Abstract
Background
The COVID-19 crisis has created unanticipated changes in health care delivery for people living with multiple sclerosis (MS). The pandemic's rapid evolution has resulted in a knowledge gap in how COVID-19 has affected MS clinical practice. Our objective was to understand how the COVID-19 pandemic has affected clinical practice patterns in a nationwide cohort of MS clinicians across the United States.
Methods
In collaboration with the National Multiple Sclerosis Society (NMSS), we developed a 28-item SurveyMonkeyTM electronic questionnaire exploring MS specialists’ perceptions of how COVID-19 has altered how they prescribe MS disease-modifying therapies (DMTs), provide telehealth and other services, and view issues affecting their own well-being including re-deployment to the front lines of COVID-19 care and availability of personal protective equipment (PPE). NMSS staff sent a recruitment email containing the electronic survey link to 188 clinicians who serve on regional NMSS Healthcare Provider Councils across the US, 86 (45.7%) of whom were MS specialist physicians.
Results
Eighty-six of 188 potential respondents (45.7%) from 32 US states completed the survey including 45 physicians (41 neurologists, 3 physiatrists and 1 family physician), 18 rehabilitation therapists, 7 psychologists, 6 nurse practitioners, 4 social workers, 2 physician assistants, 2 nurses and 2 health professionals from other disciplines. More than 80% of all respondents working on-site in a health care setting believed they had adequate PPE. More than 41% were able to distance safely from others at work. Nearly 10% of respondents reported they had been re-deployed to the front lines of COVID-19 patient care, and an additional 16.9% anticipated being re-deployed. Among the MS specialist physician subgroup, nearly one-third reported using telemedicine to provide over 75% of their clinical care. Only 16.7% believed COVID-19 had not changed how they prescribe DMTs. Therapies prescribed more often during the pandemic included β-IFNs (28.6% of prescribers), natalizumab (23.8%), glatiramer acetate (21.4%) and teriflunomide (19%). DMTs prescribed less often included alemtuzumab (64.3% of prescribers), cladribine (54.8%), ocrelizumab and rituximab (50%), and fingolimod and siponimod (40.5%). For at least some of their patients during the pandemic, some MS specialists reported suspending certain DMTs including alemtuzumab (21.4% of prescribers), ocrelizumab and rituximab (16.7%) and cladribine (11.9%). Others reported extending DMT dosing intervals for natalizumab (38.1%), fingolimod and siponimod (11.9%).
Conclusions
In this nationwide survey, MS specialist physicians and other clinicians serving on regional NMSS Healthcare Provider Councils across the US reported profound changes in how they are delivering MS care during the COVID-19 pandemic.
Keywords: multiple sclerosis, COVID-19, decision-making, health professionals, survey, telemedicine
1. Introduction
In late 2019, a novel coronavirus, SARS-CoV-2, originated in Wuhan, China and began to spread around the world. On March 11, 2020, the World Health Organization confirmed that COVID-19, the disease caused by SARS-CoV-2, had become a global pandemic. Because the novel coronavirus has a mortality rate that is approximately 2% in the general population, and even higher in elders and individuals with chronic medical conditions (Espinosa et al., 2020), clinicians who care for people with multiple sclerosis (MS) may be especially concerned about their patients’ welfare.
An emerging published literature is addressing specific questions pertinent to MS clinicians. One concern is lack of knowledge about how MS disease-modifying therapies (DMTs) may affect COVID-19 outcomes. Published research has begun to address potential beneficial and detrimental roles of various immune-modulating therapies for people with MS during the viral pandemic (e.g., Berger et al., 2020; Brownlee et al., 2020; Giovannoni et al., 2020; Laroni et al., 2020). In an era in which decreased social mobility has limited patients’ ability to visit their MS practitioners in person, MS professionals also need to know how best to use telemedicine to help their patients. Published studies specific to MS are limited, but a recent systematic review of digital technology in health care during the pandemic (Golinelli et al., 2020) summarized the current knowledge base and ongoing needs for additional research. In order to address these and other important questions, other investigators have conducted surveys of MS specialists aimed at understanding how the COVID-19 pandemic has affected clinical practice patterns. For instance, Mateen and colleagues (2020) conducted an electronic survey of North American neurologists who had seen at least 10 patients with MS in the six-month period (approximately November of 2019 to April of 2020) prior to completing the survey in mid-April to May of 2020. This study's findings documented altered prescribing habits for MS DMTs, practice disruptions and other shifts in clinical practice. Another web-based survey explored the perceptions of MS specialists in Argentina and other Latin American nations (Alonso et al., 2020), reporting varying percentages of respondents for whom COVID-19 had changed their approach to therapeutic decisions, such as when to perform MRI and laboratory tests and which DMTs they recommended during the pandemic.
This article describes a nationwide survey of MS health care providers who serve on regional NMSS Healthcare Provider Councils across the US, exploring perceptions of how COVID-19 has altered their clinical practice patterns and other issues affecting them and their patients living with MS. Questionnaire items addressed the pandemic's effects on how clinicians initiate and continue MS DMTs, provide telehealth and other services, and perceive issues affecting their own well-being including availability of PPE and re-deployment to the front lines of COVID-19 care. To our knowledge, the current study is unique in that it specifically addresses MS specialists’ perceptions of practice changes in the US during the COVID-19 pandemic.
2. Material and methods
We obtained approval for the study from the human subjects review boards at the University of California, Riverside (UCR) School of Medicine and Cleveland Clinic Lou Ruvo Center for Brain Health.
2.1. Study design and participants
This study was an anonymous electronic survey of MS specialist health care providers. Our target population for this survey was a group of 188 health care providers who participated on one of the NMSS's 22 Healthcare Provider Councils across the US. Councils are made up of volunteer health care providers from multiple disciplines who are charged with identifying MS health care issues in their local communities and developing tools, events or educational activities to help meet the identified needs. To be eligible for nomination to a Healthcare Provider Council, a clinician must be actively working in MS care. The vast majority of Council members work in outpatient MS practices, 40-50% of which are NMSS-designated Comprehensive MS Care Centers. Some Council members also work in hospital settings. A total of 86 of the 188 Council members (45.7%) were MS specialist physicians, and the remainder represented a variety of other health care disciplines including rehabilitation therapists (physical, occupational and speech therapists), psychologists, advanced practice clinicians (nurse practitioners and physician assistants), social workers, nurses and practitioners from other health care disciplines. Inclusion criteria for survey participants included being (1) 18 years of age or older, and (2) MS specialist health care providers who volunteered as NMSS Healthcare Provider Council members.
2.2. Survey procedure
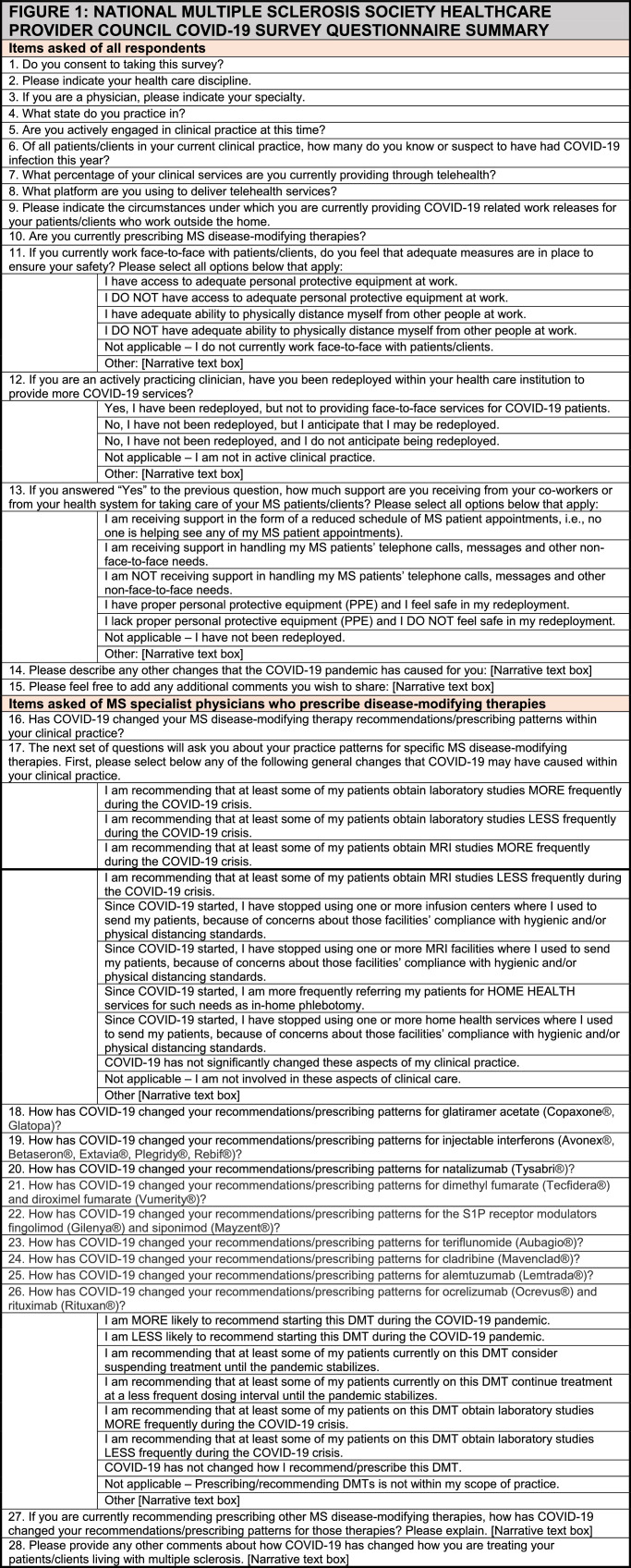
The investigators created a 28-item electronic survey using SurveyMonkeyTM. In June of 2020, NMSS staff and Council chairs sent a recruitment email containing the electronic survey link to clinicians who serve on regional NMSS Healthcare Provider Councils across the US. Fig. 1 summarizes the items included in the questionnaire. Council members and chairs who did not respond to the initial recruitment email received up to two reminder emails in July and August of 2020.
Fig. 1.
National multiple sclerosis society healthcare provider council Covid-19 survey questionnaire summary.
2.3. Statistical analysis
We obtained basic descriptive statistics for our data analysis using OpenEpi Version 3.01 (Dean et al., 2006). Because we used no scales in our questionnaire, we did not calculate reliability coefficients.
3. Results
3.1. Characteristics of survey participants
Table 1 describes the survey participants. Eighty-six of 188 potential respondents (45.7%) from 32 US states completed the survey between June and August of 2020 including 45 physicians (41 of whom were neurologists), 18 rehabilitation therapists, 7 clinical psychologists, 8 advanced practice clinicians, 4 social workers, and 4 health professionals from other disciplines (2 nurses, 1 clinical researcher and 1 pharmacist).
Table 1.
States where respondents practiced.
| n (% of row total) | ||||||
|---|---|---|---|---|---|---|
| Discipline | Total | Northeast | Southeast | Midwest | Southwest | West |
| Physicians* | 45 | 12 (26.7%) | 9 (20%) | 11 (24.4%) | 2 (4.4%) | 11 (24.4%) |
| Rehabilitation therapists⁎⁎ | 18 | 2 (11.1%) | 2 (11.1%) | 7 (38.9%) | 1 (5.6%) | 6 (33.3%) |
| Clinical psychologists | 7 | 2 (28.6%) | 2 (28.6%) | 1 (14.3%) | 0 (0%) | 2 (28.6%) |
| Advanced practice clinicians | 8 | 3 (37.5%) | 0 (0%) | 3 (37.5%) | 0 (0%) | 2 (25%) |
| Social workers | 4 | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 3 (75%) |
| Nurses | 2 | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (50%) |
| Other health professionals† | 2 | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) | 1 (50%) |
| Total | 86 | 20 (23.3%) | 14 (16.3%) | 23 (26.7%) | 3 (3.5%) | 26 (30.2%) |
42 neurologists, 3 physiatrists and 1 family physician
15 physical therapists, 2 occupational therapists and 1 speech therapist
1 clinical researcher and 1 pharmacist
3.2. Use of telehealth services
Of 82 respondents in active clinical practice at the time of the survey, 4 (4.9%) reported they were not using telehealth when providing patient care services. A total of 42 participants (53.8%) reported using telehealth less than 50% of the time, while 36 respondents (46.2%) were using telehealth less than 50-100% of the time. Within the subgroup of 45 physician respondents 16 (35.6%) used telemedicine 76-100% of the time. The most frequently reported teleconferencing platform was Zoom which was used by 35 participants (42.7%), followed by Doximity (22, or 26.8%), Facetime (10, 12.2%) and Doxy.me (10, 12.2%). A minority of respondents reported using various other commercially available telehealth platforms, internal proprietary platforms of their hospitals or health systems, and telephone only. Some respondents reported using more than one telehealth application.
3.3. Provision of work releases for patients with MS
A total of 55 of 86 survey respondents (64%) indicated that providing work releases was within their scope of practice. Thirty-one respondents (56.4%) provided work releases for patients with MS who were over age 60 and/or had significant co-morbidities such as diabetes or chronic cardiac or respiratory disease. Twenty-four respondents (43.6%) provided work releases to patients with significant MS-related disability, 9 (16.4%) to any patients with a diagnosis of multiple sclerosis or other demyelinating disease, and 7 (12.7%) on a case-by-case or other basis. For work releases based a patient's MS DMT, 26 (61.9%) of physician respondents would release from work patients on ocrelizumab or rituximab, followed by 23 (54.8%) for alemtuzumab, 15 (35.7%) for cladribine, 11 (26.2%) for fumarates regardless of lymphocyte counts, 11 (26.2%) for natalizumab, 9 (21.4%) for sphingosine-1P receptor modulators with lymphocyte counts the respondent considered unacceptable, 8 (19%) for teriflunomide, 4 (9.5%) for fumarates with lymphocyte counts they considered unacceptable, 4 (9.5%) for S1P modulators regardless of lymphocyte counts, 3 (7.1%) for β-interferons, and 3 (7.1%) for glatiramer acetate.
3.4. Safety and support in the workplace during the COVID-19 pandemic
Among the 86 study respondents, 9 (10.5%) indicated that they did not need PPE because they were not working on-site in a health care setting. Of the remaining 77 respondents (89.5% of all respondents), 62 (80.5% of those working on-site) indicated that they had access to adequate PPE, while 8 (10.4%) did not feel they had access to adequate PPE. Thirty-two (41.6%) of the participating on-site clinicians had adequate ability to physically distance themselves at work, while 11 (14.3%) did not. Fifty-seven (74.0%) had not been redeployed to the front lines of caring for COVID-19 patients and did not anticipate being redeployed. Another 13 (16.9%) had not been redeployed yet but anticipated redeployment in the future. Seven respondents (9.1%) reported that they had been redeployed, including five individuals (6.5%) to the front lines of COVID-19 care. Those clinicians who had been redeployed to work with COVID-19 patients reported inconsistent support (e.g., reduced schedules of MS patient appointments, assistance responding to patients’ messages) for handling the needs of their MS practices.
3.5. MS-specialist neurologists’ prescribing habits and monitoring for multiple sclerosis disease-modifying therapies
Physician respondents reported a mean number of 4.4 patients each who had had COVID-19 (SD = 14.1, range = 0-32, 95% CI 1.42-7.38). When we asked survey respondents whether they were currently prescribing MS DMTs, all those who did were MS specialist neurologists with the exception of one MS specialist physician who was a physiatrist. We conducted a separate subgroup analysis of the 42 participating MS specialist physicians. When asked whether COVID-19 had changed how they recommend and prescribe MS DMTs, 23 MS physicians (54.8%) responded “Yes”, 12 (28.6%) “Maybe”, and 7 (16.7%) “No”. Table 2 shows respondents’ perceptions of how the COVID-19 pandemic changed how they use various services for their patients living with MS, including laboratory draw stations, MRI facilities, infusion centers and home health services.
Table 2.
Use of services for patients with ms during the Covid-19 Pandemic, n = 42 MS specialist physicians.
| Questionnaire item | n (%) |
|---|---|
| I am recommending that at least some of my patients obtain laboratory studies MORE frequently during the COVID-19 crisis. | 11 (26.2%) |
| I am recommending that at least some of my patients obtain laboratory studies LESS frequently during the COVID-19 crisis. | 17 (40.5%) |
| I am recommending that at least some of my patients obtain MRI studies MORE frequently during the COVID-19 crisis. | 0 (0%) |
| I am recommending that at least some of my patients obtain MRI studies LESS frequently during the COVID-19 crisis. | 23 (54.8%) |
| Since COVID-19 started, I have stopped using one or more infusion centers where I used to send my patients, because of concerns about those facilities’ compliance with hygienic and/or physical distancing standards. | 3 (7.1%) |
| Since COVID-19 started, I have stopped using one or more MRI facilities where I used to send my patients, because of concerns about those facilities’ compliance with hygienic and/or physical distancing standards. | 1 (2.4%) |
| Since COVID-19 started, I have stopped using one or more laboratory draw stations where I used to send my patients, because of concerns about those facilities’ compliance with hygienic and/or physical distancing standards. | 2 (4.8%) |
| Since COVID-19 started, I am more frequently referring my patients for home health services for such needs as in-home phlebotomy. | 6 (14.3%) |
| Since COVID-19 started, I have stopped using one or more home health services where I used to send my patients, because of concerns about those facilities’ compliance with hygienic and/or physical distancing standards. | 2 (4.8%) |
| COVID-19 has not significantly changed these aspects of my clinical practice. | 12 (28.6%) |
MS specialist physicians’ prescribing patterns for individual MS DMTs are shown in Table 3 . The DMTs prescribed more often during the COVID-19 pandemic included β-IFNs (28.6% of prescribers), natalizumab (23.8%), glatiramer acetate (21.4%) and teriflunomide (19%). Those DMTs prescribed less often during the pandemic included alemtuzumab (64.3% of prescribers), cladribine (54.8%), ocrelizumab and rituximab (50%), and fingolimod and siponimod (40.5%). For at least some of their patients, some MS specialists reported suspending certain DMTs during the pandemic including alemtuzumab (21.4% of prescribers), ocrelizumab and rituximab (16.7%) and cladribine (11.9%). Others used extended DMT dosing intervals, particularly for natalizumab (38.1%), fingolimod and siponimod (11.9%).
Table 3.
Prescribing patterns for ms disease-modifying therapies during the Covid-19 Pandemic, n = 42 MS specialists.
| How has COVID-19 changed your… prescribing patterns for… | I am MORE likely to recommend starting this DMT during the COVID-19 pandemic. | I am LESS likely to recommend starting this DMT during the COVID-19 pandemic. | I am recommend-ing that at least some of my patients currently on this DMT consider suspending treatment until the pandemic stabilizes. | I am recommend-ing that at least some of my patients currently on this DMT continue treatment at a less frequent dosing interval until the pandemic stabilizes. | I am recommend-ing that at least some of my patients on this DMT obtain laboratory studies MORE frequently during the COVID-19 crisis. | I am recommend-ing that at least some of my patients on this DMT obtain laboratory studies LESS frequently during the COVID-19 crisis. | COVID-19 has not changed how I recommendor prescribe this DMT. |
|---|---|---|---|---|---|---|---|
| Glatiramer acetate (Copaxone®, Glatopa) | 9 (21.4%) | 2 (4.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 30 (71.4%) |
| Injectable interferons (Avonex®, Betaseron®, Extavia®, Plegridy®, Rebif®) | 12 (28.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 27 (64.3%) |
| Natalizumab (Tysabri®) | 10 (23.8%) | 1 (2.4%) | 0 (0%) | 16 (38.1%) | 0 (0%) | 0 (0%) | 17 (40.5%) |
| Dimethyl fumarate (Tecfidera®) and diroximel fumarate (Vumerity®) | 2 (4.8%) | 5 (11.9%) | 0 (0%) | 0 (0%) | 5 (11.9%) | 1 (2.4%) | 27 (64.3%) |
| Fingolimod (Gilenya®) and siponimod (Mayzent®) | 0 (0%) | 17 (40.5%) | 0 (0%) | 5 (11.9%) | 3 (7.1%) | 1 (2.4%) | 17 (40.5%) |
| Teriflunomide (Aubagio®) | 8 (19.0%) | 0 (0%) | 0 (0%) | 1 (2.4%) | 0 (0%) | 1 (2.4%) | 30 (71.4%) |
| Cladribine (Mavenclad®) | 2 (4.8%) | 23 (54.8%) | 5 (11.9%) | 0 (0%) | 2 (4.8%) | 0 (0%) | 7 (16.7%) |
| Alemtuzumab (Lemtrada®) | 0 (0%) | 27 (64.3%) | 9 (21.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (14.3%) |
| Ocrelizumab (Ocrevus®) and rituximab (Rituxan®) | 0 (0%) | 21 (50%) | 7 (16.7%) | 0 (0%) | 5 (11.9%) | 0 (0%) | 11 (26.2%) |
4. Discussion
In this nationwide survey, MS specialist physicians and other clinicians serving on regional NMSS Healthcare Provider Councils across the US reported profound changes in their delivery of MS care during the COVID-19 pandemic. More than 95% of respondents in active clinical practice at the time of the survey reported that they were using telehealth platforms to provide patient care. The proportion of respondents in our study using telehealth was considerably higher than the 67% reported among Latin American MS specialists in another recent published survey (Alonso et al., 2020), a discrepancy that may reflect differential access to various electronic resources in different countries.
Many of our survey respondents also reported that the COVID-19 pandemic had changed other aspects of their clinical practice. Clinicians who reported that they provided work releases also indicated using a range of criteria to make individual work release decisions for patients and clients living with MS. The majority of clinician respondents further indicated that they had changed how they were recommending various services to their patients with MS. For instance, more than 50% of respondents recommended that their patients with MS obtain MRI studies less frequently than they did before the pandemic began, and more than 40% recommended less frequent laboratory studies, presumably to decrease risk of viral exposure during testing. Fewer than 30% of participating MS specialists believed that COVID-19 had not significantly changed these aspects of their clinical practice.
The pandemic appeared to have had an even greater effect on MS specialists’ DMT prescribing habits. More than four out of five MS specialist neurologists believed that COVID-19 may have changed how they prescribe or recommend MS DMTs. Our results are fairly similar to those of a published survey of neuroimmunologists in Latin American countries (Alonso et al., 2020) in which the majority of participating MS specialists considered alemtuzumab, ocrelizumab, cladribine and fingolimod “not safe to start” during the COVID-19 pandemic, while 62% were suspending alemtuzumab for all patients, 46.5% were holding ocrelizumab, and 40.8% were suspending cladribine. In contrast, another published North American survey (Mateen et al., 2020) found a lower rate of 65% of participating neurologists who had altered their prescribing habits in response to the pandemic. Their survey's inclusion criteria (e.g., requiring participants to have seen at least 10 patients with MS in the prior six months) differed from ours, however, in that their sample may have included general neurologists in addition to MS specialists. In our study, MS specialists recommended glatiramer acetate and β-interferons at the same or higher rates than they did before the pandemic. The vast majority of our respondents continued to recommend natalizumab during the pandemic, although more than one-third of respondents reported extending dosing intervals for at least some patients. More than 40% of our respondents were less likely to recommend S1P modulators, and more than 10% were recommending extended-interval dosing of these agents. For the agents we defined as immunosuppressive, more than half of respondents were less likely to start patients on cladribine, ocrelizumab or rituximab during the pandemic, and nearly two thirds were less likely to start alemtuzumab. The published literature suggests partial support for these approaches. For example, the Musc 19 Study Group retrospectively evaluated 844 Italian adults with MS who had had suspected or confirmed COVID-19 infection (Sormani et al., 2021) and found that treatment with a B cell-depleting therapy was an independent risk factor for severe COVID-19 infection, while treatment with other MS disease-modifying therapies (including alemtuzumab or cladribine) was not.
Not surprisingly, many of our survey respondents attested to significant concerns about their personal safety and support in the workplace during the COVID-19 pandemic. More than 10% of the MS professionals who were working on-site reported that they lacked adequate PPE, and fewer than one-half felt they had adequate ability to physically distance themselves from others in the workplace. This finding is particularly concerning in light of a recent published survey of ICU clinicians in the U.S. (Sharma et al., 2020) that found insufficient PPE strongly predicted health care providers feeling that the hospital could not keep them safe. We were surprised to learn that nearly 10% of respondents in active clinical practice had already been redeployed to work with COVID-19 patients, and another 16.9% anticipated being redeployed in the future. Those redeployed to the front lines of caring for COVID-19 patients reported that they lacked consistent support from their colleagues for handling their MS patients’ ongoing needs.
One limitation of the current study was that we did not ask our respondents for their age, gender or other demographic characteristics. Another limitation was that the study included a small sample size of participants limited to MS specialist clinicians in the US serving on NMSS Healthcare Provider Councils. The response rate was also low, particularly in the southwestern region of the US. We suspect that the added pressures of the viral pandemic on the professional and personal lives of potential participants affected their ability to complete the survey.
5. Conclusion
In a nationwide survey of regional Healthcare Provider Councils of the National Multiple Sclerosis Society, MS specialist clinicians across the United States reported that the COVID-19 pandemic has caused profound changes in their clinical practices. Many MS specialists changed how they prescribe and recommend MS DMTs, and some are less likely to use the highest-efficacy therapies. Some reported that they had been redeployed to the front lines of COVID-19 care, raising concern that the pandemic has challenged the capacity of the MS health care workforce. Further research is needed to explore these trends and develop consensus guidelines on best treatment practices for people living with MS during and after the global pandemic.
Acknowledgments
Funding/support
This study was not funded by grant or contract support.
Declaration of competing interests
The authors declared the following potential conflicts of interest with respect to the research, authorship or publication of this article: Dr. Morrison has received honoraria, consulting fees or research support from AbbVie, Acorda, Biogen, EMD Serono, Genentech, Landon Pediatric Foundation, Neurosearch, Inc. and Teva Neuroscience. Dr. Hersh has received honoraria, consulting fees or research support from Biogen, Bristol-Meyers Squibb, EMD Serono, Genentech, Genzyme, Novartis and PCORI.
Acknowledgments
The authors thank the National Multiple Sclerosis Society Healthcare Provider Council members and staff, including Kathleen Costello, MS, ANP-BC, MSCN and Kaye Gooch, MSW for their assistance with this study.
References
- Alonso R., Contentti E.C., Silva B.A., Lopez P.A., Garcea O., Hamuy F., Rivera V., Gracia F., Rojas J.I. Decision-making on management of MS and NMOSD patients during the COVID-19 pandemic: a Latin American survey. Mult Scler Relat Disord. 2020;44 doi: 10.1016/j.msard.2020.102310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Brandstadter R., Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2020;7:e761. doi: 10.1212/NXI.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee W., Bourdette D., Broadley S., Killestein J., Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurol. 2020;94:949–952. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- Espinosa O.A., Zanetti A.D.S., Antunes E.F., Longhi F.G., de Matos T.A., Battaglini P.F. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2020;62:e43. doi: 10.1590/S1678-9946202062043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C., Lechner-Scott J., Levy M., Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golinelli G., Boetto E., Carullo G., Nuzzolese A.G., Landini M.P., Fantini M.P. Adoption of digital technologies in health care during the COVID-19 pandemic: systematic review of early scientific literature. J Med Internet Res. 2020;22(11) doi: 10.2196/22280. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroni A., Schiavetti I., Sormani M.P., Uccelli A. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult Scler. 2020 doi: 10.1177/1352458520971817. Nov 18;1352458520971817. [DOI] [PubMed] [Google Scholar]
- Mateen F.J., Rezaei S., Alakel N., Gazdag B., Kumar A.R., Vogel A. Impact of COVID-19 on U.S. and Canadian neurologists’ therapeutic approach to multiple sclerosis: a survey of knowledge, attitudes, and practices. J Neurol. 2020;267(12):3467–3475. doi: 10.1007/s00415-020-10045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Creutzfeldt C.J., Lewis A., Patel P.V., Hartog C., Jannotta G.E., Blissitt P., Kross E.K., Kassebaum N., Greer D.M., Curtis J.R., Wahlster S. Healthcare professionals’ perceptions of critical care resource availability and factors associated with mental well-being during COVID-19: Results from a U.S. survey. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1311. Sep 2;ciaa1311Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., the Musc-19 Study Group Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021 doi: 10.1002/ana.26028. Jan 21Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, A.G., Sullivan, K.M., Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health 2006, Version. www.OpenEpi.com, updated 2013/04/06, accessed 2021/01/02.