Highlights
-
•
Six patients underwent cytoreductive surgery plus HIPEC and EPIC for morcellated uLMS.
-
•
There were no postoperative deaths and a single patient had class 3 adverse events.
-
•
Three-year overall survival was 50% and 5-year survival 33%.
-
•
Patients with small volume uLMS or low-grade disease showed long-term survival.
Keywords: Cisplatin, Hyperthermia, Sarcomatosis, Leiomyoma, Intraperitoneal chemotherapy, Peritoneal metastases
Abstract
Uterine leiomyosarcoma (uLMS) is a rare aggressive malignant mesenchymal tumor with high risk of recurrence and poor prognosis regardless of stage. It is often diagnosed postoperatively following myomectomy, hysterectomy or supracervical hysterectomy for presumed benign disease. Primary surgery at the diagnosis of uLMS is considered to affect outcomes. If the tumor was morcellated, the oncologist will encounter special problems that require knowledgeable management of peritoneal metastases. We previously reported that six patients who successfully underwent cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) plus early postoperative intraperitoneal chemotherapy (EPIC) to manage the disease dissemination that must occur following morcellation. This is a study for long-term outcome of these patients. Six patients were treated with an absence of grade IV adverse events and no mortality. The median Peritoneal Cancer Index (PCI) was 18 and complete cytoreduction without peritoneal metastases visualized within the operative field at the completion of the surgical procedure (CC-0) was achieved in all patients. One patient was diagnosed leiomyomatosis peritonealis postoperatively. Among five patients who were confirmed uterine leiomyosarcoma, the 3-year overall survival was 40.0% and 5-year overall survival was 20.0% with the median follow-up of 18 months (range 5–73 months). The patient with PCI 0 at the time of CRS showed no evidence of disease (NED) at 73 months. We believe that prophylactic CRS contributed her favorable outcome. Therapeutic options for patients with uLMS post-morcellation are limited. Currently, CRS and HIPEC plus EPIC followed by adjuvant systemic chemotherapy may be considered an option for treatment. Further studies in a larger number of patients are needed.
1. Introduction
Uterine leiomyosarcoma (uLMS) is a rare aggressive malignant mesenchymal tumor that spreads rapidly through intraperitoneal and hematogenous pathways (Ricci et al., 2017, Sardi et al., 2017, Roberts et al., 2018). The incidence of uLMS is 0.36–0.8 per 100,000 woman-years and most occur in women over 40 years of age (Roberts et al., 2018, Toro et al., 2006). It is associated with a high risk of recurrence and poor prognosis regardless of stage at presentation (Ricci et al., 2017). In the largest study of 1396 patients with uLMS, the 5-year disease –specific survival (DSS) rates of patients with stage I, II, III, and IV disease were 75.8%, 60.1%, 44.9% and 28.7%, respectively (Kapp et al., 2008). uLMS is often diagnosed postoperatively following myomectomy, hysterectomy or supracervical hysterectomy for presumed benign disease as it is challenging to distinguish uLMS from uterine leiomyomas preoperatively due to similar clinical presentations (D'Angelo and Prat, 2010). Sagae et al. reported that 65% of the patients with uLMS were preoperatively diagnosed with benign uterine leiomyomas (Sagae et al., 2004). The incident rate of uLMS after hysterectomy or supracervical hysterectomy for presumed benign disease was 0.002–0.13% (Parker et al., 1994, Lieng et al., 2015).
If uLMS is diagnosed after myomectomy or supracervical hysterectomy, the current strategy is completion of surgery, which means either hysterectomy or trachelectomy. An individual decision is required regarding salpingo-oophorectomy (Network NCC, 2020, Einstein et al., 2008). No lymphadenectomy is indicated. Studies have shown that patients with uLMS who were diagnosed after myomectomy with leiomyoma fragmentation using a morcellation technique had a higher rate of peritoneal surface dissemination and poorer outcomes of uLMS. Fifteen to 28% of previously morcellated uLMS cases were upstaged at the time of reoperative surgery (Ricci et al., 2017, Einstein et al., 2008, George et al., 2014, Raine-Bennett et al., 2016, Oduyebo et al., 2014, Perri et al., 2009).
Hyperthermic intraperitoneal chemotherapy (HIPEC) is an intraoperative intraperitoneal chemotherapy administration under hyperthermic condition. Early postoperative intraperitoneal chemotherapy (EPIC) is a second perioperative regional chemotherapy with a goal to eradicate peritoneal metastatic disease. following aggressive cytoreduction surgery (CRS). We previously reported that CRS plus HIPEC and EPIC could be successfully completed for both newly diagnosed and recurrent uLMS following morcellation (Sugarbaker et al., 2016). In this article, we present the long-term outcome of these cases.
2. Patients and methods
2.1. Eligibility criteria
Upon obtaining Institutional Review Board approval at MedStar Health Research Institute, six cases were identified in an institutional database at the surgical oncology department between January 2010 and December 2019. The inclusion criteria included: 1) Women with diagnosis of uLMS by histopathologic examination who were initially thought to have uterine leiomyoma, 2) All six patients were treated with myomectomy or hysterectomy with morcellation technique, 3) A second intervention of CRS was followed by HIPEC in 6 of 6 patients and EPIC in 5 of 6 patients.
Patient demographics included age at CRS followed by perioperative chemotherapy, race and ethnicity. Tumor characteristics at initial diagnosis included cancer type, stage and histology. Treatment history included the date of morcellation, date of CRS followed by perioperative chemotherapy and the regimen of HIPEC and EPIC. Treatment response, adverse events including perioperative complications and survival outcomes were abstracted from medical records.
2.2. Informed consent
Prior to commencing this study, written consent to publish this clinical material was obtained from each patient.
2.3. Treatments
Methodologies for CRS, HIPEC and EPIC were described in the previous report (Sugarbaker et al., 2016). In summary, the goal of CRS is to remove all visible sarcoma deposits by visceral resection and peritonectomies. Organs or peritoneal surfaces were not resected if there was no sarcomatosis except greater omentum and gallbladder which were removed routinely. HIPEC was used for 90 min following the CRS in the operating room. The regimen consisting of cisplatin 50 mg/m2 and doxorubicin 15 mg/m2 by intraperitoneal instillation that was manually distributed into the abdominal cavity and maintained 42.5℃. Ifosfamide 1300 mg/m2 was administered as a continuous intravenous infusion over 90 min. 2-Mercaptoethanesulfoante sodium (MESNA) 256 mg/m2 was administered by bolus intravenous infusion 15 min before HIPEC, and repeated 4 and 8 h later. A Tenckhoff catheter was placed within the abdomen prior to the closure. Paclitaxel 20 mg/m2 in 1L of 1.5 hetastarch solution was administered into the peritoneal space on postoperative day one through five as EPIC.
2.4. Study definition
Peritoneal Cancer Index (PCI score) was used intraoperatively to assess the extent of peritoneal metastases at the time of surgical exploration of the abdomen and pelvis (Harmon and Sugarbaker, 2005). This assessment determines the size of peritoneal nodules in 13 abdominopelvic regions within the open abdomen at the time of cytoreductive surgery. The score ranges from 0 (no visible disease) to 39 (tumor greater than 5 cm in all abdominopelvic regions) (Harmon and Sugarbaker, 2005). The completeness of cytoreduction score was determined after the cytoreductive surgery was completed. Complete cytoreduction was indicated by CC-0; there was no peritoneal metastases visualized within the operative field at the completion of the surgical procedure. CC-1 indicated nodules persisting after cytoreduction less than 2.5 cm, CC-2 indicated nodules between 2.5 and 5 cm and CC-3 indicated nodules greater than 5 cm or unresectable tumor nodule at any site within the abdomen or pelvis (Harmon and Sugarbaker, 2005). Revised Response Evaluation Criteria in Solid Tumours (RECIST) guideline was used to assess the change in tumor burden as the clinical evaluation (Therasse et al., 2000). Clavien-Dindo classification was used to assess postoperative complications (Dindo et al., 2004). Grade 1 included minor risk events not requiring therapy, grade 2 complications were defined as adverse events that required pharmacologic intervention. Grade 3 complications were defined as complications requiring surgical, endoscopic or radiologic intervention. Grade 4 complication indicated a life-threatening complication and a grade 5 indicated death of a patient (Dindo et al., 2004).
2.5. Statistical consideration
Continuous variables were expressed with mean and standard deviation or median and range as appropriate. Categorical variables were expressed with number and percent proportion. Standard descriptive analysis was performed for this case series.
3. Results
3.1. Tumor characteristics and treatment history
Six women met the inclusion criteria and their tumor characteristics and treatment histories are summarized in Table 1. The mean age at the initial surgery with morcellation was 45 years (range 40–59), and 67% (n = 4) were younger than 50 years of age. All of them had either hysterectomy or supracervical hysterectomy with morcellation. Power morcellation technique was used for patient 1. Scalpel was used for morcellation in patient 3. No detail regarding the morcellation technique was available among other patients. Patient 1 and 2 were referred for CRS followed by HIPEC and EPIC for primary treatment after the diagnosis of morcellated leiomyosarcoma was made. Patient 3 had a robotic-assisted vaginal hysterectomy and the specimen was morcellated at the vagina. She underwent a diagnostic laparoscopy 17 days after the pathological review which revealed two nodules of metastatic leiomyosarcoma. Post laparoscopy, she received two courses of gemcitabine and paclitaxel. The regimen was changed to doxorubicin and ifosfamide plus mesna after progression of disease was observed. After completion of 5 cycles of the second regimen, she was referred to our institution for CRS plus HIPEC and EPIC. Patient 4 underwent a laparoscopic supracervical hysterectomy with morcellation and the pathology revealed that she had leiomyosarcoma. Postoperatively, she completed gemcitabine and docetaxel. Nine months after the initial surgery, she underwent exploratory laparotomy, trachelectomy and bilateral salpingo-oophorectomy for multiple nodules in the abdomen. She was referred to our institution for CRS plus HIPEC and EPIC after nodules increased in size to a maximum dimension of 17 cm. Patient 5 was diagnosed with leiomyomas after total laparoscopic hysterectomy with morcellation. Three years after the surgery, the patient was found to have a mass at right umbilical ligament that was removed via laparoscopic technique. The pathology showed leiomyomas. Four years after she was found to have multiple masses up to 10 cm throughout her abdominal cavity and pelvis. At this time she was given a diagnosis of leiomyomatosis peritonealis. She was referred to our institution after she underwent CT-guided biopsy, which again showed leiomyoma. Patient 6 had a hysterectomy with morcellation at age of 57. The pathology revealed leiomyosarcoma. Two years after the initial surgery, she underwent debulking surgery followed by adjuvant systemic chemotherapy. Details for both debulking surgery and chemotherapy were not available. She did not have any evidence of disease for 2 years then had recurrence including a 14 × 13 × 30 cm pelvic mass, which caused small bowel obstruction and prompted referral to our institution.
Table 1.
Tumor characteristics and treatment history (CRS = cytoreductive surgery, HIPEC = hyperthermic intraperitoneal chemotherapy, EPIC = early postoperative intraperitoneal chemotherapy).
| Patient No. | Age at initial surgery | Initial surgery with morcellation | Pathology | Interval from morcellation to the time of cytoreductive surgery | Indication for reoperative surgery | Surgery prior to CRS plus HIPEC and EPIC | Chemotherapy prior to HIPEC plus CRS |
|---|---|---|---|---|---|---|---|
| 1 | 46 | Supracervical hysterectomy with morcellation | Leiomyosarcoma | 28 days | Staging | None | None |
| 2 | 40 | Laparoscopic hysterectomy with morcellation | Leiomyosarcoma | 64 days | Staging | None | None |
| 3 | 59 | Robotic-assisted vaginal hysterectomy with morcellation | Leiomyosarcoma | 8 months | Staging | Diagnostic laparoscopy | 1) Gemcitabine/Paclitaxel, 2) Doxorubicin/Ifosfamide/Mesna |
| 4 | 44 | Laparoscopic supracervical hysterectomy with morcellation | Leiomyosarcoma | 26 months | Recurrence | Ex-lap, trachelectomy, BSO | 1) Gemcitabine/Docetaxel, 2) Ifosamide |
| 5 | 43 | Laparoscopic hysterectomy with morcellation | Leiomyomatosis peritonealis | 7 years | Recurrence | 1) Diagnostic laparoscopy, laparoscopic myomectomy, 2) CT guided biopsy | None |
| 6 | 57 | Hysterectomy with morcellation | Leiomyosarcoma | 5 years | Recurrence | debulking surgery | Systemic chemotherapy (detail unknown) |
3.2. Prognostic indicators and perioperative chemotherapy
Five patients underwent CRS followed by HIPEC and EPIC. A single patient had CRS and HIPEC. Mean age at CRS followed by HIPEC was 49 years (range 40–62) and mean interval from the initial surgery was 17 months (range 1 month to 7 years) (Table 2). The mean PCI score was 18 (range 0–20). CC score was 0 in all cases. Cisplatin, doxorubicin and ifosfamide were used for all 6 patients as the HIPEC regimen. All except patient 5 received EPIC with paclitaxel for 5 days after the surgery. The resections required to achieve CC-0 status are summarized in Table 3.
Table 2.
Prognostic indicators and perioperative chemotherapy (CRS = cytoreductive surgery, HIPEC = hyperthermic intraperitoneal chemotherapy, EPIC = early postoperative intraperitoneal chemotherapy).
| Patient No. | Preoperative CT/MRI | Age at CRS plus HIPEC and EPIC | Interval from the initial surgery (month) | Peritoneal Cancer Index (PCI) | CRS (see Table 3 for details) | Completeness of cytoreduction (CC score) | HIPEC regimen | EPIC |
|---|---|---|---|---|---|---|---|---|
| 1 | Negative | 46 | 1 | 0 | Y | 0 | cisplatin/doxorubicin/ifosfamide | Paclitaxel x5 days |
| 2 | Solitary nodule | 40 | 2 | 2 | Y | 0 | cisplatin/doxorubicin/ifosfamide | Paclitaxel x5 days |
| 3 | Progressive disease | 59 | 8 | 18 | Y | 0 | cisplatin/doxorubicin/ifosfamide | Paclitaxel x5 days |
| 4 | Progressive disease | 46 | 26 | 18 | Y | 0 | cisplatin/doxorubicin/ifosfamide | Paclitaxel x5 days |
| 5 | Progressive disease | 52 | 7 years | 20 | Y | 0 | cisplatin/doxorubicin/ifosfamide | None |
| 6 | Progressive disease | 62 | 62 | 20 | Y | 0 | cisplatin/doxorubicin/ifosfamide | Paclitaxel x5 days |
Table 3.
Cytoreductive surgical procedures.
| Procedures | Patient No. |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Excision of tumor at laparoscopy port site | X | X | ||||
| Excision of tumor at prior abdominal incision | X | X | X | X | ||
| Exploratory laparotomy | X | X | X | X | X | X |
| Greater omentectomy | X | X | X | X | X | X |
| lesser omentectomy | X | X | ||||
| Pelvic peritonectomy | X | X | X | X | X | X |
| Resection of iliacus muscle and psoas muscle | X | |||||
| Right upper quadrant peritonectomy | X | |||||
| Cholecystectomy | X | X | X | X | X | X |
| Resection of tumor mass > 10 cm | X | X | ||||
| Pelvic sidewall dissection | X | X | ||||
| Resection of apex of vagina | X | X | ||||
| Cytoreduction of small bowel and bowel mesentery | X | X | ||||
| Appendectomy | X | X | X | X | X | X |
| Liver resection | X | |||||
| Ureterolysis | X | X | ||||
| Small bowel resection with anastomosis | X | |||||
| Colectomy with anastomosis | X | |||||
| Bilateral salpingo-oophorectomy | X | X | X | |||
3.3. Patient characteristics, intra- and postoperative data and length of stay
The mean BMI was 26 (range 21–32), the mean length of surgery was 8.5 h (range 7–11), the mean estimated blood loss was 600 ml (range 400–3000), the mean number of red blood cell (RBC) transfusion was 2 (range 0–7), the mean number of fresh frozen plasma (FFP) transfusion was 0 (range 0–4), the mean length of ICU stay was 3 days (range 2–4), and the mean length of hospital stay after the surgery was 15 days (range 9–56) (Table 4). Prior to CRS, patient 6 had a small bowel obstruction caused by a 10 cm abdominal recurrent mass. Postoperative complications included Clavien-Dindo grade II deep vein thrombosis and portal vein thrombosis, grade II pneumonia and grade III hemoperitoneum. Her hospital stay was 54 days.
Table 4.
Patient characteristics, intra- and postoperative data and length of stay (BMI = body mass index, EBL = estimated blood loss, RBC = red blood cells, FFP = fresh frozen plasma, ICU = intensive care unit, DVT = deep vein thromobosis).
| Patient No. | Medical history | BMI | Length of surgery (hours) | EBL (ml) | RBC transfusion | FFP transfusion | Adverse event | Length of ICU stay (days) | Length of Hospital stay (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | None | 23 | 8 | 500 | 2 | 0 | None | 3 | 12 |
| 2 | None | 26 | 7 | 400 | 0 | 0 | None | 2 | 8 |
| 3 | Depression | 21 | 10 | 600 | 2 | 0 | None | 4 | 14 |
| 4 | Gastric bypass procedure | 30 | 8.5 | 3000 | 7 | 4 | None | 3 | 14 |
| 5 | Brain tumor (benign, detail unknown) | 32 | 8.5 | 600 | 0 | 0 | None | 2 | 17 |
| 6 | Hypertension, small bowel obstruction | 26 | 11 | 2000 | 4 | 4 | Grade II Penumonia, Grade II DVT, Grade III hemoperitoneum, Grade II portal vein thrombosis, | 4 | 54 |
3.4. Outcome
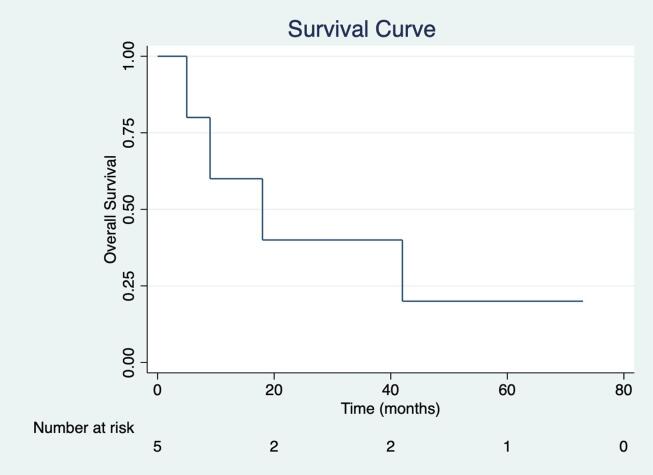
Outcome is summarized in table 5. Patient 1 did not have any metastasis at the time of cytoreductive surgery performed one month after hysterectomy plus morcellation. Patient 5 was diagnosed leiomyomatosis peritonealis postoperatively. All other patients had recurrent leiomyosarcoma. Fig. 1 shows a 3-year overall survival is 40.0% and 5-year overall survival of 20.0% with the median follow-up of 18 months (range 5 months to 73 months) among five patients.
Table 5.
Long-term outcome (CRS = cytoreductive surgery, HIPEC = hyperthermic perioperative chemotherapy, NED = no evidence of disease, DOD = dead of disease).
| Patient No. | Survival after morcellation | Survival after CRS plus HIPEC (months) | Status |
|---|---|---|---|
| 1 | 74 months | 73 | NED |
| 2 | 45 months | 42 | DOD |
| 3 | 12 months | 5 | DOD |
| 4 | 35 months | 9 | DOD |
| 5 | 25 years | 72 | NED |
| 6 | 74 months | 18 | DOD |
Fig. 1.
Overall survival of five patients who had cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) and early postoperative intraperitoneal chemotherapy (EPIC) for morcellated uterine leiomyosarcoma. Kaplan-Meier survival curve of overall survival among five patients was shown, the 3-year overall survival was 40.0% and 5-year overall survival was 20.0% with the median follow-up of 18 months (range 5–73 months).
4. Discussion
Data suggests that the primary resection surgery at the diagnosis of uLMS affects the outcome. Morcellation is a surgical technique to reduce the size of the uterus or leiomyoma to allow piecemeal removal of the specimen (Ricci et al., 2017). George et al reported that 39 women who underwent en bloc removal of uLMS via total abdominal hysterectomy had longer recurrence-free survival rates compared to 19 women who underwent myomectomy with any morcellation technique (10.8 months vs 39.6 months, p = 0.002). Also, there was prolonged survival (hazard ratio, 3.18; 95% confidence interval, 1.5–6.8; p = 0.003) (George et al., 2014). Raine-Bennett et al. reported that morcellation is associated with high risk for death at one year after the diagnosis compared to the patients without morcellation (5.12, 95% CI 1.33–19.76, p = 0.02) (Raine-Bennett et al., 2016). Perri et al. reported that 21 women with uLMS who underwent abdominal hysterectomy had better overall survival compared with 16 women who did not have en bloc surgery (OR 2.8, 95% CI, 1.02–7.67) (Perri et al., 2009). Furthermore, Oduyebo et al. reported that 28.5% (two out of seven) patients with presumed stage I uLMS had disseminated intraperitoneal disease detected at immediate surgical re-exporation (Oduyebo et al., 2014). Thus, when uLMS is diagnosed incidentally following resection with morcellation performed for presumed benign disease, prognosis is worse. Umesaki and coworkers recommended imaging of the chest, abdomen and pelvis to rule out metastatic disease prior to the completion of hysterectomy (Umesaki et al., 2001). Even though peritoneal metastases are noted, cytoreductive surgery should be strongly recommended as a complete cytoreduction is associated with better median progression-free survival (14.2 versus 6.8 months, p = 0.002) and median overall survival (31.9 versus 20.2 months, p = 0.04) in patients newly diagnosed with metastatic uLMS (Leitao et al., 2012).
Hyperthermia is defined as the temperatures of 41℃ or higher, which can cause vasodilation of the vasculature within the tumor microenvironment, directly induce tumor apoptosis via impaired DNA repair, denaturation of proteins, inhibition of oxidative mechanism and increase the penetration of chemotherapy at the peritoneal surface and thus increase the sensitivity of the cancer to chemotherapy (Sugarbaker and Van der Speeten, 2016, van de Vaart et al., 1998). Intraperitoneal administration can maximize drug delivery to the tumor, as pharmacokinetic studies have demonstrated that intraperitoneal administration of cisplatin, paclitaxel, carboplatin, and docetaxel results in high peritoneal-to-plasma ratios compared to intravenous administration of these agents (Sugarbaker and Van der Speeten, 2016).
There is emerging evidence to support HIPEC as a treatment option for epithelial ovarian cancer, however data to support HIPEC for uLMS is still anecdotal due to the rarity of disease (van Driel et al., 2018, Zivanovic et al., 2015). Sardi et al. reported that among 7 primary or recurrent uterine sarcoma patients, 3-year PFS was 38.7%, and 5-year OS was 57.1% after cytoreductive surgery and HIPEC therapy (Sardi et al., 2018). Sardi et al. also conducted a retrospective review of 36 patients from multiple international centers where HIPEC therapy was used and reported 5-year PFS was 32% and 5-year OS was 32% among patients who had complete cytoreduction plus HIPEC (Sardi et al., 2017). Those chart reviews have limitations as data included both uLMS, adenosarcoma and endometrial stromal sarcoma.
Among our five patients with uLMS, only 20.0% of patients (n = 1) survived without evidence of disease at 73 month after the CRS followed by HIPEC/EPIC. The median length of survival was 18 months (range 5–73). Although these data do not exceed that reported in the literature, 3 out of 5 patients already had large volume disease progression after morcellation and prior to CRS plus HIPEC and EPIC. Also, morcellation may add not only to intraperitoneal disease dissemination but also to systemic dissemination. Although the patient with the longest survival’s PCI was 0 at the time of surgery, Early referral after diagnosis for CRS and HIPEC plus EPIC may improve the prognosis and reduce morbidity. It is shown that PCI, the extent of disease within the abdomen and pelvis increased as the time interval between the initial surgery and CRS and HIPEC increased. Regarding the HIPEC regimen, we used cisplatin 50 mg/m2, doxorubicin 15 mg/m2 and ifosfamide 1300 mg/m2 as it has been used safely in our institution. This regimen showed better 3 years disease-free survival and 3-year overall survival if administered intravenously with radiation (Pautier et al., 2013).
Our patient 1, operated on within 28 days of morcellation had CRS and HIPEC with extensive resection of postoperative scar tissue within the abdomen and pelvis which included tissue at laparoscopy port site, greater omentectomy, pelvic peritonectomy, cholecystectomy, appendectomy and bilateral salpingo-oophorectomy. No sarcoma was found so that her PCI was 0 and pathology confirmed that there was no ecidence of metastasis at the time of surgery. Her early intervention and low PCI resulted in a 73 month survival free of disease. Another prolonged survivor, patient 2, had a low PCI of 2 and a short interval from morcellation to CRS and HIPEC. Her survival was 45 months prior to death from disease. Our patients 3, 4 and 6 had large volume disease recurrence after morcellation and prior to CRS with perioperative chemotherapy. A high PCI (18, 18, and 20) in combination with an aggressive tumor biology is, in the peritoneal metastases literature, almost always associated with palliative benefit but an absence of long-term survival.
5. Conclusion
Our case series suggests that CRS, HIPEC and EPIC is safely performed for uLMS in experienced institutions. Although further studies are needed, CRS and HIPEC plus EPIC followed by adjuvant systemic chemotherapy may be considered an option for treatment among patients with post morcellated uLMS.
Credit authorship contribution statement
Maya Yasukawa: Conceptualization, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Louis A. Dainty: Investigation, Writing - review & editing. Paul H. Sugarbaker: Conceptualization, Data curation, Formal analysis, Investigation, Writing - review & editing.
Declaration of Competing Interest
All authors declare that there is no conflict of interest.
References
- D'Angelo E., Prat J. Uterine sarcomas: a review. Gynecol. Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein M.H., Barakat R.R., Chi D.S. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int. J. Gynecol. Cancer. 2008;18:1065–1070. doi: 10.1111/j.1525-1438.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- George S., Barysauskas C., Serrano C. Retrospective cohort study evaluating the impact of intraperitoneal morcellation on outcomes of localized uterine leiomyosarcoma. Cancer. 2014;120:3154–3158. doi: 10.1002/cncr.28844. [DOI] [PubMed] [Google Scholar]
- Harmon R.L., Sugarbaker P.H. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int. Semin. Surg. Oncol. 2005;2:3. doi: 10.1186/1477-7800-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp D.S., Shin J.Y., Chan J.K. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer. 2008;112:820–830. doi: 10.1002/cncr.23245. [DOI] [PubMed] [Google Scholar]
- Leitao M.M., Jr., Zivanovic O., Chi D.S. Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecol. Oncol. 2012;125:409–413. doi: 10.1016/j.ygyno.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Lieng M., Berner E., Busund B. Risk of morcellation of uterine leiomyosarcomas in laparoscopic supracervical hysterectomy and laparoscopic myomectomy, a retrospective trial including 4791 women. J. Minim. Invasive Gynecol. 2015;22:410–414. doi: 10.1016/j.jmig.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Network NCC, 2020. NCCN Guidelines Version 1.2020 Uterine Neoplasms.
- Oduyebo T., Rauh-Hain A.J., Meserve E.E. The value of re-exploration in patients with inadvertently morcellated uterine sarcoma. Gynecol. Oncol. 2014;132:360–365. doi: 10.1016/j.ygyno.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Parker W.H., Fu Y.S., Berek J.S. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet. Gynecol. 1994;83:414–418. [PubMed] [Google Scholar]
- Pautier P., Floquet A., Gladieff L. A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study). A study of the French Sarcoma Group. Ann. Oncol. 2013;24:1099–1104. doi: 10.1093/annonc/mds545. [DOI] [PubMed] [Google Scholar]
- Perri T., Korach J., Sadetzki S., Oberman B., Fridman E., Ben-Baruch G. Uterine leiomyosarcoma: does the primary surgical procedure matter? Int. J. Gynecol. Cancer. 2009;19:257–260. doi: 10.1111/IGC.0b013e31819a1f8f. [DOI] [PubMed] [Google Scholar]
- Raine-Bennett T., Tucker L.Y., Zaritsky E. Occult Uterine Sarcoma and Leiomyosarcoma: Incidence of and Survival Associated With Morcellation. Obstet. Gynecol. 2016;127:29–39. doi: 10.1097/AOG.0000000000001187. [DOI] [PubMed] [Google Scholar]
- Ricci S., Stone R.L., Fader A.N. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol. Oncol. 2017;145:208–216. doi: 10.1016/j.ygyno.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Roberts M.E., Aynardi J.T., Chu C.S. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol. Oncol. 2018;151:562–572. doi: 10.1016/j.ygyno.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Sagae S., Yamashita K., Ishioka S. Preoperative diagnosis and treatment results in 106 patients with uterine sarcoma in Hokkaido, Japan. Oncology. 2004;67:33–39. doi: 10.1159/000080283. [DOI] [PubMed] [Google Scholar]
- Sardi A., Sipok A., Baratti D. Multi-institutional study of peritoneal sarcomatosis from uterine sarcoma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2017;43:2170–2177. doi: 10.1016/j.ejso.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Sardi A., Munoz-Zuluaga C.A., Sittig M., Diaz-Montes T. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in seven patients with peritoneal sarcomatosis from uterine sarcoma. Clin. Case Rep. 2018;6:1142–1152. doi: 10.1002/ccr3.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarbaker P., Ihemelandu C., Bijelic L. Cytoreductive Surgery and HIPEC as a Treatment Option for Laparoscopic Resection of Uterine Leiomyosarcoma with Morcellation: Early Results. Ann. Surg. Oncol. 2016;23:1501–1507. doi: 10.1245/s10434-015-4960-y. [DOI] [PubMed] [Google Scholar]
- Sugarbaker P.H., Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J. Gastrointest. Oncol. 2016;7:29–44. doi: 10.3978/j.issn.2078-6891.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Toro J.R., Travis L.B., Wu H.J., Zhu K., Fletcher C.D., Devesa S.S. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int. J. Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- Umesaki N., Tanaka T., Miyama M. Positron emission tomography with (18)F-fluorodeoxyglucose of uterine sarcoma: a comparison with magnetic resonance imaging and power Doppler imaging. Gynecol. Oncol. 2001;80:372–377. doi: 10.1006/gyno.2000.6081. [DOI] [PubMed] [Google Scholar]
- van de Vaart P.J., van der Vange N., Zoetmulder F.A. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur. J. Cancer. 1998;34:148–154. doi: 10.1016/s0959-8049(97)00370-5. [DOI] [PubMed] [Google Scholar]
- van Driel W.J., Koole S.N., Sikorska K. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- Zivanovic O., Abramian A., Kullmann M. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int. J. Cancer. 2015;136:699–708. doi: 10.1002/ijc.29011. [DOI] [PubMed] [Google Scholar]