | 0.80 | <0.01 | Modest | 103 | |
| miR-letA | Plasma, exosomes | 6 | 11 | 0.764–0.782 | 0.025–0.017 | Modest | 35 | |
| Others | ||||||||
| Adenosine | Urine | 5 | 5 | 0.971 | 0.048 | Large | ||
| Anti-carbonic anhydrase antibody | Serum plasma | 40 | 48 | 0.560 | 0.315 | None | 106 | |
| Circulating T-helper cells | Peripheral blood | 50 | 50 | - | <0.01 | Modest | Unable to impute AUROC | 107 |
| Cysteine | Plasma | 48 | 90 | 0.862–0.918 | <0.001 | Moderate | Mean plasma cysteine levels four times lower in CP than normal | 65 |
| Heat shock protein 27 | Serum | 102 | 44 | 0.799 | <0.001 | Modest | 108 | |
| Substance P | Serum | 8 | 30 | 0.752 | 0.006 | Modest | About half of CP patients had marked rise compared to controls | 109 |
| Proteomic panels | Serum | 14–30 | 9–16 | 0.96 | <0.001 | Moderate | Distinct biomarker panels described for acute, chronic, and autoimmune pancreatitis 75% sensitivity / 100% specificity |
40, 74 |
| Relative microbial abundance | Saliva | 38 | 27 | - | <0.05 | Modest | Granulicatella adiacens (increased abundance) and S mitis (decreased abundance) were validated using independent samples and showed significant variation (p < 0.05, qPCR) between CP samples and controls; unable to impute AUROC from published data | 110 |
| Des-Leu albumin | Plasma, serum | 34 | 9 | 0.996 | - | Large | Measured in patients hospitalized with acute flare-ups; no p-value presented in article | 49 |
| Receptors | ||||||||
| Serum TNFR-p75 (TNF receptor) | Serum | 28 | 34 | 0.832 | <0.05 | Moderate | 111 | |
| Soluble IL-2 receptor | Serum | 72 | 24 | 0.808 | <0.05 | Modest | 78 | |
Most of the studies compared CP cases to control cases as defined in the study. However, several studies included control cases with non-ulcer dyspepsia or chronic upper abdominal pain that were deemed by the investigators not to have pancreatic disease.33–39 Between the biofluids and tissue biomarkers, we identified 30 potential biomarkers with a moderate effect size and five potential biomarkers with a large effect size. In addition to the effect size of the biomarkers, we examined other features of the published studies against the PRoBE study design, such as the presence of a validation set. Among the studies reviewed, we found that only one included a validation set.40 Additionally, after careful analysis of the study methodologies used in the articles, we determined that all the studies fell into the Phase 1 category (initial discovery studies), that is, the first of the five biomarker-development phases defined by the PRoBE design.29
We found several studies that used the Bayes factor as the measure of distribution difference between cases and controls. The Bayes factor can be viewed as the Bayesian equivalent of the frequentist p-value. It is a positive number, defined as the ratio of the likelihoods under the null and alternative hypotheses. A Bayes factor that deviates from 1 indicates departure from the null hypothesis. In two studies, a Bayes factor greater than 10 was used as the criterion for differential protein expression between the comparison groups.12, 41 The Bayes factor can be used with sample sizes that are even smaller than those typically required by two-sample tests and that are hence suitable for small, pilot Phase 1 studies. The Bayes factor generates initial evidence for differential biomarker distributions among the comparison groups but does not provide the same clinically relevant interpretation as sensitivity and specificity. Because these studies were relevant to our analysis and their Bayes factors could not be converted into p-values or AUROCs, we could not directly compare them to the studies represented in Table 1; they are listed in Supplemental Table 1. In addition, we excluded one study of potential proteomic biomarkers that reported biomarker performance characteristics using a “leave-one-out” methodology.40 This study reported a biomarker panel AUROC of 1.0, suggesting perfect sensitivity and specificity. We were unable to determine the effect of the statistical methodology used on potential future clinical reporting of biomarker values. However, it will be of interest to validate the methodology reported in this study following the PRoBE design.
Discussion
The diagnosis of earlier stages of CP remains difficult because current diagnostic tests are specific for CP only when morphologic features are more apparent on imaging from late stages of CP or there is loss of pancreatic function.10 The aim of this systematic literature review was to identify potential biomarkers in the medical literature that merit further investigation for their ability to diagnose definitive CP as defined by advanced to moderate-severe changes on imaging. In contrast to other published reviews of CP biomarkers,42 this review focused on a quantitative analysis of available data to identify promising biofluid biomarkers worthy of further development and validation.
All the studies we identified fit the definition of Phase 1 biomarker development as per the PRoBE strategy.29 This means that the studies were exploratory in nature and focused on biomarker discovery, not on validation of the proposed biomarker.40 No proposed human CP biomarkers have been adequately validated using a clinical assay in separate discovery and validation cohorts (Phase 2) or tested for their ability to diagnose early or even preclinical disease using the PRoBE strategy (Phase 3).30 Although the current literature contains only Phase 1 studies, we were able to identify promising biomarkers on the basis of their apparent relative effect sizes. Below, we highlight the five biomarkers that had a large effect size using biofluids; these merit further investigation in additional Phase 1 studies utilizing rigorous sample collection and processing techniques and Phase 2 studies. Although these biomarkers had a large effect size, it may still be worthwhile to investigate the potential biomarkers that had a moderate effect size, particularly in combination with other potential biomarkers.
Adenosine:
Adenosine is a metabolite of ATP hydrolysis and, as such, is elevated in conditions of metabolic stress caused by disease. In patients with CP, adenosine levels were significantly increased in the urine compared to healthy controls in a study comparing urinary metabolomics using a 1H-NMR (proton nuclear magnetic resonance) assay.43 In addition to CP, this study included a group of patients with mild acute pancreatitis (AP). Although the data indicated that urine metabolites could not differentiate between AP and CP, the groups were small (n = 5) and the risk of a type II error was high. Therefore, validation of adenosine as a CP-specific biomarker needs to include comparisons between healthy controls, CP, and AP with larger cohorts.
Adiponectin:
Adiponectin is an anti-inflammatory adipokine that is secreted mainly from adipocytes and can reduce the secretion of many pro-inflammatory cytokines.44 However, the adiponectin data reviewed had heterogeneous results; that is, in terms of differentiating CP from healthy controls, adiponectin was not effective in some studies.45–47Gasiorowska et al.47 found that plasma adiponectin levels were significantly elevated in both CP and PDAC patients compared to normal controls, but no difference was found between the CP and PDAC groups. Another study found that serum levels of adiponectin were higher only in PDAC patients, compared to CP and control.48 Validation of adiponectin should include a PDAC comparison group to determine its ability to differentiate between healthy controls, CP, and PDAC cases.
Des-Leu albumin:
Des-Leu albumin is a truncated form of serum albumin that lacks the C-terminal leucine residue, likely due to the action of pancreatic carboxypeptidase-A. The des-Leu form of albumin was found to comprise 68% of circulating albumin in patients hospitalized with CP versus 5% in control patients. This form of albumin appears to have a longer serum half-life; however, samples were obtained from patients hospitalized with acute flare-ups of pancreatitis.49 Therefore, studies of albumin and its truncated variants as a potential biomarker for CP should focus on determining specificity for CP in stable outpatients, should include clinically relevant control groups, and need to assess for potential confounding factors due to acute inflammation.
Interleukin 6 (IL-6):
IL-6 is a pro-inflammatory cytokine produced by many cell types, including macrophages and adipocytes. IL-6 levels are often elevated secondary to infection, acute or chronic inflammation, and cancer. Results of IL-6 as a CP biomarker were varied, as shown in Table 1. Heterogeneity may have been introduced due to varying definitions of cases and controls, confounding from acute inflammation (e.g., acute pancreatitis), different detection limits of IL-6 assays, and IL-6 gene polymorphisms.50 Circulating IL-6 levels are also influenced by PDAC and acute alcohol ingestion, so these variables should be considered in future analyses.51, 52
Oxidized fatty acids:
Oxidized fatty acids are generated in response to increased oxidative stress and may also play a role in the pathogenesis of CP. One small study using serum samples from 16 subjects (six with mild CP, five with severe CP, and five controls) and pancreatic fluid samples from 18 subjects (nine with mild CP, nine controls) identified elevated levels of several oxidized fatty acids in patients with mild and severe CP.33 In our analyses, the large effect observed came from findings related to differentiating severe CP from healthy controls, which is not helpful for the diagnosis of early-stage CP. These levels also correlated with the severity of the EUS findings. Large differences between CP and healthy controls were reported for the arachidonic-acid-based 5-HETE:AA, 11-HETE:AA, and 15-HETE:AA. Because this was a small pilot study, a larger validation study would be needed, and it is unclear whether oxidized fatty acids could distinguish early CP and from relevant controls.
As outlined above, several promising CP-biomarker candidates that warrant further investigation were identified in the literature. Some of the biomarkers found to have moderate effect sizes could also be explored further. However, many of the articles we reviewed had methodological limitations that limit the certainty and generalizability of their findings. For example, there was a large variation in the sample size used in each study. Since many of these studies did not provide a sample size power calculation, the actual value of each biomarker for the detection of CP still needs to be determined through validation studies. Clinical definitions of CP varied across most of the studies and often relied on the judgement of the investigators rather than the guidelines of an established professional society.5, 10, 53 Baseline phenotypic definitions and characteristics for the control and CP cases were provided in only a fraction of the studies. In addition, many studies included only limited information regarding the characteristics of the control groups and did not match controls to CP subjects in terms of age and/or gender. Most studies did not indicate the percentage of patients suffering from episodes of acute pancreatitis or the length of time between the most recent episode of acute pancreatitis and the time point of the biospecimen acquisition. The biospecimen collection protocols often did not have a standard operating procedure that included important information such as the time from collection to processing and storage, centrifuge speeds and times, duration of sample storage, or the number of freeze–thaw cycles. In some cases, neither the AUROC nor biomarker diagnostic specificity and sensitivity were reported (see Supplemental Table 1), and in some reports no quantitative data was provided, with results presented in graphical form only. Many of the statistical tests used in the studies reviewed, such as t-tests, compared the mean biomarker levels between cases and controls. However, a difference in the means, regardless of statistical significance, does not necessarily translate into adequate discrimination. Finally, only a few studies reported results from benign-disease control groups that represent important differential diagnoses for early CP, such as patients with abdominal pain, peptic ulcer disease, non-ulcer dyspepsia, and functional bowel disease.
To successfully identify and develop CP biomarkers, best practices and standardized guidelines should be followed regarding study design, sample selection, sample size determination, analytical methods,30, 54 and presentation of results, even in exploratory studies. This will minimize common research biases and reduce the likelihood of false-positive findings in the early phases of biomarker discovery studies. We recommend that researchers clearly state case definitions of CP and control groups studied, using sample sizes of matched disease and controls large enough to adequately power the study and achieve reasonably precise confidence intervals.55, 56 A biomarker’s diagnostic performance should be reported in terms of the sensitivity, specificity, AUROC, and ROC curve. Moreover, data should be presented in both quantitative and graphical formats, ideally depicting median, interquartile range, and outliers.57–59 Biospecimens should be collected and processed following a standard operating procedure and should be well annotated with clinical information. The PRoBE strategy is a useful guide for performing the tasks described above.27–30
Advances are being made in the field of pancreatic disease, in part due to recent NIH-sponsored grants, workshops, and symposia focused on outlining research gaps and defining funding opportunities for innovative investigators and collaborative teams.20, 60–62 In regard to standardizing CP definitions, the following mechanistic definition of CP has been developed by Whitcomb and colleagues: “chronic pancreatitis is a pathologic fibro-inflammatory syndrome of the pancreas in individuals with genetic, environmental, and/or other risk factors who develop persistent pathologic responses to parenchymal injury or stress.”5 With this new definition, early diagnosis may be possible based on a combination of risk factors and selected biomarkers of disease activity and/or progression, once those biomarkers have been validated. Currently, the best method of validating biomarkers for diagnosis of early CP is to prospectively collect biospecimens from a large group of patients with suspected CP who lack definitive imaging or functional findings and to obtain a chronological follow-up of the cohort. This will permit retrospective identification of patients who progressed to unequivocal CP. Testing these subjects’ stored biospecimens can then be performed to identify biomarkers of early disease. Identifying mechanistic dysfunctional pathways can help define or stratify populations in which various biomarkers might have more utility. Thus, utilization of a CP-biomarker test of even moderate accuracy in these risk-stratified groups might markedly enhance diagnostic accuracy, improve the precision of pre- and post-test probabilities of having disease, and free diagnostic testing from relying solely on advanced imaging criteria.5
In regard to standardization in biospecimen collection, the NIH-sponsored PROCEED study27 within the CPDPC is currently collecting biospecimens from patients with known or suspected CP and nonpancreatic-disease controls, utilizing detailed, published standard operating procedures.28 PROCEED is the first prospective, longitudinal observational cohort study of CP in the United States. The study is innovative in several ways: it enrolls subjects representing the complete clinical spectrum of acute to chronic pancreatitis, and it establishes a robust biorepository of longitudinally collected samples consistent with the accepted principles of the PRoBE strategy to support translational studies, including biomarker testing.29, 30 At the time of this writing, PROCEED has enrolled over 1,350 subjects. One of the main goals of PROCEED is to develop a platform for conducting biomarker studies using clinical information and longitudinally collected biospecimens. The detailed phenotyping of the PROCEED cohort, along with its stringent biospecimen collection and handling procedures, addresses many of the methodological limitations and biases that frequently hamper biomarker studies in the current literature. Developing large, well-defined biorepositories like this will allow for robust and efficient validation (Phase 2) and diagnostic (Phase 3) biomarker studies.56
In conclusion, the detection of earlier stages of CP has remained elusive due to a poor understanding of pathogenic mechanisms and the dependence on obvious morphologic changes on radiologic and endoscopic imaging.60 Moreover, numerous methodological issues have hampered the search for CP biomarkers. Recent advances in our understanding of the etiologies, risk factors, genetic alterations, and fibro-inflammatory changes observed in CP has clarified our understanding of “at risk” patient populations and mechanistically defined CP phenotypes.5 The NIH-funded PROCEED study developed by the Chronic Pancreatitis Working Group of the CPDPC has established a robust biorepository of well-annotated CP samples in alignment with the guiding principles of the PRoBE strategy.27, 28 The platform has now been established for the pancreas community to conduct robust investigations in CP-biomarker discovery and validation.
Supplementary Material
Acknowledgements:
Grant Support:
Supported by the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer and by grants from the National Cancer Institute and the National Institute of Diabetes and Digestive and Kidney Diseases under the following award numbers: U01DK108314, Cedars-Sinai Medical Center; U01DK108332 and U01DK108288, Mayo Clinic; U01DK108327, The Ohio State University; U01DK108320, University of Florida; U01DK108306, University of Pittsburgh; and U01DK108328, The University of Texas MD Anderson Cancer Center. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institute of Health, or the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures:
None
References
- 1.Group BDW: Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 2.Anaizi A, Hart PA, Conwell DL: Diagnosing chronic pancreatitis. Dig Dis Sci 2017; 62: 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steer ML, Waxman I, Freedman S: Chronic pancreatitis. N Engl J Med 1995; 332: 1482–1490. [DOI] [PubMed] [Google Scholar]
- 4.Pham A, Forsmark C: Chronic pancreatitis: Review and update of etiology, risk factors, and management. F1000Res 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D et al. : Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology 2016; 16: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etemad B, Whitcomb DC: Chronic pancreatitis: Diagnosis, classification, and new genetic developments. Gastroenterology 2001; 120: 682–707. [DOI] [PubMed] [Google Scholar]
- 7.Sarles H, Adler G, Dani R, Frey C, Gullo L, Harada H et al. : The pancreatitis classification of marseilles-rome 1988. Scand J Gastroenterol 1989; 24: 641–642. [DOI] [PubMed] [Google Scholar]
- 8.Singer MV, Gyr K, Sarles H: Revised classification of pancreatitis. Report of the second international symposium on the classification of pancreatitis in marseille, france, march 28–30, 1984. Gastroenterology 1985; 89: 683–685. [PubMed] [Google Scholar]
- 9.Draganov P, Toskes PP: Chronic pancreatitis: Controversies in etiology, diagnosis and treatment. Rev Esp Enferm Dig 2004; 96: 649–654; 654–649. [DOI] [PubMed] [Google Scholar]
- 10.Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ et al. : American pancreatic association practice guidelines in chronic pancreatitis: Evidence-based report on diagnostic guidelines. Pancreas 2014; 43: 1143–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitcomb DC, Shimosegawa T, Chari ST, Forsmark CE, Frulloni L, Garg P et al. : International consensus statements on early chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the international association of pancreatology, american pancreatic association, japan pancreas society, pancreasfest working group and european pancreatic club. Pancreatology 2018. [DOI] [PMC free article] [PubMed]
- 12.Paulo JA, Kadiyala V, Lee LS, Banks PA, Conwell DL, Steen H: Proteomic analysis (gelc-ms/ms) of epft-collected pancreatic fluid in chronic pancreatitis. Journal of proteome research 2012; 11: 1897–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conwell DL, Wu BU: Chronic pancreatitis: Making the diagnosis. Clin Gastroenterol Hepatol 2012; 10: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 14.Hart PA, Conwell DL: Chronic pancreatitis: Managing a difficult disease. The American journal of gastroenterology 2020; 115: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee LS, Tabak YP, Kadiyala V, Sun X, Suleiman S, Johannes RS et al. : Diagnosis of chronic pancreatitis incorporating endosonographic features, demographics, and behavioral risk. Pancreas 2017; 46: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsmark CE: The early diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol 2008; 6: 1291–1293. [DOI] [PubMed] [Google Scholar]
- 17.Conwell DL, Zuccaro G Jr., Vargo JJ, Trolli PA, Vanlente F, Obuchowski N et al. : An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc 2003; 57: 37–40. [DOI] [PubMed] [Google Scholar]
- 18.Stevens T, Dumot JA, Zuccaro G Jr., Vargo JJ, Parsi MA, Lopez R et al. : Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol 2009; 7: 114–119. [DOI] [PubMed] [Google Scholar]
- 19.Sainani NI, Kadiyala V, Mortele K, Lee L, Suleiman S, Rosenblum J et al. : Evaluation of qualitative magnetic resonance imaging features for diagnosis of chronic pancreatitis. Pancreas 2015; 44: 1280–1289. [DOI] [PubMed] [Google Scholar]
- 20.Forsmark CE, Andersen DK, Farrar JT, Golden M, Habtezion A, Husain SZ et al. : Accelerating the drug delivery pipeline for acute and chronic pancreatitis: Summary of the working group on drug development and trials in chronic pancreatitis at the national institute of diabetes and digestive and kidney diseases workshop. Pancreas 2018; 47: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooshe M, Abdolghaffari AH, Nikfar S, Mahdaviani P, Abdollahi M: Antioxidant therapy in acute, chronic and post-endoscopic retrograde cholangiopancreatography pancreatitis: An updated systematic review and meta-analysis. World journal of gastroenterology 2015; 21: 9189–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neesse A, Ellenrieder V: Nemo-cxcl12/cxcr4 axis: A novel vantage point for antifibrotic therapies in chronic pancreatitis? Gut 2017; 66: 211–212. [DOI] [PubMed] [Google Scholar]
- 23.Bombardo M, Chen R, Malagola E, Saponara E, Hills AP, Graf R et al. : Inhibition of class i histone deacetylases abrogates tumor growth factor beta expression and development of fibrosis during chronic pancreatitis. Mol Pharmacol 2018; 94: 793–801. [DOI] [PubMed] [Google Scholar]
- 24.Kawakubo K, Ohnishi S, Kuwatani M, Sakamoto N: Mesenchymal stem cell therapy for acute and chronic pancreatitis. J Gastroenterol 2018; 53: 1–5. [DOI] [PubMed] [Google Scholar]
- 25.Kelly KA, Hollingsworth MA, Brand RE, Liu CH, Singh VK, Srivastava S et al. : Advances in biomedical imaging, bioengineering, and related technologies for the development of biomarkers of pancreatic disease: Summary of a national institute of diabetes and digestive and kidney diseases and national institute of biomedical imaging and bioengineering workshop. Pancreas 2015; 44: 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano J, Andersen DK, Forsmark CE, Pandol SJ, Feng Z, Srivastava S et al. : Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer: From concept to reality. Pancreas 2018; 47: 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav D, Park WG, Fogel EL, Li L, Chari ST, Feng Z et al. : Prospective evaluation of chronic pancreatitis for epidemiologic and translational studies: Rationale and study design for proceed from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 2018; 47: 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher WE, Cruz-Monserrate Z, McElhany AL, Lesinski GB, Hart PA, Ghosh R et al. : Standard operating procedures for biospecimen collection, processing, and storage: From the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 2018; 47: 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M et al. : Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001; 93: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 30.Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD: Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J Natl Cancer Inst 2008; 100: 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepe M: The statistical evaluation of medical tests for classification and prediction, New York, Oxford University Press, 2003. [Google Scholar]
- 32.Zhou X-h, McClish DK, Obuchowski NA: Statistical methods in diagnostic medicine, Hoboken, N.J., Wiley, 2011. [Google Scholar]
- 33.Stevens T, Berk MP, Lopez R, Chung YM, Zhang R, Parsi MA et al. : Lipidomic profiling of serum and pancreatic fluid in chronic pancreatitis. Pancreas 2012; 41: 518–522. [DOI] [PubMed] [Google Scholar]
- 34.Noh KW, Pungpapong S, Wallace MB, Woodward TA, Raimondo M: Do cytokine concentrations in pancreatic juice predict the presence of pancreatic diseases? Clin Gastroenterol Hepatol 2006; 4: 782–789. [DOI] [PubMed] [Google Scholar]
- 35.Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M: A microrna signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett 2017; 393: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cote GA, Gore AJ, McElyea SD, Heathers LE, Xu H, Sherman S et al. : A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select mirna in plasma and bile. The American journal of gastroenterology 2014; 109: 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng S, Zhu S, Wang B, Li X, Liu Y, Qin Q et al. : Chronic pancreatitis and pancreatic cancer demonstrate active epithelial-mesenchymal transition profile, regulated by mir-217-sirt1 pathway. Cancer Lett 2014; 355: 184–191. [DOI] [PubMed] [Google Scholar]
- 38.Prokopchuk O, Grunwald B, Nitsche U, Jager C, Prokopchuk OL, Schubert EC et al. : Elevated systemic levels of the matrix metalloproteinase inhibitor timp-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer 2018; 18: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manjari KS, Jyothy A, Vidyasagar A, Prabhakar B, Nallari P, Venkateshwari A: Matrix metalloproteinase-9, transforming growth factor-beta1, and tumor necrosis factor-alpha plasma levels in chronic pancreatitis. Indian J Gastroenterol 2013; 32: 103–107. [DOI] [PubMed] [Google Scholar]
- 40.Hocker JR, Postier RG, Li M, Lerner MR, Lightfoot SA, Peyton MD et al. : Discriminating patients with early-stage pancreatic cancer or chronic pancreatitis using serum electrospray mass profiling. Cancer Lett 2015; 359: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulo JA, Kadiyala V, Brizard S, Banks PA, Conwell DL, Steen H: Short gel, long gradient liquid chromatography tandem mass spectrometry to discover urinary biomarkers of chronic pancreatitis. Open Proteomics J 2013; 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komar HM, Hart PA, Cruz-Monserrate Z, Conwell DL, Lesinski GB: Local and systemic expression of immunomodulatory factors in chronic pancreatitis. Pancreas 2017; 46: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lusczek ER, Paulo JA, Saltzman JR, Kadiyala V, Banks PA, Beilman G et al. : Urinary 1h-nmr metabolomics can distinguish pancreatitis patients from healthy controls. Jop 2013; 14: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitt HA: Hepato-pancreato-biliary fat: The good, the bad and the ugly. HPB (Oxford) 2007; 9: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adrych K, Smoczynski M, Stelmanska E, Korczynska J, Goyke E, Swierczynski J: Serum adiponectin and leptin concentrations in patients with chronic pancreatitis of alcoholic and nonalcoholic origin. Pancreas 2008; 36: 120–124. [DOI] [PubMed] [Google Scholar]
- 46.Pezzilli R, Barassi A, Corsi MM, Morselli-Labate AM, Campana D, Casadei R et al. : Serum leptin, but not adiponectin and receptor for advanced glycation end products, is able to distinguish autoimmune pancreatitis from both chronic pancreatitis and pancreatic neoplasms. Scand J Gastroenterol 2010; 45: 93–99. [DOI] [PubMed] [Google Scholar]
- 47.Gasiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Malecka-Panas E: Subclinical inflammation and endothelial dysfunction in patients with chronic pancreatitis and newly diagnosed pancreatic cancer. Dig Dis Sci 2016; 61: 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dranka-Bojarowska D, Lekstan A, Olakowski M, Jablonska B, Lewinski A, Musialski P et al. : The assessment of serum concentration of adiponectin, leptin and serum carbohydrate antigen-19.9 in patients with pancreatic cancer and chronic pancreatitis. J Physiol Pharmacol 2015; 66: 653–663. [PubMed] [Google Scholar]
- 49.Ireland RD, Brennan SO, Gerrard JA, Walmsley TA, George PM, King RI: A mass-spectroscopic method for measuring des-leu albumin--a novel marker for chronic pancreatitis. Clin Biochem 2012; 45: 1664–1668. [DOI] [PubMed] [Google Scholar]
- 50.Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E: Clinical significance of interleukin-6 (il-6) gene polymorphism and il-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig Dis Sci 2009; 54: 683–689. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen N, Larsen S, Seidelin JB, Nielsen OH: Alcohol modulates circulating levels of interleukin-6 and monocyte chemoattractant protein-1 in chronic pancreatitis. Scand J Gastroenterol 2004; 39: 277–282. [DOI] [PubMed] [Google Scholar]
- 52.Hansen M, Nielsen AR, Vilsboll T, Lund A, Krarup T, Knop FK et al. : Increased levels of ykl-40 and interleukin 6 in patients with chronic pancreatitis and secondary diabetes. Pancreas 2012; 41: 1316–1318. [DOI] [PubMed] [Google Scholar]
- 53.Schneider A, Lohr JM, Singer MV: The m-annheim classification of chronic pancreatitis: Introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol 2007; 42: 101–119. [DOI] [PubMed] [Google Scholar]
- 54.Pepe MS, Feng Z: Improving biomarker identification with better designs and reporting. Clin Chem 2011; 57: 1093–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pepe MS, Li CI, Feng Z: Improving the quality of biomarker discovery research: The right samples and enough of them. Cancer Epidemiol Biomarkers Prev 2015; 24: 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng Z, Kagan J, Pepe M, Thornquist M, Ann Rinaudo J, Dahlgren J et al. : The early detection research network’s specimen reference sets: Paving the way for rapid evaluation of potential biomarkers. Clin Chem 2013; 59: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pepe M, Longton G, Janes H: Estimation and comparison of receiver operating characteristic curves. Stata J 2009; 9: 1. [PMC free article] [PubMed] [Google Scholar]
- 58.Buas MF, Li CI, Anderson GL, Pepe MS: Recommendation to use exact p-values in biomarker discovery research in place of approximate p-values. Cancer Epidemiol 2018; 56: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pepe MS, Fan J, Seymour CW: Estimating the receiver operating characteristic curve in studies that match controls to cases on covariates. Acad Radiol 2013; 20: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uc A, Andersen DK, Bellin MD, Bruce JI, Drewes AM, Engelhardt JF et al. : Chronic pancreatitis in the 21st century - research challenges and opportunities: Summary of a national institute of diabetes and digestive and kidney diseases workshop. Pancreas 2016; 45: 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lowe ME, Andersen DK, Caprioli RM, Choudhary J, Cruz-Monserrate Z, Dasyam AK et al. : Precision medicine in pancreatic disease-knowledge gaps and research opportunities: Summary of a national institute of diabetes and digestive and kidney diseases workshop. Pancreas 2019; 48: 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee LS, Andersen DK, Ashida R, Brugge WR, Canto MI, Chang KJ et al. : Endoscopic ultrasound and related technologies for the diagnosis and treatment of pancreatic disease - research gaps and opportunities: Summary of a national institute of diabetes and digestive and kidney diseases workshop. Pancreas 2017; 46: 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaur S, Chakraborty S, Baine MJ, Mallya K, Smith LM, Sasson A et al. : Potentials of plasma ngal and mic-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PLoS One 2013; 8: e55171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamath MG, Pai CG, Kamath A, Kurien A: Monocyte chemoattractant protein-1, transforming growth factor-β1, nerve growth factor, resistin and hyaluronic acid as serum markers: Comparison between recurrent acute and chronic pancreatitis. Hepatobiliary & Pancreatic Diseases International 2016; 15: 209–215. [DOI] [PubMed] [Google Scholar]
- 65.Girish BN, Vaidyanathan K, Rao NA, Rajesh G, Reshmi S, Balakrishnan V: Chronic pancreatitis is associated with hyperhomocysteinemia and derangements in transsulfuration and transmethylation pathways. Pancreas 2010; 39: e11–16. [DOI] [PubMed] [Google Scholar]
- 66.Pan S, Chen R, Crispin DA, May D, Stevens T, McIntosh MW et al. : Protein alterations associated with pancreatic cancer and chronic pancreatitis found in human plasma using global quantitative proteomics profiling. J Proteome Res 2011; 10: 2359–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adrych K, Stojek M, Smoczynski M, Sledzinski T, Sylwia SW, Swierczynski J: Increased serum chemerin concentration in patients with chronic pancreatitis. Dig Liver Dis 2012; 44: 393–397. [DOI] [PubMed] [Google Scholar]
- 68.Mroczko B, Groblewska M, Gryko M, Kedra B, Szmitkowski M: Diagnostic usefulness of serum interleukin 6 (il-6) and c-reactive protein (crp) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal 2010; 24: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manjari KS, Jyothy A, Vidyasagar A, Prabhakar B, Nallari P, Venkateshwari A: Matrix metalloproteinase-9, transforming growth factor-β1, and tumor necrosis factor-α plasma levels in chronic pancreatitis. Indian J Gastroenterol 2013; 32: 103–107. [DOI] [PubMed] [Google Scholar]
- 70.Kozak A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Malecka-Panas E et al. : Utility of different serum fibrosis markers in diagnosing patients with chronic pancreatitis and pancreatic adenocarcinoma. World J Gastrointest Oncol 2016; 8: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yasuda M, Ito T, Oono T, Kawabe K, Kaku T, Igarashi H et al. : Fractalkine and tgf-beta1 levels reflect the severity of chronic pancreatitis in humans. World journal of gastroenterology 2008; 14: 6488–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ceyhan GO, Deucker S, Demir IE, Erkan M, Schmelz M, Bergmann F et al. : Neural fractalkine expression is closely linked to pain and pancreatic neuritis in human chronic pancreatitis. Laboratory investigation; a journal of technical methods and pathology 2009; 89: 347–361. [DOI] [PubMed] [Google Scholar]
- 73.Cavestro GM, Zuppardo RA, Bertolini S, Sereni G, Frulloni L, Okolicsanyi S et al. : Connections between genetics and clinical data: Role of mcp-1, cftr, and spink-1 in the setting of acute, acute recurrent, and chronic pancreatitis. The American journal of gastroenterology 2010; 105: 199–206. [DOI] [PubMed] [Google Scholar]
- 74.Sandstrom A, Andersson R, Segersvard R, Lohr M, Borrebaeck CA, Wingren C: Serum proteome profiling of pancreatitis using recombinant antibody microarrays reveals disease-associated biomarker signatures. Proteomics Clin Appl 2012; 6: 486–496. [DOI] [PubMed] [Google Scholar]
- 75.Sliwinska-Mosson M, Milnerowicz S, Nabzdyk S, Kokot I, Nowak M, Milnerowicz H: The effect of smoking on endothelin-1 in patients with chronic pancreatitis. Applied immunohistochemistry & molecular morphology : AIMM 2015; 23: 288–296. [DOI] [PubMed] [Google Scholar]
- 76.Bamba T, Yoshioka U, Inoue H, Iwasaki Y, Hosoda S: Serum levels of interleukin-1 beta and interleukin-6 in patients with chronic pancreatitis. J Gastroenterol 1994; 29: 314–319. [DOI] [PubMed] [Google Scholar]
- 77.Paulo JA, Lee LS, Banks PA, Steen H, Conwell DL: Difference gel electrophoresis identifies differentially expressed proteins in endoscopically collected pancreatic fluid. Electrophoresis 2011; 32: 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manes G, Spada OA, Rabitti PG, Feola B, Misso S, Minerva A et al. : Neopterin serum levels in pancreatic adenocarcinoma. Int J Pancreatol 1999; 25: 31–37. [DOI] [PubMed] [Google Scholar]
- 79.Shaw VE, Lane B, Jenkinson C, Cox T, Greenhalf W, Halloran CM et al. : Serum cytokine biomarker panels for discriminating pancreatic cancer from benign pancreatic disease. Molecular cancer 2014; 13: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bartel M, Hansch GM, Giese T, Penzel R, Ceyhan G, Ketterer K et al. : Abnormal crosstalk between pancreatic acini and macrophages during the clearance of apoptotic cells in chronic pancreatitis. J Pathol 2008; 215: 195–203. [DOI] [PubMed] [Google Scholar]
- 81.Schneider A, Haas SL, Hildenbrand R, Siegmund S, Reinhard I, Nakovics H et al. : Enhanced expression of interleukin-18 in serum and pancreas of patients with chronic pancreatitis. World journal of gastroenterology 2006; 12: 6507–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Li Y, Tian H, Qi J, Li M, Fu C et al. : Macrophage inhibitory cytokine 1 (mic-1/gdf15) as a novel diagnostic serum biomarker in pancreatic ductal adenocarcinoma. BMC Cancer 2014; 14: 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhingra R, Singh N, Sachdev V, Upadhyay AD, Saraya A: Effect of antioxidant supplementation on surrogate markers of fibrosis in chronic pancreatitis: A randomized, placebo-controlled trial. Pancreas 2013; 42: 589–595. [DOI] [PubMed] [Google Scholar]
- 84.Stojek M, Adrych K, Rojek L, Smoczynski M, Sledzinski T, Szrok S et al. : Decreased serum platelet derived growth factor bb levels in acute and increased in chronic pancreatitis. World journal of gastroenterology 2014; 20: 13127–13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tahara H, Sato K, Yamazaki Y, Ohyama T, Horiguchi N, Hashizume H et al. : Transforming growth factor-alpha activates pancreatic stellate cells and may be involved in matrix metalloproteinase-1 upregulation. Laboratory investigation; a journal of technical methods and pathology 2013; 93: 720–732. [DOI] [PubMed] [Google Scholar]
- 86.Sri Manjari K, Jyothy A, Shravan Kumar P, Prabhakar B, Uma Devi M, Ramanna M et al. : A single-nucleotide polymorphism in tumor necrosis factor-alpha (−308 g/a) as a biomarker in chronic pancreatitis. Gene 2014; 539: 186–189. [DOI] [PubMed] [Google Scholar]
- 87.Berindan-Neagoe I, Burz C, Balacescu O, Balacescu L, Seicean A, Cristea V et al. : Molecular angiogenesis profile as a tool to discriminate chronic pancreatitis (cp) from pancreatic cancer (pc). Pancreas 2011; 40: 482–483. [DOI] [PubMed] [Google Scholar]
- 88.Talar-Wojnarowska R, Gasiorowska A, Olakowski M, Lekstan A, Lampe P, Malecka-Panas E: Clinical value of serum neopterin, tissue polypeptide-specific antigen and ca19–9 levels in differential diagnosis between pancreatic cancer and chronic pancreatitis. Pancreatology 2010; 10: 689–694. [DOI] [PubMed] [Google Scholar]
- 89.Lohr M, Fischer B, Weber H, Emmrich J, Nizze H, Liebe S et al. : Release of hyaluronan and laminin into pancreatic secretions. Digestion 1999; 60: 48–55. [DOI] [PubMed] [Google Scholar]
- 90.Maekawa T, Kamada Y, Ebisutani Y, Ueda M, Hata T, Kawamoto K et al. : Serum mac-2 binding protein is a novel biomarker for chronic pancreatitis. World journal of gastroenterology 2016; 22: 4403–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujiyama T, Ito T, Ueda K, Tachibana Y, Yasunaga K, Miki M et al. : Serum levels of wisteria floribunda agglutinin-positive mac-2 binding protein reflect the severity of chronic pancreatitis. Journal of digestive diseases 2017; 18: 302–308. [DOI] [PubMed] [Google Scholar]
- 92.Grunwald B, Harant V, Schaten S, Fruhschutz M, Spallek R, Hochst B et al. : Pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells via cd63 signaling to create a premetastatic niche in the liver. Gastroenterology 2016; 151: 1011–1024.e1017. [DOI] [PubMed] [Google Scholar]
- 93.Poruk KE, Firpo MA, Scaife CL, Adler DG, Emerson LL, Boucher KM et al. : Serum osteopontin and tissue inhibitor of metalloproteinase 1 as diagnostic and prognostic biomarkers for pancreatic adenocarcinoma. Pancreas 2013; 42: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ni Q, Yun L, Xu R, Shang D: Correlation between blood lipid levels and chronic pancreatitis: A retrospective case-control study of 48 cases. Medicine (Baltimore) 2014; 93: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang L, Lin B: Decreased serum paraoxonase activity in patients with chronic pancreatitis. The American journal of the medical sciences 2013; 346: 363–365. [DOI] [PubMed] [Google Scholar]
- 96.Podborska M, Sevcikova A, Trna J, Dite P, Lojek A, Kubala L: Increased markers of oxidative stress in plasma of patients with chronic pancreatitis. Neuro endocrinology letters 2009; 30 Suppl 1: 116–120. [PubMed] [Google Scholar]
- 97.Zeman M, Macasek J, Burda M, Tvrzicka E, Vecka M, Krechler T et al. : Chronic pancreatitis and the composition of plasma phosphatidylcholine fatty acids. Prostaglandins, leukotrienes, and essential fatty acids 2016; 108: 38–44. [DOI] [PubMed] [Google Scholar]
- 98.Ueda M, Kamada Y, Takamatsu S, Shimomura M, Maekawa T, Sobajima T et al. : Specific increase in serum core-fucosylated haptoglobin in patients with chronic pancreatitis. Pancreatology 2016; 16: 238–243. [DOI] [PubMed] [Google Scholar]
- 99.Sri Manjari K, Krishnaveni D, Vidyasagar A, Prabhakar B, Jyothy A, Nallari P et al. : Role of matrix metalloproteinase 3 gene promoter polymorphism in chronic pancreatitis. Indian J Gastroenterol 2011; 30: 217–220. [DOI] [PubMed] [Google Scholar]
- 100.Manjari KS, Jyothy A, Kumar PS, Prabhakar B, Nallari P, Venkateshwari A: Association of matrix metalloproteinase-7 (−181a/g) promoter polymorphism in chronic pancreatitis. Indian J Med Res 2014; 140: 609–615. [PMC free article] [PubMed] [Google Scholar]
- 101.Joshi GK, Deitz-McElyea S, Liyanage T, Lawrence K, Mali S, Sardar R et al. : Label-free nanoplasmonic-based short noncoding rna sensing at attomolar concentrations allows for quantitative and highly specific assay of microrna-10b in biological fluids and circulating exosomes. ACS nano 2015; 9: 11075–11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun B, Liu X, Gao Y, Li L, Dong Z: Downregulation of mir-124 predicts poor prognosis in pancreatic ductal adenocarcinoma patients. British journal of biomedical science 2016; 73: 152–157. [DOI] [PubMed] [Google Scholar]
- 103.Bauer AS, Keller A, Costello E, Greenhalf W, Bier M, Borries A et al. : Diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis by measurement of microrna abundance in blood and tissue. PLoS One 2012; 7: e34151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshimatsu G, Takita M, Kanak MA, Haque WZ, Chang C, Saravanan PB et al. : Mir-375 and mir-200c as predictive biomarkers of islet isolation and transplantation in total pancreatectomy with islet autotransplantation. Journal of hepato-biliary-pancreatic sciences 2016; 23: 585–594. [DOI] [PubMed] [Google Scholar]
- 105.Deng T, Yuan Y, Zhang C, Zhang C, Yao W, Wang C et al. : Identification of circulating mir-25 as a potential biomarker for pancreatic cancer diagnosis. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2016; 39: 1716–1722. [DOI] [PubMed] [Google Scholar]
- 106.Hardt PD, Ewald N, Brockling K, Tanaka S, Endo T, Kloer HU et al. : Distinct autoantibodies against exocrine pancreatic antigens in european patients with type 1 diabetes mellitus and non-alcoholic chronic pancreatitis. Jop 2008; 9: 683–689. [PubMed] [Google Scholar]
- 107.Talukdar R, Sasikala M, Pavan Kumar P, Rao GV, Pradeep R, Reddy DN: T-helper cell-mediated islet inflammation contributes to beta-cell dysfunction in chronic pancreatitis. Pancreas 2016; 45: 434–442. [DOI] [PubMed] [Google Scholar]
- 108.Liao WC, Wu MS, Wang HP, Tien YW, Lin JT: Serum heat shock protein 27 is increased in chronic pancreatitis and pancreatic carcinoma. Pancreas 2009; 38: 422–426. [DOI] [PubMed] [Google Scholar]
- 109.Mascetta G, di Mola FF, Tavano F, Selvaggi F, Giese N, Bassi C et al. : Substance p and neprilysin in chronic pancreatitis. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes 2012; 48: 131–138. [DOI] [PubMed] [Google Scholar]
- 110.Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D et al. : Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012; 61: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanck C, Rossol S, Singer MV: Immunological changes of mild acute pancreatitis in late-stage alcoholic chronic pancreatitis. Dig Dis Sci 1999; 44: 1768–1773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


