Summary
The deployment of a vaccine that limits transmission and disease likely will be required to end the coronavirus disease 2019 (COVID-19) pandemic. We recently described the protective activity of an intranasally administered chimpanzee adenovirus-vectored vaccine encoding a pre-fusion stabilized spike (S) protein (ChAd-SARS-CoV-2-S [chimpanzee adenovirus-severe acute respiratory syndrome-coronavirus-2-S]) in the upper and lower respiratory tracts of mice expressing the human angiotensin-converting enzyme 2 (ACE2) receptor. Here, we show the immunogenicity and protective efficacy of this vaccine in non-human primates. Rhesus macaques were immunized with ChAd-Control or ChAd-SARS-CoV-2-S and challenged 1 month later by combined intranasal and intrabronchial routes with SARS-CoV-2. A single intranasal dose of ChAd-SARS-CoV-2-S induces neutralizing antibodies and T cell responses and limits or prevents infection in the upper and lower respiratory tracts after SARS-CoV-2 challenge. As ChAd-SARS-CoV-2-S confers protection in non-human primates, it is a promising candidate for limiting SARS-CoV-2 infection and transmission in humans.
Keywords: vaccine, SARS-CoV-2, immunity, non-human primates, immunogenicity, mucosal, protection, adenoviral vector
Graphical abstract

Highlights
ChAd-SARS-CoV-2 is a chimpanzee adenoviral vectored vaccine against SARS-CoV-2
ChAD-SARS-CoV-2-S immunization induces neutralizing Abs and T cell responses in NHP
ChAD-SARS-CoV-2-S protects NHPs against infection in the respiratory tract
Vaccine-induced serum neutralizing antibody titers correlate with the protection
Hassan et al. show that a single-dose immunization of rhesus monkeys with ChAd-SARS-CoV-2-S via an intranasal route induces neutralizing antibodies and T cell responses against SARS-CoV-2. ChAd-SARS-CoV-2-S vaccine protects rhesus monkeys against SARS-CoV-2 infection in both the upper and lower respiratory tracts.
Introduction
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) was first isolated in late 2019 from patients with severe respiratory illness in China.1 SARS-CoV-2 infection results in a clinical syndrome, coronavirus disease 2019 (COVID-19), that can progress to respiratory failure2 and systemic inflammatory disease.3, 4, 5 The elderly, immunocompromised, and those with comorbidities (e.g., obesity, diabetes, hypertension) are at greatest risk of death from COVID-19.6 Virtually all countries and territories have been affected, with more than 125 million infections and 2.7 million deaths recorded worldwide (https://covid19.who.int/). The rapid expansion and prolonged nature of the COVID-19 pandemic and its accompanying morbidity, mortality, and destabilizing socioeconomic effects have made the development and deployment of a SARS-CoV-2 vaccine an urgent global health priority.
The spike (S) protein of the SARS-CoV-2 virion engages the cell-surface receptor angiotensin-converting enzyme 2 (ACE2) to promote coronavirus entry into human cells.7 Because the S protein is critical for viral entry, it has been targeted for vaccine development and therapeutic antibody interventions. SARS-CoV-2 S proteins are cleaved to yield S1 and S2 fragments, followed by further processing of S2 to yield a smaller S2′ protein.8 The S1 protein includes the receptor-binding domain (RBD), and the S2 protein promotes membrane fusion. The prefusion form of the SARS-CoV-2 S protein,9 which displays the RBD in an “up” position, is recognized by potently neutralizing monoclonal antibodies10, 11, 12, 13, 14 or protein inhibitors.15
Multiple academic and industry groups are developing vaccines that target the SARS-CoV-2 S protein16 using several platforms, including DNA plasmid, lipid nanoparticle-encapsulated mRNA, inactivated virion, subunit, and viral-vectored vaccines,17 with most requiring two doses. While several vaccines are in advanced clinical trials in humans to evaluate safety and efficacy, those granted emergency use authorization (Pfizer/BioNTech BNT162b2 and Moderna 1273 mRNA18,19 and Johnson & Johnson Ad26.COV2 and AstraZeneca ChAdOx1 nCoV-19 adenoviral20,21 platforms) are administered by intramuscular injection, resulting in robust systemic yet uncertain mucosal immunity. Thus, questions remain as to the ability of these vaccines to curtail both transmission and severe disease, especially if upper airway infection is not prevented or reduced. While many of the intramuscular vaccines prevent SARS-CoV-2-induced pneumonia in non-human primates, they variably protect against upper airway infection and, presumably, transmission.22, 23, 24, 25
We recently described a chimpanzee adenovirus (simian Ad-36)-based SARS-CoV-2 vaccine (ChAd-SARS-CoV-2-S) encoding for the S protein. Intranasal administration of a single dose of ChAd-SARS-CoV-2-S induced robust humoral and cell-mediated immune responses against the S protein and prevented upper and lower airway infection in mice expressing the human ACE2 receptor.26 This vaccine differs from ChAdOx1 nCoV-19, a chimpanzee Ad-23-based SARS-CoV-2 vaccine that is currently under Phase III evaluation in humans as intramuscular injections (NCT04324606) and granted emergency use approval in some countries. ChAd-SARS-CoV-2-S is derived from a different ChAd serotype, has further deletions in the backbone to enhance production, and introduces proline mutations to stabilize the S protein into a pre-fusion form.26 Here, as a next step toward clinical development of ChAd-SARS-CoV-2-S, we immunized rhesus macaques (Macaca mulatta) with a single intranasal dose of ChAd-Control or ChAd-SARS-CoV-2-S, and then challenged animals 1 month later with SARS-CoV-2 via the combined intranasal and intrabronchial routes. Immunization with ChAd-SARS-CoV-2-S resulted in the development of anti-S, anti-RBD, and neutralizing antibodies as well as T cell responses that prevented or limited infection in nasal swabs, bronchoalveolar lavage fluid, and lung tissues after SARS-CoV-2 challenge. Thus, the administration of a single dose of ChAd-SARS-CoV-2-S vaccine through a non-injection route has the potential to protect at the portal of entry and in distant tissues, which could limit both virus-induced disease and transmission.
Results
Immunogenicity analysis
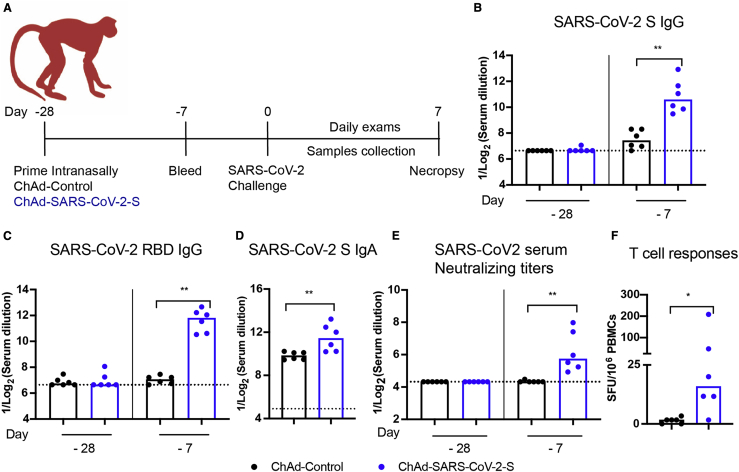
We immunized 12 adult rhesus macaques (RMs, Indian origin), aged 3 to 11 years old, with ChAd-Control or ChAd-SARS-CoV-2-S (n = 6 each; 3 females, 3 males). RMs received a single immunization of 1011 viral particles of the ChAd vectors by the intranasal route without adjuvant at day −28 (Figure 1A). All of the animals were pre-screened for the absence of neutralizing antibody responses against the simian AdV vector (Figure S1) as well as against SARS-CoV-2. Three weeks after vaccination (day −7), we detected immunoglobulin G (IgG) antibodies against the S and RBD proteins by ELISA in all RMs immunized with ChAd-SARS-CoV-2-S but not ChAd-Control vaccines (Figures 1B and 1C). We also detected low levels of SARS-CoV-2-specifc IgA in serum at this time point (Figure 1D). Neutralizing antibody responses were assessed using an infectious SARS-CoV-2 focus-reduction neutralization test27 and were detected in all RMs immunized with ChAd-SARS-CoV-2-S (Figure 1E). T cell responses specific to SARS-CoV-2 S were assessed by an interferon-γ (IFN-γ) ELISpot assay on peripheral blood mononuclear cells (PBMCs) isolated 2 weeks after vaccination (day −14). All RMs immunized with ChAd-SARS-CoV-2-S but not ChAd-Control developed T cell responses to SARS-CoV-2 S (Figure 1F).
Figure 1.
Immunogenicity of ChAd-SARS-CoV-2-S in RMs
(A) RMs (3–11 years old) were immunized via intranasal route with a single dose (1011 virus particles) of ChAd-Control or ChAd-SARS-CoV-2-S vaccine. Antibody responses in sera of immunized RMs at day −28 (day of immunization) and at day −7 (3 weeks after immunization) were evaluated.
(B–E) An ELISA measured anti-S and RBD IgG (B and C) and IgA (D) levels, and a focus neutralization reduction test (FRNT) determined neutralization activity (E) (n = 6 per group; Mann-Whitney test: ∗∗p < 0.01). Bars and columns show median values, and dotted lines indicate the limit of detection (LOD) of the assays.
(F) T cell response. PBMCs were isolated at day −14, and SARS-CoV-2-specific T cells were quantified by counting IFN-γ+ spots following stimulation with a 15-mer SARS-CoV-2 S peptide pool (2 μg/mL). Spot-forming unit counts per 106 stimulated PBMCs after deduction of background counts are shown (n = 6; Mann-Whitney test: ∗p < 0.05).
See also Figure S1.
Efficacy of ChAd-SARS-CoV-2-S in the upper respiratory tract
At 4 weeks after immunization, RMs were challenged with 1 × 106 50% tissue culture infective dose (TCID50) of SARS-CoV-2 (strain 2019 n-CoV/USA_WA1/2020) split between intranasal and intrabronchial routes of administration. The combined challenge route includes the direct infection of the bronchial tree; while not optimal for assaying vaccines that induce mucosal immunity in the upper airway, this protocol was required since intranasal infection by itself does not cause consistent lung infection in RMs.28,29 Even though clinical disease is limited in SARS-CoV-2-infected RMs,29,30 it was less in animals immunized with ChAd-SARS-CoV-2-S than ChAd-Control vaccine (Figure 2A; Table S1). Viral loads in the nasal swabs at days +1, +3, +5, and +7 were measured by quantitative reverse transcription-PCR (qRT-PCR) using primers for subgenomic (N gene, subgenomic RNA ) or genomic (nsp12) RNA; the use of both primer sets allows for distinction of actively replicating virus (subgenomic RNA) versus input and packaged virus (genomic RNA). Whereas most of the RMs immunized with ChAd-Control vaccine showed high levels of viral RNA through day +5 with some animals persisting through day +7, lower levels were observed in RMs immunized with ChAd-SARS-CoV-2-S (Figures 2B–2E). At day +1, only one RM vaccinated with ChAd-SARS-CoV-2-S showed detectable infectious virus in nasal swabs by a TCID50 assay, whereas 4 of 6 RMs immunized with ChAd-Control were positive (Figure 2F). At later time points, infectious virus was not recovered from the nasal swabs of vaccinated animals. Overall, our data suggest that ChAd-SARS-CoV-2-S vaccine protects the nasopharyngeal region from SARS-CoV-2 infection and results in reduced viral RNA levels and accelerated clearance.
Figure 2.
Viral RNA levels in the upper respiratory tract of RMs after SARS-CoV-2 challenge
RMs (3–11 years old) were immunized via intranasal route with ChAd-Control or ChAd-SARS-CoV-2-S. Four weeks later, RMs were challenged with 1 × 106 50% tissue culture infective dose (TCID50) of SARS-CoV-2 (strain 2019 n-CoV/USA_WA1/2020) split between the intranasal and intrabronchial administration routes.
(A) Clinical scores after challenge. Boxes show the 25th–75th percentiles, the center lines represent the median, and the extended whiskers from boxes indicate the minimum/maximum values.
(B–E) Nasal swabs were collected at days +1, +3, +5, and +7. Viral RNA was measured by qRT-PCR using primers specific for subgenomic mRNA (N gene) (B and D) or genomic RNA (nsp12 gene) (C and E) (n = 6, Mann-Whitney test: ∗p < 0.05; ∗∗p < 0.01; ns, not significant). (D and E) Longitudinal analysis of SARS-CoV-2 viral RNA in nasal swabs as determined by qRT-PCR showing the subgenomic (D) and genomic RNA (E). Each connected line shows the viral RNA in individual animals at the indicated time points.
(F) Levels of infectious virus (TCID50 analysis) recovered from nasal swabs of RM obtained 1 day after SARS-CoV-2 challenge (n = 6, Mann-Whitney test). Column heights (B, C, and F) indicate median values. The dotted line represents the LOD of the assay in this figure.
See also Table S1.
Efficacy of ChAd-SARS-CoV-2-S in the lower respiratory tract
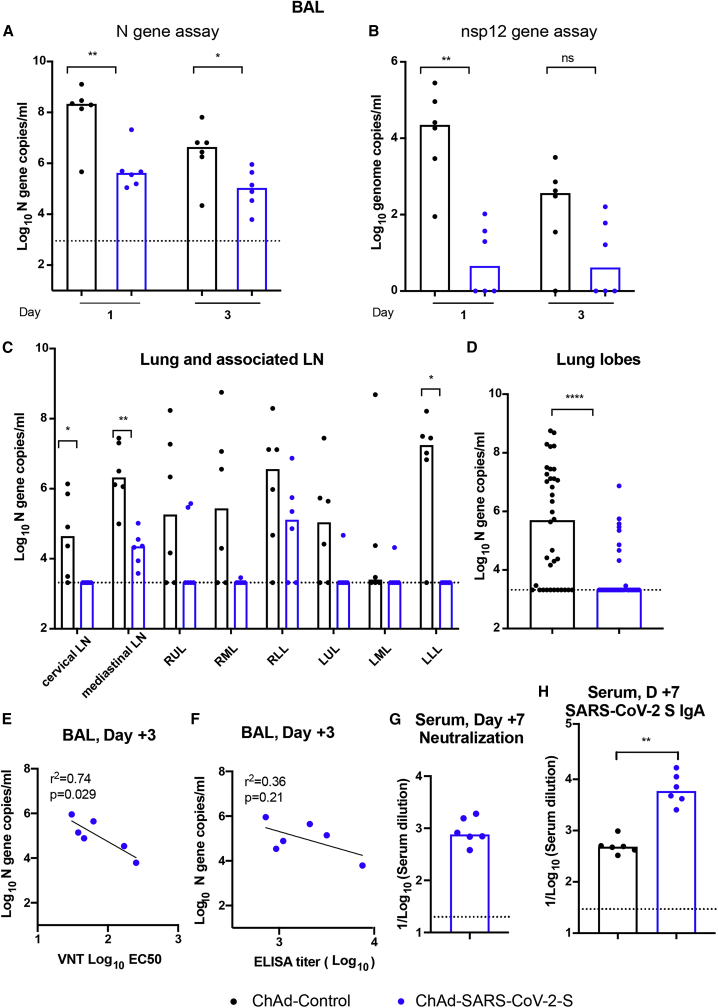
We next evaluated protective effects in the lower respiratory tract by measuring infection in bronchoalveolar lavage (BAL) fluid at days +1 and +3 and in lung tissues at day +7. For the BAL fluid obtained at day +1, all of the samples from ChAd-Control immunized RMs were positive for infectious virus, with titers reaching as high as 105 TCID/mL, whereas only 1 of 6 samples from ChAd-SARS-CoV-2-S-immunized animals was positive, with a lower titer of 4 × 102 TCID/mL (Table 1). At day +3, only 1 (from ChAd-control immunized RMs) of the 12 BAL fluid samples collected was positive for infectious virus. Consistent with these results, at days +1 and +3, substantially higher levels (100- and 50-fold, respectively) of viral RNA were detected in the BAL fluid of RMs immunized with ChAd-control compared to ChAd-SARS-CoV-2-S (Figures 3A and 3B).
Table 1.
Virus isolation from BAL fluid
| Vaccine | RM no. | BAL day 1 | BAL day 3 |
|---|---|---|---|
| ChAd-SARS-COV-2-S | 1 | − | − |
| 2 | − | − | |
| 3 | − | − | |
| 4 | − | − | |
| 5 | − | − | |
| 6 | + | − | |
| ChAd-Control | 7 | + | − |
| 8 | ++ | − | |
| 9 | +++ | ++ | |
| 10 | ++ | − | |
| 11 | + | − | |
| 12 | + | − |
−, negative; +, <102 TCID50/mL; ++, 102–104 TCID50/mL; +++, 105 TCID50/mL; BAL, bronchoalveolar lavage; RM, rhesus macaque; and TCID50, tissue culture infectious dose 50.
Figure 3.
Viral RNA levels in lower respiratory tracts of RMs after SARS-CoV-2 challenge
RMs (3–11 years old) immunized with ChAd-SARS-CoV-2-S or ChAd-Control were challenged with SARS-CoV-2, as described in Figure 2.
(A and B) BAL fluid was collected at days +1 and +3, and viral subgenomic (A) and genomic (B) RNA levels were assessed in BAL fluids by qRT-PCR (n = 6, Mann-Whitney test: ∗p < 0.05; ∗∗p < 0.01).
(C and D) Tissue samples from different lung lobes, mediastinal LNs, and cervical LNs were collected at day +7 after challenge. Subgenomic viral RNA levels were assessed by qRT-PCR (C). Viral RNA levels of the combined lung lobe specimens from ChAd-Control or ChAd-SARS-CoV-2-S-immunized RMs are shown (D) (n = 6, Mann-Whitney test: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001).
(E and F) Correlation analysis of serum neutralizing antibody titers (E) and anti-S antibody levels (F) in vaccinated RMs at 3 weeks post-immunization with viral RNA levels in BAL at day 3 after challenge. The black lines represent the linear regression fit (n = 6, Spearman correlation test: P and r2 values shown).
(G and H) Neutralizing antibody (G) and anti-S IgA (H) responses were evaluated from serum of ChAd-SARS-CoV-2-S-immunized RMs collected at day +7 after SARS-CoV-2 challenge (n = 6, Mann-Whitney test: ∗∗p < 0.01).
Column heights (A–D, G, and H) indicate median values. Dotted lines represent the LOD of the assays. See also Table S1.
At day +7, all of the animals were euthanized, and tissues were collected. Viral RNA was detected in the cervical lymph nodes (LNs), mediastinal LNs, and the lung tissues in the majority of ChAd-Control vaccinated animals. However, in ChAd-SARS-CoV-2-S-immunized animals, lower, if any, viral RNA was detected (Figure 3C). The viral RNA levels in the combined lung lobes from all of the ChAd-SARS-CoV-2-S-immunized animals were substantially lower than those measured in ChAd-Control-immunized animals (Figure 3D). To begin to establish correlates of protection, the viral RNA levels in BAL fluid at day +3 were compared to the serum-neutralizing or anti-S IgG titers obtained 3 weeks after immunization. We observed an inverse correlation between viral RNA levels in BAL fluid obtained 3 days after SARS-CoV-2 challenge and neutralizing antibody titers (Figure 3E). The neutralizing antibody levels correlated better than the anti-S IgG levels (p = 0.029, R2 = 0.74 versus p = 0.21, R2 = 0.36, respectively) (Figures 3E and 3F). Thus, serum-neutralizing antibody titers may serve as a correlate of protection for the ChAd-SARS-CoV-2-S vaccine. To determine whether antibody activity in sera could be boosted, we collected sera from ChAd-SARS-CoV-2-S-immunized RMs at day +7 after SARS-CoV-2 challenge. We observed an ~10-fold increase in serum-neutralizing titers (Figure 3G) compared to those measured 1 week before challenge (see Figure 1E). At this time point, we also observed substantially higher levels of anti-SARS-CoV-2 IgA in RMs that had been vaccinated with ChAd-SARS-CoV-2-S (Figure 3H). These findings suggest that a booster dose of ChAd-SARS-Cov-2-S may enhance the serum-neutralizing antibody, mucosal immunity, and protective activity.
Pathological analysis of lungs from vaccinated RMs
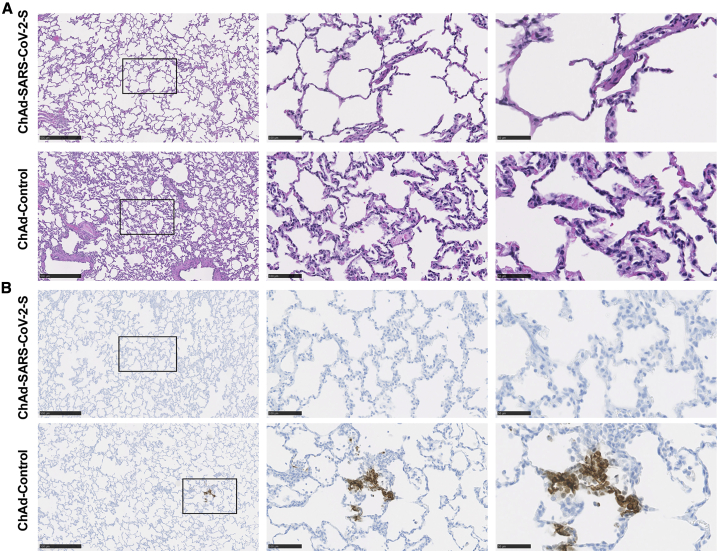
In this particular set of challenge experiments, infection in RMs was mild, and chest radiographs did not show evidence of frank consolidative pneumonia. ChAd-Control-vaccinated RMs developed changes consistent with mild pulmonary disease (Figure 4A). In two animals, we observed marked interstitial pneumonia characterized by small foci of alveolar septae, thickened by edema fluid and fibrin, with evidence of macrophage and neutrophil infiltration. Adjacent alveoli contained small numbers of foamy pulmonary macrophages and rare neutrophils and were occasionally lined by small numbers of type II pneumocytes. Perivascular infiltrates with small numbers of lymphocytes forming perivascular cuffs were observed. Immunohistochemistry revealed that 4 of the 6 ChAd-Control RMs were positive for viral antigen that principally localized to type I pneumocytes (Figure 4B). Two ChAd-SARS-CoV-2-S-vaccinated RMs also showed small microscopic pulmonary lesions that were similar to those in the ChAd-Control animals (Figure 4A). Notwithstanding these findings, none of the ChAd-SARS-CoV-2-S-vaccinated RMs showed evidence of viral antigen staining in lung tissues as analyzed by immunohistochemistry (Figure 4B).
Figure 4.
Pathological analysis of lungs of vaccinated RMs
RMs were immunized with ChAd-control and ChAd-SARS-CoV-2-S and challenged following the scheme described in Figure 2. Lungs were harvested at 7 days post-infection (dpi).
(A) Sections were stained with hematoxylin and eosin and imaged. Each image is representative of a group of 6 RMs.
(B) SARS-CoV-2 antigen was detected in lung sections from RMs for conditions described in (A). Images show low- (left; scale bars, 500 μm), medium- (center; scale bars, 100 μm), and high-power magnification (right; scale bars, 50 μm). Representative images from n = 6 RMs per group.
Discussion
In this study, we show that in RMs, a single intranasal immunization of ChAd-SARS-CoV-2-S confers protection in both the upper and lower airways against challenge with a high dose of SARS-CoV-2. These results are consistent with recent studies showing protection against SARS-CoV-2 challenge after a similar immunization strategy in mice expressing human ACE2 (hACE2) receptors26 and in hamsters.31 Within 3 weeks of intranasal vaccination, we detected S protein-specific T cell responses in PBMCs as well as anti-S IgG and IgA, anti-RBD, and neutralizing antibodies in serum. Immunization with ChAd-SARS-CoV-2-S compared to the control ChAd-Control vaccine resulted in more rapid clearance of SARS-CoV-2 RNA from nasal samples, decreased levels of viral RNA in BAL fluid, and lower levels of viral RNA in the homogenates from different regions of the lungs.
Our experiments showing protection against SARS-CoV-2 infection and pathogenesis in RMs are consistent with other studies in nonhuman primates using similar or distinct vaccine platforms. These include: (1) a 3-dose intramuscular vaccination regimen with whole inactivated SARS-CoV-2, which protected RM from SARS-CoV-2-induced pneumonia32; (2) a 2-dose intramuscular vaccination regimen with a DNA vaccine encoding the S protein, which reduced viral RNA levels in BAL fluid and nasal swabs25; (3) a priming or 2-dose intramuscular immunization with ChAdOx1 nCoV-19, a related chimpanzee adenoviral vector, resulted in reduced viral loads in BAL fluid and lung tissues without protection against upper airway infection23; (4) intramuscular immunization with a single-dose human Ad26 adenoviral vector provided near-complete protection in BAL fluid and nasal swabs after SARS-CoV-2 challenge,22 although viral RNA levels in lung tissues were not measured; and (5) 1 month after intranasal or intramuscular vaccination with a human Ad5 adenoviral vector, RMs were protected against SARS-CoV-2 challenge as evidenced by decreased viral RNA levels in oropharyngeal swabs and lung tissues.33 While all of these studies show protection in the RM model, it is nonetheless challenging to compare them directly because of the following disparities: RMs of different origin, different immunization and challenge protocols, different viral stocks and inoculation doses, different clinical and pathological scoring systems, separate assays for readout of infection, and different study locations. Moreover, the RM model generally shows less lung disease after SARS-CoV-2 infection than other animal models (e.g., hACE2 transgenic mice, mouse-adapted strains and wild-type mice, and hamsters), making it more difficult to establish conclusively vaccine protection against severe disease. The single intranasal immunization of ChAd-SARS-CoV-2-S in NHPs in this study conferred virological protection that was comparable to other viral-vectored vaccines administered to NHPs via intramuscular routes.22,23,33
The serum neutralization titers at 21 days with a single intranasal dose of ChAd-SARS-CoV-2-S were similar to those obtained with other 1- or 2-dose intramuscular vaccine platforms in RMs. However, these levels are lower than what we observed in BALB/c or C57BL/6 mice immunized with a single dose of ChAd-SARS-CoV-2-S.26 The basis for this discrepancy remains uncertain, although higher doses on a viral particle per kilogram were used in mice. As reported recently by others, the relatively modest levels of immune response in RMs after intranasal vaccination of adenoviral vectors still conferred effective protection against SARS-CoV-2,33 and our data show that serum-neutralizing antibody levels are a reasonable correlate of protection. These results are consistent with a recent study establishing serum antibody as a correlate of protection against SARS-CoV-2 infection in RMs.34 In comparison, others have used anti-RBD responses in convalescent plasma from humans to predict protective immunity.35 We also observed T cell responses to SARS-CoV-2 after vaccination, which also could contribute to protection.36 Future vaccine dose-response analysis in RM could provide further insight into the correlates of protection and potential ways to enhance the humoral and cellular responses against SARS-CoV-2. Alternatively, a homologous intranasal or intramuscular booster dose37 with ChAd-SARS-CoV-2-S or a heterologous adenoviral vector could augment immune responses and protection. Finally, a different intranasal vaccine formulation such as spray installation could also improve protective immune responses.
Vaccines for COVID-19 should protect against pneumonia and death and curtail transmission in the population. Our preclinical data in mice,26 hamsters,31 and RMs suggest that ChAd-SARS-CoV-2-S may accomplish these goals, given its ability to reduce levels of subgenomic SARS-CoV-2 RNA and infectious virus in the BAL fluid and lungs and diminish infection at nasopharyngeal portal sites. We challenged RMs with a high dose of virus (1 × 106 TCID50) using 2 routes (intranasal and intrabronchial) to test the efficacy of the vaccine. As this likely does not reflect the exposure pattern in humans, the ChAd-SARS-CoV-2-S vaccine may show better efficacy. The direct intrabronchial inoculation of SARS-CoV-2 used in our challenge model, which is necessary to establish consistent pulmonary infection,28,29 likely bypassed the nasal and pharyngeal mucosal immune barrier and limited the protective impact of the intranasal vaccine.
In contrast to results with SARS-CoV vaccines or antibodies,38, 39, 40 no enhanced infection, immunopathology, or enhanced disease in animals immunized with the ChAd-SARS-CoV-2-S vaccine were observed in our study. In the ChAd-SARS-CoV-2-S vaccinated and challenged RMs, we did not observe worsening in clinical signs or enhanced replication compared to controls at any time point in the study. Moreover, the pathological analysis did not show evidence of the enhanced immune cell infiltration or alveolar damage seen in the pulmonary tissues of vaccinated, SARS-CoV challenged RMs.
Limitations of the study
We acknowledge several limitations in this study: (1) we did not perform direct immunogenicity and efficacy comparisons with intramuscular delivery of ChAd-SARS-CoV-2-S; (2) the differential effect of route of vaccine administration on protection that we observed in mice and hamsters26,31 may not directly translate to primates; (3) we did not assess the durability of immune responses—future longitudinal studies must be conducted to monitor immune responses over time after intranasal vaccination with ChAd-SARS-CoV-2-S to establish durability; and (4) even with a high dose and invasive route of challenge, many of the control vaccinated animals did not develop severe lung pathology or disease, which limited our ability to evaluate protection against disease in this model.
In summary, our studies establish that the immunization of primates with ChAd-SARS-CoV-2-S induces neutralizing antibody and protective immune responses in both the upper and lower respiratory tracts. While several vaccine candidates (mRNA, inactivated, viral vectored) are in advanced phases of human clinical trials or recently granted emergency use authorization for the immunization of humans, their efficacy in curtailing transmission remains to be established. Based on preclinical data in multiple animal models, we suggest that intranasal delivery of ChAd-SARS-CoV-2-S or possibly other viral-vectored or subunit-based vaccines is a promising platform for preventing SARS-CoV-2 infection, disease, and transmission, and warrants further evaluation in humans.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-NP-1 | GenScript | U864YFA140-4/CB2093 |
| Anti-SARS-CoV-2 S mAb | Diamond laboratory | N/A |
| Goat anti-RM IgG HRP | Southern Biotech | Cat # 6200-05; RRID:AB_2796268 |
| Goat anti-RM IgA HRP | Sigma | Cat# SAB3700759 |
| Goat anti-mouse IgG HRP | Sigma | Cat. # A9044; RRID:AB_258431 |
| TrueBlue peroxidase substrate | KPL | 5510-0030 |
| SARS-CoV-2 RBD | Fremont laboratory | N/A |
| SARS-CoV-2 S protein | Fremont laboratory | N/A |
| Ultra TMB-ELISA | ThermoFisher | Cat. #34028 |
| Anti-human IFN-gamma | ImmunoSpot | N/A |
| SARS-CoV-2 S protein peptide pools | Feldmann laboratory | N/A |
| Virus and bacterial strains | ||
| SARS-CoV-2 (strain 2019 n-CoV/USA_WA1/2020) | CDC/BEI Resources | NR52281 |
| ChAd-SARS-CoV-2-S | 26 | N/A |
| ChAd-Control | 26 | N/A |
| Experimental models: Cell lines | ||
| Vero E6 | ATCC | CRL-1586; RRID:CVCL_0574 |
| HEK293 | ATCC | CRL-1573; RRID:CVCL_0045 |
| Experimental models: Organisms/strains | ||
| Rhesus macaques | NIH Colony | N/A |
| Oligonucleotides | ||
| SARS-CoV-2 N Probe: 5′-/56-FAM/TCAAGGAAC/ZEN/AACATTGCCAA/3IABkFQ/-3′ | 26 | N/A |
| SARS-CoV-2 nsp12 F: 5′- GTGARATGGTCATGTGTGGCGG −3′ | This paper | N/A |
| SARS-CoV-2 nsp12 R: 5′- CARATGTTAAASACACTATTAGCATA −3′ | This paper | N/A |
| SARS-CoV-2 nsp12 Probe1: 6-FAM/CCAGGTGGWACRTCATCMGGTGATGC | This paper | N/A |
| SARS-CoV-2 nsp12 Probe12 probe 2: 6-FAM/CAGGTGGAACCTCATCAGGAGATGC. | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prism | GraphPad | v 8.2.1 |
| Biorender | biorender.com | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael S. Diamond (diamond@wusm.wustl.edu).
Materials availability
All requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact author. This includes viruses, vaccines, and proteins. All reagents will be made available on request after completion of a Materials Transfer Agreement.
Data and code availability
All data supporting the findings of this study are available within the paper and are available from the corresponding author upon request.
Experimental model and subject details
Viruses and cells
Vero E6 (CRL-1586, American Type Culture Collection (ATCC), and HEK293 cells were cultured at 37°C in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES pH 7.3, 1 mM sodium pyruvate, 1X non-essential amino acids, and 100 U/ml of penicillin–streptomycin. SARS-CoV-2 strain 2019 n-CoV/USA_WA1/2020 was kindly provided by the Centers for Disease Control and Prevention as a passage 3 stock. The virus was propagated once more in Vero E6 cells in DMEM (Sigma) supplemented with 2% fetal bovine serum (GIBCO), 1 mM L-glutamine (GIBCO), 50 U/ml penicillin and 50 μg/ml of streptomycin (GIBCO). The virus stock used was passage 4, free of mycoplasma contamination, and identical to the initial deposited GenBank sequence (MN985325.1).
RM experiments
Experiments with RMs were approved by the Institutional Biosafety and Rocky Mountain Laboratories (NIAID, NIH) Animal Care and Use Committees. Twelve healthy rhesus macaques (Macaca mulatta; Indian origin, between 3 and 11 years of age and 4 – 10 kg in weight) were divided randomly into two groups of 6 animals (3 females and 3 males). All macaques were immunized 28 days prior to challenge by the intranasal route with a total dose of 1011 viral particles of ChAd-Control or ChAd-SARS-CoV-2-S in a total volume of 1 mL (0.5 mL into each nostril). Challenge was performed with a total dose of 1 x106 TCID50 of SARS-CoV-2 equally split between the two routes in a total volume of 5 mL (4 mL intrabronchial; 1 mL intranasal). Animals were monitored at least twice daily throughout the study using an established scoring sheet.30,41 Examinations were performed on days −28, −27, −25, −21, −14, −7, 0, 1, 3, 5 and 7 (euthanasia) and included clinical evaluation, thoracic radiographs, venous blood draw, and swabs. Bronchoalveolar lavage (BAL) was performed on day 1 and 3 as described.42 The study endpoint was day 7. Following euthanasia, necropsies were performed on all animals, and organs were harvested for virology, immunology and pathology. Gross lung lesions were scored by a board-certified veterinary pathologist blinded to the group assignment. Animals were sedated with either ketamine (10-12 mg/Kg) or Telazol (3.5-5 mg/Kg) by intramuscular injection for all clinical examinations and procedures. Isoflurane in oxygen was administered via face mask as needed to maintain appropriate levels of sedation and anesthesia.
Method details
Biosafety and ethics
Work with infectious SARS-CoV-2 was approved by the Institutional Biosafety Committee and performed in high biocontainment at Rocky Mountain Laboratories, NIAID, NIH. Sample removal from high biocontainment followed IBC-approved Standard Operating Protocols. Animal work was approved by the Rocky Mountain Laboratories Animal Care and Use Committee and performed by certified staff in an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility. Work followed the institution’s guidelines for animal use, the guidelines and basic principles in the NIH Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, United States Department of Agriculture and the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. RMs were single-housed in adjacent primate cages allowing social interactions, in a climate-controlled room with a fixed light-dark cycle (12-hr light/12-hr dark). They were provided with commercial monkey chow, treats, and fruit twice daily with water ad libitum. Environmental enrichment consisted of a variety of human interaction, manipulanda, commercial toys, videos, and music.
Chimpanzee adenovirus vectors
The replication-incompetent ChAd-SARS-CoV-2-S and ChAd-Control vectors were previously described.26 Vaccines were scaled up in HEK293 cells and purified by CsCl density-gradient ultracentrifugation. Viral particle concentration in each vector preparation was determined by spectrophotometry at 260 nm as described.43
Neutralization assay
Serum samples were diluted serially and incubated with 102 FFU of SARS-CoV-2 for 1 h at 37°C. The virus-serum mixtures were added to Vero E6 cell monolayers in 96-well plates and incubated for 1 h at 37°C. Subsequently, cells were overlaid with 1% (w/v) methylcellulose in MEM supplemented with 2% FBS. Plates were incubated for 30 h then fixed using 4% PFA in PBS for 1 h at room temperature. After washing, cells were sequentially incubated with murine anti-SARS-CoV-2 S mAb and a HRP-conjugated goat anti-mouse IgG (Sigma) in PBS supplemented with 0.1% (w/v) saponin (Sigma) and 0.1% BSA. TrueBlue peroxidase substrate (KPL) was used to develop the plates followed by counting the foci on a BioSpot analyzer (Cellular Technology Limited).
ELISA
Purified antigens (S or RBD) were coated onto 96-well Maxisorp clear plates at 2 μg/mL in 50 mM Na2CO3 pH 9.6 (70 μL) overnight at 4°C. Coating buffers were aspirated, and wells were blocked with 200 μL of PBS + 0.05% Tween-20 + 5% BSA (Blocking buffer, PBSTBA) overnight at 4°C. Serum samples were diluted in PBSTBA in a separate 96-well polypropylene plate. The plates then were washed thrice with 1X PBS + 0.05% Tween-20 (PBST) followed by addition of 50 μL of respective serum dilutions. Sera were incubated in the ELISA plates for at least 1 h at room temperature. Plates were again washed thrice in PBST followed by addition of 50 μL of 1:1000 goat anti-RM IgG(H+L)-HRP (Southern Biotech Cat. # 6200-05) in PBSTBA. Plates were incubated at room temperature for 1 h, washed thrice in PBST, and 100 μL of 1-Step Ultra TMB-ELISA was added (ThermoFisher Cat. #34028). Reactions were stopped with 50 μL of 2 M sulfuric acid. Optical density (450 nm) measurements were determined using a microplate reader (Bio-Rad).
Simian Ad neutralization assays
Serum samples were collected from RMs one day prior to ChAd immunizations. Sera were serially diluted prior to incubation with 102 FFU of ChAd-SARS-CoV-2-S for 1 h at 37°C. The virus-serum mixtures were added to Vero cell monolayers in 96-well plates and incubated for 1 h at 37°C. Cells were overlaid with 1% (w/v) methylcellulose in MEM supplemented with 5% FBS. Plates were incubated at 37°C for 48 h before fixation with 4% PFA in PBS for 20 min at room temperature. Subsequently, plates were washed with PBS and incubated overnight at 4°C with murine anti-SARS-CoV-2 S mAb diluted in permeabilization buffer (PBS supplemented with 0.1% (w/v) saponin and 0.1% BSA). Plates were washed again and incubated with anti-mouse-HRP (1:500; Sigma Cat. # A9044) in permeabilization buffer for 1 h at room temperature. After a final wash series, plates were developed using TrueBlue peroxidase substrate (KPL) and foci were counted on a BioSpot analyzer (Cellular Technology Limited).
T cell assay
Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation on a Ficoll gradient. Cells (3.0 × 106 PBMCs) from each RM were seeded into duplicate wells in a 96-well flat-bottom plate which pre-coated with human IFN-γ-capturing antibody (3.0 × 105 cells/well-Human IFN-γ Single-Color ELISPOT-ImmunoSpot). PBMCs were stimulated with SARS-CoV-2 spike protein peptide pools, at a final concentration of 2 μg/mL per peptide, for 24 h in a humidified incubator with 5% CO2 at 37 °C. IFN-γ spots were developed according to the manufacturer’s protocol and counted by CTL 328 ImmunoSpot® Analyzer and ImmunoSpot® Software.
Measurement of viral burden
SARS-CoV-2 infected animals were euthanized at day +7 after SARS-CoV-2 challenge, and tissues were collected. Tissues (up to 30 mg) were homogenized in RLT buffer. RNA was extracted using RNeasy kit (QIAGEN) according to manufacturer’s instructions. RNA was extracted from BAL fluid and nasal swabs using the QiaAmp Viral RNA kit (QIAGEN) according to manufacturer’s instructions. SARS-CoV-2 RNA levels were measured by one-step TaqMan RT-qPCR assay. SARS-CoV-2 nucleocapsid (N) or nsp12 specific primers and probe sets were used: (N: F primer: ATGCTGCAATCGTGCTACAA; R primer: GACTGCCGCCTCTGCTC; probe: /56-FAM/TCAAGGAAC/ZEN/AACATTGCCAA/3IABkFQ/ and nsp12: F primer: GTGARATGGTCATGTGTGGCGG; R primer: CARATGTTAAASACACTATTAGCATA; probe 1: 6-FAM/CCAGGTGGWACRTCATCMGGTGATGC; probe 2: 6-FAM/CAGGTGGAACCTCATCAGGAGATGC. Viral RNA was expressed as N or nsp12 gene copy numbers per g of tissues or per ml of fluid.
Virus isolation was performed on BAL liquid and homogenized lung tissues (approximately 30 mg in 1 mL DMEM using a TissueLyser (QIAGEN)) and inoculating Vero E6 cells in a 24 well plate with 250 μL of cleared and a 1:10 dilution of the homogenate. One hour after inoculation of cells, the supernatant was removed and replaced with 500 μL DMEM (Sigma-Aldrich) supplemented with 2% fetal bovine serum, 1 mM L-glutamine, 50 U/mL penicillin and 50 μg/mL of streptomycin. Six days after inoculation, cytopathogenic effect was scored, and the TCID50 was calculated.
Histopathology and immunohistochemistry
Histopathology and immunohistochemistry were performed on RM tissues. After fixation for a minimum of 7 days in 10% neutral-buffered formalin and embedding in paraffin, tissue sections were stained with hematoxylin and eosin (H & E). Tissues were placed in cassettes and processed with a Sakura VIP-6 Tissue Tek on a 12-hour automated schedule, using a graded series of ethanol, xylene, and ParaPlast Extra. Embedded tissues are sectioned at 5 μm and dried overnight at 42°C prior to staining. Specific anti-SARS-CoV-2 immunoreactivity was detected using GenScript U864YFA140-4/CB2093 NP-1 at a 1:1,000 dilution. The secondary antibody used was an anti-rabbit IgG polymer from Vector Laboratories ImPress VR. Tissues were processed for immunohistochemistry using the Discovery Ultra automated processor (Ventana Medical Systems) with a ChromoMap DAB kit (Roche Tissue Diagnostics).
Quantification and statistical analysis
Statistical significance was assigned when P values were < 0.05 using Prism Version 8 (GraphPad). Tests, number of animals (n), median values, and statistical comparison groups are indicated in the Figure legends.
Acknowledgments
The authors thank the staff of the Rocky Mountain Veterinary Branch, NIAID, NIH for animal care and veterinary services. This study was supported by NIH contracts and grants (R01 AI157155, 75N93019C00062, and HHSN272201400018C), as well as by the Intramural Research Program of NIAID, NIH.
Author contributions
A.O.H. generated the vaccines, performed ELISA assays, and analyzed the data. R.E.C. and J.B.C. performed the neutralization assays. H.Z. and D.H.F. designed and produced the recombinant S and RBD proteins. T.-L.T.-H. performed the T cell assays. F.F., A.O., J.L., and P.W.H. performed and evaluated the clinical examinations, sample collection, hematology and blood chemistry, and clinical scoring. F.F., K.M.-W., T.-L.T.-H., and D.P.S. performed the necropsies and organ harvest. D.P.S. performed and evaluated the histopathology. A.O.H., F.F., J.C., and K.M.-W. performed and evaluated the virological assays. H.F. and M.S.D. designed experiments and secured funding. D.T.C. provided key vaccine reagents. A.O.H., H.F., and M.S.D. wrote the initial draft, with the other authors providing editorial comments.
Declaration of interests
M.S.D. is a consultant for Inbios, Vir Biotechnology, NGM Biopharmaceuticals, and Carnival Corporation and serves on the Scientific Advisory Board of Moderna and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Moderna, Vir Biotechnology, and Emergent BioSolutions. M.S.D., D.T.C., and A.O.H. have filed a disclosure with Washington University for the possible commercial development of ChAd-SARS-CoV-2-S. D.T.C. is an equity holder in Precision Virologics, which has optioned the ChAd-SARS-CoV-2-S vaccine.
Published: March 18, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100230.
Contributor Information
Heinz Feldmann, Email: feldmannh@niaid.nih.gov.
Michael S. Diamond, Email: diamond@wusm.wustl.edu.
Supplemental information
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., China Medical Treatment Expert Group for Covid-19 Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J., Shen J., Zhu L.R., Chen Y., Iacucci M. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., Milner J.D. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182:73–84.e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zost S.J., Gilchuk P., Chen R.E., Case J.B., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Chen E.C. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med. 2020;26:1422–1427. doi: 10.1038/s41591-020-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes C.O., Jette C.A., Abernathy M.E., Dam K.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tortorici M.A., Beltramello M., Lempp F.A., Pinto D., Dang H.V., Rosen L.E., McCallum M., Bowen J., Minola A., Jaconi S. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science. 2020;370:950–957. doi: 10.1126/science.abe3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao L., Goreshnik I., Coventry B., Case J.B., Miller L., Kozodoy L., Chen R.E., Carter L., Walls A.C., Park Y.J. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science. 2020;370:426–431. doi: 10.1126/science.abd9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton D.R., Walker L.M. Rational Vaccine Design in the Time of COVID-19. Cell Host Microbe. 2020;27:695–698. doi: 10.1016/j.chom.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 18.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., C4591001 Clinical Trial Group Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;385:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 22.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V.A., Bushmaker T., Flaxman A., Ulaszewska M. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182:713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., Chen R.E., Winkler E.S., Wessel A.W., Case J.B. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell. 2020;183:169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Case J.B., Rothlauf P.W., Chen R.E., Liu Z., Zhao H., Kim A.S., Bloyet L.M., Zeng Q., Tahan S., Droit L. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe. 2020;28:475–485.e5. doi: 10.1016/j.chom.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz-Fontela C., Dowling W.E., Funnell S.G.P., Gsell P.S., Riveros-Balta A.X., Albrecht R.A., Andersen H., Baric R.S., Carroll M.W., Cavaleri M. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L., Tostanoski L.H., Yu J., Maliga Z., Nekorchuk M. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munster V.J., Feldmann F., Williamson B.N., van Doremalen N., Pérez-Pérez L., Schulz J., Meade-White K., Okumura A., Callison J., Brumbaugh B. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bricker T.L., Darling T.L., Hassan A.O., Harastani H.H., Soung A., Jiang X., Dai Y.N., Zhao H., Adams L.J., Holtzman M.J. A single intranasal or intramuscular immunization with chimpanzee adenovirus vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. bioRxiv. 2020 doi: 10.1101/2020.12.02.408823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng L., Wang Q., Shan C., Yang C., Feng Y., Wu J., Liu X., Zhou Y., Jiang R., Hu P. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020;11:4207. doi: 10.1038/s41467-020-18077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2020;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salazar E., Kuchipudi S.V., Christensen P.A., Eagar T., Yi X., Zhao P., Jin Z., Long S.W., Olsen R.J., Chen J. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J. Clin. Invest. 2020;130:6728–6738. doi: 10.1172/JCI141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sui Y., Bekele Y., Berzofsky J.A. Potential SARS-CoV-2 Immune Correlates of Protection in Infection and Vaccine Immunization. Pathogens. 2021;10 doi: 10.3390/pathogens10020138. 10. 2021/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson J.S., Pillet S., Bello A.J., Kobinger G.P. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J. Virol. 2013;87:3668–3677. doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., Tang H., Nishiura K., Peng J., Tan Z. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4:e123158. doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brining D.L., Mattoon J.S., Kercher L., LaCasse R.A., Safronetz D., Feldmann H., Parnell M.J. Thoracic radiography as a refinement methodology for the study of H1N1 influenza in cynomologus macaques (Macaca fascicularis) Comp. Med. 2010;60:389–395. [PMC free article] [PubMed] [Google Scholar]
- 42.Singletary M.L., Phillippi-Falkenstein K.M., Scanlon E., Bohm R.P., Jr., Veazey R.S., Gill A.F. Modification of a common BAL technique to enhance sample diagnostic value. J. Am. Assoc. Lab. Anim. Sci. 2008;47:47–51. [PMC free article] [PubMed] [Google Scholar]
- 43.Maizel J.V., Jr., White D.O., Scharff M.D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and are available from the corresponding author upon request.