Abstract
Equine herpesvirus type 1 (EHV-1) is an alphaherpesvirus related to pseudorabies virus (PRV) and varicella-zoster virus (VZV). This virus is one of the major pathogens affecting horses worldwide. EHV-1 is responsible for respiratory disorders, abortion, neonatal foal death and equine herpes myeloencephalopathy (EHM). Over the last decade, EHV-1 has received growing attention due to the frequent outbreaks of abortions and/or EHM causing serious economical losses to the horse industry worldwide. To date, there are no effective antiviral drugs and current vaccines do not provide full protection against EHV-1-associated diseases. Therefore, there is an urgent need to gain a better understanding of the pathogenesis of EHV-1 in order to develop effective therapies. The main objective of this review is to provide state-of-the-art information on the pathogenesis of EHV-1. We also highlight recent findings on EHV-1 immune evasive strategies at the level of the upper respiratory tract, blood circulation and endothelium of target organs allowing the virus to disseminate undetected in the host. Finally, we discuss novel approaches for drug development based on our current knowledge of the pathogenesis of EHV-1.
Keywords: pathogenesis, immune evasion, prevention, therapies, Equine Herpesvirus type 1
Equine Herpesvirus Type 1 (EHV-1)
In 1933, Dimock and Edwards documented epidemic virus abortion in mares in Kentucky (Dimock and Edwards, 1933). The virus was first designated as “equine abortion virus” and later renamed equine herpesvirus type 1. EHV-1 was first isolated in 1966 from cases of abortion and paralysis (Saxegaard, 1966). The virus was recognized by the Herpesvirus Study group of the International Committee for Taxonomy of Viruses in 1988 (Roizman et al., 1992). EHV-1 is among nine equid herpesviruses that have been identified so far (Davison et al., 2009). EHV-1 shares its classification with other animal herpesviruses of agricultural importance including bovine herpesvirus type 1 (BHV-1) and pseudorabies virus (PRV). The EHV-1 virion is 200–250 nm in diameter and consists of four main structural components characteristic of herpesviruses: genome, capsid, tegument, and envelop. The viral genome consists of a linear double stranded DNA of 150 kbp. EHV-1 virion structure, replication cycle and cell-associated spread are not covered in this review, as several excellent reviews on alphaherpesviruses already addressed these topics in more detail (Whitley and Roizman, 2001; Mettenleiter et al., 2009).
The Pathogenesis of EHV-1
Here, we provide an overview of the key steps in the pathogenesis of EHV-1 in the horse that will be relevant to thoroughly understand its immune evasive mechanisms.
Introduction
EHV-1 is a highly contagious pathogen and is usually transmitted via direct contact with infectious secretions (saliva, nasal discharge) or via inhalation of infectious aerosols (Patel et al., 1982). Fetal or placental tissues that contain high virus loads may also serve as a possible source of infection (Reed and Toribio, 2004).
EHV-1 Primary Replication in the Upper Respiratory Tract (URT)
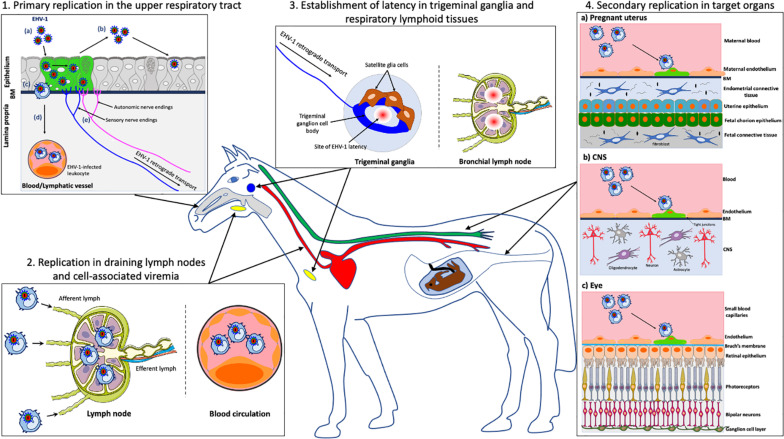
Upon entry into the host, EHV-1 first replicates in a restricted plaquewise manner in the epithelial cells lining the URT, including nasal septum, nasopharynx, and trachea (van Maanen, 2002; Gryspeerdt et al., 2010; Figure 1.1a). In vivo, EHV-1-induced plaques were observed in the epithelium of the nasal mucosa starting from 2 to 7 days post-inoculation (dpi) (Gryspeerdt et al., 2010). In ex vivo experiments, single infected epithelial cells were visible at 12 h post-inoculation (hpi) and EHV-1-induced plaques were observed in the epithelium of equine nasal and nasopharyngeal explants starting from 24 hpi (Vandekerckhove et al., 2010; Negussie et al., 2016). Primary EHV-1 infection of several tissues of the URT results in the destruction and erosion of the epithelium and in nasal shedding starting from 1 to 14 dpi (Gibson et al., 1992; Gryspeerdt et al., 2010; Figure 1.1b). Ultimately, the destruction of the respiratory epithelial cells and local inflammation causes mild clinical symptoms (e.g., serous nasal discharge and fever) in the horse after 2–10 dpi (Allen and Bryans, 1986). Most EHV-1 infections are self-limiting in adult horses and accompanying respiratory symptoms disappear from 9 to 12 dpi (Gibson et al., 1992). However, young horses can develop more severe clinical symptoms consisting of high fever, swelling of the submandibular and retropharyngeal lymph nodes (Coggins, 1979; Patel et al., 1982). The nasal discharge can become mucopurulent, due to secondary bacterial infections, and contribute to the development of rhinopneumonitis (Thomson et al., 1979; McGavin and Zachary, 2007).
FIGURE 1.
Schematic representation of the pathogenesis of EHV-1 in the horse. (1) Primary EHV-1 replication in the epithelial cells of the upper respiratory tract: (a) EHV-1 infection (green); (b) Viral spread within the respiratory epithelium and viral shedding; (c) EHV-1 crosses the basement membrane (BM) and penetrates the lamina propria via the use of single infected leukocytes; (d) EHV-1 reaches the blood circulation and draining lymph nodes; (e) EHV-1 enters nerve endings of the peripheral nervous system and spreads in the retrograde direction to the trigeminal ganglia (TG). (2) EHV-1 replication in the draining lymph nodes and establishment of a cell-associated viremia in peripheral blood mononuclear cells (PBMC). (3) Establishment of EHV-1 latency in TG neurons and respiratory lymphoid tissues. (4) Via a cell-associated viremia in PBMC, EHV-1 is transported to target organs such as the pregnant uterus (4a), the central nervous system (CNS) (4b) or the eye (4c), where it initiates a secondary replication in the endothelial cells lining the blood vessels of this organ. gray, respiratory tract; red, blood circulation; yellow, lymph nodes; blue, TG; green, spinal cord. Drawings are partially based on SMART servier medical art templates.
Recently, Van Cleemput and colleagues demonstrated that certain environmental circumstances stimulate the replication of EHV-1 in the URT. They showed that plant pollen contain proteases that selectively and irreversibly alter intercellular junctions (ICJ) of columnar equine respiratory epithelial cells (Van Cleemput et al., 2019a). The authors demonstrated that EHV-1 infection of ex vivo respiratory mucosal explants is greatly enhanced following pollen protease pretreatment, suggesting that pollen-induced loss of respiratory epithelial barrier function facilitates EHV-1 invasion. Interestingly, pollen concentrations in the outdoor air have been shown to peak between late winter and spring, a period that coincides with high incidence of seasonally observed EHV-1-associated symptoms (e.g., respiratory disease, abortion and EHM) (Goehring et al., 2006; D’Amato et al., 2007). Similarly, it was demonstrated that pretreatment of ex vivo respiratory mucosal explants and EREC with deoxynivalenol (DON), a mycotoxin mainly present in equine feeds, alters respiratory epithelial integrity and predisposes these cells to EHV-1 infection (Van Cleemput et al., 2019b). It was proposed that the inhalation of mycotoxins from contaminated hay, grains and roughage disrupts the respiratory epithelial integrity and subsequently promotes EHV-1 infection. Most importantly, the authors dissected the mechanisms of EHV-1 invasion through the respiratory epithelium. Equine mucosal explants were first treated with EGTA or N-acetylcysteine (NAC), two chelating agents, to disrupt respiratory epithelial ICJ and were subsequently inoculated with EHV-1. The number of EHV-1 plaques, the plaque latitude and infectious virus titer were significantly increased in explants pretreated with EGTA or NAC compared to untreated ones (Van Cleemput et al., 2017). Interestingly, the authors demonstrated that disruption of ICJ results in enhanced EHV-1 binding to explant basolateral surfaces. Consequently, it was hypothesized that EHV-1 binding/entry receptor is expressed basolaterally on respiratory epithelial cells and is only exposed when the integrity of the epithelium is compromised. So far, the EHV-1 basolateral receptor remains uncharacterized.
EHV-1 Replication in Draining Lymph Nodes and Cell-Associated Viremia
Following infection of the respiratory epithelium, EHV-1 crosses the basement membrane (BM) by the use of single infected leukocytes (Kydd et al., 1994; Vandekerckhove et al., 2010; Figure 1.1c). These cells were mainly identified as CD172a+ cells (myeloid origin) followed by T lymphocytes and B lymphocytes (Gryspeerdt et al., 2010; Vandekerckhove et al., 2010; Baghi et al., 2014). Upon crossing the BM, EHV-1-infected leukocytes penetrate the connective tissues and reaches the bloodstream and the draining lymph nodes (Figure 1.1d). Within 24–48 hpi, EHV-1 antigens as well as infectious virus can be detected in submandibular, retropharyngeal and bronchial lymph nodes. The infection is amplified in the draining lymph nodes with discharge of infected leukocytes, via the efferent lymph, into the blood circulation (Kydd et al., 1994). As a result, EHV-1 initiates a cell-associated viremia in peripheral blood mononuclear cells (PBMC) to disseminate in the host (Siedek et al., 1999; van der Meulen et al., 2000; Figure 1.2). Viremia can be detected starting from 1 dpi and persists for 14 days (Gryspeerdt et al., 2010). The cell-associated viremia is a prerequisite for EHV-1 spread to target organs such as the central nervous system (CNS) and/or pregnant uterus. The spread of EHV-1 to the local lymph nodes and in the bloodstream leads to clinical symptoms including lymph node swelling and fever (Patel et al., 1982; Allen and Bryans, 1986).
Establishment of a Latent EHV-1 Infection in the Peripheral Nervous System (PNS) and in Respiratory Associated Lymphoid Tissues
After primary replication in the horse respiratory epithelium, EHV-1 enters nerve endings of the PNS, including those coming from the trigeminal ganglia (TG), sympathetic and parasympathetic neurons that innervate the epithelium (Figure 1.1e; Van Cleemput et al., 2017). EHV-1 particles travel via retrograde transport to the sensory and autonomic peripheral ganglia. A hallmark of herpesviruses is the establishment of a reactivable, latent infection in its host (Grinde, 2013). Therefore, EHV-1 establishes a lifelong latent infection in horse PNS neurons (Figure 1.3). An in vivo study of Slater and colleagues presented evidence of a latent EHV-1 infection in horses. Two months following the initial intranasal EHV-1 inoculation, the authors administered a reactivation stimulus (e.g., dexamethasone) to asymptomatic ponies. EHV-1 infectious virus and viral DNA were subsequently detected in the TG of infected ponies by cocultivation and PCR assays (Slater et al., 1994a,b). These results were confirmed by another study demonstrating the presence of specific latency-associated transcripts (LAT) in TG of EHV-1-infected horses by in situ hybridization (Baxi et al., 1995). EHV-1 has also been shown to establish latency in respiratory associated lymphoid tissues (e.g., mandibular, retropharyngeal and bronchial lymph nodes). Latent EHV-1 was detected by PCR and recovered by co-cultivation from lymphoid tissues draining the respiratory tract of experimentally inoculated ponies (Welch et al., 1992; Kydd et al., 1994; Slater et al., 1994a). Additional studies stating that EHV-1 establishes latency in lymphoid tissues, failed to conclusively demonstrate the presence of LATs in these tissues (Pusterla et al., 2010, 2012; Goehring, 2017; Giessler et al., 2020). Finally, circulating T lymphocytes have been defined as a predominant site of EHV-1 latency. Using southern hybridization and PCR techniques, Chesters and colleagues first demonstrated that PBMC express a putative LAT antisense to and overlapping the 3′ end of the EHV-1 immediate-early gene (ORF64) (Chesters et al., 1997). An in vitro study showed that EHV-1 reactivates from CD8+ T lymphocytes upon mitogen stimulation (e.g., phytohaemagglutin ad pokeweed mitogen) (Smith et al., 1998). It was estimated that 1/50,000 PBMC are latently infected with EHV-1. However, these results must be interpreted with caution as the authors also failed to demonstrate the presence of LAT in these cells and/or confirm the findings obtained by Chesters and colleagues.
Upon stress-induced reactivation months or years after primary infection, EHV-1 replication occurs and particles spread back from infected T lymphocytes or from infected TG neurons through anterograde transport to the respiratory epithelium where the infection is initiated. The presence of an EHV-1 receptor located at the basolateral side of the respiratory epithelium is a key advantage for the virus to efficiently bind and infect epithelial cells upon reactivation (Van Cleemput et al., 2017). So far, there is no direct evidence of EHV-1 neuronal spread from the PNS (e.g., TG) to the CNS (e.g., brain). Cycles of EHV-1 latency and reactivation in horses lead to the shedding of infectious virus and transmission to new hosts, allowing the virus to be maintained in herds. In addition, latently infected cells are masked from immune surveillance and constitute a permanent reservoir of the virus that is difficult to eliminate by conventional antiviral therapies and vaccine strategies. It is now estimated that the majority of horses (> 60%) are latently infected by EHV-1 (Lunn et al., 2009).
Secondary EHV-1 Replication in the Pregnant Uterus, CNS and/or Eye
Once in the blood circulation, infected leukocytes can adhere and subsequently transfer EHV-1 to the endothelial cells (EC) lining the blood vessels of target organs such as the pregnant uterus or CNS. The infection of EC located in the vasculature of the late-gravid uterus or CNS is mediated by cell-to-cell contacts between infected PBMC and EC (Goehring et al., 2011). The adhesion molecules present on the surface of both EC [e.g., intracellular adhesion molecule (ICAM), E-selectin, P-selectin] and leukocyte [e.g., α4β1 (VLA-4), αLβ2 (LFA-1), and αvβ3 integrins] have been shown to play an important role in the infection of the vascular endothelium (Smith et al., 2001; Laval et al., 2015b).
Secondary replication in the EC of the pregnant uterus causes vasculitis and multifocal thrombosis that particularly affect small arteriolar branches in the glandular layer of the endometrium at the base of the microcotyledons (Edington et al., 1991; Smith et al., 1992, 1993; Figure 1.4a). This event leads to avascular necrosis and edema of the endometrium. A widespread EC infection can cause detachment of the fetal membranes, thus leading to the abortion of a virus-negative fetus. Less extensive uterine vascular pathology allows EHV-1 to invade the fetus through the uteroplacental barrier and leads to the abortion of a virus-infected fetus. In EHV-1-positive fetuses, infected EC are present in blood vessels of the allantochorion and umbilical cord (Smith et al., 1997). The aborted fetus shows multiple lesions, including subcutaneous edema, pulmonary edema and splenic enlargement (Corner et al., 1963; Machida et al., 1997). At late stage of gestation, transplacental EHV-1 infection often results in the delivery of a live infected foal that dies within a few days. Whether or not abortion occurs may depend on the hormonal activity during pregnancy. The production of high levels of cortisone, progesterone and estrogens at late stage of pregnancy may alter the immune system of the mare (Smith et al., 1996). EHV-1-induced abortion typically occurs in the last trimester of pregnancy. The incubation period of abortion following viremia, arising from either a primary infection or a reactivation of latent virus, varies between 9 days and 4 months (Allen, 1999, 2002). EHV-1-induced abortions usually occur in single mares within a group, suggesting that abortion resulted from viral reactivation rather than from newly acquired respiratory infection (Doll and Bryans, 1963). Mares may experience respiratory symptoms and fever prior to abortion but can also abort without showing any clinical symptoms. The reproductive potential of the mare is usually not affected, and the virus is rapidly cleared from the reproductive tract.
Secondary replication in the EC lining the blood vessels of the CNS can cause vasculitis with or without local hemorrhage and thrombo-ischemic necrosis, in the brain and spinal cord (Figure 1.4b; Edington et al., 1986; Wilson, 1997). The lack of nutrients and oxygen and the elevated levels of inflammatory cytokines cause neurons to degenerate, eventually leading to equine herpes myeloencephalopathy (EHM) (Jackson and Kendrick, 1971; Wilson, 1997). Neurological symptoms go from ataxia to a complete fore and hind limb paralysis. Other clinical signs comprise fecal and/or urinary incontinence, head tilting, tail paralysis, distal limb edema and blindness (Jackson and Kendrick, 1971; van Maanen et al., 2001; Borchers et al., 2006; Gryspeerdt et al., 2011). The prognosis for non-recumbent horses is favorable but full recovery can take months. However, recumbent horses usually develop fatal complications and require euthanasia. The incubation period of EHV-1-induced neurological disorders can vary between 6 and 8 days (Mumford et al., 1994). Several risks factors, including the age (elder), breed (Standardbred), gender (female), season (late autumn, winter, spring), and type of EHV-1 strain (neurovirulent) have been associated with an increased risk to develop EHM (Goehring et al., 2006; Henninger et al., 2007; Allen, 2008; Perkins et al., 2009; Pusterla et al., 2016). The neurovirulent EHV-1 strain presents a single nucleotide polymorphism (SNP) in the catalytic subunit of the viral DNA polymerase (ORF30), causing a substitution of asparagine (N) by aspartic acid (D) at amino acid position 752 (Nugent et al., 2006). It was demonstrated that more than 86% of neuropathogenic outbreaks are caused by strains encoding D752 and more than 95% of non-neuropathogenic outbreaks were caused by virus strains encoding DNA pol N752. Horses infected with neurovirulent strains also showed a higher and longer viremia compared to those infected with non-neurovirulent strains and were more likely to develop neurological disorders (Allen and Breathnach, 2006). These results were confirmed by targeted mutation of the D752 to the N752 genotype in a neurovirulent isolate which resulted in attenuation of virulence, reduced levels of viremia and reduced capacity to cause EHM (Goodman et al., 2007).
Finally, secondary replication of EHV-1 in the vasculature of the eye can cause multifocal chorioretinal lesions in infected horses (Figure 1.4c; Matthews, 2004). This type of lesion is typically caused by endothelial damage with subsequent ischemic injury to the chorioretina that may result from direct infection of the vascular endothelium following viremia. Most EHV-1-induced ocular infections are subclinical but sometimes, diffuse lesions may lead to extensive retinal destruction and blindness. The frequency of EHV-1 ocular lesions varies between 50 and 90% in experimentally infected horses (Hussey et al., 2013).
The Mechanisms of EHV-1 Immune Evasion
In this section, we discuss the current knowledge on the pathogenesis of EHV-1 with an emphasis on its immune evasive strategies developed at the level of the URT, blood circulation and endothelium of target organs in order to survive and disseminate undetected in the host.
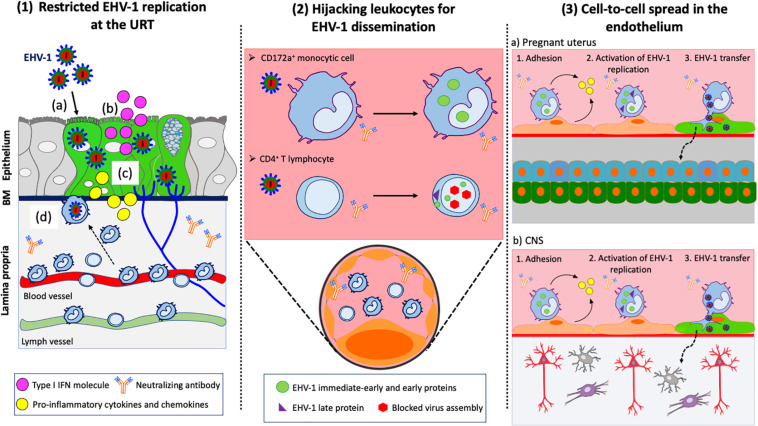
Restricted EHV-1 Replication in the URT: A Strategy to Control Innate Immune Defenses
Recently, Van Cleemput and colleagues showed that EHV-1 exploits antimicrobial equine β-defensins (eBD) to initiate viral replication and spread within the URT. Pretreatment of primary respiratory epithelial cells (EREC) with eBD increased EHV-1 virion binding and infection of these cells. It was demonstrated that EHV-1 virions are resistant to the antimicrobial properties of eBD through the action of EHV-1 gM, which stabilizes the viral envelope and protects it from eBD permeabilization (Van Cleemput et al., 2020). While EHV-1 can infect the respiratory epithelium, previous in vivo, ex vivo and in vitro studies showed that its replication is somehow restricted (Gryspeerdt et al., 2010; Vandekerckhove et al., 2010; Hussey et al., 2014). The limited replication of EHV-1 represents a finely tuned viral strategy to control the innate immune responses at the primary site of infection (Figure 2.1). On the one hand, this strategy allows a limited number of EHV-1 particles present in the epithelium to reach the cell bodies of TG neurons and to efficiently establish latency. The virus is also able to modulate the recruitment of circulating leukocytes to the URT. On the other hand, this strategy prevents the onset of a strong immune response that could be detrimental for the host. The interferon system (IFN) is the most effective mechanism of the innate response against viral infections, including EHV-1. Equine type 1 interferon (IFNα/β) has been detected in nasal secretions and serum of experimentally EHV-1-infected ponies the first 2 weeks post-inoculation (Edington et al., 1989; Chong and Duffus, 1992). The production of type I IFN was directly associated with a self-limited EHV-1 infection of the respiratory epithelium and short-duration of nasal shedding (Gryspeerdt et al., 2010). EHV-1 activated the IFN response in the URT by upregulating the expression of IFN-α mRNA and protein levels in EREC and respiratory mucosal explants (Hussey et al., 2014; Poelaert et al., 2018). However, it was demonstrated that EHV-1 modulates the IFN antiviral response in a virus strain-specific manner. The replication of EHV-1 neurovirulent strains was more efficient in explants treated with Ruxolitinib, an IFN signaling inhibitor, compared to non-treated explants. In contrast, the replication of EHV-1 non-neurovirulent strains was not altered in treated explants with plaque size similar to control group. The authors suggested that EHV-1 non-neurovirulent strains have developed an anti-IFN mechanism to focus on a more efficient replication at the level of the URT than neurovirulent strains.
FIGURE 2.
EHV-1 immune evasive strategies. (1) Restricted replication at the URT: (a) EHV-1 infects respiratory epithelial cells; (b) Infected epithelial cells produce type I IFN to limit viral spread within the upper respiratory tract (URT); (c) Infected epithelial cells produce pro-inflammatory cytokines and chemokines to recruit immune cells to the site of infection; (d) CD172a+ cells and T lymphocytes migrate to the URT. EHV-1 uses these cells to cross the basement membrane (BM) and penetrate the lamina propria. (2) Hijacking leukocytes for viral dissemination. EHV-1 silences its replication in immune cells to avoid detection by neutralizing antibodies in the blood circulation and draining lymph nodes. EHV-1 only expresses immediate-early and early proteins in CD172a+ cells. All classes of EHV-1 proteins are expressed in T lymphocyte but viral assembly is blocked, and no progeny virions are released. (3) Cell-to-cell spread in the endothelium of target organs: EHV-1 infected immune cells are transported via the blood circulation to the pregnant uterus (a) and CNS (b). The adhesion of infected immune cells to the endothelial cells lining the blood vessels of target organs activates viral replication. New EHV-1 progeny virions are released from infected immune cells and transferred to endothelial cells via (micro)fusion events. Dashed arrows represent further spread of virus particles through connective tissues or CNS. Drawings are partially based on SMART servier medical art templates.
Several ex vivo and in vitro studies further characterized the innate immune responses to EHV-1 infection in the respiratory epithelium. Soboll-Hussey and colleagues showed that EHV-1 induces the upregulation of toll-like receptors (TLR)-3 and TLR-9 as well as inflammatory cytokines (e.g., IL-1, TNF-alpha, and IL-6) and chemokines (e.g., IL-8, MCP-1) in EREC (Hussey et al., 2014). In nasal mucosal explants, EHV-1 elicited the production of chemokines (e.g., CCL2 and CCL5), already at 24 hpi, in order to attract target CD172a+ cells (Gryspeerdt et al., 2010; Zhao et al., 2017). Using a chemotaxis assay, it was demonstrated that EHV-1 upregulates the expression of CXCL9, CXCL10, eBD2, and 3 in EREC and induces the recruitment of CD172a+ cells and T lymphocytes to the site of infection (Poelaert et al., 2019c; Van Cleemput et al., 2020). Both studies indicated that primary replication of EHV-1 neurovirulent strains in the URT attracted significantly more CD172a+ cells and T lymphocytes to the infected epithelium than non-neurovirulent strains. These results are in accordance with a previous ex vivo study of Vandekerckhove and colleagues (Vandekerckhove et al., 2010). EHV-1 neurovirulent strains are likely to favor rapid infection of immune cells rather than efficient spread through the epithelium. So far, two EHV-1 glycoproteins (gG and gp2) have been shown to control the recruitment of leukocytes to the horse’s respiratory tract. EHV-1 gG is a chemokine binding protein (vCKBP), which binds to a broad range of chemokines, such as IL-8 and CXCL1, with high affinity and subsequently blocks their activity (Bryant et al., 2003). In vivo, an EHV-1 gG deleted mutant induces a more pronounced inflammatory response compared to the wild-type virus (von Einem et al., 2007). EHV-1 gG is also able to inhibit migration of equine neutrophils in response to equine recombinant IL-8 (Van de Walle et al., 2007). Using a functional cell migration assay, the infection of EREC with a EHV-1 deleted gG mutant was shown to increase the migration of monocytic cells and T lymphocytes to the infected site (Poelaert et al., 2019c). In addition, EHV-1 gp2 was identified as another viral protein with similar chemokine-binding activities as gG, that impedes the migration of immune cells to the URT. Besides, EHV-1 early protein pUL56 (encoded by EHV-1 ORF1) has also been shown to modulate the cytokine responses both in vivo and in vitro (Soboll Hussey et al., 2011; Hussey et al., 2014). pUL56 plays a major role in the downregulation of cell surface Major Histocompatibility Complex type I (MHC I) in infected cells (Ma et al., 2012; Huang et al., 2014, 2015). Soboll-Hussey and colleagues demonstrated that EHV-1 infection significantly down-regulates MHC I expression in EREC. Infection with a deleted ORF1 mutant partially restored MHC I expression and significantly increased chemokine expression and chemotaxis of immune cells to EREC (Soboll Hussey et al., 2014). Therefore, it was proposed that EHV-1 pUL56 has a dual function during infection. On one hand, this protein modulates the recruitment of leukocytes at the primary site of infection. On the other hand, it downregulates MHC I expression on infected cells and allows the virus to evade CTL-mediated cell lysis. This may explain why the virus can still cause a viremia in the presence of CTL precursors in the host (O’Neill et al., 1999; Kydd et al., 2006).
Hijacking Leukocytes: The “Trojan Horse” Mechanism of EHV-1 Dissemination
Previous in vivo and ex vivo studies of the pathogenesis of EHV-1 demonstrated that the virus misuses single leukocytes from the URT to breach the BM into the connective tissues (Gryspeerdt et al., 2010; Vandekerckhove et al., 2010; Figure 2.2). It was demonstrated that the expression of late gC and gD proteins is hampered in these cells at early stages of infection (Gryspeerdt et al., 2012). These findings are in accordance with a study from van der Meulen and colleagues, showing that the expression of EHV-1 late proteins is hampered in circulating leukocytes (van der Meulen et al., 2006). These studies suggest that an early block in the replication cycle of EHV-1 protects infected leukocytes from efficient recognition by the immune system. This “Trojan horse” mechanism allows infected leukocytes to disseminate in the blood circulation despite the presence of virus-neutralizing antibodies. Still, the minority of PBMC expressing viral glycoproteins at their cell surface were found to be resistant to antibodies, suggesting that EHV-1 uses other strategies to evade antibody-dependent cell lysis (van der Meulen et al., 2003).
Laval and colleagues further investigated the mechanisms of how EHV-1 hijacks and modulates its replication in the main carrier cells: CD172a+ cells. The authors demonstrated that the replication of EHV-1 is highly restricted in these cells with less than 10% infected compared to fully susceptible rabbit kidney (RK-13) cells (Laval et al., 2015a, 2017). In addition, EHV-1 replication is mainly non-productive in these cells. EHV-1 non-neurovirulent strains, but not neurovirulent strains, delay their replication in CD172a+ cells at a very early time of infection. The gene expression of EHV-1 non-neurovirulent strains is silenced and tightly regulated by histone deacetylases (HDAC) in these cells. Chromatin remodeling by HDAC plays a key role in the regulation of viral gene expression during herpesvirus infections (Sarkar et al., 2006; Guise et al., 2013). These findings suggest that EHV-1 non-neurovirulent and neurovirulent strains use distinct immune evasive strategies in target cells. Silencing gene expression by HDAC might be an epigenetic strategy for EHV-1 non-neurovirulent strains to slow down their replication and persist longer in CD172a+ cells than neurovirulent strains. It was proposed that mutations in the coding sequences of some regulatory proteins of EHV-1 non-neurovirulent strains might alter their capacity to bind and subsequently phosphorylate HDAC and/or HDAC-containing complexes at viral promoters in CD172a+ cells. This may result in increased levels of HDAC and repression of EHV-1 gene expression, and thus may indirectly delay viral replication in these cells. However, the fact that both viral strains can still cause a viremia in vaccinated horses indicates that manipulation of HDAC is only one part of EHV-1’s strategy to bypass detection by the immune system. Laval and colleagues further investigated the molecular mechanisms underlying the restricted infection of EHV-1 in CD172a+ cells (Laval et al., 2016). Using dioctadecyloxacarbocyanine perchlorate (dio)-labeled EHV-1 particles, the authors characterized the entry mechanisms of EHV-1 in these cells. EHV-1 bound to a limited number of CD172a+ cells compared to RK-13 cells, suggesting that a block at the virus binding level might be partially responsible for the restricted viral replication in these cells. These results also suggest the presence of specific receptor(s) at the cell surface. Previous studies have already shown that EHV-1 uses a cell surface receptor that is distinct from any of the known alphaherpesvirus entry receptors (Frampton et al., 2005). MHC I has been identified as a functional entry receptor for gD in equine dermal and brain microvascular EC (Kurtz et al., 2010; Sasaki et al., 2011). However, a study from Azab and colleagues demonstrated that MHC I antibodies do not efficiently block EHV-1 entry into PBMC, indicating that different receptors are present on equine leukocytes (Azab and Osterrieder, 2012). Enzymatic removal of sialic acids present on the cell surface, but not heparan sulfate, inhibited EHV-1 infection of CD172a+ cells by 90–100%. It was proposed that EHV-1 interacts with a sialic acid-containing cell surface receptor expressed on these cells. Future work is still needed to determine which sialylated glycans are expressed on CD172a+ cells and involved in EHV-1 binding step. Specific EHV-1 glycoprotein(s) that serve as viral glycan-binding proteins should also be identified. EHV-1 gC has hemagglutination activity against equine red blood cells and is considered a potential viral lectin candidate for the binding of sialic acid residues on CD172a+ cells (Andoh et al., 2015). Moreover, the blockage of αvβ3 integrin by neutralizing antibodies significantly reduced EHV-1 entry at a post-binding step. αvβ3 integrin acts as a co-receptor for HSV1 and HCMV (Wang et al., 2005; Gianni and Campadelli-Fiume, 2012). The interaction between αvβ3 integrin present on PBMC and the arginine, serine and aspartic acid (RSD) motif present in EHV-1 gD is known to trigger EHV-1 entry via endocytosis (Van de Walle et al., 2008). Therefore, it was proposed that EHV-1 uses αvβ3 integrin to route its entry via endocytosis into CD172a+ cells in order to shorten its exposure at the cell surface. Finally, the authors demonstrated that EHV-1 enters CD172a+ cells via an endocytic mechanism that requires cholesterol, tyrosine kinase activity, actin polymerization, dynamin activity, and endosomal acidification, pointing toward a phagocytic mechanism. Phagocytic mechanisms have already been described for the entry of HSV1 and CMV into cells (Clement et al., 2006; Tiwari and Shukla, 2012). The formation of protrusions at the surface of CD172a+ cells mediated by actin polymerization may facilitate uptake of EHV-1 into the cell and protect the virus from neutralizing antibodies.
While CD172a+ cells are considered the main “Trojan horse” in the pathogenesis of EHV-1, T lymphocytes also serve as an important vehicle for viral dissemination. EHV-1 predominantly infects T lymphocytes (CD4+) with EHV-1 non-neurovirulent strains infecting twice as much cells (~10%) than neurovirulent strains (Poelaert et al., 2019b). Both type of strains efficiently replicated in T lymphocytes with the expression of all classes of viral proteins. However, viral glycoproteins clustered at the plasma membrane of T lymphocytes and formed aggregates. Viral capsids were also found to accumulate in the cell nucleus and the release of progeny virions was hampered. The authors proposed that the aggregation of membrane-bound viral proteins at the cell surface and the blockage of virus assembly represent two new immune evasive mechanisms that EHV-1 uses to prevent efficient recognition of infected cells by antibodies. Interestingly, contact of the infected T lymphocytes with either another T lymphocyte or CD172a+ cell activated viral assembly and egress. The transfer of infectious virus from T lymphocyte to target cell was mediated by the polarization of microtubule-organizing center (MTOC) and the accumulation of lymphocyte function-associated antigen 1 (LFA1) at the site of contact. These events resulted in the formation of a virological synapse between the two cells. The authors further demonstrated that EHV-1 hijacks the dynein motor proteins to catalyze the transport of viral progeny along the microtubule network that connects the cellular nucleus to the virological synapse. These findings show that cell-to-cell spread is an essential viral mechanism to disseminate within the host and evade immunosurveillance.
EHV-1 Transfer From Leukocytes to Endothelial Cells: Unloading the Trojan Horse
EHV-1-induced abortion and neurological disease are direct results of viral spread from infected leukocytes to EC lining the blood vessels of target organs, and their subsequent infection. EHV-1 also relies on cell-to-cell spread to bypass antibody-mediated immune responses for efficient transmission to the endothelium (Figure 2.3).
Recently, several studies investigated the molecular mechanisms of EHV-1 transmission from leukocytes to EC. Laval and colleagues showed that EHV-1 infection significantly increases adhesion of CD172a+ cells to equine EC. Antibody-blocking experiments indicated that αVβ3, α4β1, and αLβ2 integrins partially mediate adhesion of infected CD172a+ cells to EC (Laval et al., 2015b). The authors proposed that the interaction of EHV-1 gD and αVβ3 integrin expressed at the surface of CD172a+ cells activates PI(3)K and ERK/MAPK signaling pathways. This event mediates EHV-1 entry into CD172a+ cells at early time of infection. Later, activated integrins expressed on infected CD172a+ cells interact with EC ligands, facilitating immune cell adhesion to the endothelium. The PI3K signaling pathway plays an important role in the inflammatory response. PI3K isoform γ is highly expressed in leukocytes and triggers chemokine-mediated recruitment and activation of innate immune cells at inflammation sites (Hawkins and Stephens, 2015). It was therefore suggested that EHV-1 infection switches CD172a+ cells to a pro-inflammatory phenotype to promote their differentiation into macrophages, migration, and subsequent adhesion. However, another study demonstrated that the release of cytokines and chemokines is significantly reduced in PBMC upon EHV-1 infection (Pavulraj et al., 2020). Besides, EHV-1 induces procoagulant activity in equine monocytic cells (Yeo et al., 2013). Tissue factor (TF) is the primary activator of coagulation and is mainly produced by monocytes in an inflammatory state (Østerud, 2010). It was proposed that increased monocyte TF expression promotes their adhesion to the endothelium and this process may be involved in EHV-1-associated thrombosis. The adhesion of EHV-1-infected CD172a+ cells to EC was found to correlate with the production of pro-inflammatory cytokines, such as TNF-α, in the microenvironment (Laval et al., 2015b). The adhesion process is likely to create an inflammatory environment that facilitates the recruitment of additional monocytic cells to the endothelium. This inflammatory cascade may promote EC infection. Additional factors present in the local environment of the endothelium may influence the adhesion of leukocytes and transfer of virus to EC. It was demonstrated that cytokines (e.g., IL-2) and hormones (e.g., 17-estradiol and equine chrorionic gonadotropin) upregulate the expression of adhesion molecules on EC (Smith et al., 2002). Consequently, the hormonal activity and immune status of the pregnant mare may impact on the efficient infection of the endothelium and the development of abortion at late stage of pregnancy. Short-chain fatty acids (SCFA), such as sodium butyrate and sodium propionate, are metabolic end-products of the fermentation of dietary fibers. Recently, SCFA were found to downregulate the expression of ICAM-1 and VCAM-1 adhesion molecules on EC. Pretreatment of EC with SCFA decreased adhesion of EHV-1 infected immune cells to EC and viral transfer (Poelaert et al., 2019a).
Cell-to-cell contacts activate the transcription and translation of viral proteins in infected CD172a+ cells and facilitate the transfer of new progeny virions to EC (Laval et al., 2015b). Infected T lymphocytes can also transfer virions to EC (Poelaert et al., 2019b). Using an in vitro flow system that mimics the rolling of PBMC to EC, Spiesschaert et al. (2015) demonstrated that EHV-1 gB and US3 proteins mediate viral transfer to EC in the presence of neutralizing antibodies. As a common strategy for herpesviruses, EHV-1 uses cell-cell (micro) fusion mechanisms for viral transmission (Laval et al., 2015b; Kamel et al., 2020). However, this transfer seems to be mainly non-productive (Laval et al., 2015b). These findings suggest that not all infected leukocytes transfer EHV-1 to EC, not every transfer leads to effective spread within the endothelium and not every successful infection of EC causes infection of surrounding tissues. Furthermore, it was shown that EHV-1 infection inhibits the type I IFN response in EC in vitro (Sarkar et al., 2015). After an early induction of type I IFN, EHV-1 suppresses the production of IFN while increasing the expression of late viral genes in EC. EHV-1 infection disrupts the interferon regulatory factor-3 (IRF-3) signaling pathway in EC through downregulation of endogenous IRF-3 protein level and inhibition of IRF3 nuclear translocation (Sarkar et al., 2016a). Additional studies showed that EHV-1 infection of EC suppresses the expression of TLR3 and TLR4 mRNA as well as the transcription of IRF7 and IRF9 mRNA in order to evade type I IFN antiviral effects. The virus also disrupts the JAK/STAT signaling pathway by degrading cellular levels of TYK2 and thus, downregulates downstream STAT1 and STAT2 phosphorylation levels (Sarkar et al., 2016b; Oladunni et al., 2019). The suppression of key factors in the induction of type I IFN only occurs after viral DNA synthesis, suggesting that late viral proteins are involved in the inhibition of STAT phosphorylation. The imbalance between antiviral and pro-inflammatory immune responses has been shown to play an important role in the clinical outcome of alphaherpesviruses infection in vivo (Laval et al., 2019). It is likely that EHV-1-mediated inhibition of type I IFN responses in EC directly promotes efficient viral replication and spread in the endothelium. In turn, this event favors the induction of a pro-inflammatory environment that contributes to the development of EHV-1-induced disease.
Therapeutic Approaches Based on Current Knowledge of the Pathogenesis of EHV-1
Despite many years of research, there are currently no fully protective vaccines against EHV-1 and no efficient antiviral drugs. Current inactivated and live-attenuated vaccines fail to fully prevent virus shedding, cell-associated viremia and EHV-1-induced abortion and neurological disorders. The virus is still able to spread via a cell-associated viremia in vaccinated horses. Over the last 5 years, knowledge on the mechanisms of EHV-1 pathogenesis has dramatically increased. We can now think about designing new therapeutics that selectively target key immune evasive strategies developed by the virus at the level of the URT, blood circulation, and endothelium of target organs to prevent or mitigate future EHV-1 infections.
The first approach would be to prevent or restrict more efficiently primary EHV-1 infection. The integrity of the respiratory epithelium is often compromised by respiratory hazards (e.g., pollens, mycotoxins, mucolytic agents) present in the horse’s environment. The disruption of respiratory ICJ facilitates EHV-1 infection of the URT. The use of aerosol drugs that strengthen the respiratory epithelium may be an interesting strategy to modulate primary EHV-1 infection. These drugs should contain calcium to allow the stabilization of ICJ as well as protease inhibitors to act on foreign incoming proteases arising from pollens and other putative sources. The efficacy of aerosol therapy in the prophylaxis and treatment of EHV-1 disease is however speculative at this point (Van Cleemput et al., 2019a). Moreover, EHV-1 infection of respiratory epithelial cells induces the production of type I IFN that limits further spread of the virus within the epithelium. Still, the IFN response is not enough to prevent virus to migrate through the epithelium and infect immune cells. The oral use of recombinant type I IFN could be considered as an antiviral drug in EHV-1-infected horses to treat primary infection. So far, the low dose administration of human type I IFN to experimentally EHV-1 infected horses did not show significant decrease in viral shedding and clinical disease (Seahorn et al., 1990). The use of an equine recombinant type I IFN at a higher dose might be more appropriate for in vivo studies. Ultimately, the identification and subsequent targeting of EHV-1 basolateral receptor in the respiratory epithelium might be the best approach to fully block viral infection of the URT.
The second approach would be to halt the migration of immune cells to the primary site of infection and/or their subsequent infection. EHV-1-infected epithelial cells produce pro-inflammatory cytokines, chemokines, eBD2, and 3 to attract immune cells. The use of anti-inflammatory aerosol drugs that reduce the cytokine and chemokine responses (e.g., corticosteroids, cytokine/chemokine neutralizing antibodies, cytokine signaling blockers) could be considered in combination with antiherpesvirus therapies. As the mechanism of EHV-1 entry into target CD172a+ cells has been partially elucidated, it would be relevant to examine the efficacy of entry blockers as antivirals to prevent cell-associated viremia. Sialic acid residues present on CD172a+ cells are essential in the initiation of EHV-1 infection. The use of carbohydrate-binding molecules specific for sialic acid demonstrated protective in vivo efficacy against influenza virus and therefore could provide similar protection against EHV-1 infection and other pathogens that recognize sialic acid receptors (Connaris et al., 2014). As the replication of EHV-1 non-neurovirulent strain is delayed and controlled by HDAC in CD172a+ cells, it could be interesting to use specific HDAC inhibitors in combination with potent antiviral drugs to prevent cell-associated viremia. Highly selective HDAC inhibitors may relieve EHV-1 temporary block in viral gene expression and accelerate viral replication in monocytic cells. Subsequently, antiviral treatment may efficiently inhibit viral replication in these cells before they reach the target epithelium. Vaccination prior to HDAC inhibitors treatment could boost the immune response and also facilitate the clearance of infected cells. However, these approaches are contingent on the availability of potent antivirals and vaccine candidates on the market. As HDAC do not control replication of EHV-1 neurovirulent strains in CD172a+ cells, it is important to keep in mind that HDAC inhibitors may not be useful for horses with EHV-1-related neurological disorders or already showing symptoms.
The third approach would be to prevent the adhesion of EHV-1-infected immune cells to the endothelium of target organs. Transient administration of anti-adhesive agents that interfere with immune cell adhesion should be explored in vivo as a potential mitigation strategy for EHV-1-induced abortion and neurological disorders. A complete characterization of the adhesion molecules present on both immune cells and EC is beforehand required. Anti-inflammatory drugs have also been shown to decrease EC infection by reducing contact between EHV-1-infected PBMC and EC (Goehring et al., 2017). The administration of corticosteroids and anti-inflammatory drugs has been recommended for a short duration in EHM treatment, but no data has demonstrated their potential efficacy so far. SCFA have been shown to reduce leukocyte adhesion to EC in vitro. SCFA have anti-inflammatory properties and hence a high-fiber diet or diet supplemented with SFCA could potentially reduce systemic inflammation and ultimately prevent EHV-1-associated disease.
Concluding Remarks
EHV-1 is able to impair horses’ health and affect equine industry all over the world. Over the last decade, the interest in EHV-1 has significantly increased due to frequent outbreaks of neurological disease and abortions caused by the virus. Within a global horse population of 58 million including 6 million in Europe, the high incidence of EHV-1 and severe associated symptoms raise serious health and economical concerns to the equine industry. Despite many years of research, no fully protective vaccines against EHV-1 or efficient antiviral drugs have been developed. Treatment is mainly limited to supportive therapy. The main reason is that EHV-1 uses a plethora of immune mechanisms to bypass the host’s immune response. A major breakthrough in our understanding of the pathogenesis of EHV-1 is therefore urgently needed. In this review, we summarize recent findings on the key immune evasive strategies used by the virus at main sites of infection: the URT, blood circulation, and endothelium of target organs. Their targeting may be a new and more effective therapeutic option to fight against EHV-1 infection.
Author Contributions
KL suggested the idea and wrote the review. HN critically reviewed and corrected the review. All other authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
KM was employed by company PERSEUS bvba. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Special Research Fund of Ghent University–Concerted Research Action 01G01311 to KL, the Institute for the promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) (141627 to KP), the Research Foundation Flanders (FWO Vlaanderen) (11Y5415N to JV), and the Special Research Fund of Ghent University–Concerted Research Action 01G01317, the China Scholarship Council (n° 201203250001) and Belspo, IAP (project BELVIR to JZ), the Institute for the promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen to AV), the Institute for the promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen to AG), the Scholarship by the Ministry of Science, Research and Technology of Iran (HB), Special Research Fund of Ghent University–Concerted Research Action 01G01317 to IZ, and Special Research Fund of Ghent University-Concerted Research Action 01D15319 to EV.
References
- Allen G. (1999). “Advances in understanding of the pathogenesis, epidemiology and immunological control of equine herpesvirus abortion,” in Equine Infectious Diseases: Proceedings of the Eighth International Conference, eds Nakajima H., Plowright W., (Newmarket: R&W Publishers; ), 129–146. [Google Scholar]
- Allen G. (2002). Epidemic disease caused by Equine herpesvirus-1: recommendations for prevention and control. Equine Vet. Educ. 14 136–142. [Google Scholar]
- Allen G., Breathnach C. (2006). Quantification by real-time PCR of the magnitude and duration of leucocyte-associated viraemia in horses infected with neuropathogenic vs. non-neuropathogenic strains of EHV-1. Equine Vet. J. 38 252–257. [DOI] [PubMed] [Google Scholar]
- Allen G. P. (2008). Risk factors for development of neurologic disease after experimental exposure to equine herpesvirus-1 in horses. Am. J. Vet. Res. 69 1595–1600. [DOI] [PubMed] [Google Scholar]
- Allen G. P., Bryans J. T. (1986). Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog. Vet. Microbiol. Immunol. 2 78–144. [PubMed] [Google Scholar]
- Andoh K., Hattori S., Mahmoud H. Y., Takasugi M., Shimoda H., Bannai H., et al. (2015). The haemagglutination activity of equine herpesvirus type 1 glycoprotein C. Virus Res. 195 172–176. 10.1016/j.virusres.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Azab W., Osterrieder N. (2012). Glycoproteins D of equine herpesvirus type 1 (EHV-1) and EHV-4 determine cellular tropism independently of integrins. J. Virol. 86 2031–2044. 10.1128/JVI.06555-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghi H. B., Laval K., Favoreel H., Nauwynck H. J. (2014). Isolation and characterization of equine nasal mucosal CD172a + cells. Vet. Immunol. Immunopathol. 157 155–163. 10.1016/j.vetimm.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Baxi M. K., Efstathiou S., Lawrence G., Whalley J. M., Slater J. D., Field H. J. (1995). The detection of latency-associated transcripts of equine herpesvirus 1 in ganglionic neurons. J. Gen. Virol. 76(Pt 12), 3113–3118. 10.1099/0022-1317-76-12-3113 [DOI] [PubMed] [Google Scholar]
- Borchers K., Thein P., Sterner-Kock A. (2006). Pathogenesis of equine herpes-associated neurological disease: a revised explanation. Equine Vet. J. 38 283–287. [DOI] [PubMed] [Google Scholar]
- Bryant N. A., Davis-Poynter N., Vanderplasschen A., Alcami A. (2003). Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO J. 22 833–846. 10.1093/emboj/cdg092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesters P. M., Allsop R., Purewal A., Edington N. (1997). Detection of latency-associated transcripts of equid herpesvirus 1 in equine leukocytes but not in trigeminal ganglia. J. Virol. 71 3437–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y. C., Duffus W. P. (1992). Immune responses of specific pathogen free foals to EHV-1 infection. Vet. Microbiol. 32 215–228. [DOI] [PubMed] [Google Scholar]
- Clement C., Tiwari V., Scanlan P. M., Valyi-Nagy T., Yue B. Y., Shukla D. (2006). A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 174 1009–1021. 10.1083/jcb.200509155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins L. (1979). Viral respiratory disease. Vet. Clin. North Am. Large Anim. Pract. 1 59–72. [DOI] [PubMed] [Google Scholar]
- Connaris H., Govorkova E. A., Ligertwood Y., Dutia B. M., Yang L., Tauber S., et al. (2014). Prevention of influenza by targeting host receptors using engineered proteins. Proc. Natl. Acad. Sci. U.S.A. 111 6401–6406. 10.1073/pnas.1404205111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner A. H., Mitchell D., Meads E. B. (1963). Equine virus abortion in Canada. I. Pathological studies on aborted fetuses. Cornell Vet. 53 78–88. [PubMed] [Google Scholar]
- D’Amato G., Cecchi L., Bonini S., Nunes C., Annesi-Maesano I., Behrendt H., et al. (2007). Allergenic pollen and pollen allergy in Europe. Allergy 62 976–990. 10.1111/j.1398-9995.2007.01393.x [DOI] [PubMed] [Google Scholar]
- Davison A., Eberle R., Ehlers B., Hayward G., McGeoch D., Minson A., et al. (2009). The order Herpesvirales. Arch. Virol. 154 171–177. 10.1007/s00705-008-0278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimock W. W., Edwards P. R. (1933). Is there a filterable virus of abortion in mares? Ky. Agric. Exp. Stn. Bull. 333 297–301. [Google Scholar]
- Doll E. R., Bryans J. T. (1963). Epizootiology of equine viral rhinopneumonitis. J. Am. Vet. Med. Assoc. 142 31–37. [PubMed] [Google Scholar]
- Edington N., Bridges C. G., Griffiths L. (1989). Equine interferons following exposure to equid herpesvirus-1 or -4. J. Interferon Res. 9 389–392. [DOI] [PubMed] [Google Scholar]
- Edington N., Bridges C. G., Patel J. R. (1986). Endothelial cell infection and thrombosis in paralysis caused by equid herpesvirus-1: equine stroke. Arch. Virol. 90 111–124. [DOI] [PubMed] [Google Scholar]
- Edington N., Smyth B., Griffiths L. (1991). The role of endothelial cell infection in the endometrium, placenta and foetus of equid herpesvirus 1 (EHV-1) abortions. J. Comp. Pathol. 104 379–387. [DOI] [PubMed] [Google Scholar]
- Frampton A. R., Jr., Goins W. F., Cohen J. B., von Einem J., Osterrieder N., O’Callaghan D. J., et al. (2005). Equine herpesvirus 1 utilizes a novel herpesvirus entry receptor. J. Virol. 79 3169–3173. 10.1128/jvi.79.5.3169-3173.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni T., Campadelli-Fiume G. (2012). alphaVbeta3-integrin relocalizes nectin1 and routes herpes simplex virus to lipid rafts. J. Virol. 86 2850–2855. 10.1128/jvi.06689-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J., Slater J., Awan A., Field H. (1992). Pathogenesis of equine herpesvirus-1 in specific pathogen-free foals: primary and secondary infections and reactivation. Arch. Virol. 123 351–366. [DOI] [PubMed] [Google Scholar]
- Giessler K. S., Samoilowa S., Soboll Hussey G., Kiupel M., Matiasek K., Sledge D. G., et al. (2020). Viral load and cell tropism during early latent equid Herpesvirus 1 infection differ over time in lymphoid and neural tissue samples from experimentally infected Horses. Front. Vet. Sci. 7:621. 10.3389/fvets.2020.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring L. S. (2017). Latency in Equid Herpesvirus-1 purpose-infected Horses. ACVIM Forum Research Report Program. J. Vet. Int. Med. 31 1598–1599. [Google Scholar]
- Goehring L. S., Brandes K., Ashton L. V., Wittenburg L. A., Olea-Popelka F. J., Lunn D. P., et al. (2017). Anti-inflammatory drugs decrease infection of brain endothelial cells with EHV-1 in vitro. Equine Vet. J. 49 629–636. 10.1111/evj.12656 [DOI] [PubMed] [Google Scholar]
- Goehring L. S., Hussey G. S., Ashton L. V., Schenkel A. R., Lunn D. P. (2011). Infection of central nervous system endothelial cells by cell-associated EHV-1. Vet. Microbiol. 148 389–395. 10.1016/j.vetmic.2010.08.030 [DOI] [PubMed] [Google Scholar]
- Goehring L. S., van Winden S. C., van Maanen C., Sloet van Oldruitenborgh-Oosterbaan M. M. (2006). Equine herpesvirus type 1-associated myeloencephalopathy in The Netherlands: a four-year retrospective study (1999-2003). J. Vet. Intern. Med. 20 601–607. [DOI] [PubMed] [Google Scholar]
- Goodman L. B., Loregian A., Perkins G. A., Nugent J., Buckles E. L., Mercorelli B., et al. (2007). A point mutation in a herpesvirus polymerase determines neuropathogenicity. PLoS Pathog. 3:e160. 10.1371/journal.ppat.0030160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinde B. (2013). Herpesviruses: latency and reactivation - viral strategies and host response. J. Oral Microbiol. 5:10.3402/jom.v3405i3400.22766. 10.3402/jom.v3405i3400.22766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryspeerdt A., Vandekerckhove A., Van Doorsselaere J., Van de Walle G., Nauwynck H. (2011). Description of an unusually large outbreak of nervous system disorders caused by equine herpesvirus 1 (EHV1) in 2009 in Belgium. Vlaams Diergeneeskd. Tijdschr. 80 147–153. [Google Scholar]
- Gryspeerdt A. C., Vandekerckhove A., Garré B., Barbé F., Van de Walle G., Nauwynck H. (2010). Differences in replication kinetics and cell tropism between neurovirulent and non-neurovirulent EHV1 strains during the acute phase of infection in horses. Vet. Microbiol. 142 242–253. [DOI] [PubMed] [Google Scholar]
- Gryspeerdt A. C., Vandekerckhove A. P., Baghi H. B., Van de Walle G. R., Nauwynck H. J. (2012). Expression of late viral proteins is restricted in nasal mucosal leucocytes but not in epithelial cells during early-stage equine herpes virus-1 infection. Vet. J. 193 576–578. 10.1016/j.tvjl.2012.01.022 [DOI] [PubMed] [Google Scholar]
- Guise A. J., Budayeva H. G., Diner B. A., Cristea I. M. (2013). Histone deacetylases in Herpesvirus replication and virus-stimulated host defense. Viruses 5 1607–1632. 10.3390/v5071607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L. R. (2015). PI3K signalling in inflammation. Biochim. Biophys. Acta 1851 882–897. 10.1016/j.bbalip.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Henninger R. W., Reed S. M., Saville W. J., Allen G. P., Hass G. F., Kohn C. W., et al. (2007). Outbreak of neurologic disease caused by equine Herpesvirus-1 at a University Equestrian Center. J. Vet. Intern. Med. 21 157–165. 10.1111/j.1939-1676.2007.tb02942.x [DOI] [PubMed] [Google Scholar]
- Huang T., Lehmann M. J., Said A., Ma G., Osterrieder N. (2014). Major histocompatibility complex class I downregulation induced by equine herpesvirus type 1 pUL56 is through dynamin-dependent endocytosis. J. Virol. 88 12802–12815. 10.1128/jvi.02079-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Ma G., Osterrieder N. (2015). Equine Herpesvirus 1 multiply inserted transmembrane protein pUL43 cooperates with pUL56 in downregulation of cell surface major histocompatibility complex class I. J. Virol. 89 6251–6263. 10.1128/jvi.00032-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey G. S., Ashton L. V., Quintana A. M., Lunn D. P., Goehring L. S., Annis K., et al. (2014). Innate immune responses of airway epithelial cells to infection with Equine herpesvirus-1. Vet. Microbiol. 170 28–38. [DOI] [PubMed] [Google Scholar]
- Hussey G. S., Goehring L. S., Lunn D. P., Hussey S. B., Huang T., Osterrieder N., et al. (2013). Experimental infection with equine herpesvirus type 1 (EHV-1) induces chorioretinal lesions. Vet. Res. 44:118. 10.1186/1297-9716-44-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T., Kendrick J. (1971). Paralysis of horses associated with equine herpesvirus 1 infection. Am. Vet. Med. Assoc. J. 158 1351–1357. [PubMed] [Google Scholar]
- Kamel M., Pavulraj S., Fauler B., Mielke T., Azab W. (2020). Equid Herpesvirus-1 exploits the extracellular matrix of mononuclear cells to ensure transport to target cells. iScience 23:101615. 10.1016/j.isci.2020.101615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz B. M., Singletary L. B., Kelly S. D., Frampton A. R., Jr. (2010). Equus caballus major histocompatibility complex class I is an entry receptor for equine herpesvirus type 1. J. Virol. 84 9027–9034. 10.1128/jvi.00287-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kydd J. H., Smith K. C., Hannant D., Livesay G. J., Mumford J. A. (1994). Distribution of Equid herpesvirus-1 (EHV-1) in respiratory tract associated lymphoid tissue: implications for cellular immunity. Equine Vet. J. 26 470–473. 10.1111/j.2042-3306.1994.tb04052.x [DOI] [PubMed] [Google Scholar]
- Kydd J. H., Townsend H. G., Hannant D. (2006). The equine immune response to equine herpesvirus-1: the virus and its vaccines. Vet. Immunol. Immunopathol. 111 15–30. 10.1016/j.vetimm.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Laval K., Favoreel H. W., Nauwynck H. J. (2015a). Equine herpesvirus type 1 replication is delayed in CD172a+ monocytic cells and controlled by histone deacetylases. J. Gen. Virol. 96(Pt 1), 118–130. 10.1099/vir.0.067363-0 [DOI] [PubMed] [Google Scholar]
- Laval K., Favoreel H. W., Poelaert K. C., Van Cleemput J., Nauwynck H. J. (2015b). Equine Herpesvirus type 1 enhances viral replication in CD172a+ monocytic cells upon adhesion to endothelial cells. J. Virol. 89 10912–10923. 10.1128/jvi.01589-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval K., Favoreel H. W., Van Cleemput J., Poelaert K. C. K., Brown I. K., Verhasselt B., et al. (2016). Entry of equid herpesvirus 1 into CD172a+ monocytic cells. J. Gen. Virol. 97 733–746. 10.1099/jgv.0.000375 [DOI] [PubMed] [Google Scholar]
- Laval K., Van Cleemput J., Poelaert K. C., Brown I. K., Nauwynck H. J. (2017). Replication of neurovirulent equine herpesvirus type 1 (EHV-1) in CD172a(+) monocytic cells. Comp. Immunol. Microbiol. Infect. Dis. 50 58–62. 10.1016/j.cimid.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Laval K., Van Cleemput J., Vernejoul J. B., Enquist L. W. (2019). Alphaherpesvirus infection of mice primes PNS neurons to an inflammatory state regulated by TLR2 and type I IFN signaling. PLoS Pathog. 15:e1008087. 10.1371/journal.ppat.1008087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn D. P., Davis-Poynter N., Flaminio M. J., Horohov D. W., Osterrieder K., Pusterla N., et al. (2009). Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 23 450–461. 10.1111/j.1939-1676.2009.0304.x [DOI] [PubMed] [Google Scholar]
- Ma G., Feineis S., Osterrieder N., Van de Walle G. R. (2012). Identification and characterization of equine herpesvirus type 1 pUL56 and its role in virus-induced downregulation of major histocompatibility complex class I. J. Virol. 86 3554–3563. 10.1128/jvi.06994-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida N., Taniguchi T., Nakamura T., Kiryu K. (1997). Cardio-histopathological observations on aborted equine fetuses infected with equid herpesvirus 1 (EHV-1). J. Comp. Pathol. 116 379–385. [DOI] [PubMed] [Google Scholar]
- Matthews A. (2004). Fundus. 2. London: Elsevier Health Sciences. [Google Scholar]
- McGavin M. D., Zachary J. F. (2007). Pathological Basis of Veterinary Disease. St. Louis, MI: Mosby Elsevier. [Google Scholar]
- Mettenleiter T. C., Klupp B. G., Granzow H. (2009). Herpesvirus assembly: an update. Virus Res. 143 222–234. 10.1016/j.virusres.2009.03.018 [DOI] [PubMed] [Google Scholar]
- Mumford J. A., Hannant D. A., Jesset D. M., O’Neill T., Smith K. C., Ostlund E. N. (1994). “Abortigenic and neurological disease caused by experimental infection with equine herpesvirus 1,” in Proceedings of the 7th International Conference of Equine Infectious Diseases, Newmarket, 261–275. [Google Scholar]
- Negussie H., Li Y., Tessema T. S., Nauwynck H. J. (2016). Replication characteristics of equine herpesvirus 1 and equine herpesvirus 3: comparative analysis using ex vivo tissue cultures. Vet Res. 47:19. 10.1186/s13567-016-0305-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent J., Birch-Machin I., Smith K. C., Mumford J. A., Swann Z., Newton J. R., et al. (2006). Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 80 4047–4060. 10.1128/jvi.80.8.4047-4060.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladunni F. S., Sarkar S., Reedy S., Balasuriya U. B. R., Horohov D. W., Chambers T. M. (2019). Equid Herpesvirus 1 targets the sensitization and induction steps to inhibit the type I interferon response in equine endothelial cells. J. Virol. 93:e01342-19. 10.1128/jvi.01342-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill T., Kydd J. H., Allen G. P., Wattrang E., Mumford J. A., Hannant D. (1999). Determination of equid herpesvirus 1-specific, CD8+, cytotoxic T lymphocyte precursor frequencies in ponies. Vet. Immunol. Immunopathol. 70 43–54. [DOI] [PubMed] [Google Scholar]
- Østerud B. (2010). Tissue factor expression in blood cells. Thromb. Res. 125(Suppl. 1), S31–S34. 10.1016/j.thromres.2010.01.032 [DOI] [PubMed] [Google Scholar]
- Patel J. R., Edington N., Mumford J. A. (1982). Variation in cellular tropism between isolates of equine herpesvirus-1 in foals. Arch. Virol. 74 41–51. [DOI] [PubMed] [Google Scholar]
- Pavulraj S., Kamel M., Stephanowitz H., Liu F., Plendl J., Osterrieder N., et al. (2020). Equine Herpesvirus type 1 modulates cytokine and chemokine profiles of mononuclear cells for efficient dissemination to target organs. Viruses 12:999. 10.3390/v12090999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins G. A., Goodman L. B., Tsujimura K., Van de Walle G. R., Kim S. G., Dubovi E. J., et al. (2009). Investigation of the prevalence of neurologic equine herpes virus type 1 (EHV-1) in a 23-year retrospective analysis (1984–2007). Vet. Microbiol. 139 375–378. 10.1016/j.vetmic.2009.06.033 [DOI] [PubMed] [Google Scholar]
- Poelaert K. C. K., Van Cleemput J., Laval K., Descamps S., Favoreel H. W., Nauwynck H. J. (2019a). Beyond gut instinct: metabolic short-chain fatty acids moderate the pathogenesis of alphaherpesviruses. Front. Microbiol. 10:723. 10.3389/fmicb.2019.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelaert K. C. K., Van Cleemput J., Laval K., Favoreel H. W., Couck L., Van den Broeck W., et al. (2019b). Equine Herpesvirus 1 bridles T lymphocytes to reach its target organs. J. Virol. 93:e02098-18. 10.1128/jvi.02098-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelaert K. C. K., Van Cleemput J., Laval K., Favoreel H. W., Soboll Hussey G., Maes R. K., et al. (2018). Abortigenic but not neurotropic equine Herpes Virus 1 modulates the interferon antiviral defense. Front. Cell. Infect. Microbiol. 8:312. 10.3389/fcimb.2018.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelaert K. C. K., Van Cleemput J., Laval K., Xie J., Favoreel H. W., Nauwynck H. J. (2019c). Equine herpesvirus 1 infection orchestrates the expression of chemokines in equine respiratory epithelial cells. J. Gen. Virol. 100 1567–1579. 10.1099/jgv.0.001317 [DOI] [PubMed] [Google Scholar]
- Pusterla N., Hussey S. B., Mapes S., Johnson C., Collier J. R., Hill J., et al. (2010). Molecular investigation of the viral kinetics of equine herpesvirus-1 in blood and nasal secretions of horses after corticosteroid-induced recrudescence of latent infection. J. Vet. Intern. Med. 24 1153–1157. 10.1111/j.1939-1676.2010.0554.x [DOI] [PubMed] [Google Scholar]
- Pusterla N., Mapes S., Akana N., Barnett C., MacKenzie C., Gaughan E., et al. (2016). Prevalence factors associated with equine herpesvirus type 1 infection in equids with upper respiratory tract infection and/or acute onset of neurological signs from 2008 to 2014. Vet. Rec. 178:70. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Mapes S., Wademan C., White A., Estell K., Swain E. (2012). Investigation of the role of mules as silent shedders of EHV-1 during an outbreak of EHV-1 myeloencephalopathy in California. Vet. Rec. 170:465. 10.1136/vr.100598 [DOI] [PubMed] [Google Scholar]
- Reed S. M., Toribio R. E. (2004). Equine herpesvirus 1 and 4. Vet. Clin. North Am. Equine Pract. 20 631–642. 10.1016/j.cveq.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Roizman B., Desrosiers R. C., Fleckenstein B., Lopez C., Minson A. C., Studdert M. J. (1992). The family Herpesviridae: an update. Arch. Virol. 123 425–449. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Balasuriya U. B., Horohov D. W., Chambers T. M. (2015). Equine herpesvirus-1 suppresses type-I interferon induction in equine endothelial cells. Vet. Immunol. Immunopathol. 167 122–129. 10.1016/j.vetimm.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Balasuriya U. B., Horohov D. W., Chambers T. M. (2016a). Equine herpesvirus-1 infection disrupts interferon regulatory factor-3 (IRF-3) signaling pathways in equine endothelial cells. Vet. Immunol. Immunopathol. 173 1–9. 10.1016/j.vetimm.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Balasuriya U. B., Horohov D. W., Chambers T. M. (2016b). The neuropathogenic T953 strain of equine herpesvirus-1 inhibits type-I IFN mediated antiviral activity in equine endothelial cells. Vet. Microbiol. 183 110–118. 10.1016/j.vetmic.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Longacre M., Tatur N., Heerboth S., Lapinska K. (2006). “Histone Deacetylases (HDACs): function, mechanism, & inhibition,” in Encyclopedia of Analytical Chemistry, ed. Meyers R. (New York, NY: John Wiley & Sons, Ltd; ). [Google Scholar]
- Sasaki M., Hasebe R., Makino Y., Suzuki T., Fukushi H., Okamoto M., et al. (2011). Equine major histocompatibility complex class I molecules act as entry receptors that bind to equine herpesvirus-1 glycoprotein D. Genes Cells 16 343–357. 10.1111/j.1365-2443.2011.01491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxegaard F. (1966). Isolation and identification of equine rhinopneumonitisvirus (equine abortionvirus) from cases of abortion and paralysis. Nord. Veterinaermedecin 18 506–516. [Google Scholar]
- Seahorn T. L., Carter G. K., Martens J. G., Crandell R. A., Martin M. T., Scrutchfield W. L., et al. (1990). Effects of human alpha interferon on experimentally induced equine herpesvirus-1 infection in horses. Am. J. Vet. Res. 51 2006–2010. [PubMed] [Google Scholar]
- Siedek E., Whelan M., Edington N., Hamblin A. (1999). Equine herpesvirus type 1 infects dendritic cells in vitro: stimulation of T lymphocyte proliferation and cytotoxicity by infected dendritic cells. Vet. Immunol. Immunopathol. 67 17–32. [DOI] [PubMed] [Google Scholar]
- Slater J. D., Baxi M., Tewari D., Gibson J. S., Dield H. J., Ludwig H., et al. (1994a). “Experimental infection of specific pathogen-free ponies with equid Herpesvirus-1: detection of infectious virus and Viral DNA,” in Equine Infectious Diseases VII, eds Plowright W., Nakajima H., (Newmarket: R&W Publications; ), 255–260. [Google Scholar]
- Slater J. D., Borchers K., Thackray A. M., Field H. J. (1994b). The trigeminal ganglion is a location for equine herpesvirus 1 latency and reactivation in the horse. J. Gen. Virol. 75(Pt 8), 2007–2016. 10.1099/0022-1317-75-8-2007 [DOI] [PubMed] [Google Scholar]
- Smith D., Hamblin A., Edington N. (2002). Equid herpesvirus 1 infection of endothelial cells requires activation of putative adhesion molecules: an in vitro model. Clin. Exp. Immunol. 129 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Hamblin A. S., Edington N. (2001). Infection of endothelial cells with equine herpesvirus-1 (EHV-1) occurs where there is activation of putative adhesion molecules: a mechanism for transfer of virus. Equine Vet. J. 33 138–142. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Iqbal J., Purewal A., Hamblin A. S., Edington N. (1998). In vitro reactivation of latent equid herpesvirus-1 from CD5+/CD8+ leukocytes indirectly by IL-2 or chorionic gonadotrophin. J. Gen. Virol. 79(Pt 12), 2997–3004. 10.1099/0022-1317-79-12-2997 [DOI] [PubMed] [Google Scholar]
- Smith K. C., McGladdery A. J., Binns M. M., Mumford J. A. (1997). Use of transabdominal ultrasound-guided amniocentesis for detection of equid herpesvirus 1-induced fetal infection in utero. Am. J. Vet. Res. 58 997–1002. [PubMed] [Google Scholar]
- Smith K. C., Mumford J. A., Lakhani K. (1996). A comparison of equid herpesvirus-1 (EHV-1) vascular lesions in the early versus late pregnant equine uterus. J. Comp. Pathol. 114 231–247. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Whitwell K. E., Binns M. M., Dolby C. A., Hannant D., Mumford J. A. (1992). Abortion of virologically negative foetuses following experimental challenge of pregnant pony mares with equid herpesvirus 1. Equine Vet. J. 24 256–259. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Whitwell K. E., Mumford J. A., Gower S. M., Hannant D., Tearle J. P. (1993). An immunohistological study of the uterus of mares following experimental infection by equid herpesvirus 1. Equine Vet. J. 25 36–40. [DOI] [PubMed] [Google Scholar]
- Soboll Hussey G., Ashton L. V., Quintana A. M., Van de Walle G. R., Osterrieder N., Lunn D. P. (2014). Equine herpesvirus type 1 pUL56 modulates innate responses of airway epithelial cells. Virology 46 76–86. 10.1016/j.virol.2014.05.023 [DOI] [PubMed] [Google Scholar]
- Soboll Hussey G., Hussey S. B., Wagner B., Horohov D. W., Van de Walle G. R., Osterrieder N., et al. (2011). Evaluation of immune responses following infection of ponies with an EHV-1 ORF1/2 deletion mutant. Vet. Res. 42:23. 10.1186/1297-9716-42-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiesschaert B., Goldenbogen B., Taferner S., Schade M., Mahmoud M., Klipp E., et al. (2015). Role of gB and pUS3 in equine Herpesvirus 1 transfer between peripheral blood mononuclear cells and endothelial cells: a dynamic in vitro model. J. Virol. 89 11899–11908. 10.1128/jvi.01809-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson G. R., Numford J. A., Smith I. M. (1979). Experimental immunization against respiratory disease due to equid herpesvirus 1 infection (rhinopneumonitis) using formalin-inactivated virus with various adjuvants. Vet. Microbiol. 4 209–222. 10.1016/0378-1135(79)90057-9 [DOI] [Google Scholar]
- Tiwari V., Shukla D. (2012). Nonprofessional phagocytosis can facilitate herpesvirus entry into ocular cells. Clin. Dev. Immunol. 2012:651691. 10.1155/2012/651691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleemput J., Poelaert K. C. K., Laval K., Impens F., Van den Broeck W., Gevaert K., et al. (2019a). Pollens destroy respiratory epithelial cell anchors and drive alphaherpesvirus infection. Sci. Rep. 9:4787. 10.1038/s41598-019-41305-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleemput J., Poelaert K. C. K., Laval K., Maes R., Hussey G. S., Van den Broeck W., et al. (2017). Access to a main alphaherpesvirus receptor, located basolaterally in the respiratory epithelium, is masked by intercellular junctions. Sci. Rep. 7:16656. 10.1038/s41598-017-16804-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleemput J., Poelaert K. C. K., Laval K., Van den Broeck W., Nauwynck H. J. (2019b). Deoxynivalenol, but not fumonisin B1, aflatoxin B1 or diesel exhaust particles disrupt integrity of the horse’s respiratory epithelium and predispose it for equine herpesvirus type 1 infection. Vet. Microbiol. 234 17–24. 10.1016/j.vetmic.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Van Cleemput J., Poelaert K. C. K., Laval K., Vanderheijden N., Dhaenens M., Daled S., et al. (2020). An alphaherpesvirus exploits antimicrobial β-Defensins to initiate respiratory tract infection. J. Virol. 94:e01676-19. 10.1128/jvi.01676-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Walle G. R., May M. L., Sukhumavasi W., von Einem J., Osterrieder N. (2007). Herpesvirus chemokine-binding glycoprotein G (gG) efficiently inhibits neutrophil chemotaxis in vitro and in vivo. J. Immunol. 179 4161–4169. [DOI] [PubMed] [Google Scholar]
- Van de Walle G. R., Peters S. T., VanderVen B. C., O’Callaghan D. J., Osterrieder N. (2008). Equine Herpesvirus 1 entry via endocytosis is facilitated by αV integrins and an RSD motif in glycoprotein D. J. Virol. 82 11859–11868. 10.1128/jvi.00868-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen K., Caij B., Pensaert M., Nauwynck H. (2006). Absence of viral envelope proteins in equine herpesvirus 1-infected blood mononuclear cells during cell-associated viremia. Vet. Microbiol. 113 265–273. 10.1016/j.vetmic.2005.11.048 [DOI] [PubMed] [Google Scholar]
- van der Meulen K., Vercauteren G., Nauwynck H., Pensaert M. (2003). A local epidemic of equine herpesvirus 1-induced neurological disorders in Belgium. Vlaams Diergeneeskd. Tijdschr. 72 366–372. [Google Scholar]
- van der Meulen K. M., Nauwynck H. J., Buddaert W., Pensaert M. B. (2000). Replication of equine herpesvirus type 1 in freshly isolated equine peripheral blood mononuclear cells and changes in susceptibility following mitogen stimulation. J. Gen. Virol. 81 21–25. [DOI] [PubMed] [Google Scholar]
- van Maanen C. (2002). Equine herpesvirus 1 and 4 infections: an update. Vet. Q. 24 58–78. [PubMed] [Google Scholar]
- van Maanen C., Sloet van Oldruitenborgh-Oosterbaan M. M., Damen E. A., Derksen A. G. (2001). Neurological disease associated with EHV-1-infection in a riding school: clinical and virological characteristics. Equine Vet. J. 33 191–196. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove A. P., Glorieux S., Gryspeerdt A. C., Steukers L., Duchateau L., Osterrieder N., et al. (2010). Replication kinetics of neurovirulent versus non-neurovirulent equine herpesvirus type 1 strains in equine nasal mucosal explants. J. Gen. Virol. 91(Pt 8), 2019–2028. 10.1099/vir.0.019257-0 [DOI] [PubMed] [Google Scholar]
- von Einem J., Smith P. M., Van de Walle G. R., O’Callaghan D. J., Osterrieder N. (2007). In vitro and in vivo characterization of equine herpesvirus type 1 (EHV-1) mutants devoid of the viral chemokine-binding glycoprotein G (gG). Virology 362 151–162. 10.1016/j.virol.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Wang X., Huang D. Y., Huong S. M., Huang E. S. (2005). Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat. Med. 11 515–521. 10.1038/nm1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch H. M., Bridges C. G., Lyon A. M., Griffiths L., Edington N. (1992). Latent equid herpesviruses 1 and 4: detection and distinction using the polymerase chain reaction and co-cultivation from lymphoid tissues. J. Gen. Virol. 73(Pt 2), 261–268. 10.1099/0022-1317-73-2-261 [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Roizman B. (2001). Herpes simplex virus infections. Lancet 357 1513–1518. 10.1016/s0140-6736(00)04638-9 [DOI] [PubMed] [Google Scholar]
- Wilson W. D. (1997). Equine herpesvirus 1 myeloencephalopathy. Vet. Clin. North Am. Equine Pract. 13 53–72. [DOI] [PubMed] [Google Scholar]
- Yeo W. M., Osterrieder N., Stokol T. (2013). Equine herpesvirus type 1 infection induces procoagulant activity in equine monocytes. Vet. Res. 44:16. 10.1186/1297-9716-44-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Poelaert K. C., Cleemput J., Nauwynck H. J. (2017). CCL2 and CCL5 driven attraction of CD172a+ monocytic cells during an equine herpesvirus type 1 (EHV-1) infection in equine nasal mucosa and the impact of two migration inhibitors, rosiglitazone (RSG) and quinacrine (QC). Vet. Res. 48:14. [DOI] [PMC free article] [PubMed] [Google Scholar]