Graphical abstract
Abbreviations: ACE2, angiotensin converting enzyme 2; DW, dry weight; ELISA, enzyme linked immunosorbent assay; HPLC, high-performance liquid chromatography; IC50, the half maximal inhibitory concentration; ISSR, inter-simple sequence repeat; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; PVPP, polyvinylpyrrolidone; TEAC, trolox equivalent anti-radical capacity; TFC, total flavonoid content; TPCC, total phenolic content
Keywords: Sambucus nigra L., Elderberry fruit, Elderberry flower, Phytochemistry, Antiviral
Abstract
Berries and flowers of Sambucus nigra L. tree are well known for their ability to mitigate symptoms of upper respiratory disorders related to reported antiviral properties. Industrial application and commercial cultivation of S. nigra is largely limited to a few widely grown cultivars. Restricted genetic diversity of cultivated S. nigra can be disadvantageous if new industrial applications are discovered. In this study wild S. nigra populations located on the north-east edge of the species natural range were explored by assessing genetic origin, berry and flower anti-oxidative potential, and berry rutin content. Best performing wild S. nigra extracts were selected for an assessment of previously unreported biological activity- inhibitory capacity against SARS-CoV2 S1 protein receptor binding domain (RBD) binding to recombinant human angiotensin -converting enzyme 2 (ACE2) receptor in vitro based on competitive enzyme linked immunosorbent assay (ELISA). Inter-simple sequence repeat (ISSR) marker-based genetic characterization suggested that explored wild S. nigra populations result from wild gene pool expanding northwards with admixture of historically introduced cultivated S. nigra. Average values of total phenolic content, anti-radical activity, and total flavonoids content of wild S. nigra populations did not exceed those of cv. ‘Haschberg’. Concentration-dependent inhibition of ACE2-SARS-CoV2 S-protein RBD binding was demonstrated in vitro for elderberry fruits and flowers extracts (IC50 of 1.66 mg DW ml−1 and 0.532 mg DW ml−1, respectively). Wild elderberry fruit extract exhibited higher inhibitory capacity than the extract from berries of cv ‘Haschberg’. This study validates the requirement for S. nigra wild germplasm bioprospecting and opens up directions for further research of new anti-SARS-CoV2 industrial applications of S. nigra.
1. Introduction
S. nigra is a medicinal tree native to western and southern parts of Europe and North Africa (Atkinson and Atkinson, 2002). The species has also been introduced into other parts of the world – including North America, South East Asia, and Australia (Charlebois et al., 2010). S. nigra forms several subspecies, although the taxonomic treatment of the species is challenging and has been reviewed several times (Bolli, 1994; Applequist, 2013). S. nigra fruit and flower extracts have mainly been studied for antiviral properties. Elderberries are shown to mitigate symptoms of respiratory disorders during influenza infection (Tiralongo et al., 2016; Hawkins et al., 2019). Industrial potential of elderberry fruits is mainly associated with high levels of anthocyanins – predominantly cyanide-3-O-glucoside, cyanidin sambubioside, cyanidin 3,5-diglucoside and flavonoids – mainly rutin, quercetin, quercetin-3-O-glucoside (Silva et al., 2017; Mota et al., 2020), whereas industrial application of elderberry flowers is related to the rich content of hydroxycinnamic acids (chlorogenic acid and neochlorogenic acid) and flavonoids – predominantly rutin, kaempferol-3-rutinoside, isorhamnetin-3-rutinoside) (Milena et al., 2019).
Cultivation of S. nigra and industrial applications are mainly limited to a few leading cultivars widely grown in western and central Europe (Salvador et al., 2016). 75 % of cultivated S. nigra acreage in Austria – one of the leading S.nigra producers in the world – is covered with cv ‘Haschberg’ (Kopper et al., 2020). Limited diversity of cultivated S. nigra imposes risks associated with increased vulnerability to phytopathogens and logistic challenges due to a narrow harvesting period (Kopper et al., 2020). Underexplored wild germplasm of S. nigra also implicates a risk in case of emergence of new previously unreported industrial applications because potentially superior genotypes are missed out.
Widely grown S. nigra cultivars ‘Haschberg’, ‘Korsor’, ‘Mammoth’, ‘Allesø’ originate from wild selections demonstrating the importance of wild S. nigra germplasm as a valuable genetic source for S. nigra breeding (Hummer et al., 2012). Despite the wide native range of the species, the majority of commercial cultivars originate from a comparatively narrow geographical regions of Austria, Germany, and Denmark. Limited wild germplasm resources are conserved and are explored in the main S. nigra germplasm collections (Bushakra et al., 2013; Nordic Baltic Genebanks Information System, 2020). Chemical composition – e.g., content of flavonols and anthocyanines can significantly vary in wild berry populations and can be influenced by inheritance, geographical origin, and environmental growth conditions among other factors (Nestby et al., 2019). Geographical location also significantly affects the chemical composition of elderberry fruits (Johnson et al., 2017; Senica et al., 2017), suggesting that S. nigra fruits and flowers from populations growing at extremes of the distribution range could hold valuable germplasm resource for S. nigra breeding. However, data on the chemical content of elderberry fruits mainly represent cultivated S. nigra (Młynarczyk et al., 2020; Csorba et al., 2020; Veberic et al., 2009) or wild populations from southern and western parts of distribution range –Turkey (Akbulut et al., 2009), Italy (Caruso et al., 2016), Slovakia (Sedláčková et al., 2018), Croatia (Jatoi and Jemrić, 2016). Due to geographical location wild S. nigra populations from Baltic region growing at extremes of distribution range could offer a valuable source of genetic resources for S. nigra breeding and for assessment of potential for new industrial applications. Nevertheless, chemical properties of wild elderberry fruits and flowers from north-east edge of the natural species range have not previously been explored.
Infectious diseases capable of causing epidemics have been named by World Health Organization among the top 13 global urgent health challenges for the next decade (World health organization, 2020). Plant-derived antiviral compounds are increasingly viewed as an important supplementary treatment (Kronbichler et al., 2020) and alternative to conventional antiviral drugs. Elderberry fruit juice and flower infusions are traditionally used for the treatment of symptoms of common colds and influenza (European medicines agency (EMA, 2014, 2018) and several industrially produced elderberry based pharmaceutical products (Rubini®, Sambucol®, Sinupret®) have been clinically tested (Ulbricht et al., 2014; Porter and Bode, 2017). Antiviral activity of S. nigra extracts has previously been associated with specific compounds belonging to flavonoids. Roschek et al. (2009) proposed that flavonoid compounds of S. nigra berry extract - 5,7,3′,4′-tetra-O-methylquercetin and 5,7-dihydroxy-4-oxo-2-(3,4,5-trihydroxyphenyl)chroman-3-yl-3,4,5-trihydroxycyclohexanecarboxylate might be acting as inhibitors of viral binding to the host cell thus prevent viral entry. Pretreatment of influenza A virus with elderberry fruit juice significantly inhibited viral entry into mammalian cells in vitro (Torabian et al., 2019). Stem extract of S. japonica significantly inhibited the attachment of coronavirus CoNL63 in mammalian cells, suggesting an action primarily at an early stage of infection (Weng et al., 2019). S. nigra berry extract significantly reduced virus titers in Vero cells infected with Infectious bronchitis virus – pathogenic poultry coronavirus (Chen et al., 2014). Recently, S. nigra products have been suggested as a potential Covid-19 supplementary treatment considering their effectiveness in the treatment of cold and influenza symptoms based on randomized, double-blind, placebo-controlled studies and meta-analyses (Harnett et al., 2020; Kronbichler et al., 2020; Silveira et al., 2020). S. nigra can be hypothesized comprising inhibitory properties against SARS-CoV2 viral entry. Nevertheless, the effect of S. nigra berry or flower extracts on SARS-CoV2 surface protein S1 binding to ACE2 has not been evaluated before.
This study explored three consequential hypotheses. First, it was expected that due to the historical events of germplasm introduction and specific geographic location, wild S. nigra populations from north-east edge of the species range represent a distinct genetic origin. Second, it was hypothesized that due to the unique origin these S. nigra populations hold valuable phytochemical properties. Third, it was expected that the superior phytochemical properties would contribute to superior biological activity of the explored wild S. nigra populations.
This study explores wild S. nigra populations from the region previously neglected in S. nigra studies and described the inhibitory activity of S. nigra fruit and flower extracts against SARS-CoV2 RBD and ACE2 receptor binding – a novel biological activity for S. nigra.
2. Materials and methods
2.1. Collection of plant material
A total of 18 wild populations of S. nigra were assessed in this study. Sampling region covered the natural distribution areas of S. nigra in Latvia (Laiviņš et al., 2009) and well-established populations comprising the largest number of individuals were selected for sampling. Geographic location of the sampling sites is depicted in Fig. 1 , and coordinates of the sites are presented in Appendix Table A1. In each population, at least five individual plants were randomly selected to cover area of the population at a particular site. Flowers and berries from wild populations were collected in mid-June and the beginning of September 2019, respectively. Ten inflorescences and ten fruit clusters were collected in a plastic food box from each individual plant. The plant material was placed on ice for transportation to a laboratory where it was immediately frozen and stored at −80 °C. Frozen plant material was lyophilized for 48 h at −50 °C and 0.04 mbar following final drying for 0.5 h at −65 °C and 0.0054 mbar using an Alpha 1−4LD plus laboratory freeze-dryer (Martin Christ Gefriertrocknungsanlagen GmbH) prior to extraction.
Fig. 1.
S. nigra sampling region. (a) Geographical location of S. nigra sampling sites in Latvia (white dots), sites located in Lithuania included for genetic analysis only (black dots) and the location of S. nigra cv ‘Haschberg’ sampling orchard (x). (b) location of sampling region in Europe. Maps were generated using ArcGIS Online (Environmental Systems Research Institute (ESRI, 2012) and MapChart (MapChart, 2020).
Cultivar ‘Haschberg’ which is the commercially most widely grown S. nigra cultivar in Europe (Schmitzer et al., 2012), was selected as the reference material. Flowers and fruits from cv ‘Haschberg’ were collected from a 10-year-old organic S. nigra orchard located in south-west of Latvia.
2.2. DNA isolation and ISSR based analysis of genetic relatedness
For DNA isolation young, immature leaves were collected in late May 2019, immediately placed on dry ice in sterile microcentrifuge tubes, and stored at −80 °C upon arrival to the laboratory. DNA isolation was carried out with the modified CTAB-alkaline PVPP extraction method (Kasajima et al., 2013). Frozen leaves were disintegrated in liquid nitrogen using a mortar and pestle. 600 μL of CTAB buffer (Tris-HCl pH 9.5 50 mM, EDTA 10 mM, NaCl 4 M, CTAB 1 % w/v, PVPP 0.5 % w/v and β-mercaptoethanol 19 % v/v) were added to approximately 300−500 mg of frozen disintegrated plant material and the samples were heated at 60 °C for an hour using dry block thermostat CH-100 (Biosan). 250 μL of chloroform:isoamyl alcohol (v/v 24:1) were added and the samples were thoroughly mixed by vortex mixing, followed by centrifugation at room temperature at 16000 rcf for 10 min. Chloroform:isoamyl alcohol step was repeated and the collected aqueous phase was precipitated with 300 μL ice-cold isopropanol followed by ethanol wash. Air-dried DNA was resuspended in sterile distilled water and stored at −20 °C. Integrity of isolated DNA was assessed by agarose gel electrophoresis. DNA concentrations were determined using microvolume spectrophotometer NanoDrop 2000c (Thermo Scientific). Concentration of all samples was normalized to 200 ng μl−1 with distilled water prior further use.
Five ISSR primers from the University of British Columbia (UBC) primer set Nr.9 amplified polymorphic bands from S. nigra DNA were used (Table 1 ). Each PCR reaction of 12.5 μL total volume contained 200 ng DNA, 0.8 μM primers, 50 μM each dNTP, 1x Dream Taq buffer supplemented with MgCl2 at 2 mM final concentration, 1.6 units of DreamTaq DNA polymerase (Thermo Scientific), and 2.5 μL trehalose-BSA based additive for relieve of PCR inhibition (Samarakoon et al., 2013). PCR amplification was conducted using GeneAmp PCR System 9700 thermocycler (Applied Biosystems) with the following PCR program: initial denaturing 5 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 52 °C, 90 s at 72 °C, and final extension for 10 min at 72 °C. PCR products were separated on a 2 % low melting point agarose gel (60 V for 90 min in 1 x TBE buffer) containing 0.1 mg ml−1 ethidium bromide. Banding patterns were visualized under UV light and scored for the presence/absence of the band using the molecular weight calibration option based on DNA molecular weight ladders using VisionWorks LS v5.5.2 software.
Table 1.
Five ISSR primers from UBC primer set Nr.9 (University of British Columbia) tested for amplification of S.nigra DNA and the number of amplified bands.
| Oligonucleotide | Oligonucleotide sequence | Total number of amplified bands | Number of polymorphic bands (estimated band size) bpa |
|---|---|---|---|
| 17899A | (CA)6AG | 8 | 5 (1790; 1224; 865; 715; 537) |
| 17898B | (AC)6GT | 5 | 2 (1256; 820; 550) |
| UBC840 | (GA)8YT | 7 | 2 (848; 534) |
| UBC844A | (CT)8AC | 5 | 4 (1224; 880; 750; 648) |
| HB11 | (GT)6CC | 7 | 3 (786; 611, 476) |
band size was estimated by VisionWorks LS v5.5.2 software with the option of molecular weight calibration based on DNA molecular weight ladders.
2.3. Preparation of S. nigra berry and flowers extracts
150 mg of lyophilized elderberry fruit powder was extracted with 1.5 mL 80 % ethanol at 60 °C for 2 h, followed by incubation at RT for 24 h under constant rotation. The obtained extract was cleared by centrifugation at 10 °C 2200 g for 15 min using microcentrifuge 5417 (Eppendorf). The extraction procedure was repeated by adding 1 mL 80 % ethanol to the obtained pellets. Supernatants from the first and the second extraction were pooled to derive the final analyzed extract of concentration 60 mg ml−1 DW of plant material. Flowers extracts were made following the same extraction procedure as applied for elderberry fruits except for the final concentration of flower extracts was 10 mg ml−1 DW of plant material.
2.4. Determination of total phenolic content (TPC), total flavonoid content (TFC), and anti-radical activity
For TPC, TFC, and TEAC assays, berry and flower extracts of each individual plant were treated as a separate sample and population means were based on data from at least three plants from each sampling site.
TPC was estimated by spectrophotometric microplate assay using Folin-Ciocalteu’s phenol reagent (Sigma-Aldrich, Cat. Nr. F9252) essentially as described by (Herald et al., 2012). Results were expressed as mg gallic acid (Sigma-Aldrich, Cat. Nr. G7384) per g DW of plant material.
Anti-radical activity was assessed using Trolox equivalent anti-radical capacity (TEAC) spectrophotometric microplate assay using DPPH reagent (Sigma-Aldrich, Cat. Nr. D9132) essentially as described by (Herald et al., 2012). Results were expressed as μmol Trolox per g DW of plant material.
TFC was estimated using the modified Davis, method modified for more precise and specific detection of major classes of flavonoids and adopted for the microplate scale as described by (Huang et al., 2018). TFC was assessed applying Davis method (Huang et al., 2018). 80 μL of sterile distilled water was added to 20 μL of extract or rutin standard (freshly prepared from rutin trihydrate (Sigma-Aldrich, Cat.Nr. 78095). 100 μL of 90 % diethylene glycol was added followed by the addition of 30 μL of 0.4 M sodium hydroxide. Samples were thoroughly mixed by pipetting and incubated for 10 min at 40 °C. The microplate was cooled to room temperature for a few minutes and absorbance measurements were performed. Results were expressed as mg rutin per g DW of plant material.
The absorbance measurements were conducted on a microplate reader Infinite200Pro (Tecan Life Sciences) at the following wavelengths: 620 nm for TPC assay, 515 nm for TEAC assay and 360 nm for TFC assay.
2.5. Estimation of rutin content using HPLC
For HPLC analyses, elderberry extracts were filtered through syringe filters of pore size 0.45 μm. Elderberry fruit extracts of each individual plant were treated as a separate sample and population means were based on data from at least five plants from each sampling site.
Chromatographic analyses were performed on the Agilent 1290 Infinity II series HPLC system (Agilent Technologies, Deutschland GmbH, Waldbronn, Germany). LC separations were achieved by using the Agilent Eclipse XDB-18 3.5 μm, 4.6 × 150 mm (Zorbax) column (Agilent Technologies Inc., Wood Dale, IL, USA) (45 °C) with a mobile phase A, composed of formic acid and water (5:95 v/v) and mobile phase B, composed of methanol (Fisher Scientific UK, Loughborough, United kingdom, HPLC grade) at a flow rate of 1.0 mL min−1 under gradient conditions. The injection volume was 5 μL. Chromatograms were obtained on the Agilent WVD detector (Agilent Technologies, Germany) at a wavelength of 350 nm. The experimental data were handled using ChemStation 32 software (Agilent Technologies, Deutschland GmbH, Waldbronn, Germany). For peak identification, the retention time (tR) for the standard solution of rutin and all analyzed samples were compared.
Amounts of rutin were quantified based on rutin trihydrate (Sigma-Aldrich, Cat.Nr. 78,095) standard curve (r2 = 0.9998) and expressed as mg per g of lyophilized elderberry fruits.
2.6. ELISA for assessment of S. nigra extract inhibitory effect on binding of ACE2 and SARS-CoV2 RBD in vitro
Elderberry fruit extract derived from individual wild S. nigra plant from site ZIS comprised the highest rutin content and TPC among the assessed berry extracts as well as high TFC and TEAC therefore it was chosen for the test of inhibitory effect of S. nigra extracts on SARS-CoV2 RBD and hACE2 binding in vitro. Assessment of SARS-CoV2 RBD and hACE2 binding inhibition was performed using COVID-19 Spike-ACE2 binding assay kit (CoV-SACE2-1, RayBiotech Inc, https://www.raybiotech.com/covid-19-spike-ace2-binding-assay-kit/) following the protocol provided by the manufacturer. Elderberry fruit and flower extracts prepared in 80 % ethanol were incubated at 40 °C for 24 h for complete evaporation of ethanol and sterile PBS was added in a volume equal to the initial ethanol extract. Each sample was tested at five concentrations (ranging from 0.8 mg ml−1 extract dry weight to 13.2 mg ml−1 extract dry weight for berry extracts and from 0.2 mg ml−1 extract dry weight to 3.6 mg ml−1 extract dry weight for flower extracts) and inhibitory capacity of each concentration was assessed in triplicate. Analyzed extracts were mixed with recombinant hACE2 protein (PBS instead of extract was added to control samples), added to ELISA plate coated with recombinant SARS-Cov2 S-protein RBD and incubated overnight at 4 °C with shaking. Unbound ACE2 was removed by washing, and binding was assessed based on anti-ACE2 antibody- HRP-conjugated anti-goat IgG reaction with 3,3’,5,5’-tetramethylbenzidine (TMB). Absorbance at 450 nm was measured with a microplate reader Infinite 200 PRO (Tecan Life Sciences).
2.7. Data analysis and applied software
Map displaying the sampling sites (Fig. 1) was generated using ArcGIS Online, ESRI and light grey canvas base map (Source: Esri, DeLorme, HERE, MapmyIndia) (Environmental Systems Research Institute (ESRI), 2012). Map displaying the location of the sampling regions in Europe was derived from MapChart (MapChart, 2020).
ISSR marker-based analysis of molecular variance (AMOVA) was performed using GenAlEx 6.51b2 software (Peakall and Smouse, 2012).
ISSR marker-based relationships between analyzed wild S. nigra populations were assessed by Nei’s standard genetic distance (Nei, 1972) using Unweighted Pair Group method with arithmetic mean (UPGMA) clustering and visualization using NTSYSpc 2.20 j software, Applied Biostatistics Inc. To assess the hypothesis of wild S. nigra population establishment as a result of species native range expansion northwards, four sites located southwards from the analyzed regions (in the territory of Lithuania) (Table S1) were added for genetic distance analysis.
Pairwise linear geographic distance matrix displaying the distances between sampling sites was obtained using GenAlEx 6.51b2 software (Peakall and Smouse, 2012).
Testing for isolation-by-distance based on linear geographic distance matrix and Nei’s genetic distance matrix of sampled sites was performed with two-way Mantel test for matrix correspondence using NTSYSpc 2.20 j software, Applied Biostatistics Inc.
PCA of ISSR data was based on variance-covariance matrix. According to the geographical location (meridian 23 °E was considered as a border value), each site was assigned to either of the two groups - west or east – roughly corresponding to the historical regions of Kurzeme and Zemgale. PCA was performed and visualized with NTSYSpc 2.20 j software, Applied Biostatistics Inc.
Data of TPC, TFC, TEAC, and rutin content were analyzed with one-way ANOVA followed by Dunnett’s multiple comparison procedure comparing the values of wild S. nigra populations with the value of the cv ‘Haschberg’. Each plant was represented by a single extract and mean value for each population was averaged from five plants. Data statistical analysis and graphic display was performed using GraphPad Prism Software version 8.4.3 for Windows, San Diego, California USA.
ELISA results were analyzed with two-way ANOVA followed by Dunnett’s multiple comparison test for assessment of differences between different applied extract concentrations within wild S. nigra data set and within cv ‘Haschberg’ dataset. ANOVA was followed by Sidak’s multiple comparison test to assess differences between both datasets (wild and cultivated) within each concentration. Statistical analysis and IC50 values were estimated using GraphPad Prism Software version 8.4.3 for Windows, San Diego, California USA.
3. Results and discussion
3.1. ISSR based genetic relationships between analyzed wild S. nigra populations
A total of 18 wild populations of S. nigra were assessed in this study (Fig. 1). Genetic relationships among S. nigra populations were assessed using ISSR (inter-simple sequence repeat) markers. Application of different types of molecular markers for genetic analysis of S. nigra has established ISSR markers as highly polymorphic and informative in comparison to SSR (simple sequence repeat) and ITS (internal transcribed spacer) based markers (Lima-Brito et al., 2013). In our study, the proportion of amplified polymorphic bands ranged from 28 % to 80 % for different primers (Table 1), confirming previously published results of high variability of markers amplified by ISSR primers (Lima-Brito et al., 2013). ISSR based analysis revealed significant molecular variation (AMOVA F(17;70) = 0.11; p = 0.003) with the majority of variability being explained by within-site genetic variability (89 %) as opposed to between-site variability (11 %). These data conform well with the generally observed low proportion of among-population genetic variability in outcrossing woody perennials dispersed through ingestion (Hamrick and Godt, 1996). Although data on genetic variability of wild S. nigra populations is scarce, studies in related species – S. palmensis Link and S. canadensis L. observed a similar trend of high within-site genetic variation compared with genetic variability between different sampling sites and sampling regions (Rodríguez-Rodríguez et al., 2018; Johnson et al., 2008; Sosa et al., 2010).
Clustering of S. nigra sampling sites based on genetic distances derived from ISSR marker analysis could be explained by the origin of the analyzed populations (Fig. 2 a). The origin of the wild S. nigra population in Latvia is ambiguous – S. nigra has been introduced to Baltic countries in the 17th century and the first events of naturalization in Latvia were recorded in the 19th century (Laiviņš, 2002). According to recent observations, the native range of S. nigra is continuously expanding and establishing naturalized populations in the Baltic region (Pratašienė et al., 2019; Laiviņš, 2002). Therefore, at least three hypotheses regarding the ancestry of S. nigra in Latvia are plausible. Wild populations of S. nigra in Latvia could have originated from old European landraces introduced as ornamental plants starting from the 17th century and escaped in the wild, or as a result of species natural range expansion from southern wild S. nigra populations, or encompass an admixture of genetic material from both gene pools. Sampling sites included populations located in close proximity to known historical sites of S. nigra introduction (BLN, PUR, EDO, BAU, RUD), as well as sites with no obvious connection to historical orchards or located remotely from human settlements (BAR, KAL, IEC, DUR). Four Lithuanian sites (LIT, PAL, JKR, PER) were added to genetic analysis to elucidate possible S. nigra native range expansion from wild southern populations. UPGMA clustering based on Nei’s genetic distances partially complies with the idea of mixed origin of wild S. nigra populations in Latvia since sites of predictably cultivated origin tended to cluster together (EDO with BAU, PUR with RUD) or stand out as genetically distant (as BLN). Clustering of Lithuanian sites with the closest sites in Latvia (LIT with sites from central Latvia and PER and JKR with sites from the western coast of Latvia) suggested that S. nigra in Latvia at least partially originated from wild S. nigra populations located southwards as a result of the species native range expansion. However, the low overall genetic variability among sites favors the hypothesis of admixture of S. nigra populations in Latvia. Correlation between genetic and geographic distance matrix was non-significant (Mantel test rm = 0.069; p = 0.783), even though ISSR data-based PCA revealed a grouping of sites into two larger clusters relatively corresponding to west-east division of the study area (Fig. 2b). In PCA, sites of putatively escaped origin (BLN, BAU, EDO) did not group with corresponding east-west groups complying with the idea of distinct origin of S. nigra located in these sites. All analyzed wild S. nigra plants differed from cv. ‘Haschberg’ in ISSR maker banding pattern in at least four out of five applied ISSR primers (data not shown) suggesting that escape from recent cultivation sites is highly unlikely.
Fig. 2.
ISSR marker-based assessment of genetic relationships among wild S. nigra sampling sites. (a) genetic distance tree based on Nei’s standard genetic distances clustered using Unweighted Pair Group method with arithmetic mean (UPGMA), (b) ISSR marker-based principal-components analysis (PCA) of sampling sites. Sites were assigned to three major groups representing geographic location – sites located in Latvia are assigned to east and west regions (meridian 23 °E was considered as a border value) and four sampling sites (JKR, PAL, PER, LIT) located in Lithuania were added to genetic analysis.
Low among-populations genetic variability, clustering of sites in east-west groups, clustering with Lithuanian sites, and distinct clustering and grouping of sites of putatively historically introduced origin suggests that wild S. nigra populations in Latvia mainly originated from wild S. nigra plants as a result of natural expansion of species range with admixture of genetic material from historically introduced cultivated S. nigra plants.
3.2. Phytochemical characteristics of the analyzed extracts - TPC, TFC, TEAC, and rutin content
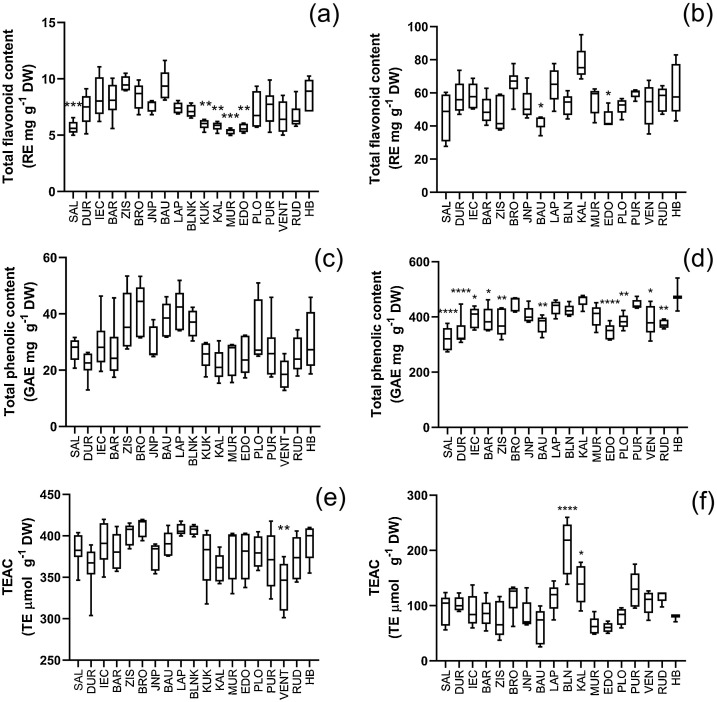
Antiviral properties of elderberries have previously been associated with a specific flavonoid belonging to a class of flavonols - 5,7,3’,4’-tetra-O-methylquercetin (Roschek et al., 2009). Flavonoids have also been proposed as target molecules for SARS-CoV2 therapeutics (Wyganowska-Swiatkowska et al., 2020), therefore, the total flavonoid content was determined in the analyzed S. nigra berry and flower extracts. Sampling sites had a significant effect on TFC of elderberry fruits (Table 2 ).
Table 2.
Summary of one-way ANOVA results indicating significant differences among sampled wild S. nigra populations regarding analyzed phytochemical indicators.
| Plant tissue type | Phytochemical indicator assessed | df | F | p |
|---|---|---|---|---|
| Berries | TPC | 18; 100 | 4.336 | <0.0001 |
| TFC | 18; 100 | 6.841 | <0.0001 | |
| TEAC | 18; 100 | 3.076 | 0.0002 | |
| Rutin content | 18; 100 | 4.859 | <0.0001 | |
| Flowers | TPC | 17; 72 | 6.602 | <0.0001 |
| TFC | 17; 74 | 4.366 | <0.0001 | |
| TEAC | 17; 71 | 8.376 | <0.0001 |
TPC – total phenolic content, TFC – total flavonoid content, TEAC - Trolox equivalent anti-radical capacity, df - degrees of freedom for “between sites” and “within sites”, p indicates the level of significance for between sites difference.
The highest average TFC of sampled wild S. nigra was 9.57 ± 0.65 mg RE g−1 DW of plant material for berry extracts and 77.59 ± 10.23 mg RE g−1 DW of plant material for flower extracts (Fig. 3 ). Significantly higher TFC values of flowers compared with berries were also reported by Viapiana and Wesolowski (2017) although reported absolute values (4.49 and 13.19 mg g−1 DW for berries and flower respectively) were slightly lower than those detected in this study, which can be explained by application of different extraction method (infusion vs ethanol extract) and TFC assay (aluminum chloride method vs Davis method).
Fig. 3.
Phenolic content, flavonoid content and anti-radical activity of extracts derived from wild elderberries and elderflowers sampled from 18 sampling sites located in Latvia (denoted with 3 letter symbols SAL to RUD). Corresponding values of cultivated S. nigra cv. ‘Haschberg’ are denoted as HB. Whisker-box plot represents the minimum and maximum value of data set representing n = 5 individual plants from particular sampling site. (a) Total flavonoid content of elderberry extracts derived from wild plants and from cv. ‘Haschberg’. Values are expressed as rutin equivalents per gram of dry weight of plant material (b) TFC of elderflower extracts derived from wild plants and from cv ‘Haschberg’, (c) Total phenolic content (TPC) of elderberry extracts derived from wild plants and from cv ‘Haschberg’. Values are expressed as gallic acid equivalents (GAE) per gram of dry weight of plant material (d) TPC of elderflower extracts derived from wild plants and from cv ‘Haschberg’, (e) Trolox equivalent anti-radical capacity (TEAC) of elderberry extracts derived from wild plants and from cv ‘Haschberg’, (f) TPC of elderflower extracts derived from wild plants and from cv ‘Haschberg’. Statistically significant differences between wild plants and cv ‘Haschberg’ based on Dunnett’s multiple range test are denoted with stars - ****p < 0.0001, **p < 0.01, *p < 0.05.
Sampling site had a significant effect on the TPC of elderberry fruits and flowers (Table 2). The highest average TPC of sampled wild S. nigra was 41.31 ± 9.44 mg gallic acid equivalent (GAE) g−1 DW for berry extracts and 451.72 ± 25.31 mg GAE g−1 DW for flower extracts (Fig. 3). Assessed values fall within the range of elderberry fruits and flowers TPC reported by other authors. Młynarczyk et al. (2020) reported TPC of 56 mg g−1 DW for wild elderbery fruits from Poland, wild S. nigra berries collected in Turkey comprised TPC 4 mg GAE g−1 FW (Akbulut et al., 2009) and wild elderberry fruits from southern Italy comprised TPC 7 mg GAE g−1 FW (Caruso et al., 2016). TPC values of wild S. nigra berries from Latvia are also comparable to cultivated S. nigra from Portugal (highest value being 14 mg g−1 FW) (Ferreira et al., 2020), Poland (70 mg g−1 DW) (Młynarczyk et al., 2020) and Hungary (10 mg g−1 FW) (Csorba et al., 2020). TPC of elderberry flowers in our study exceeded TPC of fruits by an order of magnitude. Significantly higher TPC of flowers as compared to berries has also been observed in other studies (Młynarczyk et al., 2018).
TEAC values significantly correlated with TPC values for elderberry fruits (r = 0.856; p < 0.0001) and flowers (r = 0.505; p < 0.0001). TEAC values significantly varied among different sampling sites in fruits, as well as in flowers (Table 2). The highest average TEAC value of sampled wild S. nigra was 410.39 ± 11.52 μmol Trolox equivalents g−1 DW for elderberry fruits and 205.02 ± 48.48 μmol Trolox equivalent g−1 DW for flowers (Fig. 3). Data of the present study were similar to TEAC values of wild S. nigra from Poland (327 and 397 μmol Trolox equivalent g−1 DW of flowers and fruits respectively) (Młynarczyk et al., 2020) and to cultivated S. nigra from Portugal (5.36 mmol Trolox equivalents 100 g−1 FW of elderberry fruits) (Ferreira et al., 2020). TPC and TEAC values of extracts derived from wild S. nigra plants were compared with TPC and TEAC of cv. ‘Haschberg’ which is the main source of raw material for the production of standardized antiviral food supplements. Although the average values of TFC, TPC, and TEAC of wild S. nigra populations did not exceed cv. ‘Haschberg’ cultivated in the same region, individual plants from several wild populations were superior to the standard cultivar (Fig. 3), suggesting that if thoroughly selected, wild S. nigra from Latvia can serve as a valuable genetic material for S. nigra breeding and as a source for raw material for the production of S. nigra derived products.
Rutin is a major flavonoid compound of elderberry flowers and one of the most abundant flavonols of elderberry fruits (Młynarczyk et al., 2018). Rutin was reported to exhibit antiviral activity (Savov et al., 2006; Orhan et al., 2010; Ganeshpurkar and Saluja, 2017), and has recently been suggested among potential therapeutic targets for SARS-CoV2 (Wu et al., 2020). Several in-silico based studies that are yet published as preprints (Altayeb et al., 2020; Jani et al., 2020) have identified rutin as one of the major candidates for the inhibition of ACE2-SARS-CoV2 S-protein RBD binding. HPLC based assessment of rutin content of sampled elderberry fruits (Fig. 4 ) was performed. Rutin content of wild elderberry fruits significantly differed among sampling sites (Table 2) and the average site values ranged from 1.50 ± 0.85 to 7.08 ± 2.69 mg g−1 DW. These values can be considered moderate to high in comparison to the rutin content reported in other studies (Lee and Finn, 2007; Veberic et al., 2009; Zielińska-Wasielica et al., 2019). Similarly to TPC and TEAC values, the assessed wild populations did not contain superior average amounts of rutin as compared with cv ‘Haschberg’, however, several wild S. nigra individuals comprised exceptionally high rutin content (Fig. 4).
Fig. 4.
Rutin content of extracts derived from wild elderberries sampled from 18 sampling sites located in Latvia (denoted with 3 letter symbols: SAL to RUD), determined by HPLC. Corresponding values of cultivated S. nigra of cv ‘Haschberg’ are denoted as HB. Whisker-box plot represents the minimum and maximum value of data set representing n = 5 individual plants from particular sampling site. Rutin content is expressed as mg rutin per g of lyophilised elderberries. Differences between wild S.nigra plants and cv ‘Haschberg’ were assessed as non-significant using Dunnett’s multiple range test.
3.3. Elderberry fruit and flower extracts inhibit SARS-CoV2 S-protein RBD and hACE2 binding in vitro
Angiotensin -converting enzyme 2 (ACE2) has been reported as the main entry receptor for SARS-Cov2 virus entry into the host cell (Zhou et al., 2020). Complex formation between the host cell receptor and viral particles is mediated by the receptor binding domain (RBD) of spike-protein of the virus and the extracellular peptidase domain of ACE2 receptor (Yan et al., 2020). Therefore, assays testing the inhibition of SARS-CoV2 RBD and ACE2 binding can serve as an indicative screening of plant-derived substances with potential anti-SARS-CoV2 properties before more costly and potentially hazardous assays involving infectious viral particles are undertaken.
Here, the inhibitory effect of elderberry fruit and flower extracts on SARS-CoV2 RBD and ACE2 binding was assessed because these results can single out S. nigra for the scientific community as a potentially interesting plant for more thorough anti-SARS-CoV2 testing. Inhibitory potential of elderberry fruit and flower extracts on SARS-CoV2-RBD-ACE2 protein binding in vitro was assessed using competitive ELISA. Results of ELISA assay of S. nigra extracts on ACE2-SARS CoV2 RBD binding in vitro revealed a significant concentration-dependent (Two-way ANOVA, F(1.629, 3.25) = 97.84; p = 0.001) inhibitory effect of wild elderberry fruit extracts (Fig. 5 ). IC50 of elderberry fruits was estimated at 1.66 mg dry extract ml−1 (concentration of extract added). ELISA results revealed also significant, albeit less pronounced inhibition of ACE2-SARS CoV2 RBD binding in vitro in the case of wild elderberry flower extracts probably due to comparatively lower concentrations. IC50 of flowers was estimated at 0.532 mg dry extract ml−1. Minimal inhibitory concentration of wild elderberry flower extract was 3.59 mg dry extract ml−1. IC50 values determined in our study are two magnitudes of an order higher than the values reported for Sambucus Formasan Nakai stem ethanol extract inhibitory properties towards human coronavirus NL63 virus attachment (reported IC50 value 15.75 μg ml−1) (Weng et al., 2019). This result is not surprising considering the differences in extract preparation (PBS vs DMSO as the final solvent) and the differences in the assays used for assessment of the viral attachment (ELISA involving isolated proteins vs plaque assay involving viral particles and cells). Inhibitory antiviral capacity of plant-derived extracts also significantly depends on viral infection stage inspected. For example, a study assessing the antiviral activity of Aphloia theiformis against several strains of Zika virus showed that IC50 values (100 μg ml−1) of plant-derived extracts required to inhibit plaque formation were lower than the concentration (500 μg ml−1) required for viral attachment inhibition (Clain et al., 2018).
Fig. 5.
Effect of S. nigra berry and flower extracts on binding of recombinant hACE2 protein to SARS-CoV2 S protein receptor binding domain (RBD) in competitive ELISA. Extract concentration values in ELISA are expressed as mg of dry extract per ml of solvent (PBS). Each extract was tested in five concentrations each in three replicates, and the negative control contained PBS instead of plant extract. (a) Inhibitory capacity of extract derived from wild elderberries (black bars) compared to extract derived from cv ‘Haschberg’ (grey bars), (b) Inhibitory capacity of extract derived from wild elderflowers (black bars) compared to extract derived from cv ‘Haschberg’ (grey bars). Statistically significant differences between inhibitory effects of applied extract concentrations and PBS control (denoted as 0) within each data set were assessed with Dunnett’s multiple range test and derived statistical significance values are denoted with stars - **p < 0.01, *p < 0.05. Statistically significant differences between data sets within each concentration were assessed with Sidak’s multiple range test and derived statistical significance values are denoted with stars - **p < 0.01, *p < 0.05 above line joining two compared values.
Inhibitory capacity of wild S. nigra berry extracts was also compared with extracts derived from S. nigra cultivar ‘Haschberg’. Origin of the plant material significantly affected the inhibitory efficiency of elderberry fruit extracts (two-way ANOVA F(1,2) = 43.69; p = 0.02) suggesting that wild populations of S. nigra are superior to the well-known traditional cultivar. Data derived from the inhibitory assay and data from phytochemical characterization of the extracts identified wild populations as a potentially valuable source of S. nigra genetic resources. Effect of origin was not observed in a case of flower extracts (Two-way ANOVA F(1,2) = 1.922; p = 0.30).
This is the first report of S. nigra berry and flower inhibitory activity against ACE2-SARS CoV2 RBD binding in vitro. Derived results can open new directions for novel industrial applications of S. nigra extracts but require further validation in future research of S. nigra anti-SARS-CoV2 applications. Further research comparing various extraction techniques, applying fractionation to elderberry fruit and flower extracts and evaluating direct antiviral effects using plaque assays is required for more conclusive and applied evidence regarding anti- SARS-CoV2 activity of S. nigra.
4. Conclusions
ISSR marker-based genetic characterization of wild S. nigra from north-east edge of the species native range revealed low among-populations genetic variability, east-west grouping of sites, clustering with sites in northern Lithuania and distinct clustering and grouping of sites of putatively historically introduced origin. The data suggest that wild S. nigra populations in Latvia mainly originated from wild S. nigra plants as a result of natural expansion of the species range with admixture of genetic material from historically introduced cultivated S. nigra plants.
Although the average values of TPC, TEAC, TFC, and rutin content of wild S. nigra populations did not exceed those of the leading cultivar ‘Haschberg’, individual plants from several wild populations were superior to the standard cultivar suggesting that, if thoroughly selected, wild S. nigra from Latvia can serve as valuable genetic material for breeding and as source for raw-material for production of S. nigra derived products.
Wild elderberry fruit extract exhibited higher inhibitory capacity towards ACE2 and SARS-CoV2 RBD binding than the extract derived from berries of cv ‘Haschberg’. Collectively, these results encourage further research of new anti-SARS-CoV2 industrial applications of S. nigra berry and flower extracts.
CRediT authorship contribution statement
Anete Boroduske: Conceptualization, Methodology, Formal analysis, Writing - original draft, Resources, Investigation, Writing - review & editing, Visualization, Funding acquisition. Kaspars Jekabsons: Methodology, Formal analysis, Investigation, Writing - review & editing, Visualization. Una Riekstina: Conceptualization, Resources, Writing - review & editing. Ruta Muceniece: Conceptualization, Resources, Writing - review & editing. Nils Rostoks: Conceptualization, Resources, Writing - review & editing. Ilva Nakurte: Conceptualization, Methodology, Investigation, Resources, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
This research was financially supported by ERDF, University of Latvia and Republic of Latvia post-doctoral grant number 1.1.1.2./VIAA/2/18/256 “Biotechnological solutions for control of target compounds in European elder Sambucus nigra in vitro cell cultures and in planta using endophytic microorganisms”.
We acknowledge the farmer and breeder Eglons Bruns for providing access to S. nigra cv ‘Haschberg’ orchard.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.indcrop.2021.113438.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Akbulut M., Ercisli S., Tosun M. Physico-chemical characteristics of some wild grown European elderberry (Sambucus nigra L.) genotypes. Pharmacogn. Mag. 2009;5(20):320. https://www.phcog.com/article.asp?issn=0973-1296;year=2009;volume=5;issue=20;spage=320;epage=323;aulast=Akbulut [Google Scholar]
- Altayeb H., Bouslama L., Abdulhakimc J.A., Chaieb K., Baothman O.A., Zamzami M.A. 2020. Potential Activity of a Selected Natural Compounds on SARS-CoV-2 RNA-dependent-RNA Polymerase, and Binding Affinity of the Receptor-binding Domain (RBD)https://assets.researchsquare.com/files/rs-32971/v1/5203fd5f-4f09-40e9-ae7c-46f53efcd849.pdf (Accessed 7.08.2020) [Google Scholar]
- Applequist W.L. A brief review of recent controversies in the taxonomy and nomenclature of Sambucus nigra sensu lato. In: Thomas A.L., editor. Proceedings of the I International Symposium on Elderberry; International Society of Horticultural Sciences; 2013. pp. 25–33. [Google Scholar]
- Atkinson M.D., Atkinson E. Sambucus nigra L. J. Ecol. 2002;90(5):895–923. https://www.jstor.org/stable/3072258?casa_token=W8FIvux92usAAAAA%3AhdNriwQ0qusP8XXgf6iScupDHfSOfy-k2nYxpGmtUYb9odTzIoAtMB66GCIPTBc2-UvOCkSGE0Rm3HtGvV_UA6_nuA4eUwcC2a7r3ABMsdgf2-rIL8Ey#metadata_info_tab_contents [Google Scholar]
- Bolli R. Revision of the genus Sambucus. Dissertationes botanicae 223. J. Cramer. 1994:256. https://www.schweizerbart.de/publications/detail/isbn/9783443641351/Revision-of-the-iGenus-Sambucusi Berlin, Stuttgart. [Google Scholar]
- Bushakra J.M., Bassil N., Finn C.E., Hummer K.E. Sambucus genetic resources at the US National clonal germplasm repository. I Int. Symp. Elderberry. 2013;1061:135–145. https://www.actahort.org/books/1061/1061_13.htm [Google Scholar]
- Caruso M.C., Galgano F., Tolve R., Pecora M., Tedesco I., Favati F., Condelli N. Nutraceutical properties of wild berry fruits from Southern Italy. J. Berry Res. 2016;6(3):321–332. https://content.iospress.com/articles/journal-of-berry-research/jbr140 [Google Scholar]
- Charlebois D., Byers P.L., Finn C.E., Thomas A.L. In: Horticultural Reviews. Janick J., editor. A John Wiley & Sons Inc.; Hoboken, New-Jersey: 2010. 4 Elderberry: botany, horticulture, potential, Vol 37(4) pp. 214–280.https://books.google.lv/books?hl=en&lr=&id=IOmys0frsz0C&oi=fnd&pg=PA213&dq=Elderberry:+Botany,+Horticulture,+Potentia&ots=q_5ieIRYsd&sig=-53iqkb1-KJitHfUdTvdef6IgtY&redir_esc=y#v=onepage&q=Elderberry%3A%20Botany%2C%20Horticulture%2C%20Potentia&f=false [Google Scholar]
- Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E., Pendleton A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014;10(1):1–12. doi: 10.1186/1746-6148-10-24. https://bmcvetres.biomedcentral.com/articles/10.1186/1746-6148-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clain E., Sinigaglia L., Koishi A.C., Gorgette O., Gadea G., Viranaicken W., Krejbich-Trotot P., Mavingui P., Desprès P., dos Santos C.N.D., Guiraud P. Extract from Aphloia theiformis, an edible indigenous plant from Reunion Island, impairs Zika virus attachment to the host cell surface. Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-29183-2. https://www.nature.com/articles/s41598-018-29183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csorba V., Magdolna T.Ó.T.H., Laszlo A.M., Kardos L., KováCs S. Cultivar and year effects on the chemical composition of elderberry (Sambucus nigra L.) fruits. Not. Bot. Horti Agrobo. Cluj-Napoca. 2020;48(2):770–782. https://www.notulaebotanicae.ro/index.php/nbha/article/view/11873 [Google Scholar]
- Environmental Systems Research Institute (ESRI) 2012. ArcGIS Release 10.1.https://www.arcgis.com/home/webmap/viewer.html?webmap=cb2aca672b21495e8791af408f881f73 (Accessed 25 September 2020) [Google Scholar]
- European medicines agency (EMA) 2014. Assessment Report on Sambucus nigra L., flos. EMA/HMPC/611512/2016.https://www.ema.europa.eu/en/documents/herbal-report/superseded-assessment-report-development-community-monographs-inclusion-herbal-substances_en.pdf (Accessed 8 April 2019) [Google Scholar]
- European medicines agency (EMA) 2018. European Union Herbal Monograph on Sambucus nigra L., fructus. EMA/HMPC/44208/2012.https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-sambucus-nigra-l-fructus_en.pdf (Accessed 8 April 2019) [Google Scholar]
- Ferreira S.S., Silva P., Silva A.M., Nunes F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: a three-year study. Food Chem. 2020;302 doi: 10.1016/j.foodchem.2019.125366. https://www.sciencedirect.com/science/article/pii/S0308814619314797?casa_token=q4SnJSMgPMUAAAAA:VBGlJGYMuJCdyMLdB-J-2Yx1tyI-a2YNX1KBdY1vDaX19fFvhCa1-4pklPyg5OBIQw5ce-jK1P4 [DOI] [PubMed] [Google Scholar]
- Ganeshpurkar A., Saluja A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017;25(2):149–164. doi: 10.1016/j.jsps.2016.04.025. https://www.sciencedirect.com/science/article/pii/S1319016416300263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick J.L., Godt M.W. Effects of life history traits on genetic diversity in plant species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351(1345):1291–1298. doi: 10.1098/rstb.1996.0112. [DOI] [Google Scholar]
- Harnett J., Oakes K., Carè J., Leach M., Brown D., Cramer H., Pinder T.A., Steel A., Anheyer D. The effects of Sambucus nigra berry on acute respiratory viral infections: a rapid review of clinical studies. Adv. Integr. Med. 2020;7(4):240–246. doi: 10.1016/j.aimed.2020.08.001. https://www.sciencedirect.com/science/article/pii/S2212958820301543?casa_token=AUfkskySs1EAAAAA:CQ9PI4acz9mRJmPLZuHp_EshCbgf_o_Msfu_Qj-H15lL_6ixH5sNIDItKMnra4vaZh0PX-D2sEo [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J., Baker C., Cherry L., Dunne E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: a meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019;42:361–365. doi: 10.1016/j.ctim.2018.12.004. https://www.sciencedirect.com/science/article/pii/S0965229918310240?casa_token=UUQ9ucx-Bn4AAAAA:KEbixfUTA_J2HwLaH-bBg826YYtI2so-Vap59HC2qWW5jPJ0CCWlsM3rqtGy4lvis4fcfxY2m48 [DOI] [PubMed] [Google Scholar]
- Herald T.J., Gadgil P., Tilley M. High‐throughput micro plate assays for screening flavonoid content and DPPH‐scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012;92(11):2326–2331. doi: 10.1002/jsfa.5633. [DOI] [PubMed] [Google Scholar]
- Huang R., Wu W., Shen S., Fan J., Chang Y., Chen S., Ye X. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Anal. Methods. 2018;10(22):2575–2587. https://pubs.rsc.org/--/content/articlelanding/2018/ay/c8ay00661j/unauth#!divAbstract [Google Scholar]
- Hummer K.E., Pomper K.W., Postman J., Graham C.J., Stover E., Mercure E.W., Aradhya M., Crisosto C.H., Ferguson L., Thompson M.M., Byers P. In: Fruit Breeding. Badenes M.L., Byrne D.H., editors. Springer; Boston: 2012. Emerging fruit crops; pp. 97–147. [Google Scholar]
- Jani V., Koulgi S., Uppuladinne V.N.M., Sonavane U., Joshi R. 2020. Computational Drug Repurposing Studies on the ACE2-Spike (RBD) Interface of SARS-CoV-2.https://europepmc.org/article/ppr/ppr188118 (Accessed 7 August 2020) [Google Scholar]
- Jatoi M., Jemrić T. A comparative study of some local genotypes with commercial cultivar of black elder (Sambucus nigra L.) regarding vegetative and reproductive traits. Agric. Conspec. Sci. 2016;81(3):149–153. https://hrcak.srce.hr/index.php?id_clanak_jezik=263712&show=clanak [Google Scholar]
- Johnson H.-Y., Byers P., Hu J., Thomas A., Tesfaye S. Assessment of genetic diversity among elderberry (Sambucus sp.) species, cultivars, and wild selections by TRAP technique. HortScience. 2008;43(4):1137–1138. https://www.ars.usda.gov/research/publications/publication/?seqNo115=225230 [Google Scholar]
- Johnson M.C., Dela Libera Tres M., Thomas A.L., Rottinghaus G.E., Greenlief C.M. Discriminant analyses of the polyphenol content of American elderberry juice from multiple environments provide genotype fingerprint. J. Agric. Food Chem. 2017;65(20):4044–4050. doi: 10.1021/acs.jafc.6b05675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasajima I., Sasaki K., Tanaka Y., Terakawa T., Ohtsubo N. Large-scale extraction of pure DNA from mature leaves of Cyclamen persicum Mill. and other recalcitrant plants with alkaline polyvinylpolypyrrolidone (PVPP) Sci. Hortic. 2013;164:65–72. https://www.sciencedirect.com/science/article/pii/S0304423813004706?casa_token=9sEcXKqZJLMAAAAA:b7OBg0-poBh5LjdxMrzYeE-kFLOhep_rpId9t-EIAZmN-xO5NFUYkNpgnGeMYP1IZu3rHZ54ubE [Google Scholar]
- Kopper E., Granilshchikova M., Leichtfried T., Reisenzein H. Micropropagation and pathogen elimination in elderberry (Sambucus nigra L.) Plant Cell Tissue Organ. 2020;142(3):647–652. https://link.springer.com/article/10.1007/s11240-020-01874-7 [Google Scholar]
- Kronbichler A., Effenberger M., Eisenhut M., Lee K.H., Shin J.I. Seven recommendations to rescue the patients and reduce the mortality from COVID-19 infection: an immunological point of view. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102570. https://www.sciencedirect.com/science/article/pii/S1568997220301324?casa_token=sHGJGXvQw0UAAAAA:TvYsvW9TfpoFpxMZsQpAWFP6eTTu8rgzBrZU01VwkbRUdnjSg9ZUn6KQAJbYBz24rv77OhqIIbk [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiviņš M. Melnā plūškoka sabiedrības Sambucetum nigrae Oberd.1967 Latvijā. Mežzinātne. 2002;11:92–110. [Google Scholar]
- Laiviņš M., Bice M., Krampis I., Knape D., Šmite D., Šulcs V. 2009. Latvijas kokaugu atlants.www.kurtuesi.lv/flora (Accessed 4 April 2019) [Google Scholar]
- Lee J., Finn C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007;87(14):2665–2675. doi: 10.1002/jsfa.3029. https://onlinelibrary.wiley.com/doi/full/10.1002/jsfa.3029?casa_token=H2so8oWBKbEAAAAA%3AWyc2qHKpn31WsReXB_J0K1I93WuaMf6IN6iY3RqK7W9VU-8AND27Iy9VMoIkvN7MoG_U1n0YP3rbwYH0 [DOI] [PubMed] [Google Scholar]
- Lima-Brito J., Castro L., Coutinho J., Morais F., Gomes L., Guedes-Pinto H., Carvalho A. Genetic variability in Sambucus nigra L. clones: a preliminary molecular approach. J. Genet. 2013;92(2):47–52. doi: 10.1007/s12041-011-0066-x. https://pubmed.ncbi.nlm.nih.gov/21873774/ [DOI] [PubMed] [Google Scholar]
- MapChart . 2020. Map of Europe.https://mapchart.net/ (Accessed on 15 September 2020) [Google Scholar]
- Milena V., Tatjana M., Gökhan Z., Ivana B., Aleksandra C., Mohammad M.F., Marija R. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crops Prod. 2019;136:93–101. https://www.sciencedirect.com/science/article/pii/S0926669019303085?casa_token=qf4LJ3o9sGQAAAAA:UV0CkYO_bASH82_8hObY6xEyECEmFv4fq2KH-1b94vJ6BVLeotvm2Rxk4M0_sBTEo5hfJkdRKX0 [Google Scholar]
- Młynarczyk K., Walkowiak-Tomczak D., Łysiak G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods. 2018;40:377–390. doi: 10.1016/j.jff.2017.11.025. https://www.sciencedirect.com/science/article/pii/S1756464617306977?casa_token=jLx6YE9zmt4AAAAA:h6hNlzykkbaIwFGA_HQOJ6UKyigsDj7fM9Zu2nZOLEJy-ASIlCUDDsPUnCb_jtbs3RWRYpZpVd4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Młynarczyk K., Walkowiak-Tomczak D., Staniek H., Kidoń M., Łysiak G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules. 2020;25(4):876. doi: 10.3390/molecules25040876. https://www.mdpi.com/1420-3049/25/4/876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota A.H., Andrade J.M., Rodrigues M.J., Custódio L., Bronze M.R., Duarte N., Baby A., Rocha J., Gaspar M.M., Simões S., Carvalheiro M. Synchronous insight of in vitro and in vivo biological activities of Sambucus nigra L. extracts for industrial uses. Ind. Crops Prod. 2020;154 https://www.sciencedirect.com/science/article/abs/pii/S0926669020306257 [Google Scholar]
- Nei M. Genetic distances between populations. Am. Nat. 1972;106:283–292. doi: 10.1086/282771. [DOI] [Google Scholar]
- Nestby R., Hykkerud A.L., Martinussen I. Review of botanical characterization, growth preferences, climatic adaptation and human health effects of Ericaceae and Empetraceae wild dwarf shrub berries in boreal, alpine and arctic areas. J. Berry Res. 2019;9(3):515–547. https://content.iospress.com/articles/journal-of-berry-research/jbr190390 [Google Scholar]
- Nordic Baltic Genebanks Information System . 2020. Sambucus Nigra Accessions.https://www.nordic-baltic-genebanks.org/gringlobal/search.aspx (Accessed 13 December 2020) [Google Scholar]
- Orhan D.D., Özçelik B., Özgen S., Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010;165(6):496–504. doi: 10.1016/j.micres.2009.09.002. https://www.sciencedirect.com/science/article/pii/S0944501309000743 [DOI] [PubMed] [Google Scholar]
- Peakall R., Smouse P.E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R.S., Bode R.F. A review of the antiviral properties of black elder (Sambucus nigra L.) products. Phytother. Res. 2017;31(4):533–554. doi: 10.1002/ptr.5782. [DOI] [PubMed] [Google Scholar]
- Pratašienė K., Kulbokas G., Marozas V. Distribution, composition and structure of forest communities with non-native" Sambucus" species in Lithuania. Mediterr. Bot. Madrid. 2019;40 https://www.vdu.lt/cris/handle/20.500.12259/61555 [Google Scholar]
- Rodríguez-Rodríguez P., de Castro A.G.F., Sosa P.A. The restoration of the endangered Sambucus palmensis after 30 years of conservation actions in the Garajonay National Park: genetic assessment and niche modeling. PeerJ. 2018;6:e4985. doi: 10.7717/peerj.4985. https://peerj.com/articles/4985/?utm_source=TrendMD&utm_campaign=PeerJ_TrendMD_0&utm_medium=TrendMD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschek B., Jr., Fink R.C., McMichael M.D., Li D., Alberte R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochem. 2009;70(10):1255–1261. doi: 10.1016/j.phytochem.2009.06.003. https://www.sciencedirect.com/science/article/pii/S0031942209002386?casa_token=0Yeu9hiGEREAAAAA:ZMIv9Yb7SWEJvGbNjhwlMgjEiMI5782sBqGZEJlW8M2GexYHlx1ON4Lr1tdxXZ5MR6Jn2QCobhk [DOI] [PubMed] [Google Scholar]
- Salvador A.C., Rocha S.M., Silvestre A.J.D. In: Atta-ur-Rahman, editor. Vol2. Bentham Science Publishers; USA: 2016. Sambucus nigra L.: A potential source of healthpromoting components; pp. 343–392. (Frontiers in Natural Product Chemistry). [Google Scholar]
- Samarakoon T., Wang S.Y., Alford M.H. Enhancing PCR amplification of DNA from recalcitrant plant specimens using a trehalose‐based additive. Appl. Plant Sci. 2013;1(1) doi: 10.3732/apps.1200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savov V.M., Galabov A.S., Tantcheva L.P., Mileva M.M., Pavlova E.L., Stoeva E.S., Braykova A.A. Effects of rutin and quercetin on monooxygenase activities in experimental influenza virus infection. Exp. Toxicol. Pathol. 2006;58(1):59–64. doi: 10.1016/j.etp.2006.05.002. https://www.sciencedirect.com/science/article/pii/S0940299306000571?casa_token=NBOqL32ux5kAAAAA:gZRp4tYJZUEgy4IyniQQyGEj0tMNlAe-rmJr0GhsxFZwybdQIWF_y2XwxWc-CL1SmEG7kp75xwA [DOI] [PubMed] [Google Scholar]
- Schmitzer V., Veberic R., Stampar F. European elderberry (Sambucus nigra L.) and American Elderberry (Sambucus canadensis L.): botanical, chemical and health properties of flowers, berries and their products. Berries Prop. Consum. Nutr. 2012:127–144. [Google Scholar]
- Sedláčková V.H., Grygorieva O., Fatrcová-Šramková K., Vergun O., Vinogradova Y., Ivanišová E., Brindza J. The morphological and antioxidant characteristics of inflorescences within wild-growing genotypes of elderberry (Sambucus nigra L.) Potr. S. J. F. Sci. 2018;12(1):444–453. https://www.potravinarstvo.com/journal1/index.php/potravinarstvo/article/view/919 [Google Scholar]
- Senica M., Stampar F., Veberic R., Mikulic‐Petkovsek M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Agric. Food Chem. 2017;97(8):2623–2632. doi: 10.1002/jsfa.8085. [DOI] [PubMed] [Google Scholar]
- Silva P., Ferreira S., Nunes F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017;95:227–234. https://www.sciencedirect.com/science/article/pii/S0926669016306872?casa_token=fq--5g9mTQ8AAAAA:nMe5MZMFHatmR4kU-vOZDf4d-1JiAFNI1lHEUtTET8kPyxabWBG_UNesD1rPtOK-4HvTSh_G0Q0 [Google Scholar]
- Silveira D., Prieto-Garcia J.M., Boylan F., Estrada O., Fonseca-Bazzo Y.M., Jamal C.M., Magalhães P.O., Pereira E.O., Tomczyk M., Heinrich M. COVID-19: Is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 2020;11:1479. doi: 10.3389/fphar.2020.581840. https://www.frontiersin.org/articles/10.3389/fphar.2020.581840/full?utm_source=FRN&utm_medium=EMAIL_IRIS&utm_campaign=EMI_FRN_ARTICLEPUBLISHED_COAUTHORS&utm_content=ARTICLE_TITLE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa P.A., González-Pérez M.A., Moreno C., Clarke J.B. Conservation genetics of the endangered endemic Sambucus palmensis Link (Sambucaceae) from the Canary Islands. Conserv. Genet. 2010;11(6):2357–2368. [Google Scholar]
- Tiralongo E., Wee S.S., Lea R.A. Elderberry supplementation reduces cold duration and symptoms in air-travellers: a randomized, double-blind placebo-controlled clinical trial. Nutrients. 2016;8(4):182. doi: 10.3390/nu8040182. https://www.mdpi.com/2072-6643/8/4/182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabian G., Valtchev P., Adil Q., Dehghani F. Anti-influenza activity of elderberry (Sambucus nigra) J. Funct. Foods. 2019;54:353–360. https://www.sciencedirect.com/science/article/pii/S1756464619300313?casa_token=j0PCQbN9j2EAAAAA:iyjLxGBH1q6E6Bk2x7YUlGQrMxCqOsbO3mVPPTAJlVMZy35di8sIT_xhCGVHog6GY2jNQttx6z8 [Google Scholar]
- Ulbricht C., Basch E., Cheung L., Goldberg H., Hammerness P., Isaac R., Khalsa K.P.S., Romm A., Rychlik I., Varghese M., Weissner W. An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2014;11(1):80–120. doi: 10.3109/19390211.2013.859852. [DOI] [PubMed] [Google Scholar]
- Veberic R., Jakopic J., Stampar F., Schmitzer V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009;114(2):511–515. https://www.sciencedirect.com/science/article/pii/S0308814608011710?casa_token=6e9sJa6vXCEAAAAA:hBpImMPudFODh54H4KgWV2IYN53nNeLjrrR0LZmoAeJAQuJ9fRtAoTWAf6h-638ytQYTPtMVEEU [Google Scholar]
- Viapiana A., Wesolowski M. The phenolic contents and antioxidant activities of infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017;72(1):82–87. doi: 10.1007/s11130-016-0594-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5325840/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J.R., Lin C.S., Lai H.C., Lin Y.P., Wang C.Y., Tsai Y.C., Wu K.C., Huang S.H., Lin C.W. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019;273 doi: 10.1016/j.virusres.2019.197767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World health organization (WHO) 2020. Urgent Health Challenges for the Next Decade.https://www.who.int/news-room/photo-story/photo-story-detail/urgent-health-challenges-for-the-next-decade (Accessed on 27 August 2020) [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. https://www.sciencedirect.com/science/article/pii/S2211383520302999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyganowska-Swiatkowska M., Nohawica M., Grocholewicz K., Nowak G. Influence of herbal medicines on HMGB1 release, SARS-CoV-2 viral attachment, acute respiratory failure, and Sepsis. A literature review. Int. J. Mol. Sci. 2020;21(13):4639. doi: 10.3390/ijms21134639. https://www.mdpi.com/1422-0067/21/13/4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. https://science.sciencemag.org/content/367/6485/1444.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. https://www.nature.com/articles/s41586-020-2012-7?rel=outbound [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska-Wasielica J., Olejnik A., Kowalska K., Olkowicz M., Dembczyński R. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods. 2019;8(8):326. doi: 10.3390/foods8080326. https://www.mdpi.com/2304-8158/8/8/326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.