Abstract
Pregnant women and neonates are often categorised as being at high risk during the coronavirus disease-2019 (COVID-19) pandemic. Numerous studies have demonstrated that the characteristics of COVID-19 disease in pregnant women and non-pregnant women are very similar. However, pregnant women with COVID-19 in the third trimester are more likely than their non-pregnant counterparts to require intensive care, though this may reflect a lower threshold for intervention in pregnant women rather than more serious disease. Compared with pregnant women without COVID-19, pregnant women with symptomatic COVID-19 requiring admission to hospital have worse maternal outcomes, including death, although the absolute risk remains very low. Outcomes of neonates born to women positive for COVID-19 are generally very good, though iatrogenic preterm birth is more common. Findings from these studies highlight the need for further monitoring of the outcomes of pregnant and post-partum women according to trimester during this pandemic.
Keywords: COVID-19, Maternal outcomes, Neonatal outcomes, Mortality, ICU admission
Introduction
The global number of cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) rose exponentially in 2020. As of 6th March 2021, there have been over 116 million cases and over 2.5 million deaths caused by coronavirus disease-2019 (COVID-19) [1]. The United Kingdom (UK) has faced a total of over 400,000 hospitalisations and over 122,000 deaths since March 2020 due to this disease [2]. It has become clear that this virus poses a particular threat to vulnerable individuals, including the elderly, immunosuppressed, those with certain pre-existing morbidities, and those from a Black Asian or Minority Ethnic (BAME) background; pregnant women may also be at increased risk, particularly in the third trimester [3]. In general, pregnant women are particularly susceptible to respiratory pathogens due to immunological and physical changes, which include changes to T lymphocyte immunity, a decrease in lung capacity and a decrease in functional residual capacity [4]. Initially, pregnant women were also considered a high-risk group due to concerns about the potential effect of COVID-19 on their babies, either in utero or in the neonatal period [5]. As new evidence accumulated exponentially, a clearer picture of how COVID-19 impacts pregnancy outcomes has crystallised. This review will explore the effect of COVID-19 on pregnant women and their babies.
Effect of COVID-19 in pregnant women
When assessing the effects of COVID-19 in pregnancy, it is important to distinguish studies comparing pregnant women with COVID-19 and non-pregnant women with COVID-19, from those studies comparing pregnant women with and without COVID-19. The former address the question as to whether pregnancy increases the risk of adverse outcomes in women who have the disease. The latter addresses the question as to whether contracting the disease during pregnancy increases the risk of adverse outcomes.
Early reports from China, the epicentre of the pandemic, initially suggested that pregnant women were not at an increased risk of COVID-19-related complications as compared to the general population [6]. Further case reports from countries such as the United States (US) [7] and Iran [8] produced contradictory evidence, with some reports that described women who required invasive mechanical ventilation and at an increased risk of death. Since then, further large-scale studies have added more robust evidence to these preliminary findings, though definitive answers to many of these questions are still awaited.
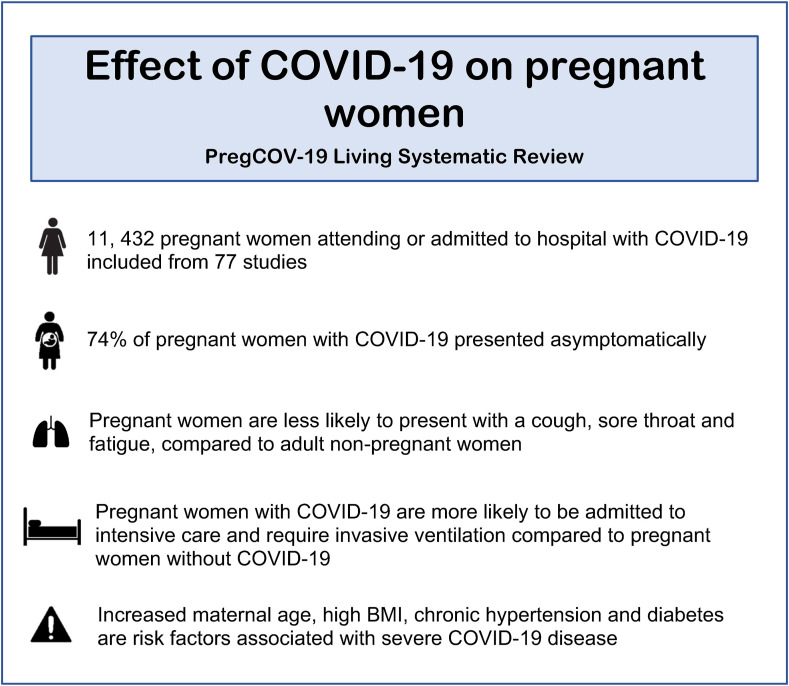
A prospective study from the UK found that the clinical symptoms of COVID-19 in pregnant women were similar to the cases reported from China and the US [9]. Pregnant women reported symptoms of fever, cough and breathlessness [10]. A recent systematic review reported that the most common symptoms were fever and cough (40% and 39%, respectively) [11]. This review of 11,432 women noted that pregnant and recently pregnant women were more likely to remain asymptomatic when compared with non-pregnant individuals [11]. Additionally, compared to non-pregnant women, pregnant women are less likely to report symptoms of fever and myalgia [11]. Similarly, another systematic review that investigated maternal outcomes discovered that pregnant women are less likely to present with cough, sore throat, fatigue, headaches and diarrhoea as compared to adult non-pregnant women [12]. These consistent observations suggest that the presentation of COVID-19 is comparable among pregnant and non-pregnant women, but asymptomatic disease may even be more common in pregnancy.
Pneumonia is one of the most common sequelae of COVID-19 in pregnant women [13]. Its presentation in pregnancy is similar to that seen in the non-pregnant adult population [14]. A meta-analysis concluded that most women had mild to moderate COVID-19 pneumonia [14]; nevertheless, severe pneumonia is one of the leading causes of hospital admission in pregnant women [15]. An early case–control study from China suggested that chest CT evidence of pneumonia was common (94%) in pregnant women with confirmed COVID-19, even though most were asymptomatic of pneumonia upon admission to hospital [16]. However, it seems unusual for such patients to progress to acute respiratory distress syndrome [17]. Another study of the presentation of pneumonia found that, in those with confirmed COVID-19, pleural effusions were seen more often in pregnant than in non-pregnant women [18].
Although many studies have confirmed that the majority of pregnant women infected with SARS-CoV-2 are asymptomatic, COVID-19 symptoms in pregnancy can range from mild to severe and critical disease, which cause acute respiratory distress syndrome and other complications such as pulmonary embolus and acute coronary syndrome. Severe illness due to COVID-19 has been reflected by the increased hospitalisation of pregnant women. In June 2020, the Centers for Disease Control and Prevention (CDC) reported that in those with COVID-19, 31.5% of pregnant women required hospitalisation when compared with 5.8% of non-pregnant women [19], though it is possible that this reflects greater caution to be taken in the management of pregnant women rather than more serious disease. The PregCOV-19 Living Systematic Review Consortium reported that pregnant women with COVID-19 are more likely to be admitted to intensive care (ICU) (OR 1.62, 95% CI 1.33–1.96) and require invasive ventilation (OR 1.88, 95% CI 1.36–2.60) as compared to non-pregnant women with COVID-19 [11]. In addition, a US study published in January 2021 that compared 22 pregnant women with symptomatic COVID-19 to 240 non-pregnant controls found that pregnant women were more likely to have severe COVID-19 [20].
The question of whether pregnant women who contract COVID-19 are at an increased risk of death as compared to their non-pregnant peers remains controversial. The US CDC reported that the risk of death is 70% higher in pregnant women than in non-pregnant women [19]. However, this study has its limitations; for example, pregnancy status was not reported for around half of the women included. ICU admission and mortality rates should be interpreted with caution due to the lower threshold for ICU admission in pregnant women than in non-pregnant women [[21], [22], [23]]. The absolute risk of maternal death remains low; for example, in the UK the risk of death associated with COVID-19 is 2.2 per 100,000 maternities.
Various studies have identified risk factors related to the prevalence of COVID-19 and severe disease in pregnant women. A multicentre retrospective cohort study found that Hispanic women are over-represented in pregnant women diagnosed with COVID-19 [24]. Another study reported that pregnant women with COVID-19 were more likely to be Black or Hispanic [22]. A US study confirmed that women of BAME background are more likely to suffer pregnancy complications from COVID-19 and require general anaesthesia [23]. These studies also identified a higher prevalence of co-morbidities in pregnant women with COVID-19 than in pregnant women without COVID-19. Possible explanations for the greater severity of COVID-19 and its pregnancy complications in BAME women have been proposed. Vitamin D deficiency, more commonly observed among the BAME population, has been associated with poor COVID-19 outcomes [25]; thus, the UK recommends vitamin D supplementation to all pregnant women [26]. The incidence of high BMI and pre-existing co-morbidities such as diabetes and chronic hypertension is also higher in certain BAME communities; these are factors known to increase the risk of severe COVID-19 [11,22,27]. An association with socio-economic status may also play a role.
Although a single-centre study from Brazil identified higher gestational age as a protective factor against severe or critical COVID-19 [28], such small studies are clearly contradicted by the large body of evidence outlined above suggesting that the third trimester is the time of greatest risk [29]. In general, the factors that increase the risk of severe COVID-19 in pregnant women mirror those that cause severe disease in the general population [30]. The association between pregnant women from BAME backgrounds and severe COVID-19 echoes multiple pre-pandemic reports that women from a BAME background have higher maternal morbidity and mortality rates than white women [31].
Effect of COVID-19 on pregnancy outcomes
Data on the complications of pregnancy associated with COVID-19 have also been accumulating (Fig. 1 ). Small studies suggest that pre-eclampsia is associated with severe COVID-19 [32]. However, further research is required to differentiate the presentation of pre-eclampsia from worsening COVID-19 [32]. The association of COVID-19 with haemostatic and thromboembolic complications is well-recognised in the general population. Pregnancy is a prothrombotic state; it is likely that COVID-19 exacerbates the already increased risk of thromboembolic complications in pregnancy [33]. A recent systematic review, including 1630 pregnant women with confirmed COVID-19 reported that 15 women were diagnosed with coagulopathy, thromboembolic disease, deep vein thrombosis or disseminated intravascular coagulopathy, suggesting that COVID-19 increases the risk of these pathologies [33]. Similarly, another study reported that the rates of venous thromboembolism and myocardial infarction were higher in pregnant women with COVID-19 than pregnant women without COVID-19 [22]. This may explain the possible increased maternal mortality rate associated with COVID-19 and highlights the importance of early thromboprophylaxis. However, a study that compares haemorrhage-related outcomes in pregnant women with and without COVID-19 found that blood loss and rates of obstetric haemorrhage were not higher in pregnant women with COVID-19 [23].
Fig. 1.
A summary of the key maternal outcomes from the PregCOV-19 Living systematic review.
Numerous case reports, cohort studies and more recently, systematic reviews have investigated the effect of COVID-19 on pregnancy outcomes, which includes caesarean section, miscarriage, preterm birth, stillbirth and fetal anomaly.
Maternal COVID-19 is associated with an increased rate of caesarean delivery. A study in Wuhan, China, which compares pregnant women with confirmed COVID-19 and pregnant women without COVID-19 reported an increased risk of caesarean section in those with COVID-19 (OR 3.34 and 95% CI 1.60–7.00) [34]. Indications for caesarean section included worsening COVID-19 symptoms such as maternal breathlessness [34]. In contrast, an early retrospective comparison of delivery methods found no significant difference between pregnant women with and without COVID-19 [35]. As a whole, however, studies indicate that caesarean section rates are higher in women with COVID-19 - a large-scale systematic review found an OR of 3 (95% CI: 2–5) [12]. In that review, the indication for caesarean section in 55.9% of cases was COVID-19 pneumonia [36]. The increased rate of caesarean section may be explained by the direct effect of COVID-19 on maternal health but also by an increased incidence of pathology indirectly caused by COVID-19. For example, a study from China suggested that excess gestational weight gain associated with reduced physical activity during the pandemic has led to an increase in caesarean sections [37]. There may be an increased rate of fetal distress during labour in women with COVID-19. A prospective study recorded that COVID-19 was not associated with preterm premature rupture of the membranes (aRR: 0.19, 95% CI: 0.02, 2.20 and p = 0.186), a possible indication for caesarean delivery [38]. Taking all of this evidence together, it seems likely that the primary cause of the higher caesarean section rate in those with COVID-19 is the worsening of maternal COVID-19 symptoms.
Although data suggest that caesarean section rates are higher in women with confirmed COVID-19, nevertheless these rates vary widely from country to country. A meta-analysis observed that the caesarean section rate, as well as adverse pregnancy outcomes, were considerably higher in Chinese studies (91% and 21%, respectively) than in European (38% and 19%) and American studies (40% and 15%) [39]. This may be related to the time period during which these studies were carried out, particularly if data collection occurred during the peaks of the pandemic or early in the pandemic when obstetricians on a steep learning curve might have had a lower threshold for delivering by caesarean section.
Caesarean delivery rates in symptomatic and asymptomatic women positive for SARS-CoV-2 were also explored in a prospective cohort study. Caesarean delivery rates were higher in symptomatic women with COVID-19 (46.7%), 45.5% in asymptomatic COVID-19 and 30.9% in pregnant women without COVID-19 [40]. The study notes that the increase in caesarean delivery rate may be associated with intrapartum fever related to COVID-19 being treated as chorioamnionitis, an indication for caesarean delivery in some cases [40].
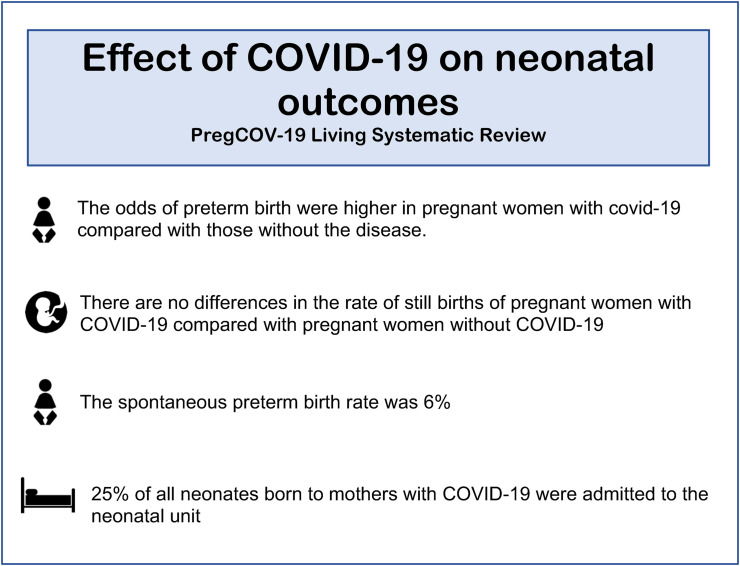
Maternal COVID-19 has been linked with iatrogenic preterm birth due to the maternal indications described above in relation to caesarean section and also for intrauterine fetal distress. The PregCOV-19 Living Systematic Review reported a risk of preterm birth in pregnant women of approximately 17%, the majority (94%) of which were iatrogenic. A UK Obstetric Surveillance System (UKOSS) study confirmed that preterm births were more common in women with COVID-19; 19% of symptomatic pregnant women and 9% of asymptomatic pregnant women with the disease gave birth before 37 weeks of gestation [41]. When comparing data with a historical cohort of pregnant women without COVID-19, women with symptomatic COVID-19 were more likely to give birth before 32 weeks of gestation (adjusted OR [aOR] 3.98 and 95% CI 1.48–10.70) and before 37 weeks of gestation (aOR 1.87 and 95% CI 1.23–2.85) [41]. Additionally, pregnant women from a BAME background are more likely to deliver preterm [23]. Although some smaller case studies described contradictory results suggesting no significant differences, larger cohort studies [34], systematic reviews and meta-analysis all reached the same conclusion [12], confirming the relationship between COVID-19 and iatrogenic preterm birth. Although the absolute rate of preterm birth varies between countries, with American studies that demonstrated lower rates (12%) than European (19%) and Chinese studies (17%), the effect of COVID-19 is consistent [39].
Unlike prematurity, there is currently no evidence to suggest that fetal growth restriction is a consequence of COVID-19. Two COVID-19 pregnancy registries reported that the number of neonates small for gestational age was similar to historical US and UK data [42]. This is despite fears early in the pandemic that COVID-19 might lead to fetal growth restriction, based largely on the knowledge that SARS, another coronavirus disease, can affect fetal growth [43]. However, one large systematic review of over 129,000 pregnant women found that low birth weight was more common in those with COVID-19 than in those without COVID-19 [12]. A study protocol that investigates pregnancy and neonatal outcomes estimates that in a sample size of 500, the proportion of small for gestational age neonates will be 15% (±2.9) as compared to the pre-COVID-19 incidence of 10% [44].
A comparison between stillbirth rates during the pandemic when compared with pre-pandemic suggests a possible increase. A small single-centre study from the UK reported an increase in the stillbirth rate during the pandemic (9.31 per 1000 births) as compared to before the pandemic (2.38 per 1000 births) [45]. None of the women suffering a stillbirth had COVID-19; therefore, these findings may reflect the changes made to the provision of maternity services rather than a direct effect of the virus. However, the Office for National Statistics provided reassurance when it described a decrease in the stillbirth rate in England and Wales from 4.0 stillbirths per 1000 (2019) to 3.9 per 1000 (January–September 2020) [46]. This is a continuation of the long-standing trend of decreasing rates of stillbirth in the UK [46].
During the initial wave of the COVID-19 pandemic, changes to maternity services were made with the aim to reduce the risk of infection among both pregnant women and healthcare staff [47]. The consequences of these changes are still being investigated. Surveys in the UK have shown that in April 2020, both antenatal and post-natal consultations were carried out online, possibly reducing the chance of detecting fetal abnormalities [47]. A study in China reported that COVID-19 has made women reluctant to attend hospital appointments when indicated. There was a significantly higher percentage of ‘never attending’ prenatal appointments reported by women living in areas with more confirmed cases of COVID-19 (8.9%) than by women living in areas with lower confirmed cases (2.3%) [37]. There have also been changes in the procedures to screen for fetal growth restriction and gestational diabetes [47,48]. In the UK, women have expressed anxiety about the changes to antenatal and intrapartum services [49]. These changes were thought to be barriers to diagnosing maternal or fetal abnormalities, possibly contributing to a rise in adverse outcomes. In contrast, a retrospective review of statistics from a large maternity unit in Dublin, Ireland, reported that service modification during the pandemic did not negatively impact maternal or neonatal outcomes, including stillbirth [50].
The recent PregCOV-19 Living Systematic Review showed no increase in stillbirth among women with COVID-19. Another systematic review reported the rates of stillbirth as less than 2.5% [51]. Comparisons in these outcomes have also been made between pregnant women with and without confirmed COVID-19. A multicentre study reported no statistical difference in stillbirth between the two groups [24]. A similar study in the US made similar observations and found no stillbirths in the population of pregnant women with confirmed COVID-19. These findings suggest that COVID-19 itself does not increase the risk of stillbirth.
Effect of COVID-19 on neonatal outcomes
A summary of the key neonatal outcomes is provided in Fig. 2 . Although women with COVID-19 were more likely to require intensive care and mechanical ventilation, neonates born to mothers with COVID-19 show milder manifestations of the disease [52]. A large study in India reported that 7 out of 65 neonates tested positive for COVID-19, six of which were asymptomatic [53]. A large meta-analysis found that most neonates born to mothers with confirmed COVID-19 did not exhibit any clinical signs of SARS-CoV-2 infection [54]. A minority (42.3%) of neonates had dyspnoea and 19.1% had a fever [54]. Moreover, neonates are less likely to experience complications or spend time in intensive care. A UK prospective study found that, of the small number of neonates testing positive for SARS-CoV-2, 88% were discharged home [55]. When comparing neonatal outcomes in pregnant women with and without COVID-19, there was no significant difference in the risk of neonatal respiratory morbidity (21.8% vs 19.6% and P = 0.692) [56]. Birth outcomes such as neonatal asphyxia were investigated; no statistical difference was found between pregnant women with and without confirmed COVID-19 [34].
Fig. 2.
A summary of the key foetal and neonatal outcomes from the PregCOV-19 Living systematic review.
While a rise in cases of prematurity and low birth weight due to COVID-19 have been reported, as described above, a study collecting data from Japanese acute care hospitals found that there has been a decline in the number of neonatal intensive care unit admissions and neonatal resuscitations during the pandemic [57]. The authors hypothesise that travel bans and a greater emphasis on infection prevention and control measures may have produced a secondary positive effect on neonatal outcomes during COVID-19 [57]. In the UK, a UKOSS study reported that 19% of neonates born to mothers with confirmed COVID-19 were admitted to the neonatal unit. However, the indications for admission were not specified so many of these admissions may have been unrelated to COVID-19 [25]. A systematic review found that neonates born to mothers who were confirmed COVID-19 positive were at an increased risk of admission as compared to mothers testing negative [11]. However, without the ascertainment of the indications for admission, it is difficult to decipher the true impact of COVID-19 on neonatal admissions. In summary, it seems that the risk of admission to the neonatal unit due to COVID-19 is low in babies born to mothers with the illness, and the overwhelming majority of those few affected have a very mild illness.
Another obstetric outcome addressed in the literature is fetal distress. A meta-analysis that investigated pregnancy outcomes found that 5.3% (15/238) of neonates were delivered because of fetal distress [58]. When neonatal outcomes were compared between pregnant women with and without confirmed COVID-19, no difference in the proportion with APGAR scores below 7 at 5 min of age was found [24], which suggests that COVID-19 does not increase respiratory distress in neonates.
Neonatal death rates have also remained relatively low during the pandemic. Of 2837 babies in the PregCOV-19 Living Systematic Review, there were six neonatal deaths, which suggest a very low risk of neonatal death in those born to women with COVID-19. Another systemic review reported the neonatal mortality rate as less than 3%, with no deaths associated with neonatal SARS-CoV-2 infection [51]. A larger meta-analysis found that the rate of neonatal death was 0.6% [58].
In spite of over 116 million COVID-19 cases worldwide, there has been no reported increase in the incidence of congenital anomalies. With growing evidence that vertical transmission of SARS-CoV-2 is rare, the likelihood that congenital anomalies can be caused by COVID-19 is low [59]. Small case studies have reported anomalies such as congenital pulmonary airway malformation, cleft palate and characteristics of Down Syndrome associated with maternal COVID-19 [60]. Collectively, however, these studies do not suggest an increased risk of congenital defects in the neonates of mothers infected with this virus [61].
Although no increase in the rate of congenital anomalies caused by COVID-19 has been observed, researchers are already investigating the pathogenesis of SARS-CoV-2 to explore whether there is any mechanism by which it may potentially cause congenital birth defects. Thus far, the published literature indicates that SARS-CoV-2 can cross the blood-brain barrier, raising the possibility that it could contribute to the pathogenesis of neural tube defects [60]. The medications used to treat COVID-19 are very unlikely to increase the risk of birth defects or preterm birth. Moreover, a study that investigated the impact of paternal intake of COVID-19 medications, such as prednisolone and hydroxychloroquine, on the risk of birth defects found no association [62].
The role of COVID-19 on rates of miscarriage is difficult to assess because of insufficient evidence in large cohort studies and systematic reviews. Additionally, many studies have not reported their outcomes by trimester, making findings difficult to evaluate. In smaller case reports where miscarriage in women with COVID-19 was recorded, all miscarriages occurred in the second or third trimester [51]. In a retrospective study in France, approximately 2% of women experienced a miscarriage [63]. Although other types of coronavirus, such as Middle East Respiratory Syndrome (MERS) and SARS, have been linked with an increased risk of miscarriage, COVID-19 has not [64].
A recent systematic review of all coronavirus-related illnesses (including Middle East Respiratory Syndrome and SARS) found that the risk of miscarriage and other perinatal outcomes was higher than the risk in non-infected pregnant women [65]. However, the study included very few studies related to SARS-CoV-2. Therefore, further research into the effect of COVID-19 on miscarriage specifically is needed. Although there is insufficient evidence available to comment clearly on the risk of miscarriage, risk factors related to miscarriage have been discussed in the literature. A study that investigated the effect of the stressful COVID-19 pandemic environment on early pregnancy concluded that in asymptomatic pregnant women, the COVID-19 environment did not seem to affect first trimester miscarriage rates [66].
While the stressful pandemic environment has not affected the rates of spontaneous miscarriage, data from the UK Office for National Statistics shows an increase in the number of terminations of pregnancy carried out during the pandemic. In April 2020, the height of the first peak of the pandemic in the UK, there were over 4500 more terminations than in April 2019 [67]. Self-isolation, along with stress and fear of this unknown but significant disease, may have caused women to experience economic stress and health concerns related to the pandemic [68].
A large-scale longitudinal survey carried out in Sweden revealed that, at the beginning of the pandemic, pregnant women experienced a considerable increase in worries regarding their own health and that of their child. These worries persisted at higher levels than usual throughout the pandemic [69]. Such feelings may have contributed to the increase in terminations seen during the COVID-19 pandemic.
Postpartum outcomes
It is known that COVID-19 is a major contributor to stress in pregnant women; this can hinder the process of postpartum adjustment [70]. A higher acute stress response associated with COVID-19 was found to be associated with less bonding with the infant as well as breastfeeding issues [70]. This highlights the importance to provide an increased level of support to pregnant women during the pandemic.
Summary
Current research indicates that the clinical presentation of COVID-19 in pregnant women resembles that of non-pregnant women, which generally includes a mild cough, breathlessness and fever. Pregnant women are more likely to remain asymptomatic than non-pregnant women and yet are more likely to be admitted to ICU. However, this may be due to a lower threshold for ICU admission in unwell pregnant women with COVID-19. Risk factors for severe disease mirror those in the general population and include diabetes, chronic hypertension, obesity and BAME background. Caesarean section rates among pregnant women with COVID-19 are higher than in those without COVID-19. Studies also demonstrate a higher frequency of preterm births among women with COVID-19; the majority are iatrogenic, most likely related to the deterioration in maternal disease.
The clinical application of these findings is already apparent. New recommendations regarding the care of pregnant and post-partum women were put in place, including multidisciplinary care and daily review, providing specific advice for pregnant women with COVID-19 regarding their risk of deterioration and addressing mental health concerns. Finally, pregnant women should not be excluded from clinical trials unless there is a clear contraindication. In return, this will increase the understanding of the effects of COVID-19 therapies on pregnant women and their outcomes.
Practice points.
-
1.
In general, COVID-19 follows a similar course in pregnant and non-pregnant women, though pregnant women may be more likely to require ICU admission in the third trimester.
-
2.
It is important to implement a multidisciplinary approach when caring for pregnant and post-partum women with COVID-19, and to provide specific advice to reduce the risk of complications and ICU admission.
-
3.
Pregnant women should be considered for inclusion in clinical drug trials unless there are clear contraindications to gain further understanding of the effects of these therapies on pregnancy outcomes.
Research agenda.
-
•
Further research on the impact of COVID-19 on post-partum outcomes.
-
•
Investigation of any prolonged signs and symptoms after SARS-CoV-2 infection (‘long COVID’) in pregnant and post-partum women.
-
•
Collection of data on pregnancy outcomes according to trimester, including miscarriage, stillbirth and fetal growth restriction. Outcomes should be compared between pregnant women with and without COVID-19.
-
•
Indications for ICU admission and mode of delivery must be recorded to ascertain the relationship of COVID-19 with these outcomes.
Declaration of competing interest
The authors have no conflicts of interest to declare.
References
- 1.WHO coronavirus disease (COVID-19) dashboard n.d. https://covid19.who.int (accessed February 13, 2021).
- 2.Official UK Coronavirus Dashboard n.d. https://coronavirus.data.gov.uk/details/deaths (accessed February 13, 2021).
- 3.Wenling Y, Junchao Q, Xiao Z, Ouyang S. Pregnancy and COVID-19: management and challenges. Rev Inst Med Trop São Paulo n.d.;62. 10.1590/S1678-9946202062062. [DOI] [PMC free article] [PubMed]
- 4.Tang P., Wang J., Song Y. Characteristics and pregnancy outcomes of patients with severe pneumonia complicating pregnancy: a retrospective study of 12 cases and a literature review. BMC Pregnancy Childbirth. 2018;18:434. doi: 10.1186/s12884-018-2070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staying alert and safe – social distancing guidance for young people. GOVUK n.d. https://www.gov.uk/government/publications/stay-alert-and-safe-social-distancing-guidance-for-young-people/staying-alert-and-safe-social-distancing-guidance-for-young-people (accessed February 22, 2021).
- 6.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirshberg A., Kern-Goldberger A.R., Levine L.D., Pierce-Williams R., Short W.R., Parry S., et al. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am J Obstet Gynecol. 2020;223:286–290. doi: 10.1016/j.ajog.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favre G., Pomar L., Musso D., Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. 2020;395:e40. doi: 10.1016/S0140-6736(20)30311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coronavirus infection and pregnancy. Roy Coll Obstet Amp Gynaecol n.d. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/covid-19-virus-infection-and-pregnancy/(accessed February 27, 2021).
- 11.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jafari M, Pormohammad A, Neshin SAS, Ghorbani S, Bose D, Alimohammadi S, et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol n.d.;n/a:e2208. 10.1002/rmv.2208. [DOI] [PMC free article] [PubMed]
- 13.Yang H., Hu B., Zhan S., Yang L.-Y., Xiong G. Effects of severe acute respiratory syndrome coronavirus 2 infection on pregnant women and their infants. Arch Pathol Lab Med. 2020;144:1217–1222. doi: 10.5858/arpa.2020-0232-SA. [DOI] [PubMed] [Google Scholar]
- 14.Kasraeian M., Zare M., Vafaei H., Asadi N., Faraji A., Bazrafshan K., et al. COVID-19 pneumonia and pregnancy; a systematic review and meta-analysis. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2020:1–8. doi: 10.1080/14767058.2020.1763952. [DOI] [PubMed] [Google Scholar]
- 15.Khan S, Peng L, Siddique R, Nabi G, Nawsherwan, Xue M, et al. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol n.d.:1–3. 10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed]
- 16.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K., et al. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71:2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215:127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 18.yang H., Sun G., Tang F., Peng M., Gao Y., Peng J., et al. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. 2020;81:e40–e44. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambrano L.D. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oakes M.C., Kernberg A.S., Carter E.B., Foeller M.E., Palanisamy A., Raghuraman N., et al. Pregnancy as a risk factor for severe coronavirus disease 2019 using standardized clinical criteria. Am J Obstet Gynecol Mfm. 2021;3:100319. doi: 10.1016/j.ajogmf.2021.100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royal Colleges update national guidance on COVID-19 and pregnancy. Roy Coll Obstet Amp Gynaecol n.d. https://www.rcog.org.uk/en/news/royal-colleges-update-national-guidance-on-covid-19-and-pregnancy/(accessed February 27, 2021).
- 22.Jering K.S., Claggett B.L., Cunningham J.W., Rosenthal N., Vardeny O., Greene M.F., et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2020.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M.J., Schapero M., Iverson R., Yarrington C.D. Obstetric hemorrhage risk associated with novel COVID-19 diagnosis from a single-institution cohort in the United States. Am J Perinatol. 2020;37:1411–1416. doi: 10.1055/s-0040-1718403. [DOI] [PubMed] [Google Scholar]
- 24.Gold S., Saeed H., Mokhtari N., Chornock R.L., Fries M., Overcash R., et al. 564 Comparison of clinical outcomes in pregnant women with and without COVID-19 based on disease severity. Am J Obstet Gynecol. 2021;224:S357. doi: 10.1016/j.ajog.2020.12.585. [DOI] [Google Scholar]
- 25.Liu N., Sun J., Wang X., Zhang T., Zhao M., Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitamin D in Pregnancy (Scientific Impact Paper No.43). Roy Coll Obstet Amp Gynaecol n.d. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/sip43/(accessed February 23, 2021).
- 27.Khoury R., Bernstein P.S., Debolt C., Stone J., Sutton D.M., Simpson L.L., et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York city medical Centers. Obstet Gynecol. 2020;136:273–282. doi: 10.1097/AOG.0000000000004025. [DOI] [PubMed] [Google Scholar]
- 28.Tutiya C., Mello F., Chaccur G., Almeida C., Galvão E., Barbosa de Souza A.C., et al. Risk factors for severe and critical Covid-19 in pregnant women in a single center in Brazil. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2021:1–4. doi: 10.1080/14767058.2021.1880561. [DOI] [PubMed] [Google Scholar]
- 29.Coronavirus (COVID-19): advice for pregnant employees. GOVUK n.d. https://www.gov.uk/government/publications/coronavirus-covid-19-advice-for-pregnant-employees/coronavirus-covid-19-advice-for-pregnant-employees (accessed February 25, 2021).
- 30.Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. 2020;395:760–762. doi: 10.1016/S0140-6736(20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using open safely. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C., Garcia-Manau P., Serrano B., et al. Preeclampsia-like syndrome induced by severe COVID-19: a prospective observational study. Br J Obstet Gynaecol. 2020 doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Servante J., Swallow G., Thornton J.G., Myers B., Munireddy S., Malinowski A.K., et al. Haemostatic and thrombo-embolic complications in pregnant women with COVID-19: a systematic review and critical analysis. BMC Pregnancy Childbirth. 2021;21:108. doi: 10.1186/s12884-021-03568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R., Mei H., Zheng T., Fu Q., Zhang Y., Buka S., et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18:330. doi: 10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L., Jiang Y., Wei M., Cheng B.H., Zhou X.C., Li J., et al. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province] Zhonghua Fu Chan Ke Za Zhi. 2020;55:166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 36.Della Gatta A.N., Rizzo R., Pilu G., Simonazzi G. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020;223:36–41. doi: 10.1016/j.ajog.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J., Zhang Y., Ma Y., Ke Y., Huo S., He L., et al. The associated factors of cesarean section during COVID-19 pandemic: a cross-sectional study in nine cities of China. Environ Health Prev Med. 2020;25:60. doi: 10.1186/s12199-020-00899-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pirjani R., Hosseini R., Soori T., Rabiei M., Hosseini L., Abiri A., et al. Maternal and neonatal outcomes in COVID-19 infected pregnancies: a prospective cohort study. J Trav Med. 2020;27 doi: 10.1093/jtm/taaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubey P., Reddy S.Y., Manuel S., Dwivedi A.K. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490–501. doi: 10.1016/j.ejogrb.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prabhu M., Cagino K., Matthews K.C., Friedlander R.L., Glynn S.M., Kubiak J.M., et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG An Int J Obstet Gynaecol. 2020;127:1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) | MedRxiv n.d. https://www.medrxiv.org/content/10.1101/2021.01.04.21249195v1 (accessed February 23, 2021). [DOI] [PMC free article] [PubMed]
- 42.Mullins E., Hudak M.L., Banerjee J., Getzlaff T., Townson J., Barnette K., et al. Pregnancy and neonatal outcomes of COVID-19 – co-reporting of common outcomes from the PAN-COVID and AAP SONPM registry. MedRxiv. 2021 doi: 10.1101/2021.01.06.21249325. 2021.01.06.21249325. [DOI] [Google Scholar]
- 43.Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East Respiratory Syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis. 2016;16:105. doi: 10.1186/s12879-016-1437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee J., Mullins E., Townson J., Playle R., Shaw C., Kirby N., et al. Pregnancy and neonatal outcomes in COVID-19: study protocol for a global registry of women with suspected or confirmed SARS-CoV-2 infection in pregnancy and their neonates, understanding natural history to guide treatment and prevention. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalil A., von Dadelszen P., Draycott T., Ugwumadu A., O'Brien P., Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Homer C.S.E., Leisher S.H., Aggarwal N., Akuze J., Babona D., Blencowe H., et al. Counting stillbirths and COVID 19—there has never been a more urgent time. Lancet Global Health. 2021;9 doi: 10.1016/S2214-109X(20)30456-3. e10–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jardine J, Relph S, Magee LA, von Dadelszen P, Morris E, Ross-Davie M, et al. Maternity services in the UK during the coronavirus disease 2019 pandemic: a national survey of modifications to standard care. BJOG An Int J Obstet Gynaecol n.d.;n/a. 10.1111/1471-0528.16547. [DOI] [PubMed]
- 48.Rimmer M.P., Al Wattar B.H., UKARCOG Members Provision of obstetrics and gynaecology services during the COVID-19 pandemic: a survey of junior doctors in the UK National Health Service. BJOG An Int J Obstet Gynaecol. 2020;127:1123–1128. doi: 10.1111/1471-0528.16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karavadra B., Stockl A., Prosser-Snelling E., Simpson P., Morris E. Women's perceptions of COVID-19 and their healthcare experiences: a qualitative thematic analysis of a national survey of pregnant women in the United Kingdom. BMC Pregnancy Childbirth. 2020;20:600. doi: 10.1186/s12884-020-03283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonnell S., McNamee E., Lindow S.W., O'Connell M.P. The impact of the Covid-19 pandemic on maternity services: a review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;255:172–176. doi: 10.1016/j.ejogrb.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papapanou M., Papaioannou M., Petta A., Routsi E., Farmaki M., Vlahos N., et al. Maternal and neonatal characteristics and outcomes of COVID-19 in pregnancy: an overview of systematic reviews. Int J Environ Res Publ Health. 2021;18 doi: 10.3390/ijerph18020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trevisanuto D., Cavallin F., Cavicchiolo M.E., Borellini M., Calgaro S., Baraldi E. Coronavirus infection in neonates: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2020 doi: 10.1136/archdischild-2020-319837. [DOI] [PubMed] [Google Scholar]
- 53.Anand P., Yadav A., Debata P., Bachani S., Gupta N., Gera R. Clinical profile, viral load, management and outcome of neonates born to COVID 19 positive mothers: a tertiary care centre experience from India. Eur J Pediatr. 2020:1–13. doi: 10.1007/s00431-020-03800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neef V., Buxmann H., Rabenau H.F., Zacharowski K., Raimann F.J. Characterization of neonates born to mothers with SARS-CoV-2 infection: review and meta-analysis. Pediatr Neonatol. 2021;62:11–20. doi: 10.1016/j.pedneo.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gale C., Quigley M.A., Placzek A., Knight M., Ladhani S., Draper E.S., et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021;5:113–121. doi: 10.1016/S2352-4642(20)30342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Izewski J., Boudova S., Rouse C.E., Ibrahim S.A., Shanks A.L., Reinhardt J.C., et al. 786 Neonatal outcomes in pregnant women with diagnosis of COVID-19. Am J Obstet Gynecol. 2021;224:S489–S490. doi: 10.1016/j.ajog.2020.12.809. [DOI] [Google Scholar]
- 57.Maeda Y., Nakamura M., Ninomiya H., Ogawa K., Sago H., Miyawaki A. Trends in intensive neonatal care during the COVID-19 outbreak in Japan. Arch Dis Child Fetal Neonatal Ed. 2020 doi: 10.1136/archdischild-2020-320521. [DOI] [PubMed] [Google Scholar]
- 58.Khalil A., Kalafat E., Benlioglu C., O'Brien P., Morris E., Draycott T., et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. Clin Med. 2020;25:100446. doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker K.F., O'Donoghue K., Grace N., Dorling J., Comeau J.L., Li W., et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. Br J Obstet Gynaecol. 2020 doi: 10.1111/1471-0528.16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan MSI, Nabeka H, Akbar SMF, Al Mahtab M, Shimokawa T, Islam F, et al. Risk of congenital birth defects during COVID-19 pandemic: draw attention to the physicians and policymakers. J Global Health n.d.;10. 10.7189/jogh.10.020378. [DOI] [PMC free article] [PubMed]
- 61.Salem D., Katranji F., Bakdash T. COVID-19 infection in pregnant women: review of maternal and fetal outcomes. Int J Gynecol Obstet. 2021;152:291–298. doi: 10.1002/ijgo.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizzi S., Wensink M.J., Lindahl-Jacobsen R., Tian L., Lu Y., Eisenberg M.L. Risk of pre-term births and major birth defects resulting from paternal intake of COVID-19 medications prior to conception. BMC Res Notes. 2020;13 doi: 10.1186/s13104-020-05358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sentilhes L., De Marcillac F., Jouffrieau C., Kuhn P., Thuet V., Hansmann Y., et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.06.022. 914.e1-914.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C.-L., Liu Y.-Y., Wu C.-H., Wang C.-Y., Wang C.-H., Long C.-Y. Impact of COVID-19 on pregnancy. Int J Med Sci. 2021;18:763–767. doi: 10.7150/ijms.49923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis n.d. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7104131/(accessed February 25, 2021). [DOI] [PMC free article] [PubMed]
- 66.Rotshenker-Olshinka K., Volodarsky-Perel A., Steiner N., Rubenfeld E., Dahan M.H. COVID-19 pandemic effect on early pregnancy: are miscarriage rates altered, in asymptomatic women? Arch Gynecol Obstet. 2020 doi: 10.1007/s00404-020-05848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abortion statistics for England and Wales during the COVID-19 pandemic. GOVUK n.d. https://www.gov.uk/government/statistics/abortion-statistics-during-the-coronavirus-pandemic-january-to-june-2020/abortion-statistics-for-england-and-wales-during-the-covid-19-pandemic (accessed February 25, 2021).
- 68.Abortion clinics: pandemic boosts demand, heightens stress. AP NEWS; 2020. https://apnews.com/article/818509e4d83c0dae8541311c593a0a33 [Google Scholar]
- 69.Naurin E., Markstedt E., Stolle D., Enström D., Wallin A., Andreasson I., et al. Pregnant under the pressure of a pandemic: a large-scale longitudinal survey before and during the COVID-19 outbreak. Eur J Publ Health. 2021;31:7–13. doi: 10.1093/eurpub/ckaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayopoulos G.A., Ein-Dor T., Dishy G.A., Nandru R., Chan S.J., Hanley L.E., et al. COVID-19 is associated with traumatic childbirth and subsequent mother-infant bonding problems. J Affect Disord. 2021;282:122–125. doi: 10.1016/j.jad.2020.12.101. [DOI] [PMC free article] [PubMed] [Google Scholar]