Abstract
Purpose
To evaluate the prognostic value of myocardial perfusion PET in patients with and patients without diabetes mellitus.
Materials and Methods
The authors performed a retrospective analysis of prospectively acquired data from a multicenter registry cohort of 7061 patients, including 1966 with diabetes mellitus, who underwent clinically indicated rest-stress rubidium 82 (82Rb) myocardial perfusion PET. The mean patient age (±standard deviation) was 63.3 years ± 13. Of the 7061 patients, 3348 were women (47.4%), 2296 (32.5%) had known coronary artery disease, and 1895 (26.8%) had previously undergone revascularization. The primary end point was cardiac death (n = 169) assessed at a mean of 2.5 years ± 1.5. The authors used Cox proportional hazards models and risk reclassification measures stratified according to diabetes status.
Results
In multivariable models adjusting for established clinical predictors, increasing magnitude of stress myocardial perfusion abnormality was associated with greater risk of cardiac death in patients with diabetes (hazard ratio [HR]: 7.2; 95% confidence interval [CI]: 3.1, 16.8) for severely abnormal myocardium compared with normal myocardium. The addition of stress myocardial perfusion imaging results significantly improved the fit of a clinical model for predicting cardiac death in patients with and patients without diabetes. Myocardial perfusion PET improved risk reclassification for cardiac death in patients with diabetes (category-based net reclassification index: 0.39; 95% CI: 0.15, 0.60, P < .001). Among diabetic patients, an abnormal myocardial perfusion PET scan was associated with increased risk of cardiac death (HR: 4.4; 95% CI: 2.0, 9.7) in all important clinical subgroups based on age, sex, obesity, or prior revascularization.
Conclusion
In a large cohort of patients referred for clinical 82Rb stress PET, myocardial perfusion imaging results provided incremental risk prediction of cardiac death in patients with and patients without diabetes mellitus.
© RSNA, 2019
Summary
In this large cohort including nearly 2000 patients with diabetes, myocardial perfusion PET results provide powerful and incremental prognostic information with appropriate reclassification improvement in 39% of patients with diabetes.
Key Points
■ Patients with diabetes derive significantly improved risk reclassification with myocardial perfusion PET.
■ Women and younger patients with diabetes remain at higher risk of cardiovascular death despite normal myocardial perfusion and might need additional risk stratification methods.
Introduction
Diabetes affects an estimated 20 million adult Americans, with a projected worldwide prevalence of 552 million by the year 2030, and is a devastating pandemic and a major risk factor for coronary artery disease and myocardial infarction. It is a leading cause of mortality from cardiovascular diseases (1) and is often considered equivalent to myocardial infarction (2). Although mortality from cardiovascular diseases has declined steeply and continuously in the general population during the past 40 years, this benefit was not constantly observed among patients with diabetes (3–5)—especially in women and younger patients (4,6). Importantly, not all patients with diabetes display a similarly heightened hazard (7), making risk stratification among patients with diabetes a clinically important goal (8,9).
SPECT is a well-established method for evaluating diagnosis and prognosis among patients suspected of having or known to have coronary heart disease (10). Abnormal SPECT myocardial perfusion is associated with worse clinical outcomes in both patients with and patients without diabetes (11,12). Myocardial perfusion PET, with superior diagnostic accuracy and lower radiation exposure, is increasingly used in more than 200 centers across the United States (13–15). Several analyses have established the diagnostic and prognostic utility of myocardial perfusion PET in patients with coronary artery disease (13,15,16). However, given the heightened risk of cardiac death among patients with diabetes, it is unknown whether myocardial perfusion PET would have incremental value for risk stratification in this higher risk group.
Our objective was to assess the prognostic value of stress myocardial perfusion PET in patients with diabetes, including clinically important subgroups based on age, sex, and obesity. We also compared the prognostic performance of myocardial perfusion PET between patients with and patients without diabetes. Our aim was to test the hypotheses that, among patients with diabetes, abnormal myocardial perfusion PET would (a) provide incremental prognostic information about cardiac death and (b) further stratify risk among women and younger patients.
Materials and Methods
This analysis is based on data from a four-center myocardial perfusion PET registry that has been previously described (17). Briefly, 7061 patients were prospectively enrolled at four expert centers participating in this registry when referred for a clinically indicated rubidium 82 (82Rb) myocardial perfusion PET and followed up for all-cause death and cardiac death. Standardized methods for data collection of clinical history, risk factors, medication usage, and stress data were applied at each center. For the purpose of this analysis, diabetes was based on self-reported diagnosis or use of antidiabetes therapy (insulin or oral agents). All participating centers had institutional review board approval for the study.
Bracco and Astellas provided partial funding for the initial PET registry study. The current analysis is an independent analysis conducted and performed by the PET registry investigators. Investigators and authors had complete control of the data and the information submitted for publication.
82Rb Myocardial Perfusion PET
All patients underwent 82Rb myocardial perfusion PET with dedicated PET or hybrid PET/CT scanners according to conventional protocols, as previously reported (17). Rest and stress images were scored using the 17-segment scoring system, and the percentage of abnormal myocardium at stress was determined from the summed stress score. The summed difference score (difference between stress and rest scores) was used to indicate the percentage of ischemic myocardium. Each stress scan was categorized according to the percentage of abnormal myocardium at stress as follows: 0%, normal; 0.1%–9.9%, mildly abnormal; 10%–19.9%, moderately abnormal; and 20% or greater, severely abnormal. This analysis is primarily based on the percentage of abnormal myocardium at stress unless specifically stated otherwise.
Follow-up and Study End Points
Follow-up findings were ascertained from medical records and Social Security Death Index. Cardiac death was the primary end point for this analysis and occurred in 169 of 6037 patients from three centers where the cause of death was ascertained. The secondary end point was all-cause death (n = 570) and was determined at all four centers.
Statistical Analysis
Standard data analysis methods were used for data description and comparison, including comparisons of categorical variables with χ2 statistics and comparisons of continuous measures with the Student t test. Two-sided P <.05 was considered indicative of a significant difference.
Event rates were estimated based on duration of follow-up and expressed per 100 patient-years of follow-up. Cox proportional hazard models were used to assess the association between myocardial perfusion imaging results and death and between diabetes and death. A multivariable model previously used in the overall cohort (17) was used as a starting point to select a more parsimonious model that would reduce overfitting. The initial multivariable model included age, sex, body mass index, hypertension, high cholesterol level, smoking, angina, or dyspnea as indications for the test, beta-blockers, resting heart rate, and prior revascularization procedure. The final model retained only variables that were statistically significant in the overall cohort and included age, sex, high cholesterol level, smoking, angina or dyspnea, and resting heart rate. We studied several interactions, including age, sex, obesity, angina symptoms, and prior revascularization.
To assess the incremental prognostic value of myocardial perfusion PET, we compared a multivariable clinical model to a model with clinical variables and percentage abnormal stress myocardium using net reclassification improvement (continuous [18] and category-based [1%, 5%, and 10% cardiac death at 2 years]), model χ2, Harrell C statistic, and Akaike information criteria.
P <.05 was considered indicative of a statistically significant difference. All analyses were performed with Stata 12.1 (StataCorp, College Station, Tex) and R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org) software.
Results
Baseline characteristics and myocardial perfusion imaging results for all patients and according to diabetes category are shown in Table 1. Of the 7061 patients, 1966 (27.8%) had diabetes. Compared with those without diabetes, patients with diabetes were older and more likely to be obese, have hypertension, have high cholesterol, have known coronary artery disease, and have undergone prior revascularization procedures. They were also more likely to be on aspirin, lipid-lowering medications, beta-blockers, and angiotensin-converting enzyme inhibitors than patients without diabetes.
Table 1:
Baseline Characteristics
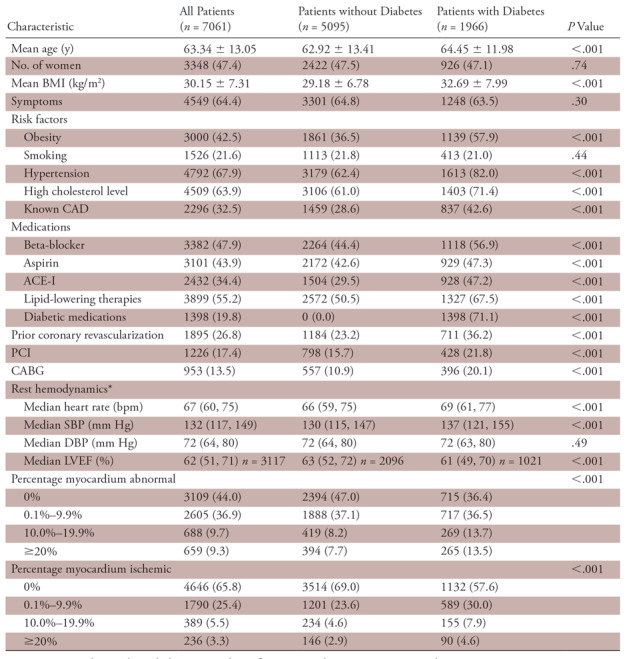
Note.—Except where indicated, data are numbers of patients, with percentages in parentheses. ACE-I = angiotensin-converting enzyme inhibitors, BMI = body mass index, CABG = coronary artery bypass graft, CAD = coronary artery disease, DBP = diastolic blood pressure, LVEF = left ventricular ejection fraction, PCI = percutaneous coronary intervention, SBP = systolic blood pressure.
*Numbers in parentheses are the 25th and 75th percentiles.
Overall, 3109 of the 7061 patients (44.0%) had a normal myocardial perfusion study and 659 (9.3%) had a severely abnormal scan (≥20% myocardium abnormal). Compared with those without diabetes, patients with diabetes were less likely to have normal scans (47.0% and 36.4%, respectively) and more likely to have severely abnormal scans (7.7% and 13.5%) (P <.001) (Table 1).
Outcomes Analysis
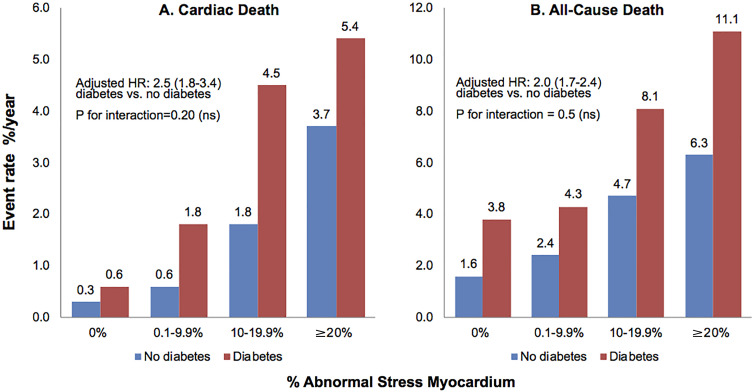
The cause of death was assessed during a mean follow-up of 2.5 years in 1651 patients with and 4386 patients without diabetes. The primary end point of cardiac death occurred in 82 of the 1651 patients with diabetes (5.0%) and 87 of the 4386 patients without diabetes (2.0%). Compared with those without diabetes, patients with diabetes had higher rates of cardiac death (2.2 vs 0.7 per 100 patient-years of follow-up) and all-cause mortality (5.4 vs 2.5 per 100 patient-years of follow-up) as well as higher adjusted hazard ratios (HRs). Annualized cardiac and all-cause death rates (adjusted or unadjusted) increased linearly as a function of scan abnormality in both patients with and patients without diabetes (Fig 1). For any degree of myocardial perfusion imaging abnormality, the cardiac death rate was uniformly higher in patients with diabetes than in those without diabetes (P for interaction = .20, Fig 1).
Figure 1:
Bar charts show annualized rates of, A, cardiac death and, B, all-cause death stratified according to percentage abnormal stress myocardium category in patients without and patients with diabetes. Within each scan category, patients with diabetes demonstrated a higher risk of cardiac death and all-cause death than did patients without diabetes. The risk of cardiac death and all-cause death increased significantly with worsening scan category. Numbers in parentheses are 95% confidence intervals. HR = hazard ratio, ns = not significant.
In univariable analyses, clinical variables that were significantly associated with a higher risk of cardiac death among patients with diabetes included older age, female sex, lower body mass index, smoking, lack of anginal symptoms, and prior revascularization. In both univariable and multivariable analyses adjusting for age, female sex, high cholesterol level, smoking, symptoms, and resting heart rate, a greater magnitude of scan abnormality was associated with a significantly higher risk of cardiac death (Table 2, Fig 1). Even a mildly abnormal scan was associated with an increased risk of cardiac death in both patients with (HR: 2.95; 95% confidence interval [CI]: 1.29, 6.75) and patients without (HR: 1.86; 95% CI: 0.97, 3.55) diabetes (Table 2). Patients with diabetes and severely abnormal scans (≥20% abnormal myocardium) had a sevenfold increase in the risk of cardiac death compared with those with normal scans in multivariable model analyses (Table 2). The results for the secondary end point of all-cause death were very similar (Fig 1). Additional analyses using the expanded multivariable models previously described also showed similar results (Tables E1, E2 [supplement]).
Table 2:
Multivariable Cox Models for Cardiac Death in Patients without and Patients with Diabetes
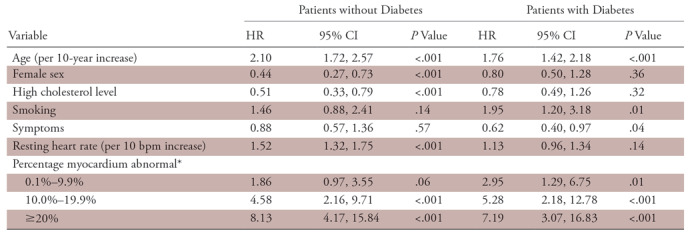
Note.—CI = confidence interval, HR = hazard ratio.
*Reference group = 0%.
Incremental Prognostic Value
The addition of the degree of stress scan abnormality to a previously validated clinical model significantly improved the goodness-of-fit and reclassification metrics in both patients with and patients without diabetes (Table 3). With use of risk categories for cardiac death of less than 1%, 1%–5%, 5%–10%, and greater than 10% at 2 years, the addition of percentage abnormal stress myocardium information to a clinical model yielded a net reclassification improvement of 39% (P <.001) in patients with diabetes as well as an increase in the Harrell C statistic and a decrease in Akaike information criterion, suggesting improved model fit (Table 3). Moreover, reclassification was improved both for events (cases) (net reclassification improvement for events, 0.27) and nonevents (controls) (net reclassification improvement for nonevents, 0.12) in patients with and patients without diabetes (net reclassification improvement for events, 0.14) and nonevents (net reclassification improvement for nonevents, 0.07).
Table 3:
Reclassification Measures Associated with Addition of Myocardial Perfusion PET Information in Multivariable Models
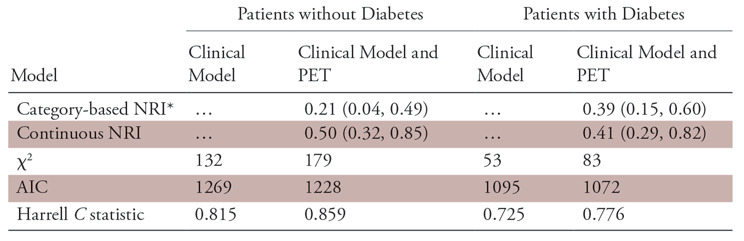
Note.—Data in parentheses are 95% confidence intervals. AIC = Akaike information criterion, NRI = net reclassification index. The variables in the clinical model are age, sex, high cholesterol, smoking, symptoms, and resting heart rate.
*Risk categories for cardiac death are less than 1%, 1%–5%, 5%–10%, and greater than 10% or more at 2 years.
Subgroup Analyses
In multivariable risk-adjusted analyses stratified according to sex, diabetes was associated with an increased risk of cardiac death in both men (HR: 1.9; 95% CI: 1.3, 2.8) and women (HR: 2.7; 95% CI: 1.5, 4.9), without evidence of a statistical interaction with scan results (P for interaction = .21). Among patients without diabetes, men consistently demonstrated a higher adjusted risk of cardiac death at each category of percentage abnormal stress myocardium (HR for men vs women: 2.2; 95% CI: 1.3, 3.6); however, diabetic women demonstrated a similar risk to diabetic men (HR for men vs women: 1.2; 95% CI: 0.8, 2.0) (Fig 2). Higher percentage myocardium abnormal at stress remained a strong predictor of higher cardiac death rate among men and women in both patients with and patients without diabetes.
Figure 2:
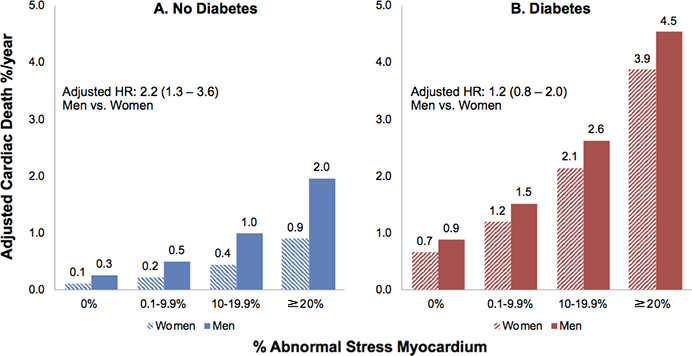
Bar charts show risk-adjusted annualized cardiac death rate in women and men, A, without and, B, with diabetes stratified according to percentage abnormal stress myocardium category. In patients without diabetes, women showed a lower risk of cardiac death than men for each scan category. In patients with diabetes, the risk of cardiac death was similar for women and men. Numbers in parentheses are 95% confidence intervals. HR = hazard ratio.
Age- and diabetes-stratified analyses showed that younger patients with diabetes (age <55 years) and a normal scan had an adjusted cardiac death rate of 0.27 per 100 patient-years of follow-up, which is similar to that of older patients with normal scan results and without diabetes (age, 65–74 years). In fact, for each category of abnormal myocardium, the cardiac death rate of young diabetic patients (<55 years) was similar to that of older patients without diabetes (65–74 years) (Fig E1 [supplement]). Among patients without diabetes, there was an interaction between age and severity of abnormal perfusion, such that there was a greater increased risk due to abnormal perfusion at younger ages than at older ages (P for interaction = .04). We did not observe this interaction among patients with diabetes; within each age group, young and old, a more abnormal myocardial perfusion scan was associated with a higher risk of cardiac death (Fig E1 [supplement]).
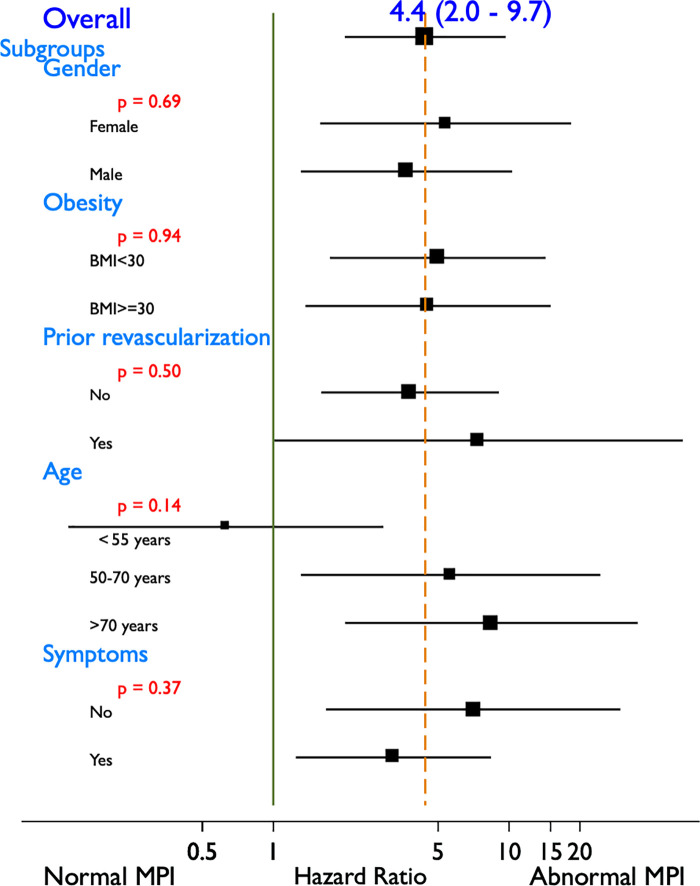
Overall, in clinically relevant diabetic subgroups an abnormal stress myocardial perfusion PET result was associated with an overall higher risk of cardiac death (HR: 4.4; 95% CI: 2.0, 9.7; P <.001) without any significant interactions with sex, obesity, prior revascularization, age category, or lack of symptoms (Fig 3).
Figure 3:
Risk of cardiac death with abnormal stress myocardial perfusion imaging (MPI) in important clinical subgroups among patients with diabetes. The overall hazard ratio (HR) for cardiac death associated with an abnormal myocardial perfusion imaging in patients with diabetes was 4.4 (95% confidence interval: 2.0, 9.7). This risk was not significantly different among the various subgroups tested, including sex, obesity, prior revascularization, age category, or lack of symptoms. BMI = body mass index. Green solid line indicates HR of 1. Yellow dashed line indicates overall HR for abnormal MPI. P value is for interaction between subgroup and abnormal MPI.
Discussion
In our study, the largest study of patients undergoing clinical 82Rb stress myocardial perfusion PET to our knowledge, we demonstrated that perfusion scan results provide powerful prognostic information, as well as similarly improved reclassification, in patients with and patients without diabetes. Our analyses show several additional findings. First, we showed that patients with diabetes were more likely to have moderately and severely abnormal PET myocardial perfusion imaging results and that the severity of abnormal perfusion is directly associated with a higher risk of cardiac death. Second, at each degree of scan abnormality, including normal scans, patients with diabetes had a twofold increased hazard of cardiac death compared to patients without diabetes. Furthermore, we showed that for each category of stress myocardial perfusion imaging abnormality, the risk of cardiac death in women with diabetes was as high as that in men with diabetes. Conversely, women without diabetes demonstrated a lower risk of cardiac death than men without diabetes with normal, mildly abnormal, moderately abnormal, or severely abnormal scans. Finally, for similar scan results, the risk of cardiac death in younger patients with diabetes was comparable to that of nondiabetic patients who were 10–15 years older.
Although myocardial perfusion PET is an established high-quality examination for risk stratification and diagnosis of coronary disease in the general population, it has not been extensively evaluated specifically in patients with diabetes. Other studies have evaluated the prognostic value of myocardial perfusion SPECT (11,19–21) in patients with diabetes, but, to our knowledge, this is the largest prognostic study of patients with diabetes (n = 1966) undergoing pharmacological stress 82Rb myocardial perfusion PET.
The risk of cardiac death is significantly higher in patients with diabetes compared to those without (22,23), and it is important to establish if myocardial perfusion PET continues to provide incremental prognostic information in this high-risk population. Accordingly, identification of ischemic heart disease and risk stratification with a goal to institute appropriate therapies or interventions to reduce the cardiac death rate may prove beneficial in this population. In our analyses, we show that in each degree of scan abnormality, diabetes was associated with a doubling in the risk of cardiac death. Giri et al (11), however, showed similar survival between patients with (n = 929, 19.5%) and patients without diabetes in multivariable models adjusting for pretest clinical risk and stress myocardial perfusion SPECT results. Despite this lack of association between diabetes and cardiac death risk in multivariable models, which could be explained by statistical model specificity, SPECT perfusion abnormalities were associated with an increased risk of cardiac death in patients with and patients without diabetes. In a study of 463 patients (44% with diabetes), Kang et al (12) showed that dual-isotope SPECT had similar sensitivity and specificity for angiographically proven coronary artery disease in patients with and patients without diabetes. These studies, however, did not specifically address the incremental prognostic value of myocardial perfusion SPECT in patients with diabetes. The results of our analyses confirm that abnormal stress myocardial perfusion PET is prognostically associated with an increased risk of cardiac death in patients with diabetes but further establish that, overall, 39% of patients with diabetes are appropriately reclassified in a better and/or lower risk category.
Reports of a differential prognostic effect of SPECT perfusion results in men and women have been previously published and justifiably enhanced our goal of better risk stratification among women. Diabetes has been described as an important prognostic marker such that women with diabetes are at higher risk of cardiac death than any other group (women without diabetes, men with and without diabetes), regardless of the degree of myocardial perfusion SPECT abnormality (19). The prognostic value of myocardial perfusion PET is similar in men and women (24). However, when further stratified according to diabetes status, our analyses showed that although nondiabetic women were at a significantly lower risk of cardiac death than nondiabetic men, this “advantage” seems to be erased in the presence of diabetes. Indeed, at every degree of abnormal myocardial perfusion, the risk of cardiac death in diabetic women was similar to that of diabetic men. Diabetes was associated with a slightly higher HR for cardiac death in women than in men, but this difference was not statistically significant. It is important to note that although the diagnostic accuracy of myocardial perfusion PET is overall higher than that of SPECT, it appears to be specifically more accurate in women as well (accuracy rate: 89% for PET and 67% for SPECT; P = .009) (16,25).
Although younger patients are expected to be at a lower risk of cardiac death than older patients, the advantage of young age appears to be obviated by diabetes. We showed that the rate of cardiac death for patients with diabetes is similar to that in patients without diabetes who are 15 years older at the same myocardial perfusion scan category. That even a normal perfusion scan in a young diabetic patient (<55 years) portends a similar cardiac death risk as a nondiabetic patient aged 55–64 years with mild ischemia may have important clinical implications. Indeed, although normal myocardial perfusion PET can predict a low event rate in patients without diabetes, it may be necessary to use other tools for further risk stratification (7,26–29) to detect subclinical atherosclerosis and potentially initiate appropriate interventions in patients with diabetes. It is important to note that, to our knowledge, no randomized clinical trial of myocardial perfusion SPECT or CT coronary angiography has shown a survival benefit based on a screening strategy in asymptomatic patients with diabetes (30,31). Admittedly, patients in these trials were optimally treated at baseline. For example, in the Screening for Asymptomatic Obstructive Coronary Artery Disease among High-Risk Diabetic Patients Using CT Angiography, Following Core 64 (faCTor-64) trial, in which more than 75% of patients had a low-density lipoprotein level of less than 100 mg/dL and a mean baseline blood pressure of 130/75 mm Hg, the cardiac event rate was relatively lower than expected, suggesting that an imaging-based screening strategy may not have significant incremental benefit in well-treated patients with diabetes (31,32). In another cohort of patients with diabetes referred for myocardial perfusion PET, coronary flow reserve, a parameter of coronary vascular dysfunction, provided incremental risk stratification beyond myocardial perfusion results (26). In addition, patients with diabetes and preserved coronary flow reserve demonstrated low cardiac death rates that were comparable to patients without known coronary artery disease or diabetes (26).
Our study had several strengths, including large sample size and proportion of patients with diabetes and long follow-up. We used currently accepted statistical methods to assess incremental prognostic value of stress myocardial perfusion PET in patients with and patients without diabetes. However, some limitations must be noted as well. Most important, duration and complications of diabetes were not well ascertained. Multiple studies have noted the importance of diabetes duration, glycated hemoglobin level, need for insulin therapy, and presence of diabetic nephropathy, neuropathy, or retinopathy as important prognostic markers in the diabetic population. Renal function and albuminuria level were not available. These important factors would have certainly improved our prediction models but not necessarily decreased the prognostic value of perfusion imaging (33). Coronary flow reserve obtained during myocardial perfusion PET was not available in this cohort and could have further enhanced risk stratification (26), especially in patients with normal myocardial perfusion images. Last, despite our large sample size and event rates, statistical power might have been limited to detect small interaction effects.
In conclusion, in this large study of 7061 patients including almost 2000 with diabetes, myocardial perfusion PET results provide powerful and incremental prognostic information with appropriate reclassification improvement in 39% of patients with diabetes. We also showed that women and younger patients with diabetes remain at significantly high risk of cardiac death despite normal perfusion. Whether these patients may benefit from further risk stratification with additional methods warrants further investigation.
SUPPLEMENTAL TABLES
SUPPLEMENTAL FIGURES
Disclosures of Conflicts of Interest: H.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the advisory board of OptimizeRx. Other relationships: disclosed no relevant relationships. M.F.D.C. Activities related to the present article: institution received a grant from Spectrum Dynamics and Gilead Sciences; receives a consulting fee or honorarium from Sanofi Aventis and GE Healthcare for scientific and medical advisory board membership; received a research grant from Toshiba. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. R.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives research support from Astellas and Amgen. Other relationships: disclosed no relevant relationships. disclosed no relevant relationships. B.J.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution has grants/grants pending from CV Diagnostix and AusculSciences; has equity interest in GE Healthcare; institution receives in-kind research/education support from TeraRecon; receives in-kind research support from Siemens. Other relationships: disclosed no relevant relationships. R.S.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives money for advisory board membership from GE Healthcare; is a paid consultant for Lantheus Medical Imaging and Jubilant DraxImage; has grants/grants pending from Lantheus Medical Imaging, Jubilant DraxImage, and GE Healthcare. Other relationships: disclosed no relevant relationships. D.S.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives software royalties from Cedars-Sinai Medical Center. Other relationships: disclosed no relevant relationships. G.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives software royalties from Cedars-Sinai Medical Center. Other relationships: disclosed no relevant relationships. J.K.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has grants/grants pending from GE Healthcare and Dalio Foundation; has equity interest in Cleerly; serves on scientific advisory boards at Arineta and GE Healthcare; is a consultant to Abbott Vascular, HeartFlow, NeoGraft Technologies, MyoKardia, and CardioDx; has ownership in MDDX and Autoplaq. Other relationships: disclosed no relevant relationships. M.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is employed by CardioNavix; receives royalties from 1951. Other relationships: disclosed no relevant relationships. B.W. disclosed no relevant relationships. E.V. disclosed no relevant relationships. L.J.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received research grants from Astellas Pharma and Bracco Diagnostics. Other relationships: disclosed no relevant relationships. S.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for GE Healthcare, Annexin, and Pfizer; institution has grants/grants pending from Pfizer. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- HR
- hazard ratio
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339(4):229–234. [DOI] [PubMed] [Google Scholar]
- 3.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA 1999;281(14):1291–1297. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed B, Davis HT, Laskey WK. In-hospital mortality among patients with type 2 diabetes mellitus and acute myocardial infarction: results from the national inpatient sample, 2000-2010. J Am Heart Assoc 2014;3(4):e001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonsen JA, Gerke O, Rask CK, et al. Prognosis in patients with suspected or known ischemic heart disease and normal myocardial perfusion: long-term outcome and temporal risk variations. J Nucl Cardiol 2013;20(3):347–357. [DOI] [PubMed] [Google Scholar]
- 6.Roche MM, Wang PP. Sex differences in all-cause and cardiovascular mortality, hospitalization for individuals with and without diabetes, and patients with diabetes diagnosed early and late. Diabetes Care 2013;36(9):2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raggi P, Bellasi A. Not all diabetic patients were created equal: how to discriminate risk? Atherosclerosis 2014;237(1):82–83. [DOI] [PubMed] [Google Scholar]
- 8.Moralidis E, Didangelos T, Arsos G, Athyros V, Mikhailidis DP. Myocardial perfusion scintigraphy in asymptomatic diabetic patients: a critical review. Diabetes Metab Res Rev 2010;26(5):336–347. [DOI] [PubMed] [Google Scholar]
- 9.Bax JJ, Bonow RO, Tschöpe D, Inzucchi SE, Barrett E; Global Dialogue Group for the Evaluation of Cardiovascular Risk in Patients With Diabetes. The potential of myocardial perfusion scintigraphy for risk stratification of asymptomatic patients with type 2 diabetes. J Am Coll Cardiol 2006;48(4):754–760. [DOI] [PubMed] [Google Scholar]
- 10.Hendel RC, Budoff MJ, Cardella JF, et al. ACC/AHA/ACR/ASE/ASNC/HRS/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SIR 2008 Key Data Elements and Definitions for Cardiac Imaging A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Cardiac Imaging). J Am Coll Cardiol 2009;53(1):91–124. [DOI] [PubMed] [Google Scholar]
- 11.Giri S, Shaw LJ, Murthy DR, et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation 2002;105(1):32–40. [DOI] [PubMed] [Google Scholar]
- 12.Kang X, Berman DS, Lewin H, et al. Comparative ability of myocardial perfusion single-photon emission computed tomography to detect coronary artery disease in patients with and without diabetes mellitus. Am Heart J 1999;137(5):949–957. [DOI] [PubMed] [Google Scholar]
- 13.Al-Mallah MH, Sitek A, Moore SC, Di Carli M, Dorbala S. Assessment of myocardial perfusion and function with PET and PET/CT. J Nucl Cardiol 2010;17(3):498–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur SR, Reddy P, Carlos RC. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol 2008;15(4):444–451. [DOI] [PubMed] [Google Scholar]
- 15.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol 2007;49(10):1052–1058. [DOI] [PubMed] [Google Scholar]
- 16.Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 2006;13(1):24–33. [DOI] [PubMed] [Google Scholar]
- 17.Dorbala S, Di Carli MF, Beanlands RS, et al. Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol 2013;61(2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Pencina KM, Janssens ACJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol 2012;176(6):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman DS, Kang X, Hayes SW, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men: impact of diabetes mellitus on incremental prognostic value and effect on patient management. J Am Coll Cardiol 2003;41(7):1125–1133. [DOI] [PubMed] [Google Scholar]
- 20.Kang X, Berman DS, Lewin HC, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography in patients with diabetes mellitus. Am Heart J 1999;138(6 Pt 1):1025–1032. [DOI] [PubMed] [Google Scholar]
- 21.Zellweger MJ, Hachamovitch R, Kang X, et al. Threshold, incidence, and predictors of prognostically high-risk silent ischemia in asymptomatic patients without prior diagnosis of coronary artery disease. J Nucl Cardiol 2009;16(2):193–200. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA 1979;241(19):2035–2038. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 24.Kay J, Dorbala S, Goyal A, et al. Influence of sex on risk stratification with stress myocardial perfusion Rb-82 positron emission tomography: results from the PET (Positron Emission Tomography) Prognosis Multicenter Registry. J Am Coll Cardiol 2013;62(20):1866–1876. [DOI] [PubMed] [Google Scholar]
- 25.Di Carli MF, Hachamovitch R. Should PET replace SPECT for evaluating CAD? The end of the beginning. J Nucl Cardiol 2006;13(1):2–7. [DOI] [PubMed] [Google Scholar]
- 26.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126(15):1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100(10):1134–1146. [DOI] [PubMed] [Google Scholar]
- 28.Yeboah J, Erbel R, Delaney JC, et al. Development of a new diabetes risk prediction tool for incident coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis and the Heinz Nixdorf Recall Study. Atherosclerosis 2014;236(2):411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman MG, Blaha MJ, Budoff MJ, et al. Potential implications of coronary artery calcium testing for guiding aspirin use among asymptomatic individuals with diabetes. Diabetes Care 2012;35(3):624–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young LH, Wackers FJT, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301(15):1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhlestein JB, Lappé DL, Lima JAC, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA 2014;312(21):2234–2243. [DOI] [PubMed] [Google Scholar]
- 32.Gibbons RJ. Optimal medical therapy vs CT angiography screening for patients with diabetes. JAMA 2014;312(21):2219–2220. [DOI] [PubMed] [Google Scholar]
- 33.Al-Mallah MH, Hachamovitch R, Dorbala S, Di Carli MF. Incremental prognostic value of myocardial perfusion imaging in patients referred to stress single-photon emission computed tomography with renal dysfunction. Circ Cardiovasc Imaging 2009;2(6):429–436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.