Abstract
SARS-CoV-2, a member of the family coronaviridae, has triggered a lethal pandemic termed coronavirus disease 2019 (COVID-19). Pediatric patients, mainly from families with a cluster of infection or a history of exposure to epidemic areas, get infected via direct contacts or air-borne droplets. Children (aged below 18 years) are susceptible to COVID-19, with an average incubation period of about 6.5 days. Most cases present asymptomatic or common cold symptoms such as fever, cough, and myalgia or fatigue, which is milder than adult patients. Besides, most abnormal laboratory and radiologic findings in children with COVID-19 are non-specific. Since no specific chemotherapeutic agents have been approved for children, timely preventive methods could effectively forestall the transmission of SARS-CoV-2. To date, mostly studied cases have been adults with COVID-19, whereas data on pediatrics patients remain poorly defined. We herein conducted a literature review for papers published in PubMed and medRxiv (preprints) between December 2019 and December 2020 that reported on pediatrics patients (aged below 18 years) with a confirmed COVID-19 diagnosis. In this review, we summarized and discussed the pathogenesis, epidemiology, and clinical management of COVID-19 in pediatrics patients to improve our understanding of this new disease in children.
Keywords: pediatrics, COVID-19, SARS-C0V-2, infection, characteristics, children
Introduction
In December 2019, a new type of pneumonia of unknown etiology quickly spread. An unknown beta-CoVs was detected by unbiased sequencing in the samples from patients with new pneumonia (1), which was later termed SARS-CoV-2 by the International Committee on Taxonomy of Viruses (ICTV) based on its close relationship with SARS-CoV. Both SARS-CoV and MERS-CoV belong to beta-CoVs and share 79.6 and 50% identity with SARS-CoV-2 (2, 3). Currently, COVID-19 has spread widely around the world, affecting more than 200 countries and territories. The population of all ages is susceptible to COVID-19 via respiratory tract infection or direct contact due to the absence of specific immunity (4).
Even though the overall mortality for children is about 1% and severe complications are less likely to occur, the possibility of children being infected is the same as that of adults (5). The difference in disease development between children and adults may lead to distinct clinical management. Therefore, we conducted a literature review for papers published in PubMed and medRxiv (preprints) between December 2019 and December 2020 that reported on pediatrics patients (aged below 18 years) with a confirmed COVID-19 diagnosis. In this review, we focus on the pathogenesis, epidemiologic features, clinical symptoms, diagnostic criteria, and prevention methods of children with COVID-19, and expect to provide systematic understanding and new insight into the diagnosis as well as management of children infected with COVID-19.
Pathogenesis
SARS-CoV-2 enters cells by binding to the receptor, angiotensin-converting enzyme 2 (ACE2) through S protein (6). ACE2 is highly expressed in alveolar epithelium, heart, renal tubules and intestinal epithelial cells (7–9). Severe complications of COVID-19, such as multiple organ dysfunction syndromes (MODS) and acute respiratory distress syndrome (ARDS), might be caused by dysregulated immune response and cytokine storms (10–12). Recently, many severe cases presenting persistent fever and the involvement of two or more organ systems in children with COVID-19 have been reported, which is termed as multisystem inflammatory syndrome (MIS-C). The symptoms of MIS-C are similar to Kawasaki Disease (KD) and toxic shock-like syndrome (13). MIS-C was firstly reported in Europe (13, 14). Pediatrics patients diagnosed with MIS-C are less common in Asia countries than in Europe, with only one case in Korea now (15). Some studies hypothesized that the pathogenesis of MIS-C might be attributable to genomic variation of virus and post-infectious immune dysregulation (16). Recently, immunophenotype studies have demonstrated that some MIC-S patients have a peculiar B cell response with an increase of plasmablast (PB) proportion (17, 18). However, the specific pathological process is still unclear, and further studies evaluating the etiopathogenesis of MIS-C are essential for developing the treatment strategies.
Epidemiologic Features
Children of all ages are susceptible to COVID-19 (19). Despite the higher incidence of COVID-19 in older children, infants (<1 year) seem to be the most vulnerable due to a high hospitalization rate (20). The average incubation period for COVID-19 in children is about 6.5 days, which is longer than the 5.4 days reported in adults (21). There is no significant gender difference in pediatric patients (19). Of the 2,135 pediatric patients, 94.1% of cases were either asymptomatic or diagnosed with a mild to moderate symptom, only 5.8% had developed severe complications compared with 18.5% in adult patients (19). Several factors could account for severe outcomes among children with COVID-19 infection, such as immunocompromised condition, pulmonary pathology, the age of infants (<3 months) and patients' underlying diseases, including asthma and obesity (22).
Even though the symptoms of pediatric patients are mild, children are as susceptible as adults. Recent virologic data indicated that viral load in the asymptomatic patient was not significantly different from that in the symptomatic patients, which suggested asymptomatic patients could be the potential source of COVID-19 infection (23, 24). In addition, nasopharyngeal SARS-CoV-2 viral loads in infected children were similar to those in other age groups, indicating that children were at a similar risk of infection to adults (25). However, all data suggest that SARS-CoV-2 transmission from children to adults or other children is infrequent (26). The incidence rate of COVID-19, though low in pediatric patients, varies from study to study in different countries (Table 1). The variation of incidence rate is probably due to the testing policy and testing availability. According to the report from the Centers for Disease Control and Prevention (CDC) in the United States, SARS-CoV-2 pediatrics patients (<18 years) have accounted for 10.2% of all reported cases by December 14, 2020 (39). However, mortality in infected children is <1% (39).
Table 1.
| Characteristics | ||||||
|---|---|---|---|---|---|---|
| Region | USA | China | Italy | Spain | Iran | Mexico |
| Incidence (%) | 1.7 | 12.3 | NA | 16.6 | NA | 1.5 |
| Number of patients | 2,572 | 171 | 100 | 58 | 30 | 51 |
| Median age (range), yr | 11 (0–17) | 6.7 (1 day−15 yr) | 3.3 (0–17.5) | 2.9 (3.3 months−12.2 yr) | 5.5 (1 day−15 yr) | 10 |
| Age distribution, No. (%) | ||||||
| <1 yr | 398 (15.5) | 31 (18.1) | 40 (40.0) | NA | 6 (20.0) | 8 (15.7) |
| 1 to <6 yr | NA | 40 (23.4) | 15 (15.0) | NA | 11 (36.7) | 8 (15.7) |
| 6–10 yr | NA | 58 (33.9) | 21 (21.0) | NA | NA | NA |
| >10 yr | NA | 42 (24.6) | 24 (24.0) | NA | NA | NA |
| Male-No./total No. (%) | 1,408/2,490 (56.5) | 104/171 (60.8) | 57/100 (57.0) | 37/58 (63.8) | 14/30 (46.7) | 26/51 (51.0) |
| Coexisting conditions-No./total No. (%) | 80/345 (23.2) | NA | 27/100 (27.0) | 23/58 (39.7) | 7/30 (23.3) | 8/51 (15.7) |
| Exposure to SARS-COV-2-No./total No. (%) | ||||||
| Family cluster | 168/184 (91.3) | 131/171 (76.6) | 45/100 (45.0) | 30/58 (51.7) | NA | NA |
| Other exposure | 16/184 (8.7) | 2/171 (1.2) | 48/100 (48.0) | NA | NA | NA |
| Unknown exposure | 0 | 15/171 (8.8) | 7/100 (7.0) | NA | 19/30 (63.3) | 18/51 (35.3) |
| Survived-No./total No. (%) | 2,569/2,572 (99.9) | 170/171 (99.4) | 100/100 (100.0) | 57/58 (98.3) | 30/30 (100.0) | 48/51 (94.1) |
| Symptoms-No./total No. (%) | ||||||
| Fever | 163/291 (56.0) | 71/171 (41.5) | 54/100 (54.0) | 41/58 (70.7) | 23/30 (76.7) | 40/51 (78.4) |
| Temperature | ||||||
| ≤37.5°C | 128/291 (44.0) | 100/171 (58.5) | 46/100 (46.0) | NA | NA | NA |
| 37.6-38.0°C | NA | 16/171 (9.4) | 15/100 (15.0) | NA | NA | NA |
| 38.1–39.0°C | NA | 39/171 (22.8) | 28/100 (28.0) | NA | NA | NA |
| >39.0°C | NA | 16/171 (9.4) | 11/100 (11.0) | NA | NA | NA |
| Cough | 158/291 (54.3) | 83/171 (48.5) | 44/100 (44.0) | 42/58 (72.4) | 16/30 (53.3) | 34/51 (66.7) |
| Diarrhea | 37/291 (12.7) | 15/171 (8.8) | 9/100 (9.0) | 7/58 (12.1) | 3/30 (10.0) | 7/51 (13.7) |
| Rhinorrhea | 21/291 (7.2) | 13/171 (7.6) | 22/100 (22.0) | 33/58 (56.9) | 0 | 10/50 (20.0) |
| Fatigue | NA | 13/171 (7.6) | 9/100 (9.0) | NA | NA | NA |
| Shortness of breath | 39/291 (13.4) | NA | 11/100 (11.0) | 10/58 (17.2) | NA | NA |
| Sore throat | 71/291 (24.4) | NA | 4/100 (4.0) | 4/58 (6.9) | 6/30 (20.0) | 10/49 (20.4) |
| Nausea/Vomiting | 31/291 (10.6) | NA | 10/100 (10.0) | 9/58 (15.5) | 8/30 (26.7) | 5/49 (10.2) |
| Abdominal pain | 17/291 (5.8) | NA | 4/100 (4.0) | NA | NA | NA |
| Headache | 81/291 (27.8) | NA | 4/100 (4.0) | 8/58 (13.8) | NA | 28/49 (57.1) |
| Myalgia | 66/291 (22.7) | NA | NA | 2/58 (3.4) | NA | NA |
| Runny nose | 21/291 (7.2) | NA | NA | NA | 0 | NA |
| Region | Argentina | Brazil | Ethiopia | Perú | UK | Korea |
| Incidence (%) | NA | 19.1 | NA | 19.9 | 0.8 | NA |
| Number of patients | 578 | 66 | 90 | 91 | 451 | 91 |
| Median age (range), yr | 4.2 (0.7–11.2) | 7.0 (24 day−18 yr) | 15 (6 months−18 yr) | 6 (3–10) | 3.9 (0.3–12.9) | 11 (0.07–18) |
| Age distribution, No. (%) | ||||||
| <1 yr | NA | 13 (19.7) | NA | NA | 162 (35.9) | 6 (6.6) |
| 1 to <6 yr | NA | NA | NA | NA | NA | 13 (14.3) |
| 6–10 yr | NA | NA | 16 (17.8) | NA | NA | 23 (25.3) |
| >10 yr | NA | NA | 64 (71.1) | NA | 150 (33.2) | 49 (53.8) |
| Male-No./total No. (%) | 315/578 (54.5) | 44/66 (66.7) | 33/90 (36.7) | 58/91 (63.7) | 256/450 (56.9) | 53/91 (58.2) |
| 49/91 (53.8) | ||||||
| Coexisting conditions-No./total No. (%) | 204/578 (35.3) | 50/66 (75.8) | 3/90 (3.3) | 195/451 (43.2) | 6/91 (6.6) | |
| Exposure to SARS-COV-2-No./total No. (%) | ||||||
| Family cluster | NA | NA | NA | 24/91 (26.4) | NA | 57/91 (62.6) |
| Other exposure | NA | NA | NA | 4/91 (4.4) | NA | 30/91 (33.0) |
| Unknown exposure | 156/578 (27.0) | 39/66 (59.0) | 49/90 (54.4) | 63/91 (69.2) | NA | 4/91 (4.4) |
| Survived-No./total No. (%) | 577/578 (99.8) | 65/66 (98.5) | 90/90 (100.0) | 82/91 (90.1) | 448/451 (99.3) | 91/91 (100.0) |
| Symptoms-No./total No. (%) | ||||||
| Fever | 207/400 (51.7) | 37/66 (56.1) | 5/90 (5.6) | 36/91 (39.6) | 306/418 (73.2) | 62/91 (68.1) |
| Temperature | ||||||
| ≤37.5°C | NA | NA | NA | NA | NA | NA |
| 37.6−38.0°C | NA | NA | NA | NA | NA | 35/91 (38.5) |
| 38.1–39.0°C | NA | NA | NA | NA | NA | NA |
| >39.0°C | NA | NA | NA | NA | NA | NA |
| Cough | 40/400 (10.0) | 23/66 (34.8) | 20/90 (22.2) | 18/91 (19.8) | 175/431 (40.6) | 37/90 (41.1) |
| Diarrhea | NA | NA | NA | NA | 58/431 (13.4) | 11/90 (12.2) |
| Rhinorrhea | 39/400 (9.7) | NA | NA | NA | NA | 24/90 (26.7) |
| Fatigue | NA | NA | 5/90 (5.6) | NA | 103/431 (23.9) | 5/89 (5.6) |
| Shortness of breath | NA | 10/66 (15.2) | NA | 12/91 (13.2) | 124/389 (31.9) | 1/77 (1.3) |
| Sore throat | 49/400 (12.2) | NA | 9/90 (10.0) | NA | 40/431 (9.3) | 22/77 (28.6) |
| Nausea/Vomiting | NA | NA | 4/90 (4.4) | 11/91 (12.1) | 120/380 (31.6) | 6/90 (6.7) |
| Abdominal pain | 15/400 (3.7) | NA | NA | NA | 66/431 (15.3) | 6/77 (7.8) |
| Headache | 37/400 (9.2) | NA | 9/90 (10.0) | NA | 43/431 (10.0) | 12/77 (15.6) |
| Myalgia | NA | NA | NA | NA | 28/431 (6.5) | 7/77 (9.1) |
| Runny nose | NA | NA | 5/90 (5.6) | NA | 61/431 (14.1) | NA |
Different from the various transmission routes of adults, pediatric patients get infected mainly from families with a cluster of infection or a history of exposure to epidemic areas (40). On January 11, 2020, in Shenzhen, Guangdong, China, one asymptomatic child (aged 10 years) was confirmed, whose parents and grandparents suffered COVID-19 earlier (4). Although schools and universities have been fully reopened, few infected cases were identified, suggesting that children play a potentially minor role in SARS-CoV-2 transmission within schools and beyond (41). On the other hand, Kang Zhang et al. reported that real-time polymerase chain reaction (RT-PCR) results in rectal swabs were persistently positive even after nasopharyngeal swabs turned negative (42). Therefore, they hypothesized the fecal-oral transmission and doubted whether children could facilitate it if they were not toilet trained. ACE2 is highly expressed in stratified epithelial cells of upper esophageal and intestinal epithelial cells in the ileum and colon (9). And the fecal-oral transmission does exist with other respiratory viruses (43). Although fecal-oral transmission has not been confirmed, it cannot be ruled out.
The reason for the lower infection rate among children might be the closure of schools and kindergartens reduces the exposure of children to the virus, and children are not tested for SARS-CoV-2 as frequently as adults due to mild or absent symptoms. For example, in a study of children (aged below 22 years) tested for SARS-CoV-2 at a community, the result showed that 28.2% had a positive PCR test, much higher than the reported incidence of COVID-19 in pediatric patients (44).
Clinical Presentation
The clinical manifestations of pediatric patients are mostly mild and non-specific. Common symptoms among these early confirmed patients included fever, cough, and myalgia or fatigue (45–47). The median duration of fever in children lasts 3 days compared to 10 days in adults (48). A few children have upper respiratory symptoms, such as nasal congestion, sore throat and runny nose (4, 46). Specifically, gastrointestinal symptoms could be initial symptoms in some cases, including nausea, vomiting, diarrhea and abdominal pain, and these pediatric patients are more likely to develop the more severe clinical condition (49, 50). And gastrointestinal symptoms are twice as common in children as in adults (51, 52). Some infected newborns may present only low spirits, loss of appetite, and shortness of breath (22, 53). Severe pediatric cases show dyspnea and cyanosis and may advance to ARDS, septic shock, refractory metabolic acidosis, MODS, and coagulation dysfunction (21, 50). According to one analysis from China, younger children, especially infants and pre-school children are more susceptible to severe symptoms (10.6% <1-year-old vs. 3%≥16 years old) (19). The potential explanation is the immaturity of the immune system in infants and pre-school children. Recently, Kawasaki-like disease was reported among children with COVID-19 (13). This disease is also named pediatric inflammatory multisystem syndrome (PIMS) or MIS-C due to its clinical manifestation associated with multisystem inflammation such as conjunctivitis, myocarditis, meningitis and coronary vessel inflammation (13, 54). Feldstein et al. reported that 33% of the diagnosed infected children had Kawasaki-like clinical symptoms (55). Intriguingly, a 14-year-old boy diagnosed and treated for orchiepididymitis, however, confirmed COVID-19 infection without respiratory symptoms, suggesting the possibility of testicular involvement in COVID-19 (56). Different clinical features of pediatrics patients among countries are summarized in Table 1.
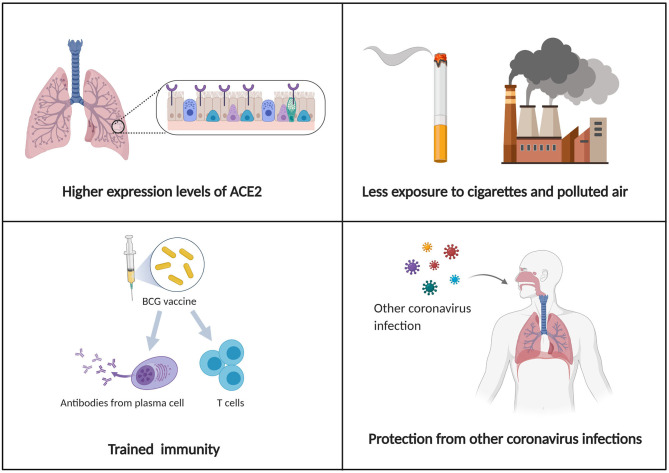
The reason why children have milder symptoms than adults remains unclear. According to recent research, several factors are worth considering (Figure 1). The first reason is the higher expression level of ACE2 in children. According to the analysis from China, older adults (>50 years) who were more likely to develop into serious pneumonia, presented decreased expression of ACE2 when compared to children. This may impact disease severity and recovery from pneumonia caused by SARS-CoV-2 infection in older patients (57). The second possibility is trained immunity, which means training innate immunity to generate immune memory for non-specific immune protection (58). Thirdly, the difference in innate and adaptive immunity between adults and children should also be taken into account. And the antibodies following other coronavirus infections may play a protective role in SARS-CoV-2 infection (59). Finally, compared with the elderly, children had no underlying diseases but a healthy respiratory tract without exposure to cigarettes and polluted air.
Figure 1.
Schematic illustration of possible mechanisms for explaining why children have milder symptoms and a better prognosis than adults.
Laboratory and Radiologic Examinations
Most abnormal laboratory findings in children with COVID-19 are non-specific. The white blood cell count is typically normal or reduced with decreased lymphocyte and/or neutrophil counts (27, 60). The levels of C-reactive protein and procalcitonin (PCT) can be normal or elevated. However, children have a much lower prevalence of increased C-reactive protein than adults, suggesting a much milder immunological response and less immune damage (61). The best markers for diagnosing the severity of the disease in children are the levels of bilirubin and hepatic enzymes (62, 63). During April 2020, a surge of PIMS cases presenting a hyper-inflammatory state including elevated levels of C-reactive protein, PCT, ferritin, and D-dimers, as well as markers of myocarditis (13).
RT-PCR is the most common to detect SARS-CoV-2 nucleic acid. Since the virus exists in serum, urine, stool, upper, and lower respiratory tract specimens, nasopharyngeal and oropharyngeal swabs, bronchoalveolar lavage, or tracheal aspirates might be helpful (4, 64). The result from Liu et al. showed that the median viral shedding duration detected in nasopharyngeal swabs, oropharyngeal swabs, and stools were 13, 4, and 43 days, respectively, suggesting the possibility of fecal-oral transmission (65). Neutralizing antibody (NAb) response is also helpful for the diagnosis. However, negative antibody tests cannot exclude COVID-19 as it needs a certain time period for the body to produce serum-specific antibodies after infection (65).
The sensitivity of chest X-ray might be lower than that of computed tomography (CT) scan. Infected pediatric patients do not commonly present abnormalities in chest X-ray at the early stage, with occasional interstitial changes (66). Chest CT abnormalities observed in pediatric patients include unilateral or bilateral multiple patchy shadows, nodular ground-glass opacities (GGO) or consolidations with a surrounding halo sign (67). The sensitivity of chest CT in diagnosing COVID-19 is greater than that of RT-PCR (98 vs. 71%) (68). In addition, lung ultrasound (LUS) is also used as a diagnostic tool. Recent research from Italy indicated LUS abnormalities including subpleural consolidations and confluent B-lines in eight children with COVID-19 infection (69).
Diagnosis
The criteria for COVID-19 diagnosis in children are based on epidemiology, clinical manifestations, and laboratory testing to confirm SARS-CoV-2 infection. The case definition and clinical classification of children are summarized in Table 2. Also, co-infections such as mycoplasma, influenza A and B, respiratory syncytial virus, Epstein-Barr virus, cytomegalovirus, parainfluenza, and adenovirus should take into consideration in diagnosis since co-infections rate are up to 79% in children (67).
Table 2.
Case definition and clinical classification of children from the Chinese updated consensus statement (March 24, 2020) (22).
| CASE DEFINITION |
| A suspected case is defined as a case that meets: one epidemiologic criterion and two clinical criteria |
|
Epidemiologic criteria:
1. Children with a travel or residence history in a community with infected cases reported in China or a country or region with a serious epidemic within 14 days prior to disease onset (with the global pandemic of COVID-19, imported cases deserve attention) 2. Children with a history of contacting patients infected with SARS-CoV-2 within 14 days prior to disease onset 3. Children with a history of contacting patients with fever or respiratory symptoms from communities with reported cases in China or countries or regions with serious epidemic within 14 days prior to disease onset 4. Clustered cases: two or more cases with fever and/or respiratory symptoms within 14 days in small groups (such as family members, school classmates, etc.) 5. Newborns delivered by mothers with confirmed infection. |
|
Clinical criteria:
1. Fever, fatigue, dry cough, and/or other respiratory symptoms; some pediatric patients may have low-grade fever or no fever 2. Patients with the following chest imaging findings: single or multiple localized ground-glass opacities in the form of light cloud or fine mesh, with thickened blood vessels shadows inside the lesions; localized consolidation, located under the pleura or near the bronchial blood vessel bundles, most in the bilateral lower lobes of the periphery of the subpleural lung; increased ground-glass shadows; large-scale consolidation; diffused consolidation of unilateral or bilateral lungs, with ground-glass opacities, bronchial inflation signs 3. In the early phase of the disease, white blood cell count is normal or decreased, or with decreased lymphocytes count 4. No other pathogens are detected which can fully explain the clinical manifestations. |
|
A confirmed case is defined as a case that meets any of the following criteria:
1. Testing positive for SARS-CoV-2 by real-time PCR 2. Genetic sequencing of respiratory tract or blood samples is highly homologous with the known SARS-CoV-2 3. Both serum-specific antibodies IgM and IgG are positive 4. Serum-specific antibody IgG changed from negative to positive or increased 4-folds or higher than that in the acute phase during the recovery period. |
| CLINICAL CLASSIFICATION |
| 1. Asymptomatic infection (silent infection) Testing positive for SARS-CoV-2, but without clinical symptoms or abnormal chest imaging findings 2. Acute upper respiratory tract infection With only fever, cough, pharyngeal pain, nasal congestion, fatigue, headache, myalgia or discomfort, etc., and without signs of pneumonia by chest imaging or sepsis 3. Mild pneumonia With or without fever, with respiratory symptoms such as cough; and chest imaging indicating changes of viral pneumonia, but not reaching the criteria of severe pneumonia 4. Severe pneumonia a. Polypnea: ≥60 times/min (<2 months), ≥50 times/ min (2–12 months), ≥40 times/min (1–5 years), ≥30 times/min (>5 years) (after ruling out the effects of fever and crying) b. Oxygen saturation <92% under a resting state c. Dyspnea: assisted breathing (moans, nasal flaring, and three concave sign), cyanosis, intermittent apnea d. Disturbance of consciousness: somnolence, coma, or convulsion e. Food refusal or feeding difficulty, with signs of dehydration f. Pulmonary high-resolution CT (HRCT) examination showing bilateral or multi-lobe infiltrates, rapid progression of disease in a short period or with pleural effusion. 5. Critical cases (require ICU care) a. Respiratory failure requiring mechanical ventilation b. Shock c. Combined with other organs failure. |
Therapy and Prevention
Until now, no concrete evidence has demonstrated the effectiveness and safety of specific drugs against COVID-19. Children with mild or absent symptoms should be isolated at home for 2 weeks. Severe cases should be admitted to the pediatric intensive care unit (PICU) as soon as possible. Antiviral drugs targeting specific sites on different stages could effectively inhibit the virus replication in the host cells. However, their efficacy and safety remain to be determined. Antibiotics and antifungal drugs can be used only in patients with secondary bacterial infections based on the culture and antibiogram results. Given the immunomodulatory effect, corticosteroids are critical for the treatment of inflammatory and immune diseases. However, the modulatory property sometimes might lead to immune suppression that hinders the virus clearance in the host. Therefore, corticosteroids should be avoided, except when required for other indications such as MIS-C, refractory shock, or asthma exacerbation. Evidence showed that the adjunctive steroid treatment was effective and safe in pediatric patients with Kawasaki-like presentations. However, they showed resistance to intravenous immunoglobulin (IVIG) (13). In November 2020, the US Food and Drug Administration (FDA) provided Emergency Use Authorizations (EUA) for two novel virus-neutralizing monoclonal antibodies (mAbs) for the treatment of mild to moderate COVID-19 in adolescents and adults in specified high-risk groups. However, the safety and efficacy of mAbs for the COVID-19 treatment among children or adolescents remains unclear (70). Most pediatric patients with COVID-19 present a good prognosis and usually recover within 1–2 weeks. Despite a higher hospitalization rate of infants, children are rarely admitted to intensive care units (ICU) in general (71).
Emergent health conditions all over the world accelerate vaccine development, clinical testing, and usage. The vaccine currently available mainly falls into six categories: inactivated virus, live attenuated virus, nucleic acid-based vaccines, protein subunit vaccines, virus-like particles (VLPs), and recombinant viral vectors (72). Until January 21, 2021, 64 vaccine candidates have been under clinical development while 10 vaccines (inactivated vaccines, RNA-based vaccines, and non-replicating viral vectors) have been adopted for “emergent use” in America, Canada, China, Russia, Brazil, etc. (73, 74).
Variability of host immune responses among populations, production of secreted IgA antibodies on the mucosal surface in the upper respiratory tract, and T-cell involvement in the immune response all play roles in eliciting successful protection against SARS-CoV-2 (75). An increasing number of clinical trials have demonstrated the safety and immunogenicity of SARS-CoV-2 vaccines among people ranging from 18 to 55 years old, with a low incidence of side effects such as fatigue and headache (76–78). However, lacking data concerning the safety and response rate in licensed clinical trials, vaccine efficacy in children remains unclear. It is noteworthy that Pfizer recently has included children aged from 12 to 17 into clinical trials of mRNA vaccine, which might feedback the assessment of its effectiveness shortly (79). Additionally, the occurrence of rare severe side effects such as vaccine-associated enhanced disease and Pediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS) suggests that researchers should further explore the different immunopathogenesis of COVID-19 between children and adults (80, 81). Vaccine-associated enhanced disease refers to severe side effects related to worse clinical outcomes after vaccination vs. without vaccination (80). In the 1960s, due to atypical measles such as fever and pneumonia observed in children after formalin-inactivated measles virus vaccination, the vaccine was prohibited (82). Despite the absence of compelling evidence regarding VAED in both animal models and human beings (80), additional attention to such rare adverse immune responses could guarantee the overall safety of populations administrated vaccines. Finally, reinfection interval or the longest possible immunity is also worth noticing since it helps indicate the optimal age for vaccination, thus providing better protective strategies for children against COVID-19 (83).
Since no specific drugs against COVID-19, it is crucial to prevent the amplification of the outbreak. Early detection of children, a possible hidden source could effectively prevent the outbreak in kindergartens or schools, especially for children who tend to develop atypical clinical characteristics but with a high virus load. Once confirmed with COVID-19 infection, the children must be quarantined at home or in the hospital to prevent close contact and human-to-human transmission. Social distance and washing hands frequently are crucial to prevent COVID-19 spread. In addition, consistent use of the mask is necessary, except for children under age two or anyone who has trouble removing it on his own. Besides, children should maintain good moods, exercise regularly and have a balanced diet to enhance their immunity (22).
Conclusions
This review summarized the updated evidence regarding the epidemiology and clinical management of COVID-19 in children. Even though most pediatric patients with COVID-19 present mild symptoms and good prognosis, children are as susceptible as adults. Besides, an increasing number of COVID-19 pediatric patients with MIS-C have been reported. Further study is of paramount importance for better prevention, diagnosis and treatment of COVID-19 in children worldwide.
Author Contributions
XH and XL were major writers of the manuscript. YX and RY designed the tables and edited the manuscript. YW researched appropriate references and reviewed the manuscript. XW developed the structure of the article, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (Grant Number 81821002).
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. (2020) 20:911–9. 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song R, Preston G, Yosypiv IV. Ontogeny of angiotensin-converting enzyme 2. Pediatr Res. (2012) 71:13–9. 10.1038/pr.2011.7 [DOI] [PubMed] [Google Scholar]
- 8.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci. (2004) 61:2738–43. 10.1007/s00018-004-4242-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Y, Rong L. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. (2020) 51:843–51. 10.1111/apt.15731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Files JK, Boppana S, Perez MD, Sarkar S, Lowman KE, Qin K, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest. (2021) 131:e140491. 10.1172/JCI140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Wang G, Tan J, Cao Y, Long X, Luo H, et al. Scoring cytokine storm by the levels of MCP-3 and IL-8 accurately distinguished COVID-19 patients with high mortality. Signal Transduct Target Ther. (2020) 5:292. 10.1038/s41392-020-00433-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. (2021) 11:316–329. 10.7150/thno.49713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 395:1771–8. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Shim JY. Multisystem inflammatory syndrome in children related to COVID-19: the first case in Korea. J Korean Med Sci. (2020) 35:e391. 10.3346/jkms.2020.35.e391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-System Inflammatory Syndrome in Children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. (2020) 7:69. 10.3390/children7070069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathew D, Giles JR. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. (2020) 369:eabc8511. 10.1126/science.369.6508.1203-l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter MJ, Fish M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. (2020) 26:1701–7. 10.1038/s41591-020-1054-6 [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. (2020) 145:e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 20.Rankin DA, Talj R, Howard LM, Halasa NB. Epidemiologic trends and characteristics of SARS-CoV-2 infections among children in the United States. Curr Opin Pediatr. (2021) 33:114–21. 10.1097/MOP.0000000000000971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen KL, Yang YH, Jiang RM, Wang TY, Zhao DC, Jiang Y, et al. Updated diagnosis, treatment and prevention of COVID-19 in children: experts' consensus statement (condensed version of the second edition). World J Pediatr. (2020) 16:232–9. 10.1007/s12519-020-00362-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. (2020) 382:1177–9. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. (2020) 581:465–9. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 25.Jones TC, Mühlemann B, Veith T, Biele G, Zuchowski M, Hofmann J, et al. An analysis of SARS-CoV-2 viral load by patient age. medRxiv. (2020). 10.1101/2020.06.08.20125484. [Epub ahead of print]. [DOI] [Google Scholar]
- 26.Lee B, Raszka WV, Jr. COVID-19 Transmission and children: the child is not to blame. Pediatrics. (2020). 146:e2020004879. 10.1542/peds.2020-004879 [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. (2020) 382:1663–5. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC COVID-19 Response Team . Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:422–6. 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parri N, Lenge M. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. (2020) 383:187–90. 10.1056/NEJMc2007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Ceano-Vivas M, Martín-Espín I, Del Rosal T. SARS-CoV-2 infection in ambulatory and hospitalised Spanish children. Arch Dis Child. (2020) 105:808–9. 10.1136/archdischild-2020-319366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soltani J, Sedighi I, Shalchi Z, Sami G, Moradveisi B, Nahidi S. Pediatric coronavirus disease 2019. (COVID-19): an insight from west of Iran. North Clin Istanb. (2020) 7:284–91. 10.14744/nci.2020.90277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez Gaxiola G, Flores Rocha R, Valadez Vidarte JC, Hernandez Alcaraz M, Herrera Mendoza G, Del Real Lugo MA. Clinical and epidemiological characteristics of children with SARS-CoV-2 infection: case series in Sinaloa. medRxiv. (2020). 10.1101/2020.07.07.20146332. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.Raiden S, Cairoli H, Potasnik J, Di Lalla S, Chiolo MJ, Torres F, et al. Children hospitalized for COVID-19 during the first winter of the pandemic in Buenos Aires, Argentina. medRxiv. (2020). 10.1101/2020.11.05.20225300. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Vieira RSR, Aguiar ELd, Evangelista NMdA, Sarrubbo SAB, Verlangieri HAR, Otsuka M. Clinical characteristics in children and adolescents with SARS-CoV-2 infection: experience in a highly complex public hospital in the city of São Paulo. medRxiv. (2020). 10.1101/2020.06.22.20136994. [Epub ahead of print]. [DOI] [Google Scholar]
- 35.Leulseged TW, Hassen IS, Maru EH, Zewde WC, Chamiso NW, Edo MG, et al. COVID-19 in Hospitalized Ethiopian Children: Characteristics and Outcome Profile. medRxiv. (2020). 10.1101/2020.10.30.20223115. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiara-Chilet C, Luna-Vilchez M, Maquera-Afaray J, Salazar-Mesones B, Portillo-Alvarez D, Priale-Miranda R, et al. Clinical-epidemiological and treatment characteristics of children with COVID-19 in a tertiary referral center in Perú. medRxiv. (2020). 10.1101/2020.09.18.20186866. [Epub ahead of print]. [DOI] [Google Scholar]
- 37.Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people hospitalised with covid-19 in the United Kingdom: prospective multicentre observational cohort study. medRxiv. (2020) 2020.2007.2014.20153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han MS, Choi EH, Chang SH, Jin BL, Lee EJ, Kim BN, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr. (2021) 175:73–80. 10.1001/jamapediatrics.2020.3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CDC . CDC COVID Data Tracker. (2020). Vol. (2020). [Google Scholar]
- 40.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease (2019). (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. (2020) 20:689–96. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. (2020) 10.1101/2020.08.21.20178574. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. (2020) 26:502–5. 10.1038/s41591-020-0817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z, Liu Y, Xu L, Guan W, Zhang X, Qi T, et al. Extra-pulmonary viral shedding in H7N9 Avian Influenza patients. J Clin Virol. (2015) 69:30–2. 10.1016/j.jcv.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 44.Simpson JN, Goyal MK, Cohen JS, Badolato GM, McGuire M, Ralph A, et al. Results of testing children for SARS-CoV-2 through a community-based testing site. J Pediatr. (2020). 10.1016/j.jpeds.2020.12.030. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. (2020) 109:1088–95. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai J, Xu J, Lin D, Yang Z, Xu L, Qu Z, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. (2020) 71:1547–51. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.See KC, Liew SM, Ng DCE, Chew EL, Khoo EM, Sam CH, et al. COVID-19: four paediatric cases in Malaysia. Int J Infect Dis. (2020) 94:125–7. 10.1016/j.ijid.2020.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (2020) 80:e1–6. 10.1016/j.jinf.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng LK, Tao XW, Yuan WH, Wang J, Liu X, Liu ZS. First case of neonate infected with novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. (2020) 58:E009. 10.3760/cma.j.issn.0578-1310.2020.0009 [DOI] [PubMed] [Google Scholar]
- 50.Chen F, Liu ZS, Zhang FR, Xiong RH, Chen Y, Cheng XF, et al. First case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. (2020) 58:179–82. 10.3760/cma.j.issn.0578-1310.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 51.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong H, Wang Y, Chung HT, Chen CJ. Clinical characteristics of novel coronavirus disease 2019. (COVID-19) in newborns, infants and children. Pediatr Neonatol. (2020) 61:131–2. 10.1016/j.pedneo.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. (2020) 25:2001010. 10.2807/1560-7917.ES.2020.25.22.2001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. Children and Adolescents. N Engl J Med. (2020) 383:334–46. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gagliardi L, Bertacca C, Centenari C, Merusi I, Parolo E, Ragazzo V, et al. Orchiepididymitis in a boy with COVID-19. Pediatr Infect Dis J. (2020) 39:e200–e2. 10.1097/INF.0000000000002769 [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z, Guo L, Huang L, Zhang C, Luo R, Zeng L, et al. Distinct disease severity between children and older adults with COVID-19: Impacts of ACE2 expression, distribution, and lung progenitor cells. Clin Infect Dis. (2021). 10.1093/cid/ciaa1911. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. (2016) 352:aaf1098. 10.1126/science.aaf1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: why children fare better than adults? Indian J Pediatr. (2020) 87:537–46. 10.1007/s12098-020-03322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang XF, Yuan J, Zheng YJ, Chen J, Bao YM, Wang YR, et al. Retracted: clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen. Zhonghua Er Ke Za Zhi. (2020) 58:E008. 10.3760/cma.j.issn.0578-1310.2020.0008 [DOI] [PubMed] [Google Scholar]
- 61.Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther. (2011) 9:71–9. 10.1586/eri.10.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. (2020) 58:1135–38. 10.1515/cclm-2020-0272 [DOI] [PubMed] [Google Scholar]
- 63.Tan YP, Tan BY, Pan J, Wu J, Zeng SZ, Wei HY. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. (2020) 127:104353. 10.1016/j.jcv.2020.104353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. (2020) 46:579–82. 10.1007/s00134-020-05967-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu P, Cai J, Jia R, Xia S, Wang X, Cao L, et al. Dynamic surveillance of SARS-CoV-2 shedding and neutralizing antibody in children with COVID-19. Emerg Microbes Infect. (2020) 9:1254–8. 10.1080/22221751.2020.1772677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia W, Shao J. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. (2020) 55:1169–74. 10.1002/ppul.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. (2020) 39:469–77. 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang Y, Zhang H. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. (2020) 296:E115–e7. 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denina M, Scolfaro C, Silvestro E, Pruccoli G, Mignone F, Zoppo M, et al. Lung ultrasound in children with COVID-19. Pediatrics. (2020) 146:e20201157. 10.1542/peds.2020-1157 [DOI] [PubMed] [Google Scholar]
- 70.Wolf J, Abzug MJ, Wattier RL, Sue PK, Vora SB, Zachariah P, et al. Initial guidance on use of monoclonal antibody therapy for treatment of COVID-19 in children and adolescents. J Pediatric Infect Dis Soc. (2021). 10.1093/jpids/piaa175. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. Jama. (2020) 323:1313–4. 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. (2020) 20:615–32. 10.1038/s41577-020-00434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.https://www.covid-19vaccinetracker.org/authorized-vaccines.
- 74.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 75.Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. (2020) 14:12522–37. 10.1021/acsnano.0c07197 [DOI] [PubMed] [Google Scholar]
- 76.Walsh EE, Frenck RW, Jr., Falsey AR, Kitchin N, Absalon J, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. (2020) 383:2439–50. 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. (2020) 383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. (2020) 395:1845–54. 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamidani S, Rostad CA, Anderson EJ. COVID-19 vaccine development: a pediatric perspective. Curr Opin Pediatr. (2021) 33:144–51. 10.1097/MOP.0000000000000978 [DOI] [PubMed] [Google Scholar]
- 80.Haynes BF, Corey L. Prospects for a safe COVID-19 vaccine. Sci Trans Med. (2020) 12:eabe0948. 10.1126/scitranslmed.abe0948 [DOI] [PubMed] [Google Scholar]
- 81.Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: the current state of play. Paediatr Respir Rev. (2020) 35:43–9. 10.1016/j.prrv.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rauh LW, Schmidt R. Measles immunization with killed virus vaccine. serum antibody titers and experience with exposure to measles epidemic. Am J Dis Child. (1965) 109:232–7. 10.1001/archpedi.1965.02090020234007 [DOI] [PubMed] [Google Scholar]
- 83.Singh T, Heston SM, Langel SN, Blasi M, Hurst JH, Fouda GG, et al. Lessons from COVID-19 in children: key hypotheses to guide preventative and therapeutic strategies. Clin Infect Dis. (2020) 71:2006–13. 10.1093/cid/ciaa547 [DOI] [PMC free article] [PubMed] [Google Scholar]