Abstract
Dysregulated neutrophil activation contributes to the pathogenesis of autoimmune diseases including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Neutrophil-derived reactive oxygen species (ROS) and granule proteases are implicated in damage to and destruction of host tissues in both conditions (cartilage in RA, vascular tissue in SLE) and also in the pathogenic post-translational modification of DNA and proteins. Neutrophil-derived cytokines and chemokines regulate both the innate and adaptive immune responses in RA and SLE, and neutrophil extracellular traps (NETs) expose nuclear neoepitopes (citrullinated proteins in RA, double-stranded DNA and nuclear proteins in SLE) to the immune system, initiating the production of auto-antibodies (ACPA in RA, anti-dsDNA and anti-acetylated/methylated histones in SLE). Neutrophil apoptosis is dysregulated in both conditions: in RA, delayed apoptosis within synovial joints contributes to chronic inflammation, immune cell recruitment and prolonged release of proteolytic enzymes, whereas in SLE enhanced apoptosis leads to increased apoptotic burden associated with development of anti-nuclear auto-antibodies. An unbalanced energy metabolism in SLE and RA neutrophils contributes to the pathology of both diseases; increased hypoxia and glycolysis in RA drives neutrophil activation and NET production, whereas decreased redox capacity increases ROS-mediated damage in SLE. Neutrophil low-density granulocytes (LDGs), present in high numbers in the blood of both RA and SLE patients, have opposing phenotypes contributing to clinical manifestations of each disease. In this review we will describe the complex and contrasting phenotype of neutrophils and LDGs in RA and SLE and discuss their discrete roles in the pathogenesis of each condition. We will also review our current understanding of transcriptomic and metabolomic regulation of neutrophil phenotype in RA and SLE and discuss opportunities for therapeutic targeting of neutrophil activation in inflammatory auto-immune disease.
Keywords: neutrophils, rheumatoid arthritis, systemic lupus erythematosus, NETs, low density granulocytes, immunometabolism
Introduction
Systemic Lupus Erythematosus (SLE) is the archetypal autoimmune connective tissue disease, characterized by the production of multiple auto-antibodies [anti-nuclear antibodies (ANA), anti-double stranded DNA (dsDNA), anti-Sm/RNP, anti-Ro/La] and the consumption of complement (1, 2). It has the capacity to involve almost any organ system of the body resulting in protean and sometimes catastrophic consequences for patients. SLE disproportionately affects young women and is a condition with a broad spectrum of severity, ranging from mild joint involvement to life-threatening organ failure (3). Typical manifestations include systemic complaints, such as overwhelming and intrusive fatigue, brain fog, fever, swollen lymph nodes, mouth ulcers, chilblains, and weight loss (4). Rashes are common in SLE and may be transient or disfiguring; rashes are often triggered by sunlight and may be associated with hair loss and scarring. The characteristic malar flush of redness over the cheeks gives the name lupus (from wolf) and is likened to the appearance of a butterfly. Inflammation of joints, tendons, and muscle may cause arthritis, nodules, or contractures and give rise to disability and pain. Kidney disease including inflammation and immune complex deposition may occur which, if untreated, can lead to kidney failure and the need for dialysis and transplant (5, 6). Inflammation can develop in the pleural lining of the lungs, around the heart and may even affect the heart muscle and valves. SLE may also affect any part of the nervous system from brain and spinal cord to the peripheral nerves resulting in neurological problems, such as strokes, neuropathy, headache, visual loss, migraine, confusion, and acute psychosis (7). The disease may cause a fall in blood counts involving specific cell lines or indeed pancytopenia and may be associated with serious abnormalities of both clotting and bleeding. Repeated inflammatory insults, abnormal blood clotting and the consequence of treatment with high dose steroids and immunosuppression can also lead to chronic illness through damage accrual and increase the likelihood of infection, osteoporosis, premature ovarian failure, cardiovascular events such as atherosclerosis, stroke or heart attack, and malignancy. SLE is incurable and with modern treatment is still associated with an increased risk of mortality, and shortened life expectancy (3).
Rheumatoid arthritis (RA) is a chronic, autoimmune, systemic inflammatory condition associated typically with antibodies to rheumatoid factor (RF) and cyclic citrullinated peptides (anti-CCP or ACPA) (8). It is the commonest form of inflammatory arthritis and is characterized by inflammation of the tendon sheaths (tenosynovitis) and joint lining (synovitis) leading to growth of an inflammatory pannus which quickly erodes the joint cartilage and bone, causing recognizable deformities that were once commonplace in rheumatology clinics (9). Untreated or resistant to therapy, RA results in a symmetrical, deforming polyarthropathy. This leads to physical disability, progressive loss of function and as well as stiffness and pain. Extra-articular complications of the disease include interstitial lung disease, vasculitis, nodules, eye disease and an increased risk of cardiovascular disease, malignancy and osteoporosis (10, 11). Modern management is focused on a prompt diagnosis and early use of immunosuppressive treatments, including traditional and biologic disease-modifying anti-rheumatic drugs (DMARDs), with the aim of targeting disease remission. This has led to a reduction in the need for orthopedic surgery for patients with this form of arthritis, a move to out-patient based care as opposed to long hospitalizations, and a reduction in the systemic complications that can occur. However, RA still remains an incurable disease with treatments that rely on the long-term suppression of the immune system resulting in side effects and complications, including an increased risk of infection (9, 12).
Both RA and SLE are caused by a dysregulation of the innate and adaptive immune systems, including clonal expansion of auto-reactive lymphocytes, production of auto-antibodies and elevated production of multiple cytokines and other inflammatory mediators. Research into the underlying cause of both diseases focusses heavily on dysregulated T- and B-cell responses (9, 12, 13). However, it is inappropriately activated neutrophils that have the greatest potential to cause damage to local tissues, both due to their presence in high numbers at sites of inflammation and through release of their cytotoxic contents directly onto host tissues. Neutrophils are specialist cells of the innate immune system that normally play a major role in host defense against microorganisms through phagocytosis and generation of reactive oxygen species (ROS). Production of ROS within the phagosome occurs via the action of NADPH oxidase (NOX2) and myeloperoxidase (MPO) which, together with release of proteases from granules and vesicles into the phagosome, provide a defensive arsenal against a broad spectrum of microscopic pathogens. During infection, ROS and proteases may be released extracellularly causing local tissue damage at the site of infection (14). This damage is normally resolved by resident macrophages, which remove apoptotic neutrophils and damaged tissue as part of the normal process of inflammation resolution (15). Neutrophils are the most abundant leukocyte in humans, being produced by the bone marrow in huge numbers daily (estimated to be in the region of 5–10 × 1010 per day) (16, 17). Whilst the majority of neutrophils circulate in the blood (both free-flowing and marginated to the endothelial vessel walls), several populations of tissue neutrophils exist within healthy homeostasis, including within the lung, spleen and liver (18). These tissue neutrophils play a major role in surveillance and host defense, B-cell Ig-class switching, and phagocytosis of circulating bacteria, respectively. The liver is also a major site for efferocytosis and removal of neutrophils that have been involved in bacterial killing (18). Neutrophil release from the bone marrow, and subsequent homing back to bone marrow for efferocytosis at the end of their normal life span, is regulated by circadian expression of CXCR2 (receptor for CXCL2), the central clock gene BMAL1 and CXCR4 (receptor for CXCL12) (19), with granule content and the ability to produce NETs being the highest in the morning, decreasing throughout the day to reach the lowest levels by mid-afternoon in human neutrophils (20).
Neutrophils contribute to inflammation and tissue damage in inflammatory disease, when they become inappropriately activated by cytokines, chemokines and auto-antibodies (14). Auto-immune neutrophils function in a multitude of ways to direct the inflammatory response, including release of proteases which damage host tissue and activate soluble proteins (21), secretion of cytokines and chemokines which direct both the innate and adaptive immune responses (22), shedding of receptors, such as the interleukin-6 receptor to initiate trans-signaling (23, 24), release of neutrophil extracellular traps (NETs) providing a source of auto-antigens (25), and production of ROS (8, 26). This review will discuss the dysregulation of neutrophil activation in RA and SLE, two auto-immune diseases characterized by aberrant neutrophil activation. We will highlight how uncontrolled neutrophil activation contributes to the development of auto-immunity and disease progression, and summarize how inappropriate neutrophil activation might be targeted with therapeutics.
Apoptosis
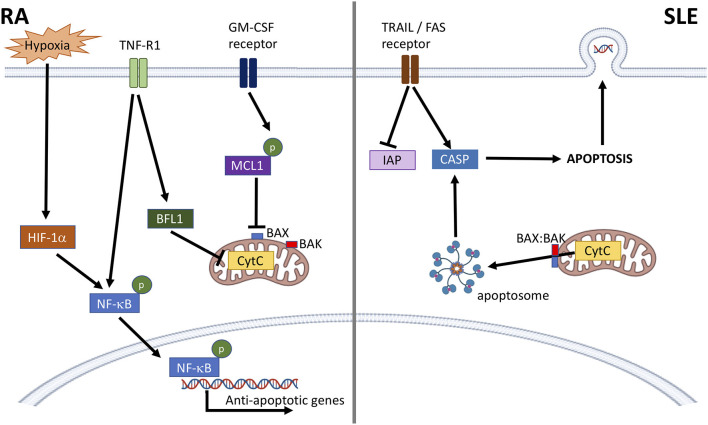
Neutrophils are terminally differentiated cells which, in the absence of inflammation, circulate in the blood for around 24–48 h before returning to the bone marrow and undergoing apoptosis (27). Constitutive neutrophil apoptosis is regulated by Bcl-2 family proteins: anti-apoptotic MCL1 and A1/BFL1, and pro-apoptotic BAX, BAK, and BID. Loss of MCL1 and BFL1 causes BAX:BAK pore formation in the mitochondrial membrane, releasing cytochrome c which along with APAF1 forms the apoptosome, leading to cleavage of caspases initiating apoptosis (14, 27–29). Neutrophil apoptosis may be delayed during inflammation by cytokines (e.g., GM-CSF, TNFα), bacterial lipopolysaccharide (LPS) and leukotrienes (e.g., leukotriene B4), which extend the life-span of neutrophils through stabilization or up-regulation of MCL1 and/or BFL1 (30–35). Aging neutrophils can be identified in circulation by decreased expression of L-selectin (CD62L) and CXCR2, and increased expression of CXCR4, the receptor for CXCL12; it is the increased signaling via CXCL12:CXCR4 which enables aged neutrophils to home back to the bone marrow to undergo apoptosis and clearance (19). Neutrophil apoptosis may be induced through activation of death-receptor signaling pathways, such as FASL, TRAIL, and TNFα which directly activate caspase-8 and caspase-3, and induce degradation of BID and BAX leading to mitochondrial release of cytochrome c (28, 36). Intriguingly, TNFα may be pro- or anti-apoptotic in vitro depending upon the concentration in media, with low concentrations delaying apoptosis and higher concentrations promoting apoptosis (37). Whilst not completely understood, this effect is believed to be mediated via the two TNF receptors (TNFR1 and TNFR2), with TNFR1 signaling anti-apoptotic activation of NF-κB, and TNFR2 activating death receptor signaling (38, 39).
In SLE, neutrophil apoptosis is enhanced (40–42) leading to increased apoptotic burden associated with development of anti-nuclear auto-antibodies (25, 43). A dysregulation between pro-apoptotic caspases and inhibitors of apoptosis (IAP1, IAP2, XIAP) may explain the enhanced apoptosis seen in SLE neutrophils in vitro (Figure 1) (41, 42, 44). Levels of pro-apoptotic TRAIL and FASL are also significantly higher in SLE serum, which can induce apoptosis in healthy neutrophils (41). Levels of GM-CSF are lower in SLE serum, and supplementation of SLE serum with physiological levels of GM-CSF can rescue the pro-apoptotic effect of SLE serum on healthy neutrophils (42). Incubation of apoptotic neutrophils with SLE PBMCs induces expression of interferon-alpha (IFNα) via a toll-like receptor (TLR)-dependent mechanism (45). SLE neutrophils also express nuclear antigens (dsDNA) at the plasma membrane, and this effect can be induced in healthy neutrophils incubated with SLE serum (46). As well as an increase in neutrophil apoptosis, defects in clearance of apoptotic cells by macrophages can also contribute to the accumulation of apoptotic debris in SLE (47).
Figure 1.
Dysregulation of neutrophil apoptosis in RA and SLE. In RA, anti-apoptotic factors, such as cytokines and hypoxia activate NF-κB and prevent mitochondrial cytochrome c (CytC) driven apoptosis through activation of BFL1, and stabilization of MCL1. This prevents BAX:BAK pore formation, CytC leakage and formation of the apoptosome. In SLE, activation of death receptors (e.g., TRAIL, FAS receptors) lowers levels of cellular inhibitors of apoptosis (IAP) and activates caspase-8 (CASP). BAX:BAK pore formation in the mitochondrial membrane releases CytC leading to apoptosome formation and activation of CASP-9 and CASP-3 leading to apoptosis. Nuclear antigens, including DNA, are also expressed at the plasma membrane.
Apoptosis is delayed in both blood and synovial fluid neutrophils from patients with RA (48, 49). The likely cause of this is inflammatory cytokines, such as GM-CSF, TNFα, IL1β, and interferons, elevated in both blood and synovial fluid (50), which have been demonstrated to delay neutrophil apoptosis in in vitro experiments (32–34, 37, 51–55). RA blood neutrophils express higher levels of MCL1, higher levels of phosphorylated NF-κB and lower levels of active caspase-9 compared to healthy controls (48). Delayed apoptosis in synovial fluid has also been attributed to other factors including lactoferrin and adenosine (56, 57). The hypoxic environment in RA synovial joints also plays a key role in delaying neutrophil apoptosis, via increased expression of MCL1 (Figure 1) (58). Hypoxia can also delay apoptosis via stabilization of hypoxia-inducible factor 1-alpha (HIF1-α) and activation of NF-κB (59), and regulates neutrophil retention at sites of inflammation, prolonging inflammation (60).
Microparticles (MPs) are small (0.2–2 μM) extracellular vesicles containing cargos of protein, lipids and nucleotides (e.g., RNA, miRNA) which are released from activated cells via budding and shedding of the cell membrane (61). MPs are distinct from other extracellular vesicles, i.e., exosomes and apoptotic bodies, by virtue of their size, cargo, membrane content, and mode of formation (61). MPs are taken up by neighboring cells through endocytosis, and their protein and RNA cargo can alter the recipient cell phenotype once released into the cytoplasm. They represent a novel form of paracellular communication and differ in composition depending upon the functional state of the originating cell. In SLE, MPs are annexin V+ and contain cell markers indicating both endothelial cell and neutrophil origins (62). SLE MPs activate plasmacytoid dendritic cells to produce a range of cytokines, including IFNα (62), and stimulate ROS production in autologous and healthy neutrophils (63). Acetylated histones within chromatin in MPs suggests the MPs are derived from apoptotic cells (62) and may be central to the stimulation of NOX2-independent NETs (64). The population of annexin V+/acetylated histone+ MPs appear to be specific to SLE and are not present in sera of healthy controls or individuals with RA (62). MPs released from RA neutrophils may have anti-inflammatory properties, activated through the pro-resolving protein Annexin-A1 (65, 66). Annexin-A1 is released from neutrophil granules following extravasation and is found in RA synovial fluid. This protein exerts its pro-resolving effect by promoting apoptosis and decreasing neutrophil:endothelial cell adhesion and extravasation (66, 67).
ROS Mediated Tissue Damage
Neutrophils produce ROS via activation of the NADPH oxidase (NOX2) (68). NOX2 is a multi-component enzyme, which is assembled at the phagosomal and plasma membrane following cytokine priming (69, 70). Priming induces phosphorylation and mobilization of granular and cytosolic NOX2 components [p22phox and gp91phox which together comprise cytochrome b558, p40phox, p47phox, p67phox, and Rac (Rac-1 or Rac-2)] to the phagosomal and plasma membranes in readiness for phagosomal killing (70–73). Assembly of NOX2 at the plasma membrane leads to the release of ROS into the extracellular environment and is a major cause of damage to host tissue in RA and SLE. ROS production via NOX2 in primed neutrophils is triggered by activation of cell receptors [e.g., FcγR, complement receptors, f-Met-Leu-Phe (fMLP) receptor]. Activated NOX2 catalyses the reduction of oxygen to superoxide (O2-), an unstable oxygen radical which rapidly forms hydrogen peroxide (H2O2), the hydroxyl free radical (HO•) and/or peroxynitrite () depending on cellular conditions (68, 74). Hydrogen peroxide is the major substrate of myeloperoxidase (MPO), a neutrophil azurophilic granule enzyme implicated in the production of highly-reactive, secondary oxidants, such as hypochlorous, hypobromous, and hypothiocyanous acids which are potent anti-microbial agents that damage proteins, lipids and DNA (68, 75).
SLE neutrophils exhibit aberrant ROS production, with O2-, H2O2, and HO• being produced more rapidly and in higher levels than healthy individuals (76, 77). DNA, protein and lipid markers of intracellular oxidative stress are increased in SLE neutrophils (76), including 8-hydroxyguanosine, an oxidized self-DNA which may function as a damage-associated molecular pattern (DAMP) (78) and which is present in NETs (79). Neutrophils from SLE patients with circulating immune complexes or cytotoxic antibodies produce the highest O2- response to FcγR/complement receptor stimulation (80), and unstimulated ex vivo neutrophils from lupus nephritis patients have the highest levels of ROS production (77). SLE serum induces O2- generation in healthy neutrophils, with O2- production correlating positively with the presence of immune complexes and negatively with the concentration of complement (81). Many patients with SLE experience both flares and periods of inactive or quiet disease. Neutrophils from SLE patients in active flare often produce lower levels of ROS in vitro than those with inactive disease, possibly due to exhaustion of the neutrophils in vivo (76), or lower expression of Fcγ and complement receptors (82). As yet unidentified factors within SLE serum induce production of cytokines by human renal glomerular endothelial cells which promote neutrophil chemotaxis and adhesion (83). In patients with lupus nephritis, immune complexes become deposited within tissues in the kidney (6). This may arise through the binding of anti-nucleosomes and anti-C1q auto-antibodies to nucleosomes and C1q captured on the surface of glomerular endothelial cells by heparan sulfate (84). Neutrophils expressing FcγR2A adhere to immune complex deposits on the surface of glomerular capillaries, activating ROS production and release directly onto host tissue (85). This release of ROS is directly responsible for damage to glomeruli, including oxidation of DNA, lipids and proteins, and induction of apoptosis (86). Oxidized high-density lipoprotein (HDL), commonly found in SLE patients, is pro-inflammatory, driving production of IL-6 and TNF by macrophages, and lacking its normal, cardioprotective properties (87). A polymorphism in the gene for neutrophil cytosolic factor 1 (NCF1, rs201802880) in a sub-set of SLE patients is associated with lower ROS production by neutrophils (88). This polymorphism also increases expression of type 1 interferon-regulated genes (89), the importance of which will be discussed later in this review. Interestingly, the NCF1 (m1J) mutated mouse model of SLE has demonstrated a link between ROS deficiency and interferon-driven autoimmunity downstream of a deficient NOX2 complex (90).
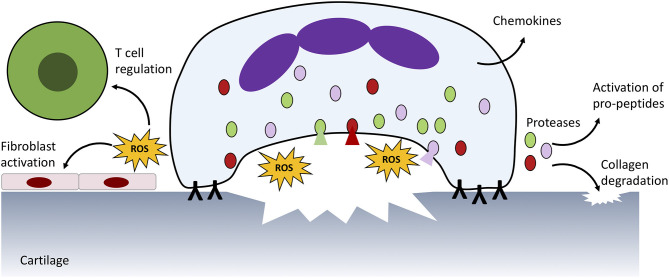
In RA, both blood and synovial fluid neutrophils have an increased capacity to produce ROS (91, 92). ROS production within RA blood leads to modifications of immunoglobulin G (IgG) which are associated with increased immunogenicity and production of rheumatoid factor immune complexes (93). Within the RA joint IgG complexes, both soluble and embedded within synovial tissue, activate further ROS production by neutrophils via activation of FcγR2a and FcγR3b (Figure 2) (94, 95), and trigger degranulation of proteolytic enzymes including elastase and cathepsin G (8, 26, 56, 96–98). When this occurs at the articular surface a microenvironment of concentrated ROS, proteases and cytotoxic factors is formed, damaging the underlying structures (8). As well as damaging collagen fibers within cartilage, neutrophil granule proteases cause proteolytic cleavage and activation of proteins (matrix metalloproteinases, pro-cytokines/chemokines) and cleavage of soluble receptors to initiate trans-signaling (such as the IL-6 receptor) (8, 21, 23, 99–102). Additionally, ROS production within the joint disrupts oxidative homeostasis and drives adaptive immune responses to the synovial environment (68). ROS-induced MAPK and NF-κB activation in synovial fibroblasts activates production of pro-inflammatory prostaglandins by cyclooxygenase (COX)-2 (103). ROS also exert profound effects on the local T cell population, regulating differentiation, apoptosis and cytokine production (104, 105).
Figure 2.
Neutrophil ROS production and protease release damages host tissue. In RA, immune complexes on the surface of the joint activate ROS production and protease release from granules (shaded red, green, and pink) which damages underlying cartilage and activates neighbouring immune cells and fibroblasts. Proteases also activate pro-peptides, such as cytokines produced by neutrophils and other infiltrating immune cells. A similar process is responsible for damage to blood vessels in SLE.
Neutrophil Extracellular Traps
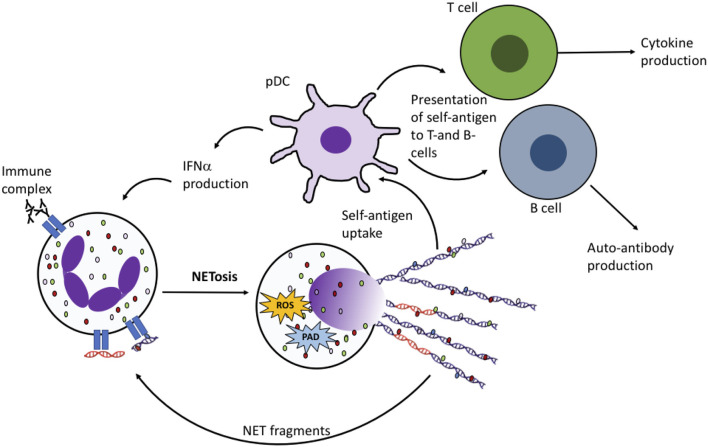
Neutrophil extracellular traps (NETs) are mesh like DNA structures decorated with histones, MPO and other antimicrobial proteins expelled from neutrophils in response to infectious or inflammatory stimuli (106). They are an alternative defense mechanism by which neutrophils trap and possibly kill microbes (106, 107). Whilst the early events that signal NET production rather than phagocytosis are unclear, at least two methods of chromatin decondensation leading to NET formation (NETosis) have been described: NOX2-dependent and NOX2-independent (108). NOX2-dependent NETosis, also termed suicidal NETosis, occurs via activation of NOX2 and production of intra-phagosomal ROS. This causes increased intracellular membrane permeability, movement of elastase to the nucleus and degradation of histones leading to chromatin decondensation and NET release (109). ROS production by NOX2 promotes the morphological changes that occur during NETosis (110) and inactivates caspases to block apoptosis pathways (109). NOX2-independent NETosis does not require the production of ROS by NOX2. In this case, mitochondrial ROS combine with increased intracellular calcium levels to activate peptidyl arginase deiminase (PAD) enzymes (e.g., PAD4) leading to hypercitrullination of histones, chromatin decondensation, and NET release (111, 112). Several inflammatory agents have been reported to induce NET release, including fMLP, IL-8, LPS, nitric oxide, and TNFα (113). Many proteins decorating NET are post-translationally modified, in particular histones which have been found to be methylated, acetylated (114) and citrullinated (111, 115–117) leading to speculation that NETs may be a source of auto-antigens in auto-immune disease (8). Recent work suggests that NETs (MPO:DNA and/or elastase:DNA complexes) detected in up to 79% of RA and up to 100% of SLE sera are generated in a NOX2-independent manner (118).
There is an ever increasing body of evidence to support the hypothesis that the externalization of double-stranded DNA and post-translationally modified proteins on NETs is implicated in the pathogenesis of SLE, through activation of interferon-producing plasmacytoid dendritic cells (pDCs) (Figure 3) (119) and damage to endothelial tissues and organs (25, 120). SLE sera cross-react in vitro with NET components (114, 121) and spontaneous NET production (NETosis) by SLE neutrophils ex vivo is also observed (120, 122). NET structures staining positive for DNA, elastase, MPO, and citrullinated histone H3 are found in cutaneous SLE lesions (123) and the kidney (120). Most strikingly the molecular targets of many of the almost 100 auto-antibodies associated with SLE, including those directed at nuclear DNA and nuclear proteins, can be detected in NETs (124). The majority of SLE patients will be positive for antibodies against ANA and/or dsDNA at some point in their disease course (2). Antibodies against histones (including acetylated and/or methylated histones) are also common (125–127). Recent proteomics analysis of NETs from SLE patients identified a number of histone proteins with acetylated, methylated and/or citrullinated residues (128), particularly in NOX2-independent NETs. A number of these, for example acetylated histone H2B (K21, K20), methylated histone H3 (K27) and acetylated histone H4 (K5, K8, K12, K16), correspond to known SLE auto-antibodies (114, 121, 129) and serum NET debris (130). Aside from histones, a number of rarer SLE auto-antibodies correspond to proteins identified on SLE NETs (128) including HMG-17 (131), catalase (132), lamin B1 and B2 (133–135), apolipoprotein A1 (136), cathelicidin (LL37) (137–139), annexin AI and α-enolase (140, 141). When directly compared with RA NETs, the levels of MPO, leukocyte elastase inhibitor and thymidine phosphorylase were higher in SLE NOX2-dependent NETs, whereas histones H1.0, H2B (type 1-J), H2B (type 2-F), and H4 were higher in SLE NOX2-independent NETs (128).
Figure 3.
NET production in RA and SLE drives the auto-immune response. Fragments of DNA and proteins (including histones, MPO, elastase, HMGB1, LL37) are taken up by plasmacytoid dendritic cells (pDCs) and presented to auto-reactive B- and T-cells, leading to production of cytokines including interferon alpha (IFNα) and autoantibodies. NET fragments, including oxidized DNA (shown in orange) may also activate NET production by neighboring neutrophils.
Many NET proteins also correlate with SLE disease activity and play a direct role in tissue damage. MMP9 contained within SLE NETs activates endothelial MMP2, inducing endothelial dysfunction and apoptosis (142). Neutrophil gelatinase-associated lipocalin (NGAL) is both a urinary biomarker of lupus nephritis and a predictor of disease flare, although it is not known whether degranulating neutrophils or NETs are the source of NGAL in SLE urine (143–145). Auto-antibodies to NET proteins that become deposited within the glomeruli of patients with lupus nephritis (141) attract complement and leukocytes expressing Fcγ and complement receptors (including other neutrophils), activating the infiltrating cells causing further tissue damage, e.g., via ROS and protease degranulation (86, 146). In addition, excess NET production within the vasculature and glomeruli promotes vascular leakage and endothelial-to-mesenchymal transition (EndMT), a process that is associated with pathogenic fibrosis of tissues (147). NET production via PAD2 and PAD4 has also been shown to contribute to the development of atherosclerosis and vascular stiffness in murine lupus models (148, 149). Auto-antibodies against apolipoprotein A1 may neutralize the cardioprotective effect of the HDL complex, causing cardiovascular disease in SLE patients with anti-apolipoprotein A1 antibodies (136, 150). Whilst cathelicidin/LL37 may not play a direct role in tissue damage in SLE, its indirect roles include activation of type-I interferon production, activation of the inflammasome and further activation of NET production (137). The abundance of NET debris in SLE sera may be due to impairments in clearance mechanisms associated with tissue homeostasis. Both inhibitors of DNase-I, and anti-NET antibodies in SLE serum, have been proposed as mechanisms impairing the dismantling of NET structures. Impairment of DNase-I is also associated with kidney involvement in SLE (151).
Immune complexes in the sera of SLE patients may contain nucleic acids (Sm RNP RNA or DNA), which activate neutrophils to produce ROS and IL-8 via FcγR2a (152). This phenomenon is inhibited by chloroquine, a DMARD commonly used to treat SLE. SLE immune complexes often contain LL37:DNA complexes derived from NETs. LL-37:DNA complexes activate B cells via TLR9 leading to expansion of self-reactive memory B cells and IgG production (153). B cell activation by LL37:DNA can also be inhibited by chloroquine, suggesting an important role for endosomal TLR activation by NETs in the pathology of SLE. In a recent clinical study of 16 SLE patients, excessive NET production by was abrogated by combination therapy with rituximab (a B cell targeted therapy) and belimumab (anti-BLyS/BAFF) (154). Low disease activity, including a significant reduction in auto-antibody titers, was achieved in over 50% of patients in the study. Further analysis of sera from the patients in this study revealed that the combination of rituximab and belimumab significantly decreased anti-dsDNA, anti-histones, anti-nucleosomes and anti-C1q titers (155). In addition, the ability of patient sera to induce NET formation in vitro was decreased by around 75% following combination therapy with rituximab and belimumab (155).
In RA it is the exposure of citrullinated proteins on NETs that is a key driver of auto-immunity, leading to the development of anti-citrullinated peptide auto-antibodies (ACPA) (8, 156). NET debris can be detected in both serum and synovial fluid from RA patients (98, 118) and NET structures staining positive for CD15, elastase, MPO and citrullinated (cit) histone H3 can be seen in synovial biopsy tissues (156, 157). Many proteins in RA patient neutrophils are citrullinated via activation of PAD2 and PAD4 (158), including known auto-antibody targets: cit-actin, cit-histone H1.3, cit-histone H3, cit-vimentin (156, 158). PAD enzymes are present in synovial biopsies, localized with MPO in necrotic areas of synovial tissue that also contain large areas of citrullinated proteins (159), and PAD2 and PAD4 are present in NETs generated from ex vivo RA neutrophils (128). PADs are also found in high concentrations in synovial fluid alongside citrullinated proteins, such as α-enolase (160, 161). Proteomics analysis of RA NETs identified citrullinated forms of known auto-antibody targets, such as cit-α-enolase, cit-histone H2A, cit-histone H4, cit-vimentin (128), as well as acetylated and methylated histones in line with analysis of NET debris in RA serum (118). Proteomic analysis of synovial fluid from RA and spondyloarthritis (SpA) patients identified many neutrophil proteins present at significantly elevated concentrations in RA synovial fluid, including MPO, cathepsin G, annexin-A1, and NGAL. Although no difference was observed in the amount of cell-free DNA between RA and SpA synovial fluid, the levels of 21 NET proteins were elevated in RA SF, including histones H2A, H2B and H4, MMP9, elastase, and α-enolase (98). Whilst the levels of ACPA in RA serum do not appear to correlate with NET material, antibodies to NET material (ANETA) are significantly higher in seropositive RA patients (162).
Several mechanisms have been proposed explaining how NETs and NET proteins contribute to joint damage and disease activity in RA. Elastase within in NETs has been demonstrated to disrupt the cartilage matrix, triggering PAD2 release from fibroblast-like synoviocytes (FLS). Activated PAD2 causes citrullination of cartilage fragments which are then internalized by FLS and presented via MHC Class II to antigen specific T-cells leading to the production of ACPA in HLA-DRB1*04:01 transgenic mice (163, 164). Both MMP8 and MMP9, found in RA NETs, contribute to degradation of the cartilage matrix and are associated with increased mortality (100, 165, 166). NETs also contain enzymes which degrade aggrecan, another key structural component of cartilage (164). Citrullinated vimentin and aggrecan are preferentially recognized by T cells expressing the HLA-DRB1*04:01/04 allele (known as the “shared epitope”) (163, 167) inducing auto-antibody production (164). Auto-antibodies secreted by RA synovial B cells cross react with cit-fibrinogen, cit-histones H2A/H2B, and cit-vimentin, as well as NETs generated from RA blood and joint neutrophils (168). Presentation of citrullinated antigens to autoreactive T cells provides a molecular explanation for the strong association between the HLA-DRB1*04:01/04 allele and the development of RA (164, 167).
Gene Expression and Cell Signalling
For many years, mature neutrophils were wrongly believed to be transcriptionally silent, and any changes in protein levels during activation were believed to be solely due to mobilization of internal stores during priming and/or membrane shedding rather than synthesis of new protein. However, there is now an increasing body of work demonstrating that neutrophil gene expression is dynamic, being rapidly regulated over short time points by exposure to inflammatory agents, such as TNFα, GM-CSF, IL-1β, LPS, and opsonized micro-particles (169–171), and over several hours by chromatin remodeling, for example in response to the TLR8 agonist resiquimod (R848) (172). Neutrophils have the capacity to express and secrete a wide range of inflammatory mediators, including interleukins (including IL-1α and−1β, IL-1RA, IL-6, IL-12, and IL-23), chemokines (CCL and CXCL family members), TNF superfamily members (including TNFα, BLyS/BAFF, APRIL, TRAIL, and RANKL), metabolites of arachidonic acid (leukotriene B4, prostaglandin E2, and thromboxane A2), and angiogenic factors, such as VEGF and HGF (8, 22, 173–177).
Neutrophil gene expression is highly regulated during granulopoiesis (178–180), with transcripts for granule proteins (e.g., MPO, elastase, lactoferrin) becoming depleted once the mature protein has been packaged within granules in the maturing neutrophil (181). The content of neutrophil granules is determined by the order in which the granules develop: azurophilic granules first, followed by specific granules, then gelatinase granules, and finally secretory vesicles (182). Expression of neutrophil receptors (integrins, FcγR, cytokine receptors) is also highly controlled during granulopoiesis, with neutrophil markers CD16 (FcγR3b) and CD10 (neprilysin) for example only being expressed as the mature neutrophil is ready to exit the bone marrow (181, 183). Many neutrophil genes, including those coding for granule proteins, migratory proteins, chemokines, and chemokine receptors are also regulated by circadian rhythms (19). Dynamic changes in neutrophil gene expression also take place as cells migrate from the blood into inflamed tissues during inflammation, with transcripts for adhesion molecules decreasing and chemokine expression increasing at sites of inflammation (49, 184). Polymorphisms within expression quantitative trait loci (eQTLs) have been found in over 160 genes which play a fundamental role in every stage of neutrophil biology, from granulopoiesis to activation during infection and ultimately apoptosis. Several of these eQTLs are associated with known Mendelian disorders and inflammatory diseases (185).
Transcriptomic analysis of RA and SLE neutrophils has revealed a strong IFNα-induced gene expression signature in both conditions (186, 187). In RA, a high IFNα-regulated gene expression signature is a predictor of response to TNF inhibitor therapy (188). IFNα has recently been shown to regulate neutrophil activation in the presence of inflammatory cytokines (173) and in particular is involved in a switch in expression of chemokine genes. Whilst expression of genes for CCL and CXCL family chemokines is relatively low in blood neutrophils from RA patients with a high interferon gene expression signature, the expression of these chemokines increases significantly in RA synovial fluid neutrophils following migration to inflamed joints (49, 189–191). RA blood neutrophils also express mRNA for MHC Class II, with both RNA and MHC Class II protein being detected in RA synovial fluid neutrophils (192). Together this activated, synovial fluid neutrophil phenotype is responsible for driving activation of innate and adaptive immune cells in RA tissues. A polymorphism in the leukocyte phosphatase PTPN22 (R620W, rs2476601) enhances migration of RA neutrophils, and causes increased production of ROS following TNF-priming in vitro (193). Neutrophils from RA patients also express significantly higher levels of membrane proteinase-3 (mPR3) compared to healthy controls, an observation that is found in other neutrophil-driven autoimmune diseases, such as vasculitis, but not in T-cell driven type-I diabetes (194). In some RA patients, mRNA levels of granule genes (e.g., elastase, MPO) remain elevated even in mature blood neutrophils despite these transcripts normally being down-regulated during granulopoiesis (188). Expression of neutrophil granule genes is associated with non-response to TNF inhibitor therapy in RA but does not correlate with intracellular levels of granule proteins (188). Expression of membrane TNFα correlates with DAS28 and is decreased following successful treatment with TNF inhibitor therapy (48).
A global down-regulation of miRNA expression has been shown in RA blood and synovial fluid neutrophils compared to healthy controls (191). Decreased miRNA levels correlate with clinical parameters of disease including ACPA titer and DAS28 scores and is more pronounced in synovial fluid neutrophils. Targets of these down-regulated miRNAs include genes associated with cell migration, cell survival and inflammation, suggesting that a defect in miRNA expression may drive neutrophil-mediated inflammation within RA joints (191). An eQTL in the gene encoding PAD4 (PADI4, rs2240335-A) is associated with increased expression of PAD4 in neutrophils and is in almost complete linkage disequilibrium with the rs230188 SNP in PADI4 (LD r2 = 0.93) that confers an elevated risk of developing RA (185, 195).
In SLE, the IFNα-induced gene expression signature is believed to result from activation of pDCs by nuclear debris and NET fragments. The IFNα-induced gene expression signature can be detected in neutrophils, PBMCs and whole blood transcriptome analysis (187, 196–198), and in some studies has been shown to correlate with organ involvement and/or disease activity (198). However, a subset of SLE patients do not display this signature and indeed SLE patients are often described as being interferon “high” or “low” (196, 199). SLE neutrophil DNA is hypomethylated, especially near interferon-response genes (200). Some success in stratifying interferon-high patients to anti-IFNα therapy (anifrolumab) is evident (201), although the largest clinical trials of anifrolumab (TULIP-1 and TULIP-2) found no significant differences in the response to anti-interferon therapy between the interferon-high and interferon-low patient groups (202, 203). Expression of neutrophil genes within SLE whole blood has been attributed to the increase in the population of low density granulocytes (LDGs), discussed below (198), although proteomics analysis of SLE neutrophils and LDGs suggests that protein levels of MPO and other granule proteins is higher in SLE neutrophils (204). The expression of neutrophil granule protein genes in SLE whole blood is strongly associated with lupus nephritis (197, 205), vascular inflammation and cardiovascular involvement (206). SLE neutrophils also express high levels of BAFF/BLyS (198), the molecular target of belimumab which is the first biologic therapy developed to specifically target SLE (207).
Immunometabolism
Cellular metabolism is key regulator of neutrophil energy production, activation and function under conditions of both homeostasis and inflammation. The complex interplay of metabolic pathways and the utilization of shared metabolic intermediates has been the subject of great focus in immunology research as well as in diseases of metabolic dysfunction and aging (208–210). Complex changes in metabolic regulation within leukocytes occur during activation, when migration from blood to tissue or differentiation into tissue resident immune cells causes dramatic changes in nutrient and oxygen availability, placing sudden and high metabolic demands upon immune cells (211, 212). Fine-tuning of metabolism during an inflammatory response is a key for the generation of small molecule metabolites, such as ATP, NADPH, nucleotides, and amino acids, which are required rapidly and in high abundance during cellular activation (211, 212). Dysregulation of metabolic control has been described in inflammatory diseases, such as RA (213), where changes in T-cell glycolytic activity drives differentiation, hyperproliferation, and hypermigration of T-cell subsets (213–215).
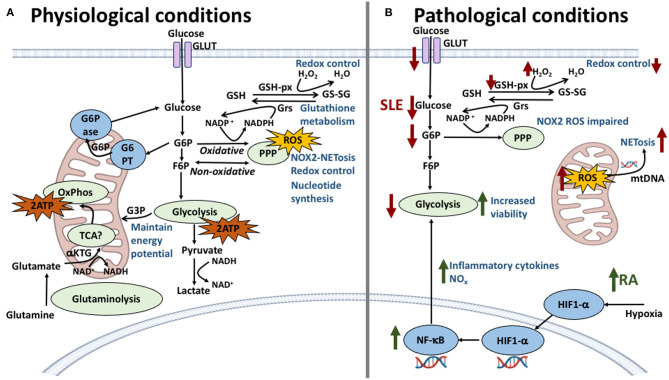
Glucose metabolism has its greatest role in neutrophil energy production (Figure 4); neutrophils are known to rely on glycolysis to fuel their energy requirements (216), where the multi-step enzymatic conversion of glucose into pyruvate in the cytosol provides relatively low levels of ATP and NADH. Pyruvate would normally be oxidized by mitochondria through the tricarboxylic acid (TCA) cycle in aerobic conditions, however in neutrophils it is converted instead into lactate, enabling the generation of NAD+ for re-use in the glycolytic pathway. The first intermediate of glycolysis, glucose 6-phosphate (G6P), fuels the pentose phosphate pathway (PPP). NAPDH is produced in the oxidative phase of the PPP, maintaining NOX2 activity and ROS production, and is necessary for chromatin decondensation, NOX2-dependent NET formation and NET release (217). The non-oxidative step of the PPP generates nucleic acids and glycolytic precursors. Tight control over glucose metabolism and PPP activity is achieved by the glucose-6-phosphate transporter (G6PT)/G6Pase-β complex which maintains cellular energy homeostasis and functionality in neutrophils by limiting G6P availability in the cytoplasm. Dysfunction in glucose homeostasis due to defective G6PT activity in neutrophils impairs ROS production, calcium mobilization and chemotaxis (218, 219).
Figure 4.
Neutrophil metabolic pathways and the effect of their dysregulation in SLE and RA. (A) In physiological conditions, glycolysis is the main energy producing pathway utilized by neutrophils; intermediate metabolites G6P and F6P are starter molecules for the pentose phosphate pathway (PPP), important in redox control, NOX2-dependent NETosis and ROS production. The oxidative stage of PPP produces NADPH which is used in the glutathione metabolism to reduce glutathione providing further redox capacity. Other energy producing pathways have been described, previously thought to be non-functional, such as the TCA cycle which connects glutaminolysis pathway and further regulates NOX2 complex by generating the reducing equivalents of NADPH. (B) In SLE and RA dysregulation of these pathways is responsible for the conditions observed at the cellular level. In SLE (red arrows), a lower expression of glucose transporters is met with lower levels of intracellular glucose, diminishing the energy output by glycolysis and compromising cellular viability. Furthermore, a decrease of G6P intermediates correlates with lower levels of NOX2-dependent ROS and decreased redox capacity. NOX2-independent ROS production in mitochondria is amplified in SLE neutrophils with increasing release of NETs containing mitochondrial DNA. In RA (green arrows), the inflammatory environment and hypoxic conditions increase the expression of HIF1-α which upregulates key glycolytic enzymes increasing energy production and viability. HIF1-α also is an upstream regulator of NF-κB which increases pro-inflammatory cytokine production therefore maintaining the inflammatory environment.
A number of other metabolic pathways have been described in neutrophils, including the Krebs/TCA cycle, oxidative phosphorylation (OXPHOS), and a fatty acid oxidation (FAO) pathway, all taking place within the mitochondria. Early experiments suggested that neutrophil mitochondria were not fully functionally active, having a dispensable role in neutrophil respiration and energy production. Mitochondrial density in human neutrophils is low and inhibitors of OXPHOS do not alter the rates of oxygen consumption or H2O2 production (220–222). However, the notion of metabolically inactive mitochondria was recently challenged by a study in mice which suggested that a metabolic shift from glycolysis to OXPHOS for energy sustainability was possible in neutrophils, both during changes that occur within the cancer tumor microenvironment, and in a number of neutrophil subsets (223). Mitochondria also have a wider and more important role in the regulation of aerobic glycolysis by maintaining the energy potential through complex III of the respiratory chain. Glycerol-3-phosphate (G3P), a by-product of glycolysis, can enter mitochondria where it is re-oxidized on the outer surface of the inner membrane, effectively maintaining membrane potential (224, 225). A further role for neutrophil mitochondrial metabolism in neutrophil function has been demonstrated, with mitochondrial activity being central to processes including ROS production, chemotaxis, and apoptosis (226–230). There is also increasing evidence of the utilization of the TCA cycle by neutrophils for energy metabolism under glucose-limited conditions (231) and during granulopoiesis (232). In addition, LDGs convert glutamate or proline to alpha-ketoglutarate (α-KG) to feed the TCA cycle and enable them to perform metabolically demanding neutrophil functions under conditions of glucose deprivation (233).
Parallel to glucose and mitochondrial metabolism, which are restricted to the cytosol and the mitochondria, glutaminolysis is a metabolic pathway that encompasses both environments and is tightly correlated with the phagocytic and bacterial killing ability of cultured neutrophils. Interestingly, neutrophils have been found to utilize more glutamine than other leukocytes including lymphocytes and macrophages (234). The precursor glutamine is not completely oxidized to generate ATP, and instead plays a role in the regulation and activation of the NOX2 complex, joining glucose metabolism in providing the means of rapidly generating the reducing equivalents of NADPH for the microbicidal NADPH oxidase (NOX2) system (235, 236). Glutaminolysis converts glutamine into TCA cycle metabolites, linking the two pathways together. Glutamine goes through a series of biochemical reactions where it is initially converted to glutamate, and then into α-ketoglutarate, which oxidizes NAD+ into NADH. At this point α-ketoglutarate enters the TCA cycle and is converted to malate which is then transported out of the mitochondria to be converted into pyruvate, which just like in glucose metabolism is converted into lactate to generate NAD+ (234, 237). Under conditions when glucose supply is limited, neutrophils can switch to the utilization of glutamine to meet their energetic needs (238). Furthermore, glutamine addition to cell culture medium of isolated neutrophils increases their phagocytic activity and rate of superoxide production (239). Glutamine has also been found to indirectly affect the functionality of neutrophils by modulating the production of IL-8, a neutrophil chemoattractant, in various cell types during activation (240, 241).
Within RA synovial fluid, a dynamic environment exists that controls neutrophil fate by a variety of anti- and pro-apoptotic factors. Low oxygen levels in the RA joint trigger a delay in apoptosis (58). HIF1-α is upregulated in hypoxic conditions by the family of oxygen sensing proteins known as prolyl hydroxylase domain enzymes (PHD1-3). HIF1-α exerts control over glycolysis by regulating the expression of key glycolytic enzymes, G3PDH and triosephosphate isomerse-1, providing a mechanism for the continued generation of ATP. This mechanism is an essential requirement for neutrophil functional responses to inflammation (242–244) and, by additionally directing the expression of the leukocyte β2 integrin CD18, it is critical for innate and adaptive immune responses (245). Studies have revealed that NF-κB is an important downstream effector of the HIF1-α-dependent response to hypoxia and that knockdown of the HIF1-α gene decreases glycolytic metabolism and induces cell death (59). RA synovial fluid contains significantly lower levels of glucose than OA synovial fluid, reflecting the high levels of cellular metabolism by infiltrating leukocytes and synovial tissues (246). Other metabolites within RA synovial fluid, including citrate, itaconate, and succinate, have been shown to regulate ROS production, cytokine expression and inflammasome activation in leukocytes (247–249), although the direct effect of these individual metabolites on synovial neutrophil function remains to be determined.
The abundance of apoptotic neutrophils in the blood along with their defective clearance and the propensity to form NETs contribute to the pathogenesis of SLE. Consistent studies reporting increased apoptosis, inflammatory phenotypes and mitochondrial defects in SLE neutrophils suggest that their cellular metabolism might be highly skewed (218, 250). SLE neutrophils have been found to have a decreased capacity for glucose uptake via defective expression of glucose transporters (GLUT-3 and GLUT-6) on the cell membrane compared to healthy neutrophils (250, 251). Limited glucose availability may pose a threat to cellular viability due to decreased glycolytic flux. This is true for other immune cells, such as lymphocytes, which enter BCL-2-regulated apoptosis when glycolytic flux decreases to levels that no longer sustain viability (252). Glucose availability is essential for the two primary pathways in activated neutrophils (glycolysis and PPP), and impaired glycolytic flux could explain the impaired NOX2-dependent ROS production seen in some SLE neutrophils, where glucose would be prioritized for energy production over ROS/NET production. Indeed, impairment of the G6PD/glucose flux in experimental models is directly associated with less NOX2 activity and with lower ROS production (253). Decreased levels of NOX2 ROS production may be compensated for by hyper-functional mitochondrial ROS production, which causes oxidation of unprotected mitochondrial DNA which is then extruded through NETosis (122). Increased mitochondrial ROS production and higher levels of O2-, H2O2, and HO• in SLE neutrophils (76, 77) are met by a decreased redox capacity compared to healthy controls (68, 251, 254). This is due to lower concentrations of glutathione which is critical for adequate redox capacity (255, 256). Neutrophils have a large capacity to keep glutathione in the reduced form via the activity of glutathione reductase (GRs) (257). A deficiency in this enzyme produces a more transient oxidative burst in response to bacteria (258). On the other hand, age-related impairment of glutathione peroxidase activity accounts for increased intracellular accumulation of hydrogen peroxide (259). Decreased levels of intracellular glutathione peroxidase (GSH-px), due to impaired GRs activity, leads to neutrophil dysfunction during conditions that are associated with chronic inflammation (260, 261). NOX2-independent ROS production in neutrophils is amplified by reagents affecting glutathione homeostasis (262). The wider impact of circulating SLE neutrophils in the modulation of other leukocyte activation is emphasized by the increased release of NETs containing mitochondrial DNA when compared to healthy donors; this was linked to chronic activation of pDCs and amplification of IFNα production (119, 263).
Neutrophil Subsets
The peripheral blood population of neutrophils is not a homogeneous pool (264); several populations of neutrophils have been identified which circulate alongside mature neutrophils, including low-density granulocytes (LDGs, CD15high/CD14low/CD10+/−/CD16+), granulocytic myeloid-derived suppressor cells (G-MDSCs, CD11chigh/CD62Llow/CD11bhigh/CD16high/CD33low), and reverse migrated neutrophils (RM, CD54+/CD18high/CXCR1low) (265– 267). Tumor associated neutrophils (TANs), which may have a pro- or anti-tumor phenotype, are present in many cancers (266, 268, 269); their function is outside the scope of this review.
G-MDSCs are an immune regulatory neutrophil subtype that inhibit the activation and expansion of autologous T cells, via the production of ROS at the immune synapse (270). Expression of MAC-1 (integrin αMβ2) is essential to the suppressor function of G-MDSCs (270). G-MDSCs with the ability to inhibit T cell proliferation are present in RA blood and synovial fluid (271, 272) and have been studied to greater effect in murine models of auto-immune arthritis, where they have been shown to inhibit both T cell proliferation and differentiation of Th1 and Th17 cells, and promote Treg numbers (273–275). G-MDSCs are found at a higher proportion in the blood of SLE patients than in healthy controls (272). SLE G-MDSCs produce high levels of ROS and also have the ability to impair T cell expansion (276). G-MDSCs from lupus-prone mice produce more ROS and NETs than healthy mice, however the population of G-MDSCs is unable to expand under inflammatory conditions, suggesting the loss of G-MDSCs due to NETosis may contribute to the impaired resolution of inflammation in SLE (277).
Reverse migration of neutrophils from sites of inflammation back into the circulation has been observed in zebrafish, mice and humans (267). Zebrafish RM neutrophils remain functional and able to respond to a second inflammatory challenge (278). RM neutrophils express high levels of ICAM-1 (CD54) (279), and in mice have been shown to contribute to systemic inflammation (280). RM neutrophils represent around 1–2% of circulating blood neutrophils in RA patients and only around 0.25% of blood neutrophils in healthy individuals (279). RM RA neutrophils have lower levels of constitutive apoptosis and produce higher amounts of ROS than circulating blood neutrophils (279).
Low-density granulocytes (LDGs) were first reported in the blood of SLE patients in 1986 (281) but their function and pathological significance has only recently been explored. These cells, remaining in the peripheral blood mononuclear cell (PBMC) layer after density-gradient centrifugation, express cell-surface markers specific to mature neutrophils (CD15high/CD14low/CD10+/−/CD16+) (282, 283), whilst expressing mRNA transcripts characteristic of immature neutrophils (e.g., MPO, elastase) (120, 283, 284). High numbers of LDGs in SLE blood correlate with skin involvement, vasculitis, dsDNA titers and SLEDAI scores (120, 284, 285), and SLE LDGs have an increased tendency to form NETs in vitro (120). Un-stimulated SLE LDGs secrete increased amounts of IL-8 and IL-6 and have impaired phagocytic capacity (282). SLE LDGs also stimulate production of TNFα, TNFβ, and IFNγ by T cells (286). Recent work has revealed that the SLE LDG population is heterogeneous (mature CD10+ or immature CD10−), with significant differences in transcriptomic and epigenomic regulation of function and phenotype that correlates with clinical manifestations of the disease (187, 284). Mature CD10+ SLE LDGs express high amounts of mRNA and protein for interferon-regulated genes, whereas immature CD10− SLE LDGs express high amounts of mRNA for cell cycle genes (187, 204). CD10+ SLE LDGs undergo phagocytosis, chemotaxis and NETosis at higher levels than CD10− LDGs, which release MPO at higher amounts than CD10+ LDGs and normal density SLE neutrophils (187). It has been suggested that SLE LDGs undergo NETosis in response to the production of mtROS, with SLE LDG NETs containing mitochondrial DNA including oxidized DNA (8-oxo-2'-deoxyguanosine) which is strongly interferogenic (122). This phenomenon is also observed in chronic granulomatous disease LDGs, which lack functional NOX2 but can produce mtROS and NETs (122). SLE LDGs have stiffer biomechanical properties and are slower to migrate through microvascular mimetics in vitro (204). This may explain why LDGs are not found in affected tissues in SLE (284).
RA LDGs have a distinct transcriptome profile compared to RA neutrophils, expressing high levels of transcripts for granule proteins and cell cycle checkpoint genes, and lower levels of expression of apoptotic genes, cytokines, chemokines, and signaling receptors (283). The presence of LDGs in RA blood is unaffected by therapy, and LDG counts correlate with measures of disease activity (DAS28) (283). RA LDGs undergo lower levels of apoptosis in vitro after overnight culture; however whilst LDG apoptosis can be further delayed by GM-CSF, LDG apoptosis is unaffected by TNF-α. In addition, ROS production by TNFα-primed RA LDGs is lower than paired blood neutrophils, likely due to their lower expression of TNF-receptors (283). NET production by RA LDGs is not significantly different from paired neutrophils (283).
Whilst the main focus of investigation into the phenotype of LDGs has focused on SLE and to a lesser extent RA, their presence in the blood is not exclusive to rheumatic disease. LDGs have been identified in many other disease settings, including asthma, vasculitis, multiple sclerosis and chronic kidney disease (287–290). Indeed, they are even present in low numbers in healthy controls (291). Isolation of LDGs from blood is highly dependent upon the density of the isolation medium used (e.g., Ficoll, Percoll, Polymorphprep) (292) and this raises the question as to whether studies using different isolation protocols for preparation of neutrophils and LDGs from whole blood can be directly compared. There are mixed reports on the functionality of LDGs from healthy controls, and whether they have different immunological properties (e.g., T cell suppression) to normal density neutrophils and LDGs from inflammatory disease (291, 292). Another key question is whether LDGs represent a novel subset of neutrophils, or whether their phenotype reflects one of spectra of phenotypes that blood neutrophils may exhibit through functional plasticity. Evidence from RA and SLE (CD10− LDGs), where LDGs express cell cycle genes and transcripts for neutrophil granule proteins, suggests that these cells may have arisen from emergency granulopoiesis due to chronic inflammation (187, 283). Administration of LPS in vivo to healthy volunteers appears to support this conclusion, with an increase in immature CD16dim band cells being observed in blood 3h after LPS challenge (292). However, it is also possible to induce a low density phenotype from normal density neutrophils in vitro by activation with agents, such as fMLP, platelet activating factor, TNFα and LPS (291, 292), suggesting that LDGs may represent a subset of primed or activated neutrophils (292). Further work needs to be carried out to determine the true origin, phenotype and nature of LDGs and other neutrophil subsets, such as G-MDSCs (264).
Neutrophils as a Therapeutic Target
In this review we have discussed the multitude of ways that inflammatory neutrophils drive inflammation in RA and SLE. This raises the potential to target dysregulated neutrophil activation with therapeutics in both diseases (293). In RA, neutrophil activation can be targeted by biologic DMARDs (bDMARDs), such as anti-TNF therapy, which has been demonstrated to decrease neutrophil membrane TNF expression and NF-κB activation (48). Newer orally available, small molecule therapies, such as JAK inhibitors have shown good efficacy in RA, as they target intracellular signaling via a number of cytokine receptors, including IFNα, IFNγ, GM-CSF, and IL-6 (294, 295). JAK inhibitors baricitinib (JAK1/2) and tofacitinib (JAK3/1) inhibit cytokine priming in neutrophils and can inhibit RA neutrophil migration and ROS production (296). In SLE, the bDMARD belimumab inhibits the cytokine BLyS/BAFF, a major source of which is activated neutrophils and LDGs (175, 198). Belimumab is one of only two drugs specifically licensed to treat SLE in the UK, the other being hydroxychloroquine.
Hydroxychloroquine, an anti-malarial already widely used to treat both RA and SLE, is a potent modulator of neutrophil function. It has been shown to inhibit neutrophilic inflammation into inflamed kidneys (297), inhibit NET production via inhibition of TLR9 (298), and block ROS and IL-8 production in response to RNA-containing immune complexes (152). Methotrexate, commonly used in both SLE and RA, inhibits cytokine-delayed neutrophil apoptosis, ROS production and leukotriene B4 synthesis (299–301). Glucocorticoids are frequently used in both RA and SLE to control disease flares. Prednisolone for example, rapidly disarms pro-inflammatory neutrophils and inhibit both ROS release and production of pro-inflammatory mediators (14, 302, 303).
Several newer therapies under development or in clinical trial also target neutrophil activation. Major activators of neutrophil production and priming, the colony stimulating factors (CSFs), are exciting targets for treatment of neutrophil-driven inflammatory diseases. Anti-GM-CSF (mavrilimumab) therapy has had success in RA clinical trials (294), and anti-G-CSF therapy is effective in treating murine arthritis, both inhibiting neutrophil migration into joints, and suppressing cytokine production (304). Neutrophil migration into inflammatory murine joints is also significantly decreased by inhibitors of CXCR1/CXCR2, the receptor for CXCL8 (IL-8) (305). This decrease in neutrophil infiltration is mirrored by lower disease activity and TNFα production within the joint (306). Bosutinib, an Abl/Src kinase inhibitor currently used to treat patients with chronic myeloid leukemia, inhibits neutrophil FcγR2A-induced ROS production, recruitment to glomerular capillaries and kidney injury in an immune complex-driven model of kidney disease (85) suggesting this may be a promising therapy to target neutrophilic damage in lupus nephritis.
Excess NET production represents an exciting prospect for therapeutic development, with the potential to break the chain leading to auto-antigen recognition, activation of pDCs, interferon production, auto-antibody production and damage to local tissues, such as cartilage and microvessels within the kidney. As mentioned earlier, a clinical trial of rituximab and belimumab inhibited NET production in SLE, and this was associated with lower auto-antibody titers (including lower anti-dsDNA and anti-histones) and a decrease in disease activity (154, 155). NET production may also be targeted by inhibitors of PAD4, MPO and neutrophil elastase. MPO and elastase inhibitors reduce neutrophil-driven inflammation in animal models of inflammatory disease and human respiratory disease (307, 308). PAD4 is an enzyme that catalyses the conversion of arginine to citrulline (116, 309). It plays an important role in chromatin decondensation during NETosis and is physically associated with the cytosolic subunits of the oxidative burst machinery in a way that regulates assembly of the active NOX2 complex (116, 309). Over the last few years, PAD4 has emerged as a potential therapeutic for the treatment of RA and SLE. Initially irreversible inhibitor compounds, such as F- and Cl-amide were found to inhibit PAD4 (310), and showed efficacy in RA and SLE models through the inhibition of NET production. Cl-amidine prevented development of atherosclerotic plaques, interferon production and immune complex deposition in the kidney in lupus-prone mice (148, 149, 311). However, their poorly understood involvement in this process was the driving force for discovering reversible inhibitors, such as GSK484 and GSK199. GSK484 is the more potent of the two and selectively targets PAD4. It inhibits citrullination in primary neutrophils and NET formation in both mouse and human neutrophils (312, 313). Currently novel agents targeting PAD4 are being developed, which have shown to decrease the levels of circulating NET DNA in serum (313, 314). However, complete inhibition of NET production may block an important neutrophil function that provides protection against infection (315). Indeed, PAD4 knock out mice are highly susceptible to developing systemic inflammation from bacterial keratitis, where NETs normally function to protect the host from infection at the expense of the cornea (316). PAD enzymes also have a key physiological role in regulating gene expression and cellular differentiation, therefore a more targeted approach to PAD/NET inhibition may be required for development of therapeutics (315).
Final Summary
In this review we have highlighted the way in which dysregulated neutrophil activation can contribute to the development and progression of RA and SLE. In particular, dysregulated apoptosis and NETosis lead to exposure of intracellular post-translationally modified proteins and DNA activating the adaptive immune response (interferon release, auto-antibody production) and inducing damage to tissues either directly or by activating neighboring cells. Neutrophil degranulation and ROS production damage local tissues and contribute to systemic inflammation. Aberrant neutrophil activation in RA and SLE is caused, in part, by a dysregulation of gene expression and metabolism, via different mechanisms specific to each disease. Targeting unwanted neutrophil activation in RA and SLE may be a promising avenue for investigation and may have fewer side effects than the broad-spectrum immunosuppressants often used to treat these life-limiting auto-immune conditions.
Author Contributions
MF, ZM, and HW wrote, edited, and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. MF was funded by a Versus Arthritis and Masonic Charitable Foundation Ph.D. scholarship (Grant No. 22193). HW was funded by a Versus Arthritis Career Development Fellowship (Grant No. 21430).
References
- 1.Bengtsson AA, Ronnblom L. Systemic lupus erythematosus: still a challenge for physicians. J Intern Med. (2017) 281:52–64. 10.1111/joim.12529 [DOI] [PubMed] [Google Scholar]
- 2.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey's end? Rheumatology (Oxford). (2007) 46:1052–6. 10.1093/rheumatology/kem112 [DOI] [PubMed] [Google Scholar]
- 3.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. (1999) 42:338–46. [DOI] [PubMed] [Google Scholar]
- 4.Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. (2014) 384:1878–88. 10.1016/S0140-6736(14)60128-8 [DOI] [PubMed] [Google Scholar]
- 5.Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol. (2016) 12:143–53. 10.1038/nrrheum.2015.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishi H, Mayadas TN. Neutrophils in lupus nephritis. Curr Opin Rheumatol. (2019) 31:193–200. 10.1097/BOR.0000000000000577 [DOI] [PubMed] [Google Scholar]
- 7.Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol. (2014) 10:338–47. 10.1038/nrrheum.2014.15 [DOI] [PubMed] [Google Scholar]
- 8.Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. (2014) 10:593–601. 10.1038/nrrheum.2014.80 [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. (2016) 388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 10.Bucknall RC. Arthritis and inflammatory eye disease. Rheumatology (Oxford). (2005) 44:1207–9. 10.1093/rheumatology/kei027 [DOI] [PubMed] [Google Scholar]
- 11.Roman MJ, Salmon JE. Cardiovascular manifestations of rheumatologic diseases. Circulation. (2007) 116:2346–55. 10.1161/CIRCULATIONAHA.106.678334 [DOI] [PubMed] [Google Scholar]
- 12.McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. (2017) 389:2328–37. 10.1016/S0140-6736(17)31472-1 [DOI] [PubMed] [Google Scholar]
- 13.Zharkova O, Celhar T, Cravens PD, Satterthwaite AB, Fairhurst AM, Davis LS. Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology (Oxford). (2017) 56:i55–66. 10.1093/rheumatology/kew427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford). (2010) 49:1618–31. 10.1093/rheumatology/keq045 [DOI] [PubMed] [Google Scholar]
- 15.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. (2011) 13:e23. 10.1017/S1462399411001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. (2018) 9:113. 10.3389/fphys.2018.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. (2010) 31:318–24. 10.1016/j.it.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidalgo A, Chilvers ER, Summers C, Koenderman L. The neutrophil life cycle. Trends Immunol. (2019) 40:584–97. 10.1016/j.it.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 19.Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. (2019) 50:390–402 e310. 10.1016/j.immuni.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 20.Adrover JM, Aroca-Crevillen A, Crainiciuc G, Ostos F, Rojas-Vega Y, Rubio-Ponce A, et al. Programmed ‘disarming' of the neutrophil proteome reduces the magnitude of inflammation. Nat Immunol. (2020) 21:135–44. 10.1038/s41590-019-0571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. (2006) 6:541–50. 10.1038/nri1841 [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. (2011) 11:519–31. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 23.Desgeorges A, Gabay C, Silacci P, Novick D, Roux-Lombard P, Grau G, et al. Concentrations and origins of soluble interleukin 6 receptor-alpha in serum and synovial fluid. J Rheumatol. (1997) 24:1510–6. [PubMed] [Google Scholar]
- 24.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. (2001) 14:705–14. 10.1016/s1074-7613(01)00151-0 [DOI] [PubMed] [Google Scholar]
- 25.Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. (2011) 7:691–9. 10.1038/nrrheum.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V. Human neutrophils in auto-immunity. Semin Immunol. (2016) 28:159–73. 10.1016/j.smim.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 27.McCracken JM, Allen LA. Regulation of human neutrophil apoptosis and lifespan in health and disease. J Cell Death. (2014) 7:15–23. 10.4137/JCD.S11038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Kebir D, Filep JG. Modulation of neutrophil apoptosis and the resolution of inflammation through beta2 integrins. Front Immunol. (2013) 4:60. 10.3389/fimmu.2013.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. (2007) 109:1620–6. 10.1182/blood-2006-03-013771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein JB, Buridi A, Coxon PY, Rane MJ, Manning T, Kettritz R, et al. Role of extracellular signal-regulated kinase and phosphatidylinositol-3 kinase in chemoattractant and LPS delay of constitutive neutrophil apoptosis. Cell Signal. (2001) 13:335–43. 10.1016/s0898-6568(01)00151-6 [DOI] [PubMed] [Google Scholar]
- 31.Klein JB, Rane MJ, Scherzer JA, Coxon PY, Kettritz R, Mathiesen JM, et al. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J Immunol. (2000) 164:4286–91. 10.4049/jimmunol.164.8.4286 [DOI] [PubMed] [Google Scholar]
- 32.Derouet M, Thomas L, Cross A, Moots RJ, Edwards SW. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J Biol Chem. (2004) 279:26915–21. 10.1074/jbc.M313875200 [DOI] [PubMed] [Google Scholar]
- 33.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. (1998) 92:2495–502. [PubMed] [Google Scholar]
- 34.Cross A, Moots RJ, Edwards SW. The dual effects of TNFalpha on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood. (2008) 111:878–84. 10.1182/blood-2007-05-087833 [DOI] [PubMed] [Google Scholar]
- 35.Petrin D, Turcotte S, Gilbert AK, Rola-Pleszczynski M, Stankova J. The anti-apoptotic effect of leukotriene B4 in neutrophils: a role for phosphatidylinositol 3-kinase, extracellular signal-regulated kinase and Mcl-1. Cell Signal. (2006) 18:479–87. 10.1016/j.cellsig.2005.05.021 [DOI] [PubMed] [Google Scholar]
- 36.Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. (2011) 18:1457–69. 10.1038/cdd.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Berg JM, Weyer S, Weening JJ, Roos D, Kuijpers TW. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. (2001) 69:467–73. 10.1189/jlb.69.3.467 [DOI] [PubMed] [Google Scholar]
- 38.Murray J, Barbara JA, Dunkley SA, Lopez AF, Van Ostade X, Condliffe AM, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. (1997) 90:2772–83. [PubMed] [Google Scholar]
- 39.Nolan B, Kim R, Duffy A, Sheth K, De M, Miller C, et al. Inhibited neutrophil apoptosis: proteasome dependent NF-kappaB translocation is required for TRAF-1 synthesis. Shock. (2000) 14:290–4. 10.1097/00024382-200014030-00008 [DOI] [PubMed] [Google Scholar]
- 40.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. (1994) 153:1254–63. [PubMed] [Google Scholar]
- 41.Midgley A, McLaren Z, Moots RJ, Edwards SW, Beresford MW. The role of neutrophil apoptosis in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. (2009) 60:2390–401. 10.1002/art.24634 [DOI] [PubMed] [Google Scholar]
- 42.Chiewchengchol D, Midgley A, Sodsai P, Deekajorndech T, Hirankarn N, Beresford MW, et al. The protective effect of GM-CSF on serum-induced neutrophil apoptosis in juvenile systemic lupus erythematosus patients. Clin Rheumatol. (2015) 34:85–91. 10.1007/s10067-014-2800-2 [DOI] [PubMed] [Google Scholar]
- 43.Courtney PA, Crockard AD, Williamson K, Irvine AE, Kennedy RJ, Bell AL. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. (1999) 58:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Midgley A, Mayer K, Edwards SW, Beresford MW. Differential expression of factors involved in the intrinsic and extrinsic apoptotic pathways in juvenile systemic lupus erythematosus. Lupus. (2011) 20:71–9. 10.1177/0961203310382128 [DOI] [PubMed] [Google Scholar]
- 45.Midgley A, Thorbinson C, Beresford MW. Expression of Toll-like receptors and their detection of nuclear self-antigen leading to immune activation in JSLE. Rheumatology (Oxford). (2012) 51:824–32. 10.1093/rheumatology/ker400 [DOI] [PubMed] [Google Scholar]
- 46.Midgley A, Beresford MW. Cellular localization of nuclear antigen during neutrophil apoptosis: mechanism for autoantigen exposure? Lupus. (2011) 20:641–6. 10.1177/0961203310392421 [DOI] [PubMed] [Google Scholar]
- 47.Mahajan A, Herrmann M, Munoz LE. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front Immunol. (2016) 7:35. 10.3389/fimmu.2016.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright HL, Chikura B, Bucknall RC, Moots RJ, Edwards SW. Changes in expression of membrane TNF, NF-{kappa}B activation and neutrophil apoptosis during active and resolved inflammation. Ann Rheum Dis. (2011) 70:537–43. 10.1136/ard.2010.138065 [DOI] [PubMed] [Google Scholar]
- 49.Wright HL, Lyon M, Chapman EA, Moots RJ, Edwards SW. Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species and neutrophil extracellular traps. Front Immunol. (2021) 11:584116. 10.3389/fimmu.2020.584116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright HL, Bucknall RC, Moots RJ, Edwards SW. Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatology (Oxford). (2012) 51:451–9. 10.1093/rheumatology/ker338 [DOI] [PubMed] [Google Scholar]
- 51.Watson RW, Rotstein OD, Parodo J, Bitar R, Marshall JC. The IL-1 beta-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1 beta. J Immunol. (1998) 161:957–62. [PubMed] [Google Scholar]
- 52.Maianski NA, Roos D, Kuijpers TW. Bid truncation, bid/bax targeting to the mitochondria, and caspase activation associated with neutrophil apoptosis are inhibited by granulocyte colony-stimulating factor. J Immunol. (2004) 172:7024–30. 10.4049/jimmunol.172.11.7024 [DOI] [PubMed] [Google Scholar]
- 53.Salamone G, Trevani A, Martinez D, Vermeulen M, Gamberale R, Fernandez-Calotti P, et al. Analysis of the mechanisms involved in the stimulation of neutrophil apoptosis by tumour necrosis factor-alpha. Immunology. (2004) 113:355–62. 10.1111/j.1365-2567.2004.01973.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakamoto E, Hato F, Kato T, Sakamoto C, Akahori M, Hino M, et al. Type I and type II interferons delay human neutrophil apoptosis via activation of STAT3 and up-regulation of cellular inhibitor of apoptosis 2. J Leukoc Biol. (2005) 78:301–9. 10.1189/jlb.1104690 [DOI] [PubMed] [Google Scholar]
- 55.Parsonage G, Filer A, Bik M, Hardie D, Lax S, Howlett K, et al. Prolonged, granulocyte-macrophage colony-stimulating factor-dependent, neutrophil survival following rheumatoid synovial fibroblast activation by IL-17 and TNFalpha. Arthritis Res Ther. (2008) 10:R47–59. 10.1186/ar2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong SH, Francis N, Chahal H, Raza K, Salmon M, Scheel-Toellner D, et al. Lactoferrin is a survival factor for neutrophils in rheumatoid synovial fluid. Rheumatology (Oxford). (2009) 48:39–44. 10.1093/rheumatology/ken412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ottonello L, Cutolo M, Frumento G, Arduino N, Bertolotto M, Mancini M, et al. Synovial fluid from patients with rheumatoid arthritis inhibits neutrophil apoptosis: role of adenosine and proinflammatory cytokines. Rheumatology (Oxford). (2002) 41:1249–60. 10.1093/rheumatology/41.11.1249 [DOI] [PubMed] [Google Scholar]
- 58.Cross A, Barnes T, Bucknall RC, Edwards SW, Moots RJ. Neutrophil apoptosis in rheumatoid arthritis is regulated by local oxygen tensions within joints. J Leukoc Biol. (2006) 80:521–8. 10.1189/jlb.0306178 [DOI] [PubMed] [Google Scholar]
- 59.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. (2005) 201:105–15. 10.1084/jem.20040624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, et al. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. (2011) 118:712–22. 10.1182/blood-2010-12-324186 [DOI] [PubMed] [Google Scholar]
- 61.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. (2016) 228:R57–71. 10.1530/JOE-15-0201 [DOI] [PubMed] [Google Scholar]
- 62.Dieker J, Tel J, Pieterse E, Thielen A, Rother N, Bakker M, et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. (2016) 68:462–72. 10.1002/art.39417 [DOI] [PubMed] [Google Scholar]
- 63.Winberg LK, Jacobsen S, Nielsen CH. Microparticles from patients with systemic lupus erythematosus induce production of reactive oxygen species and degranulation of polymorphonuclear leukocytes. Arthritis Res Ther. (2017) 19:230. 10.1186/s13075-017-1437-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rother N, Pieterse E, Lubbers J, Hilbrands L, van der Vlag J. Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis. Front Immunol. (2017) 8:1136. 10.3389/fimmu.2017.01136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. (2015) 7:315ra190. 10.1126/scitranslmed.aac5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Headland SE, Norling LV. The resolution of inflammation: principles and challenges. Semin Immunol. (2015) 27:149–60. 10.1016/j.smim.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 67.Perretti M, Solito E. Annexin 1 and neutrophil apoptosis. Biochem Soc Trans. (2004) 32:507–10. 10.1042/BST0320507 [DOI] [PubMed] [Google Scholar]
- 68.Glennon-Alty L, Hackett AP, Chapman EA, Wright HL. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic Biol Med. (2018) 125:25–35. 10.1016/j.freeradbiomed.2018.03.049 [DOI] [PubMed] [Google Scholar]
- 69.Dang PM, Dewas C, Gaudry M, Fay M, Pedruzzi E, Gougerot-Pocidalo MA, et al. Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47(phox). J Biol Chem. (1999) 274:20704–8. [DOI] [PubMed] [Google Scholar]
- 70.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. (2008) 30:279–89. 10.1007/s00281-008-0118-3 [DOI] [PubMed] [Google Scholar]
- 71.Ward RA, Nakamura M, McLeish KR. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J Biol Chem. (2000) 275:36713–9. 10.1074/jbc.M003017200 [DOI] [PubMed] [Google Scholar]
- 72.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. (1983) 97:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kjeldsen L, Sengelov H, Lollike K, Nielsen MH, Borregaard N. Isolation and characterization of gelatinase granules from human neutrophils. Blood. (1994) 83:1640–9. [PubMed] [Google Scholar]
- 74.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. (2014) 20:1126–67. 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nauseef WM. Myeloperoxidase in human neutrophil host defence. Cell Microbiol. (2014) 16:1146–55. 10.1111/cmi.12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elloumi N, Ben Mansour R, Marzouk S, Mseddi M, Fakhfakh R, Gargouri B, et al. Differential reactive oxygen species production of neutrophils and their oxidative damage in patients with active and inactive systemic lupus erythematosus. Immunol Lett. (2017) 184:1–6. 10.1016/j.imlet.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 77.Perazzio SF, Salomao R, Silva NP, Andrade LE. Increased neutrophil oxidative burst metabolism in systemic lupus erythematosus. Lupus. (2012) 21:1543–51. 10.1177/0961203312461060 [DOI] [PubMed] [Google Scholar]
- 78.McConnell JR, Crockard AD, Cairns AP, Bell AL. Neutrophils from systemic lupus erythematosus patients demonstrate increased nuclear DNA damage. Clin Exp Rheumatol. (2002) 20:653–60. [PubMed] [Google Scholar]
- 79.Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. (2013) 39:482–95. 10.1016/j.immuni.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 80.Alves CM, Marzocchi-Machado CM, Louzada-Junior P, Azzolini AE, Polizello AC, de Carvalho IF, et al. Superoxide anion production by neutrophils is associated with prevalent clinical manifestations in systemic lupus erythematosus. Clin Rheumatol. (2008) 27:701–8. 10.1007/s10067-007-0768-x [DOI] [PubMed] [Google Scholar]
- 81.Shingu M, Oribe M, Todoroki T, Tatsukawa K, Tomo-oka K, Yasuda M, et al. Serum factors from patients with systemic lupus erythematosus enhancing superoxide generation by normal neutrophils. J Invest Dermatol. (1983) 81:212–5. [DOI] [PubMed] [Google Scholar]
- 82.Marzocchi-Machado CM, Alves CM, Azzolini AE, Polizello AC, Carvalho IF, Lucisano-Valim YM. Fcgamma and complement receptors: expression, role and co-operation in mediating the oxidative burst and degranulation of neutrophils of Brazilian systemic lupus erythematosus patients. Lupus. (2002) 11:240–8. 10.1191/0961203302lu172oa [DOI] [PubMed] [Google Scholar]
- 83.Russell DA, Markiewicz M, Oates JC. Lupus serum induces inflammatory interaction with neutrophils in human glomerular endothelial cells. Lupus Sci Med. (2020) 7:418. 10.1136/lupus-2020-000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O'Flynn J, Flierman R, van der Pol P, Rops A, Satchell SC, Mathieson PW, et al. Nucleosomes and C1q bound to glomerular endothelial cells serve as targets for autoantibodies and determine complement activation. Mol Immunol. (2011) 49:75–83. 10.1016/j.molimm.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 85.Nishi H, Furuhashi K, Cullere X, Saggu G, Miller MJ, Chen Y, et al. Neutrophil FcgammaRIIA promotes IgG-mediated glomerular neutrophil capture via Abl/Src kinases. J Clin Invest. (2017) 127:3810–26. 10.1172/JCI94039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flores-Mendoza G, Sanson SP, Rodriguez-Castro S, Crispin JC, Rosetti F. Mechanisms of tissue injury in lupus nephritis. Trends Mol Med. (2018) 24:364–78. 10.1016/j.molmed.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 87.Smith CK, Seto NL, Vivekanandan-Giri A, Yuan W, Playford MP, Manna Z, et al. Lupus high-density lipoprotein induces proinflammatory responses in macrophages by binding lectin-like oxidised low-density lipoprotein receptor 1 and failing to promote activating transcription factor 3 activity. Ann Rheum Dis. (2017) 76:602–11. 10.1136/annrheumdis-2016-209683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urbonaviciute V, Luo H, Sjowall C, Bengtsson A, Holmdahl R. Low production of reactive oxygen species drives systemic lupus erythematosus. Trends Mol Med. (2019) 25:826–35. 10.1016/j.molmed.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 89.Olsson LM, Johansson AC, Gullstrand B, Jonsen A, Saevarsdottir S, Ronnblom L, et al. A single nucleotide polymorphism in the NCF1 gene leading to reduced oxidative burst is associated with systemic lupus erythematosus. Ann Rheum Dis. (2017) 76:1607–13. 10.1136/annrheumdis-2017-211287 [DOI] [PubMed] [Google Scholar]
- 90.Kelkka T, Kienhofer D, Hoffmann M, Linja M, Wing K, Sareila O, et al. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxid Redox Signal. (2014) 21:2231–45. 10.1089/ars.2013.5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kundu S, Ghosh P, Datta S, Ghosh A, Chattopadhyay S, Chatterjee M. Oxidative stress as a potential biomarker for determining disease activity in patients with rheumatoid arthritis. Free Radic Res. (2012) 46:1482–9. 10.3109/10715762.2012.727991 [DOI] [PubMed] [Google Scholar]
- 92.El Benna J, Hayem G, Dang PM, Fay M, Chollet-Martin S, Elbim C, et al. NADPH oxidase priming and p47phox phosphorylation in neutrophils from synovial fluid of patients with rheumatoid arthritis and spondylarthropathy. Inflammation. (2002) 26:273–8. 10.1023/a:1021460517468 [DOI] [PubMed] [Google Scholar]
- 93.Rasheed Z. Hydroxyl radical damaged immunoglobulin G in patients with rheumatoid arthritis: biochemical and immunological studies. Clin Biochem. (2008) 41:663–9. 10.1016/j.clinbiochem.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 94.Fossati G, Bucknall RC, Edwards SW. Insoluble and soluble immune complexes activate neutrophils by distinct activation mechanisms: changes in functional responses induced by priming with cytokines. Ann Rheum Dis. (2002) 61:13–9. 10.1136/ard.61.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robinson J, Watson F, Bucknall RC, Edwards SW. Activation of neutrophil reactive-oxidant production by synovial fluid from patients with inflammatory joint disease. Soluble and insoluble immunoglobulin aggregates activate different pathways in primed and unprimed cells. Biochem J. (1992) 286:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edwards SW, Hughes V, Barlow J, Bucknall R. Immunological detection of myeloperoxidase in synovial fluid from patients with rheumatoid arthritis. Biochem J. (1988) 250:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nzeusseu Toukap A, Delporte C, Noyon C, Franck T, Rousseau A, Serteyn D, et al. Myeloperoxidase and its products in synovial fluid of patients with treated or untreated rheumatoid arthritis. Free Radic Res. (2014) 48:461–5. 10.3109/10715762.2014.886327 [DOI] [PubMed] [Google Scholar]
- 98.Birkelund S, Bennike TB, Kastaniegaard K, Lausen M, Poulsen TBG, Kragstrup TW, et al. Proteomic analysis of synovial fluid from rheumatic arthritis and spondyloarthritis patients. Clin Proteomics. (2020) 17:29. 10.1186/s12014-020-09292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baici A, Salgam P, Cohen G, Fehr K, Boni A. Action of collagenase and elastase from human polymorphonuclear leukocytes on human articular cartilage. Rheumatol Int. (1982) 2:11–6. [DOI] [PubMed] [Google Scholar]
- 100.Van den Steen PE, Proost P, Grillet B, Brand DD, Kang AH, Van Damme J, et al. Cleavage of denatured natural collagen type II by neutrophil gelatinase B reveals enzyme specificity, post-translational modifications in the substrate, and the formation of remnant epitopes in rheumatoid arthritis. FASEB J. (2002) 16:379–89. 10.1096/fj.01-0688com [DOI] [PubMed] [Google Scholar]
- 101.Burkhardt H, Schwingel M, Menninger H, Macartney HW, Tschesche H. Oxygen radicals as effectors of cartilage destruction. Direct degradative effect on matrix components and indirect action via activation of latent collagenase from polymorphonuclear leukocytes. Arthritis Rheum. (1986) 29:379–87. [DOI] [PubMed] [Google Scholar]
- 102.Shabani F, McNeil J, Tippett L. The oxidative inactivation of tissue inhibitor of metalloproteinase-1 (TIMP-1) by hypochlorous acid (HOCI) is suppressed by anti-rheumatic drugs. Free Radic Res. (1998) 28:115–23. [DOI] [PubMed] [Google Scholar]
- 103.Onodera Y, Teramura T, Takehara T, Shigi K, Fukuda K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio. (2015) 5:492–501. 10.1016/j.fob.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cemerski S, Cantagrel A, Van Meerwijk JP, Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem. (2002) 277:19585–93. 10.1074/jbc.M111451200 [DOI] [PubMed] [Google Scholar]
- 105.Abimannan T, Peroumal D, Parida JR, Barik PK, Padhan P, Devadas S. Oxidative stress modulates the cytokine response of differentiated Th17 and Th1 cells. Free Radic Biol Med. (2016) 99:352–63. 10.1016/j.freeradbiomed.2016.08.026 [DOI] [PubMed] [Google Scholar]
- 106.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 107.Brinkmann V, Goosmann C, Kuhn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Front Immunol. (2012) 3:413. 10.3389/fimmu.2012.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosazza T, Warner J, Sollberger G. NET formation–mechanisms and how they relate to other cell death pathways. FEBS J. (2020). 10.1111/febs.15589. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 109.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. (2010) 191:677–91. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. (2011) 21:290–304. 10.1038/cr.2010.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. (2008) 180:1895–902. 10.4049/jimmunol.180.3.1895 [DOI] [PubMed] [Google Scholar]
- 112.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci USA. (2015) 112:2817–22. 10.1073/pnas.1414055112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. (2012) 3:360. 10.3389/fimmu.2012.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu CL, Tangsombatvisit S, Rosenberg JM, Mandelbaum G, Gillespie EC, Gozani OP, et al. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther. (2012) 14:R25. 10.1186/ar3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. (2009) 184:205–13. 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. (2010) 207:1853–62. 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS ONE. (2011) 6:e22043. 10.1371/journal.pone.0022043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pieterse E, Rother N, Yanginlar C, Gerretsen J, Boeltz S, Munoz LE, et al. Cleaved N-terminal histone tails distinguish between NADPH oxidase (NOX)-dependent and NOX-independent pathways of neutrophil extracellular trap formation. Ann Rheum Dis. (2018) 77:1790–8. 10.1136/annrheumdis-2018-213223 [DOI] [PubMed] [Google Scholar]
- 119.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. (2011) 3:73ra19. 10.1126/scitranslmed.3001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. (2011) 187:538–52. 10.4049/jimmunol.1100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dieker J, Berden JH, Bakker M, Briand JP, Muller S, Voll R, et al. Autoantibodies against modified histone peptides in SLE patients are associated with disease activity and lupus nephritis. PLoS ONE. (2016) 11:e0165373. 10.1371/journal.pone.0165373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. (2016) 22:146–53. 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Safi R, Al-Hage J, Abbas O, Kibbi AG, Nassar D. Investigating the presence of neutrophil extracellular traps in cutaneous lesions of different subtypes of lupus erythematosus. Exp Dermatol. (2019) 28:1348–52. 10.1111/exd.14040 [DOI] [PubMed] [Google Scholar]
- 124.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. (2004) 34:501–37. 10.1016/j.semarthrit.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 125.Gripenberg M, Helve T, Kurki P. Profiles of antibodies to histones, DNA and IgG in patients with systemic rheumatic diseases determined by ELISA. J Rheumatol. (1985) 12:934–9. [PubMed] [Google Scholar]
- 126.Muller S, Bonnier D, Thiry M, Van Regenmortel MH. Reactivity of autoantibodies in systemic lupus erythematosus with synthetic core histone peptides. Int Arch Allergy Appl Immunol. (1989) 89:288–96. [DOI] [PubMed] [Google Scholar]
- 127.Schett G, Steiner G, Smolen JS. Nuclear antigen histone H1 is primarily involved in lupus erythematosus cell formation. Arthritis Rheum. (1998) 41:1446–55. [DOI] [PubMed] [Google Scholar]
- 128.Chapman EA, Lyon M, Simpson D, Mason D, Beynon RJ, Moots RJ, et al. Caught in a trap? Proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front Immunol. (2019) 10:423. 10.3389/fimmu.2019.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Bavel CC, Dieker JW, Kroeze Y, Tamboer WP, Voll R, Muller S, et al. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis. (2011) 70:201–7. 10.1136/ard.2010.129320 [DOI] [PubMed] [Google Scholar]
- 130.Pieterse E, Hofstra J, Berden J, Herrmann M, Dieker J, van der Vlag J. Acetylated histones contribute to the immunostimulatory potential of neutrophil extracellular traps in systemic lupus erythematosus. Clin Exp Immunol. (2015) 179:68–74. 10.1111/cei.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tzioufas AG, Boumba VA, Seferiadis K, Tsolas O, Moutsopoulos HM. Autoantibodies to HMG-17 nucleosomal protein in autoimmune rheumatic diseases. Correlation with systemic lupus erythematosus clinical activity and with antibodies to double-stranded DNA. Arthritis Rheum. (1993) 36:955–61. [DOI] [PubMed] [Google Scholar]
- 132.Mansour RB, Lassoued S, Gargouri B, El Gaid A, Attia H, Fakhfakh F. Increased levels of autoantibodies against catalase and superoxide dismutase associated with oxidative stress in patients with rheumatoid arthritis and systemic lupus erythematosus. Scand J Rheumatol. (2008) 37:103–8. 10.1080/03009740701772465 [DOI] [PubMed] [Google Scholar]
- 133.Brito J, Biamonti G, Caporali R, Montecucco C. Autoantibodies to human nuclear lamin B2 protein. Epitope specificity in different autoimmune diseases. J Immunol. (1994) 153:2268–77. [PubMed] [Google Scholar]
- 134.Reeves WH, Chaudhary N, Salerno A, Blobel G. Lamin B autoantibodies in sera of certain patients with systemic lupus erythematosus. J Exp Med. (1987) 165:750–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Senecal JL, Rauch J, Grodzicky T, Raynauld JP, Uthman I, Nava A, et al. Strong association of autoantibodies to human nuclear lamin B1 with lupus anticoagulant antibodies in systemic lupus erythematosus. Arthritis Rheum. (1999) 42:1347–53. [DOI] [PubMed] [Google Scholar]
- 136.Dinu AR, Merrill JT, Shen C, Antonov IV, Myones BL, Lahita RG. Frequency of antibodies to the cholesterol transport protein apolipoprotein A1 in patients with SLE. Lupus. (1998) 7:355–60. 10.1191/096120398678920262 [DOI] [PubMed] [Google Scholar]
- 137.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. (2013) 191:4895–901. 10.4049/jimmunol.1302005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun CL, Zhang FZ, Li P, Bi LQ. LL-37 expression in the skin in systemic lupus erythematosus. Lupus. (2011) 20:904–11. 10.1177/0961203311398515 [DOI] [PubMed] [Google Scholar]
- 139.Kienhofer D, Hahn J, Schubert I, Reinwald C, Ipseiz N, Lang SC, et al. No evidence of pathogenic involvement of cathelicidins in patient cohorts and mouse models of lupus and arthritis. PLoS ONE. (2014) 9:e115474. 10.1371/journal.pone.0115474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kretz CC, Norpo M, Abeler-Dorner L, Linke B, Haust M, Edler L, et al. Anti-annexin 1 antibodies: a new diagnostic marker in the serum of patients with discoid lupus erythematosus. Exp Dermatol. (2010) 19:919–21. 10.1111/j.1600-0625.2010.01145.x [DOI] [PubMed] [Google Scholar]
- 141.Bruschi M, Sinico RA, Moroni G, Pratesi F, Migliorini P, Galetti M, et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: alpha-enolase and annexin AI. J Am Soc Nephrol. (2014) 25:2483–98. 10.1681/ASN.2013090987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. (2015) 74:1417–24. 10.1136/annrheumdis-2013-204837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aragon CC, Tafur RA, Suarez-Avellaneda A, Martinez MT, Salas AL, Tobon GJ. Urinary biomarkers in lupus nephritis. J Transl Autoimmun. (2020) 3:100042. 10.1016/j.jtauto.2020.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.El Shahawy MS, Hemida MH, Abdel-Hafez HA, El-Baz TZ, Lotfy AM, Emran TM. Urinary neutrophil gelatinase-associated lipocalin as a marker for disease activity in lupus nephritis. Scand J Clin Lab Invest. (2018) 78:264–8. 10.1080/00365513.2018.1449242 [DOI] [PubMed] [Google Scholar]
- 145.Rubinstein T, Pitashny M, Levine B, Schwartz N, Schwartzman J, Weinstein E, et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology (Oxford). (2010) 49:960–71. 10.1093/rheumatology/kep468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mayadas TN, Rosetti F, Ernandez T, Sethi S. Neutrophils: game changers in glomerulonephritis? Trends Mol Med. (2010) 16:368–78. 10.1016/j.molmed.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pieterse E, Rother N, Garsen M, Hofstra JM, Satchell SC, Hoffmann M, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. (2017) 37:1371–9. 10.1161/ATVBAHA.117.309002 [DOI] [PubMed] [Google Scholar]
- 148.Knight JS, Zhao W, Luo W, Subramanian V, O'Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. (2013) 123:2981–93. 10.1172/JCI67390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Y, Carmona-Rivera C, Moore E, Seto NL, Knight JS, Pryor M, et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front Immunol. (2018) 9:1680. 10.3389/fimmu.2018.01680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O'Neill SG, Giles I, Lambrianides A, Manson J, D'Cruz D, Schrieber L, et al. Antibodies to apolipoprotein A-I, high-density lipoprotein, and C-reactive protein are associated with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. (2010) 62:845–54. 10.1002/art.27286 [DOI] [PubMed] [Google Scholar]
- 151.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. (2010) 107:9813–8. 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bonegio RG, Lin JD, Beaudette-Zlatanova B, York MR, Menn-Josephy H, Yasuda K. Lupus-associated immune complexes activate human neutrophils in an FcgammaRIIA-dependent but TLR-independent response. J Immunol. (2019) 202:675–83. 10.4049/jimmunol.1800300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gestermann N, Di Domizio J, Lande R, Demaria O, Frasca L, Feldmeyer L, et al. Netting neutrophils activate autoreactive B cells in lupus. J Immunol. (2018) 200:3364–71. 10.4049/jimmunol.1700778 [DOI] [PubMed] [Google Scholar]
- 154.Kraaij T, Kamerling SWA, de Rooij ENM, van Daele PLA, Bredewold OW, Bakker JA, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun. (2018) 91:45–54. 10.1016/j.jaut.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 155.van Dam LS, Osmani Z, Kamerling SWA, Kraaij T, Bakker JA, Scherer HU, et al. A reverse translational study on the effect of rituximab, rituximab plus belimumab, or bortezomib on the humoral autoimmune response in SLE. Rheumatology (Oxford). (2020) 59:2734–45. 10.1093/rheumatology/kez623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. (2013) 5:178ra140. 10.1126/scitranslmed.3005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spengler J, Lugonja B, Ytterberg AJ, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. (2015) 67:3135–45. 10.1002/art.39313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. (2013) 5:209ra150. 10.1126/scitranslmed.3006869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Turunen S, Huhtakangas J, Nousiainen T, Valkealahti M, Melkko J, Risteli J, et al. Rheumatoid arthritis antigens homocitrulline and citrulline are generated by local myeloperoxidase and peptidyl arginine deiminases 2, 3 and 4 in rheumatoid nodule and synovial tissue. Arthritis Res Ther. (2016) 18:239. 10.1186/s13075-016-1140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kinloch A, Lundberg K, Wait R, Wegner N, Lim NH, Zendman AJ, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. (2008) 58:2287–95. 10.1002/art.23618 [DOI] [PubMed] [Google Scholar]
- 161.Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. (2005) 7:R1421–9. 10.1186/ar1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Bont CM, Stokman MEM, Faas P, Thurlings RM, Boelens WC, Wright HL, et al. Autoantibodies to neutrophil extracellular traps represent a potential serological biomarker in rheumatoid arthritis. J Autoimmun. (2020) 113:102484. 10.1016/j.jaut.2020.102484 [DOI] [PubMed] [Google Scholar]
- 163.Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. (2017) 2:eaag3358. 10.1126/sciimmunol.aag3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carmona-Rivera C, Carlucci PM, Goel RR, James EA, Brooks SR, Rims CR, et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight. (2020) 5:e139388. 10.1172/jci.insight.139388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mattey DL, Nixon NB, Dawes PT. Association of circulating levels of MMP-8 with mortality from respiratory disease in patients with rheumatoid arthritis. Arthritis Res Ther. (2012) 14:R204. 10.1186/ar4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. (2002) 169:2643–7. 10.4049/jimmunol.169.5.2643 [DOI] [PubMed] [Google Scholar]
- 167.Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med. (2013) 210:2569–82. 10.1084/jem.20131241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis. (2016) 75:1866–75. 10.1136/annrheumdis-2015-208356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wright HL, Thomas HB, Moots RJ, Edwards SW. RNA-seq reveals activation of both common and cytokine-specific pathways following neutrophil priming. PLoS ONE. (2013) 8:e58598. 10.1371/journal.pone.0058598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.de Kleijn S, Kox M, Sama IE, Pillay J, van Diepen A, Huijnen MA, et al. Transcriptome kinetics of circulating neutrophils during human experimental endotoxemia. PLoS ONE. (2012) 7:e38255. 10.1371/journal.pone.0038255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci USA. (2002) 99:6901–6. 10.1073/pnas.092148299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zimmermann M, Aguilera FB, Castellucci M, Rossato M, Costa S, Lunardi C, et al. Chromatin remodelling and autocrine TNFalpha are required for optimal interleukin-6 expression in activated human neutrophils. Nat Commun. (2015) 6:6061. 10.1038/ncomms7061 [DOI] [PubMed] [Google Scholar]
- 173.Glennon-Alty L, Moots RJ, Edwards SW, Wright HL. Type-I interferon regulates cytokine-delayed neutrophil apoptosis, reactive oxygen species production and chemokine expression. Clin Exp Immunol. (2020) 203:151–9. 10.1111/cei.13525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. (2006) 203:837–42. 10.1084/jem.20052371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, et al. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. (2005) 105:830–7. 10.1182/blood-2004-02-0564 [DOI] [PubMed] [Google Scholar]
- 176.Gilbert C, Poubelle PE, Borgeat P, Pouliot M, Naccache PH. Crystal-induced neutrophil activation: VIII. Immediate production of prostaglandin E2 mediated by constitutive cyclooxygenase 2 in human neutrophils stimulated by urate crystals. Arthritis Rheum. (2003) 48:1137–48. 10.1002/art.10851 [DOI] [PubMed] [Google Scholar]
- 177.Yang CW, Unanue ER. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J Exp Med. (2013) 210:375–87. 10.1084/jem.20122183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hoogendijk AJ, Pourfarzad F, Aarts CEM, Tool ATJ, Hiemstra IH, Grassi L, et al. Dynamic transcriptome-proteome correlation networks reveal human myeloid differentiation and neutrophil-specific programming. Cell Rep. (2019) 29:2505–19 e2504. 10.1016/j.celrep.2019.10.082 [DOI] [PubMed] [Google Scholar]
- 179.Rorvig S, Ostergaard O, Heegaard NH, Borregaard N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J Leukoc Biol. (2013) 94:711–21. 10.1189/jlb.1212619 [DOI] [PubMed] [Google Scholar]
- 180.Pedersen CC, Borup R, Fischer-Nielsen A, Mora-Jensen H, Fossum A, Cowland JB, et al. Changes in gene expression during G-CSF-induced emergency granulopoiesis in humans. J Immunol. (2016) 197:1989–99. 10.4049/jimmunol.1502690 [DOI] [PubMed] [Google Scholar]
- 181.Theilgaard-Monch K, Jacobsen LC, Borup R, Rasmussen T, Bjerregaard MD, Nielsen FC, et al. The transcriptional program of terminal granulocytic differentiation. Blood. (2005) 105:1785–96. 10.1182/blood-2004-08-3346 [DOI] [PubMed] [Google Scholar]
- 182.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. (1997) 89:3503–21. [PubMed] [Google Scholar]
- 183.Elghetany MT. Surface antigen changes during normal neutrophilic development: a critical review. Blood Cells Mol Dis. (2002) 28:260–74. 10.1006/bcmd.2002.0513 [DOI] [PubMed] [Google Scholar]
- 184.Lakschevitz FS, Visser MB, Sun C, Glogauer M. Neutrophil transcriptional profile changes during transit from bone marrow to sites of inflammation. Cell Mol Immunol. (2015) 12:53–65. 10.1038/cmi.2014.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Naranbhai V, Fairfax BP, Makino S, Humburg P, Wong D, Ng E, et al. Genomic modulators of gene expression in human neutrophils. Nat Commun. (2015) 6:7545. 10.1038/ncomms8545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wright HL, Thomas HB, Moots RJ, Edwards SW. Interferon gene expression signature in rheumatoid arthritis neutrophils correlates with a good response to TNFi therapy. Rheumatology (Oxford). (2015) 54:188–93. 10.1093/rheumatology/keu299 [DOI] [PubMed] [Google Scholar]
- 187.Mistry P, Nakabo S, O'Neil L, Goel RR, Jiang K, Carmona-Rivera C, et al. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci USA. (2019) 116:25222–8. 10.1073/pnas.1908576116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wright HL, Cox T, Moots RJ, Edwards SW. Neutrophil biomarkers predict response to therapy with tumor necrosis factor inhibitors in rheumatoid arthritis. J Leukoc Biol. (2017) 101:785–95. 10.1189/jlb.5A0616-258R [DOI] [PubMed] [Google Scholar]
- 189.Schlenk J, Lorenz HM, Haas JP, Herrmann M, Hohenberger G, Kalden JR, et al. Extravasation into synovial tissue induces CCL20 mRNA expression in polymorphonuclear neutrophils of patients with rheumatoid arthritis. J Rheumatol. (2005) 32:2291–8. [PubMed] [Google Scholar]
- 190.Hatano Y, Kasama T, Iwabuchi H, Hanaoka R, Takeuchi HT, Jing L, et al. Macrophage inflammatory protein 1 alpha expression by synovial fluid neutrophils in rheumatoid arthritis. Ann Rheum Dis. (1999) 58:297–302. 10.1136/ard.58.5.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Arias de la Rosa I, Perez-Sanchez C, Ruiz-Limon P, Patino-Trives A, Torres-Granados C, Jimenez-Gomez Y, et al. Impaired microRNA processing in neutrophils from rheumatoid arthritis patients confers their pathogenic profile. Modulation by biological therapies. Haematologica. (2020) 105:2250–61. 10.3324/haematol.2018.205047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cross A, Bucknall RC, Cassatella MA, Edwards SW, Moots RJ. Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis Rheum. (2003) 48:2796–806. 10.1002/art.11253 [DOI] [PubMed] [Google Scholar]
- 193.Bayley R, Kite KA, McGettrick HM, Smith JP, Kitas GD, Buckley CD, et al. The autoimmune-associated genetic variant PTPN22 R620W enhances neutrophil activation and function in patients with rheumatoid arthritis and healthy individuals. Ann Rheum Dis. (2015) 74:1588–95. 10.1136/annrheumdis-2013-204796 [DOI] [PubMed] [Google Scholar]
- 194.Witko-Sarsat V, Lesavre P, Lopez S, Bessou G, Hieblot C, Prum B, et al. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J Am Soc Nephrol. (1999) 10:1224–33. [DOI] [PubMed] [Google Scholar]
- 195.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. (2014) 506:376–81. 10.1038/nature12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. (2003) 100:2610–5. 10.1073/pnas.0337679100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. (2016) 165:551–65. 10.1016/j.cell.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Petri M, Fu W, Ranger A, Allaire N, Cullen P, Magder LS, et al. Association between changes in gene signatures expression and disease activity among patients with systemic lupus erythematosus. BMC Med Genomics. (2019) 12:4. 10.1186/s12920-018-0468-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brohawn PZ, Streicher K, Higgs BW, Morehouse C, Liu H, Illei G, et al. Type I interferon gene signature test-low and -high patients with systemic lupus erythematosus have distinct gene expression signatures. Lupus. (2019) 28:1524–33. 10.1177/0961203319885447 [DOI] [PubMed] [Google Scholar]
- 200.Coit P, Yalavarthi S, Ognenovski M, Zhao W, Hasni S, Wren JD, et al. Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J Autoimmun. (2015) 58:59–66. 10.1016/j.jaut.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, et al. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. (2017) 69:376–86. 10.1002/art.39962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. (2020) 382:211–21. 10.1056/NEJMoa1912196 [DOI] [PubMed] [Google Scholar]
- 203.Furie R, Morand EF, Bruce IN, Manzi S, Kalunian K, Vital EM, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. (2019) 1:E208–19. 10.1016/S2665-9913(19)30098-0 [DOI] [PubMed] [Google Scholar]
- 204.Bashant KR, Aponte AM, Randazzo D, Rezvan Sangsari P, Wood AJ, Bibby JA, et al. Proteomic, biomechanical and functional analyses define neutrophil heterogeneity in systemic lupus erythematosus. Ann Rheum Dis. (2020) 80:209–18. 10.1136/annrheumdis-2020-218338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wither JE, Prokopec SD, Noamani B, Chang NH, Bonilla D, Touma Z, et al. Identification of a neutrophil-related gene expression signature that is enriched in adult systemic lupus erythematosus patients with active nephritis: clinical/pathologic associations and etiologic mechanisms. PLoS ONE. (2018) 13:e0196117. 10.1371/journal.pone.0196117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Carlucci PM, Purmalek MM, Dey AK, Temesgen-Oyelakin Y, Sakhardande S, Joshi AA, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight. (2018) 3:e99276. 10.1172/jci.insight.99276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dubey AK, Handu SS, Dubey S, Sharma P, Sharma KK, Ahmed QM. Belimumab: First targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. (2011) 2:317–9. 10.4103/0976-500X.85930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. (2011) 11:81–3. 10.1038/nri2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. (2014) 32:609–34. 10.1146/annurev-immunol-032713-120236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Medzhitov R. Origin and physiological roles of inflammation. Nature. (2008) 454:428–35. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- 211.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. (2016) 16:553–65. 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gaber T, Strehl C, Buttgereit F. Metabolic regulation of inflammation. Nat Rev Rheumatol. (2017) 13:267–79. 10.1038/nrrheum.2017.37 [DOI] [PubMed] [Google Scholar]
- 213.Weyand CM, Goronzy JJ. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol. (2017) 13:291–301. 10.1038/nrrheum.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. (2013) 210:2119–34. 10.1084/jem.20130252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yang Z, Shen Y, Oishi H, Matteson EL, Tian L, Goronzy JJ, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. (2016) 8:331ra338. 10.1126/scitranslmed.aad7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sbarra AJ, Karnovsky ML. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. (1959) 234:1355–62. [PubMed] [Google Scholar]
- 217.Azevedo EP, Rochael NC, Guimarães-Costa AB, De Souza-Vieira TS, Ganilho J, Saraiva EM, et al. A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-Acetate-induced neutrophil extracellular trap (NET) formation. J Biol Chem. (2015) 290:22174–83. 10.1074/jbc.M115.640094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen LY, Shieh JJ, Lin B, Pan CJ, Gao JL, Murphy PM, et al. Impaired glucose homeostasis, neutrophil trafficking and function in mice lacking the glucose-6-phosphate transporter. Hum Mol Genet. (2003) 12:2547–58. 10.1093/hmg/ddg263 [DOI] [PubMed] [Google Scholar]
- 219.Cooper MR, DeChatelet LR, McCall CE, LaVia MF, Spurr CL, Baehner RL. Complete deficiency of leukocyte glucose-6-phosphate dehydrogenase with defective bactericidal activity. J Clin Invest. (1972) 51:769–78. 10.1172/JCI106871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Edwards SW. Biochemistry and Physiology of the Neutrophil. Cambridge: Cambridge University Press; (1994). [Google Scholar]
- 221.Wilson PD, Rustin GJS, Smith GP, Peters TJ. Electron microscopic cytochemical localization of nucleoside phosphatases in normal and chronic granulocytic leukaemic human neutrophils. Histochem J. (1981) 13:73–84. 10.1007/BF01005841 [DOI] [PubMed] [Google Scholar]
- 222.Hirsch JG, Fedorko ME. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and “postfixation” in uranyl acetate. J Cell Biol. (1968) 38:615–27. 10.1083/jcb.38.3.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. (2018) 9:5099. 10.1038/s41467-018-07505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS ONE. (2008) 3:e2013. 10.1371/journal.pone.0002013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zmijewski JW, Lorne E, Banerjee S, Abraham E. Participation of mitochondrial respiratory complex III in neutrophil activation and lung injury. Am J Physiol Lung Cell Mol Physiol. (2009) 296:L624–34. 10.1152/ajplung.90522.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fossati G, Moulding DA, Spiller DG, Moots RJ, White MRH, Edwards SW. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol. (2003) 170:1964–72. 10.4049/jimmunol.170.4.1964 [DOI] [PubMed] [Google Scholar]
- 227.Maianski NA, Geissler J, Srinivasula SM, Alnemri ES, Roos D, Kuijpers TW. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Differ. (2004) 11:143–53. 10.1038/sj.cdd.4401320 [DOI] [PubMed] [Google Scholar]
- 228.Vorobjeva N, Prikhodko A, Galkin I, Pletjushkina O, Zinovkin R, Sud'ina G, et al. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur J Cell Biol. (2017) 96:254–65. 10.1016/j.ejcb.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 229.Bao Y, Ledderose C, Seier T, Graf AF, Brix B, Chong E, et al. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem. (2014) 289:26794–803. 10.1074/jbc.M114.572495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bao Y, Ledderose C, Graf AF, Brix B, Birsak T, Lee A, et al. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol. (2015) 210:1153–64. 10.1083/jcb.201503066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kumar S, Dikshit M. Metabolic insight of neutrophils in health and disease. Front Immunol. (2019) 10:2099. 10.3389/fimmu.2019.02099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Six E, Lagresle-Peyrou C, Susini S, De Chappedelaine C, Sigrist N, Sadek H, et al. AK2 deficiency compromises the mitochondrial energy metabolism required for differentiation of human neutrophil and lymphoid lineages. Cell Death Dis. (2015) 6:e1856. 10.1038/cddis.2015.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hsu BE, Tabariès S, Johnson RM, Andrzejewski S, Senecal J, Lehuédé C, et al. Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Rep. (2019) 27:3902–15.e3906. 10.1016/j.celrep.2019.05.091 [DOI] [PubMed] [Google Scholar]
- 234.Pithon-Curi TC, De Melo MP, Curi R. Glucose and glutamine utilization by rat lymphocytes, monocytes and neutrophils in culture: a comparative study. Cell Biochem Func. (2004) 22:321–6. 10.1002/cbf.1109 [DOI] [PubMed] [Google Scholar]
- 235.Pithon-Curi TC, Trezena AG, Tavares-Lima W, Curi R. Evidence that glutamine is involved in neutrophil function. Cell Biochem Func. (2002) 20:81–6. 10.1002/cbf.954 [DOI] [PubMed] [Google Scholar]
- 236.Sasaki E, Umeda T, Takahashi I, Arata K, Yamamoto Y, Tanabe M, et al. Effect of glutamine supplementation on neutrophil function in male judoists. Luminescence. (2013) 28:442–9. 10.1002/bio.2474 [DOI] [PubMed] [Google Scholar]
- 237.Yang L, Venneti S, Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. (2017) 19:163–94. 10.1146/annurev-bioeng-071516-044546 [DOI] [PubMed] [Google Scholar]
- 238.Injarabian L, Devin A, Ransac S, Marteyn BS. Neutrophil metabolic shift during their lifecycle: impact on their survival and activation. Int J Mol Sci. (2020) 21:287. 10.3390/ijms21010287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Furukawa S, Saito H, Inoue T, Matsuda T, Fukatsu K, Han I, et al. Supplemental glutamine augments phagocytosis and reactive oxygen intermediate production by neutrophils and monocytes from postoperative patients in vitro. Nutrition. (2000) 16:323–9. 10.1016/s0899-9007(00)00228-8 [DOI] [PubMed] [Google Scholar]
- 240.Huang Y, Li N, Liboni K, Neu J. Glutamine decreases lipopolysaccharide-induced IL-8 production in Caco-2 cells through a non-NF-κB p50 mechanism. Cytokine. (2003) 22:77–83. 10.1016/S1043-4666(03)00115-7 [DOI] [PubMed] [Google Scholar]
- 241.De Beaux AC, O'Riordain MG, Ross JA, Jodozi L, Carter DC, Fearon KCH. Glutamine-supplemented total parenteral nutrition reduces blood mononuclear cell interleukin-8 release in severe acute pancreatitis. Nutrition. (1998) 14:261–5. 10.1016/S0899-9007(97)00477-2 [DOI] [PubMed] [Google Scholar]
- 242.Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, et al. The transcription factor HIF-1 plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. (2007) 21:1037–49. 10.1101/gad.1529107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Watts ER, Walmsley SR. Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol Med. (2019) 25:33–46. 10.1016/j.molmed.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 244.Harris AJ, Mirchandani AS, Lynch RW, Murphy F, Delaney L, Small D, et al. IL4RA signaling abrogates hypoxic neutrophil survival and limits acute lung injury responses in vivo. Am J Respir Crit Care Med. (2019) 200:235–46. 10.1164/rccm.201808-1599OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kong T, Eltzschig HK, Karhausen J, Colgan SP, Shelley CS. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of β2 integrin gene expression. Proc Natl Acad Sci USA. (2004) 101:10440–5. 10.1073/pnas.0401339101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Anderson JR, Chokesuwattanaskul S, Phelan MM, Welting TJM, Lian LY, Peffers MJ, et al. (1)H NMR metabolomics identifies underlying inflammatory pathology in osteoarthritis and rheumatoid arthritis synovial joints. J Proteome Res. (2018) 17:3780–90. 10.1021/acs.jproteome.8b00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. (2018) 556:113–7. 10.1038/nature25986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ryan DG, O'Neill LAJ. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. (2017) 591:2992–3006. 10.1002/1873-3468.12744 [DOI] [PubMed] [Google Scholar]
- 249.Williams NC, O'Neill LAJ. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol. (2018) 9:141. 10.3389/fimmu.2018.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, et al. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. (2007) 37:282–90. 10.1111/j.1365-2362.2007.01786.x [DOI] [PubMed] [Google Scholar]
- 251.Li KJ, Wu CH, Hsieh SC, Lu MC, Tsai CY, Yu CL. Deranged bioenergetics and defective redox capacity in T lymphocytes and neutrophils are related to cellular dysfunction and increased oxidative stress in patients with active systemic lupus erythematosus. Clin Dev Immunol. (2012) 2012:548516. 10.1155/2012/548516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.MacIver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leuk Biol. (2008) 84:949–57. 10.1189/jlb.0108024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Perner A, Nielsen SE, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. (2003) 29:642–5. 10.1007/s00134-002-1628-4 [DOI] [PubMed] [Google Scholar]
- 254.Huang WN, Tso TK, Huang HY. Enhanced oxidative status but not corresponding elevated antioxidative status by anticardiolipin antibody and disease activity in patients with systemic lupus erythematosus. Rheumatol Int. (2007) 27:453–8. 10.1007/s00296-006-0233-1 [DOI] [PubMed] [Google Scholar]
- 255.Bilzer M, Lauteburg BH. Glutathione metabolism in activated human neutrophils: stimulation of glutathione synthesis and consumption of glutathione by reactive oxygen species. Eur J Clin Invest. (1991) 21:316–22. 10.1111/j.1365-2362.1991.tb01376.x [DOI] [PubMed] [Google Scholar]
- 256.Pravda J. Systemic lupus erythematosus: pathogenesis at the functional limit of redox homeostasis. Oxidat Med Cell Longev. (2019) 2019:1651724. 10.1155/2019/1651724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Voetman AA, Loos JA, Roos D. Changes in the levels of glutathione in phagocytosing human neutrophils. Blood. (1980) 55:741–7. [PubMed] [Google Scholar]
- 258.Yan J, Meng X, Wancket LM, Lintner K, Nelin LD, Chen B, et al. Glutathione reductase facilitates host defense by sustaining phagocytic oxidative burst and promoting the development of neutrophil extracellular traps. J Immunol. (2012) 188:2316–27. 10.4049/jimmunol.1102683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ito Y, Kajkenova O, Feuers RJ, Udupa KB, Desai VG, Epstein J, et al. Impaired glutathione peroxidase activity accounts for the age-related accumulation of hydrogen peroxide in activated human neutrophils. J Gerontol A Biol Sci Med Sci. (1998) 53:M169–75. 10.1093/gerona/53a.3.m169 [DOI] [PubMed] [Google Scholar]
- 260.NaveenKumar SK, Hemshekhar M, Jagadish S, Manikanta K, Vishalakshi GJ, Kemparaju K, et al. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J Pineal Res. (2020) 69:e12676. 10.1111/jpi.12676 [DOI] [PubMed] [Google Scholar]
- 261.Stojkov D, Amini P, Oberson K, Sokollik C, Duppenthaler A, Simon HU, et al. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J Cell Biol. (2017) 216:4073–90. 10.1083/jcb.201611168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Reshetnikov V, Hahn J, Maueröder C, Czegley C, Munoz LE, Herrmann M, et al. Chemical tools for targeted amplification of reactive oxygen species in neutrophils. Front Immunol. (2018) 9:1827. 10.3389/fimmu.2018.01827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. (2011) 3:73ra20. 10.1126/scitranslmed.3001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. (2016) 273:48–60. 10.1111/imr.12448 [DOI] [PubMed] [Google Scholar]
- 265.Grieshaber-Bouyer R, Nigrovic PA. Neutrophil heterogeneity as therapeutic opportunity in immune-mediated disease. Front Immunol. (2019) 10:346. 10.3389/fimmu.2019.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. (2019) 19:255–65. 10.1038/s41577-019-0141-8 [DOI] [PubMed] [Google Scholar]
- 267.Nourshargh S, Renshaw SA, Imhof BA. Reverse migration of neutrophils: where, when, how, and why? Trends Immunol. (2016) 37:273–86. 10.1016/j.it.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 268.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lecot P, Sarabi M, Pereira Abrantes M, Mussard J, Koenderman L, Caux C, et al. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol. (2019) 10:2155. 10.3389/fimmu.2019.02155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. (2012) 122:327–36. 10.1172/JCI57990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kurko J, Vida A, Glant TT, Scanzello CR, Katz RS, Nair A, et al. Identification of myeloid-derived suppressor cells in the synovial fluid of patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord. (2014) 15:281. 10.1186/1471-2474-15-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhu J, Chen S, Wu L, Wang R, Zheng S, Zhao D, et al. The expansion of myeloid-derived suppressor cells is associated with joint inflammation in rheumatic patients with arthritis. BioMed Res Int. (2018) 2018:5474828. 10.1155/2018/5474828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang W, Jiao Z, Duan T, Liu M, Zhu B, Zhang Y, et al. Functional characterization of myeloid-derived suppressor cell subpopulations during the development of experimental arthritis. Eur J Immunol. (2015) 45:464–73. 10.1002/eji.201444799 [DOI] [PubMed] [Google Scholar]
- 274.Park MJ, Lee SH, Kim EK, Lee EJ, Baek JA, Park SH, et al. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci Rep. (2018) 8:3753. 10.1038/s41598-018-21856-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Li M, Zhu D, Wang T, Xia X, Tian J, Wang S. Roles of myeloid-derived suppressor cell subpopulations in autoimmune arthritis. Front Immunol. (2018) 9:2849. 10.3389/fimmu.2018.02849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ji J, Xu J, Zhao S, Liu F, Qi J, Song Y, et al. Myeloid-derived suppressor cells contribute to systemic lupus erythaematosus by regulating differentiation of Th17 cells and Tregs. Clin Sci (Lond). (2016) 130:1453–67. 10.1042/CS20160311 [DOI] [PubMed] [Google Scholar]
- 277.Vlachou K, Mintzas K, Glymenaki M, Ioannou M, Papadaki G, Bertsias GK, et al. Elimination of granulocytic myeloid-derived suppressor cells in lupus-prone mice linked to reactive oxygen species-dependent extracellular trap formation. Arthritis Rheumatol. (2016) 68:449–61. 10.1002/art.39441 [DOI] [PubMed] [Google Scholar]
- 278.Ellett F, Elks PM, Robertson AL, Ogryzko NV, Renshaw SA. Defining the phenotype of neutrophils following reverse migration in zebrafish. J Leukoc Biol. (2015) 98:975–81. 10.1189/jlb.3MA0315-105R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol. (2006) 79:303–11. 10.1189/jlb.0905496 [DOI] [PubMed] [Google Scholar]
- 280.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. (2011) 12:761–9. 10.1038/ni.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. (1986) 29:1334–42. [DOI] [PubMed] [Google Scholar]
- 282.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. (2010) 184:3284–97. 10.4049/jimmunol.0902199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wright HL, Makki FA, Moots RJ, Edwards SW. Low-density granulocytes: functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J Leukoc Biol. (2017) 101:599–611. 10.1189/jlb.5A0116-022R [DOI] [PubMed] [Google Scholar]
- 284.Kegerreis BJ, Catalina MD, Geraci NS, Bachali P, Lipsky PE, Grammer AC. Genomic identification of low-density granulocytes and analysis of their role in the pathogenesis of systemic lupus erythematosus. J Immunol. (2019) 202:3309–17. 10.4049/jimmunol.1801512 [DOI] [PubMed] [Google Scholar]
- 285.Midgley A, Beresford MW. Increased expression of low density granulocytes in juvenile-onset systemic lupus erythematosus patients correlates with disease activity. Lupus. (2016) 25:407–11. 10.1177/0961203315608959 [DOI] [PubMed] [Google Scholar]
- 286.Rahman S, Sagar D, Hanna RN, Lightfoot YL, Mistry P, Smith CK, et al. Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann Rheum Dis. (2019) 78:957–66. 10.1136/annrheumdis-2018-214620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fu J, Tobin MC, Thomas LL. Neutrophil-like low-density granulocytes are elevated in patients with moderate to severe persistent asthma. Ann Allergy Asthma Immunol. (2014) 113:635–40 e632. 10.1016/j.anai.2014.08.024 [DOI] [PubMed] [Google Scholar]
- 288.Ui Mhaonaigh A, Coughlan AM, Dwivedi A, Hartnett J, Cabral J, Moran B, et al. Low density granulocytes in ANCA vasculitis are heterogenous and hypo-responsive to anti-myeloperoxidase antibodies. Front Immunol. (2019) 10:2603. 10.3389/fimmu.2019.02603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ostendorf L, Mothes R, van Koppen S, Lindquist RL, Bellmann-Strobl J, Asseyer S, et al. Low-density granulocytes are a novel immunopathological feature in both multiple sclerosis and neuromyelitis optica spectrum disorder. Front Immunol. (2019) 10:2725. 10.3389/fimmu.2019.02725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Rodriguez-Carrio J, Carrillo-Lopez N, Ulloa C, Seijo M, Rodriguez-Garcia M, Rodriguez-Suarez C, et al. A subset of low density granulocytes is associated with vascular calcification in chronic kidney disease patients. Sci Rep. (2019) 9:13230. 10.1038/s41598-019-49429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hardisty GR, LLanwarne F, Minns D, Gillan JL, Davidson DJ, Gwyer Findley E, et al. Ultra-pure isolation of low density neutrophils casts doubt on their exceptionality in health and disease. bioRxiv. (2020). 10.1101/2020.06.17.156588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hassani M, Hellebrekers P, Chen N, van Aalst C, Bongers S, Hietbrink F, et al. On the origin of low-density neutrophils. J Leukoc Biol. (2020) 107:809–18. 10.1002/JLB.5HR0120-459R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nemeth T, Sperandio M, Mocsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. (2020) 19:253–75. 10.1038/s41573-019-0054-z [DOI] [PubMed] [Google Scholar]
- 294.Burmester GR, McInnes IB, Kremer J, Miranda P, Korkosz M, Vencovsky J, et al. A randomised phase IIb study of mavrilimumab, a novel GM-CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis. Ann Rheum Dis. (2017) 76:1020–30. 10.1136/annrheumdis-2016-210624 [DOI] [PubMed] [Google Scholar]
- 295.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. (2017) 376:652–62. 10.1056/NEJMoa1608345 [DOI] [PubMed] [Google Scholar]
- 296.Mitchell TS, Moots RJ, Wright HL. Janus kinase inhibitors prevent migration of rheumatoid arthritis neutrophils towards interleukin-8, but do not inhibit priming of the respiratory burst or reactive oxygen species production. Clin Exp Immunol. (2017) 189:250–8. 10.1111/cei.12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tang TT, Lv LL, Pan MM, Wen Y, Wang B, Li ZL, et al. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. (2018) 9:351. 10.1038/s41419-018-0378-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhang S, Zhang Q, Wang F, Guo X, Liu T, Zhao Y, et al. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin immunol. (2020) 216:108461. 10.1016/j.clim.2020.108461 [DOI] [PubMed] [Google Scholar]
- 299.Laurindo IM, Mello SB, Cossermelli W. Influence of low doses of methotrexate on superoxide anion production by polymorphonuclear leukocytes from patients with rheumatoid arthritis. J Rheumatol. (1995) 22:633–8. [PubMed] [Google Scholar]
- 300.Sperling RI, Benincaso AI, Anderson RJ, Coblyn JS, Austen KF, Weinblatt ME. Acute and chronic suppression of leukotriene B4 synthesis ex vivo in neutrophils from patients with rheumatoid arthritis beginning treatment with methotrexate. Arthritis Rheum. (1992) 35:376–84. [DOI] [PubMed] [Google Scholar]
- 301.Weinmann P, Moura RA, Caetano-Lopes JR, Pereira PA, Canhao H, Queiroz MV, et al. Delayed neutrophil apoptosis in very early rheumatoid arthritis patients is abrogated by methotrexate therapy. Clin Exp Rheumatol. (2007) 25:885–7. [PubMed] [Google Scholar]
- 302.Wittkowski H, Foell D, af Klint E, De Rycke L, De Keyser F, Frosch M, et al. Effects of intra-articular corticosteroids and anti-TNF therapy on neutrophil activation in rheumatoid arthritis. Ann Rheum Dis. (2007) 66:1020–5. 10.1136/ard.2006.061507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Liu L, Wang YX, Zhou J, Long F, Sun HW, Liu Y, et al. Rapid non-genomic inhibitory effects of glucocorticoids on human neutrophil degranulation. Inflamm Res. (2005) 54:37–41. 10.1007/s00011-004-1320-y [DOI] [PubMed] [Google Scholar]
- 304.Campbell IK, Leong D, Edwards KM, Rayzman V, Ng M, Goldberg GL, et al. Therapeutic targeting of the G-CSF receptor reduces neutrophil trafficking and joint inflammation in antibody-mediated inflammatory arthritis. J Immunol. (2016) 197:4392–402. 10.4049/jimmunol.1600121 [DOI] [PubMed] [Google Scholar]
- 305.Cunha TM, Barsante MM, Guerrero AT, Verri WA, Jr, Ferreira SH, Coelho FM, et al. Treatment with DF 2162, a non-competitive allosteric inhibitor of CXCR1/2, diminishes neutrophil influx and inflammatory hypernociception in mice. Br J Pharmacol. (2008) 154:460–70. 10.1038/bjp.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Coelho FM, Pinho V, Amaral FA, Sachs D, Costa VV, Rodrigues DH, et al. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheum. (2008) 58:2329–37. 10.1002/art.23622 [DOI] [PubMed] [Google Scholar]
- 307.Zheng W, Warner R, Ruggeri R, Su C, Cortes C, Skoura A, et al. PF-1355, a mechanism-based myeloperoxidase inhibitor, prevents immune complex vasculitis and anti-glomerular basement membrane glomerulonephritis. J Pharmacol Exp Ther. (2015) 353:288–98. 10.1124/jpet.114.221788 [DOI] [PubMed] [Google Scholar]
- 308.Stockley R, De Soyza A, Gunawardena K, Perrett J, Forsman-Semb K, Entwistle N, et al. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir Med. (2013) 107:524–33. 10.1016/j.rmed.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 309.Zhou Y, An LL, Chaerkady R, Mittereder N, Clarke L, Cohen TS, et al. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci Rep. (2018) 8:15228. 10.1038/s41598-018-33385-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Teo CY, Shave S, Chor AL, Salleh AB, Rahman MB, Walkinshaw MD, et al. Discovery of a new class of inhibitors for the protein arginine deiminase type 4 (PAD4) by structure-based virtual screening. BMC Bioinformatics. (2012) 13:S4. 10.1186/1471-2105-13-S17-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. (2014) 114:947–56. 10.1161/CIRCRESAHA.114.303312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Koushik S, Joshi N, Nagaraju S, Mahmood S, Mudeenahally K, Padmavathy R, et al. PAD4: pathophysiology, current therapeutics and future perspective in rheumatoid arthritis. Expert Opin Ther Targets. (2017) 21:433–47. 10.1080/14728222.2017.1294160 [DOI] [PubMed] [Google Scholar]
- 313.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. (2015) 11:189–91. 10.1038/nchembio.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li M, Lin C, Deng H, Strnad J, Bernabei L, Vogl DT, et al. A novel peptidylarginine deiminase 4 (PAD4) inhibitor BMS-P5 blocks formation of neutrophil extracellular traps and delays progression of multiple myeloma. Mol Cancer Ther. (2020) 19:1530–8. 10.1158/1535-7163.MCT-19-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Curran AM, Naik P, Giles JT, Darrah E. PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets. Nat Rev Rheumatol. (2020) 16:301–15. 10.1038/s41584-020-0409-1 [DOI] [PubMed] [Google Scholar]
- 316.Thanabalasuriar A, Scott BNV, Peiseler M, Willson ME, Zeng Z, Warrener P, et al. Neutrophil extracellular traps confine pseudomonas aeruginosa ocular biofilms and restrict brain invasion. Cell Host Microbe. (2019) 25:526–36 e524. 10.1016/j.chom.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]