Abstract
Introduction
The world is witnessing the spread of one of the members of Coronaviruses (CoVs) family, called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the 21st century. Considering the short time spent after its prevalence, limited information is known about the effect of the virus mechanism on different organs of the body; meanwhile the lack of specific treatment and vaccine for this virus has exposed millions of people to a big challenge.
Areas covered
The review article aims to describe the general and particular characteristics of CoVs, their classification, genome structure, host cell infection, cytokine storm, anti-viral treatments, and inhibition of COVID-19-related ER-mitochondrial stress. In addition, it refers to drugs such as Chloroquine/Hydroxychloroquine, Lopinavir/Ritonavir, darunavir, ribavirin, remdesivir, and favipiravir, which have undergone clinical trials for coronavirus disease 2019 (COVID-19) treatment. This analysis was derived from an extensive scientific literature search including Pubmed, ScienceDirect, and Google Scholar performed.
Expert opinion
The effectiveness rate and complications of these drugs can reveal new insights into the potential therapeutic goals for the disease. Moreover, lifestyle change can effectively prevent SARS-CoV-2 infection.
Keywords: Anti-viral treatments, Cytokine storm, MERS, Pathogenesis, SARS-CoV-2, SARS
Graphical Abstract
1. Introduction
A century after the influenza pandemic in 1918, we have faced a new pandemic called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The number of the confirmed cases and deaths from SARS-CoV-2 worldwide is increasing daily, and this has raised concerns of the World Health Organization (WHO) [2], [3]. SARS-CoV-2 was first found in December 2019 in Wuhan, China, which cause coronavirus disease 2019 (COVID-19). Coronaviruses (CoVs) were first discovered in the 1960s [4], [5]. CoVs have a wide range of clinical symptoms, including mild symptoms such as cold to the acute respiratory distress syndrome (ARDS) [5], [6], [7]. Among the two recent outbreaks of CoV pneumonia, we can refer to SARS and MERS [8], [9], [10]. In 2002 and 2003, the outbreak of SARS began in China, and then, affected the whole world. SARS mortality rate was recorded to be about 11% [8], [9], [11]. After 10 years, the outbreak of MERS began in Saudi Arabia and spread to other countries of the world. Its mortality rate was reported to be about 37% [8], [9], [11]. CoVs are the largest RNA viruses that are capable of infecting animals and mammals [12]. They have been identified in various hosts such as avian, camels, bats, masked palm civets, mice, dogs, and cats [13], [14]. CoVs transmission from animals to humans has made them a zoonotic virus [15], [16].
2. Coronavirus classification
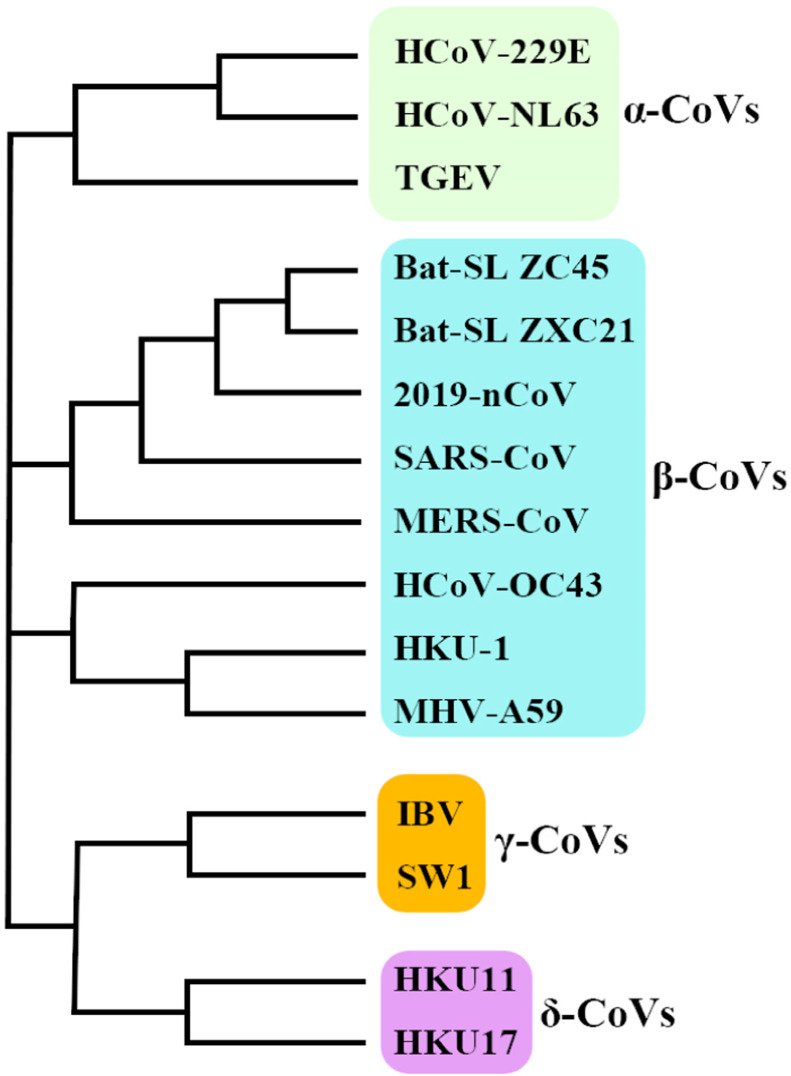
CoVs belong to the subfamily Coronavirinae, in the family Coronaviridae, to order Nidovirales, and this subfamily is divided into 4 groups α, β, γ, and δ ( Fig. 1) [17], [18]. So far, seven CoVs have been found that infect humans and cause respiratory diseases. In fact, four of the seven are common human CoVs, causing common upper respiratory illness: HCoV-229E, HCoV-OC43, HCoV-NL63, and HKU1 [4], [18]. The sequence analysis of SARS-CoV-2 revealed that this virus belongs to the beta CoVs category, including Bat‐SARS‐like (SL) -ZC45, Bat‐ SL ZXC21, SARS‐CoV, and MERS‐CoV. According to the CoV phylogenetic tree, SARS-CoV-2 lies close to the Bat‐SL‐CoV ZC45 and Bat‐SL‐CoV ZXC21 and farther is related to SARS‐CoV (Fig. 1) [18], [19]. In the 1960s, the first two cases of human CoVs i.e., HCoV-229E and HCoV-OC43 were identified. In fact, with the advent of SARS in 2002, new Beta-CoVs received attention. Moreover, HCoV-NL63 and HCoV-HKU1 were identified in 2004 and 2005, respectively [4]. MERS-CoV was discovered in 2012. Like SARS-CoV, it was able to infect the lower respiratory system, which causes severe respiratory syndrome in human [20]. In fact, SARS-CoV-2 was identified by separating the human airway epithelial cells in January 2020, using next-generation sequencing (NGS) method, and it was found that it is a new member of Beta-CoVs [21]. Additionally, SARS-CoV-2 is able to infect the lower respiratory system [22].
Fig. 1.
The phylogenetic tree of coronaviruses.
3. Genome structure
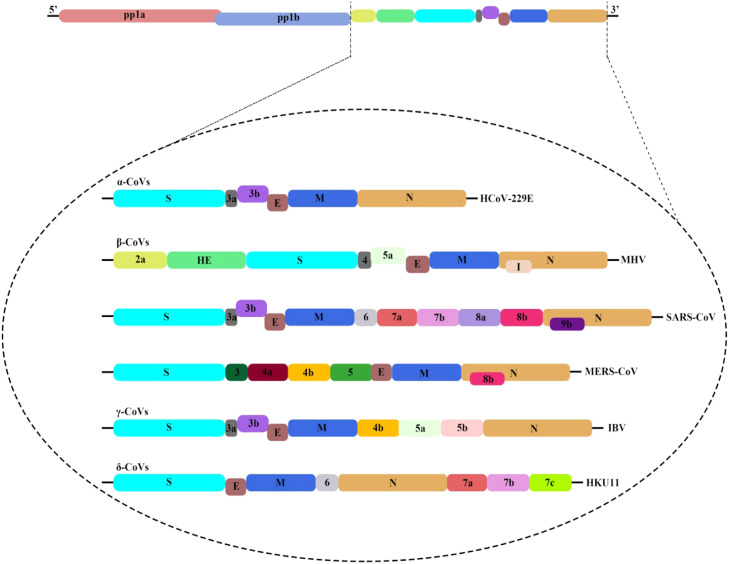
The genome of CoVs is a single‐stranded positive‐sense RNA (+ssRNA) with an approximately a length of 30 kb. Generally, a nested set of subgenomic RNAs (sgRNAs) are synthesized by the replication‐transcription complex (RTC) in a discontinuous transcription way [17], [23]. According to the observations, the common genome and subgenomes of CoV have at least six open reading frames (ORFs). The first ORF is ORF1a/b, forming about two third of the whole length of the genome. Additionally, it contains 16 non-structural proteins (non-structural proteins 1–16). Gamma CoV lacks the non-structural protein 1. It is worth mentioning that there is a frameshift between ORF1a and ORF1b, leading to the production of two polypeptides pp1a and pp1ab [24]. These polypeptides are processed by chymotrypsin-like protease (3CLpro) or the main protease (Mpro) [25], [26], [27]. However, other ORFs form one third of the genome, which is located near the 3′- terminus. It is also able to code four main structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) [28]. It is interesting to note that in addition to encoding these four main structural proteins, different CoVs encode specific and sub-structural proteins, such as hemagglutinin-esterase (HE) protein, 3a/b protein, and 4a/b protein ( Fig. 2) [18]. According to research studies, all structural and sub-structural proteins are transcribed from subgenomic RNAs of CoV [29]. The alignment of the CoVs genome sequence shows that 58% of the match exists in the non-structural protein encoding region and 43% in the structural protein encoding region between different CoVs (Fig. 2) [18]. Non-structural proteins are more protected, and structural proteins are more diverse with regard to the need for adaptation to new hosts. As the mutations rate in RNA viruses' replication is higher than DNA viruses, the RNA viruses usually have a length of less than 10 kb, while according to the observations, the CoV genome is very large, with a length of about 30 kb, and is known as one of the longest RNA viruses [30]. According to the reports, most non-structural proteins 1–16 have been found to play their specific roles in CoVs replication. In fact, the performance of some non-structural proteins has not yet been well understood [18], [31], [32], [33], [34], [35].
Fig. 2.
The genome structure of four genera of coronaviruses. Pp1a and pp1b represent the two long polypeptides that are processed into 16 nonstructural proteins. S, E, M, and N indicate the four structural proteins spike, envelope, membrane,and nucleocapsid. 2019-nCoV, 2019 novel coronavirus; CoVs, coronavirus; HE, hemagglutinin-esterase. Viral names: HKU, coronaviruses identified by Hong Kong University; HCoV, human coronavirus; IBV, infectious bronchitis virus; MHV, murine hepatitis virus; TGEV, transmissible gastroenteritis virus.
Homotrimers of the S protein make up spikes on the virus surface and bind to host receptors [36]. On the other hand, M protein has transmembrane domains (TMDs) and is also responsible for the formation of viral particle [37]. According to research studies, protein E plays an important role in the release of the virus, and in fact leads to the pathogenesis of the virus [38]. Additionally, Protein N has two domains; both of them are capable of connecting to RNA virus genome through different mechanisms. Protein N is also useful for virus replication [39], [40].
4. Host cell infection
As soon as the host is exposed to the virus, the virus binds to the virus receptor expressing cells, which can cause infection this way. SARS can bind to the host cell through angiotensin-converting enzyme 2 (ACE2), which is one of the main receptors, and thus target it. The other receptor is the CD209, which is an alternative very low-dependent receptor [41]. In fact, ACE2 regulates the renin system of angiotensin [2], [41], [42]. SARS infection decreases the expression of the ACE2 receptors in both lung tissue and target cells. Therefore, it disrupts the function of the renin system of angiotensin, and causes an increase in inflammation on the other hand [2], [43]. In the respiratory tract, ACE2 is widely expressed on alveolar epithelial cells (AEC), trachea, bronchi, bronchial serous glands, and monocytes and alveolar macrophages [44], [45]. Moreover, as a surface molecule, it is focused on the endothelial cells of blood vessels and veins, intestinal mucosal cells, epithelial tubular cells of the kidneys, epithelial cells of renal tubules, cerebral nerve cells, and immune cells of the body [46], [47]. The virus enters these target cells and then replicates. The viruses are then released from primary cells, and finally, infect the new target cells [48]. Considering the similarity of the SARS-CoV-2 and SARS-CoV genetic sequences, the SARS-CoV-2 is believed to use the same ACE2 receptor to infect target cells [11], [49], [50]. Dipeptidyl peptidase 4 (DPP4), also known as CD26, is the causative agent of MERS-CoV infection in humans [51]. The receptor is widely expressed on epithelial cells in the kidneys, alveolus, small intestine, liver, prostate, and leukocytes [52].
However, research studies have shown that MERS-CoV can infect several human cell lines, including respiratory, kidney, intestine, and liver cells, as well as histiocytes. In the following, it was observed that the in vitro tissue's tendency domain of this virus is broader than any other CoV [53], [54], [55]. Additionally, acute MERS-CoV can cause highly fatal pneumonia and renal dysfunction with a variety of clinical symptoms, including fever, cough, sore throat, muscle aches, chest pain, diarrhea, vomiting, and abdominal pain [53], [54], [55]. Lung infection in the MERS animal model showed the infiltration of neutrophils and macrophages and alveolar edema [56]. It is important to note that the DPP4 receptor for MERS-CoV is also highly expressed in the kidney. Thus it causes renal disorders with hypoxia or direct infection of the epithelium, and it is worth mentioning that MERS-CoV has the ability to infect human dendritic cells and macrophages in vitro, thus helps the virus disrupt the immune system [57], [58], [59]. Due to their high CD26 levels, T cells can also be alternative targets for MERS-CoV [60]. The virus may deregulate T-cell antiviral responses of T cell as a result of the simulation of T-cell apoptosis [60], [61], [62], [63], [64], [65].
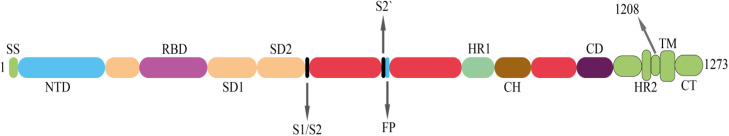
The above mentioned receptors bind to the virus spike protein, and the virus enters the host cells this way [11]. In fact, spike protein is a protein fusion that undergoes a structural rearrangement to fuse the virus cell membrane with the host cell membrane [66]. The structure of spike protein is shown in ( Fig. 3) [66]. This structure consists of two sub-units s1 and s2, where N-terminal domain (NTD) and receptor binding domain (RBD) are placed in unit s1. Below the S2 unit, there are FP section, S2′ protease cleavage, HR1 and HR2 [2], [66], [67], [68]. The infection process is actually begun as the RBD binds to the ACE2 receptor of the host cell. This binding leads to the SARS-CoV-2 endocytosis. Then they are exposed to endosomal proteases, and finally, the viral pack is released within the host cytoplasm [2], [69]. Research studies have shown that the sequences of the RBDs in SARS-CoV and SARS-CoV-2 are similar by more than 70%. On the other hand, the transmission and increase rate of SARS-CoV-2 infection was higher than SARS-CoV, which may indicate the difference in the RBDs down conformation positions in their respective S structures. Therefore, the tendency of SARS-CoV-2 RBD to bind to ACE2 receptor will be about 10–20-fold higher than SARS-CoV [2], [66].
Fig. 3.
Spike protein structure in SARS-CoV-2. SS, signal sequence; S2′, protease cleavage site; FP, fusion peptide; HR1, heptad repeat 1; CH, central helix; CD, connector domain; HR2, heptad repeat 2; TM, transmembrane domain; CT, cytoplasmic tail.
Following the entry of SARS-CoV-2 into the lung cells, a severe immune response is generated, which leads to a phenotype, called cytokine storm syndrome. Cytokine storm syndrome causes ARDS and severe failures in various organs such as lung tissue [70], [71]. According to the pathology of COVID-19, two important factors ARDS and dysfunctional immune responses account for the highest death rates (above 97%), with ARDS having the main role [72], [73], [74]. It is also now clear that patients with COVID-19-related ARDS in the intensive care unit (ICU) have lower numbers of T cells (CD4+ and CD8+), B cells, and natural killer cells (NKs) than patients who are not admitted to the ICU [75], [76], [77].
The immune responses to COVID-19-related ARDS will be extensive and out of balance, which would then lead to over-release, especially IL-6, resulting in lymphocyte death [76]. ACE2 receptor is not present on T cells, whereas the decrease in the number of these cells is quite evident [76]. Thus, it can be assumed that T cells are indirectly attacked by SARS-CoV-2 [76]. Elevated serum levels of cytokines including IL-6, IL-10, and TNF-α in elderly COVID-19 patients (over 60 years), as well as age-related diseases with one or more co-morbidities, have been widely reported [76].
More important than the decrease of T cell numbers is the exhaustion of these cells, which has been associated with the disease severity and the prolongation of the ICU term [76]. Excessively increased cytokines mentioned above are inversely related to the number of T cells, which may indicate its effect on T cell exhaustion [76]. Today, numerous markers of T cell exhaustion have been identified. These markers increase during the course of infectious diseases and even cancers [78].
Recently, a number of studies have been devoted to measuring the levels of T cell exhaustion markers in COVID-19 patients and comparing them to other chronic infections. As a result, patients with COVID‐19 admitted to the ICU were associated with increased levels of lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin domain and mucin domain 3 (TIM-3), and programmed death-1 (PD-1) markers [75], [79], [80]. In summary, irregular secretion of cytokines causes an up-regulation of exhaustion markers, which leads to apoptosis or necrosis and the decline of T cells [75].
Therefore, ARDS reduces the immune system function, and then, the body will become vulnerable to secondary infections and respiratory disorders [11], [81], [82], [83], [84], [85].
5. Cytokine storm
The innate immune system detects viral infections using different pattern recognition receptors (PRR) to detect pathogen-associated molecular patterns (PAMPs) [2], [86]. Therefore, SARS-CoV, MERS-CoV, and SARS-CoV-2 infections produce localized inflammatory responses, including the increased uncontrolled secretion of cytokines (e.g., IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGFβ, etc.) and chemokines (e.g., CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) [2], [87], [88]. Following the formation of this cytokine storm, the immune system launches a broad attack against the infected organs, and subsequently leads to the organs failure and ARDS [87]. First of all, the aim of releasing these cytokines is to generate a defensive and anti-inflammatory response, but the increased uncontrolled secretion of these cytokines leads to the disruption of the immune response and makes the disease worse [89]. Secretory cytokines such as IL-2, IL-7, IL-10, GCSF, IP10, MCP1, MIP1, and TNF-α were observed in the serum of COVID-19 patients. [2], [89]. Additionally, the higher amounts of inflammatory monocytes CD14+ and CD16+ were quite evident in these patients, causing in fact the secretion of cytokines MCP1, IP10, and MIP1-α, which play a significant role in the cytokine storm [2]. Studies have shown that SARS-CoV, MERS-CoV, and SARS-CoV-2 infections are able to infect human respiratory epithelial cells, macrophages, T cells, and dendritic cells, affecting the ability to produce and induce pre-inflammatory cytokines and chemokines [2], [90]. In another study, patients with low IFN-α level died, but in contrast, patients with high IFN-α level survived [75]. Given the issues raised above, one of the research methods could be to focus on reducing immune damage in patients with COVID-19 to prevent further damage to lung tissue.
6. Anti-viral treatments
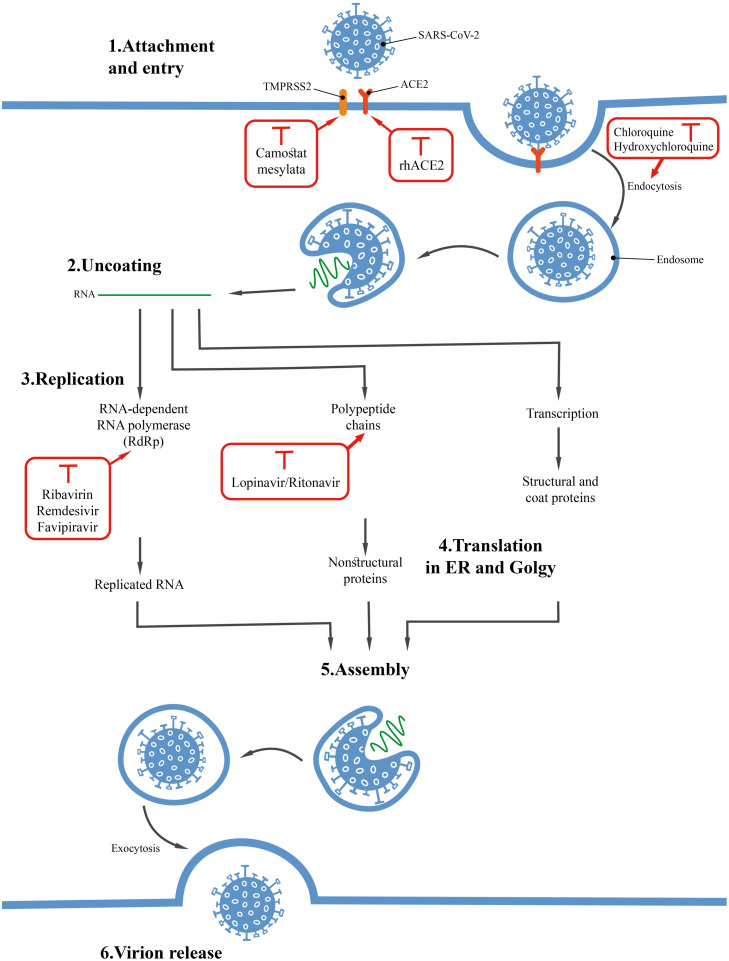
Fig. 4 shows the life cycle stages for SARS-CoV-2 virus, which can specifically be considered as the therapeutic objectives [91]. In this section, some of the results of drugs used to treat COVID-19 are investigated.
Fig. 4.
The effect of the mechanism of some drugs on different stages of the SARS-CoV-2 life cycle.
6.1. Chloroquine(CQ)/hydroxychloroquine (HCQ)
Chloroquine (CQ) and hydroxychloroquine (HCQ) are in fact among the 70-year-old drugs used to prevent and treat malaria. Additionally, these drugs are used as an anti-inflammatory substance to treat rheumatoid arthritis and lupus arrhythmia [92], [93], [94], [95], [96], [97]. According to in vitro studies, HCQ has anti-SARS activity. That is why HCQ can also be investigated as a potential drug to treat COVID-19 [94], [95], [96].
Among the functional mechanisms of these drugs, can refer to the increased endosomal pH, interference with cellular receptor glycosylation, inhibition of virus replication, decreased cytokine secretion, autophagy inhibition, lysosomal activity, and immunomodulatory effects [92], [93], [95], [96], [98], [99], [100].
The results from 100 patients with COVID-19 in China showed that CQ phosphate could effectively control and inhibit pneumonia [93]. The study conducted on 36 COVID-19 patients showed that the addition of azithromycin to HCQ provides better viral clearance compared to HCQ alone [92], [101]. The dose of HCQ used to treat COVID-19 is 500 mg orally once or twice a day [102]. Of course, this dose can vary depending on the clinical experience, available protocols, as well as with a physician's opinion [102]. On the other hand, the complications of these drugs should also be considered [91], [95], [103].
6.2. Lopinavir/ritonavir
Lopinavir/ritonavir is used to treat the human immunodeficiency virus (HIV). According to research studies, Lopinavir has been able to inhibit the protease activity of SARS and MERS. In fact, proteinases are among the key and important enzymes in CoVs' polyproteins processing [104], [105], [106]. The prescribed doses of lopinavir/ritonavir to treat COVID-19 are 100 mg-/-400 mg twice daily for 14 days, respectively [107]. In order to confirm the effects of lopinavir/ritonavir in treating COVID-19, we need to wait for valid clinical evidence. Among complications of this drug are nausea, diarrhea, increased bilirubin, triglyceride, and liver enzyme levels [106], [107], [108], [109].
6.3. Darunavir
Darunavir is used as the second generation of HIV-1 protease inhibitors. The research studies conducted in China showed that darunavir can inhibit SARS-CoV-2 infection in laboratory conditions [108]. A recent study on 30 COVID-19 patients showed that the addition of cobicistat to darunavir (combination therapy) had no significant effect on the treatment of the patients [93], [110].
6.4. Ribavirin
In fact, ribavirin inhibits viral RNA-dependent RNA polymerase. Research studies indicate this compound has been able to inhibit SARS replication in vitro conditions. Due to its antiviral activity, this drug can also be investigated as a treatment for COVID-19 [111]. Clinical trials of ribavirin to treat SARS showed that the drug can cause complications such as liver and hematological toxicity [111], [112]. There is currently very little information about the therapeutic potential of ribavirin to treat COVID-19. On the other hand, the experiments indicate the high toxicity of this compound. Therefore, to use this compound, all the existing aspects should be well investigated and the appropriate combined therapies should be used to increase its clinical effectiveness and decrease its complications if possible. This drug was first used to treat Ebola [113].
6.5. Remdesivir
Remdesivir drug is a nucleoside analogue used to treat a wide range of viruses. The effects of this drug on lung tissue of mice infected with MERS-CoV showed that it can reduce the viral load in lung tissue [100], [108], [114]. The drug was examined by Holshue et al. to treat patients with COVID-19 and the experiment revealed promising results [108]. Its dose is in the form of a single dose of 200 mg, and then, the daily injection of 100 mg is used.
6.6. Favipiravir
Favipiravir is a new type of viral RNA-dependent RNA polymerase inhibitors. The drug is able to inhibit the proliferation of a wide range of RNA viruses. In addition, favipiravir drug has recently received attention in the COVID-19 treatment protocol in some countries. A clinical trial with favipiravir was conducted in Shenzhen hospital on 80 patients to treat COVID-19. The obtained results showed that the drug has a stronger antiviral effect than lopinavir and ritonavir. However, no significant complication was observed on 80 patients participated in this clinical trial with favipiravir [108], [115], [116], [117].
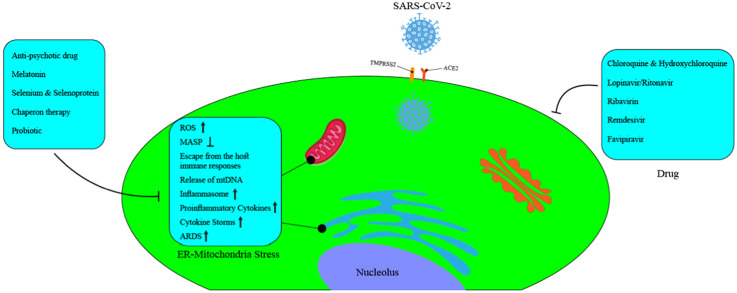
7. Inhibition of endoplasmic reticulum-mitochondrial stress as a novel strategy to combat COVID-19
There are several studies that have identified a range of interactions between SARS-CoV-2 spike proteins and host cell receptors. In general, the high ability of SARS-CoV-2 spike protein to identify and bind to several host cell receptors including, ACE2, membrane serine proteinase (TMPRSS2), glucose-78 regulated protein (GRP78), DPP4, basigin or CD147, protein tyrosine kinase (AXL) receptor, and neuropilin-1 (Nrp-1) is well-established [118], [119], [120], [121].
It is possible that genetic diversity and different isoforms of receptors mentioned in individuals affected the susceptibility to COVID-19. It can be speculated that one of the reasons that compared with other receptors, ACE2 is targeted more by the SARS-CoV-2 is its prominent role in regulating mitochondrial function [119]. It now appears that, during evolution, many respiratory viruses have evolved strategies to escape immune system responses by targeting two vital organelles of the host cells, including endoplasmic reticulum (ER) and mitochondria [118], [122].
ER and mitochondria are closely related to each other, and their main tasks are Ca++ homeostasis, autophagy, and apoptosis, in addition to the regulation of innate and adaptive immune responses during viral infections [119], [123], [124], [125]. Studies have identified several SARS-CoV proteins with an ability to manipulate ER and mitochondria proteins, and thus enabling the virus to escape from host cell defense and continue to replicate [119], [122].
For instance, SARS-CoV orf9b localizes inside mitochondria, leading to degradation of dynamin-like protein 1, which subsequently causes the suppression of mitochondria antiviral signaling protein [118], [119]. The role of SARS-CoV orf3a in the control of mitophagy function by targeting a mitochondria ubiquitin specific peptidase 30 (USP30) has been documented. It is possible that SARS-CoV-2 could diminish or suppress host immune responses by altering ubiquitination [119]. SARS-CoV E protein, apart from can disorder intracellular Ca++, it also appears that induce mitogen activated protein (MAP) kinase, which eventually activates of Inflammasome [118].
On the other hand, SARS-CoV NSP3/4/6 complexes are localized into the ER and mitochondria membranes. They subsequently participate in forming double-membrane vesicles (DMV) derived from both organelles, leading to the escape from the immune system responses and continue to replicate safely [73], [119], [122]. These events can be explained by the encoding of similar proteins in SARS-CoV-2. Recent evidence suggests the interaction of SARS-CoV-2 with a mitochondrial import receptor Tomm70, which exerting a possible role in the down-regulate of antiviral cellular defense pathways [119].
After the organelles are infected with SARS-CoV, it causes stress in both of them. Endoplasmic reticulum-mitochondrial stress increases reactive oxygen species (ROS) and produces proinflammatory cytokines. Interestingly, proinflammatory cytokines in turn, further increase ROS. For instance, proinflammatory cytokines such as TNF-α, IL-6, IL-10, and CXCL-8 hinder oxidative phosphorylation and lead to cell death (apoptosis) by increasing ROS production [126]. There is some evidence that patients with COVID-19-related ARDS who suffer from low oxygen levels are associated with mitochondrial dysfunction in oxidative phosphorylation and electron transport chain [73], [119].
Also, ER-mitochondrial stress promotes ARDS by disrupting the function of regulatory T cells (Tregs) [127]. Besides, reduced oxidative phosphorylation would lead to T cell exhaustion [118]. Furthermore, by affecting Ca++ homeostasis, ROS causes Ca++ to leakage from the ER into the mitochondria, which ultimately leads to the destruction of the inner mitochondrial membrane [120], [123], [126]. Based on the pathology evidence of COVID-19 patients, viral particles in ER and mitochondria were observed to be dilated and swollen, respectively [120].
As the viral infection progresses, the ER-mitochondrial stress gradually increases, causing mitochondrial DNAs (mtDNAs) to be secreted into the cytoplasm. Immune cells detect mtDNAs, resulting in extensive local and systematic inflammatory responses [119], [128]. It is possible that mtDNA secretion may be involved in the formation of cytokine storms in COVID-19 patients [73]. Meanwhile, ER-mitochondrial stress is associated with diabetes, cancer, viral infections, neurological diseases, and gastrointestinal disorders [120], [123], [126].
New and compelling evidence suggests that ICU-related high mortality in elderly COVID-19 patients with co-morbidities can be attributed to ER-mitochondrial stress. In the next section, we will discuss some of the compounds that may be effective in reducing ER-mitochondrial stress in COVID-19 patients.
7.1. Anti-psychotic drug
SARS-CoV-2 NSP6, infects both organelles by binding to the sigma receptor in the ER membrane [129], [130]. Haloperidol, which has been used to treat people with psychosis, has recently gained attention due to its anti-inflammatory role in the treatment of COVID-19. In fact, it blocks the binding of NSP6 to the Sigma receptor. Another drug that was thought to have a similar mechanism was sigma-1 benzomorphan agonist, dextromethorphan.
It is prescribed to reduce cough in adults with viral infections of the upper respiratory tract. However, the conflicting results and biphasic activity led to the drug being further investigated. Pandey et al. determined the molecular mechanism of both drugs haloperidol and dextromethorphan using computational bioinformatics methods that only proved the inhibitory effects of haloperidol SARS-CoV-2 [129].
7.2. Melatonin
Decreased melatonin levels, often more common in elderly patients with viral infections, can put the ER and mitochondrial more stressed. With its unique properties, such as anti-inflammatory and antioxidant and, most importantly, rearrangement of ER and mitochondrial functions, melatonin can be considered an attractive option in treating COVID-19 by strengthening and modulating immune responses [125].
The protective effects of melatonin in many viral infections have already been well documented [131], [132], [133]. It is possible that melatonin relieves ER-mitochondrial stress and thus modulates signaling pathways associated with apoptosis and autophagy [125]. It is also suggested that it can manage the cytokine storm in COVID-19 patients by suppressing the inflammatory pathway of CD147 and reducing macrophage inflammation [118], [125].
7.3. Selenium and selenoprotein
As a rare organic and mineral element, the deficiency of selenium can contribute to the pathogenicity of infectious and non-infectious diseases [134]. Studies have shown that COVID-19 patients are selenium deficient. Interestingly, in the human genome, 25 genes encode selenoproteins, some of which play a significant role in regulating inflammatory cytokines [134], [135].
It is possible that CoVs could cause ER-mitochondrial stress by manipulating host selenoproteins and subsequently increase ROS. Following an increase in ROS, the NFKB pathway is activated, and eventually, inflammation develops. Its deficiency has been reported to cause macrophages to penetrate the lungs and promote cytokine storms [134], [135], [136].
Preclinical and clinical studies on COVID-19 patients have shown that taking selenium supplements at a dose of 400 µg-/-500 µg daily increases the number of CD4+ cells. Consumption of higher doses may cause toxicity and even help to aggravate the disease [134], [135]. It is interesting to note that its consumption can be associated with reduced ER-mitochondrial stress, ROS, IL-6, and immune responses in elderly COVID-19 patients [135], [136].
7.4. Chaperone therapy
It has now been shown that chaperone therapy can be considered in the treatment of COVID-19. For instance, tauroursodeoxycholic acid (TUDCA) and 4-phenyl butyric acid (PBA) are used to treat ER-mitochondria stress-related illnesses. Previous studies have shown that these compounds have anti-inflammatory effects in viral respiratory infections [120].
7.5. Probiotic
Another exciting treatment strategy in COVID-19 treatment is related to probiotics. SARS-CoV-2 penetrates the gastrointestinal tract through the ACE2 receptor and changes the microbial population by inducing ER-mitochondrial stress. These changes lead to the release of toxic gases, which in turn increase ER-mitochondrial stress. This data does suggest that abnormal immune responses induced by ER-mitochondrial stress lead to microbiota dysbiosis. It is concluded that probiotics modulate intestinal homeostasis by inhibiting ER-mitochondrial stress and preventing the exacerbation of COVID-19. It is expected that probiotics can relieve gastrointestinal symptoms such as diarrhea in COVID-19 patients by relevant stress reduction [126].
8. Expert opinion
Over recent years, COVs have spread periodically and every few years. Considering their high prevalence rate, they are spreading rapidly all around the world, causing very serious infectious diseases. Therefore, it is not unexpected that we will encounter a new epidemic of other members of the COVs family in the future years. Phylogenetic studies have shown that COVs have transmitted from natural hosts to humans.
The rate of SARS-CoV-2 transmission from human to human is very high, and it is mainly transmitted through small respiratory droplets. Thus it is of significant importance that human activities such as modern farming methods, urbanization, event cancellations, social distancing, wearing a face mask, and frequent hand-washing should be reconsidered in all around the world.
COVID-19 develops a variety of clinical symptoms and signs. Older adults and those who have one or more than one underlying medical conditions are at higher risk for developing this illness.
There is currently no specific approved vaccine or medicine for treatment of COVID-19 disease. Most of the available information has been obtained through the studies on other members of this family called SARS and MERS. Many researchers are currently working on developing various types of specific drugs to treat this disease all around the world. Extensive research is being conducted all around the world to produce medicines and vaccines to treat COVID-19, which is generally very time consuming. A large number of studies are being conducted to investigate the effectiveness of medicines such as Lopinavir/Ritonavir, Darunavir, Ribavirin, Remdesivir, and Favipiravir as potential therapeutic methods for COVID-19. The investigation of this effectiveness and the complications of antiviral medicines in clinical studies will be a great help in the production of medicines in the near future for treatment of COVID-19. Moreover, information such as the structure of SARS-CoV-2 genome, how the host immune system responds to the virus, and how to control cytokine storm are of considerable importance in the vaccines' design and production.
Conflict of interest statement
The authors declare that they have no conflict of interests.
References
- 1.Shi Y., et al. Nature Publishing Group; 2020. COVID-19 Infection: The Perspectives on Immune Responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanaei S., Rezaei N. COVID-19: developing from an outbreak to a pandemic. Arch. Med. Res. 2020;51:582–584. doi: 10.1016/j.arcmed.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo P.C.Y., Lau S.K.P., Lam C.S.F., Lau C.C.Y., Tsang A.K.L., Lau J.H.N., Bai R., Teng J.L.L., Tsang C.C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valencia D.N. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12(3) doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotfi M., Rezaei N. SARS-CoV-2: a comprehensive review from pathogenicity of the virus to clinical consequences. J. Med. Virol. 2020;92:1864–1874. doi: 10.1002/jmv.26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization, W.H., Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. http://www.who.int/csr/sars/country/table2004_04_21/en/index. html, 2003.
- 9.Al-Rabiaah A., Temsah M.H., Al-Eyadhy A.A., Hasan G.M., Al-Zamil F., Al-Subaie S., Alsohime F., Jamal A., Alhaboob A., Al-Saadi B., Somily A.M. Middle East Respiratory Syndrome-Corona Virus (MERS-CoV) associated stress among medical students at a university teaching hospital in Saudi Arabia. J. Infect. Public Health. 2020;13:687–691. doi: 10.1016/j.jiph.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabbari P., Taraghikhah N., Jabbari F., Ebrahimi S., Rezaei N. Adherence of the general public to self-protection guidelines during the COVID-19 pandemic. Disaster Med. Public Health Prep. 2020:1–12. doi: 10.1017/dmp.2020.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X., Wang X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genom. 2020;47(2):119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): a lesson from animal coronaviruses. Vet. Microbiol. 2020;244 doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackenzie J.S., Smith D.W. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t. Microbiol. Aust. 2020;41(1):45–50. doi: 10.1071/MA20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotfi M., Hamblin M.R., Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J.M., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80(12):5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith E.C., Blanc H., Vignuzzi M., Denison M.R. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013;9(8) doi: 10.1371/journal.ppat.1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P., Fan H., Lan T., Yang X.L., Shi W.F., Zhang W., Zhu Y., Zhang Y.W., Xie Q.M., Mani S., Zheng X.S., Li B., Li J.M., Guo H., Pei G.Q., An X.P., Chen J.W., Zhou L., Mai K.J., Wu Z.X., Li D., Anderson D.E., Zhang L.B., Li S.Y., Mi Z.Q., He T.T., Cong F., Guo P.J., Huang R., Luo Y., Liu X.L., Chen J., Huang Y., Sun Q., Zhang X.L.L., Wang Y.Y., Xing S.Z., Chen Y.S., Sun Y., Li J., Daszak P., Wang L.F., Shi Z.L., Tong Y.G., Ma J.Y. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556(7700):255–258. doi: 10.1038/s41586-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P.K., Wong D.A., Tong L.K., Ip S.M., Lo A.C., Lau C.S., Yeung E.Y., Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sola I., Almazán F., Zúñiga S., Enjuanes L. Continuous and discontinuous RNA synthesis in coronaviruses. Annu. Rev. Virol. 2015;2:265–288. doi: 10.1146/annurev-virology-100114-055218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain S., Pan J., Chen Y., Yang Y., Xu J., Peng Y., Wu Y., Li Z., Zhu Y., Tien P., Guo D. Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79(9):5288–5295. doi: 10.1128/JVI.79.9.5288-5295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J. Virol. 2007;81(1):20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81(4):853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 29.Eckerle L.D., Becker M.M., Halpin R.A., Li K., Venter E., Lu X., Scherbakova S., Graham R.L., Baric R.S., Stockwell T.B., Spiro D.J., Denison M.R. Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathog. 2010;6(5) doi: 10.1371/journal.ppat.1000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogando N.S., Ferron F., Decroly E., Canard B., Posthuma C.C., Snijder E.J. The curious case of the nidovirus exoribonuclease: its role in RNA synthesis and replication fidelity. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beniac D.R., Andonov A., Grudeski E., Booth T.F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 2006;13(8):751–752. doi: 10.1038/nsmb1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64(11):5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nal B., Chan C., Kien F., Siu L., Tse J., Chu K., Kam J., Staropoli I., Crescenzo-Chaigne B., Escriou N., van der Werf S., Yuen K.Y., Altmeyer R. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J. Gen. Virol. 2005;86(5):1423–1434. doi: 10.1099/vir.0.80671-0. [DOI] [PubMed] [Google Scholar]
- 34.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., Droese B., Klaus J.P., Makino S., Sawicki S.G., Siddell S.G., Stamou D.G., Wilson I.A., Kuhn P., Buchmeier M.J. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174(1):11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeDiego M.L., Álvarez E., Almazán F., Rejas T., Lamirande E., Roberts A., Shieh W.J., Zaki S.R., Subbarao K., Enjuanes L. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J. Virol. 2007;81(4):1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieto-Torres J.L., DeDiego M.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Fernandez-Delgado R., Castaño-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fehr A.R., Perlman S. Springer; 2015. Coronaviruses: An Overview of Their Replication and Pathogenesis, in Coronaviruses; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang C., Sue S.C., Yu T., Hsieh C.M., Tsai C.K., Chiang Y.C., Lee S., Hsiao H., Wu W.J., Chang W.L., Lin C.H., Huang T. Modular organization of SARS coronavirus nucleocapsid protein. J. Biomed. Sci. 2006;13(1):59–72. doi: 10.1007/s11373-005-9035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurst K.R., Koetzner C.A., Masters P.S. Identification of in vivo-interacting domains of the murine coronavirus nucleocapsid protein. J. Virol. 2009;83(14):7221–7234. doi: 10.1128/JVI.00440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui L., Wang H., Ji Y., Yang J., Xu S., Huang X., Wang Z., Qin L., Tien P., Zhou X., Guo D., Chen Y. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells. J. Virol. 2015;89(17):9029–9043. doi: 10.1128/JVI.01331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., DeMartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. 2004;101(44):15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6(3):271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L., Wei Q., Alvarez X., Wang H., Du Y., Zhu H., Jiang H., Zhou J., Lam P., Zhang L., Lackner A., Qin C., Chen Z. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011;85(8):4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Y., Korteweg C., McNutt M.A., Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133(1):4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirato K., Kanou K., Kawase M., Matsuyama S. Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J. Virol. 2017;91(1) doi: 10.1128/JVI.01387-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qinfen Z., Jinming C., Xiaojun H., Huanying Z., Jicheng H., Ling F., Kunpeng L., Jingqiang Z. The life cycle of SARS coronavirus in Vero E6 cells. J. Med. Virol. 2004;73(3):332–337. doi: 10.1002/jmv.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. J. Pathol. Soc. G. B. Irel. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyerholz D.K., Lambertz A.M., McCray P.B., Jr. Dipeptidyl peptidase 4 distribution in the human respiratory tract: implications for the Middle East respiratory syndrome. Am. J. Pathol. 2016;186(1):78–86. doi: 10.1016/j.ajpath.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Widagdo W., Raj V.S., Schipper D., Kolijn K., van Leenders G.J.L.H., Bosch B.J., Bensaid A., Segalés J., Baumgärtner W., Osterhaus A.D.M.E., Koopmans M.P., van den Brand J.M.A., Haagmans B.L. Differential expression of the Middle East respiratory syndrome coronavirus receptor in the upper respiratory tracts of humans and dromedary camels. J. Virol. 2016;90(9):4838–4842. doi: 10.1128/JVI.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S., Alkhaldi K.Z., Almohammadi E.L., Alraddadi B.M., Gerber S.I., Swerdlow D.L., Watson J.T., Madani T.A. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. New Engl. J. Med. 2015;372(9):846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F., Fung K.S., Tang H.L., Yan W.W., Chan H.W.H., Lai T.S.T., Tong K.L., Lai K.N. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A., Selim M.A.A., Mutairi M.A., Nakhli D.A., Aidaroos A.Y.A., Sherbeeni N.A., Al-Khashan H.I., Memish Z.A., Albarrak A.M. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int. J. Infect. Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D., de Wit E., Munster V.J., Hensley L.E., Zalmout I.S., Kapoor A., Epstein J.H., Karesh W.B., Daszak P., Mohammed O.B., Lipkin W.I. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5(2) doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambeir A.-M., Durinx C., Scharpé S., De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003;40(3):209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 59.Chu H., Zhou J., Ho-Yin Wong B., Li C., Cheng Z.S., Lin X., Kwok-Man Poon V., Sun T., Choi-Yi Lau C., Fuk-Woo Chan J., Kai-Wang To K., Chan K.H., Lu L., Zheng B.J., Yuen K.Y. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou J., Chu H., Li C., Wong B.H.Y., Cheng Z.S., Poon V.K.M., Sun T., Lau C.C.Y., Wong K.K.Y., Chan J.Y.W., Chan J.F.W., To K.K.W., Chan K.H., Zheng B.J., Yuen K.Y. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. 2014;209(9):1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu H., Zhou J., Wong B.H.Y., Li C., Chan J.F.W., Cheng Z.S., Yang D., Wang D., Lee A.C.Y., Li C., Yeung M.L., Cai J.P., Chan I.H.Y., Ho W.K., To K.K.W., Zheng B.J., Yao Y., Qin C., Yuen K.Y. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016;213(6):904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeung M.-L., Yao Y., Jia L., Chan J.F.W., Chan K.H., Cheung K.F., Chen H., Poon V.K.M., Tsang A.K.L., To K.K.W., Yiu M.K., Teng J.L.L., Chu H., Zhou J., Zhang Q., Deng W., Lau S.K.P., Lau J.Y.N., Woo P.C.Y., Chan T.M., Yung S., Zheng B.J., Jin D.Y., Mathieson P.W., Qin C., Yuen K.Y. MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2. Nat. Microbiol. 2016;1(3):1–8. doi: 10.1038/nmicrobiol.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W.J., Lan J., Liu K., Deng Y., Yao Y., Wu S., Chen H., Bao L., Zhang H., Zhao M., Wang Q., Han L., Chai Y., Qi J., Zhao J., Meng S., Qin C., Gao G.F., Tan W. Protective T cell responses featured by concordant recognition of Middle East respiratory syndrome coronavirus–derived CD8+ T cell epitopes and host MHC. J. Immunol. 2017;198(2):873–882. doi: 10.4049/jimmunol.1601542. [DOI] [PubMed] [Google Scholar]
- 64.Chan R.W.Y., Chan M.C.W., Agnihothram S., Chan L.L.Y., Kuok D.I.T., Fong J.H.M., Guan Y., Poon L.L.M., Baric R.S., Nicholls J.M., Peiris J.S.M. Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. J. Virol. 2013;87(12):6604–6614. doi: 10.1128/JVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lau S.K.P., Lau C.C.Y., Chan K.H., Li C.P.Y., Chen H., Jin D.Y., Chan J.F.W., Woo P.C.Y., Yuen K.Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J. Gen. Virol. 2013;94(12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 66.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marra M.A. The genome sequence of the SARS-associated coronavirus. Science. 2003;300(5624):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 68.Qu X.-X., Hao P., Song X.J., Jiang S.M., Liu Y.X., Wang P.G., Rao X., Song H.D., Wang S.Y., Zuo Y., Zheng A.H., Luo M., Wang H.L., Deng F., Wang H.Z., Hu Z.H., Ding M.X., Zhao G.P., Deng H.K. Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J. Biol. Chem. 2005;280(33):29588–29595. doi: 10.1074/jbc.M500662200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS‐coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus - a perspective. Expert Rev. Clin. Immunol. 2020;16(5):465–470. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hashemian S.M., Shafigh N., Afzal G., Jamaati H., Tabarsi P., Marjani M., Malekmohammad M., Mortazavi S.M., Khoundabi B., Mansouri D., Moniri A., Hajifathali A., Roshandel E., Mortaz E., Adcock I.M. Plasmapheresis reduces cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS) Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holder K., Reddy P.H. The Covid-19 effect on the immune system and mitochondrial dynamics in diabetes, obesity, and dementia. Neuroscientist. 2020 doi: 10.1177/1073858420960443. [DOI] [PubMed] [Google Scholar]
- 74.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M., Bortolotti P., Martinez L., Dubucquoi S., Dessein R., Gosset P., Mathieu D., Guery B. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PloS One. 2014;9(2) doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Notz Q., Schmalzing M., Wedekink F., Schlesinger T., Gernert M., Herrmann J., Sorger L., Weismann D., Schmid B., Sitter M., Schlegel N., Kranke P., Wischhusen J., Meybohm P., Lotz C. Pro-and anti-inflammatory responses in severe COVID-19-induced acute respiratory distress syndrome—an observational pilot study. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.581338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tahaghoghi-Hajghorbani S., Zafari P., Masoumi E., Rajabinejad M., Jafari-Shakib R., Hasani B., Rafiei A. The role of dysregulated immune responses in COVID-19 pathogenesis. Virus Res. 2020;290 doi: 10.1016/j.virusres.2020.198197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McLane L.M., Abdel-Hakeem M.S., Wherry E.J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 79.Herrmann M., Schulte S., Wildner N.H., Wittner M., Brehm T.T., Ramharter M., Woost R., Lohse A.W., Jacobs T., Schulze zur Wiesch J. Analysis of co-inhibitory receptor expression in COVID-19 infection compared to acute plasmodium falciparum malaria: lAG-3 and TIM-3 correlate with t cell activation and course of disease. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Z., Wherry E.J. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Villar J., Zhang H., Slutsky A.S. Lung repair and regeneration in ARDS: role of PECAM1 and Wnt signaling. Chest. 2019;155(3):587–594. doi: 10.1016/j.chest.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Channappanavar R., Perlman S. Seminars in Immunopathology. Springer; 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H., Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 2008;26(6):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 84.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cong Y., Hart B.J., Gross R., Zhou H., Frieman M., Bollinger L., Wada J., Hensley L.E., Jahrling P.B., Dyall J., Holbrook M.R. MERS-CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte-derived antigen-presenting cells. PloS One. 2018;13(3) doi: 10.1371/journal.pone.0194868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajaei S., Dabbagh A. The immunologic basis of COVID-19: a clinical approach. J. Cell. Mol. Anesth. 2020;5(1):37–42. [Google Scholar]
- 90.Zhou J., Chu H., Chan J.F.W., Yuen K.Y. Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis. Virol. J. 2015;12(1) doi: 10.1186/s12985-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 92.Bhatti J.S., Bhatti G.K., Khullar N., Reddy A.P., Reddy P.H. Therapeutic strategies in the development of anti-viral drugs and vaccines against SARS-CoV-2 infection. Mol. Neurobiol. 2020;57(11):4856–4877. doi: 10.1007/s12035-020-02074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kandimalla R., John A., Abburi C., Vallamkondu J., Reddy P.H. Current status of multiple drug molecules, and vaccines: an update in SARS-CoV-2 therapeutics. Mol. Neurobiol. 2020;57:4106–4116. doi: 10.1007/s12035-020-02022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vallamkondu J., John A., Wani W.Y., Ramadevi S.P., Jella K.K., Reddy P.H., Kandimalla R. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 97.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou D., Dai S.-M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020;75(7):1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kalil A.C. Treating COVID-19—off-label drug use, compassionate use, and randomized clinical trials during pandemics. Jama. 2020;323(19):1897–1898. doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 104.Chu C.M. Role of iopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease‐19 treatment option. J. Med. Virol. 2020;92(6):556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. New Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 109.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J., et al. Open Forum Infectious Diseases. Oxford University Press; US: 2020. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9) doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K., Abdulmomen A., Qushmaq I., Sindi A.A., Mady A., Solaiman O., Al-Raddadi R., Maghrabi K., Ragab A., Al Mekhlafi G.A., Balkhy H.H., Al Harthy A., Kharaba A., Gramish J.A., Al-Aithan A.M., Al-Dawood A., Merson L., Hayden F.G., Fowler R. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin. Infect. Dis. 2020;70(9):1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jacobs M., Rodger A., Bell D.J., Bhagani S., Cropley I., Filipe A., Gifford R.J., Hopkins S., Hughes J., Jabeen F., Johannessen I., Karageorgopoulos D., Lackenby A., Lester R., Liu R.S.N., MacConnachie A., Mahungu T., Martin D., Marshall N., Mepham S., Orton R., Palmarini M., Patel M., Perry C., Peters S.E., Porter D., Ritchie D., Ritchie N.D., Seaton R.A., Sreenu V.B., Templeton K., Warren S., Wilkie G.S., Zambon M., Gopal R., Thomson E.C. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388(10043):498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sissoko D., Laouenan C., Folkesson E., M’Lebing A.B., Beavogui A.H., Baize S., Camara A.M., Maes P., Shepherd S., Danel C., Carazo S., Conde M.N., Gala J.L., Colin G., Savini H., Bore J.A., Le Marcis F., Koundouno F.R., Petitjean F., Lamah M.C., Diederich S., Tounkara A., Poelart G., Berbain E., Dindart J.M., Duraffour S., Lefevre A., Leno T., Peyrouset O., Irenge L., Bangoura N., Palich R., Hinzmann J., Kraus A., Barry T.S., Berette S., Bongono A., Camara M.S., Chanfreau Munoz V., Doumbouya L., Souley Harouna, Kighoma P.M., Koundouno F.R., Réné Lolamou, Loua C.M., Massala V., Moumouni K., Provost C., Samake N., Sekou C., Soumah A., Arnould I., Komano M.S., Gustin L., Berutto C., Camara D., Camara F.S., Colpaert J., Delamou L., Jansson L., Kourouma E., Loua M., Malme K., Manfrin E., Maomou A., Milinouno A., Ombelet S., Sidiboun A.Y., Verreckt I., Yombouno P., Bocquin A., Carbonnelle C., Carmoi T., Frange P., Mely S., Nguyen V.K., Pannetier D., Taburet A.M., Treluyer J.M., Kolie J., Moh R., Gonzalez M.C., Kuisma E., Liedigk B., Ngabo D., Rudolf M., Thom R., Kerber R., Gabriel M., Di Caro A., Wölfel R., Badir J., Bentahir M., Deccache Y., Dumont C., Durant J.F., El Bakkouri K., Gasasira Uwamahoro M., Smits B., Toufik N., Van Cauwenberghe S., Ezzedine K., Dortenzio E., Pizarro L., Etienne A., Guedj J., Fizet A., Barte de Sainte Fare E., Murgue B., Tran-Minh T., Rapp C., Piguet P., Poncin M., Draguez B., Allaford Duverger T., Barbe S., Baret G., Defourny I., Carroll M., Raoul H., Augier A., Eholie S.P., Yazdanpanah Y., Levy-Marchal C., Antierrens A., Van Herp M., Günther S., de Lamballerie X., Keïta S., Mentre F., Anglaret X., Malvy D. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13(3) doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chinello P., et al. Public Library of Science San Francisco; CA USA: 2017. QTc Interval Prolongation During Favipiravir Therapy in an Ebolavirus-Infected Patient. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumagai Y., Murakawa Y., Hasunuma T., Aso M., Yuji W., Sakurai T., Noto M., Oe T., Kaneko A. Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults. Int. J. Clin. Pharmacol. Ther. 2015;53(10):866–874. doi: 10.5414/CP202388. [DOI] [PubMed] [Google Scholar]
- 118.Nunn A.V.W., Guy G.W., Brysch W., Botchway S.W., Frasch W., Calabrese E.J., Bell J.D. SARS-CoV-2 and mitochondrial health: implications of lifestyle and ageing. Immun. Ageing. 2020;17(1):1–21. doi: 10.1186/s12979-020-00204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell Physiol. 2020;319:C258–C267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aoe T. Pathological aspects of COVID-19 as a conformational disease and the use of pharmacological chaperones as a potential therapeutic strategy. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang S., et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021:1–15. doi: 10.1038/s41422-020-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santerre M., Arjona S.P., Allen C.N., Shcherbik N., Sawaya B.E. Why do SARS-CoV-2 NSPs rush to the ER? J. Neurol. 2020:1–10. doi: 10.1007/s00415-020-10197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martucciello S., Masullo M., Cerulli A., Piacente S. Natural products targeting ER stress, and the functional link to mitochondria. Int. J. Mol. Sci. 2020;21(6) doi: 10.3390/ijms21061905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J., Zhang D., Brundel B.J.J.M., Wiersma M. Imbalance of ER and mitochondria interactions: prelude to cardiac ageing and disease? Cells. 2019;8(12) doi: 10.3390/cells8121617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Banerjee A., Czinn S.J., Reiter R.J., Blanchard T.G. Crosstalk between endoplasmic reticulum stress and anti-viral activities: a novel therapeutic target for COVID-19. Life Sci. 2020;255 doi: 10.1016/j.lfs.2020.117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saleh J., Peyssonnaux C., Singh K.K., Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Acosta M.A.T., Singer B.D. Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur. Respir. J. 2020;56(3) doi: 10.1183/13993003.02049-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shenoy S. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020:1–9. doi: 10.1007/s00011-020-01389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pandey P., Prasad K., Prakash A., Kumar V. Insights into the biased activity of dextromethorphan and haloperidol towards SARS-CoV-2 NSP6: in silico binding mechanistic analysis. J. Mol. Med. 2020;98(12):1659–1673. doi: 10.1007/s00109-020-01980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vela J.M. Repurposing sigma-1 receptor ligands for Covid-19 therapy? Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Silvestri M., Rossi G.A. Melatonin: its possible role in the management of viral infections-a brief review. Ital. J. Pediatr. 2013;39(1):1–5. doi: 10.1186/1824-7288-39-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang S.H., Cao X.J., Liu W., Shi X.Y., Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 2010;48(2):109–116. doi: 10.1111/j.1600-079X.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 133.San-Miguel B., Crespo I., Vallejo D., Álvarez M., Prieto J., González-Gallego J., Tuñón M.J. Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J. Pineal Res. 2014;56(3):313–321. doi: 10.1111/jpi.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Di Renzo L., Gualtieri P., Pivari F., Soldati L., Attinà A., Leggeri C., Cinelli G., Tarsitano M.G., Caparello G., Carrano E., Merra G., Pujia A.M., Danieli R., De Lorenzo A. COVID-19: is there a role for immunonutrition in obese patient? J. Transl. Med. 2020;18(1):1–22. doi: 10.1186/s12967-020-02594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]