Abstract
Night shift work is a risk factor for viral infection, suggesting that night shift schedules compromise host defense mechanisms. Prior studies have investigated changes in the temporal profiles of circulating cytokines important for priming and restraining the immune response to infectious challenges from night shift work, but not by way of a 24-h constant routine of continuous wakefulness devoid of behavioral or environmental influences. Hence the true endogenous pattern of cytokines, and the combined effect of sleep loss and circadian misalignment on these cytokines remains unknown. Here, 14 healthy young men and women underwent three days of either a simulated night shift or a simulated day shift schedule under dim light in a controlled in-laboratory environment. This was followed by a 24-h constant routine protocol during which venous blood was collected at 3-h intervals. Those who had been in the night shift schedule showed lower mean circulating TNF-α (t13 = -6.03, p < 0.001), without any significant differences in IL-1β, IL-8 and IL-10, compared with those who had been in the day shift (i.e., control) schedule. Furthermore, circulating IL-6 increased with time awake in both shift work conditions (t13 = 6.03, p < 0.001), such that temporal changes in IL-6 were markedly shifted relative to circadian clock time in the night shift condition. These results indicate that night shift work compromises host defense by creating cytokine conditions that initially impede anti-viral immunity (lower TNF-α) and may eventually promote autoimmunity (mistimed rise in IL-6).
Keywords: Inflammation, Night work, Infectious disease, COVID-19, Temporal regulation, Cytokine storm
Highlights
-
•
Night shift schedules compromise host defense mechanisms to infection.
-
•
This could be from dysregulated cytokine responses.
-
•
We show by a novel constant routine paradigm, that cytokine dysregulation occurs.
-
•
Cytokine dysregulation exposes night shift workers to increased risk for SARS-CoV-2.
1. Introduction
Millions of individuals experience caregiver duties, medical conditions, and/or lifestyle factors that lead to wakefulness at night, and it is estimated that about 20% of the U.S. workforce is engaged in work schedules that involve circadian misalignment (Institute of Medicine (IOM), 2006; Liu, 2019). Circadian misalignment is characterized by desynchrony between the central circadian pacemaker (biological clock) and behavioral cycles (sleep/wake, feeding/fasting, activity/rest). It is associated with adverse health consequences including increased susceptibility to infection, and risk of inflammation-related disorders including cardiovascular and cardiometabolic diseases (Besedovsky et al., 2019; Irwin, 2019; James et al., 2017; Liu, 2019).
A tightly regulated and integrated immune system is required for effective host defense against infection, and to limit auto-immune responses. This system comprises a cascade of cytokines, particularly key effectors such as tumor necrosis factor alpha (TNF-α) and interleukin (IL) 1β (IL-1β), IL-6 and IL-8, and the anti-inflammatory cytokine IL-10. The importance of these particular cytokines amongst the cascade of cytokines is as follows. Firstly, the three key cytokines of the acute phase response are IL-1β, TNF-α and IL-6, and these are each representatives of 3 important superfamilies of cytokines that coordinate host defenses, and also orchestrate sleep. Secondly, systemic administration of IL-1 and TNF-α induces, through vagal afferents, brain production of IL-1 and TNF-α mRNA, respectively. Accordingly, levels of IL-1 and TNF-α in blood are highly relevant for the regulation of sleep, whereas evaluation of other cytokines in this context is ongoing. Thirdly, IL-1β and TNF-α each interferes with the expression of clock genes by altering CLOCK-BMAL1-induced activation of E-box regulatory elements, thereby implicating a role for these cytokines in circadian disruption. Fourthly, there is an important interplay amongst these cytokines as part of the immune response to infection, and in the regulation of sleep. For example, circulating IL-6 increases markedly with infection and mediates IL-1β, TNF-α and IL-10 production (Zielinski and Krueger, 2012). The interplay of these pro and anti-inflammatory cytokines, and how they regulate sleep, is complex and has been reviewed (Krueger et al., 2008). As a simplification, pro-inflammatory cytokines generally promote sleep, whereas anti-inflammatory cytokines inhibit sleep.
The cytokine cascade is regulated by the central nervous system in part through processes that govern sleep as discussed in the preceding paragraph, as well as by activation of the hypothalamo-pituitary-adrenal axis and enteric nervous system (Irwin, 2019). Adaptive immune responses contain infection and limit self-injury by coordinating the temporal profile, magnitude, and duration of activation of pro- and anti-inflammatory cytokines, according to the type, degree, and duration of the insult (Besedovsky et al., 2019). Accordingly, the assessment of anti-inflammatory cytokines such as IL-10 is critical to further our understanding of the coordination of the cytokine response. It is thought that when the early proinflammatory response is weak, the risk for infectious disease is magnified, whereas when the late inflammatory response is excessive, there is increased risk for tissue injury, autoimmune disorders, and associated morbidity from inflammatory activation or even death from cytokine storm as occurs following infection with COVID-19.
Sleep disruption is associated with increased risk of viral infection and inflammatory disorders (Besedovsky et al., 2019; Irwin, 2019), but knowledge of the endogenous immune consequences of circadian misalignment from night shift work is limited (Besedovsky et al., 2019; Irwin, 2019; Morris et al., 2016; Wright et al., 2015; Zielinski and Krueger, 2012). Sleep has a homeostatic role in the regulation of the immune system (Irwin, 2019); and an increased inflammatory response during nighttime sleep is thought to prepare host defenses to infectious exposure during the day (Irwin, 2019; Redwine et al., 2000). Loss of sleep shifts the temporal profile of inflammation, leading to increased inflammation during the day rather than at night (Irwin, 2019). When sleep disruption is persistent, inflammatory activation becomes sustained and excessive (Irwin et al., 2016).
Studying the effects of night shift schedules on immune responses is complicated by the confounding effects of external environmental or behavioral influences – particularly the acute masking effect of sleep (Irwin, 2019). Therefore the sleep-wake cycle likely amplifies the diurnal rhythmicity observed for many cytokines. Use of a 24-h constant routine protocol, with constant wakefulness and devoid of external mediators, is needed to interrogate the endogenous temporal patterns of circulating cytokines (Duffy and Dijk, 2002). The constant routine protocol is the gold-standard method to remove or uniformly distribute external and behavioral influences that may affect the expression of endogenous rhythms (Duffy and Dijk, 2002; Mills et al., 1978; Rahman et al., 2015). The rhythms so observed under constant routine conditions are therefore not passive responses to changes in external or behavioral factors such as sleep-wake, feeding-fasting, posture, activity and light, but are internally generated by the circadian clock. We therefore conducted an in-laboratory study where healthy volunteers (aged 22–34) were assigned to three days of either a simulated night shift schedule (7 participants) or a simulated day shift (i.e., control) schedule (7 participants) – followed by a 24-h constant routine protocol, during which blood was collected at 3-h intervals through an intravenous catheter (see Fig. 1). Blood samples were assayed to compare the endogenous 24-h patterns of circulating cytokines after the central circadian pacemaker and behaviorally induced rhythms had been experimentally misaligned (night shift condition) or aligned (day shift condition).
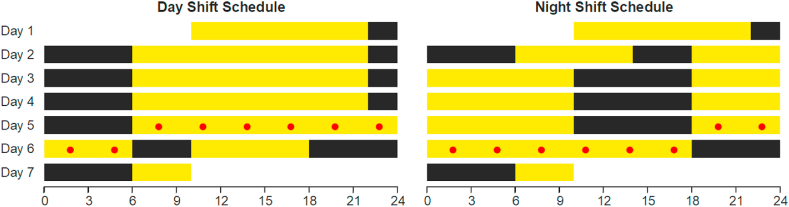
Fig. 1.
In-laboratory 7 day study protocol comprising a baseline day and night adaptation period in both conditions (day 1 and 2), a transition nap in the night-shift condition (sleep opportunity 14:00 to 18:00 on day 2), and then in both conditions 3 days of simulated day shift or night shift schedule (days 2–5), 24 h under constant routine (days 5–6), and a recovery period (days 6–7) (Skene et al., 2018). The 24-h constant routine condition consisted of sustained wakefulness in constant ambient temperature and dim light, fixed posture, and hourly identical snacks. Yellow: scheduled wakefulness under dim light (<50 lux); black: scheduled sleep. Red dots: blood draws at 3-h intervals during 24-h constant routine. Abscissas: clock time (in h). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. Materials and methods
2.1. Participants and experimental design
Fourteen healthy, drug- and medication-free young adults (10 men, 4 women) aged 22–34 y (mean ± SD 25.8 ± 3.2 y) with a body mass index (BMI) of 25.7 ± 3.2 kg/m2 completed the study. They were assigned to either the simulated day shift condition (7 participants, including 4 men) or the simulated night shift condition (7 participants, including 6 men) which required a rapid 12-h shift of the behavioral cycle; see Fig. 1. No subjects were replaced and all available data were included in the analyses. Further details of the study sample and study design have been described previously (Skene et al., 2018). In brief, the study was a 7 day protocol consisting of a baseline day and night followed by assignment to either a 3 day simulated day-shift work schedule (sleep opportunity from 22:00 to 06:00) or, after a transition nap with sleep opportunity of 14:00 to 18:00, to a 3 day simulated night-shift schedule (sleep opportunity from 10:00 to 18:00). Subject assignment to condition was determined random, through dice roll, in groups of two or three. Wake during scheduled wake periods was maintained by interactive conversation and by continuous observation under low light (<50 lux). Sleep during scheduled sleep periods was monitored by means of polysomnography (Nihon Kohden, Foothill Ranch, CA, USA). Digital sleep records were visually scored according to contemporaneous American Academy of Sleep Medicine standards (Iber et al., 2007). As previously reported, the night shift condition involved no substantive sleep loss or sleep disorder and had minimal effect on the central circadian pacemaker (Skene et al., 2018; Skornyakov et al., 2019). During this timetable of sleep/wake, meals were provided at 1.5 (breakfast), 7 (lunch), and 13.5 (dinner) h of scheduled wakefulness, and illuminance set below 50 lux during scheduled wakefulness. This was followed by a 24-h constant routine protocol, which controlled environmental and behavioral conditions with fixed semirecumbent posture, hourly identical snacks, constant temperature (21 ± 1 °C) and dim light (<50 lux), and sustained wakefulness. During the constant routine, wakefulness was again confirmed by continuous observation and maintained by interactive conversation. The protocol concluded with a recovery day.
2.2. Measurements
During the 24-h constant routine protocol, blood samples were collected at 3-h intervals in serum-separation and EDTA-containing vacutainers through an intravenous catheter. All tubes were inverted 10 times, then plasma samples were immediately stored at 4 °C, whereas serum samples were first allowed to clot at room temperature for 15 min. Serum and plasma were then separated together by centrifugation of 2200 revolutions per minute for 10 min at 4 °C and then immediately frozen at −80 °C until assayed. All plasma samples of a given individual were assayed together in the same 96-well plate to minimize the effect of inter-assay variability for cytokines. Circulating levels of TNF-α, IL-1β, IL-6, IL-8 and IL-10 were assayed simultaneously with a high–sensitivity, antibody-coated, magnetic bead-based, multiplex assay (Catalog FCSTM09-05 Lot 1573866, R&D Systems) with a Luminex 100/200 Instrument, proprietary Xponent software, and a 5-parameter logistic curve fit (Reed et al., 2016). IL-10 could not be calculated for 7 samples (6 from one woman and 1 from one man, both randomized to the night shift condition); statistical analyses were not predicated on complete data sets. The lowest detectable concentrations were 0.02 pg/ml (IL-10), 0.2 pg/ml (IL-1β), and 0.5–0.9 pg/ml (IL-6, IL-8 and TNF-α). Cortisol was measured in one run of all samples by platform immunoassay (Access Cortisol, Catalog 33600) using a Beckmann Access-2 instrument. The lowest detectable concentration for cortisol was 0.4 mcg/dL.
2.3. Statistical analyses
Cytokine and cortisol concentrations measured across the 24-h constant routine were analyzed with cosinor analysis for 24-h rhythmicity with a trend for time awake, implemented as non-linear mixed-effect regression as previously described (Mikulich et al., 2003; Olofsen et al., 2004; Skene et al., 2018; Van Dongen et al., 1999). Parameter differences between conditions (simulated night shift versus simulated day shift) were estimated for overall 24-h level (mesor); amplitude of 24-h rhythmicity; and timing (phase) of 24-h rhythmicity (provided the amplitude was significant at p < 0.05 for each condition). The estimated slope of change across time awake was shared between the two conditions. Parameter differences between conditions and the slope of change across time awake were evaluated using two-sided t-test with a two-tailed Bonferroni-corrected type I error threshold of 0.01. Primary analyses included sex as a covariant. Secondary analyses added baseline obesity (BMI), and age, and preceding sleep quality (transition index) and sleep duration (total sleep time), as covariants to the aforementioned non-linear mixed-effect models. These secondary analyses were conducted to confirm that the findings of the primary analyses were not due to differences in these covariants between conditions. Analyses were performed using SAS statistical package version 9.4 and proc nlmixed (SAS Institute, Cary, NC).
3. Results
Table 1 shows baseline characteristics.
Table 1.
Baseline demographics.
| Day Shift Condition N = 7 mean ± SD Count (percent) |
Night Shift N = 7 mean ± SD Count (percent) |
|
|---|---|---|
| Age (yr) | 24.0 ± 2.2 | 27.6 ± 3.2 |
| Height (m) | 1.8 ± 0.1 | 1.9 ± 0.1 |
| Weight (kg) | 81.1 ± 12.3 | 90.8 ± 19.6 |
| BMI (kg/m2) | 25.7 ± 3.4 | 25.6 ± 3.3 |
| Gender Male Female |
4 (57) 3 (63) |
6 (86) 1 (14) |
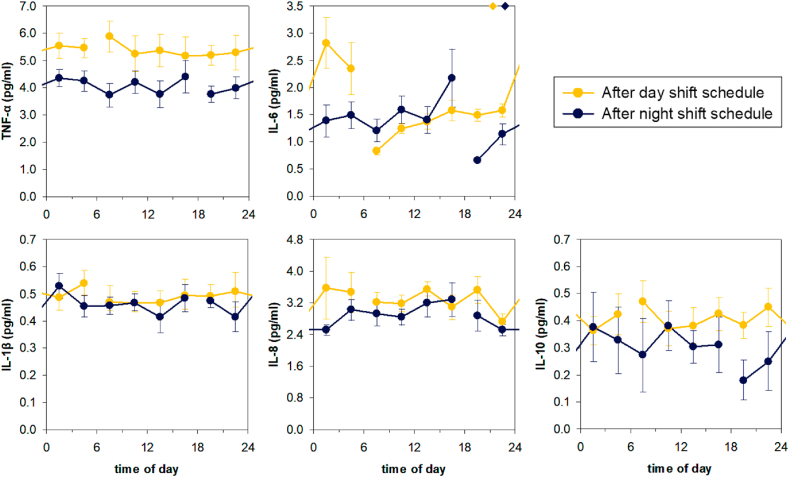
Fig. 2 shows the effects of the simulated day and night shift schedules on the endogenous 24-h patterns of five circulating cytokines: TNF-α, IL-1β, IL-6, IL-8 and IL-10. There were no significant differences between the day and night shift conditions in the amplitude or timing of circadian rhythmicity for any of the measured cytokines. However, overall 24-h levels of TNF-α were reduced after three days on the night shift schedule as compared to the day shift schedule (t13 = -6.03, p < 0.0001). Furthermore, IL-6 increased progressively with time awake irrespective of shift condition (t13 = 6.03, p < 0.0001), such that temporal changes in IL-6 were substantially shifted relative to the rhythm in the central circadian pacemaker after the night shift schedule. When analyses were repeated with measures of sleep quality, sleep quantity, BMI, age, and sex added as covariates, these main findings remained unaltered.
Fig. 2.
Effects of three days of a simulated day or night shift schedule on the temporal patterns of circulating cytokines measured under constant routine. Data are shown as group means and standard error. The gap in the line show where the 24-h constant routine protocol began/ended. Overall 24-h levels of TNF-α were reduced in the night shift condition relative to the day shift condition (top left). Additionally, IL-6 increased progressively with time awake in both conditions, but since wakefulness started 12 h later in the night shift condition, the temporal changes in IL-6 were shifted relative to the day shift condition and relative to the central circadian pacemaker (top middle). For reference, diamonds on the top of the IL-6 graph indicate the timing of the dim light melatonin onset, a marker of the timing of the central circadian pacemaker, in each condition (Skene et al., 2018).
Fig. 3 shows the effects of simulated day and night shift schedules on the endogenous 24-h pattern of cortisol. Circadian rhythmicity is apparent under both shiftwork conditions, and there is a small delay in acrophase during night shift of 26.5 min (t13 = 2.25, p = 0.04), but no difference in amplitude or mesor. Adjusting for sleep quality, sleep quantity, BMI, age, and sex did not alter this finding.
Fig. 3.
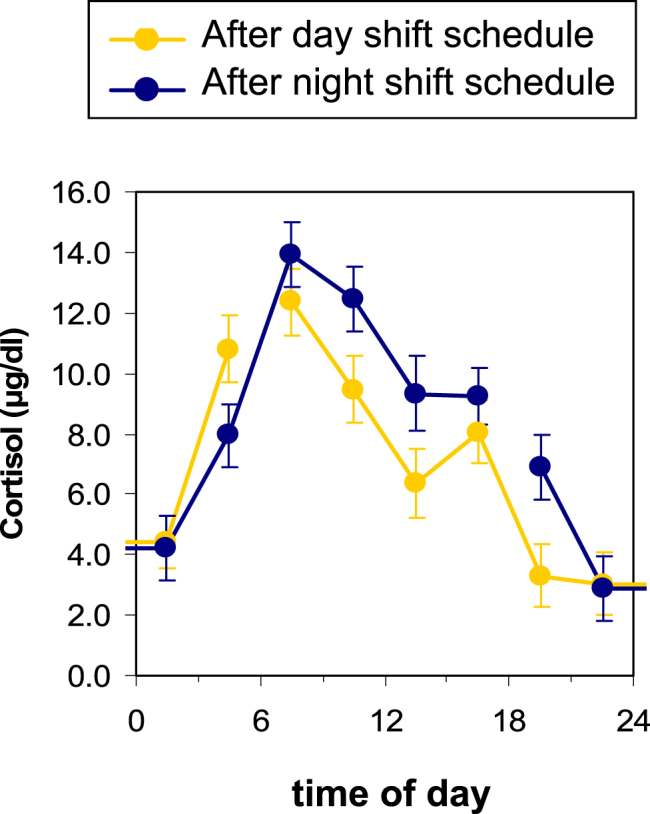
Effects of three days of a simulated day or night shift schedule on the temporal patterns of cortisol measured under constant routine. Data are shown as group means and standard error. The gap in the line show where the 24-h constant routine protocol began/ended. There was a small delay in acrophase with the night shift condition compared with the day shift condition.
4. Discussion
In this highly controlled laboratory study of healthy young adults, we found that circadian misalignment from simulated night shift altered the endogenous 24-h patterns of circulating cytokines. As the night shift condition involved no significant sleep loss (Skene et al., 2018), resulted in no substantive alteration in sleep quality (Skornyakov et al., 2019) and had minimal effect on the central circadian pacemaker (Fig. 2, top right), these results indicate imbalance in the endogenous regulation of cytokines after the night shift schedule, free of the influence of bright light. This is because TNF-α typically increases as an early response to insult, and then induces IL-6 causing pleiotropic effects in multiple organ systems (Besedovsky et al., 2019; Irwin, 2019; Zielinski and Krueger, 2012). Therefore, the overall decrease in circulating TNF-α in combination with the shifted temporal pattern of IL-6 points to cytokine conditions that increase initial susceptibility to infection, and also increase the risk of an excessive subsequent autoimmune response to infection.
Our finding that IL-6 progressively increased with time awake is consistent with prior interventional studies showing that sustained sleep loss will subsequently increase IL-6 (Haack et al., 2007; Shearer et al., 2001; Vgontzas et al., 1999, 2004); a recognized finding that occurs because the priming effect of sleep on IL-6 has not occurred (Irwin, 2019; Redwine et al., 2000). Furthermore, all these earlier studies assessed sleep loss under conditions that were not designed to cause sustained or substantial circadian misalignment (Haack et al., 2007; Lekander et al., 2013; Shearer et al., 2001; Vgontzas et al., 1999, 2004). Accordingly, our finding extends these earlier studies by now showing that sleep loss progressively increases IL-6 irrespective of the presence or absence of circadian misalignment – in other words that circadian misalignment itself does not alter IL-6. This finding is consistent with another study that misaligned the environment using a protocol that was identical in magnitude and duration to ours (12-h inverted behavioral cycles for 3 days) and also reported no difference in IL-6 concentrations at 3 days (Morris et al., 2016). However, that study (Morris et al., 2016) did not utilize a constant routine, and hence could not ensure that the observed temporal patterns of IL-6 were internally generated by the circadian clock, and not due to changes in external or behavioral factors such as sleep-wake, feeding-fasting, posture, activity and light.
Our findings of the relationship between sleep loss and circadian misalignment on TNF-α builds upon a small number of differing studies that have examined the effect of each condition separately. We did not find a relationship between time awake and TNF-α concentrations, and this is consistent with one study that reported no change with 5 days of sleep loss (Lekander et al., 2013), but not another that reported an increase after 7 days of sleep restriction (Vgontzas et al., 2004). Although a significant increase was reported in the latter study, the increase only occurred during a relatively short time period at 6–8AM.
The most striking finding was that circadian misalignment (as occurred during night shift work) resulted in TNF-α levels that were lower than in the control day shift work condition, and this contrasts with two prior studies that reported an increase in TNF-α with circadian misalignment (Morris et al., 2016; Wright et al., 2015). However, one of these studies did not assess 24-h TNF-α during a constant routine (Morris et al., 2016), and the other utilized a forced desynchrony protocol that caused sustained circadian misalignment for 21 consecutive days. Although the forced desynchrony protocol has merit, sustained circadian misalignment in dim light enforced by such protocols do not reflect real-life shift work – a limitation recognized by others (Morris et al., 2016). A parsimonious explanation of these differing findings is that circadian misalignment likely decreases TNF-α, but prolonged exposure to circadian misalignment increases TNF-α. Other explanations include differences in study design, including parallel group versus fixed order, and the masking effect of sleep.
These regulatory changes in TNF-α and IL-6 occurred without detectable changes in IL-1β, IL-8 and IL-10. These are novel findings since the effects of acute circadian misalignment on the endogenous patterns of IL-1β, IL-8 and IL-10 have not been investigated previously. In fact to the best of our knowledge, documentation of the temporal rhythms of blood cytokine levels under constant routine has not been reported. This is important because much of the day/night variation in circulating cytokines previously described is attributable to wake/sleep-related effects, which is not surprising as many cytokines serve important roles as sleep regulatory substances (Irwin, 2019; Krueger et al., 2008; Zielinski and Krueger, 2012). When measured under constant routine to expose the circadian (i.e., biological clock-driven) variation in cytokine levels in the absence of sleep/wake state changes, it should not be assumed a priori that diurnal patterns previously described will persist. Indeed, as our novel data show, not all cytokines are endogenously rhythmic in the absence of alternating sleep/wake patterns.
On the other hand, one study examined the cytokine response of whole blood (presumably white cells) collected under constant routine to ex-vivo lipopolysaccharide stimulation (Rahman et al., 2015). Interestingly, rhythms in IL-8 and TNF-α response to lipopolysaccharide stimulation were present suggesting that endogenous rhythms in cytokines exist, and could be detectable when amplified. However, an important caveat is that the endogenous rhythms exhibited in this study were entrained in a light-dark schedule typical for day shift work prior to the constant routine (Rahman et al., 2015). In contrast, bright light was avoided throughout the preceding simulated day and night shift work conditions for our study.
Limitations of our study include the relatively small sample size consisting of healthy young adults with little or no shift work experience – although we note that the repeated-measures design of the study provided sufficient statistical power to detect group differences in TNF-α and IL-6, and furthermore that this study is comparable in size with other studies (Morris et al., 2016; Wright et al., 2015). We also mimicked patterns of day and night shift work that occurs in modern society. These patterns was performed under dim light conditions to control for the impact of the light-dark cycle on the circadian misalignment of simulated night shift work compared with simulated day shift work. Our study also only examined the effects of 3 days of circadian misalignment, and future studies will need to investigate if these effects persist or mitigate prolonged exposure to circadian misalignment. These limitations notwithstanding, our results may help explain why night shift work is a risk factor for infection (Besedovsky et al., 2019; Irwin, 2019; James et al., 2017; Liu, 2019; Zielinski and Krueger, 2012). Disrupted coordination of circulating cytokines is particularly relevant for responses to certain viral infections (Belec et al., 1994). Furthermore, an IL-6 receptor inhibitor (tocilizumab) is currently being evaluated as a therapy for cytokine release syndrome in severe SARS-CoV-2 infection (Sciascia et al., 2020).
5. Implications
Our findings suggest that during times of viral pandemic, when overburdened frontline healthcare and other workers are required to perform more night shift work, diminished anti-viral response due to lower priming by TNF-α predisposes them to infection, and mistimed elevation of IL-6 predisposes them to indiscriminate inflammatory activation when responding to the infectious challenge. Further studies are warranted to examine the prevalence of SARS-CoV-2 infection in night shift workers and the adverse consequences of dysregulated inflammation, including cytokine storm, in response to COVID-19 (Silva et al., 2020).
Funding
This work was supported by start-up funds from Washington State University and the North Carolina State University to S.G.; and in part by Congressionally Directed Medical Research Program award W81XWH-16-1-0319 and United States Army Medical Research and Development Command award W81XWH-18-1-0100 to H.P.A.V.D.; National Institutes of Health (NIH) grants R01ES030113 and R21CA227381 to S.G., K24HL13632 and R01HL124211 to P.Y.L., and NS025378 to JMK; and by the National Center for Advancing Translational Sciences through UCLA CTSI grant UL1TR001881. The funding sources had no role in the study and the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CRediT authorship contribution statement
Peter Y. Liu: Conceptualization, Methodology, Formal analysis, Resources, Writing – original draft, Funding acquisition. Michael R. Irwin: Resources, Conceptualization, Writing – review & editing. James M. Krueger: Resources, Writing – review & editing. Shobhan Gaddameedhi: Methodology, Investigation, Writing – review & editing, Funding acquisition. Hans P.A. Van Dongen: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Funding acquisition.
Declaration of competing interest
P.Y.L., M.R.I., J.M.K., S.G. and H.P.A.V.D have no financial or non-financial disclosures.
Acknowledgements
We thank the staff of the Sleep and Performance Research Center at Washington State University Spokane for their help conducting the clinical study; Matthew Layton for serving as physician of record; and Fiona Yuen, Scott Filler and Norma Solis for technical support.
References
- Belec L., Meillet D., Hernvann A., Gresenguet G., Gherardi R. Differential elevation of circulating interleukin-1 beta, tumor necrosis factor alpha, and interleukin-6 in AIDS-associated cachectic states. Clin. Diagn. Lab. Immunol. 1994;1:117–120. doi: 10.1128/cdli.1.1.117-120.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L., Lange T., Haack M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019;99:1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.F., Dijk D.J. Getting through to circadian oscillators: why use constant routines? J. Biol. Rhythm. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Haack M., Sanchez E., Mullington J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C., Ancoli-Israel S., Chesson A.L., Quan S.F. American Academy of Sleep Medicine; Westchester, IL: 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- Institute of Medicine (IOM) In: Sleep Disorders and Sleep Deprivation: an Unmet Public Health Problem. Colten H.R., Altevogt B.M., editors. National Academies of Science; Washington D.C: 2006. [PubMed] [Google Scholar]
- Irwin M.R. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol. 2019;19:702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatr. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S.M., Honn K.A., Gaddameedhi S., Van Dongen H.P.A. Shift work: disrupted circadian rhythms and sleep-implications for health and well-being. Curr Sleep Med Rep. 2017;3:104–112. doi: 10.1007/s40675-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J.M., Rector D.M., Roy S., Van Dongen H.P., Belenky G., Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat. Rev. Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander M., Andreasson A.N., Kecklund G., Ekman R., Ingre M., Akerstedt T., Axelsson J. Subjective health perception in healthy young men changes in response to experimentally restricted sleep and subsequent recovery sleep. Brain Behav. Immun. 2013;34:43–46. doi: 10.1016/j.bbi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Liu P.Y. A clinical perspective of sleep and andrological health: assessment, treatment considerations and future research. J. Clin. Endocrinol. Metab. 2019;104:4398–4417. doi: 10.1210/jc.2019-00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulich S.K., Zerbe G.O., Jones R.H., Crowley T.J. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat. Med. 2003;22:3195–3211. doi: 10.1002/sim.1560. [DOI] [PubMed] [Google Scholar]
- Mills J.N., Minors D.S., Waterhouse J.M. Adaptation to abrupt time shifts of the oscillator(s) controlling human circadian rhythms. J. Physiol. 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.J., Purvis T.E., Hu K., Scheer F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsen E., Dinges D.F., Van Dongen H.P. Nonlinear mixed-effects modeling: individualization and prediction. Aviat Space Environ. Med. 2004;75:A134–A140. [PubMed] [Google Scholar]
- Rahman S.A., Castanon-Cervantes O., Scheer F.A., Shea S.A., Czeisler C.A., Davidson A.J., Lockley S.W. Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain Behav. Immun. 2015;47:4–13. doi: 10.1016/j.bbi.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine L., Hauger R.L., Gillin J.C., Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metabol. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- Reed R.G., Weihs K.L., Sbarra D.A., Breen E.C., Irwin M.R., Butler E.A. Emotional acceptance, inflammation, and sickness symptoms across the first two years following breast cancer diagnosis. Brain Behav. Immun. 2016;56:165–174. doi: 10.1016/j.bbi.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia S., Apra F., Baffa A., Baldovino S., Boaro D., Boero R., Bonora S., Calcagno A., Cecchi I., Cinnirella G., Converso M., Cozzi M., Crosasso P., De Iaco F., Di Perri G., Eandi M., Fenoglio R., Giusti M., Imperiale D., Imperiale G., Livigni S., Manno E., Massara C., Milone V., Natale G., Navarra M., Oddone V., Osella S., Piccioni P., Radin M., Roccatello D., Rossi D. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin. Exp. Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- Shearer W.T., Reuben J.M., Mullington J.M., Price N.J., Lee B.N., Smith E.O., Szuba M.P., Van Dongen H.P., Dinges D.F. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J. Allergy Clin. Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Silva F.R.D., Guerreiro R.C., Andrade H.A., Stieler E., Silva A., de Mello M.T. Does the compromised sleep and circadian disruption of night and shiftworkers make them highly vulnerable to 2019 coronavirus disease (COVID-19)? Chronobiol. Int. 2020:1–11. doi: 10.1080/07420528.2020.1756841. [DOI] [PubMed] [Google Scholar]
- Skene D.J., Skornyakov E., Chowdhury N.R., Gajula R.P., Middleton B., Satterfield B.C., Porter K.I., Van Dongen H.P.A., Gaddameedhi S. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl. Acad. Sci. U. S. A. 2018;115:7825–7830. doi: 10.1073/pnas.1801183115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skornyakov E., Gaddameedhi S., Paech G.M., Sparrow A.R., Satterfield B.C., Shattuck N.L., Layton M.E., Karatsoreos I., Hpa V.A.N.D. Cardiac autonomic activity during simulated shift work. Ind. Health. 2019;57:118–132. doi: 10.2486/indhealth.2018-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen H.P., Olofsen E., VanHartevelt J.H., Kruyt E.W. A procedure of multiple period searching in unequally spaced time-series with the Lomb-Scargle method. Biol. Rhythm. Res. 1999;30:149–177. doi: 10.1076/brhm.30.2.149.1424. [DOI] [PubMed] [Google Scholar]
- Vgontzas A.N., Papanicolaou D.A., Bixler E.O., Lotsikas A., Zachman K., Kales A., Prolo P., Wong M.L., Licinio J., Gold P.W., Hermida R.C., Mastorakos G., Chrousos G.P. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Vgontzas A.N., Zoumakis E., Bixler E.O., Lin H.M., Follett H., Kales A., Chrousos G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Wright K.P., Jr., Drake A.L., Frey D.J., Fleshner M., Desouza C.A., Gronfier C., Czeisler C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015;47:24–34. doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski M.R., Krueger J.M. Inflammation and sleep. In: Barkoukis T.J., Matheson J.K., Ferber R., Doghramji K., editors. Therapy in Sleep Medicine. Elsevier; Philadelphia, PA: 2012. pp. 607–616. [Google Scholar]