Abstract
AIM
To study the diagnostic accuracy and utility of triphasic abdominal computed tomography (CT) in the diagnosis and grading of oesophageal varices (OVs) as an alternative to endoscopy during the COVID-19 pandemic.
MATERIALS AND METHODS
A prospective analysis was undertaken of retrospective data from cirrhotic patients who underwent oesophago-gastro-duodenoscopy (OGD) and a triphasic abdominal CT from January to December 2019. Endoscopists and radiologists provided their respective independent assessment of OV grading after being blinded to the clinical details. Performance of CT grading of OVs was compared with the reference standard endoscopic grading using weighted kappa (k). Non-invasive scores such, as aspartate transaminase (AST)-to-platelet ratio index (APRI), Fibrosis-4 (FIB-4) Index, platelet: spleen (PS) ratio were correlated between the two techniques.
RESULTS
OV grading between endoscopists and radiologists showed 81.73% agreement (85 out of 104 patients) in the comparative analysis of 104 cirrhotic patients, of which no varices (57.1%, n=4), small (85.1%, n=23), medium (72.2%%, n=26), and large varices (94.1%, n=32) with a weighted k score of 0.88 (95% confidence interval 0.82–0.94). Overall, the sensitivity of CT in the diagnosis of no, small, medium, and large OVs was 66.6%, 79.3%, 89.6%, and 94.1%, respectively, with an area under the receiver operating curve (AUROC) score of 0.775, 0.887, 0.839, and 0.914. Performance of APRI, FIB-4, and PS ratio correlated well with the severity of OVs with no difference between OGD and CT grading.
CONCLUSION
Triphasic abdominal CT can be an invaluable tool in the diagnosis and grading of OVs during the COVID-19 pandemic.
Introduction
The COVID-19 pandemic has transformed the way healthcare professionals function. The risk of disease transmission to healthcare professionals can be as high as 10–20%.1 For this reason, several centres across the world have deferred elective surgeries and procedures during the pandemic.2 A number of cases of peri-operative transmission of SARS-CoV-2 via patients undergoing surgeries or procedures have been reported around the world.3
Patients with liver cirrhosis are at risk of oesophageal varices (OVs) related to the severity of liver disease and the degree of portal hypertension. At least 40% of patients with compensated cirrhosis and 80% with decompensated cirrhosis have OVs.4 Once developed, the rate of small to large varices progression occurs at 5–12% per year in Child A cirrhosis but faster progression (22%) in patients with Child B and C cirrhosis.5 , 6 The risk of bleeding is also higher in patients with large varices leading to significant morbidity and mortality of around 20%. The size of the OVs determines the risk of index variceal bleed.7 Therefore, patients with liver cirrhosis undergo variceal surveillance with oesophago-gastro-duodenoscopy (OGD) as the best standard procedure at regular intervals, depending on the degree of liver disease. Patients with small varices and high-risk features, such as red signs or advanced liver disease, will require non-specific beta-blockers, preferably carvedilol, whereas patients with medium and large varices can be treated with non-specific beta-blockers or endoscopic variceal band ligation depending on local resources and expertise.8
Unfortunately, endoscopy is considered a risky procedure as this can generate considerable aerosols, potentially spreading SARS-CoV-2 to the operating team and other patients. With the median incubation period of 5.5 days, many patients can be in the presymptomatic phase, and recent evidence showed as many as 45% of patients are asymptomatic carriers.9 In addition, COVID-19 can affect patients with chronic liver disease. These patients are considered at high risk of disease progression leading to significant morbidity and mortality.10 Therefore, several healthcare professional bodies advise against routine endoscopic procedures.2 , 11 Hence, there is certainly an immediate need for an alternative technique for OV diagnosis and screening during this pandemic. Identification of an alternative technique for OV screening in the absence of endoscopy can be challenging. The test should be comparable to endoscopy, cost-effective, and should have no risk of SARS-CoV-2 transmission in order to protect the healthcare professionals and other patients.
Non-invasive tests for liver fibrosis, such as aspartate transaminase (AST)-to-platelet ratio index (APRI), Fibrosis-4 (FIB-4) Index, platelet: spleen (PS) ratio, and Fibroscan, are well studied and validated in patients with cirrhosis as well as in portal hypertension but cannot replace OGD. Triphasic abdominal computed tomography (CT) is a commonly performed investigation in patients with liver cirrhosis. It provides better information about liver anatomy, vascularity, and importantly, the presence of hepatocellular carcinoma (HCC) in most cases. In addition, triphasic abdominal CT provides valuable information about portal hypertension and may be a useful tool in this current pandemic for the diagnosis of OVs. The aim of the present study was to investigate the diagnostic accuracy and utility of triphasic abdominal CT as an alternate investigative tool for OGD in the diagnosis and grading of OVs in patients with liver cirrhosis.
Materials and methods
Prospective scoring was undertaken of all adult cirrhotic patients who underwent OGD for OV screening and concurrent triphasic abdominal CT within 6 months before or after endoscopy from January 2019 to December 2019. Patients who did not undergo CT or endoscopy and patients with suboptimal endoscopic images were excluded. This study was approved by the hospital internal ethical committee.
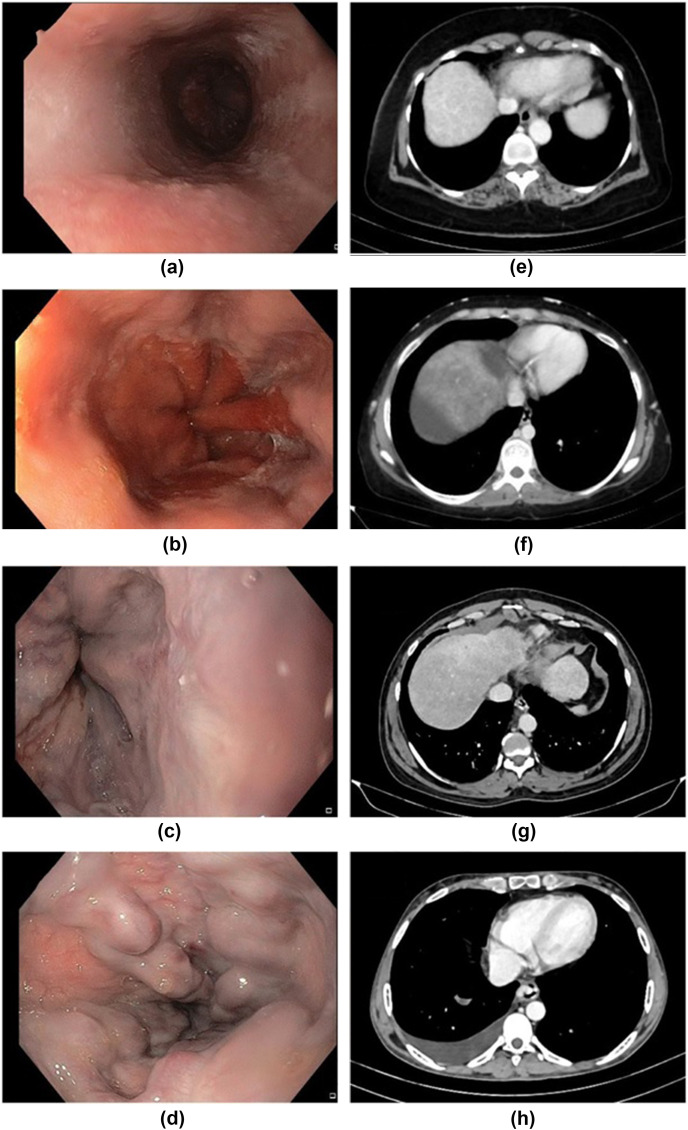
Screening OGD (reference standard) was performed with an Olympus Evis Exera III GIF HQ-190 scope. Respective endoscopic images were presented to the endoscopists (endoscopist 1 and endoscopist 2) with >10 years of experience, after blinding clinical details and original reports. Each endoscopist graded the OVs independently as described in the Baveno classification.12 Small varices were defined as minimally elevated varices above the oesophageal mucosa; medium: varices occupying less than one-third of the oesophageal lumen; large: varices occupying more than one-third of the lumen as shown in Fig 1 a–d. Grading discrepancy between the two endoscopists was reviewed by a third endoscopist (endoscopist 3) with 10 years of experience, for independent grading.
Figure 1.
(a–d) Endoscopic and (e–h) CT images for OV grading. Endoscopy: (a) no varices, (b) small varices, (c) medium varices, and (d) large varices. CT images: (e) no varices, (f) small varices, (g) medium varices, and (h) large varices.
A triphasic contrast-enhanced CT of the abdomen (index test) was performed using a 128-section CT system (Siemens Perspective, Germany) after injecting 90 ml of intravenous non-ionic iodinated contrast material (Ultravist, Bayer Healthcare). Three sets of axial images were acquired at 25, 60, and 180 seconds after contrast medium administration. In the second acquisition, the portal venous phase was used to evaluate the varices. In CT, OVs were considered to be present when there was contrast medium filled tubular structures in the wall protruding into the lumen of the oesophagus in the portal venous phase. The varices were considered small when their diameters were <3 mm, medium when they were >3 mm and <5 mm, large when >5 mm (Fig 1e–h). Two radiologists (radiologist 1 and radiologist 2) with 9 and 5 years of experience, respectively, independently reviewed the CT images after patient details were blinded. When there was a discrepancy in grading between these two reviewers, a third radiologist (radiologist 3) with 7 years of experience was asked to review those cases for an independent grading. Spleen size was measured in the longest craniocaudal dimension on coronal images and longest anteroposterior and transverse dimension on axial images. The largest values of these three measurements were taken as the maximum bipolar diameter of the spleen.
Data analyses were carried out using SPSS v 21.0. Performance of CT grading of OVs was compared with OGD grading using weighted kappa (k). Cohen's kappa is a coefficient that measures the inter- and intra-rater reliability for qualitative data. It is a robust measure that accounts for the possibility of agreement occurring by chance rather than a simple percentage agreement calculation. Weighted kappa is a variant of Cohen's kappa that considers the different levels or degrees of disagreement between observers and uses weighting schemes to calculate the closeness between categories. Quadratic weightage was used because it summarises the agreement on an ordinal scale. The interpretation of the weighted kappa score was defined as <0, 0.40–0.60, 0.60–0.80, and 0.80–1 indicating no agreement, moderate agreement, substantial agreement, and almost perfect agreement, respectively.
The area under the receiver operating characteristic (ROC) curve (AUROC) was calculated to assess the performance and measurement of a classification model for endoscopy and CT. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of CT diagnosis and grading of OV were calculated. Further, non-invasive scores such as APRI ([(AST/ULN AST)×100]/platelet count), FIB-4 (age (years)×AST (U/l)/[platelet (109/l)×ALT (U/l)]), PS ratio (platelet count (109/l)/spleen maximum bipolar diameter (mm) ratio) were calculated and the mean differences in endoscopic and CT grading of OVs were determined using the t-test. A p-value of <0.05 was considered statistically significant.
Results
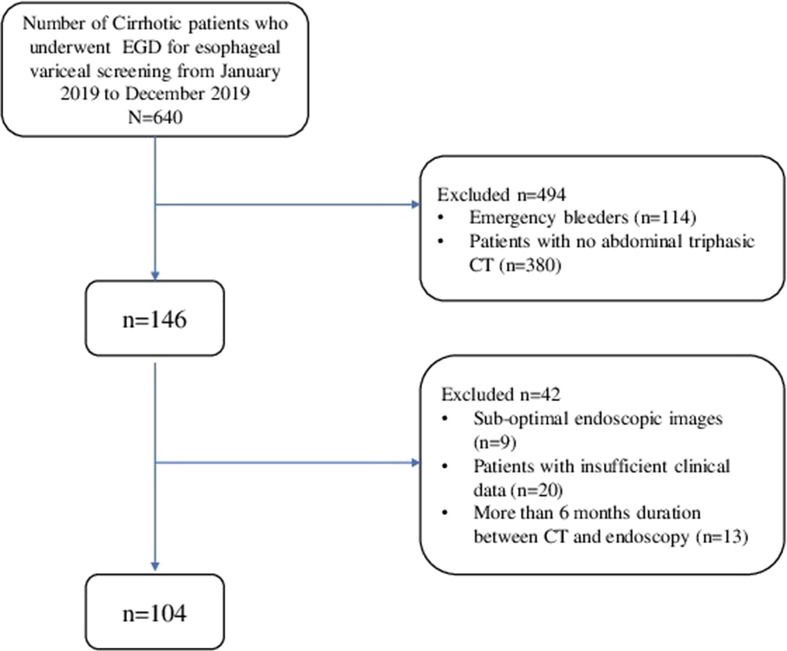
The flow chart of patient selection is illustrated in Fig 2 . In total, 104 cirrhotic patients underwent both endoscopy and triphasic abdominal CT during the study period with a median age of 53.5±7.54 years, male: female ratio 8.5 : 1, Model for end-stage liver disease (MELD) score 12 (mean, normal range: 6–40). Disease aetiology showed non-alcoholic steatohepatitis, hepatitis B, hepatitis C, alcohol-related liver disease, and others in 43 (41.3%), 16 (15.4%), six (5.8%), 24 (23.1%), and 13 (12.5%), respectively.
Figure 2.
Illustrates the flow of patient selection for the study.
The number of observed agreements for OVs between the two endoscopists was 92.3% (96 out of 104 patients) of which no varices (100% agreement, n=5 out of 5), small varices (82.8%, n=24 out of 29), medium varices (88.6%, n=31 out of 35) and large varices (97.1%, n=34 out of 35) with a weighted k score 0.90 (95% confidence interval [CI]=0.78–0.94).
The number of observed agreements for OVs between the two radiologists was 81.7% (85 out of 104 patients) of which no varices (100% agreement, n=4 out of 4), small (71.9%, n=23 out of 32), medium (62.1%, n=18 out of 29), large varices (97.4%, n=38 out of 39), with a weighted k score was 0.89 (95% CI=0.85–0.94).
The discrepancy in OV grading was noted in eight patients between the endoscopists and 19 between the radiologists, which were reviewed by an independent endoscopist and a radiologist for rescoring.
The comparative analysis of OV grading between all the endoscopists and radiologists showed 81.73% agreement (85 out of 104 patients) of which no varices (57.1%, n=4), small (85.1%, n=23), medium (72.2%, n=26) and large varices (94.1%, n=32) with a weighted k score 0.88 (95% CI: 0.82–0.94) as illustrated in Table 1 . The overall endoscopic distribution of no varices, small, medium, and large varices was 6.7%, 26%, 34.6%, and 32.7%, respectively.
Table 1.
Agreement (%) between endoscopists and radiologists for different grades of oesophageal varices.
| CT variceal grading | Endoscopic variceal grading |
|||
|---|---|---|---|---|
| No varices n=7 |
Small varices n=27 |
Medium varices n=36 |
Large varices n=34 |
|
| No varices | 4 (57.1%) | 2 | 0 | 0 |
| Small varices | 3 | 23 (85.1%) | 2 | 1 |
| Medium varices | 0 | 2 | 26 (72.2%) | 1 |
| Large varices | 0 | 0 | 8 | 32 (94.1%) |
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of CT diagnosis in predicting no, small, medium, and large OVs compared to OGD variceal grading is shown in Table 2 .
Table 2.
Sensitivity, specificity, PPV∗, NPV∗ and AUROC of CT in the diagnosis of oesophageal variceal grading.
| CT grading | No varices | Small | Medium | Large |
|---|---|---|---|---|
| Sensitivity 95% CI |
66.6% 22.3–95.7 |
79.3% 60.3–92 |
89.6% 72.7–97.8 |
94.1% 80.3–99.3 |
| Specificity 95% CI |
96.9% 91.3–99.4 |
94.6% 86.9–98.5 |
86.6% 76.8–93.4 |
88.5% 78.7–94.9 |
| PPV∗ 95% CI |
57.1% 27.7–82.3 |
85.1% 68.5–93.8 |
72.2% 59–82.4 |
80% 67.5–88.5 |
| NPV∗ 95% CI |
97.9% 93.9–99.3 |
92.2% 85.3–96 |
95.5% 88.1–98.5 |
96.8% 89–99.2 |
| AUROC 95% CI |
0.775 0.54–1 |
0.887 0.80–0.97 |
0.839 0.75–0.93 |
0.913 0.85–0.98 |
| p-Value | 0.015 | 0.00 | 0.00 | 0.00 |
PPV, positive predictive value; NPV, negative predictive value; AUROC, area under the receiver operating curve.
Correlation between non-invasive markers (APRI, FIB-4, and PS ratio) to endoscopy and CT-guided oesophageal variceal grading
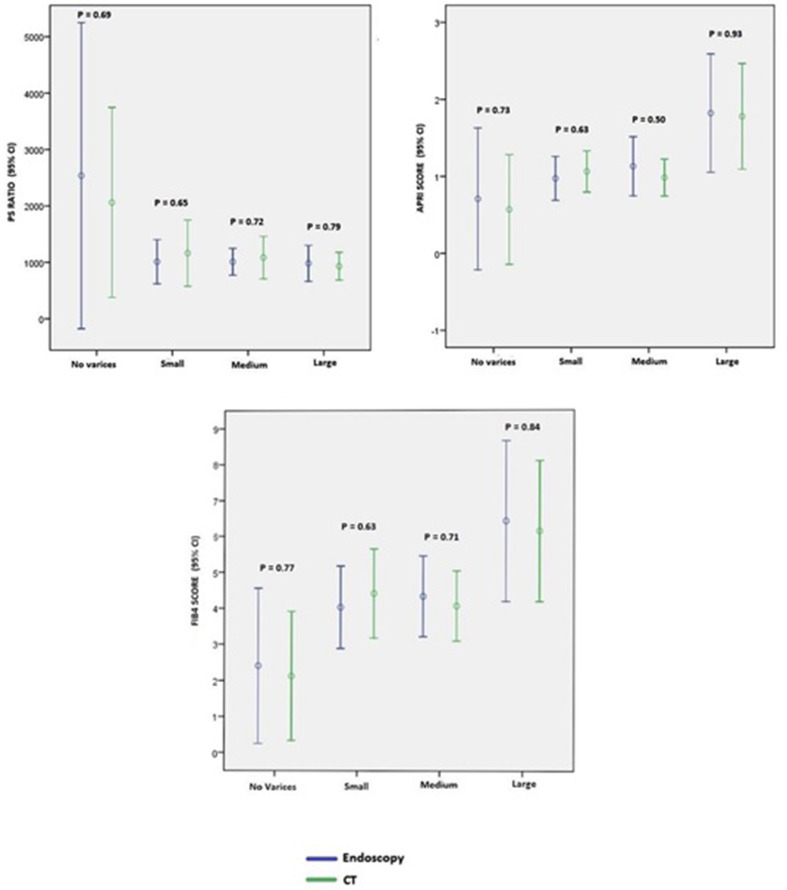
Non-invasive markers of liver fibrosis such as APRI, FIB-4, and PS ratio were calculated (n=104) and correlated with endoscopy and CT OV grading. The overall mean APRI, FIB-4, and PS ratio was 1.36 (95% CI: 1.01–1.70), 5 (95% CI: 4–6), and 1,089 (95% CI: 881–1297.9), respectively.
The mean APRI, FIB-4, PS ratio for endoscopic diagnosis of no varices, small, medium, and large varices was 0.70, 0.97, 1.13, 1.82; 2.4, 4.02, 4.32, 6.42, and 2,538, 1,012, 1,010, 983, respectively. Similarly, the mean APRI, FIB-4, PS ratio for CT diagnosis of no varices, small, medium, and large varices was 0.57, 1.06, 0.99, 1.8; 2.12, 4.41, 4.06, 6.14 and 2,062, 1,164, 1,085, 933, respectively.
The comparative analysis of the performance of these non-invasive markers between different grades of varices showed no significant difference for endoscopy and CT as shown in Fig 3 .
Figure 3.
Comparison on non-invasive markers (APRI, FIB-4 and PS ratio) to endoscopy and CT-guided OV grading.
Discussion
Routine endoscopy can be risky during the COVID-19 pandemic, because of the increased risk of disease transmission to healthcare professionals. An Italian survey identified that the majority of endoscopy units reduced their elective endoscopy services and modified their approach for emergency procedures.13 Endoscopy generates a higher amount of aerosol particles during the scope intubation, insertion, and during the usage of scope channels posing risk to the endoscopy team.
The present study clearly demonstrates 81.73% agreement between the endoscopists and radiologists in the diagnosis of OVs, indicating that venous phase images of triphasic abdominal CT is comparable to OGD for screening and grading OVs with a weighted k score of 0.88 (95% CI=0.82–0.94). In addition, the agreement between the endoscopists and radiologists was better for all grades of varices: no varices (57.1%), small (85.1%), medium (72.2%), and large (94.1%) OVs. A previous study of 137 cirrhotic patients showed that triphasic abdominal CT is as good as endoscopy for diagnosis and grading of OVs; however, the distribution of large varices in that study was only 9.7% in comparison, 32.7% in the present study.14 Moreover, only the radiologists were blinded in that study, whereas, both the operators in the present study were blinded for the reports, providing a complete independent grading assessment.
In a study of 109 cirrhotic patients with triphasic abdominal CT-guided detection of OVs, the interobserver agreement between the radiologists was k=0.64 indicating substantial agreement.15 Whereas it was 0.89 in the present study equating to near-perfect agreement.
The sensitivity of CT for OV grading in the present study was between 66.6 to 94.1% from no to large varices, which is much better than previously quoted studies. The accuracy of CT in detecting OVs in the present study improved with the severity of varices (AUROC 0.775 for no varices to 0.913 for large varices). A study from 2007 on 67 cirrhotic patients showed a sensitivity of 53–90% for all varices16; however, the quality of CT imaging has considerably improved over the last decade. With the currently available 128-section systems and better quality images, the sensitivity is much higher as shown in the present study, once again indicating that CT is probably a better alternative for screening OVs. A large meta-analysis of 11 studies with 807 patients showed a pooled sensitivity and specificity for identifying OVs as 89% and 72.3%, respectively, with an AUROC of 0.86 for CT for grading OVs.17
Non-invasive markers of fibrosis have been evaluated in portal hypertension. The present study compared APRI, FIB-4, and PS ratio with OV grading and subsequently correlated with CT grading of varices. The present study showed all three non-invasive markers correlated well with no difference between the two techniques, strengthening the present finding that CT grading of OVs is comparable to endoscopic grading. A study by Civan et al., showed that the APRI score was a better predictor of variceal bleed (AUROC 0.89).18
In a study of 139 cirrhotic patients using APRI, FIB-4, and PS ratio, FIB-4 strongly correlated with OVs with a sensitivity of 72% and an AUC 0.68.19 Similarly, analysis of 153 patients with cirrhosis, the AUROC values of APRI and FIB-4 for the prediction of OVs were 0.681 and 0.642 indicative of good predictors of disease progression.20
In the largest dataset analysis of 620 cirrhotic patients by Sebastiani et al., APRI and FIB-4 were identified as reliable non-invasive markers of OVs with AUC and sensitivity values of 0.63, 54% and 0.64, 70%, respectively.21
The outcome of the present study is further supported by a 2017 Cochrane database review that recommends a PS ratio <909 n/mm3 for the detection of OVs with a sensitivity of 85% indicating the utility of these non-invasive markers in portal hypertension.22 Similar studies showed AUC, sensitivity, and specificity of 0.9, 88.7%, and 81.4%, respectively, for PS ratio correlating with portal hypertension.23 Likewise, a meta-analysis showed APRI and FIB-4 correlated “moderately” with endoscopic large varices.19
In the absence of routine endoscopy during the COVID-19 pandemic, these non-invasive markers may have an indirect role in the diagnosis of portal hypertension. CT-guided varices diagnosis and grading, however, has a direct correlation with OGD as shown in the present study.
A 2014 budget impact analysis by Lotfipour et al., showed that abdominal CT was less expensive and sensitive tool in the detection of OVs.24 The cost of an upper GI endoscopy may vary between US$100–2,750 and the cost of a triphasic abdominal CT can be between US$135–1,500 depending on the country; however, given the current COVID-19 pandemic additional personal protective equipment for patients and the endoscopy staff members should be added to the endoscopy cost. Although triphasic abdominal CT is useful in patients with liver cirrhosis, it should be considered only after weighing its benefits and risks such as radiation exposure, cost and the possibility of contrast allergy; however, it has the added benefit of investigating other indications in cirrhotic patients with hepatic decompensation, to assess vascular anatomy, or for HCC, where it can provide additional information about OVs.
Limitations of the present study include the small number of patients, that it is a single-centre study, and the inability of CT in detecting OVs with high-risk signs of bleeding such as red spots, haemorrhagic areas, and varices in collapsed oesophagus.
In conclusion, venous phase images of triphasic contrast-enhanced abdominal CT is probably an optimal non-invasive tool for diagnosing and grading of OVs in situations where routine upper GI endoscopy is not recommended, such as during the COVID-19 pandemic. The utility of triple phase-contrast CT-guided oesophageal variceal grading is comparable to the reference standard upper GI endoscopy.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ng K., Poon B.H., Kiat Puar T.H. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020;172(11):766–767. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu P.W.Y., Ng S.C., Inoue H. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69(6):991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Balas M., Al-Balas H.I., Al-Balas H. Surgery during the COVID-19 pandemic: a comprehensive overview and perioperative care. Am J Surg. 2020;219(6):903–906. doi: 10.1016/j.amjsurg.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turon F., Casu S., Hernández-Gea V. Variceal and other portal hypertension related bleeding. Best Pract Res Clin Gastroenterol. 2013;27(5):649–664. doi: 10.1016/j.bpg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Merli M., Lucidi C., Giannelli V. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8(11):979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Groszmann R.J., Garcia-Tsao G., Bosch J. Beta-blockers to prevent gastr oesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 7.D'Amico G., Pagliaro L., Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach [published correction appears in Semin Liver Dis 2000; 20(3):399] Semin Liver Dis. 1999;19(4):475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 8.de Franchis R., Baveno VI Faculty Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173(5):362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jothimani D., Venugopal R., Abedin M.F. COVID-19 and the liver. J Hepatol. 2020;73(5):1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro Filho E.C., Castro R., Fernandes F.F. Gastrointestinal endoscopy during the COVID-19 pandemic: an updated review of guidelines and statements from international and national societies. Gastrointest Endosc. 2020;92(2):440–445. doi: 10.1016/j.gie.2020.03.3854. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szakács Z., Erőss B., Soós A. Baveno criteria safely identify patients with compensated advanced chronic liver disease who can avoid variceal screening endoscopy: a diagnostic test accuracy meta-analysis. Front Physiol. 2019;10:1028. doi: 10.3389/fphys.2019.01028. Published 2019 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repici A., Pace F., Gabbiadini R. Endoscopy units and the coronavirus disease 2019 outbreak: a multicenter experience from Italy. Gastroenterology. 2020;159(1):363–366. doi: 10.1053/j.gastro.2020.04.003. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H., Qi X., Peng Y. Diagnostic accuracy of APRI, AAR, FIB-4, FI, and King scores for diagnosis of oesophageal varices in liver cirrhosis: a retrospective study. Med Sci Monit. 2015;21:3961–3977. doi: 10.12659/msm.895005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu N.C., Margolis D., Hsu M. Detection and grading of OVs on liver CT: comparison of standard and thin-section multiplanar reconstructions in diagnostic accuracy. AJR Am J Roentgenol. 2011;197(3):643–649. doi: 10.2214/AJR.10.5458. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y.J., Raman S.S., Yu N.C. Oesophageal varices in cirrhotic patients: evaluation with liver CT. AJR Am J Roentgenol. 2007;188(1):139–144. doi: 10.2214/AJR.05.1737. [DOI] [PubMed] [Google Scholar]
- 17.Tseng Y.J., Zeng X.Q., Chen J. Computed tomography in evaluating gastr oesophageal varices in patients with portal hypertension: a meta-analysis. Dig Liver Dis. 2016;48(7):695–702. doi: 10.1016/j.dld.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Civan J.M., Lindenmeyer C.C., Whitsett M. A clinical decision rule based on the AST-to-platelet ratio index improves adherence to published guidelines on the management of acute variceal bleeding. J Clin Gastroenterol. 2015;49(7):599–606. doi: 10.1097/MCG.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 19.Deng H., Qi X., Guo X. Diagnostic accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex scores in predicting the presence of oesophageal varices in liver cirrhosis: a systemic review and meta-analysis. Medicine(Baltimore) 2015;94(42) doi: 10.1097/MD.0000000000001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F., Liu T., Gao P. Predictive value of a noninvasive serological hepatic fibrosis scoring system in cirrhosis combined with oesophageal varices. Can J Gastroenterol Hepatol. 2018;2018:7671508. doi: 10.1155/2018/7671508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastiani G., Tempesta D., Fattovich G. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: results of a multicenter, large-scale study. J Hepatol. 2010;53(4):630–638. doi: 10.1016/j.jhep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Colli A., Gana J.C., Yap J. Platelet count, spleen length, and platelet count-to-spleen length ratio for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev. 2017;4(4):CD008759. doi: 10.1002/14651858.CD008759.pub2. Published 2017 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrera F., Riquelme A., Soza A. Platelet count/spleen diameter ratio for non-invasive prediction of high risk oesophageal varices in cirrhotic patients. Ann Hepatol. 2009;8(4):325–330. [PubMed] [Google Scholar]
- 24.Lotfipour A.K., Douek M., Shimoga S.V. The cost of screening oesophageal varices: traditional endoscopy versus computed tomography. J Comput Assist Tomogr. 2014;38(6):963–967. doi: 10.1097/RCT.0000000000000147. [DOI] [PubMed] [Google Scholar]