Abstract
Anomalous patterns of brain gyrification have been reported in major psychiatric disorders, presumably reflecting their neurodevelopmental pathology. However, previous reports presented conflicting results of patients having hyper-, hypo-, or normal gyrification patterns and lacking in transdiagnostic consideration. In this article, we systematically review previous magnetic resonance imaging studies of brain gyrification in schizophrenia, bipolar disorder, major depressive disorder, and autism spectrum disorder at varying illness stages, highlighting the gyral pattern trajectory for each disorder. Patients with each psychiatric disorder may exhibit deviated primary gyri formation under neurodevelopmental genetic control in their fetal life and infancy, and then exhibit higher-order gyral changes due to mechanical stress from active brain changes (e.g., progressive reduction of gray matter volume and white matter integrity) thereafter, representing diversely altered pattern trajectories from those of healthy controls. Based on the patterns of local connectivity and changes in neurodevelopmental gene expression in major psychiatric disorders, we propose an overarching model that spans the diagnoses to explain how deviated gyral pattern trajectories map onto clinical manifestations (e.g., psychosis, mood dysregulation, and cognitive impairments), focusing on the common and distinct gyral pattern changes across the disorders in addition to their correlations with specific clinical features. This comprehensive understanding of the role of brain gyrification pattern on the pathophysiology may help to optimize the prediction and diagnosis of psychiatric disorders using objective biomarkers, as well as provide a novel nosology informed by neural circuits beyond the current descriptive diagnostics.
Subject terms: Neuroscience, Predictive markers, Psychiatric disorders, Diagnostic markers
Introduction
Previous studies demonstrated that established psychiatric disorders, such as schizophrenia (SCZ), bipolar disorder (BD), major depressive disorder (MDD), and autism spectrum disorder (ASD), are generally associated with neurodevelopmental pathology1–4, leading the debate as to whether they signify distinct entities that possess overlapping foundations or are different variants of one underlying disease. These major psychiatric disorders are presently differentiated based on their specific clinical representations, but a part of their symptoms transcends the diagnostic categories. A transdiagnostic approach may be useful for accurate diagnosis in the initial stage of illnesses and for clarifying the nature of their relationships among disorders (especially common and distinct mechanisms under their respective semiotics)5. Although preceding studies revealed shared genetic variations within a range of psychiatric disorders6, including common genetic-risk loci between SCZ and BD reported in a recent large-scale genome-wide association study (GWAS)7, it remains unclear how the disorders are trans-diagnostically characterized by neuroanatomical features, i.e., inter alia gyrification patterns. Thus, the common and distinct alterations in gyrification pattern across major psychiatric disorders have yet to be established.
Anomalous patterns of brain gyrification are assumed to be a consequence of pre/perinatal neurodevelopmental insult because cortical folding starts at 10 weeks of gestation to the third trimester of pregnancy (primary gyri emerge until 28 weeks of gestation and higher-order gyri develop thereafter) and its pattern remains rather stable after birth8,9. Very preterm children and adults showed significant changes in brain gyrification pattern10,11 that were associated with cognitive and psychopathology scores11, suggesting the impact of obstetric complications for the development of brain gyrification. As obstetric complications such as perinatal hypoxia and preterm birth were associated with locally reduced gyrification in psychosis12,13, the abnormal microenvironment in utero may impact the development of gyrification deficits which might be related to future psychopathology. Contrary to gray matter volume, which can be affected by age14, disease process15, and psychotropic drugs16, cortical gyrification patterns may be a more static neurodevelopmental marker. Therefore, it may be useful for feasible early intervention17. Although the gyral patterns may be a static neurodevelopmental marker, recent studies propounded a putative gyral pattern trajectory model that healthy individuals have a non-linear decline of gyrification index (GI) with age18,19. Patients with schizophrenia may also exhibit GI reduction over time20, suggesting multiple pathological processes at varying clinical stages. Although it is largely unknown whether patients with other psychiatric disorders exhibit gyral pattern changes during the course of the illness, such a potential longitudinal change may partly explain the conflicting results of patients with psychiatric disorders having hyper-, hypo-, or normal gyrification patterns18,19,21–36.
We systematically reviewed recent neuroimaging findings of anomalous gyral patterns in major psychiatric disorders at varying illness stages. This review article aims to integrally understand the gyral findings by focusing on the gyral pattern trajectories. Moreover, we propose an overarching model that spans the diagnoses through the transdiagnostic considerations, which may aid in clinical diagnosis and prediction of the disorders, and also transcend the limitations of the established nosological systems.
Magnetic resonance imaging (MRI) findings of brain gyrification
Study selection
We aimed to systematically review the studies published between May 1, 1997 and June 30, 2019 that investigated the gyral pattern in major psychiatric disorders, including the high-risk state both clinically and genetically for psychosis, by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement37. We searched for studies in PubMed and Google Scholar using the terms (schizophrenia OR bipolar disorder OR major depressive disorder OR autism spectrum disorder OR high risk) AND (gyrification OR local gyrification index). We further performed a cited reference search starting with the identified studies in Web of Science, and added several relevant reports. The searches were rerun just before the final analyses in June 2020. Two of the authors (D.S. and Y.T.) independently conducted a hand search and resolved their disagreement in study selection by consensus.
Only full-length or short articles written in English were selected. We included case-control studies (cross-sectional or longitudinal designs) with MRI data in patients with major psychiatric disorders, individuals at high-risk for psychosis, and healthy controls. Patients met the Diagnostic and Statistical Manual of Mental Disorders (DSM), 3rd, 4th, or 5th edition criteria or the International Classification of Disease diagnostic (ICD), 10th revision criteria for each psychiatric disorder. In addition, high-risk individuals were siblings of the patients or met the diagnosis criteria, such as the Comprehensive Assessment of At-Risk Mental States (CAARMS)38 and the Structured Interview for Prodromal Symptoms /the Scale of Prodromal Symptoms (SIPS/SOPS)39. All studies that investigated psychiatric or physical conditions other than the aforementioned states were excluded. Studies using non-T1-weighted protocols were also excluded. In all, we included 71 articles (Fig. 1), which formed the empirical basis of this review.
Fig. 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram for study search.
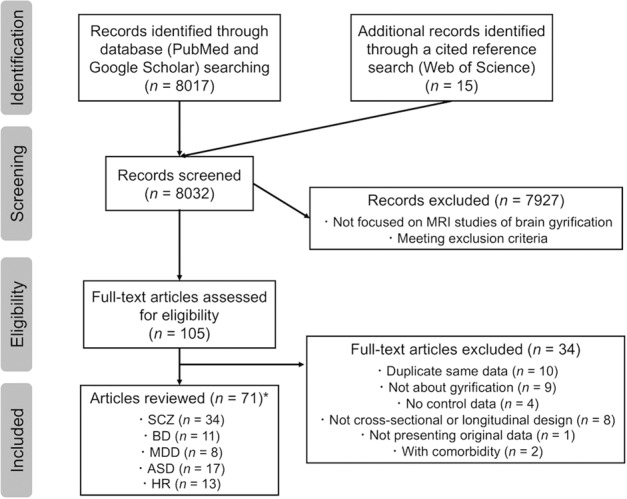
*Ten studies investigated the gyrification patterns in two or three psychiatric conditions (major psychiatric disorder or high-risk state for psychosis). ASD autism spectrum disorder, BD bipolar disorder, HR high risk for psychosis, MDD major depressive disorder; MRI magnetic resonance imaging, SCZ schizophrenia.
The quality assessment of the literature was conducted by using the Newcastle-Ottawa Quality Assessment Scale (NOS)40 arranged for a case-control study design. This scale contains six items, categorized into two dimensions including selection and comparability, ranging between zero (the lowest quality) up to six (the highest quality) stars. In this review, the NOS stars ranged from four to six and the average was 5.6 (Supplemental Table S1), suggesting that the quality of the included studies was relatively high on average.
Development of measures to assess cortical folding
In earlier studies, the GI, the ratio of the total folded cortical surface over the perimeter of the brain that was manually traced on 2-D coronal sections41, was standardly used to quantify cortical folding due to its easy interpretation and implemental simplicity. After that, the automated GI (A-GI) was developed by adapting the GI to a fully automated 2-D method in order to improve reliability and reproducibility in larger cohorts42. However, these methods were two-dimensional analyses for limited brain areas, which may be biased by slice orientation, buried sulci, and anomalies of sublobe regions. With the recent technological developments in 3-D image reconstruction and surface-based morphometry, the measurement of local GI (LGI) has widely been accepted in psychiatric researches. The index is calculated by dividing the pial surface area by the corresponding outer surface area for each vertex43. The measurement of LGI has methodological advantages over other methods by taking into account the inherent three-dimensional nature of the entire cortical surface. Several studies employed other approaches, such as the mean curvature computed by normalized summation of the amplitude and frequency of the simulated folding44 and the sulcal index (SI), which is calculated by the ratio between the sulcal area and the area of the corresponding outer cortex45, while using the same 3-D mesh of the cortical surface.
Lifetime trajectory of brain gyrification pattern in healthy subjects
MRI studies in healthy subjects, including longitudinal assessments, revealed the typical developmental trajectory of the GI. After birth, the entire cortical GI increased and reached its peak by 2 years of age (approximately 20% increase at 2 years)46, and then decreased with decreasing velocity thereafter18,47. During adolescence, the GI was reported to decline steadily with an annual decrease of around 1%, especially in the frontal and occipital cortices, where sulcal widening and reduced sulcal depth occurred together48.
Findings in individuals at high risk for psychosis
Several MRI studies have reported anomalous patterns of brain gyrification prior to the onset of overt psychotic symptoms (Supplemental Table S2). An increased GI in the prefrontal cortex, especially on the right hemisphere, was reported in siblings of SCZ patients, especially in those who subsequently developed SCZ, compared with healthy controls49–51. Individuals with at-risk mental state (ARMS) have an increased GI in widespread cortical areas regardless of the outcome (i.e., future transition or non-transition to psychosis)17,24, whereas the left occipital hyper-gyrification pattern may be specific to the ARMS subgroup who later develop psychosis17. Subjects with schizotypal features, who are also at increased risk for developing psychosis, exhibit an increased GI in the prefrontal and parietal cortices52,53.
Findings in patients with major psychiatric disorders
Based on previous MRI findings being weighted according to the number of studies, sample size for studies, representativeness of cases (one of the NOS components), and methodological advantage to assess gyral patterns [priority to the measures using 3-D mesh model of the cortical surface (e.g., LGI, mean curvature, SI)], the gyrification pattern trajectories in each illness were summarized in Fig. 2 and Supplemental Tables S3-6. We combined the results of region-of-interest (ROI) -based studies, which could have overlooked anomalous gyral patterns outside the ROI, and whole-brain voxel- or vertex- wised studies, but the summary findings remained essentially the same even when we excluded 8 ROI-based studies and assessed only 53 whole-brain analyses.
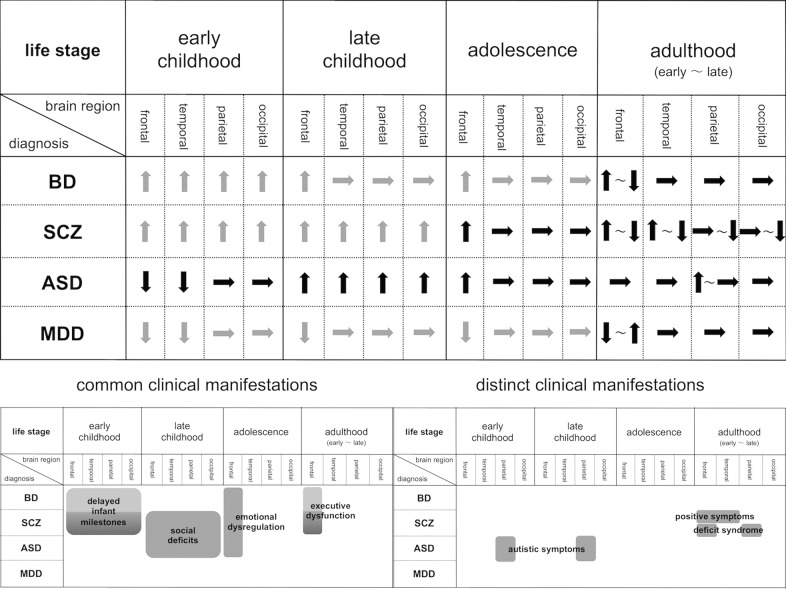
Fig. 2. Putative gyral pattern trajectories of the frontal cortex in major psychiatric disorders.
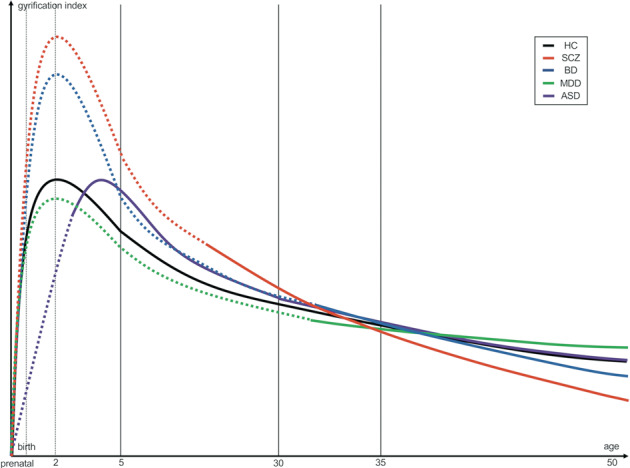
Dotted line: Presumed trajectory of gyrification pattern suggested from findings reported in neighboring developmental periods and other indicators (e.g., gene expression, gray matter volume, and white mater integrity); Solid line: Dominant trajectory of gyrification pattern based on previous gyral findings being weighted according to the number of studies, sample size for studies, representativeness of cases, and methodological advantage to assess gyral patterns. ASD autism spectrum disorder, BD bipolar disorder, HC healthy controls, MDD major depressive disorder, SCZ schizophrenia.
Adolescent and young adult patients with SCZ exhibit an increased GI or curvature in the frontal and other widespread regions compared with healthy controls23,24,28,32,36,51,54–58, whereas patients in their mid-thirties and older mostly exhibit a decreased GI or SI in widespread cortical areas, particularly on the frontal cortices18,26,45,59–64. Although few MRI studies have examined the anomalous gyral patterns in mood disorders during adolescence and young adulthood, patients with BD likely exhibit increased frontal curvature during their mid-thirties compared with healthy controls28, but have reduced frontal GI or SI in their early forties and afterwards21,61. Compared with healthy controls, patients with MDD exhibit reduced frontal GI during their thirties22,33, and then increased frontal GI around their forties and afterwards27,31,65. Patients with ASD have delayed gyral development compared with typically developing controls during infancy19, transition to an increased GI in widespread cortical regions during childhood25,30,35,66–68, and lastly approach normal gyrification pattern in adulthood and thereafter4,29,34. Given the lack of longitudinal evidence, this framework is not able to explain why different trajectories should occur in disorders with differing phenotypes, and our model (Fig. 2) is amenable for further revisions.
In addition, we divided the patients with major psychiatric disorders (except the ASD) into those with early (illness duration <3 years), middle (3–10 years), and late (10 years and afterwards) illness stages, and then conducted a subgroup analysis on the basis of 33 relevant MRI studies to see how the disease process could impact on gyrification pattern changes (Supplemental Fig. S1); the results supported the role of illness stages on gyrification pattern for these psychiatric disorders.
Relationship of brain gyrification pattern with clinical symptoms and cognitive performance (Supplemental Table S7)
The severity of positive psychotic symptoms, including verbal hallucination, in SCZ was reported to be correlated with LGI in the right frontal and temporo-limbic region32,57. SCZ patients with persistent negative symptoms (i.e., deficit subtype) are characterized by greater frontal and lesser parietal hyper-gyrification patterns compared with those without deficit syndrome36. Neurological soft signs (NSS) in SCZ patients, which are subtle neurological deficits in the execution of motor or sensory tasks69 and potentially link to cerebellar gyral changes70, are negatively associated with LGI in widespread cortical areas29. In patients with MDD, the severity of depressive mood and number of depressive episodes are inversely correlated with LGI in the lateral frontal cortices, lateral parietal cortices, and fusiform gyri33,65. Autistic symptoms, especially communication impairments and repetitive behaviors, are significantly correlated with the right parietal GI in patients with ASD and in their unaffected co-twins19,67,71. The right prefrontal GI is correlated not only with general cognition in patients with different mental states52,61,67, but also with specific cognitive subdomains such as executive function in SCZ32, working memory in BD61, and metacognition in ASD35.
Mechanism of brain gyrification
Developmental mechanisms of cortical folding, which are necessary for obtaining higher brain functions72, remain elusive. However, rapid and efficient genetic manipulation techniques in animals, in addition to computational neuroscience approaches, have been employed to clarify the mechanisms73. Moreover, neuroimaging studies in patients with neuropsychiatric disorders have reported associations of gyral pattern changes with various genotypes31,74,75. Here, we propose possible mechanisms of brain gyrification by dividing the gyral constructions into (1) primary gyri and (2) higher-order gyri. We further discuss how the upstream factors controlling cortical folding are involved in forming disease-specific gyral pattern trajectories.
Primary gyri—molecular-level model
As the positions of primary gyri are roughly the same among individuals76, their folding patterns may be mainly determined by intrinsic genetic mechanisms. Although previous studies have shown that main gyral formation can be modulated by several genes such as Cdk577 and Tbr278, we focus on the fibroblast growth factor (FGF) gene in the present review, because of a relative abundance of literature that reported its relevance to psychiatric disorders. However, the genetic mechanism of FGF-2 gene should be considered with caution, because this gene was not hit by GWAS7. Altered expression of FGF-2 gene could be a consequence of other upstream processes and it would also have more downstream consequences. FGF-2 and its coupled FGF receptors (FGFRs), whose genes were differentially expressed throughout the central nervous system tissues, were reported to regulate neuronal proliferation and differentiation (on neuronal precursor cells) as well as axonal sprouting and ensheathment (on oligodendrocytes) during a developmental stage79,80. Later in life, FGF-2 and FGFRs are thought to be involved in the maintenance of neuronal tissue (on tissue lesions) as well as learning and memory (on hippocampal pyramidal cells)79. Mice lacking FGF-2, FGFR-1, and FGFR-2 expressions showed psychosis-related behavior, cognitive deficits, and increased anxiety that coincided with cortical volume reduction, while administration of FGF-2 could ameliorate depression-like behaviors in animals81. A series of experimental approaches using gyrencephalic mammal ferrets82–85 led to a hypothesized pathway, where FGF signaling induces progenitor cell proliferation in the subventricular zone (SVZ), resulting in a neuronal increase preferentially in cortical upper layers that is responsible for protrusion of the cortex (Fig. 3).
Fig. 3. Assumed mechanisms of brain gyrification.
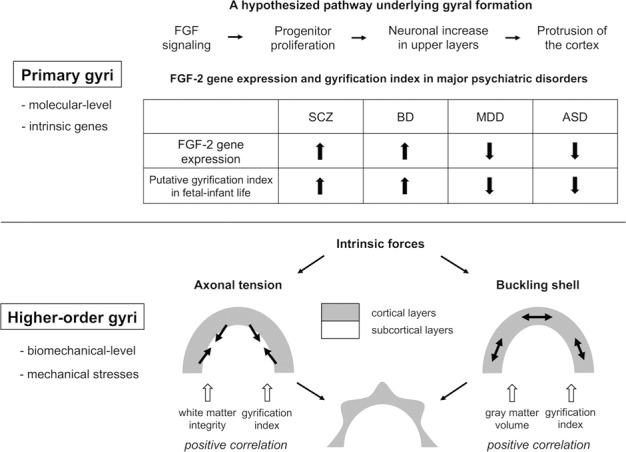
Upper: Molecular-level mechanisms in primary gyri (mainly affected by intrinsic genes). Below: Biomechanical-level mechanisms in higher-order gyri (mainly affected by mechanical stress). ASD autism spectrum disorder, BD bipolar disorder, FGF fibroblast growth factor, MDD major depressive disorder, SCZ schizophrenia.
While multiple genes regulate SVZ progenitors in the developing cerebral cortex86, overexpression of the FGF-2 gene in the dorsolateral prefrontal cortex87 commonly observed in patients with SCZ and BD may be implicated in increased GI during their cortical development. Some findings of elevated FGF-2 serum protein level in both SCZ and BD88–90, which could be directly related to FGF-2 level in brain capillaries through blood-brain barrier91, might also reinforce the evidence suggestive of an increased FGF-2 signaling in brain. In contrast, reduced FGF-2 transcripts in the frontal cortex and hippocampus in patients with MDD92,93, and lower level of serum FGF-2 in children with ASD94 may be related to reduced GI during gyral formation.
Higher-order gyri—biomechanical-level model
The positions of higher-order gyri are less uniformly distributed among individuals, suggesting that they are affected by mechanical stress rather than genetic loads. Two mechanistic explanations focusing on the intrinsic forces may be mainly involved in gyral pattern changes after childhood (Fig. 3). One is a “buckling shell” model: if the surface shell (e.g., cortical layers) expands more rapidly than the core shell (e.g., subcortical layers), then the shell eventually buckles95. Another is an “axonal tension” model: tension along obliquely oriented axonal trajectories between adjacent cortical regions may cause tangential force components that induce the cortical folds96. These potential mechanisms may also be modulated by a number of genes implicated in several developmental processes of the brain97.
As gray matter volume98 and white matter integrity71,99 are positively correlated with GI under both healthy and pathological conditions, the gyrification pattern trajectory unique to each disorder may be partly explained by longitudinal changes in cortical morphology and/or white matter pathology.
Patients with SCZ during adolescence and adulthood likely exhibit progressive gray matter reduction in the fronto-temporo-parietal region100–102 in addition to white matter integrity disruption in the peri-sylvian area103. BD patients also exhibit prefrontal gray matter volume loss over time104, although the degree of loss is smaller than that in SCZ105. These findings may reflect the longitudinal GI decrease in the corresponding regions. Faster global cortical enlargement during childhood106 and slower frontotemporal gray matter expansion thereafter107 in ASD patients than in typically developing controls may affect the gyral pattern trajectory of ASD. Patients with MDD during late adulthood demonstrate neither gray matter reduction over time108 nor white matter microstructural alterations109, supporting the low reduction rate of GI around the frontal region.
Implication of anomalous gyral pattern
Gyrification pattern and neural connectivity
An experimental study on the consequences of prefrontal resection revealed the eruption of additional fissures around the lesion and rerouting of intact cortico-striatal projections110. Recent multimodal approaches demonstrated that LGI alterations and changes in the degree centrality (DC)64 or radial diffusivity99 were located in the overlapping region in SCZ patients, and further that speech connectedness correlating with LGI and DC explained a thought disturbance, cognitive impairment, and functional outcome, irrespective of diagnostic boundaries111. Taken together, it is suggested that abnormalities in local GI are related to brain dysconnectivity of the corresponding area as neural substrates for various clinical symptoms and cognitive performance.
Brain dysconnectivity in major psychiatric disorders
Previous neuroimaging studies demonstrated disrupted functional or structural connectivity in numerous psychiatric disorders by adopting a transdiagnostic approach5.
Both SCZ and BD patients have compromised connectivity centered on the frontal region112,113, but the hypo-connectivity within the frontoparietal executive network may be more marked in SCZ patients than in BD patients114. Young patients with SCZ and ASD likely exhibit shared atypical default mode network (DMN) and salience network (SN) functional connectivities, which are related to the social functioning score115. A large-scale multidiagnostic comparison of diffusion tensor imaging (DTI) data suggested white matter microstructural abnormalities of the corpus callosum commonly in SCZ, BD, and ASD patients, possibly reflecting disturbed extensive networks subserving emotional regulation116, but alterations of the tracts connecting neocortical areas may be specific to SCZ patients109. On the other hand, MDD patients do not have altered white matter connectivity109. Functional disconnectivity in the frontal and temporo-limbic regions may underlie positive psychotic symptoms in SCZ117,118, whereas altered intracortical couplings in frontoparietal brain circuits may be associated with prominent negative symptoms119. Children with ASD were reported to have increased short-range connectivities within the frontal lobe120 but reduced long-range connectivities across different brain areas121. Among them, altered functional connectivities of the somatosensory cortex, which are considered to be disease-specific changes, may be associated with autistic symptoms such as social communication deficits122.
In summary, patients with major psychiatric disorders may have both common and distinct connectivity abnormalities in neural circuits involving several brain regions, some of which can explain certain clinical features.
Overarching model that spans the diagnoses
Based on the patterns of local connectivity and variations in neurodevelopmental gene expression across disorders described above, commonly and differentially altered gyrification patterns in patients with major psychiatric disorders may represent common and distinct clinical manifestations. We propose an overarching model that spans the diagnoses to clarify how deviated gyral pattern trajectories map onto clinical manifestations (Fig. 4).
Fig. 4. Overarching model that spans the diagnoses.
Upper: The gyrification findings in patients with major psychiatric disorders at varying stages of life compared with healthy controls. Gray arrow: Presumed direction of gyral pattern changes suggested from findings reported in neighboring developmental periods and other indicators (e.g., gene expression, gray matter volume, and white mater integrity); Black arrow: Dominant direction of gyral pattern changes based on previous gyral findings being weighted according to the number of studies, sample size for studies, representativeness of cases, and methodological advantage to assess gyral patterns. Below: Concept maps showing common or distinct clinical manifestations mapped onto deviated gyrification patterns across psychiatric disorders. ASD autism spectrum disorder, BD bipolar disorder, MDD major depressive disorder, SCZ schizophrenia.
Common clinical manifestations
SCZ and BD
As the NSS in SCZ patients is correlated with both LGI29 and delayed childhood development123, the widespread hyper-gyrification pattern in early childhood presumed in SCZ and BD patients may underlie their delayed infant milestones (e.g., not walking or speaking at year 1)124. Such a delay is greater in SCZ patients than in BD patients125, probably due to the greater genetic burden of neurodevelopmental risk in SCZ patients126. Longitudinal reductions of the frontal GI around early adulthood and thereafter commonly observed in SCZ and BD patients (more prominent changes in SCZ patients than in BD patients) may be associated with progressive frontal gray matter reduction that is also commonly observed in SCZ102 and BD104, and is related to executive dysfunction127. As transdiagnostic impairments mainly in the anterior-cingulo-insular network in chronic SCZ and BD patients link to general executive dysfunction128, the cognitive deficit may result from common frontal-related disconnection.
SCZ and ASD
The shared widespread hyper-gyrification pattern suggested in SCZ and ASD patients during late childhood is expected to be related to social deficits (e.g., impaired social interaction, disabilities in communication, and restricted interests). The oxytocin system is known to play a key role in social cognition and behavior129. The oxytocin receptor (OXTR) genotype was reported to affect brain functional connectivity130. As a neural correlate of social deficits shared in ASD and SCZ114, disturbed functional connectivities in diverse cortical regions, including DMN/SN-related brain regions, may partly be derived from genetic variations in the OXTR gene130, which are associated with the risk of both disorders131,132.
SCZ, BD, and ASD
Emotional dysregulation during adolescence, which is commonly observed in conventionally defined diagnoses, may be explained in part by the hyper-gyrification pattern common to SCZ, BD, and ASD patients in the frontal region. Such dysregulation may be mediated by abnormal emotional regulation circuitry (e.g., uncinate fasciculus and forceps minor)133. A common relationship between right prefrontal GI and general cognition in a range of psychiatric disorders suggests a neural mechanism shared across diseases134, whereas distinct relationships between right prefrontal GI and cognitive subdomains by each disorder can reflect a set of discrete dysfunctions135. Thus, further studies are required to examine which hypotheses explain the associations between anomalous patterns of focal gyrification and cognitive impairments in major psychiatric disorders.
Distinct clinical manifestations
SCZ
Disturbed functional connectivity of the dorsal prefrontal cortex and anterior temporal or posterior inferior parietal cortices are reported as schizophrenia-specific impairments (especially in adulthood), possibly underlying their unique symptomatology128. As supported by the relationships of fronto-temporo-limbic disconnectivity with delusions, hallucinations, and disorganization symptoms in SCZ patients117,118, anomalous gyral patterns in the corresponding region may lead to the generation of positive psychotic symptoms. For verbal hallucinations in particular, aberrant connectivity of the arcuate fasciculus136 may lead to a series of abnormal activation, mainly in the language-related area as follows:137 (1) Hypofunction of the frontal midline structure [i.e., poor supplementary motor area activation138] activates the speech area; (2) increased activation in the primary auditory cortex139 converts the generated inner speech into external language stimuli; and (3) altered neural circuit of self-reflective processing [i.e., deviant activity in the right inferior parietal lobule140] gives otherness to the voice, resulting in the verbal hallucination. In addition, deviated patterns of frontoparietal gyrification may be involved in deficit syndrome in SCZ patients. The medial prefrontal and lateral parietal regions constitute the principal part of the DMN141, whose abnormalities can induce severe primary negative symptoms (e.g., anhedonia and avolition or apathy)142,143. However, a recent study demonstrates that functional dysconnectivity seen in SCZ patients seems to be driven by genetic diathesis rather than clinical expression144. Hence, these maps should be interpreted with caution.
BD
Relationships between the specific clinical symptoms of BD and gyrification pattern or brain connectivity have not been well documented.
ASD
Although ASD patients do not meet developmental milestones within typical limits145, they exhibit the frontotemporal hypo-gyrification pattern in early childhood. ASD patients have different developmental signs (e.g., pragmatic aspects of language use and lack of imagination) than SCZ patients146, which may be due to a distinct genetic mechanism. Autistic symptoms, such as impaired social reciprocity and communication, in ASD children may partly be explained by parietal gyral pattern changes. The findings of disturbed connectivity and deactivation in the somatosensory cortex122,147, which are correlated with autistic symptoms, support this postulation. Increased bottom-up and reduced top-down processing in the somatosensory cortex in ASD, presumed by increased long-range feedforward connectivity and reduced long-range feedback connectivity in the comparable area, can be implicated in some core features of ASD148.
MDD
Depressive symptoms in MDD patients were reported to correlate with gyral pattern changes in the lateral frontal cortices, lateral parietal cortices, and fusiform gyri33,65. Although depression and its-related pathopsychological characteristics (e.g., depressive rumination and over-general autobiographical memory) in MDD patients are associated with compromised connectivity in the affective network and DMN149,150, attenuated frontal-striatal reward network connectivity in proportion to depression severity is common in BD and MDD patients151. A recent cluster analysis demonstrated that a melancholia component is distributed equivalently across conventional psychiatric diagnoses152. Further studies are warranted to clarify whether depressive symptoms and their neural substrate are transdiagnostic or disease-specific.
Future direction
Reproducibility and generalizability of the findings
Although increasing neuroimaging evidence supports anomalous patterns of brain gyrification in several psychiatric disorders, there have been few longitudinal studies investigating the gyral pattern trajectory in each disorder or direct comparisons of gyrification patterns among major psychiatric disorders. Sample sizes in previous studies on cortical gyrification were small [hitherto largest sample of patients (n = 207)26] as opposed to those on other cortical indices [hitherto largest sample of patients (n > 4000)153 in a previous study investigating cortical thickness]. Thus, confirmatory studies using large-scale multi-site longitudinal brain MRI datasets are needed.
While we have adopted one-illness-one-pattern approach throughout this review, potential anatomical heterogeneity within each psychiatric disorder154 should also be tested in future gyrification studies using distinct subgroups.
Although the LGI is a widely accepted measure of brain gyrification, its linkage with other indicators remains unclear and the methodology has room for improvement. As the gyrification patterns may be closely interrelated with white matter integrity and implicate connectional characteristics of corresponding regions, confirming the relationship between LGI and advanced DTI measures [myelin content155 and corrected DTI indices156] may be useful. As detecting the disturbance of coordinated maturation among brain areas can provide more information about neurodevelopmental integrity than just exploring the localized changes in the brain157, gyrification-based connectomes adopting a graph theoretical approach are being investigated158. A novel indicator focusing on the gyral hinges159, considering a hierarchical organization within the gyral system, is also being developed.
Common and distinct biotypes on the basis of brain structural features among psychiatric disorders may provide the biological basis for making appropriate animal models. Together with recent progress in experimental biomedical research [e.g., Ferret models73] that will enable us to assess the models, we may be able to further our understanding of the pathophysiology of psychiatric disorders from the perspective of anomalous gyral patterns.
Clinical significance
A previous study demonstrated that the deviated gyrification patterns can differentiate patients with schizophrenia from healthy controls with a favorable classification accuracy160. However, there have been only a few group comparisons of the gyrification pattern among psychiatric disorders [SCZ vs BD64; SCZ vs ASD29]. There are also only a few studies that examined the association between gyral pattern changes and later psychosis onset17,158 or antipsychotic treatment response in psychoses161. As mentioned above, establishing the relationships between deviated gyrification patterns and clinical phenotypes may aid in the development of biomarkers for both prediction (e.g., onset and treatment response) and diagnosis (e.g., diagnostic assistance and discrimination among psychiatric disorders). Such biomarkers will help optimize the prediction and diagnosis of psychiatric disorders.
Current diagnostic criteria for psychiatric disorders rely only on descriptive syndromes based upon a consensus of experts. In contrast, the Psychiatric Genomics Consortium previously reported cross-disorder effects of genetic variants, highlighting shared and distinct biological causes of major psychiatric disorders6. Similarly, as anomalous gyral patterns play a pleiotropic role in psychopathology, gyral findings may provide a novel nosology informed by neural circuits, which is beyond the present classifications in the psychiatric field.
Conclusion
This review provides the transdiagnostic considerations of anomalous gyrification patterns in major psychiatric disorders. Diverse alterations of gyrification patterns at varying stages of major psychiatric disorders may reflect both molecular and biomechanical mechanisms. Patients with major psychiatric disorders exhibit commonly and differentially altered gyrification patterns, which suggests corresponding neural circuit changes involving the frontal and other brain regions, representing common (e.g., delayed infant milestones, social deficits, emotional dysregulation, and executive dysfunction) and distinct (e.g., autistic symptoms, psychotic symptoms, and deficit syndrome) clinical manifestations. A comprehensive understanding of the role of brain gyrification pattern in the pathophysiology of major psychiatric disorders may aid in the development of objective biomarkers for both prediction and diagnosis, as well as provide a novel nosology informed by neural circuits.
Supplementary information
Acknowledgements
This study was funded by grants to D.S. (Wakate No. 18K15509, Kiban B No. 19H03579), T.T. (Kiban C No. 18K07550), Y.T. (Kiban C No. 18K07549), M.S. (Kiban B No. 20H03598) from the Japanese Society for the Promotion of Science, and Health and Labour Sciences Research Grants for Comprehensive Research on Persons with Disabilities from the Japan Agency for Medical Research and Development (AMED) to M.S. (No. 16dk0307029h0003). D.S. and Y.T. were supported by grants from the SENSHIN Medical Research Foundation. D.S. was also supported by THE HOKURIKU BANK Grant-in-Aid for Young Scientists. The authors would like to thank Professor. Hiroshi Kawasaki (Department of Medical Neuroscience, Graduate School of Medical Sciences, Kanazawa University, Ishikawa, Japan) for his advice about the mechanisms of gyrus formation. The authors also wish to appreciate Dr. Sho Moriguchi and Dr. Shinichiro Nakajima (Department of Neuropsychiatry, Keio University School of Medicine, Tokyo, Japan) for their advice about methodology in conducting a systematic review.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01297-8.
References
- 1.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 2.Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr. Opin. Pharmacol. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Fornito A, et al. In vivo evidence for early neurodevelopmental anomaly of the anterior cingulate cortex in bipolar disorder. Acta Psychiatr. Scand. 2007;116:467–472. doi: 10.1111/j.1600-0447.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 4.Koolschijn P, Geurts HM. Gray matter characteristics in mid and old aged adults with ASD. J. Autism Dev. Disord. 2016;46:2666–2678. doi: 10.1007/s10803-016-2810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitelman SA. Transdiagnostic neuroimaging in psychiatry: a review. Psychiatry Res. 2019;277:23–38. doi: 10.1016/j.psychres.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173:1705–1715.e1716. doi: 10.1016/j.cell.2018.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larroche, J. C. in Development of the Nervous System in Early Life (ed Falkner, F.) 257–296 (WS Sanders, Philadelphia, 1966).
- 9.White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Cortical structural abnormalities in very preterm children at 7 years of age. Neuroimage. 2015;109:469–479. doi: 10.1016/j.neuroimage.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papani C, et al. Altered cortical gyrification in adults who were born very preterm and its associations with cognition and mental health. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:640–650. doi: 10.1016/j.bpsc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Smith GN, et al. Cortical morphology and early adverse birth events in men with first-episode psychosis. Psychol. Med. 2015;45:1825–1837. doi: 10.1017/S003329171400292X. [DOI] [PubMed] [Google Scholar]
- 13.Haukvik UK, et al. Cortical folding in Broca’s area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol. Med. 2012;42:1329–1337. doi: 10.1017/S0033291711002315. [DOI] [PubMed] [Google Scholar]
- 14.Sowell ER, et al. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 15.Cannon TD, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? a meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol. Psychiatry. 2015;78:403–412. doi: 10.1016/j.biopsych.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Sasabayashi D, et al. Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol. Psychiatry. 2017;82:737–745. doi: 10.1016/j.biopsych.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Cao B, et al. Lifespan gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci. Rep. 2017;7:511. doi: 10.1038/s41598-017-00582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libero LE, Schaer M, Li DD, Amaral DG, Nordahl CW. A longitudinal study of local gyrification index in young boys with autism spectrum disorder. Cereb. Cortex. 2019;29:2575–2587. doi: 10.1093/cercor/bhy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palaniyappan L, et al. Gyrification of Broca’s region is anomalously lateralized at onset of schizophrenia in adolescence and regresses at 2 year follow-up. Schizophr. Res. 2013;147:39–45. doi: 10.1016/j.schres.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Penttilä J, et al. Cortical folding difference between patients with early-onset and patients with intermediate-onset bipolar disorder. Bipolar Disord. 2009;11:361–370. doi: 10.1111/j.1399-5618.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, et al. Decreased gyrification in major depressive disorder. Neuroreport. 2009;20:378–380. doi: 10.1097/WNR.0b013e3283249b34. [DOI] [PubMed] [Google Scholar]
- 23.Schultz CC, et al. Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophr. Res. 2010;123:137–144. doi: 10.1016/j.schres.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Tepest R, et al. Morphometry of structural disconnectivity indicators in subjects at risk and in age-matched patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:15–24. doi: 10.1007/s00406-012-0343-6. [DOI] [PubMed] [Google Scholar]
- 25.Wallace GL, et al. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136:1956–1967. doi: 10.1093/brain/awt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesvåg R, et al. Reduced brain cortical folding in schizophrenia revealed in two independent samples. Schizophr. Res. 2014;152:333–338. doi: 10.1016/j.schres.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Nixon NL, et al. Biological vulnerability to depression: linked structural and functional brain network findings. Br. J. Psychiatry. 2014;204:283–289. doi: 10.1192/bjp.bp.113.129965. [DOI] [PubMed] [Google Scholar]
- 28.Nenadic I, et al. Prefrontal gyrification in psychotic bipolar I disorder vs. schizophrenia. J. Affect Disord. 2015;185:104–107. doi: 10.1016/j.jad.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Hirjak D, et al. Neuroanatomical markers of neurological soft signs in recent-onset schizophrenia and asperger-syndrome. Brain Topogr. 2016;29:382–394. doi: 10.1007/s10548-015-0468-9. [DOI] [PubMed] [Google Scholar]
- 30.Yang DY, Beam D, Pelphrey KA, Abdullahi S, Jou RJ. Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Mol. Autism. 2016;7:11. doi: 10.1186/s13229-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han KM, et al. Local gyrification index in patients with major depressive disorder and its association with tryptophan hydroxylase-2 (TPH2) polymorphism. Hum. Brain Mapp. 2017;38:1299–1310. doi: 10.1002/hbm.23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasabayashi D, et al. Increased frontal gyrification negatively correlates with executive function in patients with first-episode schizophrenia. Cereb. Cortex. 2017;27:2686–2694. doi: 10.1093/cercor/bhw101. [DOI] [PubMed] [Google Scholar]
- 33.Depping MS, et al. Common and distinct patterns of abnormal cortical gyrification in major depression and borderline personality disorder. Eur. Neuropsychopharmacol. 2018;28:1115–1125. doi: 10.1016/j.euroneuro.2018.07.100. [DOI] [PubMed] [Google Scholar]
- 34.Maier S, et al. Cortical properties of adults with autism spectrum disorder and an IQ > 100. Psychiatry Res. Neuroimaging. 2018;279:8–13. doi: 10.1016/j.pscychresns.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Kohli JS, et al. Local cortical gyrification is increased in children with autism spectrum disorders, but decreases rapidly in adolescents. Cereb. Cortex. 2019;29:2412–2423. doi: 10.1093/cercor/bhy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takayanagi Y, et al. Altered brain gyrification in deficit and non-deficit schizophrenia. Psychol. Med. 2019;49:573–580. doi: 10.1017/S0033291718001228. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yung AR, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust. N. Z. J. Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 39.Miller TJ, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 40.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 41.Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat. Embryol. (Berl.). 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- 42.Moorhead TW, et al. Automated computation of the Gyrification Index in prefrontal lobes: methods and comparison with manual implementation. Neuroimage. 2006;31:1560–1566. doi: 10.1016/j.neuroimage.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 43.Schaer M, et al. A surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- 44.Luders E, et al. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage. 2006;29:1224–1230. doi: 10.1016/j.neuroimage.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 45.Cachia A, et al. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage. 2008;39:927–935. doi: 10.1016/j.neuroimage.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 46.Li G, et al. Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. J. Neurosci. 2014;34:4228–4238. doi: 10.1523/JNEUROSCI.3976-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raznahan A, et al. How does your cortex grow? J. Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alemán-Gómez Y, et al. The human cerebral cortex flattens during adolescence. J. Neurosci. 2013;33:15004–15010. doi: 10.1523/JNEUROSCI.1459-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogeley K, et al. Right frontal hypergyria differentiation in affected and unaffected siblings from families multiply affected with schizophrenia: a morphometric mri study. Am. J. Psychiatry. 2001;158:494–496. doi: 10.1176/appi.ajp.158.3.494. [DOI] [PubMed] [Google Scholar]
- 50.Harris JM, et al. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol. Psychiatry. 2004;56:182–189. doi: 10.1016/j.biopsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Falkai P, et al. Disturbed frontal gyrification within families affected with schizophrenia. J. Psychiatr. Res. 2007;41:805–813. doi: 10.1016/j.jpsychires.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Stanfield AC, et al. Increased right prefrontal cortical folding in adolescents at risk of schizophrenia for cognitive reasons. Biol. Psychiatry. 2008;63:80–85. doi: 10.1016/j.biopsych.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Sasabayashi D, et al. Increased brain gyrification in the schizophrenia spectrum. Psychiatry Clin. Neurosci. 2020;74:70–76. doi: 10.1111/pcn.12939. [DOI] [PubMed] [Google Scholar]
- 54.Harris JM, et al. Gyrification in first-episode schizophrenia: a morphometric study. Biol. Psychiatry. 2004;55:141–147. doi: 10.1016/S0006-3223(03)00789-3. [DOI] [PubMed] [Google Scholar]
- 55.Narr KL, et al. Abnormal gyral complexity in first-episode schizophrenia. Biol. Psychiatry. 2004;55:859–867. doi: 10.1016/j.biopsych.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 56.Schultz CC, et al. The visual cortex in schizophrenia: alterations of gyrification rather than cortical thickness-a combined cortical shape analysis. Brain Struct. Funct. 2013;218:51–58. doi: 10.1007/s00429-011-0374-1. [DOI] [PubMed] [Google Scholar]
- 57.Kubera KM, et al. Cortical folding abnormalities in patients with schizophrenia who have persistent auditory verbal hallucinations. Eur. Neuropsychopharmacol. 2018;28:297–306. doi: 10.1016/j.euroneuro.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 58.Spalthoff R, Gaser C, Nenadić I. Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophr. Res. 2018;202:195–202. doi: 10.1016/j.schres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Sallet PC, et al. Reduced cortical folding in schizophrenia: an MRI morphometric study. Am. J. Psychiatry. 2003;160:1606–1613. doi: 10.1176/appi.ajp.160.9.1606. [DOI] [PubMed] [Google Scholar]
- 60.Bonnici HM, et al. Pre-frontal lobe gyrification index in schizophrenia, mental retardation and comorbid groups: an automated study. Neuroimage. 2007;35:648–654. doi: 10.1016/j.neuroimage.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 61.McIntosh AM, et al. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr. Scand. 2009;119:192–198. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- 62.Ronan L, et al. Consistency and interpretation of changes in millimeter-scale cortical intrinsic curvature across three independent datasets in schizophrenia. Neuroimage. 2012;63:611–621. doi: 10.1016/j.neuroimage.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nanda P, et al. Local gyrification index in probands with psychotic disorders and their first-degree relatives. Biol. Psychiatry. 2014;76:447–455. doi: 10.1016/j.biopsych.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr. Bull. 2014;40:675–684. doi: 10.1093/schbul/sbt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmitgen MM, et al. Aberrant cortical neurodevelopment in major depressive disorder. J. Affect Disord. 2019;243:340–347. doi: 10.1016/j.jad.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 66.Hardan AY, Jou RJ, Keshavan MS, Varma R, Minshew NJ. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res. 2004;131:263–268. doi: 10.1016/j.pscychresns.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Kates WR, Ikuta I, Burnette CP. Gyrification patterns in monozygotic twin pairs varying in discordance for autism. Autism Res. 2009;2:267–278. doi: 10.1002/aur.98. [DOI] [PubMed] [Google Scholar]
- 68.Jou RJ, Minshew NJ, Keshavan MS, Hardan AY. Cortical gyrification in autistic and Asperger disorders: a preliminary magnetic resonance imaging study. J. Child Neurol. 2010;25:1462–1467. doi: 10.1177/0883073810368311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schröder J, et al. Neurological soft signs in schizophrenia. Schizophr. Res. 1991;6:25–30. doi: 10.1016/0920-9964(91)90017-L. [DOI] [PubMed] [Google Scholar]
- 70.Schmitt A, et al. Reduction of gyrification index in the cerebellar vermis in schizophrenia: a post-mortem study. World J. Biol. Psychiatry. 2011;12:99–103. doi: 10.3109/15622975.2011.598379. [DOI] [PubMed] [Google Scholar]
- 71.Schaer M, et al. Decreased frontal gyrification correlates with altered connectivity in children with autism. Front Hum. Neurosci. 2013;7:750. doi: 10.3389/fnhum.2013.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun T, Hevner RF. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat. Rev. Neurosci. 2014;15:217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawasaki H. Molecular investigations of the development and diseases of cerebral cortex folding using gyrencephalic mammal ferrets. Biol. Pharm. Bull. 2018;41:1324–1329. doi: 10.1248/bpb.b18-00142. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi T, et al. The disrupted-in-schizophrenia-1 Ser704Cys polymorphism and brain neurodevelopmental markers in schizophrenia and healthy subjects. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;56:11–17. doi: 10.1016/j.pnpbp.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Palaniyappan L, et al. Reduced prefrontal gyrification in carriers of the dopamine d4 receptor 7-repeat allele with attention deficit/hyperactivity disorder: a preliminary report. Front Psychiatry. 2019;10:235. doi: 10.3389/fpsyt.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welker, W. in Cerebral Cortex (eds Jones, E. G., Peters, A.) 3–136 (Springer, Boston, M. A., 1990).
- 77.Magen D, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with a loss-of-function mutation in CDK5. Hum. Genet. 2015;134:305–314. doi: 10.1007/s00439-014-1522-5. [DOI] [PubMed] [Google Scholar]
- 78.Hevner RF. Intermediate progenitors and Tbr2 in cortical development. J. Anat. 2019;235:616–625. doi: 10.1111/joa.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- 80.Furusho M, Dupree JL, Nave KA, Bansal R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J. Neurosci. 2012;32:6631–6641. doi: 10.1523/JNEUROSCI.6005-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terwisscha van Scheltinga AF, Bakker SC, Kahn RS, Kas MJ. Fibroblast growth factors in neurodevelopment and psychopathology. Neuroscientist. 2013;19:479–494. doi: 10.1177/1073858412472399. [DOI] [PubMed] [Google Scholar]
- 82.Masuda K, et al. Pathophysiological analyses of cortical malformation using gyrencephalic mammals. Sci. Rep. 2015;5:15370. doi: 10.1038/srep15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toda T, Shinmyo Y, Dinh Duong TA, Masuda K, Kawasaki H. An essential role of SVZ progenitors in cortical folding in gyrencephalic mammals. Sci. Rep. 2016;6:29578. doi: 10.1038/srep29578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsumoto N, Shinmyo Y, Ichikawa Y, Kawasaki H. Gyrification of the cerebral cortex requires FGF signaling in the mammalian brain. Elife. 2017;6:e29285. doi: 10.7554/eLife.29285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shinmyo Y, et al. Folding of the cerebral cortex requires Cdk5 in upper-layer neurons in gyrencephalic mammals. Cell Rep. 2017;20:2131–2143. doi: 10.1016/j.celrep.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 86.Betizeau M, et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron. 2013;80:442–457. doi: 10.1016/j.neuron.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 87.Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol. Psychiatry. 2008;64:89–97. doi: 10.1016/j.biopsych.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hashimoto K, et al. Increased levels of serum basic fibroblast growth factor in schizophrenia. Psychiatry Res. 2003;120:211–218. doi: 10.1016/S0165-1781(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 89.Liu X, et al. Elevated serum levels of FGF-2, NGF and IGF-1 in patients with manic episode of bipolar disorder. Psychiatry Res. 2014;218:54–60. doi: 10.1016/j.psychres.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 90.Li XS, et al. Increased serum FGF2 levels in first-episode, drug-free patients with schizophrenia. Neurosci. Lett. 2018;686:28–32. doi: 10.1016/j.neulet.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 91.Deguchi Y, et al. Blood-brain barrier transport of 125I-labeled basic fibroblast growth factor. Pharm. Res. 2000;17:63–69. doi: 10.1023/A:1007570509232. [DOI] [PubMed] [Google Scholar]
- 92.Evans SJ, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc. Natl Acad. Sci. USA. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res. Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 94.Esnafoglu E, Ayyıldız SN. Decreased levels of serum fibroblast growth factor-2 in children with autism spectrum disorder. Psychiatry Res. 2017;257:79–83. doi: 10.1016/j.psychres.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 95.Tallinen T, Chung JY, Biggins JS, Mahadevan L. Gyrification from constrained cortical expansion. Proc. Natl Acad. Sci. USA. 2014;111:12667–12672. doi: 10.1073/pnas.1406015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- 97.Atkinson EG, Rogers J, Mahaney MC, Cox LA, Cheverud JM. Cortical folding of the primate brain: an interdisciplinary examination of the genetic architecture, modularity, and evolvability of a significant neurological trait in pedigreed baboons (Genus Papio) Genetics. 2015;200:651–665. doi: 10.1534/genetics.114.173443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gautam P, Anstey KJ, Wen W, Sachdev PS, Cherbuin N. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav. Brain Res. 2015;287:331–339. doi: 10.1016/j.bbr.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 99.Schultz CC, et al. Increased white matter radial diffusivity is associated with prefrontal cortical folding deficits in schizophrenia. Psychiatry Res. Neuroimaging. 2017;261:91–95. doi: 10.1016/j.pscychresns.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi T, et al. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr. Res. 2009;108:49–56. doi: 10.1016/j.schres.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 101.Takahashi T, et al. A follow-up MRI study of the superior temporal subregions in schizotypal disorder and first-episode schizophrenia. Schizophr. Res. 2010;119:65–74. doi: 10.1016/j.schres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Asami T, et al. Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage. 2012;59:986–996. doi: 10.1016/j.neuroimage.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun Y, et al. Disruption of brain anatomical networks in schizophrenia: a longitudinal, diffusion tensor imaging based study. Schizophr. Res. 2016;171:149–157. doi: 10.1016/j.schres.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 104.Kalmar JH, et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J. Int. Neuropsychol. Soc. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohtani T, et al. Progressive symptom-associated prefrontal volume loss occurs in first-episode schizophrenia but not in affective psychosis. Brain Struct. Funct. 2018;223:2879–2892. doi: 10.1007/s00429-018-1634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hazlett HC, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry. 2011;68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 108.Carceller-Sindreu M, et al. Altered white matter volumes in first-episode depression: evidence from cross-sectional and longitudinal voxel-based analyses. J. Affect Disord. 2019;245:971–977. doi: 10.1016/j.jad.2018.11.085. [DOI] [PubMed] [Google Scholar]
- 109.Koshiyama D, et al. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol. Psychiatry. 2020;25:883–895. doi: 10.1038/s41380-019-0553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goldman-Rakic PS. Morphological consequences of prenatal injury to the primate brain. Prog. Brain Res. 1980;53:1–19. [PubMed] [Google Scholar]
- 111.Palaniyappan L, et al. Speech structure links the neural and socio-behavioural correlates of psychotic disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;88:112–120. doi: 10.1016/j.pnpbp.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 112.Sui J, et al. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage. 2011;57:839–855. doi: 10.1016/j.neuroimage.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baker JT, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brandl F, et al. Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biol. Psychiatry. 2019;85:573–583. doi: 10.1016/j.biopsych.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Chen H, et al. Shared atypical default mode and salience network functional connectivity between autism and schizophrenia. Autism Res. 2017;10:1776–1786. doi: 10.1002/aur.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paul LK, et al. Emotional arousal in agenesis of the corpus callosum. Int J. Psychophysiol. 2006;61:47–56. doi: 10.1016/j.ijpsycho.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 117.Asami T, et al. Abnormalities of middle longitudinal fascicle and disorganization in patients with schizophrenia. Schizophr. Res. 2013;143:253–259. doi: 10.1016/j.schres.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oertel-Knöchel V, et al. Association between symptoms of psychosis and reduced functional connectivity of auditory cortex. Schizophr. Res. 2014;160:35–42. doi: 10.1016/j.schres.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 119.Wheeler AL, et al. Further neuroimaging evidence for the deficit subtype of schizophrenia: a cortical connectomics analysis. JAMA Psychiatry. 2015;72:446–455. doi: 10.1001/jamapsychiatry.2014.3020. [DOI] [PubMed] [Google Scholar]
- 120.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 121.Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J, et al. Increased gray matter volume and resting-state functional connectivity in somatosensory cortex and their relationship with autistic symptoms in young boys with autism spectrum disorder. Front Physiol. 2017;8:588. doi: 10.3389/fphys.2017.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Papiol S, Fatjo-Vilas M, Schulze TG. Neurological soft signs in patients with schizophrenia: current knowledge and future perspectives in the post-genomics era. Transl. Dev. Psychiatry. 2016;4:30071. doi: 10.3402/tdp.v4.30071. [DOI] [Google Scholar]
- 124.Isohanni M, et al. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr. Res. 2001;52:1–19. doi: 10.1016/S0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- 125.Parellada M, Gomez-Vallejo S, Burdeus M, Arango C. Developmental differences between schizophrenia and bipolar disorder. Schizophr. Bull. 2017;43:1176–1189. doi: 10.1093/schbul/sbx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Owen MJ, O’Donovan MC. Schizophrenia and the neurodevelopmental continuum:evidence from genomics. World Psychiatry. 2017;16:227–235. doi: 10.1002/wps.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ho BC, et al. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 128.Huang CC, et al. Transdiagnostic and illness-specific functional dysconnectivity across schizophrenia, bipolar disorder, and major depressive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:542–553. doi: 10.1016/j.bpsc.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 129.Kanat M, Heinrichs M, Domes G. Oxytocin and the social brain: neural mechanisms and perspectives in human research. Brain Res. 2014;1580:160–171. doi: 10.1016/j.brainres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 130.Seeley SH, Chou YH, O’Connor MF. Intranasal oxytocin and OXTR genotype effects on resting state functional connectivity: a systematic review. Neurosci. Biobehav. Rev. 2018;95:17–32. doi: 10.1016/j.neubiorev.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 131.LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol. Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 132.Uhrig S, et al. Reduced oxytocin receptor gene expression and binding sites in different brain regions in schizophrenia: a post-mortem study. Schizophr. Res. 2016;177:59–66. doi: 10.1016/j.schres.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 133.Versace A, et al. White matter structure in youth with behavioral and emotional dysregulation disorders: a probabilistic tractographic study. JAMA Psychiatry. 2015;72:367–376. doi: 10.1001/jamapsychiatry.2014.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamashita M, et al. A prediction model of working memory across health and psychiatric disease using whole-brain functional connectivity. Elife. 2018;7:e38844. doi: 10.7554/eLife.38844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yahata N, et al. A small number of abnormal brain connections predicts adult autism spectrum disorder. Nat. Commun. 2016;7:11254. doi: 10.1038/ncomms11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hubl D, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch. Gen. Psychiatry. 2014;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 137.Kurachi M. Understanding of ‘endogeneity’ from biological psychiatry. Rinsho Seishin Igaku. 2011;40:1089–1096. [Google Scholar]
- 138.Raij TT, Riekki TJ. Poor supplementary motor area activation differentiates auditory verbal hallucination from imagining the hallucination. Neuroimage Clin. 2012;1:75–80. doi: 10.1016/j.nicl.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49:3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 140.Furuichi A, et al. Altered neural basis of self-reflective processing in schizophrenia: An fMRI study. Asian J. Psychiatr. 2019;45:53–60. doi: 10.1016/j.ajp.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 141.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee JS, Jung S, Park IH, Kim JJ. Neural basis of anhedonia and amotivation in patients with schizophrenia: the role of reward system. Curr. Neuropharmacol. 2015;13:750–759. doi: 10.2174/1570159X13666150612230333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaffer JJ, et al. Neural correlates of schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol. Neuropsychiatry. 2015;1:191–200. doi: 10.1159/000440979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo S, et al. Brain-wide functional dysconnectivity in schizophrenia: parsing diathesis, resilience, and the effects of clinical expression. Can. J. Psychiatry. 2020;65:21–29. doi: 10.1177/0706743719890174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhat AN, Landa RJ, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 2011;91:1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- 146.Anomitri C, Lazaratou H. Asperger syndrome and schizophrenia: Νeurodevelopmental continuum or separated clinical entities? Psychiatriki. 2017;28:175–182. doi: 10.22365/jpsych.2017.282.175. [DOI] [PubMed] [Google Scholar]
- 147.Christakou A, et al. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Mol. Psychiatry. 2013;18:236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khan S, et al. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain. 2015;138:1394–1409. doi: 10.1093/brain/awv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu X, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol. Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 150.Avery JA, et al. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Satterthwaite TD, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258–2268. doi: 10.1038/npp.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grisanzio KA, et al. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2018;75:201–209. doi: 10.1001/jamapsychiatry.2017.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Erp TGM, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol. Psychiatry. 2018;84:644–654. doi: 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pan Y, et al. Morphological profiling of schizophrenia: cluster analysis of MRI-based cortical thickness data. Schizophr. Bull. 2020;46:623–632. doi: 10.1093/schbul/sbz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Glasser MF, et al. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 2011;31:11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn. Reson Med. 2009;62:717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- 157.Palaniyappan L, et al. Globally efficient brain organization and treatment response in psychosis: a connectomic study of gyrification. Schizophr. Bull. 2016;42:1446–1456. doi: 10.1093/schbul/sbw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Das T, et al. Disorganized gyrification network properties during the transition to psychosis. JAMA Psychiatry. 2018;75:613–622. doi: 10.1001/jamapsychiatry.2018.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang, T. et al. Cortical 3-hinges could serve as hubs in cortico-cortical connective network. Brain Imaging Behav. (in the press). [DOI] [PMC free article] [PubMed]
- 160.Liang S, et al. Classification of first-episode schizophrenia using multimodal brain features: a combined structural and diffusion imaging study. Schizophr. Bull. 2019;45:591–599. doi: 10.1093/schbul/sby091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Palaniyappan L, et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013;70:1031–1040. doi: 10.1001/jamapsychiatry.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.