Abstract
This work focusses on a novel technique of producing bioethanol from fermented pomegranate fruits waste by using Saccharomyces cerevisiae, commonly known as baker's yeast. Four different blends of bioethanol, namely PE10, PE15, PE20, and PE25 were experimented at various operating speeds. It was inferred that the addition of ethanol enhanced the consumption of fuel as well as braking capacity. However, thermal performance was observed to be declined. PE15 blend exhibited optimum brake thermal efficiency at full load condition when compared with unleaded fuel. Brake specific fuel consumption of PE15 was noticed to be lower at different operating speeds among all the blends. Oxides of nitrogen as well as carbon dioxide emissions were increased as the proportion of ethanol in pure fuel was increased. Hydrocarbon and carbon monoxide emissions were reduced, while increasing the ratio of ethanol relative to pure gasoline, except PE10 blend. The combustion characteristics were also studied. Lower value of coefficient of variation revealed stable combustion. This study conclude that PE15 can be used as an alternative fuel.
Keywords: Biofuel, Combustion performance, Emission characteristics, SI Engine, Pollution control
Biofuel, Combustion performance, Emission characteristics, SI Engine, Pollution control
1. Introduction
Energy crisis is one of the major concern prevailing in this world, particularly in developed countries. Increased population, industrialisation, improved transportation, continuous exploitation of fossil fuels in various sectors are the major cause of energy shortages. This implies a huge challenge for mankind to overcome them [1]. Steep rise in needs, fuel costs, depleting petrochemical resources and harmful emissions have necessitated the need for an alternative to the conventionally used fossil fuels [2, 3]. Many agro-based countries discard about 20–30% of the harvested fruits and vegetables due to inadequate cold storage facilities and poor storage facility while in transit. Since the population grows day by day, the need for food also increases. In the markets, huge quantities of fruits and vegetables waste are produced in day to day life, which are not useful to humankind. Utilising these wastages more efficiently at different stages from farm handling, storing, processing and delivery to consumption is the real challenge in front of the production engineers. The waste so created also poses a threat to global warming in the food processing industry. At the same time it will resolve the energy crisis as an inherent gift, renewable energy may be one of the solution to this problem. Biofuels has shown a popular replacement for fossil fuels due to their low emission contaminants, renewability and oxygenation. Alcohol as an alternative fuel appeal has grown in the 19th and early 20th century because of an industrial revolution. The 10% strategy of ethanol mixing in commercial vehicles has already been implemented in many countries and the potential goal is 20% mixing. The demand and availability of ethanol in the last couple of decades have nearly threefold [4]. Biomass produces ethanol, generated from alcoholic sugar fermentation. Sugar cane, corn, cassava, sugar grass, beet sugar, grape seed, wood, sunflower and soybean are widely used as biomass in the processing of ethanol [5]. Fermentation processes are used primarily for the processing and segregation of ethanol, but it requires further distillation for purification. Other distillation options are ultrasound irradiation, different ozone contaminants depletion and adsorption of contaminants by activated charcoal or zeolites [6]. Various ethanol fermentation technologies were tested by starch and bacterial sugar feedstock [7]. In comparison to bacteria of Saccharomyces Cerevisiae yeast, ethanol production and productivity in Zymomonas mobilis were found to be much higher but cannot replace Saccharomyces Cerevisiae because of its specific range of substrates. A thorough analysis of the scope and the possibilities to incorporate ethanol-fuel blends and the engine output for lower and higher formulations with different feedstocks was carried out. The fluctuation in thermal performance, braking fuel consumption and torque implied in braking differed depending on the amount of ethanol mixing as well as engine working conditions. Owing to the increased volume efficiency and ethanol ratios, CO and hydrocarbon particulates substantially decreased. CO2 exhaust were higher for ethanol, and nitrogen oxides emissions fluctuated based on engine working conditions [8, 9, 10]. Multicylinder 4-stroke, full-load, spark ignition engines with 10–30% blended ethanol with gasoline were found to be less exhaust emissions and increased hydrocarbons [11]. With ethanol mixing and a higher compression ratio, modest torque, engine power and reasonable improvements in fuel consumption were observed. HC and CO exhaust have dramatically decreased while emissions of NOx and CO2 have reduced significantly [12]. The addition of butanol and ethanol as a fuel mixer at different engine speeds, reduced load has increased engine efficiency, and emissions were detected. Carbon monoxide, hydrocarbons and gasoline consumption plummeted while the power from the engine improved to 11.1% for mixed fuels [13]. Few researchers reported enhanced braking strength, thermal braking efficacy, special fuel consumption and reduced hydrocarbons as well as carbon emissions with ethanol addition. However, with ethanol mixing, the NOx contaminants were increased. With incorporating ethanol, the added advantage of the hybrid fuel-ethanol and the rise in octane numbers is accomplished with 20% ethanol added to pure petrol [14, 15]. There has been dramatic rise in torque, gasoline consumption, CO2 emissions, decreased carbon emissions, and hydrocarbon emissions. NOx emissions prevailed over ethanol content under the working conditions of the engine [16]. Power, torque, thermal and volumetric performance have been enhanced by potato peels extracted ethanol –petroleum blends. The concentrations of hydrocarbons, brake petrol, carbon monoxide levels have been lowered and the accumulation of CO2 and NOx has increased. The findings were verified by the development of the artificial neural network pattern, which confirmed a detrimental influence on engine efficiency and exhaust emissions at higher ethanol mixing levels, on gasoline mixtures with lower mixtures (3–10% ethanol and methanol by volume) [17, 18]. Addition of Pistacia khinjuk methyl ester along with oxidants resulted in higher engine performance with better exhaust excluding nitrogen oxides for diesel engine. Similar results were obtained by applying various techniques which include preheating of fuel, difference in the injection timing, varying compression ratio, thermal resistive coating and YSZ layering [19, 20, 21, 22, 23]. When fuelled with Mesua ferrea Linn oil blends with gasoline, improved compression engine performance and emission was noticed [24]. The blending of butanol into petrol has improved performance, improved combustion efficiency and reduced contaminants. Compared to pure gasoline, the B30 blend has been reported to be best [25]. Table 1 outlines the blending effect of bioethanol on the spark-ignited engine output and exhaust emissions. It is primarily because of the abundance and cost of pomegranate fruits and their by-products for bioethanol production. India produces 28.65 tonnes of pomegranate fruits, of which about 30%–40% of its fruits are discarded for various reasons are stated in various literature. This organic waste and leftover of pomegranate fruits including peels, seeds and pulp can be used for the manufacture of low cost bioethanol [27, 28, 29, 30]. In this work, pomegranate waste collected from the local market was used as a feedstock to extract pomegranate ethanol, resulting in lower production costs of bioethanol compared with traditional fuel sources. The waste of pomegranates was fermented and steam distilled to produce ethanol. This study invites the researchers to explore the prospect of manufacturing pomegranate bioethanol on large scale for commercial use.
Table 1.
Impact of bioethanol blends on the engine performance and exhaust emissions.
| Fuel and alcohol | Blends | Test Engine | Performance |
Emission |
Outcome | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BTE | BSFC | BP | HC | CO | NOX | CO2 | |||||
| Ethanol + gasoline | E0, E10, E20,E30 | FORD VSG 413 4-cylinder 4-stroke SI |
Ethanol enrichment reduces exhaust emissions with a lower cylinder temperature than petrol, contributing to hydrocarbon emissions. | [11] | |||||||
| Ethanol + gasoline | E10, E20, E30 | Tech ED Equipment company, Single Cylinder, 4-stroke | -- | Moderate torque, engine power increases and fuel economy increases equally. In comparison, the exhausts of HC and carbon dioxide have been greatly decreased and levels of NOx and CO2 reduced. | [12] | ||||||
| butanol + ethanol + gasoline | E2, E5, E10,E15, E20 |
Hyundai G4eh, 4-cylinder, 4-stroke, water-cooled, port-fuel injection SI engines | -- | Carbon monoxide, hydrocarbons, gasoline consumption declined, and the engine power for fuel hybrids of ethanol and butanol increased by 11.1 percent at various speeds. | [13] | ||||||
| Diesel + ethanol + biodiesel | DBE5, DBE10, DBE15, DBE20 | 1 cylinder,4 stroke diesel engine | -- | -- | -- | -- | Braking capacity, basic fuel consumption and heat braking performance improved by ethanol enrichment in petrol. | [14] | |||
| Ethanol + gasoline | E0,E5, E10,E15 |
Maruti 800 water-cooled, 3- cylinder, spark ignition engine | -- | Better braking capacity, thermal braking, fuel consumption, lower hydrocarbons and carbon dioxide emissions with enrichment of ethanol. However, with ethanol mixing, NOx emission was increased. | [15] | ||||||
| Ethanol + gasoline | E0,E5,E10,E20, E30 | New Sentra GA16DE, multi-point injection gasoline engine | -- | -- | Significant gain in torque, fuel consumption, decreased CO2 and hydrocarbon emissions with NOx emissions prevailing over the ethanol content under engine operating conditions. | [16] | |||||
| Potato peel ethanol + gasoline | E5, E7.5, E10, E12.5, E15 | KIA 1.3 SOHC, 4 cylinder, and 4 stroke SI gasoline engine. |
-- | The ethanol-gasoline blend improved the torque, power and emissions. | [17] | ||||||
| Ethanol + Methanol + gasoline | EM3, EM7 EM10 | Spark-ignition engine with four-stroke and air cooled type | -- | -- | -- | The ethanol blending improved the brake power and lowered pollutant emissions compared to methanol as well as butanol blending. | [18] | ||||
| Pistacia khinjuk methyl ester + diesel | PB20, PB40, PB50, PB100 | Kirloskar/TV1 1 cylinder, 4 stroke water cooled, vertical, naturally aspirated DI diesel engine |
-- | -- | -- | The addition of Pyrogallol antioxidant as an alternative to traditional diesel fuel to the 20% ester offers better efficiency, combustion and emission features than the Geraniol antioxidant. | [19] | ||||
| orange oil methyl ester + diesel | OME5DEE, OME10DEE, diesel, OME5E, OME10E and OME | Kirloskar TV1, One cylinder,4- stroke, direct injection, diesel engine, water cooled | -- | Improved engine performance and emission compared to other fuel mixtures. | [20] | ||||||
| curry leaf oil+ diesel |
B25,B50,B75,B100 | Kirloskar/TV1 model, Single cylinder, four stroke, DI diesel engine | -- | -- | Improved performance and emission characteristics with B25 fuel blend. | [21] | |||||
| fish oil ethyl ester+ diesel |
FOEE25,FOEE50, FOEE75, FOEE | Kirloskar/TV1, Direct injection, water cooled, vertical, diesel, naturally aspired engine | -- | -- | Fish Oil ethyl ester exhibited improved engine performance results complying with the limits set up by CPCB, India | [22] | |||||
| lemon grass oil and diesel | -- | Kirloskar/TV1, Direct injection, water cooled, vertical, naturally aspirated diesel engine | -- | -- | Modified engine with coating showed reduced emissions. | [23] | |||||
| Diesel + Mesua ferrea linn oil | VO30, VO30E05,VO30E10 | Kirloskar/TV1, Four-stroke, single-cylinder, water cooled, DI, constant speed, naturally aspirated, CI engine | -- | The addition of ethanol increases both the thermal brake efficiency and the consumption of brake petrol. Additionally, CO and HC are rising while NOx emissions are dropping. | [24] | ||||||
| Butanol +gasoline | B10, B30, B60 | single-cylinder engine with cylinder geometry identical to the V8 engine assembled in a 2000 Ford Mustang Cobra. |
-- | -- | Use of Butanol as a fuel enhances combustion efficiency and reduces contaminants emissions. | [25] | |||||
| honge/jatropha oil methyl ester | JOME5,JOME10,JOME15 | Kirlosker TV1, Single Cylinder, 4 stroke | -- | -- | -- | Emission characteristics were improved except NOx emissions. | [26] | ||||
The literature study infers that much research has been conducted on spark-ignited engines fuelled by bioethanol blends derived from a variety of feedstocks for various analysis, emission and combustion. However, very little research work have been carried out with blends of pomegranate ethanol. The objective of this work is to extract bioethanol from pomegranate waste and to analyze the effects on the combustion, performance and exhaust characteristics of SI engine by its various blends.
2. Materials and methods
Ethanol was extracted from pomegranates fruits waste and used as biofuel. The raw juice was fermented using baker's yeast to produce ethanol. Various blends of ethanol and gasoline were prepared and experimented in the spark ignition engine. The chemical composition of ethanol was derived by gas chromatography technology.
2.1. Biological background
Punica Granatum is a genus of lythraceans with Punicoideae subfamily, which is commonly known as pomegranates. It is a fruit-bearing shrub that grows between 5 to 10 m (16–33 ft.) tall. It is abundantly grown in Asian countries, which include India, Afaganisthan, Pakistan, and Northern Himalayas up to China. It is also available in African and European countries. In India, it has large plantation in Maharashtra followed by Gujrat, Karnataka, Andhra Pradesh, Madhya Pradesh, Rajasthan and Himachal Pradesh. The varieties of grenade available in the market are Ganesh, Mrudula, Arakta and Bhagwa. Bhagwa variety was chosen for this study due to its high sugar content, which accelerates the fermentation.
2.2. Preparation of ethanol
The methods of ethanol production involve the distillation of steam; sugar and starch fermentation by yeasts and biomass waste using bacteria. Waste pomegranate fruit was used as a feedstock for bioethanol extraction for this study. Figure 1 shows the various steps involved in development of bioethanol.
Figure 1.
Steps involved in ethanol production from waste pomegranate fruits.
Pomegranate waste fruits were collected from the local market and the juice was extracted by a household mixer into the large container for further processing. The sugar content was calculated as 112.56 mg/l and pH value was 3.22. The juice was kept in a small container for fermentation. The use of Saccharomyces cerevisiae yeast yielded the fermentation of high efficiency. The starters were propagated and preserved in the fridge using MGYP (1% Malt-Glucose 1% -Yeast-extract 03%-Peptone 1%) broth. The fermenting agents and the juice were properly mixed and preserved for 72 h at a temperature of 37 °C. The stored fermented liquid was stirred properly every six hours of the day in order to boost the microbial function and maximize fermentation activity. Filter papers were used to filter the fermented pomegranate juice mix. The distillation technique for steam-water was employed to separate ethanol from the mixture. The mixture was heated in a container. The evaporation of ethanol begins at a temperature ranging from 75 to 85 °C. Ethanol has a lower boiling point than water so it evaporates first. The ethanol vapour is then cooled and condensed inside the condenser to form a pure liquid. With a rise in temperature, the water evaporates and combines into a different jar along with some portion of ethanol. A distillation of the remaining solvent containing water vapours and ethanol yielded anhydrous alcohol. When fermented for 72 h, the ethanol yield of one litre/10 kg pomegranate waste was achieved.
2.3. Chemical analysis pomegranate ethanol
Ethanol was collected in the sealed container and a gas chromatography technique was employed for the chemical analysis of the sample. Results of chromatography chemical analysis are shown in Table 2.
Table 2.
Pomegranate ethanol chemical composition.
| Test | Specifications | Result |
|---|---|---|
| Assay (by GC) (v/v) | Ethanol: 89.5–91.5% Methanol: 4.0–5.0% IPA:4.5–5.5% |
Ethanol: 90.54% Methanol: 4.54% Isopropyl Alcohol: 4.92% |
| Water, max | 0.2% | 0.14% |
| Residue after evaporation, max | 10 ppm | <5 ppm |
| Appearance | Clear | Pass |
| Specific Gravity | 0.7902–0.7912 @ 20 C | 0.7904 |
| Color (Pt–Co) | 10 max | <10 |
| Odor | Pass | Pass |
| Titrable Acid | 0.0003 meq/g | 0.0001 meq/g |
| Titrable Base | 0.0002 meq/g | <0.0001 meq/g |
| Fluorescent Background | Pass | Pass |
| Fluorescent Background | Pass | Pass |
2.4. Ethanol blends preparation
Four blends of fuel were prepared by blending ethanol with pure petrol at 10%, 15%, 20%, and 25% on a volumetric basis at a room temperature of 35 °C. Heating values of pure ethanol and its various blends were calculated using a bomb calorimeter. The research octane number of gasoline used for preparation of blends is 91. Table 3 offers heating values of pure and mix fuels.
Table 3.
The heating value of PE and its variations.
| Specification | Percentage of Ethanol | Percentage of Gasoline | Heating Value (kJ/kg) |
|---|---|---|---|
| PE00 | 0 | 100 | 44200 |
| PE10 | 10 | 90 | 42185 |
| PE15 | 15 | 85 | 41235 |
| PE20 | 20 | 80 | 4043 |
| PE25 | 25 | 75 | 39578 |
| PE100 | 100 | 0 | 29500 |
2.5. Experimental set-up
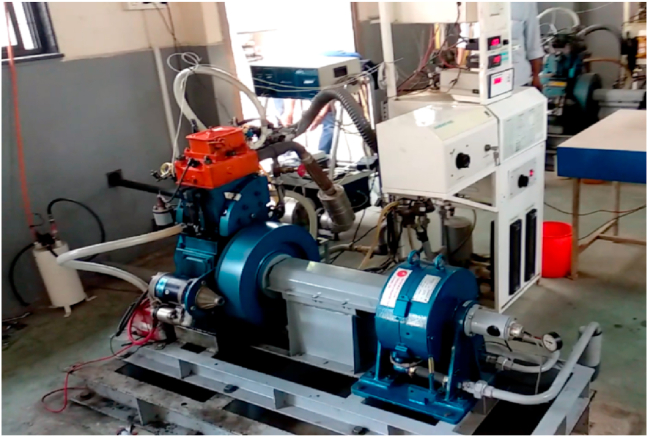
The specifications of the SI engine employed for this study are listed in Table 4. Figure 2 shows the experimental set up adopted for this study. It consists of a fuel gauge unit, air box, air handler, fuel gauge, a twin fuel tank, and a piezo power unit. The engine is coupled with high speed eddy current dynamometer. With the help of the strain gauge, the load on the engine was recorded and the rotary sensor mounted of the shaft recorded the engine speed. The rotameter measured water flow rate in calorimeter and coolers.
Table 4.
Test Engine specifications.
| Particulars | Specifications |
|---|---|
| Engine Make | Kirloskar |
| Type | Four stroke, single cylinder, Spark Ignition, Multi-fuel, VCR with open ECU Engine |
| Bore | 87.5mm |
| Stroke | 110 mm |
| Compression ratio | 10:1 |
| Engine Torque | 11.5 Nm |
| Inlet valve open | 4.5° before TDC |
| Inlet valve close | 35.5° after BDC |
| Exhaust valve open | 35.5° before BDC |
| Exhaust Valve Close | 4.5° after TDC |
| Fuel Injection Pressure | 210 bar |
| Ignition timing | 23° before TDC |
Figure 2.
The experimentation set up.
To analyse the emission and efficiency characteristics and compare them to pure gasoline, the bioethanol derived from pomegranate waste mixed with pure petrol was used. In order to avoid water reaction, the fuel mixture was prepared just before the start of experimentation to have a homogenous mixture. The engine was powered by pure petrol for obtaining baseline data generation in a steady state. Engine speed is set by 100 rotations, from 1300 to 1800 rpm. The fuel consumption and engine speed were recorded and thermal performance and fuel usage were monitored. The fuel injection timing was set and controlled by software provided by the supplier. The exhaust gas temperature, engine oil temperature and cooling water jacket temperature was recorded by thermocouples. In order to track exhaust emissions, exhaust gas analyser (Hg-540) was used. Emissions of carbon dioxide, carbon monoxide and oxygen particles were measured as fraction of volume, and the overall unburnt hydrocarbon and nitrogen oxide emissions were calculated by ppms per volume. Inside the exhaust vent, a probe was positioned to detect the exhaust particulates. The duct was sealed correctly to prevent any leakage prior to each test. The flow of cooling water was regulated over entire experimentation at a constant temperature.
All tests were carried out at full load. The prepared fuel blends were fed to the engine by the multifuel tank. Initial testing was performed at variable speed (1300–1800 rpm) with unleaded fuel at a fixed injection pressure and angle in order to produce reference results. The relative air-fuel ratio was measured. The tests for different ethanol blends were experimented. All tests were carried out in accordance with the SAE J1312 standards. All tests were carried out in a steady state condition and the results were analysed. For each blend three experimentations were done and the average reading was taken for the analysis. All the measurements have been compiled and analysed by a computerized data collection system.
2.6. Uncertainity estimation
The uncertainities of different parameters involved during the experimentation were estimated using the sequential perturbation method. Table 5 shows the projected uncertainities. The measured precision for a performance at a stage of ±4 percent was found due to uncertainty correlated with the measurement instruments and ±2.8 percent for a combustion analysis.
Table 5.
Estimated performance and combustion analysis uncertainties.
| Measured Parameter | Uncertainity (%) | Measured Parameter | Uncertainity (%) |
|---|---|---|---|
| Flow rate of air | ±1.1 | BTE | ±1.6 |
| Fuel flow rate | ±0.1 | Fuel Consumption | ±1.3 |
| Operating Speed | ±1.3 | CO | ±0.03 |
| Flow rate of cooling water | ±1.1 | CO2 | ±0.5 |
| In-Cylinder Pressure | ±1.5 | Braking Power | ±1.3 |
| Volumetric Efficiency | ±1.7 | HC and NOx | ±10 ppm |
3. Results and discussion
3.1. Performance parameters
3.1.1. Brake thermal efficiency
Figure 3 shows the variation of thermal brake thermal efficiency (BTE) of different ethanol gasoline blends at different operating speeds. There was a slight variation of the BTE with the same engine torque. When the engine was operated at 1500 rpm, the maximum brake thermal efficiency of PE15 mix was 28.33 percent. With the exception of the PE10 blend, at 1500-rpm speed, the highest performance of each blend was observed. The rise in efficiency is due to faster flame speed. The BTE increased to maximum at 1500 rpm for PE15 and PE25 blends then declined. For pure fuel, the same effect was noticed. The addition of ethanol led to increased compression, but the compression ratio was duely controlled and consistent with petrol, lower BTE at high motor speed was noticed [31]. Incomplete combustion can also lead to low thermal efficiency. Full combustion for PE10 and PE15 due to an adequate level of air that could increase BTE respectively after 1700 and 1600 rpm.
Figure 3.
Variation of Brake thermal efficiency with engine speed.
3.1.2. Brake specific fuel consumption
Brake Specific Consumption of Fuel (BSFC) is a measure of the power output of fuel consumption. It implies the ability of the engine to produce useful work. The calorific value and the oxygen content in the fuel governs BSFC. The higher the heating value lower the oxygen content and BSFC. Variation in the BSFC values for different pomegranate ethanol and gasoline blends are exhibited in Figure 4. BSFC decreased at all operating speeds with the addition of ethanol, except for the PE25 mix. However PE25, resulted in the highest BSFC at higher speeds (attributed due to a lower heating value and a lack of air supply) and PE15 has lowest fuel consumption at 1500 rpm. The fuel consumption for the PE15 blend was lowest among all the blends when the engine was operated at higher speed. This has resulted in less fuel consumption at higher speeds with ethanol enrichment.
Figure 4.
Variation of brake specific fuel consumption with engine speed.
3.1.3. Braking power
The effect of fuel blends on brake power is shown in Figure 5. When the blending ratio is higher, braking strength is enhanced by average output pressure at all speeds. The latent heat of blended fuel, which exceeds the oil evaporation, cools the air/fuel load and contributes to lower density, increased volume and braking power of the engine. Enrichment of ethanol supplies more oxygen increases the brake torque and boosts engine efficiency [14]. The brake power achieved with PE15 and PE25 exceeds all blends and was maximum (4.73 kW) at 1700 rpm rotational speed.
Figure 5.
Brake power variation with engine speed.
3.2. Emission characteristics
3.2.1. Hydrocarbon emissions
Figure 6 shows the emission of unburned hydrocarbons (HCs). It is inferred that they are in decreasing trend in comparison to pure fuel with the increase in ethanol content. Enhanced fuel and air mixture contribute to increased combustion leading to lower HC emissions. This is attributed due to the ethanol/oil mixtures increased air-fuel ratio, caused by their oxygen content. Relative to pure fuel and other blends, PE20 has low HC emissions among all blends. The fine atomization and improved combustion of petrol resulted in a high combustion chamber temperature, which lowers hydrocarbons emissions. Lean blends cause the fuel to evaporate slowly, which lead to rise in hydrocarbons.
Figure 6.
Fluctuation of Hydrocarbon emissions with engine speed.
3.2.2. Carbon monoxide emissions
For the different ethanol-gasoline blends, Figure 7 shows the difference in CO with respect to the engine speed. Inadequate air mixture volume can lead to carbon monoxide emissions. Ethanol enriched fuel can mix more oxygen with petrol and improves the engine combustion. Suitable combustion chamber temperature results in fine atomization, improved mixing, dispersing high molecular distribution, high flame speed and increased combustion that reduces carbon monoxide emissions. Carbon dioxide emissions are minimized by increased ethanol mixing with the exception of PE10 blend compared to pure gasoline. For PE15, PE20 and PE25 blends the total reduction of CO emissions was 90.32% and 90.89%, compared with gasoline at 1500-rpm speed and full load respectively. The increase of ethanol led to a leaner air-fuel mixture. In case of PE10, much faster combustion prevailed over the lean mixture. However, when the concentration of ethanol was increased, the combustion was not in the proximity, but it was leaner. Consequently, more oxygen was available after the combustion, thus by increasing the CO formation.
Figure 7.
Carbon Monoxide emissions fluctuation with engine speed.
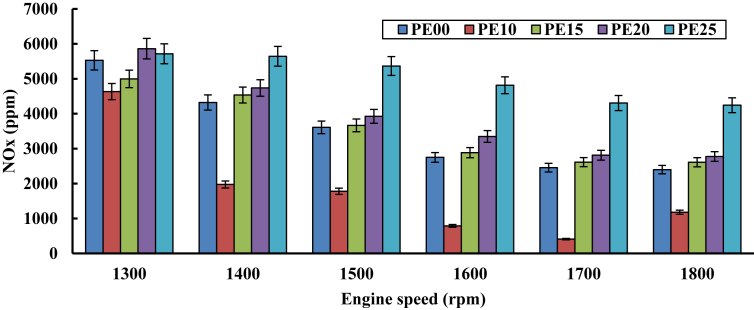
3.2.3. Oxides of nitrogen emissions
Figure 8 shows the emission of oxides of nitrogen (NOx). It can be noticed, decrease in emission at all operating speed when the ethanol proportion is increased in gasoline. The level of oxygen and the temperature of the combustion chamber decide the amount of nitrogen oxides. As ethanol levels increase, the combustion is accelerated due to a significant volume of oxygen and the temperature of the combustion chamber decreases, leading to less NOx emissions with lower blends. In case of PE10 blend, NOx is less than 30 percent compared to pure gasoline at 1700 rpm. With rising ethanol content in pure gasoline, NOx emissions have increased. Also, PE15, PE20 and PE25 blends show 8.88%, 15.72% and 76.93% increase compared to 1800 rpm. This is due to the similarity of combustion with the stoichiometric process, which raises the flame temperature and thus the emission of NOx.
Figure 8.
Nitrogen oxide emission variation with engine speed.
3.2.4. Carbon dioxide emissions
Figure 9 shows the carbon dioxide (CO2) emission of various fuel blends at different operating speeds. It was clear that the greater the ethanol content in the gasoline, increased the CO2 emissions. Caron dioxide emissions depend on the carbon monoxide concentration and combustion. Increasing the ratio of ethanol will improve engine fuel consumption and contribute to increased emissions of carbon dioxide. Pure gasoline exhaust gas emissions values were 12.22%, compared to 12,73% for PE15, 12,71% and 12,85% for PE20 and PE25 respectively. Carbon dioxide levels of PE15, PE20 and PE25 blends increased at 1700 rpm by 4.17%, by 4 and 5.15%, respectively, compared to unleaded fuel.
Figure 9.
Carbon dioxide emissions fluctuation with an engine speeds.
3.3. Combustion characteristics
The combustion analysis was performed by capturing in-cylinder pressure by piezoelectric pressure sensor (Apex, AX-409) installed in the combustion chamber. The data was captured with 1-degree crank angle for prepared fuel blends.
3.3.1. In-cylinder pressure
Figure 10 depicts the effect on engine cylinder pressure of different fuel mixtures. As the content of bioethanol of the mixed fuel is raised, the engine cylinder pressure for all blends increases slightly with addition of ethanol. The increase in cylinder pressure can be due to a higher oxygen content than pure fuel for higher ethanol blends. However, PE25 combination has highest in-cylinder pressure followed by PE20, PE15 and PE10 blends. The higher heating value of ethanol than gasoline, facilitates fuel/air charged cooling and an increase in the charge density, resulting in higher performance. The enhancement of the bioethanol percentage leads to an increased mixture density and the volumetric performance of the engine. The high laminar flame speed decreased the ignition time and raised the cylinder pressure early.
Figure 10.
In-cylinder pressure variation with crank angle.
3.3.2. Cyclic variations
The combustion quality and stability is evaluated by comparing the cyclic variability of a series of consecutive combustion events. The cyclic variations are measured in terms of coefficient of variation (COV). The COV of the mean effective pressure (IMEP) is used to compare the stability of combustion and is defined as the ratio of standard deviation in the mean effective pressure divided by the mean IMEP. Vehicle drivability issues may arise if COVIMEP exceeds around 10 % [32]. Figure 11 illustrates the coefficient of variation of the specific mean effective pressures at 1800 rpm for the different fuel mixtures. As the coefficient of variation is well below 3.5, the combustion is stable with ethanol addition for all fuel blends. The fluctuation in coefficient of variation is attributed to changes in exhaust gas recirculation rate. The rise in COV for PE10 and PE15 blends may be due to the lower temperature at the time of spark in turn adversely affecting the ignition.
Figure 11.
Coefficient of Variation with % of ethanol.
3.3.3. Combustion efficiency
Combustion efficiency variation with different operating speed for all prepared fuel blends are shown in Figure 12. The combustion efficiency decreases with increase in engine operating speed for all fuel blends. The marginal drop in combustion efficiency reduces with ethanol mixing. For pure gasoline and PE10 blend, considerable drop in combustion efficiency was observed. This is attributed to the reduction in combustion time available with increasing operating speed resulting in incomplete combustion. The combustion efficiency increases slightly with addition of ethanol. This is due to the availability of oxygen with ethanol content, which helps in complete combustion of fuel. Table 6 exhibits the comparision of present study with available literature.
Figure 12.
Combustion Efficiency Variation with % of ethanol.
Table 6.
Comparison of present study with available literature.
| Sample Details | Performance |
Emission |
Summary | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| BTE | BSFC | BP | HC | CO | NOx | CO2 | |||
| Pomegranate ethanol gasoline blends PE10,PE15, PE20 and PE25 | 15% ethanol addition gave optimal values, improved performance, CO2 and CO emissions with ethanol addition. | Present Study | |||||||
| Ethanol + gasoline blends E0, E10, E20,E30 | 10% ethanol addition was optimal with improved performance, CO2 and CO emissions. | [12] | |||||||
| Ethanol gasoline blends E10, E20, E30 | Slightly higher energy torques, engine power and moderate fuel consumption, Hydrocarbon and oxides of nitrogen emissions are minimized. | [13] | |||||||
| Potato peel ethanol + gasoline E5, E7.5, E10, E12.5, E15 | -- | Improved torque, power and emissions with ethanol addition. E10 mixture produced optimal values. | [18] | ||||||
4. Conclusions
In the work presented here, ethanol was produced from pomegranate fruit waste by fermentation and steam distillation. Four blends of ethanol and gasoline were prepared and tested in a multi-cylinder TV1 Kirloskar spark ignition engine for differing speeds and constant loading. PE15 blend produced the best result among all fuel blends. The maximum thermal efficiency of 28.33 percent for the PE15 combination was noticed when the engine was operating at 1500 rpm. PE 25 blend exhibited the highest BSFC at lower speeds, while PE10 had the most at higher speeds. As the ethanol concentration in fuel increases at all engine running levels, the braking power also increases. Maximum brake power was obtained with PE15 and PE25 is more than all other fuel blends and is the maximum (4.73 kW) at 1700 rpm. For higher blends, the braking capacity increases at all rotational speeds with increasing ethanol in the gasoline because of an improved effective overall pressure. The increased proportion of ethanol in pure fuel has resulted an increase in NOx emission. Concentration of CO2 increases as ethanol is added at higher levels. CO2 emissions are minimized by increasing ethanol mixture in comparison to pure petrol except for PE10 combination. The rise in ethanol in gasoline increases the nitrogen oxides emission, but decreases at all operating speeds for all combinations. Unburnt hydrocarbon emissions have decreased relative to pure gasoline, with the increase in ethanol proportion. PE20 pure petrol mixture has the lowest HC emissions. The maximum HC reduction was observed for both the PE15 and PE20 combinations at 1400–1600 rpm speed compared to pure gasoline. The PE15 blend produced the lowest HC emissions among all blends at higher rotational speeds. In-cylinder pressure increased with addition of ethanol. The lower values of coefficient of variation indicated good combustion stability.
4.1. Scope for future work
The current study reflects the extraction of novel pomegranate ethanol by fermentation followed by steam distillation and its blends on a spark ignition engine performance. The study presented here has some limitations. Pre-treatments like preheating and esterification were not included. For commercial production of pomegranate ethanol, post-combustion treatment methodologies like catalytic converters, Exhaust gas recirculation should be studied further to use pomegranate ethanol as alternative fuel on large scale.
Declarations
Author contribution statement
D. Y. Dhande: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kiran B. Dahe: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Nazaruddin Sinaga: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to Ms. Apex Innovations Pvt.Ltd., Sangali, Maharashtra state, India for providing resources for carrying out this research work.
References
- 1.Balat M., Balat H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy. 2009;86:2273–2282. [Google Scholar]
- 2.Shafiee S., Topal E. When will fossil fuel reserves be diminished? Energy Pol. 2009;37:181–189. [Google Scholar]
- 3.Manieniyan V., Thambidurai M., Selvakumar R. 2009. Study on Energy Crises and the Future of Fossil Fuels. Epub ahead of print. [Google Scholar]
- 4.Bae C., Kim J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017;36:3389–3413. [Google Scholar]
- 5.Onuki Shinnosuke, Koziel Jacek A., van Leeuwen J.(Hans) 2008 Providence, Rhode Island, June 29 - July 2, 2008. American Society of Agricultural and Biological Engineers. 2008. Ethanol production, purification, and analysis techniques: a review. Epub ahead of print. [Google Scholar]
- 6.Pimentel D., Patzek T.W. Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower. Nat. Resour. Res. 2005;14:65–76. [Google Scholar]
- 7.Bai F.W., Anderson W.A., Moo-Young M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008;26:89–105. doi: 10.1016/j.biotechadv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar A., Chowdhuri A.K., Bhowal A.J., Mandal B.K. The performance and emission characteristics of SI engine running on different ethanol- gasoline blends. Int. J. Sci. Eng. Res. 2012;3:1–7. [Google Scholar]
- 9.Thakur A.K., Kaviti A.K., Mehra R. Progress in performance analysis of ethanol-gasoline blends on SI engine. Renew. Sustain. Energy Rev. 2017;69:324–340. [Google Scholar]
- 10.Thakur A.K., Kaviti A.K., Mehra R. Performance analysis of ethanol–gasoline blends on a spark ignition engine: a review. Biofuels. 2017;8:91–112. [Google Scholar]
- 11.Doğan B., Erol D., Yaman H. The effect of ethanol-gasoline blends on performance and exhaust emissions of a spark ignition engine through exergy analysis. Appl. Therm. Eng. 2017;120:433–443. [Google Scholar]
- 12.Manikandan K., Walle M. The effect of gasoline –ethanol blends and compression ratio on SI engine performance and exhaust emissions. Int. J. Eng. Res. Technol. 2013;2:3142–3154. [Google Scholar]
- 13.Mourad M., Mahmoud K. Investigation into SI engine performance characteristics and emissions fuelled with ethanol/butanol-gasoline blends. Renew. Energy. 2019;143:762–771. [Google Scholar]
- 14.Al-Hasan M. Effect of ethanol–unleaded gasoline blends on engine performance and exhaust emission. Energy Convers. Manag. 2003;44:1547–1561. [Google Scholar]
- 15.Saikrishnan V., Karthikeyan A., Jayaprabakar J. Analysis of ethanol blends on spark ignition engines. Int. J. Ambient Energy. 2018;39:103–107. [Google Scholar]
- 16.Hsieh W.-D., Chen R.-H., Wu T.-L. Engine performance and pollutant emission of an SI engine using ethanol–gasoline blended fuels. Atmos. Environ. 2002;36:403–410. [Google Scholar]
- 17.Najafi G., Ghobadian B., Yusaf T. Optimization of performance and exhaust emission parameters of a SI (spark ignition) engine with gasoline–ethanol blended fuels using response surface methodology. Energy. 2015;90:1815–1829. [Google Scholar]
- 18.Elfasakhany A. Investigations on the effects of ethanol–methanol–gasoline blends in a spark-ignition engine: performance and emissions analysis. Eng. Sci. Technol. Int. J. 2015;18:713–719. [Google Scholar]
- 19.Karthickeyan V., Ashok B., Thiyagarajan S. Comparative analysis on the influence of antioxidants role with Pistacia khinjuk oil biodiesel to reduce emission in diesel engine. Heat Mass Tran. 2020;56:1275–1292. [Google Scholar]
- 20.Wang S., Viswanathan K., Esakkimuthu S. Experimental investigation of high alcohol low viscous renewable fuel in DI diesel engine. Environ. Sci. Pollut. Res. 2020 doi: 10.1007/s11356-020-08298-y. Epub ahead of print 1 May. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan K., Ashok B., Pugazhendhi A. Comprehensive study of engine characteristics of novel biodiesel from curry leaf (Murraya koenigii) oil in ceramic layered diesel engine. Fuel. 2020;280:118586. [Google Scholar]
- 22.Viswanathan K., Wang S. Experimental investigation on the application of preheated fish oil ethyl ester as a fuel in diesel engine. Fuel. 2021;285:119244. [Google Scholar]
- 23.Viswanathan K., Wang S., Esakkimuthu S. Impact of yttria stabilized zirconia coating on diesel engine performance and emission characteristics fuelled by lemon grass oil biofuel. J. Therm. Anal. Calorim. 2020 Epub ahead of print 20 November. [Google Scholar]
- 24.Dabi M., Saha U.K. Influence of ethanol on the performance, combustion and emission characteristics of a stationary diesel engine run on diesel–Mesua ferrea linn oil blend. Sādhanā. 2020;45:285. [Google Scholar]
- 25.Li Y., Gong J., Yuan W. Experimental investigation on combustion, performance, and emissions characteristics of butanol as an oxygenate in a spark ignition engine. Adv. Mech. Eng. 2017;9 168781401668884. [Google Scholar]
- 26.Banapurmath N.R., Tewari P.G. Performance, combustion, and emissions characteristics of a single-cylinder compression ignition engine operated on ethanol—biodiesel blended fuels. Proc. IME J. Power Energy. 2010;224:533–543. [Google Scholar]
- 27.Shinde P.N., Mandavgane S.A., Karadbhajane V. Process development and life cycle assessment of pomegranate biorefinery. Environ. Sci. Pollut. Res. 2020;27:25785–25793. doi: 10.1007/s11356-020-08957-0. [DOI] [PubMed] [Google Scholar]
- 28.Demiray E., Karatay S.E., Dönmez G. Evaluation of pomegranate peel in ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Energy. 2018;159:988–994. [Google Scholar]
- 29.Demiray E., Karatay S.E., Dönmez G. Improvement of bioethanol production from pomegranate peels via acidic pretreatment and enzymatic hydrolysis. Environ. Sci. Pollut. Res. 2019;26:29366–29378. doi: 10.1007/s11356-019-06020-1. [DOI] [PubMed] [Google Scholar]
- 30.Demiray E., Ertuğrul Karatay S., Dönmez G. Efficient bioethanol production from pomegranate peels by newly isolated Kluyveromyces marxianus. Energy Sources, Part A Recovery, Util. Environ. Eff. 2020;42:709–718. [Google Scholar]
- 31.Zaharin M.S.M., Abdullah N.R., Masjuki H.H. Evaluation on physicochemical properties of iso-butanol additives in ethanol-gasoline blend on performance and emission characteristics of a spark-ignition engine. Appl. Therm. Eng. 2018;144:960–971. [Google Scholar]
- 32.Heywood J.B. McGraw-Hill; 1988. Internal Combustion Engine Fundamentals. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.