Abstract
Background
In multiple sclerosis loss of myelin and oligodendrocytes impairs saltatory signal transduction and leads to neuronal loss and functional deficits. Limited capacity of oligodendroglial precursor cells to differentiate into mature cells is the main reason for inefficient myelin repair in the central nervous system. Drug repurposing constitutes a powerful approach for identification of pharmacological compounds promoting this process.
Methods
A phenotypic compound screening using the subcellular distribution of a potent inhibitor of oligodendroglial cell differentiation, namely p57kip2, as differentiation competence marker was conducted. Hit compounds were validated in terms of their impact on developmental cell differentiation and myelination using both rat and human primary cell cultures and organotypic cerebellar slice cultures, respectively. Their effect on spontaneous remyelination was then investigated following cuprizone-mediated demyelination of the corpus callosum.
Findings
A number of novel small molecules able to promote oligodendroglial cell differentiation were identified and a subset was found to foster human oligodendrogenesis as well as myelination ex vivo. Among them the steroid danazol and the anthelminthic parbendazole were found to increase myelin repair.
Interpretation
We provide evidence that early cellular processes involved in differentiation decisions are applicable for the identification of regeneration promoting drugs and we suggest danazol and parbendazole as potent therapeutic candidates for demyelinating diseases.
Funding
This work was supported by the Jürgen Manchot Foundation, Düsseldorf; Research Commission of the Medical Faculty of Heinrich-Heine-University Düsseldorf; Christiane and Claudia Hempel Foundation; Stifterverband/Novartisstiftung; James and Elisabeth Cloppenburg, Peek and Cloppenburg Düsseldorf Stiftung and International Progressive MS Alliance (BRAVEinMS).
Keywords: Remyelination, CDKN1C, Nuclear protein shuttling, Drug repurposing, Cell differentiation, Toxin-mediated demyelination
Research in context.
Evidence before this study
The currently used therapeutic approaches for multiple sclerosis are mainly focused on the immunological aspects of the pathology and they can slow down the progression of the disease, however, without inversion of the already existed damage. Compound screening and drug repurposing approaches led to the identification of compounds which promote oligodendroglial cell differentiation as means of myelin repair and neuroregeneration. And although some of them have already entered clinical trials, enhancement of regeneration still remains an unmet clinical need. Better understanding of the regulatory cues of regeneration processes might lead the way to new therapeutic approaches. In this respect, we previously described p57kip2 as potent nuclear inhibitor of oligodendroglial differentiation the subcellular re-localization of which allows maturation to proceed..
Added value of this study
This study used the capacity of p57kip2 protein's subcellular localization to serve as early differentiation competence marker. We identified four compounds with oligodendroglial differentiation promoting competence in the rat system, namely danazol, parbendazole, methiazole and nocodazole, with the first three also being able to promote human oligodendroglial cell maturation as well as developmental myelination of organotypic slice cultures. Moreover, danazol and parbendazole also promoted remyelination in the cuprizone-mediated mouse model of demyelination..
Implications of all of the available evidence
The use of p57kip2’s subcellular localization as readout of our screening suggests its use as early oligodendroglial cell differentiation competence marker and supports the notion that early cellular processes involved in differentiation are applicable for the identification of myelin repair promoting drugs. In this context, we detected regenerative properties of the steroid danazol and the antihelminthic parbendazole. In addition, our study includes rat, mouse, and human systems as well as different brain regions, suggesting targeted mechanisms which are evolutionary conserved and are therefore suitable for biomedical translation
Alt-text: Unlabelled box
1. Introduction
Multiple sclerosis (MS) is a disabling demyelinating disorder of the central nervous system (CNS) primarily arising from a misguided immune reaction. Specifically, immune cell infiltration in the CNS results in loss of myelin and oligodendrocytes, the myelin producing cells of the CNS [1,2]. Loss of myelin leads to impaired saltatory nerve conduction, neurodegeneration and eventually to irreversible neurological deficits [3,4]. In the adult CNS, a widespread population of resident oligodendroglial progenitor cells (OPCs) retains the capacity to generate myelinating oligodendrocytes throughout adulthood, thus contributing to myelin repair [5]. Despite this regenerative potential, the overall myelination capacity of these cells remains limited and further decreases over the course of the disease which is likely due to inhibitory signals and lack of stimulatory cues [6], [7], [8], [9].
While the currently used MS therapies successfully address the autoimmune reaction no effective treatment in order to boost and stabilize existing remyelination activities yet exists – rendering neuroregeneration an unmet clinical need [10,11]. Oligodendrogenesis is dependent on many extrinsic and intrinsic cues the investigation of which is promising in opening new therapeutic avenues [12]. In our study, we focused on the p57kip2 protein, which we initially identified as intrinsic inhibitor of myelinating glial cell differentiation [13], [14], [15]. In a follow-up investigation we then demonstrated that the subcellular localization of this protein is associated with and necessary for the competence of OPCs to undergo differentiation [16]. Specifically, it was shown that p57kip2 translocation from the nucleus is critical for these cells to proceed in differentiation, maturation, and to generate myelin sheaths. Drug repurposing can serve as effective means for the treatment of yet unmet clinical conditions, including neurodegeneration [17], [18], [19]. This is exemplified by our recent demonstration that teriflunomide, an immunomodulatory disease-modifying treatment, which is approved as a first-line treatment for relapsing MS, can also promote p57kip2 shuttling, OPC differentiation as well as myelination in vitro [20].
During the past years, a number of studies aiming at identifying (small) molecules able to promote oligodendroglial cell differentiation and remyelination have emerged. Most of them are based on pharmacological compound screenings using myelin marker expression [21], [22], [23], [24]. Many of these approaches revealed novel regulatory roles of known cellular mechanisms such as the cholesterol synthesis pathway [25], histamine receptor- [26], and muscarinic acetylcholine receptor signaling [21,22] and some substances already entered clinical trials, reviewed by [10,27,28]. Despite these recent developments a clinical application of an effective remyelinating treatment has still not been achieved. In order to provide an alternative approach for the identification of molecules that positively modulate myelin repair we performed a phenotypic compound screening based on p57kip2’s protein subcellular distribution in primary rodent OPCs. This analysis was undertaken step-wise starting with primary rodent OPCs, then applying an ex vivo myelination model and using primary human OPCs with eventually testing active compounds in an in vivo de- and remyelination model.
2. Methods
2.1. Ethics statements for animal and human tissue experiments
The generation of rodent primary oligodendroglial cell and organotypic cerebellar slice cultures were approved by the ZETT (Zentrale Einrichtung für Tierforschung und wissenschaftliche Tierschutzaufgaben; O69/11, V54/09). Cuprizone mediated demyelination experiments were approved by the authorities at LANUV (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen; Az.: 81–02.04.2019.A203) and carried out according to ARRIVE guidelines. These experimental procedures are characterised by mild severity grade and therefore no interventions to reduce pain, suffering and distress were needed. Human OPCs were isolated from second trimester (14–17 weeks) fetal samples obtained from the University of Washington Birth Defects Research Laboratory (MP-37–2014–540; 13–244-PED; eReviews_3345).
2.2. Rat oligodendroglial cell culture and immunocytochemistry
Primary OPC cultures from postnatal day zero or one cerebral rat cortices (Wistar rats of either sex) were generated as previously described [14,16,29]. Briefly, freshly removed cortices were cut in small pieces and placed in Minimum Essential Medium (MEM, Thermo Fisher Scientific, Darmstadt, Germany). After centrifugation at 2000 rpm for one minute the pellet was resuspended in 1 ml of digestion medium (MEM; 40 μg/ml DNAse; 0.24 mg/ml l-cystein; 30 U/ml papain, all Sigma‐Aldrich, Taufkirchen, Germany). Following incubation (5% CO2, 90% relative humidity, 37 °C) for 45 minutes, 1 ml of trypsin inhibitor solution [Leibvitz's medium l-15, Thermo Fisher Scientific; 1 mg/ml trypsin inhibitor, Sigma‐Aldrich; 50 mg/ml biotin-free bovine serum albumin (BSA), Carl Roth, Karlsruhe, Germany; 40 μg/ml DNase I type IV, Sigma‐Aldrich] was added. The solution was incubated for four minutes at room temperature (RT) and then the supernatant was removed. Subsequently, 1 ml of trypsin inhibitor solution was added to the pellet, followed by resuspension and addition of 10 ml Dulbecco´s Modified Eagle Medium (DMEM, Thermo Fisher Scientific) containing 10% fetal bovine serum (Brazilian origin, Lonza, Basel, Switzerland; USA origin, Capricorn Scientific, Palo Alto, CA, USA); 4 mM l-glutamine; 100 U/ml penicillin/0.1 mg/ml streptomycin (both Thermo Fisher Scientific). The solution was centrifuged for five minutes at 1200 rpm and the supernatant was removed. In the next step, the pellet was resuspended in 1 ml of the same medium. Cell suspension from two animals was added to each uncoated T-75 cell culture flask (Greiner Bio-One, Kremsmünster, Austria). Mixed glial cultures were maintained for ten days (5% CO2, 90% relative humidity, 37 °C) before OPC purification was performed. The medium was changed after the first four days and two times a week thereafter. Upon OPC purification, purity of the culture (~98%) was determined via anti‐A2B5 staining (Merck Millipore, Darmstadt, Germany; data not shown). OPCs were seeded either onto 1 mg/ml poly-d-lysine coated (PDL, Sigma‐Aldrich) 96-well plates (96-well Black/Clear Flat Bottom TC-treated Imaging Microplate with Lid, Corning, Glendale, AZ, USA) for the compound screening or onto 0.25 mg/ml PDL coated glass coverslips (13 mm) in 24-well plates for each step of validation and initially kept in DMEM based Sato medium [5 μg/ml bovine insulin; 50 μg/ml human transferrin; 100 μg/ml bovine serum albumin fraction V (BSA, Thermo Fisher Scientific); 6.2 ng/ml progesterone; 16 μg/ml putrescine, 5 ng/ml sodium selenite; 400 ng/ml T3 (tri-iodo-thyronine); 400 ng/ml T4 (thyroxin; all Sigma‐Aldrich unless stated otherwise); 4 mM l-glutamine; 100 U/ml penicillin/0.1 mg/l streptomycin (both Thermo Fisher Scientific)]. Cell differentiation was induced 1.5 h after plating by addition of Sato medium supplemented with 0.5% fetal bovine serum (referred to as differentiation medium hereafter). At the same time corresponding chemical compounds were applied.
Cells were fixed with 4% paraformaldehyde (PFA) for ten minutes at RT and non-specific staining was prevented by blocking with 10% normal goat serum (NGS, Sigma‐Aldrich) for 45 min at RT. Rabbit anti-p57kip2 primary antibody (Sigma-Aldrich Cat# P0357, RRID:AB_260,850) diluted 1:250 in blocking solution was applied overnight at 4 °C. Staining with rat anti-myelin basic protein (MBP, 1:250, Bio-Rad, Hercules, CA, USA Cat# MCA409S, RRID:AB_325,004) was performed in blocking solution containing 0.02% Triton X-100 (Sigma‐Aldrich) overnight at 4 °C. Cells stained using the mouse anti-myelin oligodendrocyte glycoprotein antibody (MOG, 1:500, Merck Millipore Cat# MAB5680, RRID:AB_1,587,278) were permeabilised with 0.1% Triton X-100 in blocking solution and 0.01% in antibody solution. Following washing steps with PBS, coverslips were incubated with the secondary antibodies goat anti-rabbit Alexa Fluor 594 (1:500, Thermo Fisher Scientific Cat# A-11,037, RRID:AB_2,534,095) or goat anti-rat Alexa Fluor 488 (1:500, Thermo Fisher Scientific Cat# A-11,006, RRID:AB_2,534,074) both diluted in PBS and incubated for two hours at RT. Nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI, 1:100, Roche, Basel, Switzerland). Finally, the coverslips were washed with PBS and mounted using Citifluor AF1 mountant solution (Electron Microscopy Sciences, Hatfield, PA, USA). Staining specificity was confirmed upon application of the same secondary antibodies without prior application of the corresponding primary antibodies (data not shown). For image acquisition the Zeiss Axionplan2 microscope (Carl Zeiss Microscopy, Jena, Germany) was used and the analysis was performed with the ImageJ BioVoxxel software (BioVoxxel Toolbox, RRID:SCR_015825). Nine images per coverslip were captured using 20x magnification and the same exposure times throughout each experiment. Two coverslips were analysed per condition. The total cell number per field was assessed via DAPI staining. For quantification, the number of protein marker-positive cells in relation to the total cell number was calculated and expressed as percentage.
2.3. Western blotting
For western blot analysis, 2.4 × 106 OPCs per well were seeded in 0.25 mg/ml PDL coated 6-well plates and after incubation in Sato medium for 1.5 h, cells were treated either with 0.1 µM parbendazole or DMSO for control in differentiation medium for one hour. Afterwards, cells were detached with pre-warmed trypsin-EDTA (Capricorn Scientific; 5% CO2, 90% relative humidity, 37 °C, for three to five minutes). Prior to centrifugation (1500 rpm, ten minutes, 4 °C), the enzymatic reaction was stopped with differentiation medium. Cell pellets were immediately frozen on dry ice and stored at −80 °C. Cell lysis was carried out on ice with radioimmunoprecipitation assay buffer (RIPA buffer, Cell Signaling Technology, Danvers, MA, USA) supplemented with HALT™ Protease-/Phosphatase inhibitor cocktail and EDTA (both Thermo Fisher Scientific). Specimens were subjected to ten seconds of sonication with an ultrasound homogenizer (SonopulsHD2070; 50% power, pulse 0.5 seconds on and 0.5 seconds off) and subsequently centrifuged (14,000 rpm, ten minutes, 4 °C) to proceed with the supernatant. Protein concentrations were determined using the DC Protein Assay (BioRad, Cat# 5,000,112). Samples were subjected to standard sodium dodecyl sulfate (SDS) gel electrophoresis and semi-dry western blotting using Bolt 12% Bis-Tris Plus gels and nitrocellulose membranes (both Thermo Fisher Scientific). Proteins were stained using the Pierce™ Reversible Protein Stain Kit (Thermo Fisher Scientific, Cat# 24,580) for protein normalization prior blocking with 1% milk powder for one hour at RT and applying the following primary antibodies: rabbit anti-p38 (1:1000, Cell Signaling Technology Cat# 9212, RRID:AB_330,713), rabbit anti-p-p38 (1:1000, Cell Signaling Technology Cat# 9211, RRID:AB_331,641), mouse anti-GAPDH (1:5000, Merck Millipore Cat# MAB374, RRID:AB_2,107,445) and the secondary antibodies anti-rabbit IgG, HRP-linked (1:2000, Cell Signaling Technology Cat# 7074, RRID:AB_2,099,233) and anti-mouse IgG (H + L), made in horse (1:5000, Vector Laboratories, Burlingame, CA, USA Cat# PI-2000, RRID:AB_2,336,177). Signals were visualized using Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Cat# 34,579) applied for five minutes. Membranes were first used to detect p-p38 and afterwards stripped with 10 ml ReBlot Plus Strong Solution (1x, Merck Millipore) for the detection of p38 and the housekeeping protein (GAPDH), all in a sequential manner on the same membrane to ensure reliable quantification. Protein bands were quantified using the Fusion FX software (Vilber Lourmat, Eberhardzell, Germany). The intensity for each band was determined and normalized to the total amount of the loaded protein amount and the intensity of the GAPDH band of the corresponding sample.
2.4. Chemical compound library screening
For the compound screening the Prestwick Chemical Library (Prestwick, Illkirch, France, Cat# PCL1280.10–50-G96), consisting of 1280 small molecules with high chemical and pharmacological diversity and 99% approved by the FDA, EMA and other agencies, was chosen and handled according to specifications from the providers. Compounds were tested on primary rat OPCs seeded onto PDL coated 96-well plates at a density of 5000 cells/well. All compounds were tested at a concentration of 10 µM and the cells were allowed to differentiate for 24 hours. As positive control 1 µM of the S1P agonist FTY720/Fingolimod [Echelon Biosciences, Salt Lake City, UT, USA, CAS 402,616–26–6; [30] and as negative controls differentiation medium +/- 0.5% DMSO were used. Each substance was tested in triplicates within each individual experiment and each experiment was repeated twice. For the determination of the subcellular localization the rabbit anti-p57kip2 primary antibody [p57kip2, 1:250, Sigma-Aldrich Cat# P0357, RRID:AB_260,850; [16] and the goat anti-rabbit Alexa Fluor 594 secondary antibody (1:500, Thermo Fisher Scientific Cat# A-11,037, RRID:AB_2,534,095) were used while nuclei were counterstained with DAPI. Nine images per well with a 20x magnification were automatically captured using the BD Pathway 855 High-Content Cell Analyzer (BD Biosciences, Rockville, MD, USA) and the signal intensity of the anti-p57kip2 staining was measured in each region of interest (nucleus vs. cytoplasm) using the Attovision v.1.7.1 software package (BD Biosciences Systems, RRID:SCR_014315). Analysis of the acquired data was conducted via an algorithm designed in house using the Matlab Software R2015a (Mathworks, Inc, RRID:SCR_001622), which provides the percentage of cells with prevailed nuclear p57kip2 localization in each well. As criterion for hit compound identification a ≥ 0.75 fold change in nuclear localization of the p57kip2 protein was used.
2.5. Organotypic cultures of cerebellar slices
For the preparation of slice cultures, a previously described protocol [31] with few adaptations was used. Briefly, freshly prepared intact cerebelli from Wistar rats aged seven days of both sexes, were embedded in 4% agarose (UltraPure Low Melting Point, Thermo Fisher Scientific) and mounted vertically to the flat metal chuck of the HM 650 V microtome (Thermo Fisher Scientific) using superglue (UHU, Mannheim, Germany). Sagittal sections, 350 μm thick, were made (cutting frequency: 50 Hz, cutting amplitude: 1 mm, cutting speed: 12 mm/s) and plated onto cell culture inserts (hydrophilic PTFE, pore size: 0.4 μm, diameter 30 mm; Merck Millipore, Darmstadt, Germany) in 6‐well plates upon addition of 1 ml of culture medium [50% MEM; 25% Hank's Balanced Salt Solution (HBSS) +/+; 25% heat inactivated horse serum; containing 100 U/ml penicillin/0.1 mg/ml streptomycin (all Thermo Fisher Scientific), and 5 mg/ml D‐glucose monohydrate (Merck Millipore)] underneath the membrane. Compounds were diluted in the same medium and applied four hours later as medium change. A total number of six to eight slices per animal were plated onto two inserts and kept in culture for three days (5% CO2, 90% relative humidity, 37 °C). Slices were then washed once with PBS both on the top and underneath the insert and fixed with 4% PFA for 20 minutes at RT.
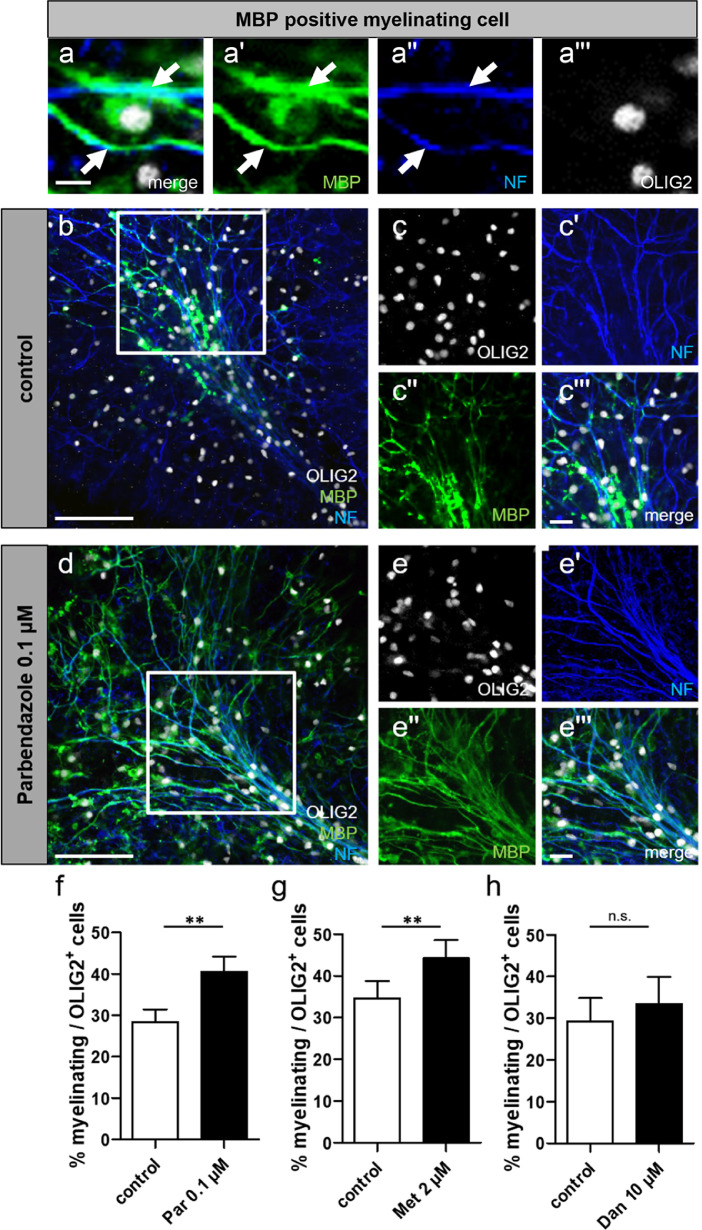
For the immunofluorescence staining slices were permeabilised with 0.5% Triton X-100 in PBS and non-specific binding was prevented by incubation in blocking solution [10% NGS, 1% bovine serum albumin fraction V (BSA; Thermo Fisher Scientific), 0.2% Triton X-100 in PBS]. Primary antibodies rabbit anti-neurofilament (NF, 1:1000, Abcam, Cambridge, UK Cat# ab8135, RRID:AB_306,298), rat anti-MBP (1:250, Bio-Rad Cat# MCA409S, RRID:AB_325,004), and mouse anti-OLIG2 (OLIG2, 1:500, Merck Millipore Cat# MABN50, RRID:AB_10,807,410) were diluted in antibody solution [10% NGS, 1% BSA, 0.1% Triton X-100 in PBS]. Secondary antibodies goat anti-rabbit Alexa Fluor 405 (1:500, Thermo Fisher Scientific Cat# A-31,556, RRID:AB_221,605), goat anti-rat Alexa Fluor 488 (1:500, Thermo Fisher Scientific Cat# A-11,006, RRID:AB_2,534,074), and goat anti-mouse Alexa Fluor 647 (1:500, Thermo Fisher Scientific Cat# A32728, RRID:AB_2,633,277) were diluted in secondary antibody solution (1% NGS, 1% BSA, 0.1% Triton X-100 in PBS). All steps were performed overnight at 4 °C and slices were then mounted using Citifluor. Staining specificity was confirmed upon application of the same secondary antibodies without prior application of the corresponding primary antibodies (data not shown). Image acquisition was performed using a confocal microscope (CLSM 510, Carl Zeiss Microscopy) and analysed with the Zen 2 Blue Edition (Carl ZEISS Microscopy, ZEN Digital Imaging for Light Microscopy, RRID:SCR_013672) and ImageJ BioVoxxel software packages. Briefly, three images per field were obtained using 40x magnification via Z-stack scanning covering 2 µm depth in total and prior the analysis they were projected onto a single plane via orthogonal projection. To assess the number of myelinating oligodendrocytes in relation to the total number of OLIG2-positive cells 12–16 fields from similar structures were analysed for all conditions. The typical morphology of myelinating cells in organotypic slices is presented in Fig. 4a-a’’’.
Fig. 4.
Parbendazole and methiazole significantly promote ex vivo developmental myelination. Organotypic cerebellar slices from P7 rats were subjected to a three-day long substance application. For each substance medium with the corresponding DMSO concentration was used as control. Exemplary pictures of a myelinating oligodendrocyte revealing morphological criteria used for the analysis (a-a’’’). Representative triple staining for OLIG2, MBP, and neurofilament for DMSO (b-c’’’) and parbendazole- (d-e''') treated slices. For the overview images (b,d) four images per field were obtained using 20x magnification via Z-stack scanning covering 3 µm depth in total and prior to the analysis they were projected onto a single plane via orthogonal projection. Scale bars for the exemplary images (a-a’’’): 10 µm. Scale bars for the overview images (b,d): 100 µm. Scale bars for the detailed images (c-c'''; e-e'''): 20 µm. Immunohistochemical analysis assessing the number of MBP-positive, myelinating oligodendrocytes in relation to the total number of OLIG2-positive cells in slices treated with parbendazole (f), methiazole (g), danazol (h), and their corresponding DMSO concentrations (controls). Data are shown as means, and error bars represent SEM. Mann-Whitney U test, for unpaired data was used and data were considered statistically significant at *p < 0.05, **p < 0 .01, ***p < 0.001. Number of experiments: n = 5 in all cases. Par, parbendazole; Met, methiazole; Dan, danazol; NF, neurofilament; MBP, myelin basic protein.
2.6. Human fetal oligodendroglial cell culture and immunocytochemistry
Human fetal second-trimester (14–17 weeks) telencephalon tissue samples collected from elective abortions were provided by the University of Washington Birth Defects Research Laboratory – an NIH supported program (MP-37–2014–540; 13–244-PED; eReviews_3345) (Seattle, Washington, USA). The tissue was digested with 0.25% trypsin (Thermo Fisher Scientific) and 25 µg/mL DNase I (Roche, Laval, Canada) to obtain dissociated cells. OPCs that comprise ~0.1% of the total cells were isolated using immunomagnetic beads coated with O4 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany Cat# 130–096–670, RRID:AB_2,847,907) [32], [33], [34]. After selection, cells were plated in 96-wells plates coated with poly-l-lysine (Sigma-Aldrich) and extracellular matrix (ECM-gel from Engelbreth-Holm-Swarm murine sarcoma, Sigma-Aldrich) at a density of 1 × 104 cells per well. Cells were cultured in DMEM-F12 media supplemented with N1 (Sigma-Aldrich), B27 supplement (Sigma-Aldrich), PDGF-AA (10 ng/ml) and bFGF (10 ng/ml, both Sigma-Aldrich) for four days before treatment with reagents as triplicates for four and six days, media with reagents were changed every two days. Cells were live stained with mouse anti-O4 (1:200, R&D Systems, Minneapolis, MN, USA Cat# MAB1326, RRID:AB_357,617), and hybridoma anti-GalC IgG3 (GC, 1:50, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada) monoclonal antibodies for 15 minutes at 37 °C and then fixed with 4% PFA for ten minutes in RT. Secondary antibodies goat anti-mouse IgM-Alexa Fluor 647 (1:500, SouthernBiotech, Birmingham, AL, USA Cat# 1021–31, RRID:AB_2,794,254) to O4 and goat anti-mouse IgG3-Alexa Fluor 488 (1:500, Thermo Fisher Scientific Cat# A-21,151, RRID:AB_2,535,784) for GC were used for 30 minutes at RT. Cell nuclei were stained with Hoechst 33,258 (1:1000, Thermo Fisher Scientific Cat# H3569, RRID:AB_2,651,133). Cells were imaged and the O4- and GC-positive cells were counted.
2.7. Cuprizone mediated demyelination and remyelination
Eight-week-old female C57BL/6 mice (Janvier Labs, Le Genest Saint Isle, Mayenne, France) were used and all experiments were performed in the animal facility of the Heinrich-Heine-University (Zentrale Einrichtung für Tierforschung und wissenschaftliche Tierschutzaufgaben; ZETT) under pathogen-free conditions and in accordance with ethical care. Only mice of the same age and with body weight between 17 and 19 gr were included and as additional exclusion criterion >10% weight loss during the experiments was used. No animals were excluded from the analysis. Animals were distributed equally into cages (groups) upon delivery and were given one week as acclimatization period before the initiation of the experiments. Using the software G*Power a required size of six animals per group with effect size d 2.6 and confidence interval 95% was computed. Demyelination was induced by feeding 0.2% (w/w) cuprizone [bis(cyclohexanone)oxaldihydrazone]-containing diet [either from Envigo (Indianapolis, IN, USA Cat# TD.140803) or from SSNIFF Spezialdiäten GmbH (Soest, Germany; maintenance diet pellets 10 mm, V1534 implemented with 0.2% cuprizone, Sigma-Aldrich, CAS 370–81–0)] for six weeks. Thereafter animals were given standard rodent chow (SSNIFF, Cat# V1534). Parbendazole (MedChem Express, Monmouth Junction, NJ, USA, CAS 14,255–87–9) was applied at a concentration 1.14 mg/ml and using 20 mg/kg body weight as dose. Parbendazole stock concentration consisted of 13.33 mg/ml in DMSO which was further diluted using sterile saline solution with 2% Tween 80 (Sigma-Aldrich). A total volume of 350 µl of the parbendazole solution or the corresponding vehicle solution (8.6% DMSO in sterile saline solution with 2% Tween 80) was administered daily via intraperitoneal (i.p.) injections for the last 17 days of cuprizone treatment. Danazol (Sigma-Aldrich, CAS 17,230–88–5; stock concentration 30 mg/ml in DMSO) was diluted first 1:1 in Tween 80 in order to increase the stability in aqueous solutions and then further diluted down to a final concentration of 0.4 mg/ml in drinking water for oral application (5 ml per animal and per day, 100 mg/kg body weight to be applied daily). One week upon cuprizone withdrawal animals were deeply anesthetized using isoflurane inhalation and transcardially perfused with 20 ml PBS followed by 20 ml 4% PFA in PBS, brains were then removed and post-fixation was performed overnight in 4% PFA at 4 °C.
Following post-fixation, cryoprotection of mouse brains was performed in 30% sucrose (in PBS) at 4 °C for 48 hours. Brains were then embedded in Tissue-Tek OCT medium (Sakura Finetek Europe, Netherlands), frozen and stored at −80 °C until sectioning with cryostat (Leica CM3050S, Wetzlar, Germany). Coronal 10 µm sections were prepared and stored at −80 °C. Regions of interest, medial (Bregma −0.82 to −1.22 mm) and caudal (Bregma −1.94 to −2.34 mm) parts of corpus callosum, were assessed according to [35].
For immunohistochemical staining, sections were thawed and air-dried for at least ten minutes at RT. Rehydration was performed for five minutes in distilled water, followed by transfer to −20 °C acetone for five minutes and two consecutive washing steps first in 1x TBS (pH 7.6) and then 1x TBS-T (TBS implemented with 0.02% Triton X-100; pH 7.6) for five minutes each. Non-specific staining was blocked with 10% biotin-free bovine serum albumin (BSA, Carl Roth) in TBS-T or 10% normal donkey serum (NDS, Sigma‐Aldrich) in TBS-T for 30 minutes at RT, followed by primary antibody solution application (10% BSA in 1x TBS or 10% NDS in 1x TBS) and incubation overnight at 4 °C. The following primary antibodies were used: mouse anti-APC (CC1, 1:500, GeneTex Cat# GTX16794, RRID:AB_422,404), rabbit anti-glutathione-S-transferase pi (GSTpi, 1:2000, Enzo Life Sciences, Farmingdale, NY, US Cat# ADI-MSA-101-E, RRID:AB_2,039,147), goat anti-PDGFR (alpha/CD140A, 1:250, Neuromics, Edina, MN, US Cat# GT15150, RRID:AB_2,737,233), and rat anti-PLP [PLP, 1:250, kind gift from B. Trapp and R. Dutta, Dept. of Neurosciences, Cleveland Clinic, OH, USA [36]. Slices were then washed twice in 1x TBS for five minutes each and secondary antibodies were diluted 1:200 and applied for 30 minutes along with DAPI (1:50) in PBS. The following secondary antibodies were used: goat anti-rabbit Alexa Fluor 594 (1:200, Thermo Fisher Scientific Cat# A-11,037, RRID:AB_2,534,095), goat anti-rat Alexa Fluor 488 (1:200, Thermo Fisher Scientific Cat# A-11,006, RRID:AB_2,534,074), goat anti-rabbit Alexa Fluor 488 (1:200, Thermo Fisher Scientific Cat# A-11,008, RRID:AB_143,165), goat anti-mouse Alexa Fluor 488 (1:200, Thermo Fisher Scientific Cat# A32728, RRID:AB_2,633,277), and donkey anti-goat Alexa Fluor 488 (1:200, Thermo Fisher Scientific Cat# A-11,055, RRID:AB_2,534,102). Two final washing steps were performed with 1x TBS-T and 1x TBS for five minutes prior to mounting with Shandon™ Immu-Mount (Thermo Fisher Scientific). Staining specificity was confirmed upon application of the same secondary antibodies without prior application of the corresponding primary antibodies (data not shown). For image acquisition and analysis a CLSM confocal microscope and the Zen 2 Blue edition and ImageJ BioVoxxel software packages were used, respectively. Animal handling and tissue processing were performed by the same person (AM) to ensure stability and control of all parameters and cofounders. Data analysis was conducted blindly.
2.8. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Graph design and statistical analyses were performed using the GraphPad Prism 8.0.2 software (GraphPad Prism, San Diego, CA, RRID:SCR_002798). The absence of Gaussian distribution of our data was assessed via Shapiro-Wilk normality test and statistical significance via unpaired Mann-Whitney U test or Tukey's range test following one-way ANOVA. Data were considered statistically significant at *p < 0.05, **p < 0.01, ***p < 0.001. n represents the number of independent experiments. A priori sample size calculation for the in vivo experiments was performed using the G*Power 3.1.9.2 [37] software and the Wilcoxon-Mann-Whitney test (two groups).
2.9. Role of funders
The funding source had no role in study design, collection, analysis and interpretation of data or in manuscript writing.
3. Results
3.1. Phenotypic screening for compounds that promote nuclear exclusion of the p57kip2 protein
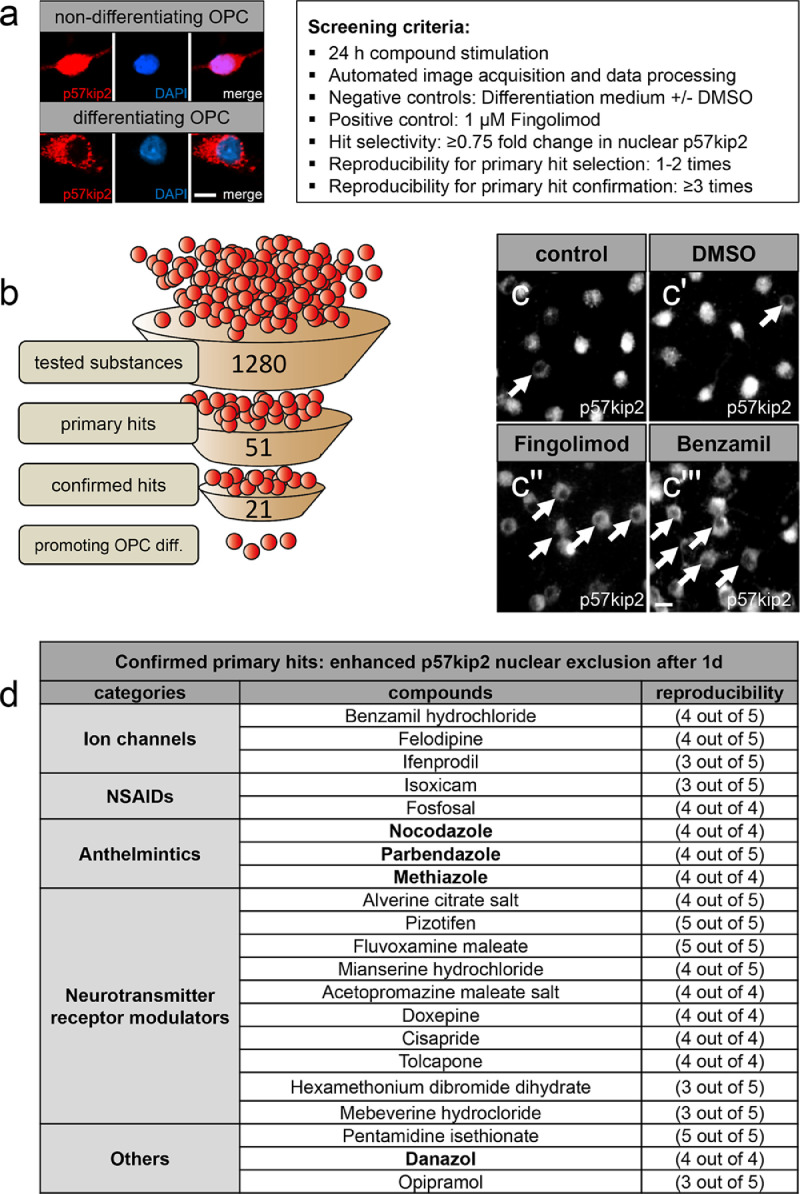
Nucleocytoplasmic shuttling of the p57kip2 protein is an early event during the course of spontaneous OPC differentiation and we could previously demonstrate that alteration of its gene expression or of its subcellular protein localization severely impacts cellular maturation [14,16]. We therefore chose the nuclear vs. cytoplasmic localization of this protein to establish a phenotypic screening for pharmacological compounds with the potential to promote oligodendrogenesis (Fig. 1a). As opposed to the majority of other screening approaches we decided to test compounds using primary OPC culture [16,20], which recapitulates closer the in vivo situation than cell lines or stem cell derivatives, in order to increase the translational potential of this study. The sphingosine-1-phosphate receptor FTY720P/Fingolimod (1 µM) was used as positive control and differentiation medium with or without 0.5% DMSO served as negative controls (Fig. 1c-c''). For determination of the subcellular localization of the p57kip2 protein, stained cells were automatically captured using the BD Pathway 855 High-Content Cell Analyzer. The staining protocol used for this study is well established [16,20] and staining specificity was regularly confirmed. In an automated way, signal intensities of the anti-p57kip2 staining in nucleus and cytoplasm were measured using the Attovision v.1.7.1 software and analysed with an algorithm designed in house. The output of the analysis is the percentage of cells with prevailed nuclear p57kip2 localization in each well, given that the p57kip2 signal intensity in the two regions (nuclear vs. cytoplasmic) is provided as input. Hit compounds were considered those which induced a ≥ 0.75 fold change in nuclear localization of the p57kip2 protein in comparison to the negative controls. This threshold corresponds to the maximum increase in nuclear exclusion between days one and three upon initiation of spontaneous differentiation that has been detected previously [16], [20]. Of note, this is also the time frame when significant transition of OPCs to oligodendrocytes is taking place and importantly this threshold was kept constant throughout the whole screening.
Fig. 1.
In vitro phenotypic screening revealed compounds that promote nuclear exclusion of the p57kip2 protein in primary OPCs. Presentation of the readout and the experimental setup of the primary screening procedure (a). Funnel illustration showing the complete progression of the screening (b). Representative images taken using the BD Pathway 855 High-Content Cell Analyzer visualizing p57kip2’s subcellular localization upon treatment with the negative controls, differentiation medium (control) and DMSO, the positive control Fingolimod (1 µM) and a profound primary hit benzamil hydrochloride (c-c'''). List of the confirmed primary hits (d). Arrows in c-c''' point at cells with cytoplasmic p57kip2 localization. Scale bars: 10 µm.
Out of 1280 compounds tested on primary rat OPCs, 21 substances (Fig. 1d) were found to reproducibly boost p57kip2’s nuclear shuttling (as represented for benzamil hydrochloride, CAS 161,804–20–2 in Fig. 1c'''). Of note, this analysis also revealed doxorubicin (CAS 25316-40-9) to induce an exclusive nuclear localization of the p57kip2 protein (data not shown). Whereas ifenprodil (CAS 23,210–56–2) [25,39,40] and isoxicam (CAS 34,552–84–6) [41] already emerged from other pro-oligodendroglial screenings, the majority of our confirmed hits were unknown in this context. However, some of the identified compounds are related to pathways that have previously been shown to regulate OPC differentiation and survival such as the histamine receptor-3 (H3R) inverse agonist GSK247246 [26] and benztropine acting on muscarinic receptors [21], respectively, thus proving the efficacy of our screening approach.
3.2. Secondary screening for compounds that promote OPC differentiation in vitro
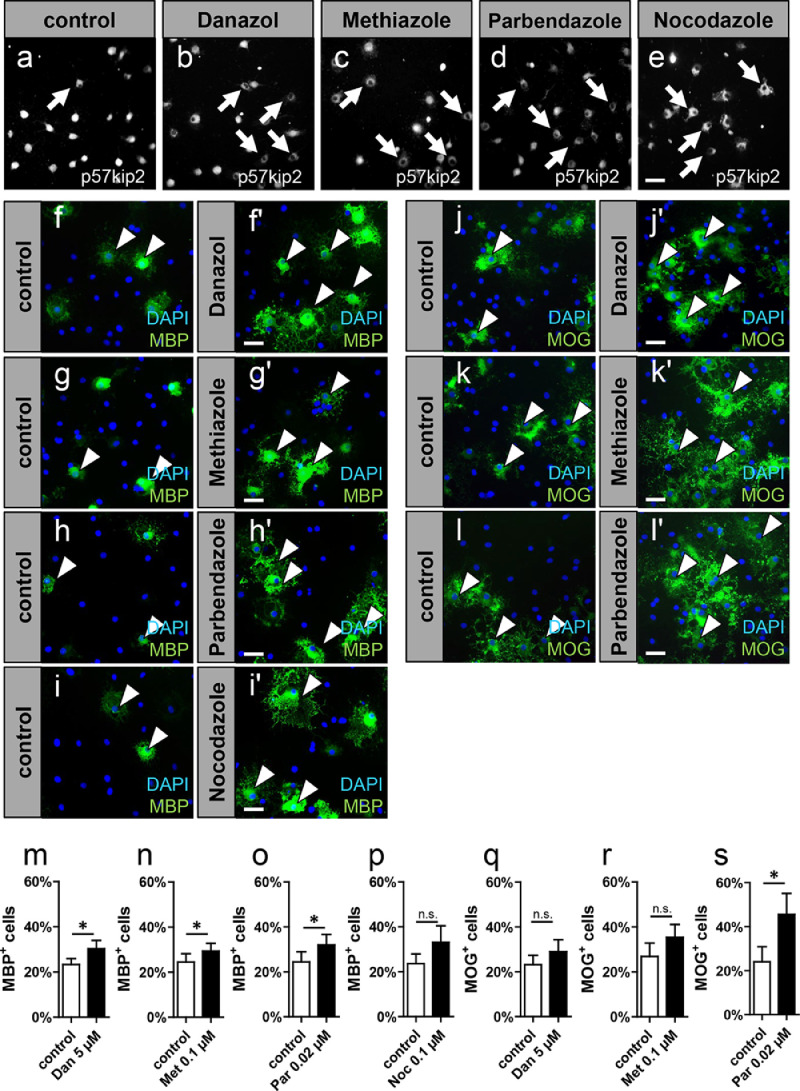
For further validation of the confirmed hits their impact on OPC differentiation was determined by means of myelin protein expression. To this end, rat primary OPCs were cultured and stimulated using compound concentrations ranging from 10 to 0.001 µM. Cells were allowed to differentiate for three days upon compound stimulation and immunofluorescence staining against the late myelin marker MBP was performed. Potential cytotoxic effects were accessed via evaluation of total cell numbers (data not shown). From this secondary screening four substances emerged with the capacity to positively modulate OPC differentiation and the most efficient and well-tolerated concentrations were determined (Fig. 2f-i'; m-p).
Fig. 2.
Selected hit compounds promote p57kip2’s protein nuclear exclusion and OPC differentiation in vitro. Representative images taken using the BD Pathway 855 High-Content Cell Analyzer visualizing the subcellular localization of the p57kip2 protein upon one-day long stimulation with 0.5% DMSO (a), danazol (b), methiazole (c), parbendazole (d), and nocodazole (e) each at a concentration of 10 µM. Immunocytochemical analysis for the mature myelin marker MBP upon a three-day long stimulation of primary rat OPCs with 5 µM danazol (m), 0.1 µM methiazole (n), 0.02 µM parbendazole (o), and 0.1 µM nocodazole (p). Immunocytochemical analysis for the mature myelin marker MOG upon a six-day long stimulation of primary rat OPCs with 5 µM danazol (q), 0.1 µM methiazole (r), and 0.02 µM parbendazole (s). For each substance medium with the corresponding DMSO concentration was used as control. Representative images for MBP (f-i') and MOG staining (j-l'). Arrows in representative pictures point at cells with cytoplasmic p57kip2 localization in a-e and arrowheads point to MBP-positive cells in f-i’ or MOG-positive cells in j-l'. Nuclei were counterstained with DAPI. Scale bars: 20 μm. Data are shown as mean values and error bars represent SEM. Numbers of independent experiments n = 6 for (m), n = 4 for (n, p, q), n = 5 for (o, s), n = 3 for (r). Statistical significance was assessed using Mann-Whitney U test, unpaired data: *p < 0.05; **p < 0.01; ***p < 0.001. Dan, danazol; Par, parbendazole; Met, methiazole; Noc, nocodazole; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein.
Treatment with either 5 µM danazol, 0.02 µM parbendazole, or 0.1 µM methiazole (CAS 74,239–55–7) significantly induced the number of MBP-positive cells (Fig. 2m-o). Treatment with 0.1 µM nocodazole (CAS 31,430–18–9) induced only a non-significant increase in MBP-positive cells but reproducibly resulted in more complex morphological phenotypes (Fig. 2i-i'). Moreover, using nocodazole a 31.43% decrease in the number of (DAPI positive) cells was observed, indicating that this substance might exert cytotoxicity and thus explaining the absence of statistically significant observations on myelin expression (Fig. 2p).
For those hits which promoted transition from OPCs to mature oligodendrocytes (excluding nocodazole due to cytotoxicity) we additionally investigated the time point day six using MOG as marker and we similarly detected a significant beneficial impact of parbendazole on cell maturation (Fig. 2s). Cells treated with danazol and methiazole induced similar changes in MOG protein expression, however, the effects were less prominent (Fig. 2q-r). Treatment with the benzimidazole derivatives, parbendazole and methiazole, not only led to increased numbers of MOG-positive cells but also accelerated morphological maturation (Fig. 2k-l'). At this point, our data provided therefore strong evidence that using the p57kip2 nuclear shuttling process as a screening read-out is efficient and successful for the identification of novel drugs that can positively modulate already early oligodendroglial cell stages.
3.3. Selected hit compounds promote oligodendroglial cell differentiation in primary human OPCs
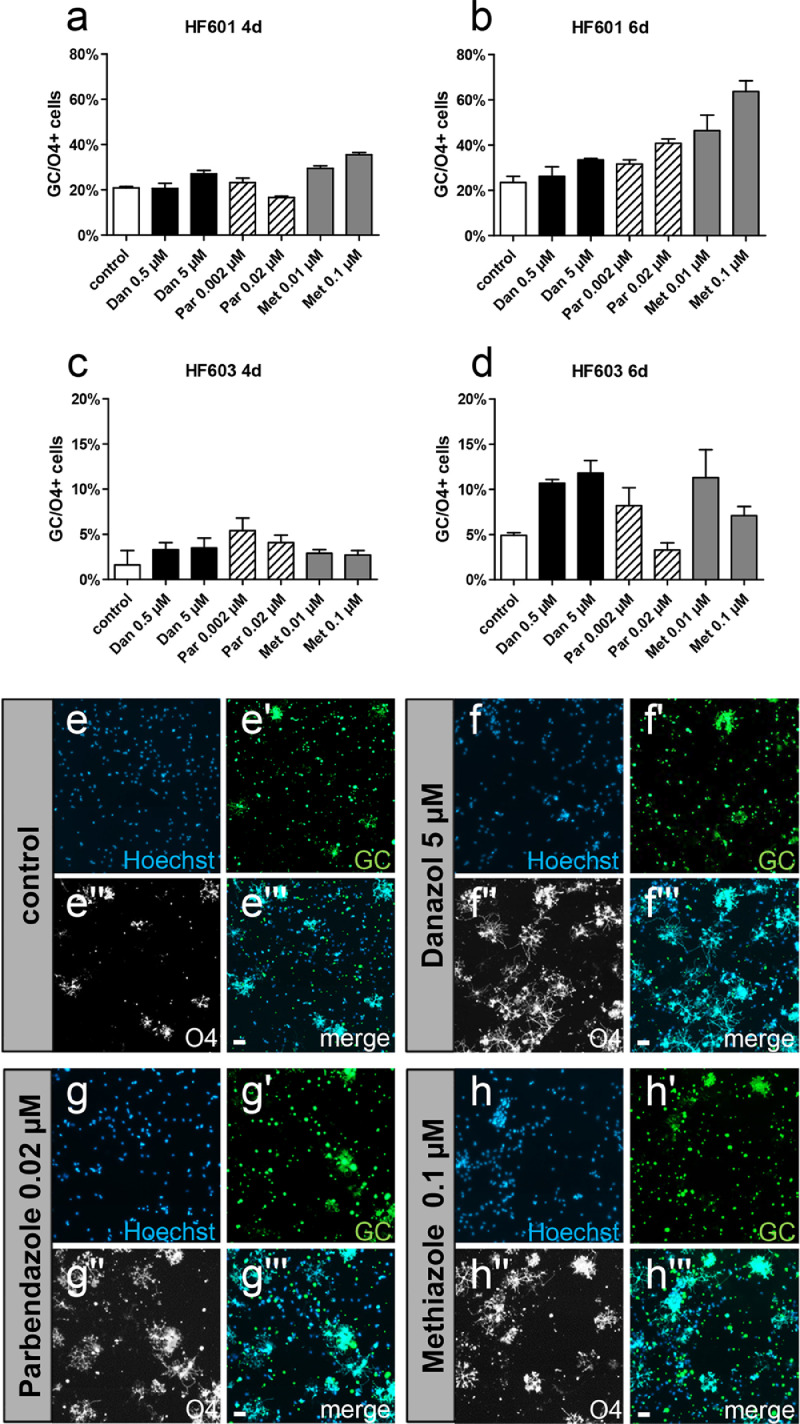
As this study aimed at the identification of promyelinating drugs with a translational potential, it was of interest to explore the activities of selected compounds on human cells. For this purpose, we used human fetal brain-derived O4-selected oligodendroglial progenitor preparations [[32], [33], [34],42] which were grown for four days in culture and then treated for another four or six days in the presence of 0.002 µM and 0.02 µM parbendazole, 0.01 µM and 0.1 µM methiazole, or 0.5 µM and 5 µM danazol. Due to the suspected toxic effect of nocodazole, this drug was excluded from the technically limited experiments with primary human cells. For technical reasons two completely different preparations from human donor tissues over a period of more than six months were used (HF601 and HF603) which resulted in quantitatively diverging expression levels. Nevertheless, double staining using anti-O4 and anti-galactocerebroside (GC) antibodies revealed that all three substances clearly elevated the percentage of morphologically matured human oligodendroglial cells (compare Fig. 3e'',3f'',3g'',3h'') as well as their ability to express GC. Note that the most consistent effects were observed for 5 µM danazol at both time points, whereas the higher concentrations of parbendazole and methiazole resulted in declining values in cells of the second preparation (HF603), particularly at the later time point. This suggests additional titration and long-term toxicology investigations to be conducted along the course of a future biomedical translation process.
Fig. 3.
Selected hit compounds enhance primary human OPC differentiation in vitro. Human OPCs of two different preparations (HF601, HF603) were cultured in the presence of 0.5 µM and 5 µM danazol, 0.002 µM, and 0.02 µM parbendazole, or 0.01 µM and 0.1 µM methiazole with DMSO-treated cells serving as controls. The results of each preparation are presented separately (HF601 in a, b and HF603 in c, d) and correspond in total to two independent experiments. Human OPCs of batch HF601 consistently showed increased degrees of anti-galactocerebroside (GC)-positivity at both time points and using all tested substance concentrations (a,b). Human OPCs of batch HF603 appeared generally less mature, showed increased GC-positivity after four days of treatment but appeared sensitive to high parbendazole and methiazole concentrations (c,d). Representative pictures of HF601 cells after six days of treatment (e-h'''). Note that all three substances evoked a clear morphological maturation of cells. Data are shown as means (derived from three wells per condition) but due to large variations and the still rather low number of technical replicates no statistical significance was calculated. Scale bars 20 µm.
3.4. Members of the benzimidazole class of compounds promote ex vivo myelination
As a next step we investigated the impact of treatment with selected active compounds on endogenous developmental myelination. To this end, organotypic cerebellar slice cultures were prepared from postnatal day seven Wistar rats. Stimulation with either 0.1 µM parbendazole or 2 µM methiazole over three days induced a significant increase in the myelination capacity of the endogenous oligodendroglial cell population as shown by the improved potential of OLIG2-positive cells to make up MBP-positive myelin sheaths when compared to the control (DMSO) conditions (Fig. 4b-g). Danazol revealed to exert a similar trend regarding the generation of myelinated segments, however, no significant differences were found compared with controls (Fig. 4h).
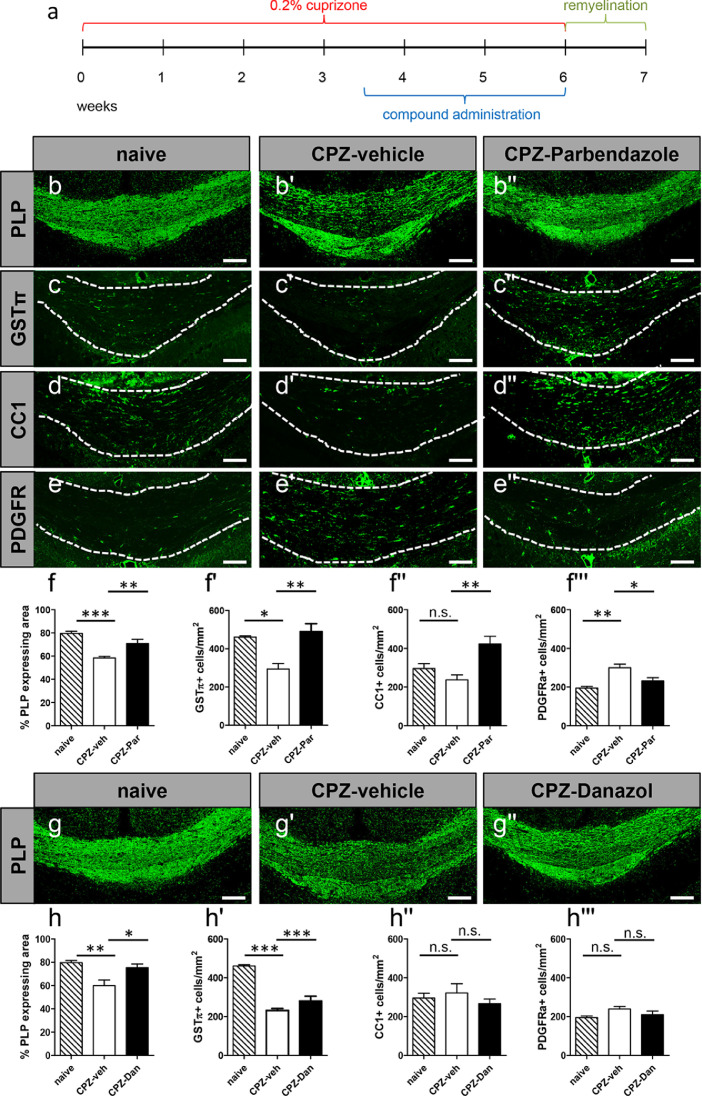
3.5. Parbendazole and danazol enhance remyelination in vivo
To assess the impact on spontaneous remyelination of those compounds with strongest and most wide-spread activities the cuprizone mediated mouse model of de- and remyelination was applied [43]. We selected danazol and parbendazole for in vivo evaluation, as representatives of steroid and benzimidazole compound classes, respectively. To achieve demyelination of the corpus callosum mice were fed with 0.2% cuprizone diet for a total of six weeks. During the last 17 days of cuprizone treatment, animals were either receiving daily intraperitoneal injections of parbendazole (20 mg/kg) or oral administration of danazol (100 mg/kg). For each substance the same number of cuprizone-treated animals were administered the corresponding vehicles. To assess the efficiency of both, cuprizone treatment and the extent of remyelination, naïve (non-cuprizone-treated) animals were also included in the analysis. The effect on remyelination was investigated one week upon cuprizone withdrawal (Fig. 5a). Immunohistochemical staining specificity was confirmed everywhere in order to exclude false positive signal detection.
Fig. 5.
Parbendazole and danazol positively affect spontaneous remyelination in a cuprizone-induced demyelination mouse model. Schematic representation of the experimental setup for cuprizone treatment and substance administration (a). Representative images of the oligodendroglial protein marker expression in corpus callosum after one week of remyelination (b-e''; g-g''). Image magnification 10x. The extent of remyelination was revealed as the percentage of PLP-expressing area in the defined region of interest between naïve, CPZ/vehicle-treated and CPZ/substance-treated groups. In a similar manner the impact of parbendazole and danazol on oligodendroglial cell differentiation and maturation was assessed as revealed by the number/mm2 of mature (GSTπ, f’,h’; CC1, f'',h'') and early (PGFRα, f''',h''') oligodendroglial protein marker-positive cells along the same area of the corpus callosum. Naïve group n = 6 (n = 4 for the GSTπ analysis); residual groups n = 5 each. For each mouse four coronal sections were analysed (two from the medial and two from the caudal region) and the data are shown as mean values while error bars represent SEM. Significance was assessed using Tukey's range test following one-way ANOVA. Data were considered statistically significant (95% confidence interval) at *p < 0.05, **p < 0.01, ***p < 0.001. CPZ, cuprizone; veh, vehicle; Par, parbendazole; Dan, danazol; PLP, myelin proteolipid protein; GSTπ, glutathione-S-transferase Pi; CC1, adenomatous polyposis coli protein (APC) clone CC1; PDGFRα, platelet-derived growth factor receptor alpha. Scale bars: 100 µm.
Significant differences in the percentage of PLP-positive area (myelinated) within the region of interest were observed upon comparison of naïve with vehicle-treated groups, confirming the efficiency of cuprizone-dependent demyelination (Fig. 5b,b’,f,g,g’,h). Remarkably, parbendazole enhanced spontaneous remyelination as revealed by PLP density along the midline of the medial and caudal compartments of the corpus callosum, as compared with vehicle-treated counterparts (Fig. 5b-b'',f). This observation was supported by corresponding differences in the numbers of cells expressing mature oligodendroglial markers such as GSTπ (Fig. 5c-c'',f') and CC1 (Fig. 5d-d'',f''). In accordance, the early oligodendroglial marker PDGFRα exerted an opposite pattern, with the vehicle-treated group demonstrating significantly increased numbers of PDGFRα-positive cells as compared to the other two groups (naïve and parbendazole-treated mice, Fig. 5e-e'',f'''). Interestingly, oral administration of danazol also significantly enhanced the PLP-positive area hence remyelination in the same experimental setup (Fig. 5g-g'',h). However, only a small but statistically significant positive impact on the generation of GSTπ-positive cells was observed, whereas numbers of CC1- and PDGFRα-positive cells remained unaffected (Fig. 5h'-h''').
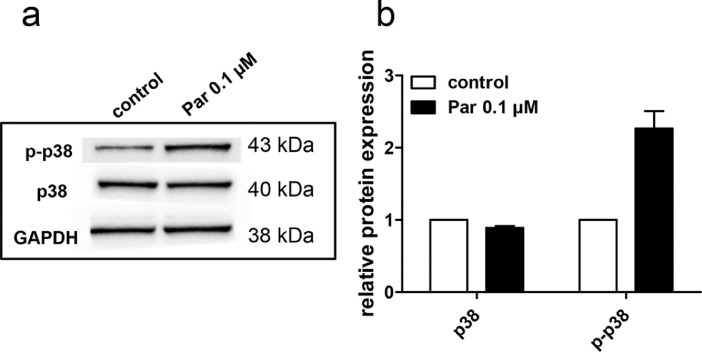
3.6. Parbendazole treatment activates p38MAPK in vitro
We then used available information on identified hit compounds to shed light onto the underlying mechanism of p57kip2 nuclear exclusion. p38MAPK activation is associated with regulation of nuclear protein export [44], [45], [46]. Moreover, omeprazole, another benzimidazole derivative, was shown to induce OPC differentiation involving p38MAPK and ERK1/2 activation [47]. In order to investigate whether p38MAPK is also activated in response to parbendazole, we stimulated primary OPCs with this substance for 60 minutes and indeed detected 2.3 times more phosphorylated p38MAPK (Fig. 6) protein in relation to the DMSO-treated control.
Fig. 6.
Parbendazole treatment induces phosphorylation of p38MAPK. Rat primary OPCs were treated either with 0.01 µM parbendazole or the corresponding DMSO concentration (control) for one hour and harvested for Western blot analysis for the detection of p-p38, p38, and GAPDH proteins (a). Quantification of the relative protein expression (b). The relative protein expression of both the phosphorylated and non-phosphorylated forms of p38 was normalized over the total amount of the protein in the lysate and GAPDH levels. Data represent means of two independent experiments. Error bars represent SEM.
4. Discussion
We established and successfully performed a phenotypic drug screening based on the p57kip2 dependent regulation of OPC differentiation. On the one hand, this analysis provided information on new pharmacological compounds with the potential to boost myelin repair processes and on the other hand, we shed light on the upstream regulatory mechanisms. Up to now it was revealed that the intracellular p57kip2 shuttling process regulated gene expression (via Ascl1 and Hes5), cytoskeletal dynamics (via LIMK-1), cell cycle exit (CDK2; all described in [16]) as well as vascular ATPase activity [48], however, corresponding signaling cascades or involved surface receptors were not known.
We nevertheless took advantage of this potent mechanism in order to identify compounds that can modulate early oligodendroglial cell stages in contrast to the majority of other screenings which were mainly based on MBP expression and which also addressed later stages and larger time windows [[21], [22], [23], [24], [25], [26],38,39,49,50]. Moreover, the 1280 compounds tested here mainly consisted of either FDA/EMA- (US Food and Drug Administration/Europeans Medicines Agency) approved substances or substances with a clinical trial history, with 98% of them being predicted to have a high blood-brain-boundary (BBB) penetrance. In addition, we tested all substances using primary OPCs which creates a condition closer to the in vivo situation as compared to cell lines or stem cell derivates that were used in most related screenings [[23], [24], [25],38,39,[49], [50], [51], [52]]. Such unique selling points, however, included the backdrop of larger variations when using primary cells as well as the fact that p57kip2 translocation appears to be an early and fast process, the detection of which can be missed. Additional variations were likely deriving from the fact that in our study cells of three different species (rat, human, mouse) as well as OPCs deriving from cortex, cerebelli, or the corpus callosum were examined.
Among the hits we identified ion channel modulators (e.g. benzamil hydrocloride), nonsteroidal anti-inflammatory drugs (NSAIDs; e.g. fosfosal, CAS 6064–83–1) and neurotransmitter receptor modulators (e.g. alverine citrate salt, CAS 5560–59–8). Given that these mechanisms have previously been implicated in oligodendrogenesis [53], [54], [55], [56] our screening procedure and the selected parameters could be validated. Moreover, a minor overlap with hits detected by other screenings, namely ifenprodil [25,39,40] and isoxicam [41], was observed. Of note, out of the identified hit compounds, only a small number was indeed able to promote transition of OPCs to mature oligodendrocytes. Possible explanations may include inappropriate concentrations or time-points of application/investigation or that additional, adverse pathways were elicited which interfered with cell differentiation.
Notably, three benzimidazoles were found to exert positive effects on several parameters of oligodendrogenesis. This compound family has been associated with anti-microbial, anti-viral, anti-cancer, anti-protozoal, anti-helminthic, anti-inflammatory, and analgesic activities [57]. Whereas their broad application in veterinary medicine has revealed drawbacks related to teratogenic and cytotoxic effects [58], their numerous pharmacological properties led to the development of drugs, such as the anti-ulcer medications pantoprazole and omeprazole, which are broadly subscribed in human medicine without the implication of severe side effects. Of note, omeprazole was recently proposed as promoter of oligodendroglial cell differentiation and remyelination [47].
We instead found parbendazole, methiazole, and nocodazole to clearly promote oligodendroglial cell differentiation and proceeded with parbendazole and methiazole, two less-characterized members of the compound family. As the methiazole cell cycle profile linked this compound to parbendazole and two other tubulin destabilizing agents [59] we believe that their similar effects could be attributed to alterations of the microtubule dynamics via a p38MAPK-related mechanism which was indeed substantiated by our observation of a parbendazole dependent induction of phosphorylated p38MAPK protein. Similar phosphorylation events were also shown to occur in response to omeprazole [47]. Nocodazole, on the other hand, a known microtubuli destabilizer, was described to act in a pro-oligodendroglial way and manner via increased microtubule arborization when applied acutely at nanomolar concentrations [60]. However, the authors reported increased cytotoxicity at micromolar levels, which is in accordance with our results, as well as deleterious effects on in vitro myelination even at low concentrations, thus justifying its exclusion from our further investigations.
Given their similar mode of action and based on the fact that in vivo experimentations are restricted parbendazole was selected for application on cuprizone demyelinated mice, taking into account that all three here investigated benzimidazole derivatives are predicted to be able to penetrate the BBB according to ADMET evaluation [61]. Moreover, teratogenic effects of parbendazole were only reported in sheep and cattle when administered at concentrations higher or equal to 60 mg/kg body weight [62], so the here administered daily doses of 20 mg/kg suggested a safe treatment, and finally confirmed a positive impact on the number of maturing oligodendroglial cells as well as on myelin reconstitution in vivo.
To what degree a similar positive impact of i.p. administered parbendazole on myelin lesion repair can also been observed in an inflammatory background (e.g. in experimental autoimmune encephalomyelitis) needs to be addressed in future experiments. Likewise, possible prolonged effects due to alternative dosing and application routes will also have to be determined.
The fourth studied substance, danazol, is an extensively studied testosterone derivative with anti-gonadotropic and anti-estrogenic properties, which has initially been approved by the FDA for the treatment of endometriosis [63]. Danazol has been studied in cancer research in view of its potential to induce toxic effects on, among others, leukemic and multidrug-resistant cancer cells [64,65]. Recently, danazol was reported to rescue MBP gene expression in a zebrafish mutant featuring reduction or complete loss of MBP expression in the peripheral nervous system [66]. Here, we show prominent promotion of oligodendroglial cell differentiation using both rat and human primary OPCs, minor improvement of ex vivo myelination using rat tissue and significantly increased in vivo remyelination in cuprizone challenged mice. The latter of which appears to be restricted to a net positive effect on the size of the myelin-positive area and to a small significant increase in the number of GSTπ-positive cells, as opposed to the clear-cut changes on OPC and oligodendrocyte numbers upon parbendazole application. Nevertheless, it has to be taken into account that danazol was applied orally thus limiting control over the consumed doses. In addition, its limited effects on myelin repair could also result from its poor aqueous solubility and dissolution [67]. But given the advantage that it can be applied as oral drug, future in vivo tests are warranted. This could include either the use of direct drug delivery into the stomach (via gastric gavage), the application of higher concentration and/or of different surfactant and emulsifier solution in order to find out whether danazol's effect on spontaneous remyelination could be improved. The fact that other steroids have also been reported to exert a myelin repair potential additionally supports our findings. Specifically, progesterone and nestorone were shown to upkeep oligodendroglial cell maturation and myelin repair in cuprizone-induced chronic demyelinating regions [68]. Other studies highlight the prominent effect of testosterone and its synthetic analogue 7α-methyl-19-nortestosterone on myelin repair by oligodendrocytes in chronic and acute demyelinated lesions via the androgen receptor [69,70].
Given that substances were administrated while microgliosis, astrogliosis, OPC proliferation, and oligodendrocyte formation were still ongoing with the latter ones being vulnerable to cuprizone once they have reached a certain maturation state [71], we can therefore not fully exclude additional cytoprotective effects of parbendazole and danazol to occur in vivo. However, based on our cell- and tissue culture experiments we nevertheless suggest that the here observed positive effects on remyelination are mainly resulting from an increased OPC differentiation potential. Specific compound related cell proliferation effects were not determined i.e. animals were not killed at corresponding earlier time points. However, in the danazol- and parbendazole-treated mice we looked at the number of OLIG2-positive cells among the different groups and observed similar numbers in both substance and vehicle treated-mice (data not shown) thus speaking against a strong proliferation effect.
Finally, from a biological point of view, the here presented analysis has yielded important insights into signaling cascades controlling the p57kip2 dependent nuclear blockade of oligodendroglial differentiation. Our data suggest that external signals acting on ion channels and neurotransmitter receptors, microtubule dynamics (nocodazole, methiazole, parbendazole), steroid hormone receptors (danazol), sigma-1 receptors (opipramol, CAS 315–72–0), COX1/2, and STAT5 (isoxicam and fosfosal) are involved in the p57kip2 shuttling/OPC differentiation process, with most of the impact possibly resulting from microtubule modulation and activation of steroid hormone receptors (Fig. 1). It also revealed mitogen-activated protein kinases (MAPK) such as p38 to be implicated in the regulatory role in this process, which is additionally supported by our previous observations that CXCL12 chemokine stimulation resulted in MAPK phosphorylation and promoted oligodendroglial differentiation [72]. Moreover, p57kip2 nuclear exclusion and the subsequent promotion of cell differentiation were found to occur in an exportin 1 (CRM1) dependent manner [16]. And then, p38MAPK activation has previously been associated with nuclear export of different proteins such as E2F1, MAPK-activated protein kinase 5 (MK5), and NFAT, in all cases with the involvement of CRM1 [44], [45], [46].
Taken together, the here presented small scale library screening revealed to be a sensitive and powerful method for the detection of early promoters of the oligodendroglial cell differentiation process, which nevertheless fostered long-lasting impacts such as myelin production and axon wrapping. We applied a limited number of pharmacologically active compounds to primary oligodendroglia of three different species, of different CNS origins and in different environments thus looking for conserved molecular mechanisms. This alternative screening, particularly the short-term read-out based on protein translocation, proved to be applicable and successful, which is why application to larger compound libraries is suggested. Our findings on parbendazole and danazol are of great interest when it comes to white matter repair, particularly in light of them also being active in human oligodendroglial cells. Nevertheless, it must be considered that these hit compounds may only act as leading substances of which derivates shall be developed and tested.
Declaration of Competing Interest
Dr Healy reports consultancy fees from Merck, and funding from Canadian Stem Cell Network, CDQM, Quantum Leap and Bouchard Foundation outside of the submitted work. The rest of the authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgments
Contributors
Anastasia Manousi-Methodology; software; validation; formal analysis; investigation; data curation; writing-original draft; writing – review & editing; visualization; funding acquisition; data verification
Peter Göttle-Conceptualization; methodology; data curation; visualization; funding acquisition; resources
Laura Reiche-Methodology; formal analysis; investigation
Qiao-Ling Cui-Methodology; investigation
Luke M. Healy-Methodology; funding acquisition
Rainer Akkermann-Methodology
Joel Gruchot-Investigation; formal analysis
Jessica Schira-Heinen-Investigation
Jack P. Antel-Funding acquisition; resources
Hans-Peter Hartung-Supervision
Patrick Küry-Conceptualization; methodology; validation; data curation; writing-original draft; writing – review & editing; visualization; supervision; project administration; funding acquisition; resources, data verification
All authors read and approved the final version of the manuscript.
Acknowledgements
We thank Brigida Ziegler and Birgit Blomenkamp for technical assistance and Dr. Michael Dietrich for scientific consultancy and assistance. We thank Dr. David Kremer for logistical support. The anti-PLP antibody was kindly provided by the Department of Neurosciences at the Cleveland Clinic, OH.
AM was supported by the Jürgen Manchot Foundation, Düsseldorf. This work was also supported by a grant from the Research Commission of the Medical Faculty of Heinrich-Heine-University Düsseldorf (to PG), by the Christiane and Claudia Hempel Foundation for clinical stem cell research (to PK and PG), Stifterverband/Novartisstiftung (to PK) and the James and Elisabeth Cloppenburg, Peek and Cloppenburg Düsseldorf Stiftung (to PK and HPH). Work with human fetal oligodendroglial cells was supported by a grant from the International Progressive MS Alliance (BRAVEinMS, to LH and JPA).
Data and Code Availability
The code generated during this study is available at https://github.com/anastasiamanousi/p57kip2-translocation-Manousi-et-al.−2020. All other data are available upon request from the corresponding author.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103276.
Appendix. Supplementary materials
References
- 1.Sospedra M., Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23(1):683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Becher B., Bechmann I., Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med (Berl) 2006;84(7):532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 3.Lee J.Y., Taghian K., Petratos S. Axonal degeneration in multiple sclerosis: can we predict and prevent permanent disability? Acta Neuropathol Commun. 2014;2(1):97. doi: 10.1186/s40478-014-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassmann H. Multiple sclerosis pathology. Cold Spring Harb Perspect Med. 2018;8(3) doi: 10.1101/cshperspect.a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin R.J.M., Ffrench-Constant C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18(12):753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 6.Kotter M.R., Stadelmann C., Hartung H.P. Enhancing remyelination in disease–can we wrap it up? Brain. 2011;134(Pt 7):1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- 7.Kremer D., Göttle P., Hartung H.-.P., Küry P. Pushing forward: remyelination as the new frontier in CNS diseases. Trends Neurosci. 2016;39(4):246–263. doi: 10.1016/j.tins.2016.02.004. a. [DOI] [PubMed] [Google Scholar]
- 8.Gruchot J., Weyers V., Göttle P., Förster M., Hartung H.-.P., Küry P. The molecular basis for remyelination failure in multiple sclerosis. Cells. 2019;8(8) doi: 10.3390/cells8080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kremer D., Aktas O., Hartung H.-.P., Küry P. The complex world of oligodendroglial differentiation inhibitors. Ann. Neurol. 2011;69(4):602–618. doi: 10.1002/ana.22415. [DOI] [PubMed] [Google Scholar]
- 10.Kremer D., Akkermann R., Küry P., Dutta R. Current advancements in promoting remyelination in multiple sclerosis. Multiple Sclerosis J. 2018;25(1):7–14. doi: 10.1177/1352458518800827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer D., Göttle P., Flores-Rivera J., Hartung H.-.P., Küry P. Remyelination in multiple sclerosis. Curr. Opin. Neurol. 2019;32(3):378–384. doi: 10.1097/WCO.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 12.Zuchero J.B., Barres B.A. Intrinsic and extrinsic control of oligodendrocyte development. Curr. Opin. Neurobiol. 2013;23(6):914–920. doi: 10.1016/j.conb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinen A., Kremer D., Göttle P., Kruse F., Hasse B., Lehmann H. The cyclin-dependent kinase inhibitor p57kip2 is a negative regulator of Schwann cell differentiation and in vitro myelination. Proc Natl Acad Sci U S A. 2008;105(25):8748–8753. doi: 10.1073/pnas.0802659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer D., Heinen A., Jadasz J., Göttle P., Zimmermann K., Zickler P. p57kip2 is dynamically regulated in experimental autoimmune encephalomyelitis and interferes with oligodendroglial maturation. Proc Natl Acad Sci U S A. 2009;106(22):9087–9092. doi: 10.1073/pnas.0900204106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadasz J.J., Rivera F.J., Taubert A., Kandasamy M., Sandner B., Weidner N. p57kip2 regulates glial fate decision in adult neural stem cells. Development. 2012;139(18):3306–3315. doi: 10.1242/dev.074518. [DOI] [PubMed] [Google Scholar]
- 16.Göttle P., Sabo J.K., Heinen A., Venables G., Torres K., Tzekova N. Oligodendroglial maturation is dependent on intracellular protein shuttling. Journal of Neuroscience. 2015;35(3):906–919. doi: 10.1523/JNEUROSCI.1423-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobinick E.L. The value of drug repositioning in the current pharmaceutical market. Drug News Perspect. 2009;22(2):119–125. doi: 10.1358/dnp.2009.22.2.1303818. [DOI] [PubMed] [Google Scholar]
- 18.Gasparini F., Di Paolo T. Drug repurposing: old drugs, new tricks to fast track drug development for the brain. Neuropharmacology. 2019;147:1–3. doi: 10.1016/j.neuropharm.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Küry P., Kremer D., Göttle P. Drug repurposing for neuroregeneration in multiple sclerosis. Neural Regen Res. 2018;13(8):1366–1367. doi: 10.4103/1673-5374.235242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Göttle P., Manousi A., Kremer D., Reiche L., Hartung H.-.P., Küry P. Teriflunomide promotes oligodendroglial differentiation and myelination. J Neuroinflammation. 2018;15(1) doi: 10.1186/s12974-018-1110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshmukh V.A., Tardif V., Lyssiotis C.A., Green C.C., Kerman B., Kim H.J. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502(7471):327–332. doi: 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei F., Fancy S.P.J., Shen Y.-.A.A., Niu J., Zhao C., Presley B. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 2014;20(8):954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y.E., Suo N., Cui X., Yuan Q., Xie X. Vitamin C promotes oligodendrocytes generation and remyelination. Glia. 2018;66(7):1302–1316. doi: 10.1002/glia.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suo N., Guo Y.E., He B., Gu H., Xie X. Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia. 2019;67(7):1320–1332. doi: 10.1002/glia.23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubler Z., Allimuthu D., Bederman I., Elitt M.S., Madhavan M., Allan K.C. Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature. 2018;560(7718):372–376. doi: 10.1038/s41586-018-0360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Zhen W., Guo T., Zhao Y., Liu A., Rubio J.P. Histamine Receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS ONE. 2017;12(12) doi: 10.1371/journal.pone.0189380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kremer D., Küry P., Dutta R. Promoting remyelination in multiple sclerosis: current drugs and future prospects. Multiple Sclerosis J. 2015;21(5):541–549. doi: 10.1177/1352458514566419. [DOI] [PubMed] [Google Scholar]
- 28.Cunniffe N., Coles A. Promoting remyelination in multiple sclerosis. J. Neurol. 2019 doi: 10.1007/s00415-019-09421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy K.D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miron V.E., Jung C.G., Kim H.J., Kennedy T.E., Soliven B., Antel J.P. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann. Neurol. 2008;63(1):61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 31.Jadasz J.J., Tepe L., Beyer F., Samper Agrelo I., Akkermann R., Spitzhorn L.S. Human mesenchymal factors induce rat hippocampal- and human neural stem cell dependent oligodendrogenesis. Glia. 2018;66(1):145–160. doi: 10.1002/glia.23233. [DOI] [PubMed] [Google Scholar]
- 32.Cui Q.L., Fragoso G., Miron V.E., Darlington P.J., Mushynski W.E., Antel J. Response of human oligodendrocyte progenitors to growth factors and axon signals. J Neuropathol Exp Neurol. 2010;69(9):930–944. doi: 10.1097/NEN.0b013e3181ef3be4. [DOI] [PubMed] [Google Scholar]
- 33.Cui Q.-.L., D'Abate L., Fang J., Leong S.Y., Ludwin S., Kennedy T.E. Human fetal oligodendrocyte progenitor cells from different gestational stages exhibit substantially different potential to myelinate. Stem Cells Dev. 2012;21(11):1831–1837. doi: 10.1089/scd.2011.0494. [DOI] [PubMed] [Google Scholar]
- 34.Leong S.Y., Rao V.T.S., Bin J.M., Gris P., Sangaralingam M., Kennedy T.E. Heterogeneity of oligodendrocyte progenitor cells in adult human brain. Ann Clin Transl Neurol. 2014;1(4):272–283. doi: 10.1002/acn3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3 ed. New York: Elsevier2008.
- 36.Chen Z., Chen J.T., Johnson M., Gossman Z.C., Hendrickson M., Sakaie K. Cuprizone does not induce CNS demyelination in nonhuman primates. Ann Clin Transl Neurol. 2015;2(2):208–213. doi: 10.1002/acn3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 38.Allimuthu D., Hubler Z., Najm F.J., Tang H., Bederman I., Seibel W. Diverse chemical scaffolds enhance oligodendrocyte formation by inhibiting CYP51, TM7SF2, or EBP. Cell Chemical Biology. 2019;26(4):593. doi: 10.1016/j.chembiol.2019.01.004. -9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najm F.J., Madhavan M., Zaremba A., Shick E., Karl R.T., Factor D.C. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522(7555):216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lariosa-Willingham K.D., Rosler E.S., Tung J.S., Dugas J.C., Collins T.L., Leonoudakis D. A high throughput drug screening assay to identify compounds that promote oligodendrocyte differentiation using acutely dissociated and purified oligodendrocyte precursor cells. BMC Res Notes. 2016;9(1) doi: 10.1186/s13104-016-2220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckley C.E., Marguerie A., Roach A.G., Goldsmith P., Fleming A., Alderton W.K. Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects. Neuropharmacology. 2010;59(3):149–159. doi: 10.1016/j.neuropharm.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Kremer D., Cui Q.-.L., Göttle P., Kuhlmann T., Hartung H.-.P., Antel J. CXCR7 is involved in human oligodendroglial precursor cell maturation. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0146503. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsushima G.K., Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11(1):107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seternes O.M., Johansen B., Hegge B., Johannessen M., Keyse S.M., Moens U. Both binding and activation of p38 mitogen-activated protein kinase (MAPK) play essential roles in regulation of the nucleocytoplasmic distribution of MAPK-activated protein kinase 5 by cellular stress. Mol. Cell. Biol. 2002;22(20):6931–6945. doi: 10.1128/MCB.22.20.6931-6945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gómez del Arco P., Martínez-Martínez S., Maldonado J.L., Ortega-Pérez I., Redondo J.M. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J Biol Chem. 2000;275(18):13872–13878. doi: 10.1074/jbc.275.18.13872. [DOI] [PubMed] [Google Scholar]
- 46.Ivanova I.A., Dagnino L. Activation of p38- and CRM1-dependent nuclear export promotes E2F1 degradation during keratinocyte differentiation. Oncogene. 2006;26(8):1147–1154. doi: 10.1038/sj.onc.1209894. [DOI] [PubMed] [Google Scholar]
- 47.Zhu K., Sun J., Kang Z., Zou Z., Wu X., Wang Y. Repurposing of omeprazole for oligodendrocyte differentiation and remyelination. Brain Res. 2019;1710:33–42. doi: 10.1016/j.brainres.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 48.Göttle P., Förster M., Gruchot J., Kremer D., Hartung H.P., Perron H. Rescuing the negative impact of human endogenous retrovirus envelope protein on oligodendroglial differentiation and myelination. Glia. 2019;67(1):160–170. doi: 10.1002/glia.23535. [DOI] [PubMed] [Google Scholar]
- 49.Porcu G., Serone E., De Nardis V., Di Giandomenico D., Lucisano G., Scardapane M. Clobetasol and halcinonide act as smoothened agonists to promote myelin gene expression and RxRγ receptor activation. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0144550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui X., Guo Y-e, Fang J-h, Shi C-j, Suo N., Zhang R. Donepezil, a drug for Alzheimer's disease, promotes oligodendrocyte generation and remyelination. Acta Pharmacol. Sin. 2019;40(11):1386–1393. doi: 10.1038/s41401-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joubert L., Foucault I., Sagot Y., Bernasconi L., Duval F., Alliod C. Chemical inducers and transcriptional markers of oligodendrocyte differentiation. J. Neurosci. Res. 2010 doi: 10.1002/jnr.22434. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 52.Peppard J.V., Rugg C.A., Smicker M.A., Powers E., Harnish E., Prisco J. High-content phenotypic screening and triaging strategy to identify small molecules driving oligodendrocyte progenitor cell differentiation. J Biomol Screen. 2014;20(3):382–390. doi: 10.1177/1087057114559490. [DOI] [PubMed] [Google Scholar]
- 53.Chen J., Zuo S., Wang J., Huang J., Zhang X., Liu Y. Aspirin promotes oligodendrocyte precursor cell proliferation and differentiation after white matter lesion. Front Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheli V.T., Santiago González D.A., Spreuer V., Paez P.M. Voltage-gated Ca++ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp Neurol. 2015;265:69–83. doi: 10.1016/j.expneurol.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan L-W, Bhatt A., Tien L-T, Zheng B., Simpson K.L., Lin R.C.S. Exposure to serotonin adversely affects oligodendrocyte development and myelinationin vitro. J Neurochem. 2015;133(4):532–543. doi: 10.1111/jnc.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preisner A., Albrecht S., Cui Q.-.L., Hucke S., Ghelman J., Hartmann C. Non-steroidal anti-inflammatory drug indometacin enhances endogenous remyelination. Acta Neuropathol. 2015;130(2):247–261. doi: 10.1007/s00401-015-1426-z. [DOI] [PubMed] [Google Scholar]
- 57.Salahuddin Shaharyar M, Mazumder A. Benzimidazoles: a biologically active compounds. Arabian J Chem. 2017;10:S157–SS73. [Google Scholar]
- 58.Radostits O., Done S. 10 ed. Elsevier London; 2007. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats; pp. 674–762. [Google Scholar]
- 59.Lo Y.-.C., Senese S., France B., Gholkar A.A., Damoiseaux R., Torres J.Z. Computational cell cycle profiling of cancer cells for prioritizing FDA-approved drugs with repurposing potential. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-11508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee B.Y., Hur E.M. A role of microtubules in oligodendrocyte differentiation. Int J Mol Sci. 2020;21(3) doi: 10.3390/ijms21031062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong J., Wang N.-.N., Yao Z.-.J., Zhang L., Cheng Y., Ouyang D. ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminform. 2018;10(1) doi: 10.1186/s13321-018-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szabo K.T. Academic Press; San Diego: 1989. Congenital malformations in laboratory and farm animals /Kalman T. Szabo. [Google Scholar]
- 63.Dmowski W.P., Scholer H.F.L., Mahesh V.B., Greenblatt R.B. Danazol—a synthetic steroid derivative with interesting physiologic properties *†. Fertil. Steril. 1971;22(1):9–18. doi: 10.1016/s0015-0282(16)37981-x. [DOI] [PubMed] [Google Scholar]
- 64.Podhorecka M., Macheta A., Chocholska S., Bojarska-Junak A., Szymczyk A., Goracy A. Danazol induces apoptosis and cytotoxicity of leukemic cells alone and in combination with purine nucleoside analogs in chronic lymphocytic leukemia. Ann. Hematol. 2015;95(3):425–435. doi: 10.1007/s00277-015-2579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang Y.-.T., Teng Y.-.N., Lin K.-.I., Wang C.C.N., Morris-Natschke S.L., Lee K.-.H. Danazol mediates collateral sensitivity via STAT3/Myc related pathway in multidrug-resistant cancer cells. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-48169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diamantopoulou E., Baxendale S., de la Vega de León A., Asad A., Holdsworth C.J., Abbas L. Identification of compounds that rescue otic and myelination defects in the zebrafish adgrg6 (gpr126) mutant. Elife. 2019;8 doi: 10.7554/eLife.44889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X., Vaughn J.M., Yacaman M.J., Williams R.O., Johnston K.P. Rapid dissolution of high-potency danazol particles produced by evaporative precipitation into aqueous solution. J Pharm Sci. 2004;93(7):1867–1878. doi: 10.1002/jps.20001. [DOI] [PubMed] [Google Scholar]
- 68.El-Etr M., Rame M., Boucher C., Ghoumari A.M., Kumar N., Liere P. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia. 2015;63(1):104–117. doi: 10.1002/glia.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hussain R., Ghoumari A.M., Bielecki B., Steibel J., Boehm N., Liere P. The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain. 2013;136(Pt 1):132–146. doi: 10.1093/brain/aws284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bielecki B., Mattern C., Ghoumari A.M., Javaid S., Smietanka K., Abi Ghanem C. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc Natl Acad Sci. 2016;113(51):14829–14834. doi: 10.1073/pnas.1614826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vega-Riquer J.M., Mendez-Victoriano G., Morales-Luckie R.A., Gonzalez-Perez O. Five decades of cuprizone, an updated model to replicate demyelinating diseases. Curr Neuropharmacol. 2019;17(2):129–141. doi: 10.2174/1570159X15666170717120343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Göttle P., Kremer D., Jander S., Ödemis V., Engele J., Hartung H.-.P. Activation of CXCR7 receptor promotes oligodendroglial cell maturation. Ann. Neurol. 2010;68(6):915–924. doi: 10.1002/ana.22214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code generated during this study is available at https://github.com/anastasiamanousi/p57kip2-translocation-Manousi-et-al.−2020. All other data are available upon request from the corresponding author.