Abstract
In recent years, a substantial number of tissue microbiome studies have been published, mainly due to the recent improvements in the minimization of microbial contamination during whole transcriptome analysis. Another reason for this trend is due to the capability of next-generation sequencing (NGS) to detect microbiome composition even in low biomass samples. Several recent studies demonstrate a significant role for the tissue microbiome in the development and progression of cancer and other diseases. For example, the increase of the abundance of Proteobacteria in tumor tissues of the breast has been revealed by gene expression analysis. The link between human papillomavirus infection and cervical cancer has been known for some time, but the relationship between the microbiome and breast cancer (BC) is more novel. There are also recent attempts to investigate the possible link between the brain microbiome and the cognitive dysfunction caused by neurological diseases. Such studies pointing to the role of the brain microbiome in Huntington’s disease (HD) and Alzheimer’s disease (AD) suggest that microbial colonization is a risk factor. In this review, we aim to summarize the studies that associate the tissue microbiome, rather than gut microbiome, with cancer and other diseases using whole-transcriptome analysis, along with 16S rRNA analysis. After providing several case studies for each relationship, we will discuss the potential role of transcriptome analysis on the broader portrayal of the pathophysiology of the breast, brain, and vaginal microbiome.
Keywords: neurodegenerative, vagina, tissue microbiome, whole transcriptome, RNA-seq, breast cancer, brain microbiome, 16S RNA analysis
Introduction
The human body hosts a microbiome of microbes, bacteria, and viruses (Morgan and Huttenhower, 2012) that reside in human tissues and biofluids accompanied by various anatomical sites (e.g., mammary glands, placenta, and ovarian follicles; Marchesi and Ravel, 2015). The majority of the studies on the human microbiome are focused on microbial diversity and interactions only with the surface or the epithelial layer. Sequencing analyses of microbiomes have mostly focused on taxonomy profiling using 16S-rRNA amplicon sequencing, which efficiently covers the biodiversity of the samples using minimal sequencing by directly characterizing the microbiome taxonomy (Shakya et al., 2019). The whole-transcriptome analysis offers an alternative to 16S-rRNA sequencing by detecting and quantifying the low expression levels including non-coding RNAs (Shakya et al., 2019).
The link between the human microbiome and some specific diseases/cancer types has been tackled by several comprehensive reviews (e.g., vaginal microbiome, Ma et al., 2012; Muls et al., 2017; Champer et al., 2018; Xu et al., 2020; breast microbiome, Chadha et al., 2020; and brain microbiome, Piacentini et al., 2014; Harris and Harris, 2018).
In this review, rather than the commonly studied gut microbiome, we summarize the recent but less frequent microbiome studies analyzing the important role of the whole tissue microbiome not limited to but including the epithelial microbiome, providing a more wholesome picture of the association of dysbiosis with cancer, neurodegenerative, and inflammatory diseases.
Various omics technologies such as transcriptomics, proteomics, metabolomics, metagenomics, and their combinations provide new insights into the understanding of the human microbiome and its role in cancer/disease development (Komorowski and Pezo, 2020). As most of the microorganisms can not be practically cloned and cultured by the conventional methods, most of the recent microbiome studies utilize 16S/18S/ITS Amplicon sequencing or whole transcriptome analysis. Among these techniques, our mini-review focuses on 16S-rRNA and whole transcriptome analysis; both of which are demonstrated to be equally sensitive in bacterial genus detection (Razzauti et al., 2015). Of 16S-rRNA and whole transcriptome analysis, whole transcriptome analysis is more robust and cost-effective in both capturing the coding and non-coding RNA and quantifying the heterogeneity in gene expressions of cells, tissues, and organs (Ozsolak and Milos, 2011; Sancesario and Bernardini, 2018). The advantages of the whole transcriptome analysis compared to other omic methods are vast, the most fundamental one being its contribution to the determination of new strategies for drug discoveries and therapeutic interventions (Ozsolak and Milos, 2011; Jiang et al., 2015).
This mini-review aims to provide a summary of the recent studies related to the role of the issue microbiome changes in cancer/unhealthy tissues of the brain, breast, and vagina using 16S-RNA and whole transcriptome analysis. The studies considering the tissue microbiome as originating not only from the surface but all parts of the aforementioned tissues are included within the scope of this article.
Breast Intracellular Microbiome
Breast cancer (BC) microbiome (Figure 1) is hypothesized to be affected by bacteria-related inflammation in the mammary ducts and glands disrupting the hierarchy of stem cells. Several recent studies focus on the intra-tissue microbial and the viral composition in BC [e.g., human papillomavirus (HPV); Nejman et al., 2020; Sher et al., 2020]. HPVs are more abundant in BCs compared to benign breast and normal breast controls (Lawson et al., 2016). Some studies claim that HPV may play a role in the formation of breast ductal carcinoma, together with the ability to immortalize human epithelial cells (Di Lonardo et al., 1992; Kan et al., 2005; Heng et al., 2009). In addition to the epithelial tissue microbiome studies, several recent studies point to the presence of intra-tissue bacteria in healthy controls (Nejman et al., 2020).
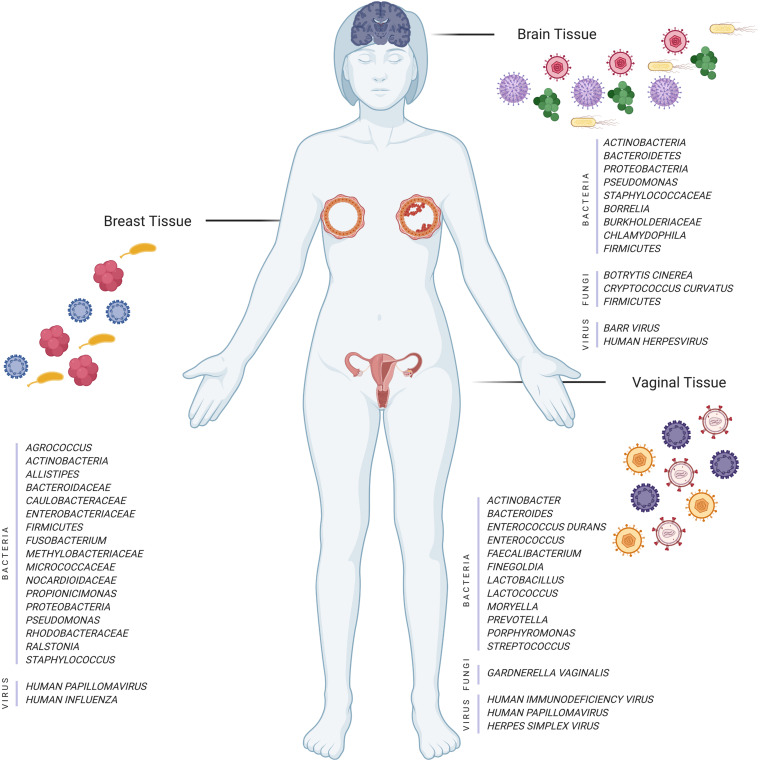
FIGURE 1.
Illustration of different tissues and their microorganisms (bacteria, fungi, and viruses) in normal and disease cases (Created with BioRender.com).
Some studies have shown that pregnancy and breastfeeding might reduce the risk of BC due to the protective behavior of the lactose fermenting bacterial flora in the mammary ducts (Marwaha et al., 2020). Another important finding related to BC is that some patients with hormone receptor-positive (HR+) tend to have more aggressive BC possibly due to the dysbiosis that triggers the early inflammation in the mammary gland during the progression of HR+ breast tumor by disrupting the mammary tissue homeostasis (Rosean et al., 2019).
The presence of mucosal-associated invariant T (MAIT) cells in the breast ducts intervenes T-helper 17 cell responses that might be regulated during breast carcinogenesis by the indications of breast microbiome and the expression of stress-related ligands by neoplastic breast duct epithelial cells (Zumwalde et al., 2018).
Live and metabolically active Proteobacteria, Firmicutes, and Actinobacteria are discovered in breast tumors (Nejman et al., 2020). Thompson et al. (2017) pointed to the increase of Proteobacteria in tumor tissues and Actinobacteria in normal tissues. Additionally, the correlation of expression profiles with the microbiome data indicates that H. influenza is significantly correlated with genes in the G2M checkpoint, E2F transcription, and mitotic spindle assembly pathways (Thompson et al., 2017) (Table 1).
TABLE 1.
Summary of recent findings on the breast, brain and vagina tissue microbiome.
| Tissue | Sample type and size | Methods | Finding | References |
| Breast | 13 fresh breast tissue benign or 45 cancerous tumors, and 23 controls | 16S-rRNA | Enterobacteriaceae, Bacillus, Staphylococcus species are abundant in breast cancer. | Urbaniak et al., 2016 |
| Breast | Breast tumor tissues (668) and non-cancerous adjacent tissues (72) from Cancer Genomics Hub (CGHuB) | 16S-rRNA | Proteobacteria showed abundant shifts in tumor samples. It appears that H. influenza strain is associated with genes in the G2M control point, E2F transcription, and mitotic spindle assembly pathways, and L. fleischmannii strain is associated with genes including epithelial to mesenchymal transition. The relationship between S. pyogenes abundance and GUSBP4, GUSBP9, and GPA2 expression levels shows the role of this species in the exposure of the local breast environment to higher estrogen levels. | Thompson et al., 2017 |
| Breast | 50 endocrine receptor (BRER) positive, 34 human epidermal growth factor receptor 2 (HER2) positive (BRHR), 24 triple positive (BRTP), and 40 triple-negative (BRTN) breast cancer tissues plus 20 healthy controls | Whole-genome and transcriptome | BRER: Bifidobacterium, Arcanobacterium, Citrobacter, Cardiobacterium, Escherichia, Filobasidilla, Brugia, Mucor, Trichophyton, Paragonimus BRHR: Streptococcus, Nodaviridae, Epidermophyton, Fonsecaea, Pseudallescheria, Balamuthia BRTP: Trichostrongylus, Chlamydophila, Hepeviridae, Bordetella, Campylobacter, Chlamydia, Birnaviridae, Legionella, Pasteurella, Penicillium, Ancylostoma, Angiostrongylus, Echinococcus, Trichomonas, Sarcocystis, BRTN: Geobacillus, Orientia, Rothia, Arcobacter, Alternaria, Malassezia, Aerococcus, Rhizomucor, Piedraia, Centrocestus, Trichuris, Contracaecum, Leishmania, Necator, Toxocara Onchocerca, Trichinella. | Banerjee et al., 2018 |
| Breast | 22 benign and 72 malignant breast cancer samples | 16S-rRNA | Micrococcaceae, Caulobacteraceae, Propionicimonas, Rhodobacteraceae, Nocardioidaceae, and Methylobacteriaceae are abundant in malignant disease. Methylobacteriaceae family is the only common biomarker/signature in the tissue microbiomes between tumor/malignant and normal/benign. | Meng et al., 2018 |
| Breast | 19 breast tissues (non-Hispanic Black women –NHB), 62 total breast samples (non-Hispanic White women-NHW); 11 donors from breast cancer and adjacent normal breast tissue | 16S-rRNA | Proteobacteria is the most abundant in normal, normal adjacent to tumor, and breast tumors from NHB and NHW women, with fewer Firmicutes, Bacteroidetes, and Actinobacteria. The racial difference is reported in breast tissue microbiome (e.g., higher abundance of genus Ralstonia in NHB women compared to NHW tumors). | Smith et al., 2019 |
| Breast | 15 breast tumor tissue from women who underwent neoadjuvant chemotherapy, 18 women with no prior therapy at the time of surgery | 16S-rRNA | The chemotherapy administration increases the breast tumor Pseudomonas spp. Treatment of breast cancer cells with Pseudomonas aeruginosa conditioned media differentially effected proliferation in a dose-dependent manner and modulated doxorubicin-mediated cell death. | Chiba et al., 2020 |
| Brain | All samples from a brain bank | Internal Transcribed Spacer (ITS) | Botrytis cinerea and Cryptococcus curvatus are common to all four central nervous system regions. Five genera are common to all nine AD patients: Alternaria, Botrytis, Candida, Cladosporium, and Malassezia. | Alonso et al., 2017 |
| Brain | Consortium to Establish a Registry for AD (CERAD) criteria and Braak stage on 24 AD and 18 age-matched controls. | DNA sequences | Lipopolysaccharide and E. coli K99 are greater in AD compared to control brains. Gram-negative bacterial molecules are related to AD neuropathology. | Zhan et al., 2016 |
| Brain | Control = 12, AD = 14 | 16S-rRNA | The control brain has similar bacterial profiles with the blood, but the AD brain displays a larger proportion of Actinobacteria. | Emery et al., 2017 |
| Brain | Accelerating Medicines Partnership AD; Knowledge Portal | 16S-rRNA | The increase in the abundance of HHV-6A, HHV-7, and herpes simplex virus (HSV-1) genomes in banked postmortem brains from subjects with AD compared to controls. | Readhead et al., 2019 |
| Brain | Postmortem hippocampal formation specimens from 10 neurological control and 10 AD cases, 22 AD specimens, and 19 neurological controls from the hippocampus; 12 control and 20 AD cerebellum samples | 16S-rRNA | The most abundant phyla in both cancer and normal cases are Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes. There is a significant role of brain region on the presence of microbial DNA revealed by the variations in beta diversity in hippocampal and cerebellum samples. | Westfall et al., 2020 |
| Brain | Examination of the olfactory bulbs from autopsy of two AD cases | Bacterial DNA | The HSV1 is a strong risk factor when it is present in the brain of carriers of the type 4 allele of the gene for APOE-ε4. Another important risk factor is the bacterium, Chlamydia pneumoniae identified and localized in the AD brain. | Itzhaki et al., 2004 |
| Brain | Samples of tissue from brain donors, AD, and control individuals | 16S-rRNA | No evidence for expression of early (ICP0) or late (ICP5) proteins of the HSV-1 in brain. A polyclonal antibody against Borrelia detected structures that appeared not related to spirochetes, but rather to fungi, revealing the presence of several bacteria in brain tissue of AD patients. | Pisa et al., 2017 |
| Brain | 118 human viruses, and PCR from 711 AD and non-AD control brains | Whole transcriptome | There is no clear relation between HHV-6 and AD, whereas the Epstein–Barr virus (EBV) and cytomegalovirus (CMV) are comparable. | Allnutt et al., 2020 |
| Vagina | 151 healthy women (65 HPV+ and 86 HPV−) | 16S rDNA | Bacteroides plebeius, Acinetobacter lwoffii, and Prevotella buccae are significantly seen in HPV-positive women. | Chao et al., 2019 |
| Vagina | Women with the age of 18–29 who have applied to the sexually transmitted infections (STIs) clinic have symptoms | Whole transcriptome | The samples from women with STI infection only contain pathogen-specific sequences (3–38% transcriptome coverage). | O’Connell et al., 2019 |
Although the microbiome profiles of malignant and benign breast tumors are different (Costantini et al., 2018; Meng et al., 2018), there are some significant similarities in the profiles of normal and tumor breast tissues revealed by 16S rRNA amplicon sequencing (Urbaniak et al., 2016) (Table 1). The genus Propionicimonas and families Micrococcaceae, Caulobacteraceae, Rhodobacteraceae, Nocardioidaceae, and Methylobacteriaceae in malignant tissues are the enriched microbial biomarkers, and the development of malignancy results in the decrease in Bacteroidaceae and the increase in Agrococcus (Meng et al., 2018) (Table 1). Additionally, the genus Fusobacterium, Bacteroides, and Allistipes are especially related to BC (Philley et al., 2019.). Another important finding is the racial differences in the microbiome of breast tissue first identified by Smith et al. (2019), i.e., relatively higher abundance of the genera Ralstonia in non-Hispanic Black women (Table 1).
The effect of HPV on BC has been investigated for years (Di Lonardo et al., 1992; Widschwendter et al., 2004; Sher et al., 2020). The presence of HPV in BC tissue has resulted in increased histopathological activity in the tumor (Al-Badry et al., 2019). This situation may also be presented with the pathologic nipple discharge (PND; Balcı et al., 2019). Also, the serum derived-extracellular vesicles (EVs) from the BC patients containing HPV DNA reveal the role of HPV as a potential trigger for aggressive BC (De Carolis et al., 2019). The expressions of P53, RB, BRCA1, and BRCA2 in HPV positive BC patients are reduced compared to those in HPV negative ones, possibly indicating the positive relationship between the increase in the inflammatory cytokines (e.g., IL-1 and IL-6) and tumor progression (Khodabandehlou et al., 2019).
Chemotherapy, unfortunately, has significantly exacerbated the disease progression by shifting the microbiome of breast tumors and increasing the Pseudomonas spp. (Chiba et al., 2020) (Table 1). There is a need for further studies that cover a larger and more racially diverse data population, and any findings will provide new insights into the role of the microbiome in the therapy stage and the investigation of novel bacterial biomarkers (Chiba et al., 2020). In summary, the recent developments related to the role of the microbiome in BC reveal the importance of this relationship for the investigation of BC (Sher et al., 2020).
Brain Microbiome
An interesting indication of tissue microbiome is its association with neurodegenerative diseases. The polymicrobial infections are comprised of fungi and bacteria found in the brain tissues of AD patients (Pisa et al., 2015, 2017). Many other studies claimed that the repeated activation of herpes simplex virus 1 (HSV-1) promotes the neurodegeneration aspect of AD (Deatly et al., 1990; Mori et al., 2004; Mancuso et al., 2014). Microbial colonization is also considered as a possible risk factor for Huntington’s disease (HD) (Alonso et al., 2019). Alzheimer’s disease (AD) is a neurodegenerative disorder whose pathogenesis is not only limited to the neuronal compartment but also accompanied by a significant interaction with the immunological mechanisms in the brain (Heneka et al., 2015). Even though the etiopathogenesis of AD has not been well documented, some investigations point to the role of the microbiome (Figure 1) (gut, Vogt et al., 2017; oral, Shoemark and Allen, 2015), and the etiology of AD has been considered as microbial (Bhattacharjee and Lukiw, 2013; Pisa et al., 2017).
The role of human herpesvirus (HHV) in the etiology of AD has been evident recently, yet studies on the different viruses of the herpes family [e.g., HHV-6, -7, cytomegalovirus (CMV), Epstein–Barr virus (EBV)] are limited (Carbone et al., 2014). Nevertheless, there are some recent additional attempts to reveal the relation between HHV and AD (Eimer et al., 2018; Readhead et al., 2018, 2019). For instance, HHV-6A and 7 have been detected in AD patients with a higher viral abundance of amyloid precursor protein (APP) (O’Brien and Wong, 2011) metabolism (i.e., induction of APBB2, APPBP2, BIN1, BACE1, and CLU) (Readhead et al., 2018). The oligomers of Amyloid-β (Aβ) peptide bind HHV surface glycoproteins and accelerate the deposition of β-amyloid (Eimer et al., 2018). This leads to protective viral entrapment activity against neurotropic HSV-1 and also HHV6A-B that are linked to AD (Eimer et al., 2018). Besides, the increase of the KIR2DL2/C1 genotype in AD patients and the lower anti-herpetic activity of KIR2DL2 positive natural killer (NK) cells support the role of HHV infection in AD development and also increase the susceptibility to HHV-6A infection (Rizzo et al., 2019). Most studies also suggest the prevalence of HHV-6A in the AD brain compared to others (Eimer et al., 2018; Readhead et al., 2019; Rizzo et al., 2019). Besides, the effect of HSV-1 in the development of AD among people with the genetic susceptibility factor of the apolipoprotein E (APOE4) allele has been evident (Lindman et al., 2019; Linard et al., 2020).
The contribution of infectious microbial components and also virulence factors rhamnolipids (RLs) to the pathophysiology of the human central nervous system (CNS), including AD, has been potentially important (Andreadou et al., 2017). The presence of RLs is attributed to chronic bacterial infections forming bacterial virulence factors secreted by a wide variety of pathogens (Andreadou et al., 2017). The fungi in the samples of the frontal cortex in AD brains are distinct, indicating the varying microbial compositions among brain regions (Alonso et al., 2018; Westfall et al., 2020) (Table 1). A polyclonal antibody against Borrelia has been identified in structures associated with fungi (Pisa et al., 2017). Two independent Chlamydophila antibodies have revealed several structures similar to fungal cells and hyphae and prokaryotic cells, but are most likely unrelated to Chlamydophila spp. Several bacteria in the AD patients suggested that the polymicrobial infections are comprised of fungi and bacteria occurred in their brain tissues (Pisa et al., 2017) (Table 1). The fungal species found in the CNS of AD patients have been investigated by next-generation sequencing (NGS) that revealed the most common species, namely, Botrytis cinerea and Cryptococcus curvatus (Alonso et al., 2017). Burkholderiaceae and Staphylococcaceae are more abundant in AD brains compared to normal brains (Alonso et al., 2018). Studies on that subject aiming at investigating the link between fungal species with the AD are rather essential for providing antifungal therapy and also for the evolution and severity stages of clinical symptoms in AD patients (Alonso et al., 2017) (Table 1). On the other hand, microbiological attack or change is thought to be one of the factors causing CNS disorders, also evident for the AD that shows an increase in bacterial populations (e.g., large amount of Actinobacteria; Emery et al., 2017) (Table 1).
Huntington’s disease is caused by a triplet expansion in the Huntingtin (HTT) gene (Vonsattel and Difiglia, 1998). Alonso et al. (2019) first identified the role of some prevalent bacterial genera (Pseudomonas, Acinetobacter, and Burkholderia) in the brain microbiome of HD patients. RNA-seq analysis of human neurodegenerative disease tissues (except for AD) reported no significant difference compared to cytotoxic T-lymphocytes (CTL) tissues, indicating that the sub-clinical infections do not result in the inflammation related to the tissue of many neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) (Bennett et al., 2019).
Vaginal Microbiome
The relation between the vaginal microbiome (Figure 1) and the high-risk HPV infection has been propounded by several studies (Chao et al., 2019, 2020; Keller et al., 2019; Liu et al., 2019; Nené et al., 2019; Zhou et al., 2019; Abudula et al., 2020; Jiang et al., 2020). The sequencing of 16S rRNA genes reveals that some anaerobic bacteria (e.g., Bacteroides plebeius and Acinetobacter lwoffii) are significantly more common in HPV positive women, suggesting a specific microbiome as a biomarker to detect changes in the cervical microenvironment indicating HPV infection (Chao et al., 2019). The genus Prevotella, Porphyromonas, and Enterococcus are the highest in the cervical permanent HPV infection, whereas the Bacteroides genus is the lowest (Chao et al., 2020).
The expression studies on cervical lesions to explore the possible relation of HPV with cervical cancer are also crucial. Toll-like receptor 4 (TLR4) expression supports the claim of a distinct relation between tumor formation and HPV-positive cervical cancer (Jiang et al., 2020), suggesting that TLR4 somehow enables the formation of a local immunosuppressive microenvironment.
Human papillomavirus, human immunodeficiency virus (HIV), and HSV have been associated with the growth of genital-related cancers. Keller et al. (2019) proclaim that the increase in microbial diversity and cervicovaginal inflammation in women with HIV+ and HSV+ significantly perturbs genital health. It is thought that neither the inception of antiretroviral therapy (ART) nor the restructuring of the immune system affects the vagina microbiome of HIV-infected women (Liu et al., 2019). Several 16S rRNA sequencing studies (Nené et al., 2019; Zhou et al., 2019) demonstrate a decrease in bacterial diversity in ovarian cancer tissues compared to normal ones. Some bacterial implications are suggested as biomarkers for the early detection of ovarian tumors, such as higher ratios of two phyla for Proteobacteria/Firmicutes, and the increase of genus Acinetobacter and decrease of genus Lactococcus (Zhou et al., 2019).
Discussion and Conclusion
In this mini-review, we mainly discuss the possible effects of human tissue microbiome in the development of some common cancers and neurodegenerative diseases. There might be two important implications of our comprehensive literature compilation: the first implication being the presentation of the limited number of studies that deal with the microbiome differences in healthy and unhealthy/cancer tissues, for example, AD is more commonly studied for its relationship with the oral and gastrointestinal microbiome. The second implication of our literature compilation is to list the shared bacteria (e.g., Proteobacteria, Bacteroides, and Firmicutes) that display either positive or negative anomalies common to all cancer tissues discussed above (Figure 1).
Many of the recent findings summarized in Table 1 are now possible due to the ability of the whole transcriptome analysis to (1) provide abundance information along with the taxonomic diversity and (2) provide a vaster picture of the expression profile of the microbiome comprising of fungal, viral, and bacterial along with host expression profile. In some of the cases summarized in Table 1, observed differences in each patient are due to fungal species which could not be discovered if a whole transcriptomic approach is not taken (Alonso et al., 2017, 2019).
Variations in the microbiome of cancer patients with different cancer stages have already been known in the literature. For instance, the species of Firmicutes and Bacteroides are dominant in the invasive and benign BC, whereas some of the species such as Fusobacterium, Atopobium, and Lactobacillus are enriched in malignant breast tissues (e.g., Hieken et al., 2016; Chadha et al., 2020). The recent studies about the role of the microbiome in different cancers and neurodegenerative diseases reveal that there are some common species observed in all cancer tissues. For instance, some of these species (e.g., Bacteroides) are enriched in the AD tissues, while they are not abundant in cervical and BC tissues. Additionally, Proteobacteria and Firmicutes are enriched in all types of cancer tissues. Such similarities and/or differences may be attributed to differences in tissue structure. Our mini-review will attract more attention to the reprocessing of the publicly available RNA-seq data to distinguish microbial contamination from tissue microbiome via in silico techniques to clarify the relative composition, abundance, and impact of microbiome in cancer tissues.
Most of the studies on the possible link between microbiome and diseases associating the presence of many potential microbial biomarkers and their pathways with the advanced stage of the diseases offer new insights into the diagnostic staging (e.g., Wozniak et al., 2005; Alonso et al., 2017; Meng et al., 2018; Rizzo et al., 2019). There is also some evidence that the profiles of microbiome in healthy and unhealthy tissues (e.g., for BC; Urbaniak et al., 2016) are identical, and hence the role of tissue microbiome for the development of diseases/cancers should be further investigated by increasing the number of available studies. As the Next-Generation Sequence (NGS) technology becomes more precise and novel in silico techniques are getting developed to discard the studies with bacterial contamination and non-standardized analysis pipelines, the re-analysis of the existing transcriptome data offers a huge potential for the future in silico data-mining microbiome studies from the vast amount of publicly available transcriptomic data.
Author Contributions
RS wrote the manuscript and TÖS supervised the project. Both authors discussed the results and contributed to the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the NIH Fellows Editorial Board (FEB) for editorial assistance.
Footnotes
Funding. RS was funded by YOK 100/2000 program and TÖS was funded by NIH Intramural program. The open-access publication cost was funded by the corresponding author.
References
- Abudula A., Rouzi N., Xu L., Yang Y., Hasimu A. (2020). Tissue-based metabolomics reveals potential biomarkers for cervical carcinoma and HPV infection. Bosn. J. Basic Med. Sci. 20 78–87. 10.17305/bjbms.2019.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Badry B. J., Al-Muswie R. T., Jameel S. (2019). Histopathological study of Human Papillomavirus (HPV) in breast cancer patients. J. Phys. 1294:062080. 10.1088/1742-6596/1294/6/062080 [DOI] [Google Scholar]
- Allnutt M. A., Johnson K., Bennett D. A., Connor S. M., Troncoso J. C., Pletnikova O., et al. (2020). Human herpesvirus 6 detection in Alzheimer’s disease cases and controls across multiple cohorts. Neuron 105 D1027–D1035.e2. 10.1016/j.neuron.2019.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R., Pisa D., Aguado B., Carrasco L. (2017). Identification of fungal species in brain tissue from Alzheimer’s disease by next-generation sequencing. J. Alzheimer Dis. 58 55–67. 10.3233/JAD-170058 [DOI] [PubMed] [Google Scholar]
- Alonso R., Pisa D., Carrasco L. (2019). Brain microbiota in Huntington’s disease patients. Front. Microbiol. 10:2622. 10.3389/fmicb.2019.02622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R., Pisa D., Fernández-Fernández A. M., Carrasco L. (2018). Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer’s disease. Front. Aging Neurosci. 10:159. 10.3389/fnagi.2018.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadou E., Pantazaki A. A., Daniilidou M., Tsolaki M. (2017). Rhamnolipids, microbial virulence factors, in Alzheimer’s disease. J. Alzheimer Dis. 59 209–222. 10.3233/JAD-161020 [DOI] [PubMed] [Google Scholar]
- Balcı F. L., Uras C., Feldman S. M. (2019). Is human papillomavirus associated with breast cancer or papilloma presenting with pathologic nipple discharge? Cancer Treat. Res. Commun. 19:100122. 10.1016/j.ctarc.2019.100122 [DOI] [PubMed] [Google Scholar]
- Banerjee S., Tian T., Wei Z., Shih N., Feldman M. D., Peck K. N., et al. (2018). Distinct microbial signatures associated with different breast cancer types. Front. Microbiol. 9:951. 10.3389/fmicb.2018.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Jr., Keeney P. M., Brohawn D. G. (2019). RNA sequencing reveals small and variable contributions of infectious agents to transcriptomes of postmortem nervous tissues from amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson’s disease subjects, and increased expression of genes from disease-activated microglia. Front. Neurosci. 13:235. 10.3389/fnins.2019.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S., Lukiw W. J. (2013). Alzheimer’s disease and the microbiome. Front. Cell. Neurosci. 7:153. 10.3389/fncel.2013.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I., Lazzarotto T., Ianni M., Porcellini E., Forti P., Masliah E., et al. (2014). Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol. Aging 35 122–129. 10.1016/j.neurobiolaging.2013.06.024 [DOI] [PubMed] [Google Scholar]
- Chadha J., Nandi D., Atri Y., Nag A. (2020). Significance of human microbiome in breast cancer: tale of an invisible and an invincible. Semin. Cancer Biol. (in press). 10.1016/j.semcancer.2020.07.010 [DOI] [PubMed] [Google Scholar]
- Champer M., Wong A. M., Champer J., Brito I. L., Messer P. W., Hou J. Y., et al. (2018). The role of the vaginal microbiome in gynaecological cancer. Intern. J. Obstet. Gynaecol. 125 309–315. 10.1111/1471-0528.14631 [DOI] [PubMed] [Google Scholar]
- Chao X., Sun T., Wang S., Tan X., Fan Q., Shi H., et al. (2020). Research of the potential biomarkers in vaginal microbiome for persistent high-risk human papillomavirus infection. Ann. Transl. Med. 8:100. 10.21037/atm.2019.12.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X. P., Sun T. T., Wang S., Fan Q. B., Shi H. H., Zhu L., et al. (2019). Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Intern. J. Gynecol. Cancer 29:32. 10.1136/ijgc-2018-000032 [DOI] [PubMed] [Google Scholar]
- Chiba A., Bawaneh A., Velazquez C., Clear K. Y., Wilson A. S., Howard-McNatt M., et al. (2020). Neoadjuvant chemotherapy shifts breast tumor microbiota populations to regulate drug responsiveness and the development of metastasis. Mol. Cancer Res. 18 130–139. 10.1158/1541-7786.MCR-19-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini L., Magno S., Albanese D., Donati C., Molinari R., Filippone A., et al. (2018). Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 8:16893. 10.1038/s41598-018-35329-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carolis S., Storci G., Ceccarelli C., Savini C., Gallucci L., Sansone P., et al. (2019). HPV DNA associates with breast cancer malignancy and it is transferred to breast cancer stromal cells by extracellular vesicles. Front. Oncol. 9:860. 10.3389/fonc.2019.00860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Haase A. T., Fewster P. H., Lewis E., Ball M. J. (1990). Human herpes virus infections and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 16 213–223. 10.1111/j.1365-2990.1990.tb01158.x [DOI] [PubMed] [Google Scholar]
- Di Lonardo A., Venuti A., Marcante M. L. (1992). Human papillomavirus in breast cancer. Breast Cancer Res. Treat. 21 95–100. 10.1007/BF01836955 [DOI] [PubMed] [Google Scholar]
- Eimer W. A., Kumar D. K. V., Shanmugam N. K. N., Rodriguez A. S., Mitchell T., Washicosky K. J., et al. (2018). Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99 56–63. 10.1016/j.neuron.2018.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery D. C., Shoemark D. K., Batstone T. E., Waterfall C. M., Coghill J. A., Cerajewska T. L., et al. (2017). 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front. Aging Neurosci. 9:195. 10.3389/fnagi.2017.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. A., Harris E. A. (2018). Molecular mechanisms for herpes simplex virus type 1 pathogenesis in Alzheimer’s disease. Front. Aging Neurosci. 10:48. 10.3389/fnagi.2018.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng B., Glenn W. K., Ye Y., Tran B., Delprado W., Lutze-Mann L., et al. (2009). Human papilloma virus is associated with breast cancer. Br. J. Cancer 101 1345–1350. 10.1038/sj.bjc.6605282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieken T. J., Chen J., Hoskin T. L., Walther-Antonio M., Johnson S., Ramaker S., et al. (2016). The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 6:30751. 10.1038/srep30751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki R. F., Wozniak M. A., Appelt D. M., Balin B. J. (2004). Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol. Aging 25 619–627. 10.1016/j.neurobiolaging.2003.12.021 [DOI] [PubMed] [Google Scholar]
- Jiang N., Xie F., Chen L., Chen F., Sui L. (2020). The effect of TLR4 on the growth and local inflammatory microenvironment of HPV-related cervical cancer in vivo. Infect. Agents Cancer 15 1–10. 10.1186/s13027-020-0279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Zhou X., Li R., Michal J. J., Zhang S., Dodson M. V., et al. (2015). Whole transcriptome analysis with sequencing: methods, challenges and potential solutions. Cell. Mol. Life Sci. 72 3425–3439. 10.1007/s00018-015-1934-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan C. Y., Iacopetta B. J., Lawson J. S., Whitaker N. J. (2005). Identification of human papillomavirus DNA gene sequences in human breast cancer. Br. J. Cancer 93 946–948. 10.1038/sj.bjc.6602778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M. J., Huber A., Espinoza L., Serrano M. G., Parikh H. I., Buck G. A., et al. (2019). Impact of herpes simplex virus type 2 and human immunodeficiency virus dual infection on female genital tract mucosal immunity and the vaginal microbiome. J. Infect. Dis. 220 852–861. 10.1093/infdis/jiz203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodabandehlou N., Mostafaei S., Etemadi A., Ghasemi A., Payandeh M., Hadifar S., et al. (2019). Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC Cancer 19:61. 10.1186/s12885-019-5286-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski A. S., Pezo R. C. (2020). Untapped “-omics”: the microbial metagenome, estrobolome, and their influence on the development of breast cancer and response to treatment. Breast Cancer Res. Treat. 179 287–300. 10.1007/s10549-019-05472-w [DOI] [PubMed] [Google Scholar]
- Lawson J. S., Glenn W. K., Whitaker N. J. (2016). Human papilloma viruses and breast cancer-assessment of causality. Front. Oncol. 6:207. 10.3389/fonc.2016.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linard M., Letenneur L., Garrigue I., Doize A., Dartigues J. F., Helmer C. (2020). Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer’s disease. Alzheimer Dement. 16 200–208. 10.1002/alz.12008 [DOI] [PubMed] [Google Scholar]
- Lindman K. L., Weidung B., Olsson J., Josefsson M., Kok E., Johansson A. (2019). A genetic signature including apolipoprotein Eε4 potentiates the risk of herpes simplex-associated Alzheimer’s disease. Alzheimer Dement. 5 697–704. 10.1016/j.trci.2019.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. M., Packman Z. R., Abraham A. G., Serwadda D. M., Nalugoda F., Aziz M., et al. (2019). The effect of antiretroviral therapy initiation on the vaginal microbiome in HIV-infected women. Open Forum Infect. Dis. 6:ofz328. 10.1093/ofid/ofz328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Forney L. J., Ravel J. (2012). Vaginal microbiome: rethinking health and disease. Ann. Rev. Microbiol. 66 371–389. 10.1146/annurev-micro-092611-150157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R., Baglio F., Agostini S., Cabinio M. C., Laganà M. M., Hernis A., et al. (2014). Relationship between herpes simplex virus-1-specific antibody titers and cortical brain damage in Alzheimer’s disease and amnestic mild cognitive impairment. Front. Aging Neurosci. 6:285. 10.3389/fnagi.2014.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J. R., Ravel J. (2015). The vocabulary of microbiome research: a proposal. Marchesi Ravel Microb. 3:31. 10.1186/s40168-015-0094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha A. K., Morris J. A., Rigby R. J. (2020). Hypothesis: bacterial induced inflammation disrupts the orderly progression of the stem cell hierarchy and has a role in the pathogenesis of breast cancer. Med. Hypothes. 136:109530. 10.1016/j.mehy.2019.109530 [DOI] [PubMed] [Google Scholar]
- Meng S., Chen B., Yang J., Wang J., Zhu D., Meng Q., et al. (2018). Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front. Oncol. 8:318. 10.3389/fonc.2018.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan X. C., Huttenhower C. (2012). Human microbiome analysis. PLoS Comput. Biol. 8:e1002808. 10.1371/journal.pcbi.1002808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Kimura Y., Naiki H., Matsubara R., Takeuchi T., Yokochi T., et al. (2004). Reactivation of HSV-1 in the brain of patients with familial Alzheimer’s disease. J. Med. Virol. 73 605–611. 10.1002/jmv.20133 [DOI] [PubMed] [Google Scholar]
- Muls A., Andreyev J., Lalondrelle S., Taylor A., Norton C., Hart A. (2017). Systematic review: the impact of cancer treatment on the gut and vaginal microbiome in women with a gynecological malignancy. Intern. J. Gynecol. Cancer 27 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejman D., Livyatan I., Fuks G., Gavert N., Zwang Y., Geller L. T., et al. (2020). The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368 973–980. 10.1126/science.aay9189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nené N. R., Reisel D., Leimbach A., Franchi D., Jones A., Evans I., et al. (2019). Association between the cervicovaginal microbiome, BRCA1 mutation status, and risk of ovarian cancer: a case-control study. Lancet Oncol. 20 1171–1182. 10.1016/S1470-2045(19)30340-7 [DOI] [PubMed] [Google Scholar]
- O’Brien R. J., Wong P. C. (2011). Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 34 185–204. 10.1146/annurev-neuro-061010-113613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C. M., Brochu H., Girardi J., Harrell E., Jones A., Darville T., et al. (2019). Simultaneous profiling of sexually transmitted bacterial pathogens, microbiome, and concordant host response in cervical samples using whole transcriptome sequencing analysis. Microb. Cell 6:177. 10.15698/mic2019.03.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsolak F., Milos P. M. (2011). RNA sequencing: advances, challenges and opportunities. Nat. Rev. Genet. 12 87–98. 10.1038/nrg2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philley J. V., Kannan A., Olusola P., McGaha P., Singh K. P., Samten B., et al. (2019). Microbiome diversity in sputum of Nontuberculous Mycobacteria infected women with a history of breast cancer. Cell. Physiol. Biochem. 52 263–279. 10.33594/00000002 [DOI] [PubMed] [Google Scholar]
- Piacentini R., De Chiara G., Li Puma D. D., Ripoli C., Marcocci M. E., Garaci E., et al. (2014). HSV-1 and Alzheimer’s disease: more than a hypothesis. Front. Pharmacol. 5:97. 10.3389/fphar.2014.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa D., Alonso R., Fernández-Fernández A. M., Rábano A., Carrasco L. (2017). Polymicrobial infections in brain tissue from Alzheimer’s disease patients. Sci. Rep. 7 1–14. 10.1038/s41598-017-05903-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa D., Alonso R., Rábano A., Rodal I., Carrasco L. (2015). Different brain regions are infected with fungi in Alzheimer’s disease. Sci. Rep. 5:15015. 10.1038/srep15015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzauti M., Galan M., Bernard M., Maman S., Klopp C., Charbonnel N., et al. (2015). A Comparison between transcriptome sequencing and 16S metagenomics for detection of bacterial pathogens in wildlife. PLoS Negl. Trop. Dis. 9:e0003929. 10.1371/journal.pntd.0003929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readhead B., Haure-Mirande J. V., Ehrlich M. E., Gandy S., Dudley J. T. (2019). Further evidence of increased human Herpesvirus in Alzheimer’s disease. bioRxiv [Preprint], 10.1101/858050 [DOI] [Google Scholar]
- Readhead B., Haure-Mirande J. V., Funk C. C., Richards M. A., Shannon P., Haroutunian V., et al. (2018). Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 99 64–82. 10.1016/j.neuron.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo R., Bortolotti D., Gentili V., Rotola A., Bolzani S., Caselli E., et al. (2019). KIR2DS2/KIR2DL2/HLA-C1 haplotype is associated with Alzheimer’s disease: implication for the role of herpesvirus infections. J. Alzheimer Dis. 67 1379–1389. 10.3233/JAD-180777 [DOI] [PubMed] [Google Scholar]
- Rosean C. B., Bostic R. R., Ferey J. C., Feng T. Y., Azar F. N., Tung K. S., et al. (2019). Preexisting Commensal Dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 79 3662–3675. 10.1158/0008-5472.CAN-18-3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancesario G. M., Bernardini S. (2018). Alzheimer’s disease in the omics era. Clin. Biochem. 59 9–16. 10.1016/j.clinbiochem.2018.06.011 [DOI] [PubMed] [Google Scholar]
- Shakya M., Lo C. C., Chain P. S. (2019). Advances and challenges in metatranscriptomic analysis. Front. Genet. 10:904. 10.3389/fgene.2019.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher G., Salman N. A., Kulinski M., Fadel R. A., Gupta V. K., Anand A., et al. (2020). Prevalence and type distribution of high-risk human Papillomavirus (HPV) in breast cancer: a qatar based study. Cancers 12:1528. 10.3390/cancers12061528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemark D. K., Allen S. J. (2015). The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J. Alzheimer Dis. 43 725–738. 10.3233/JAD-141170 [DOI] [PubMed] [Google Scholar]
- Smith A., Pierre J. F., Makowski L., Tolley E., Lyn-Cook B., Lu L., et al. (2019). Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci. Rep. 9 1–10. 10.1038/s41598-019-48348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. J., Ingle J. N., Tang X., Chia N., Jeraldo P. R., Walther-Antonio M. R., et al. (2017). A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS One 12:e0188873. 10.1371/journal.pone.0188873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak C., Gloor G. B., Brackstone M., Scott L., Tangney M., Reid G. (2016). The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 82 5039–5048. 10.1128/AEM.01235-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt N. M., Kerby R. L., Dill-McFarland K. A., Harding S. J., Merluzzi A. P., Johnson S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7 1–11. 10.1038/s41598-017-13601-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel J. P. G., Difiglia M. (1998). Huntington disease. J. Neuropathol. Exper. Neurol. 57:369. [DOI] [PubMed] [Google Scholar]
- Westfall S., Dinh D. M., Pasinetti G. M. (2020). Investigation of potential brain microbiome in Alzheimer’s Disease: implications of study bias. J. Alzheimer Dis. 75 559–570. 10.3233/JAD-191328 [DOI] [PubMed] [Google Scholar]
- Widschwendter A., Brunhuber T., Wiedemair A., Mueller-Holzner E., Marth C. (2004). Detection of human papillomavirus DNA in breast cancer of patients with cervical cancer history. J. Clin. Virol. 31 292–297. 10.1016/j.jcv.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Wozniak M. A., Shipley S. J., Combrinck M., Wilcock G. K., Itzhaki R. F. (2005). Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer’s disease patients. J. Med. Virol. 75 300–306. 10.1002/jmv.20271 [DOI] [PubMed] [Google Scholar]
- Xu J., Peng J. J., Yang W., Fu K., Zhang Y. (2020). Vaginal microbiomes and ovarian cancer: a review. Am. J. Cancer Res. 10:743. [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Stamova B., Jin L. W., DeCarli C., Phinney B., Sharp F. R. (2016). Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87 2324–2332. 10.1212/WNL.0000000000003391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Sun C., Huang J., Xia M., Guo E., Li N., et al. (2019). The biodiversity composition of microbiome in ovarian carcinoma patients. Sci. Rep. 9 1–11. 10.1038/s41598-018-38031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumwalde N. A., Haag J. D., Gould M. N., Gumperz J. E. (2018). Mucosal associated invariant T cells from human breast ducts mediate a Th17-skewed response to bacterially exposed breast carcinoma cells. Breast Cancer Res. 20:111. 10.1186/s13058-018-1036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]