Abstract
The liver is a key organ that performs diverse functions such as metabolic processing of nutrients or disposal of dangerous substances (xenobiotics). Accordingly, it seems to be protected by several mechanisms throughout the life of organisms, one of which is compensatory hyperplasia, also known as liver regeneration. This review is a recapitulation of the scientific reports describing the different ways in which the various classes of vertebrates deal with liver injuries, where since mammals have an improved molecular toolkit, exhibit optimized regeneration of the liver compared to lower vertebrates. The main molecules involved in the compensatory process, such as proinflammatory and inhibitory cytokines, are analyzed across vertebrates with an evolutionary perspective. In addition, the possible significance of this mechanism is discussed in the context of the long life span of vertebrates, especially in the case of mammals.
Keywords: Liver regeneration, Compensatory hyperplasia, Vertebrates, Cytokines, Evolution
Liver regeneration, Compensatory hyperplasia, Vertebrates, Cytokines, Evolution.
1. Introduction
In the last decade, over five hundred articles related to different aspects of the liver regeneration process are added to databases such as PubMed every year. This review is motivated by the question concerning what might be the evolutionary significance of liver regeneration. Although the literature reveals a wide range of views, it is not easy to find this kind of approach. Undoubtedly, scientists are interested in uncovering the deepest secrets and determining the mechanisms involved in the complex process of liver regeneration, but as a biologist, I am also interested in the evolutionary steps −no less complex, of course− that led to this extremely interesting and important phenomenon. Therefore, this is an attempt to put together the puzzle pieces in order to understand the main signaling molecules and the similarities among different species that possess the ability to regenerate their liver. This review focuses first on describing the main stages and concepts related to liver regeneration observed in mammals after partial hepatectomy. Then, it presents a compilation of the current knowledge regarding liver regeneration in vertebrate classes other than mammals and the evolutionary events necessary to achieve the sophisticated toolkit for the signaling of liver regeneration in mammals. The present work, also addresses the cellular mechanisms used in order to regenerate the liver, and it concludes with a discussion of the overall purpose of liver regeneration and the intrinsic characteristics that explain the robustness of the liver.
2. Liver regeneration is actually a compensatory hyperplasia process
Greek mythology alludes to the possibility of an eternal punishment, when Zeus condemns Prometheus to be chained to a rock in the Caucasus Mountains for stealing fire and giving it to humanity. On the rock he would be exposed to attacks by eagles directly on his liver. Due to the high clonogenic potential of hepatocytes, it has been reported that the constant renewal of liver tissue is possible (Michalopoulos and DeFrances, 1997). The first formal works studying what kind of processes are involved in liver regeneration date back to the end of the nineteenth century (Felekouras et al., 2010). Some of the contributions made to our current knowledge are described below.
The most accepted model for the study of liver regeneration in rats is the 70% partial hepatectomy (PH) where 2/3 of the liver tissue, represented by the two largest lobes, are removed (Higgins and Anderson, 1931). Nancy Bucher discovered using parabiotic rats, where the signals that address liver regeneration come from (Bucher et al., 1951). In mice and rats, after 5–7 days the liver is fully regenerated through a combination of the mechanisms of hypertrophy (increased cell size) and hyperplasia (increased number of cells). Hypertrophy is observed early after hepatectomy or when the resection of liver involves only 30%; it seems to be sufficient to recover the liver mass (Miyaoka et al., 2012; Miyaoka and Miyajima, 2013). The observed hypertrophy in mice after 70% PH is partially explained by the transient steatosis produced by the cytoplasmic accumulation of triglycerides in the hepatocytes. In contrast, experiments in rats previously transplanted with hepatocytes expressing GFP suggest that cell hypertrophy plays a secondary role in liver regeneration (Marongiu et al., 2017).
One of the advantages of the PH model is that life is not compromised, unlike when hepatic tissue removal reaches 90%. The property of liver tissue as being basically quiescent has been recognized for many years (Brues and Marble, 1937), and it was experimentally determined that hepatocytes, the most abundant cell type in the liver, show a low response to mitogens in vitro. In this regard, the PH model allows for the study of hepatocytes that synchronously enter the G1 stage of the cell cycle, and it does not involve acute inflammatory or necrotic processes (Michalopoulous, 2010).
To date, it is well accepted that “compensatory hyperplasia” is a term that more accurately describes what we previously referred to as “liver regeneration” (Columbano and Shinosuka, 1996). Liver size is regulated by a hepatostat, where a proportion close to 3% of the body mass in mammals is strictly maintained; thus, when a loss of hepatic mass occurs, all cell types change their quiescent stage to an active stage, which makes them able to divide and recover the liver's size, although the initial structure comprising different lobes is not recovered. It is also clear that compensatory hyperplasia is completely different respect from regeneration by animals that have lost a limb. Nevertheless, the term “liver regeneration” has been historically used in the literature, so I will do the same in this review unless otherwise indicated.
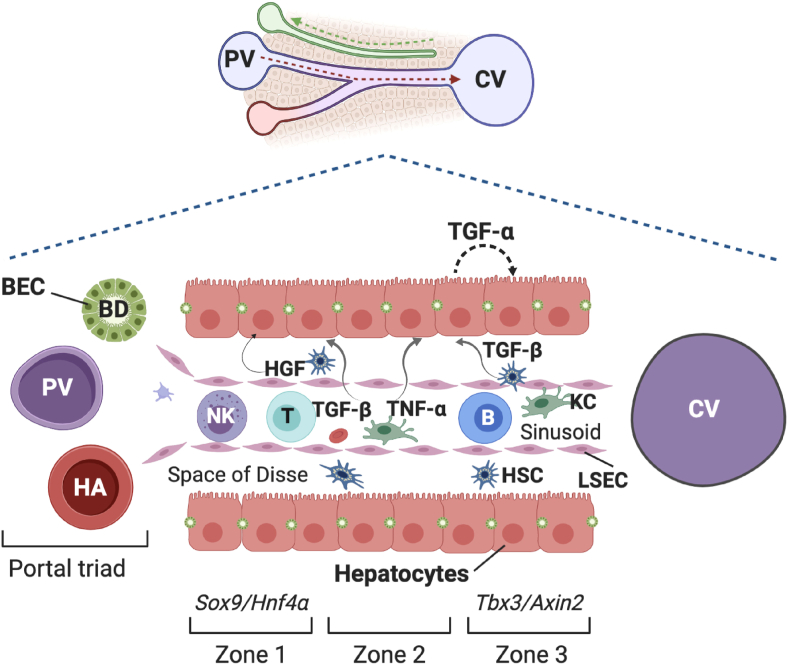
Liver tissue comprises parenchymal cells (hepatocytes) and non-parenchymal cells, where hepatic stellate cells (HSCs), which under normal conditions store droplets of vitamin A, Kupffer cells (KCs, resident macrophages of the liver), liver sinusoidal endothelial cells (LSECs), lymphocytes, and biliary epithelial cells (BECs) are included. The communication among these cell types is basic throughout the process of regeneration, since hepatocytes can respond to molecules that they themselves produce (autocrine communication) or they can respond to cytokines produced by HSCs or KCs (paracrine communication), as illustrated in Figure 1.
Figure 1.
Communication among different hepatic cell types mediating signals to achieve compensatory hyperplasia after partial hepatectomy. During the priming phase, Kupffer cells (KCs) produce TNF-α and IL-6, which through paracrine communication stimulate hepatocytes to respond to growth factors such as the hepatocyte growth factor (HGF) and proliferate. Hepatocytes respond by autocrine mechanisms to TGF-α and HGF in the proliferative phase. Finally, TGF-β synthesized by hepatic stellate cells (HSCs) and KCs stimulate the hepatocytes to counteract the effects of TGF-α and return to the G0 phase. Liver sinusoidal endothelial cells (LSECs) also communicate through paracrine pathways with hepatocytes and HSCs. Different types of lymphocytes are shown in the sinusoid (T and B cells from the adaptive immune system; and Natural killer cells -NK-, from the innate immune system). The portal triad comprises the bile duct (BD) composed by biliary epithelial cells (BECs), the hepatic artery (HA), and the portal vein (PV). Based on functional/metabolic differences and specific markers, hepatocytes are grouped in zones 1 to 3.
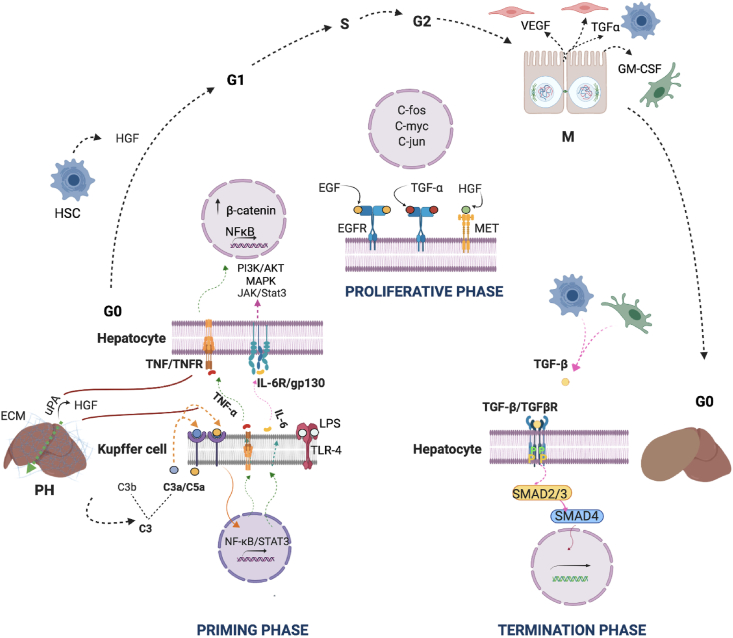
Liver regeneration involves a complex network where diverse signaling pathways from different cell types regulate the precise control of genes encoding transcription factors needed to recover the hepatic mass. The stages of the process after PH have been extensively described in excellent reviews (Taub, 2004; Fausto et al., 2006; Michalopoulous, 2010; Stanger, 2015; Mao et al., 2014; Tao et al., 2017; Michalopoulos and Bushan, 2021). The priming phase is the switch of the quiescent liver tissue to a stage where all hepatic cell types become activated and able to respond to growth factors. Only 1 min after PH, the urokinase-type plasminogen activator increases its activity and then activates the hepatocyte growth factor (HGF) in the extracellular matrix, which is released to the peripheral blood approximately 1 h later (Mars et al., 1995; Lindroos et al., 1991). In the hepatocyte, a few minutes after PH, the Wnt/β-catenin pathway promotes increased levels and translocation of β-catenin to the nucleus, and in cooperation with Yap and Hedgehog pathways, regulates proliferation, survival, and differentiation (Gilgenkratz and Collin de l’Hortet, 2018). There is evidence that several effector molecules of the complement system and lipopolysaccharide activate Kupffer cells to release the proinflammatory cytokines tumor necrosis factor (TNF-α) and interleukin-6 (IL-6), which both reach high levels in the peripheral blood within 2–5 h after PH (Strey et al., 2003; De Angelis et al., 2006). In the hepatocyte, TNF-α activates the transcription factor NF-kB; while IL-6 activates STAT3, increasing its own response to growth factors such as HGF (through MET, the HGF receptor), transforming growth factor (TGF-α, which is also a ligand for EGFR), and epidermal growth factor (EGF, and EGFR its receptor), among others. Once the hepatocytes respond to growth factors and early genes become activated, they enter the S phase of the cell cycle (S, for synthesis of DNA), where protein synthesis is increased and mitosis occurs. When hepatocytes proliferate, they produce growth factors such as the vascular endothelial growth factor (VEGF), fibroblast growth factors 1 and 2 (FGF1; FGF2), which induce mitosis in other hepatic cells (Michalopoulos and Bushan, 2021). In the final phase, inhibitory cytokines, mainly of the transforming growth factor beta (TGF-β) superfamily, produced in the liver by hepatocytes and non-parenchymal cells, and also outside of the liver by platelets and in the spleen (Tao et al., 2017) counteract the TGF-α′s effects and return the cells to the G0 phase (Figure 2). However, the relationship between TGF-β and the termination of liver regeneration is not yet clear (Michalopoulos and Bushan, 2021). In addition to the different cytokines and growth factors signaling the entire regeneration process, other relevant molecules such as cholesterol and signaling lipids play important roles from the first stages of liver regeneration (Delgado-Coello et al., 2011).
Figure 2.
Main events of the regeneration phases after partial hepatectomy (PH). At the priming stage, soon after PH the urokinase-type plasminogen activator (uPA) increases its activity and then activates the hepatocyte growth factor (HGF) in the extracellular matrix (ECM) and it is released into peripheral blood. The complement system is activated through proteolysis of C3 and it prompts Kupffer cells to release TNF-α and IL-6 which after binding to their receptors activate several signaling pathways (JAK/STAT, MAPK, and PI3/AKT) and hepatocytes become responsive to several growth factors (HGF, TGF-α, and EGF) making the transition to the G1 phase of the cell cycle (proliferative phase) that permit mitogenesis. When hepatocytes proliferate, they produce growth factors that are mitogenic for other hepatic cell types. Once the liver tissue reaches its original mass, in the termination phase, inhibitory cytokines of the TGF-β superfamily (TGF-β and activins) produced by hepatic stellate cells (HSCs) and Kupffer cells, bind their receptors in hepatocytes to phosphorylate and activate SMADs, which in this state bind to SMAD4, forming a complex that is translocated to the nucleus where it activates the transcription of another set of target genes, which facilitates the return of the hepatic cells to the G0 phase and finally, ECM and liver vasculature are reestablished.
3. Liver regeneration in non-mammalian vertebrates
550 million years is the approximate time range separating the common ancestor from the evolutionary steps that developed invertebrate and vertebrate species (Rosselló et al., 2013). Although this period of time may be quite short on the geological time scale, many important events led first to the origin of the vertebrate liver (reviewed by Subbotin, 2017) and all along the way to the complex liver regeneration process we can observe in higher vertebrates.
In reference to the origin of the liver, the midgut diverticulum of amphioxus or lancelets, which diverged from vertebrates 520 million years ago as precursors of the first vertebrates, has also been suggested as the homologous precursor of the vertebrate liver (Subbotin, 2017). Previous findings show that CCl4, used to study hepatic damage associated with the fibrotic process in mammals, is able to affect digestive caecum in the amphioxus, suggesting that this structure might be homologous to the liver of rosy barb fish in which CCl4 also showed effects (Bhattacharya et al., 2008).
Vertebrates comprise five classes of animals, where fish, amphibians, and reptiles belong to the poikilotherm or ectothermic group (formerly referred to as “cold-blooded”) which exhibits a variable and environment-dependent body temperature. Birds and mammals are endotherms, and therefore almost always able to keep their internal body temperature constant. Among others advantages, endothermy provided a strict control of metabolism, high muscular power output, and fast growth (Clarke and Pörtner, 2010). Based on a large body of evidence, it seems possible that an increase in body temperature might be the mechanism through which enhanced aerobic capacity was achieved.
Although it has been claimed that liver regeneration occurs in all vertebrates from fish to humans, most of the basic research is performed in mammals and the literature covering this phenomenon in other vertebrate species, is still scarce. However, the following section offers a brief account of the scientific reports that describe the structure of the normal liver, and the liver regeneration process in non-mammalian vertebrates.
3.1. Liver regeneration in fish
A comprehensive study carried out in two hundred fish species shows that they do not share a common structure; the authors distinguish the following arrangements of the hepatocytes and sinusoids: cord-like (hepatocytes in monolayers), tubular (double-layered hepatocytes), and solid (multi-layered hepatocytes) (Akiyoshi and Inoue, 2004). Phylogenetic analysis of these structures suggests that solid and tubular arrangements correspond to primitive species, while those with a higher phylogenic category show a cord-like disposition similar to the mammalian arrangement (Akiyoshi and Inoue, 2004). In rainbow trout (Salmo gairdneri), whose liver shows a tubular arrangement, the regeneration caused by PH or by a bile duct ligation procedure have been morphologically described (Okihiro and Hinton, 2000). The liver structure of the zebrafish (Danio rerio) shows a different arrangement from that known in other fish and mammal species (Yao et al., 2012). The parenchyma exhibits a cord-like structure, but double-layered hepatocytes are distributed around a central vein and resident macrophages are interspersed between hepatocytes (Cheng et al., 2020). The zebrafish has attracted attention because ca. 70% of human genes have orthologue representatives in this species (Howe et al., 2013), and PH has also been successfully performed (Sadler et al., 2007; Cox and Goessling, 2015). Given the trilobar structure present in the zebrafish, only the ventral lobe (1/3) is removed in this version of the PH model, and in contrast to that which is observed in mammals, the original lobular structure is recovered after 7 days. As in mammals, the remaining hepatocytes are in charge of recovering the liver tissue to 100% after hepatectomy (Kan et al., 2009).
3.2. Liver regeneration in amphibians
Both in amphibians and reptiles, the capacity for epimorphic regeneration related to the restitution of specific body regions is much better understood (Galis et al., 2003; Zhao et al., 2016; Alibardi, 2010), and this process is virtually absent in birds and mammals.
The amphibian class comprises the orders Gymnophiona (worm-like species), Caudata or Urodele (newts and salamanders), and Anura (frogs). In general, the liver in amphibians takes the form of the body shape, and most of them have a bilobate liver, although there are species with three or up to five lobes and the hepatocyte-sinusoidal structure can include, one, two, or several layers of hepatocytes (Akiyoshi and Inoue, 2012). Interestingly, the anuran liver shows an identical arrangement to that shown by mammals, while the liver of members of the order Caudata is similar to that present in teleosts. The abundant presence of melanomacrophages where primary melanogenesis seems to take place is typical in the liver of the newt (Bernabò et al., 2014; Vaissi et al., 2017), but they have also been observed in reptiles and in some fish (Agius and Roberts, 2003).
A study performed in Xenopus laevis can be found in the literature, where the goal was to analyze the effect of the production of the adrenocorticotropic hormone after 75% PH on the interrenal tissues of the kidneys (Fritsch et al., 1977). In this work, the experimental groups were observed from 3 up to 106 days after hepatectomy, but details regarding the liver regeneration process were not provided.
Recently, the liver structure of the axolotl (Ambistoma mexicanum) which belongs to the order Caudata, was described, and for the first time, the PH technique was developed in the axolotl (Oyashi et al., 2020). The axolotl liver displays shape variability as well as differences in color, number of multinucleated cells, and DNA content, depending on age. PH was safely performed by resecting 30% of the liver tissue. Observations for up to 30 days showed that hepatocytes proliferated with a trend towards accumulation, but the liver did not restore its original mass. The authors suggest a “compensatory congestion” mechanism, in which ERK signaling is involved in hepatocyte proliferation.
A peculiar phenomenon known as “emergency regeneration”, is observed in green frogs and the Italian newt, that structurally and functionally restores liver tissue after necrotic injuries caused by different hepatotoxic compounds (Bernabò et al., 2014; Akulenko, 2015). The liver shows the presence of fat droplets, degradation of connective tissue, anisocytosis, among others effects, which are comparable to the observations reported in liver regeneration after partial hepatectomy in other species of frog (Karapetian and Dzhivanian, 2006) and in mammals.
3.3. Liver regeneration in reptiles
As for the histology of reptiles, there is little information available, but it is known that they show different liver arrangements, even in members of the same clade. For example, in snakes the liver adopts the body shape, while lizard livers have a right and a left lobe. In the common lizard (Tropidurus torquatus) of Brazil, an arrangement similar to that of mammals is described, where highly vacuolated hepatocytes are located in cords following the sinusoids and those that are near the centrolobular vein show a radial arrangement (Firmiano et al., 2011). The liver parenchyma also shows many melanomacrophages. In the Nile monitor, it has been observed that the right lobe is the largest lobe, with few melanomacrophages, and the hepatocytes are arranged in tubules surrounded by a sinuous network of sinusoids (Ahmed et al., 2018), as reported by others in the liver of freshwater and desert turtles (Moura et al., 2009; Kassab et al., 2009). Interestingly, the liver of the freshwater turtle has four lobes and melanomacrophages are present, while the terrestrial tortoise has two lobes and has no melanomacrophages.
The garden lizard has been used as a model to study metabolic parameters after being partially hepatectomized in the context of the possible influence of age in the process (Rao and Patnaik, 1971a, 1971b, 1973). These works used short observation periods (24 h), so the authors did not document whether the liver recovered its original size and shape.
Several reports describe the surgical procedures performed in snakes where coelomic masses were removed by partial hepatectomy (Lawton, 1998; Knotek et al., 2012). According to their veterinary approach, these works report the adequate recovery of the snakes after 5–7 months.
In Python species, during their long periods of starvation, the metabolism is deeply downregulated and several organs including the liver, remain atrophied. However, once the snake feeds, post-feeding organ regeneration occurs (Secor and Diamond, 2000; Andrew et al., 2017). Interestingly, in all organs analyzed only the gene encoding coagulation factor X showed significant changes. At four days after feeding, the liver was the last organ to reset to fasting status (Andrew et al., 2017). In the regeneration of heart tissue, it seems that only hypertrophic mechanisms are involved, while in the liver, kidney, and small intestine, hyperplasia occurs as well. In general, this phenomenon shows differences in the liver regeneration produced by different stimuli due to the activation of non-canonical pathways such as those associated with the oxidative stress response (NRF2) or growth (mTOR).
3.4. Liver regeneration in birds
The liver of avian species has two lobes, and the whole organ represents a larger proportion of body size than that of mammals. The general arrangement of hepatocytes is similar to that which is observed in mammals (Zaefarian et al., 2019).
Regarding liver regeneration in birds, there are few formal reports following the process. However, Higgins et al. (1932) reported that the liver in the adult domestic fowl did not recover to the same extent as that which occurs in mammals after ablation of the left lobe. In contrast, liver regeneration observed in young chicks was almost complete after 30 days (Kornblith and Kalman, 1964).
In 1962, a study was conducted in roosters where 10–12.5% of the liver was removed and the process was followed for 3–90 days after hepatectomy (Sidorova, 1962). Several differences were described depending on the operated lobe; also, the hypertrophy was considered to be the mechanism explaining the regeneration and it was found that the liver did not recover its original shape. It should be noted that in this work the author considered that the regenerative hypertrophy involves proliferation of cells in the remaining liver, which is contrary to the current concept (Miyaoka and Miyajima, 2013). Several groups have described the activity of relevant enzymes (Dzhivanian and Ter-Oganian, 1979) or the role of binucleate cells in the regeneration of chicken liver (Dzhivanian and Ter-Oganian, 1983). Other researchers who were focused on the activity of hepatic glycosidases have used the regenerating chicken liver as a model for comparative purposes with highly proliferative cells from different types of tumors where cells seem to grow in an uncontrolled manner (Chelibonova-Lorer et al., 1985). In the goose, the effect of insulin on the decrease of plasma triglycerides observed after hepatectomy has been reported (de Oya et al., 1976). Another approach has been to use the PH model in chickens to correlate the performance of the hepatic function through the determination of galactose clearance, where the reduction of this parameter was proportional to the liver damage of 13% (Jaensch et al., 2000).
Importantly, in the mature liver of mammals and birds, oval cells and small hepatocytes represent progenitor cells, playing a crucial role when the hepatic tissue is severely damaged by different reagents (Lemire et al., 1991; Mitaka et al., 1992; Michalopoulous et al., 2005).
4. From multicellularity to the establishment of a sophisticated signaling of liver regeneration
4.1. Conditions for the establishment of multicellularity
In order to analyze the origin of the complex molecules that signal liver regeneration, it is necessary to go back to the origin of metazoans, the group that includes all multicellular and bilaterian animals. To date, it is accepted that multicellularity has evolved several times and through independent paths in the history of life (Sebé-Pedrós et al., 2017). According to fossil recordings, the first multicellular eukaryotes date from 1,200 million years ago, while metazoans appeared ca. 650 million years ago.
Consistent with the diversity of multicellular organisms comprising plants, fungi, and animals, different mechanisms evolved in order to promote functions such as cell adhesion and communication. The toolkit for cell adhesion included extracellular glues (mainly carbohydrates), extracellular glycoproteins, and integral membrane proteins. Together with the presence of more complex proteins in membranes, a diversity of genes encoding for transcription factors developed in different multicellular lineages shaped the evolution of eukaryotes (de Mendoza et al., 2013). In fact, it has been suggested that the hypoxia inducible factor-1α (HIF-1α) made the transition from unicellular to multicellular organisms possible (Heber-Katz and Messersmith, 2018). This factor has been relevant since ancient times because the first eukaryotic single-cell organisms had to regulate high (otherwise toxic) oxygen levels made available by the first photosynthetic organisms (Heber-Katz and Messersmith, 2018). Interestingly, the expression of HIF-1α has been studied in hepatectomized rats showing that both transcripts and protein levels increase at 24 h and remain high for up to 5 days after PH (Maeno et al., 2005). Although HIF-1α is a constitutive factor, under normal O2 levels it is degraded but when a hypoxic environment is present, as its name suggests, it is overexpressed. After hepatectomy, a transient hypoxia is observed when the massive proliferation of hepatocytes exceeds the number of sinusoidal cells where blood flow is usually very active (Maeno et al., 2005). In addition to transcription factors, molecules such as growth factors and signaling intermediaries, involved since the embryonic development of vertebrates (Schoenwolf and Smith, 2000), are also relevant to the regeneration process.
The following section describes the development of the physiological bases necessary for the evolution of the articulated responses that allow liver regeneration.
4.2. Emergence of an improved immune system
One of the relevant factors associated with the regenerative ability in vertebrates is the concomitant evolution of more complex nervous and immune systems at the same time as amniotes (reptiles, birds, and mammals) changed to adapt to a terrestrial environment, losing their restitutive regeneration (Alibardi, 2019). This dramatic change is related to the loss or modification of genes involved in the control of larval stages and metamorphosis. While the nervous system became more specialized, a trend towards increased telencephalon size was observed, and the immune system was improved.
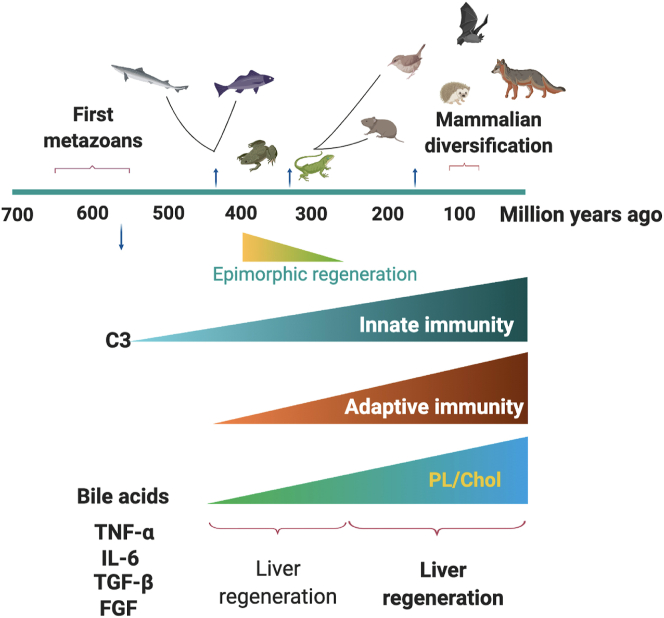
The innate immune system was the first defense mechanism that appeared more than 500 million years ago, while the adaptive immune system emerged 450 million years ago (Figure 3). C3, the main component of the complement system, is present in invertebrates as part of the innate immune system which eliminates microbes through opsonization. It has been hypothesized that a primitive version of C3, performed its functions in single-cell organisms intracellularly and on the membrane. In multicellular organisms, the mechanisms of defense were improved, thus the complement system evolved new components, such as intracellular components, proteins regulating its function, and mechanisms to secrete C3 into the surrounding milieu (Elvington et al., 2016). The family of regulators of complement activation was present in the first vertebrates (cyclostomes). When a circulatory system was developed, the liver was able to synthesize and secrete C3, protecting the intravascular space. The appearance of immunoglobulins in cartilaginous fish marked the emergence of the classical and lytic pathways of complement activation (Sunyer and Lambris, 1998). Although complement system is conserved in vertebrates, fish present a more complex complement cascade most likely in order to compensate for the lack of developed acquired immune mechanisms (Najafpour et al., 2020). In higher vertebrates, the complement system participates as effector of innate and adaptive immune responses.
Figure 3.
Summary of the evolution of vertebrates and their different mechanisms for the repair of tissues. Epimorphic regeneration is characteristic of amphibians and reptiles but it is absent in birds and mammals. Liver regeneration takes place in all vertebrate classes; however, the improved molecular toolkit present in mammals supports optimized regeneration of the liver compared to lower vertebrates. Several studies show that C3, a component of the complement system (innate immunity) as well as the cytokines TNF, IL-6, and TGF-β along with growth factors such as fibroblast growth factor (FGF) have been present since the appearance of the first metazoans (ca. 550 million years ago). In addition, it has been shown a different balance of lipids in bile acids from mammal species, with a notably high content of phosphatidylcholine and cholesterol. PL: phospholipids; CHOL: cholesterol.
As previously mentioned, the complement system plays a main role during the priming phase of liver regeneration, which will be discussed later in this work.
4.3. Evolution of signaling related to liver regeneration
The evolution of the complete set of molecules signaling for liver regeneration has not been sufficiently studied. However, work performed in different vertebrate models makes it possible to infer several interesting points. For example, the Wnt/β-catenin pathway, essential for liver homeostasis and a regulator of liver zonation, is involved in the priming phase, and now it is known that it participates in the termination of liver regeneration (Yang et al., 2015). This pathway, along with those involving bone morphogenetic proteins (BMPs) and the fibroblast growth factor (FGF), plays a role during liver regeneration in zebrafish, rodents, and humans (Kan et al., 2009; Gilgenkratz and Collin de l’Hortet, 2018). Although these findings suggest conservation of the basic pathways, it is possible to believe, as has been observed in FGF, that during evolution the ancestor molecule diverged, creating more members and more complex functions (Li, 2019).
In the priming phase of liver regeneration, some of the most significant molecules are the cytokines TNF-α and IL-6. Both cytokines show high versatility in their functions; for example, TNF can be related to cell death or to proliferation. The evolutionary analysis of TNF sequences of different vertebrates (and cartilaginous fish) shows that the most conserved regions correspond to the cell death function. Thus, some authors have hypothesized that this may be its original function (Lu et al., 2016). The comparison of the TNF and its receptor (TNFR) among invertebrate and vertebrate species, shows that they belong to old cytokines that arose ca. 550 million years ago and are highly conserved throughout evolution (Quistad et al., 2014; Lu et al., 2016). In chickens, TNF-α was recently described (Rohde et al., 2018), but TNF-like activity (Kaiser et al., 2004), ligands, and TNFR were described several years ago (Wiens and Glenney, 2011). Phylogenetic analysis of TNF-α shows that avian sequences differ from those found in fish, reptiles, and mammalian species, while TNFR1 and TNFR2 cluster with high bootstrap support with respect to the corresponding receptors described in reptiles and mammals (Rohde et al., 2018).
Several proinflammatory interleukins, have been described in fish, amphibians, birds, and mammals, but not in reptiles (Kaiser et al., 2004). However, IL-8 was later successfully cloned from a Chinese turtle species (Zhou et al., 2009). So far, 39 different interleukins have been described in mammals where they are key in the regulation of immune responses. After PH, at least 40% of approximately 100 early genes activated, respond specifically to the expression of IL-6 (Li et al., 2001). Of all the members, IL-6 and IL-31 are those that are the most variable (Gorshkova et al., 2016). In contrast to the TNF family, IL-6 seems to be poorly conserved and highly polymorphic across populations (Gorshkova et al., 2016). This plasticity might be associated with the wide range of signaling functions in which IL-6 participates. IL-6 is one of several interleukins positively selected throughout evolution and it shows the highest rate of positively selected codons (Neves et al., 2014a). In general, positive selection favoring beneficial traits tends to increase their frequency in a population, as well as their permanence through generations. In addition, coevolution events have been observed between close species of lagomorphs, where larger versions of IL-6 have been isolated (Neves et al., 2014b).
Cytokines of TGF-β superfamily involved in the termination phase of liver regeneration, share high homology across different organisms and their signaling pathways are regulated according to the physiological context (Ayyaz et al., 2017). Interestingly, TGF-β has been present since the appearance of the first metazoans, but absent in protozoans (Huminiecki et al., 2009; Zheng et al., 2018). A single TGF-β is present in lower deuterostomes (sea urchins, tunicates, and hemichordates), but not in early bilaterians (Hinck et al., 2016). The complex signaling network led by the TGF-β was relevant to specify axes defining bilateral symmetry, features of different organs and regulation of development and homeostasis. It is remarkable that three TGF-βs are found in mammals, more than 30 ligands, 5 and 7 type II and III receptors, respectively, and 8 Smad proteins (Ayyaz et al., 2017) resulting in a more complex network, that involves signaling in the intracellular and extracellular environments (Hinck et al., 2016). Gene duplication is a mechanism which can produce copies of genes in short periods of time (Magadum et al., 2013). At least two rounds of duplications have been detected in vertebrates, which seem to have occurred in several ligands or receptors of the TGF-β family.
Undoubtedly, the trend towards an increased complexity in signaling pathways and the diversification of growth factors, at least partially explains how liver regeneration has been improved in mammals.
5. Possible role of bile salts and cholesterol in the evolution of liver regeneration
The role of cholesterol and other bioactive lipids during liver regeneration has been previously reviewed (Delgado-Coello et al., 2011). At 12–24 h after PH, occurs the transitory accumulation of triglycerides and cholesterol known as steatosis, while these lipids are decreased in the serum.
In the liver, cholesterol is a precursor for the synthesis of bile acids, which contribute to the digestion and absorption of fats. In rat, mouse, and human hepatocytes, it has been widely documented that during the 24 h post-hepatectomy period, there is a transitory overload of serum and hepatic bile acids while hepatocytes display mechanisms to avoid the subsequent toxicity (Merlen et al., 2017; de Haan et al., 2018). Therefore, bile acids are considered coregulators of the priming phase of liver regeneration when they bind to nuclear receptors (farnesoid X receptor or FXR) where different signaling pathways are activated in order to inhibit the bile salt synthesis and to quickly remove bile acids from the small intestine cells (enterocytes) by stimulating the expression of a specific bile acid transporter. In FXR−/− mice (Huang et al., 2006) or when FXR is conditionally deleted in the liver (Borude et al., 2012), as observed in mice lacking different growth factors or cytokines, regeneration is delayed (Böhm et al., 2010). Interestingly, FXR−/− mice 24 h after hepatectomy, show significant increases in serum and hepatic bile acids concentration compared to normal mice (Huang et al., 2006). To date, more researchers are considering the relevant role of bile acids during liver regeneration and exploring the function of several G-protein coupled receptors involved in signaling and also activated by bile acids (van de Laarschot et al., 2016; Merlen et al., 2017).
The quantitative analysis of biliary lipids, which comprise bile salts, phospholipids, and cholesterol in a wide range of vertebrates, and cartilaginous fish, has been reported and relevant conclusions can be drawn in the context of this review (Moschetta et al., 2005) (Table 1). In mammal species, a notably high content of phosphatidylcholine and cholesterol is observed. In general terms, these data suggest that in species showing low cholesterol/bile salt ratios, cholesterol is eliminated when it is used in the synthesis of bile acids, but in mammals such as humans, cholesterol is eliminated directly (Moschetta et al., 2005). The enrichment of phospholipids and cholesterol observed in mammals may be significant from an evolutionary point of view, due to the protective role observed against cytotoxic effects produced by bile acids (Puglielli et al., 1994; Benedetti et al., 1997), and when high levels are observed in the short term after hepatectomy. However, an antagonist effect of cholesterol on the cytoprotective effects of phosphatidylcholine in primary human hepatocytes has also been reported (Ikeda et al., 2017). The significance of bile salt composition from the evolutionary point of view has been previously discussed (Hofmann et al., 2010).
Table 1.
Lipid/bile salt ratios observed in vertebrates and sharks.
| Class | Phospholipid/Bile salt | Cholesterol/Bile salt |
|---|---|---|
| Cartilaginous fish | 0.050 | 0.003 |
| Fish | 0.040 | 0.005 |
| Amphibians∗ | 0.009 | 0.0016 |
| Reptiles | 0.020 | 0.004 |
| Birds | 0.060 | 0.003 |
| Mammals: (Rodents) |
0.090 | 0.010 |
| (Primates) | 0.150 | 0.022 |
| (Humans) | 0.300 | 0.066 |
Table constructed with mean values reported by Moschetta et al. (2005). ∗For amphibians only one liver sample was considered, therefore, the lowest ratios among vertebrates cannot be considered final.
Another relevant role of cholesterol is as a structural molecule that together with other lipids, is part of dynamic regions in the cell membrane known as lipid rafts, in which many proteins are recruited to signal processes such as liver regeneration. For example, it has been reported that this kind of domains is needed in the case of the insulin receptor which has a main role in signaling for hepatocyte proliferation (Fonseca et al., 2018). A deeper analysis regarding the role of these membrane domains is far from the scope of this work, but it should be borne in mind.
6. From phenotypic fidelity to cell plasticity in the liver
The toolkit comprising a diversity of transcription factors undoubtedly evolved in parallel with the origin and evolution of metazoans, making them more complex at the same time as physiological processes were precisely signalized in time and space. Some of the mechanisms for production of new cells in order to regenerate any kind of tissue are dedifferentiation (in which differentiated cells become progenitor cell types), transdifferentiation (the process of conversion of one tissue cell into another cell type), and activation of pools of different stem cells (Zhao et al., 2016). Since these mechanisms are not present in the majority of adult mammals, they show a low regenerative ability. However, it seems that given the longer life span of vertebrates in comparison to lower species, they were supplied in the course of evolution with different mechanisms for the repair of tissues, such as liver regeneration to preserve integrity (Stanger, 2015).
Nowadays, it is widely recognized that all liver cell types under homeostatic conditions or when the liver is stimulated to regenerate after PH, are guided by phenotypic fidelity, according to which, hepatocytes produce hepatocytes, and HSCs produce HSCs (Michalopoulos and Bhushan, 2021). Thus far, the consensus is that after 70% PH in rodents or 30% PH in the zebrafish, the hepatocytes are able to replenish the liver tissue. In contrast, when the liver is exposed to a chronic or severe injury, hepatocytes and BECs (cholangiocytes) become surpassed and instead show plasticity, which means that both cells are reprogramed, showing “regenerative altruism” and they can transdifferentiate into each other, functioning as facultative stem cells (Raven et al., 2017; Schaub et al., 2018; Li et al., 2020). This alternative mechanism has been observed when the liver of zebrafish is challenged through an aggressive hepatocyte ablation by treatment with metronidazole (He et al., 2014, 2019; Khlalik et al., 2018), or in mice and rats by treatment with thioacetamide (Wallace et al., 2015; Deng et al., 2018), or in humans with chronic liver damage (Deng et al., 2018). Several years ago, a population of oval cells located in the canals of Hering was described, and it was thought that they participate in regenerating the liver when it is severely damaged. Now, lineage tracing techniques have revealed that BECs dedifferentiate into liver progenitor cells (LPCs), which are none other than the oval cells, and then, they differentiate into hepatocytes (So et al., 2020). This mechanism is known as LPC-driven liver regeneration. Thus, the current view is that stem cells do not participate in liver replenishment, and it is also thought that this would be a slow process and that cholangiocytes and hepatocytes are an abundant source of cells for liver reconstruction (Michalopoulos and Bhushan, 2021). Interestingly, it has been described the presence of proliferative stem cells expressing the receptor LGR5 (stem-cell marker) after liver injury by CCl4 treatment (Cao et al., 2017).
In this context, I concur with Cox and Goessling (2015) who established that “liver regeneration is an adaptive response to liver injury”. Conversely, liver responses to chronic injuries such as extracellular matrix overproduction and scarring are considered maladaptive because together they impair the liver regeneration process.
Another aspect that is not discussed here, but which is important nevertheless, is related to the subjacent role that epigenetic mechanisms play each time that different genes or transcription factors change their expression patterns when liver tissue is injured. In this context, it has been shown that a mechanism of epigenetic compensation given by a reposition of repressive marks in a specific residue of a histone (a trimethylation) to hypomethylated transposons (sequences abundant in intergenic segments corresponding to remains of viruses), allowed the activation of genes promoting regeneration (Wang et al., 2019).
7. Factors contributing to the high regenerative capacity of the liver
In general, it is assumed that since the liver performs diverse tasks crucial for survival, it evolved its great intrinsic ability to regenerate. But one could ask, why if other organs are also vital, do they lack the ability to regenerate to a degree comparable to that of the liver? There are some peculiarities observed in the liver that may contribute to the explanation of its robustness, as described below.
The structure of the liver shows common characteristics across vertebrates: it is composed of a solid mass of cells with strong intercommunication among them, and it is highly vascularized with sinusoids. All vertebrates also share a highly conserved evolution of the hepatic portal-liver system (Subbotin, 2017). A fact is that the liver is the only parenchymal organ that regenerates up to the proper ratio with respect to body size, in contrast with other organs, which only partially regenerate (Michalopoulous and Bhushan, 2021).
The liver, as a multitask organ, is able to perform detoxification of exogenous agents such as toxins, drugs, food additives, and viral infections. But it also has a privileged and strategic location that permits its function as a guardian of the immune system, detecting and promoting an adequate immune response against lipopolysaccharide derived from Gram-negative bacteria living in the gut. The liver can also respond to tissue damage or tissue loss.
It should be mentioned that the liver must perform all its functions, while it rebuilds itself after being partially hepatectomized. This is possible because the hepatocytes show functional and metabolic heterogeneity, such that those located close to the portal vein (zone 1) are specialized in gluconeogenesis, β-oxidation, and cholesterol synthesis, while those in zone 3 near to the close vein are engaged in glycolysis, lipogenesis, and detoxification (Figure 1). Under homeostatic conditions, pericentral hepatocytes are responsible for hepatocyte renewal (Wang et al., 2015). For a time, it has been considered that after PH, hepatocytes of zone 1 are the first in proliferate, followed by the zone 2 and zone 3 hepatocytes. Nevertheless, recent studies have concluded that hepatocytes proliferate regardless of their location, although diploidy (2N) provides a growth advantage (Chen et al., 2020). In addition, the liver's robustness is supported by a continuous crosstalk between hepatocytes and other hepatic cells. Ultimately, the final purpose of liver regeneration may be to maintain body homeostasis and allow for survival (López-Luque and Fabregat, 2018).
I believe that the key to explaining the robustness of the liver depends largely on its quality as an immunological organ, since it is provided with innate and adaptive response mechanisms and the highest content of immune cells specialized in those functions. Liver immune responses are finely regulated to distinguish antigens present in the alimentary tract; they modulate the response after receive a transplant or trigger responses to local injuries, generalized inflammation, and regeneration (Thorgersen et al., 2019). When the liver is exposed to an acute injury such as PH, it reacts by producing a rapid regenerative response that is partially mediated by immune responses.
Among the non-parenchymal cells of the liver, ca. 25% are represented by KCs and lymphocytes (natural killer cells, natural killer T cells, and B cells), while LSECs account for 50% (Racanelli and Rehermann 2006). Immune cells such as KCs, dendritic cells, and natural killer T cells, with the help of infiltrating myeloid and lymphoid cells, can respond to pathogen- and damage-associated molecular patterns (PAMPs and DAMPS, respectively). A central mechanism of the innate immune system, is the complement system of higher vertebrates, comprising among 50–60 soluble and membrane proteins. The hepatocytes synthesize ca. 90% of the soluble proteins (Qin and Gao, 2006). The main component of the complement present in plasma is C3, whose proteolysis by C3 convertases produces C3a (the α-chain) and C3b (the ß-chain), leads to the activation of the three complement pathways. C3a is known as a mediator of inflammation, and together with C5a, it has been shown to participate in the priming phase of liver regeneration when it binds to KCs which in turn release TNF-α. It has also been shown that C3 activates c-fos and promotes TNF-α signaling, which activates acute phase genes during the priming phase (Min et al., 2016). The redundancy of pathways involved in liver regeneration explains the fact that PH in knockout animals that lack different growth factors, cytokines, and others, results in a delayed but not terminated regeneration. However, in C3−/− or C5−/− mice, liver regeneration was shown to be abnormal and mortalities were observed of 40% and 23%, respectively (Strey et al., 2003). In C3−/−C5−/− mice it was even worse because C3 deficiency prevents C3 and C5 activation.
It is important to note that in the course of evolution, biological complexity was suddenly increased in a first stage when eukaryotes arose and secondly, when vertebrates arose (Bird, 1995). Genetic redundancy is commonly observed in higher organisms, and it contributes to the resilience considered as characteristic of the living beings (Gómez-Romero et al., 2020). The liver is a good example of this resilience, observed when it is exposed to acute or chronic damages.
Liver regeneration is an extremely complex process that is a matter of intense study mainly in mammals. However, research performed in lower vertebrates such as zebrafish has provided important information regarding conserved mechanisms also present in higher vertebrates. Hopefully more studies will help to fill the knowledge gaps, not only with regard to comparative liver histology, but also about the detailed mechanisms of liver regeneration present in mammalian and non-mammalian species.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests Statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
All bibliographic resources used in this review were provided by the Universidad Nacional Autónoma de México. I wish to express my gratitude to the Universidad Nacional Autónoma de México, my alma mater, for being an inspiring place to develop my academic work for 29 years. I thank Dr. Marco A. Briones-Orta (Imperial College London, UK) and Dr. Nalú Navarro-Alvarez (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán) for their critical reading of the manuscript. I also wish to recognize the professional editorial work done by Mr. Patrick Weill. The figures presented in this review were designed with BioRender.com.
References
- Agius C., Roberts R.J. Melano-macrophage centres and their role in fish pathology. J. Fish. Dis. 2003;26(9):499–559. doi: 10.1046/j.1365-2761.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- Ahmed Y.A., Abdelsabour-Khalaf M., Mohammed E. Histological insight into the hepatic tissue of the Nile monitor (Varanus niloticus) J. Exp. Appl. Anim. Sci. 2018;2(3):240–250. [Google Scholar]
- Akiyoshi H., Inoue A. Comparative histological study of teleost livers in relation to phylogeny. Zool. Sci. 2004;21(8):841–850. doi: 10.2108/zsj.21.841. [DOI] [PubMed] [Google Scholar]
- Akiyoshi H., Inoue A. Comparative histological study of hepatic architecture in the three orders amphibian livers. Comp. Hepatol. 2012;11:2. doi: 10.1186/1476-5926-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akulenko N.M. Changes in liver parenchyma of green frogs (Pelophylax esculentus complex) under conditions of anthropogenic pollution and their use in monitoring of water bodies. Vestn. Zool. 2015;49(5):453–458. [Google Scholar]
- Alibardi L. Vol 207. Springer; Berlin, Heidelberg: 2010. Regeneration in reptiles and its position among vertebrates. (Morphological and Cellular Aspects of Tail and Limb Regeneration in Lizards. Advances in Anatomy, Embriology and Cell Biology). [PubMed] [Google Scholar]
- Alibardi L. Organ regeneration evolved in fish and amphibians in relation to metamorphosis: speculations on a post-embryonic developmental process lost in amniotes after the water to land transition. Ann. Anat. 2019;222:114–119. doi: 10.1016/j.aanat.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Andrew A.L., Perry B.W., Card D.C., Schield D.R., Ruggiero R.P., McGaugh S.E. Growth and stress response mechanisms underlying post-feeding regenerative organ growth in Burmese python. BMC Genom. 2017;18:338. doi: 10.1186/s12864-017-3743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyaz A., Attisano L., Wrana J.L. Recent advances in understanding contextual TGFß signaling. F1000Res. 2017;6:749. doi: 10.12688/f1000research.11295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A., Alvaro D., Bassotti C., Gigliozzi A., Ferretti G., La Rosa T. Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductile fragments and isolated perfused liver. Hepatology. 1997;26(1):9–21. doi: 10.1002/hep.510260102. [DOI] [PubMed] [Google Scholar]
- Bernabò I., Biasone P., Macirella R. Liver histology and ultrastructure of the Italian newt (Lissotriton italicus): normal structure and modifications after acute exposure to nonylphenol ethoxylates. Exp. Toxicol. Pathol. 2014;66(9-10):455–468. doi: 10.1016/j.etp.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Bhattacharya H., Zhang S., Xiao Q. Comparison of histopathological alterations due to sublethal CCl4 on Rosy Barb (Puntius conchonius) and Amphioxus (Branchiostoma belcheri) with implications on liver ontogeny. Toxicol. Mech. Methods. 2008;18(8):627–633. doi: 10.1080/15376510701623540. [DOI] [PubMed] [Google Scholar]
- Bird A.P. Gene number, noise reduction and biological complexity. Trends Genet. 1995;11(3):94–100. doi: 10.1016/S0168-9525(00)89009-5. [DOI] [PubMed] [Google Scholar]
- Böhm F., Köhler U.A., Speicher T., Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol. Med. 2010;2(8):294–305. doi: 10.1002/emmm.201000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borude P., Edwards G., Walesky C., Li F., Ma X., Guo G.L., Apte U. Hepatocyte specific deletion of farnesoid X receptor delays, but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012;56(6):2344–2352. doi: 10.1002/hep.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brues A.M., Marble B.B. An analysis of mitosis in liver restoration. J. Exp. Med. 1937;65(1):15–27. doi: 10.1084/jem.65.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher N.R.L., Scott J., Aub J.C. Regeneration of the liver in parabiotic rats. Can. Res. 1951;11(6):457–465. [PubMed] [Google Scholar]
- Cao W., Chen K., Bolkestein M., Yin Y., Verstegen M.M.A., Bijvelds M.J.C. Dynamics of proliferative and quiescent stem cells on liver homeostasis and injury. Gastroenterology. 2017;153(4):1133–1147. doi: 10.1053/j.gastro.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Chen F., Jimenez R.J., Sharma K., Luu H.Y., Hsu B.Y., Ravindranathan A. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell. 2020;26(1):27–33. doi: 10.1016/j.stem.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D., Morsch M., Shami G.J., Chung R.S., Braet F. Observation and characterisation of macrophages in zebrafish liver. Micron. 2020;132:102851. doi: 10.1016/j.micron.2020.102851. [DOI] [PubMed] [Google Scholar]
- Chelibonova-Lorer H., Ivanov S., Gavazova E., Antonov M. Glycosidases in normal and regenerating chicken liver, hepatomas Mc-29, Rous sarcoma, in Turkey poult liver and hemocytoblastomes, provoked by the leukosis virus strain Mc.31. Int. J. Biochem. 1985;17(4):541–544. doi: 10.1016/0020-711x(85)90154-5. [DOI] [PubMed] [Google Scholar]
- Clarke A., Pörtner H.O. Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 2010;85(4):703–727. doi: 10.1111/j.1469-185X.2010.00122.x. [DOI] [PubMed] [Google Scholar]
- Columbano A., Shinosuka H. Liver regeneration versus direct hyperplasia. Faseb. J. 1996;10(10):1118–1128. doi: 10.1096/fasebj.10.10.8751714. [DOI] [PubMed] [Google Scholar]
- Cox A.G., Goessling W. The lure of zebrafish in liver research: regulation of hepatic growth in development and regeneration. Curr. Opin. Genet. Dev. 2015;32:153–161. doi: 10.1016/j.gde.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis R.A., Markiewski M.M., Lambris J.D. Liver regeneration: a link to inflammation through complement. Adv. Exp. Med. Biol. 2006;586:17–34. doi: 10.1007/0-387-34134-X_2. [DOI] [PubMed] [Google Scholar]
- De Haan L., van der Lely S.J., Warps A.-L.K., Hofsink Q., Olthof P.B., de Keijzer M.J. Post-hepatectomy liver regeneration in the context of bile acid homeostasis and the gut-liver signaling axis. J. Clin. Transl. Res. 2018;4(1):1–46. doi: 10.18053/jctres.04.201801.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oya M., Cifuentes I., Larrodera L., Serrano-Ríos M. Effect of insulin on plasma triglyceride concentration in hepatectomized geese. Rev. Esp. Fisiol. 1976;32(2):99–102. [PubMed] [Google Scholar]
- De Mendoza A., Sebé-Pedrós A., Sestak M.S., Matejcic M., Toruella G., Domazet-Loso T., Ruiz-Trillo I. Transcription factor evolution in eukaryotes and the assembly of the regulatory toolkit in multicellular lineages. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(50):E4858–E4866. doi: 10.1073/pnas.1311818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Coello B., Briones-Orta M.A., Macías-Silva M., Mas-Oliva J. Cholesterol: recapitulation of its active role during liver regeneration. Liver Int. 2011;31(9):1271–1284. doi: 10.1111/j.1478-3231.2011.02542.x. [DOI] [PubMed] [Google Scholar]
- Deng X., Zhang X., Li W., Feng R.-X., Li L., Yi G.-R. Chronic liver injury induces conversion of biliary epithelial cells into hepatocytes. Cell Stem Cell. 2018;23(1):114–122. doi: 10.1016/j.stem.2018.05.022. [DOI] [PubMed] [Google Scholar]
- Dzhivanian K.A., Ter-Oganian K.S. Nonspecific esterase and alpha-glycerolphosphate dehydrogenase activity and the fat content in the regenerating chicken liver. Byulleten’ Eksperimental’noi Biologii i Meditsiny. 1979;87(6):547–550. [PubMed] [Google Scholar]
- Dzhivanian K.A., Ter-Oganian K.S. Binuclear cells in postembryonal development and during regeneration in chickens. Byulleten’ Eksperimental’noi Biologii i Meditsiny. 1983;95(1):91–93. [PubMed] [Google Scholar]
- Elvington M., Liszewski M.K., Atkinson J.P. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol. Rev. 2016;274(1):9–15. doi: 10.1111/imr.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N., Campbell J.S., Riehle K.J. Liver regeneration. Hepatology. 2006;43(2 Suppl 1):S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Felekouras E.E., Kapaerlos D.C., Papalampros E. The history of liver surgery hepatectomy and haemostasis. Hellenic J. Surg. 2010;82:280–296. [Google Scholar]
- Firmiano E.M.S., Cardoso N.N., Vieira D.A., Slaes A., Santos M.A.J., Mendes A.L.S., Nascimento A.A. Histological study of the liver of the lizard Tropidurus torquatus wied 1820, (squamata: tropiduridae) J. Morphol. Sci. 2011;28(3):165–170. [Google Scholar]
- Fonseca M.C., Franςa A., Florentino R.M., Fonseca R.C., Lima Filho, Vidigal A.C.M., P.T.V Cholesterol-enriched membrane microdomains are needed for insulin signaling and proliferation in hepatic cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G80–G94. doi: 10.1152/ajpgi.00008.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch H.A.R., Pehlemann F.-W., Faltz H. Effect of partial hepatectomy on the interrenal tissues of Xenopus laevis (Daudin) Cell Tissue Res. 1977;179(2):197–209. doi: 10.1007/BF00219796. [DOI] [PubMed] [Google Scholar]
- Galis F., Wagner G.P., Jockusch E.L. Why is limb regeneration possible in amphibians but not in reptiles, birds, and mammals? Evol. Dev. 2003;5(2):208–220. doi: 10.1046/j.1525-142x.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- Gilgenkratz H., Collin de l’Hortet A. Understanding liver regeneration. From mechanisms to regenerative medicine. Am. J. Pathol. 2018;188(6):1316–1327. doi: 10.1016/j.ajpath.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Gómez-Romero L., López-Reyes K., Hernández-Lemus E. The large scale structure of human metabolism reveals resilience via extensive cross-talk. Front. Physiol. 2020;11:588012. doi: 10.3389/fphys.2020.588012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorshkova E.A., Nedospasov S.A., Shilov E.S. Evolutionary plasticity of IL-6 cytokine family. Mol. Biol. 2016;50(6):918–926. doi: 10.7868/S0026898416060069. [DOI] [PubMed] [Google Scholar]
- He J., Lu H., Zou Q., Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789–800. doi: 10.1053/j.gastro.2013.11.045. [DOI] [PubMed] [Google Scholar]
- He J., Chen J., Wei X., Leng L., Mu H., Cai P., Luo L. Mammalian target of rapamycin complex 1 signaling is required for the dedifferentiation from biliary cell to bipotential progenitor cell in zebrafish liver regeneration. Hepatology. 2019;70(6):2092–2106. doi: 10.1002/hep.30790. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Messersmith P. Drug delivery and epimorphic salamander-type mouse regeneration: a full parts and labor plane. Adv. Drug Deliv. Rev. 2018;129:254–261. doi: 10.1016/j.addr.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins G.M., Anderson R.M. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931;12:186–202. [Google Scholar]
- Higgins G.M., Mann F.C., Priestly J.T. Experimental pathology of the liver. X. Restoration of the liver of the domestic fowl. Arch. Pathol. 1932;14:491–497. [Google Scholar]
- Hinck A.P., Mueller T.D., Springer T.A. Structural biology and evolution of the TGF-β family. Cold Spring Harb. Perspect. Biol. 2016;8:a022103. doi: 10.1101/cshperspect.a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A.F., Hagey L.R., Krasowski M.D. Bile salt of vertebrates: structural variation and possible evolutionary significance. J. Lipid Res. 2010;51(2):226–246. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ma K., Zhang J., Qatanani M., Cuvillier J., Liu J. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- Huminiecki L., Goldovsky L., Freilich S., Moustakas A., Ouzounis C., Helding C.H. Emergence, development and diversification of the TGF-ß signaling pathway within the animal kingdom. BMC Evol. Biol. 2009;9:28. doi: 10.1186/1471-2148-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Morita S.-Y., Terada T. Cholesterol attenuates cytoprotective effects of phosphatidylcholine against bile salts. Sci. Rep. 2017;7(1):306. doi: 10.1038/s41598-017-00476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaensch S.M., Cullem L., Raidal S.R. Assessment of liver function in chickens using galactose and indocyanine green clearances. Avian Pathol. 2000;29:109–116. doi: 10.1080/03079450094135. [DOI] [PubMed] [Google Scholar]
- Kaiser P., Rothwell L., Avery S., Balu S. Evolution of the interleukins. Dev. Comp. Immunol. 2004;28(5):375–394. doi: 10.1016/j.dci.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kan N.G., Junghans D., Izpisua Belmonte J.C. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. Faseb. J. 2009;23:3516–3525. doi: 10.1096/fj.09-131730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapetian A.F., Dzhivanian K.A. Liver regenerative potential of the lake frog Rana ridibunda after partial hepatectomy. Tsitologiia. 2006;48(4):436–454. [PubMed] [Google Scholar]
- Kassab A., Shousha S., Fargani A. Morphology of blood cells, liver and spleen of the desert tortoise (Testudo graeca) Open Anat. J. 2009;1:1–10. [Google Scholar]
- Khalik M., Ko S., Liu Y., Wang H., Sun Y., Solnica-Krezel L., Shin D. Stat 3 regulates liver progenitor cell-driven liver regeneration in zebrafish. Gene Expr. 2018;18:157–170. doi: 10.3727/105221618X15242506133273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotek Z., Grabensteiner E., Knotková Z., Kübber-Heiss A., Benyr G. Partial hepatectomy in a Plains garter snake (Thamnophonis sirtalis radix) with biliary cystadenoma: case report. Acta Vet. 2012;81(4):433–437. [Google Scholar]
- Kornblith S.-J., Kalman S.M. Regeneration of the liver in the young chick. Poultry Sci. 1964;43(4):908–911. [Google Scholar]
- Lawton M.P.C. Proceedings of the Annual Conference Association of Reptilian and Amphibian Veterinarians. 1998. Partial hepatectomy in a snake; pp. 127–129. [Google Scholar]
- Lemire J.M., Shiojori N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am. J. Pathol. 1991;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Li W., Liang X., Leu J.I., Kovalovich K., Ciliberto G., Taub R. Global changes in interleukin-6-dependent gene expression patterns in mouse livers after partial hepatectomy. Hepatology. 2001;33(6):1377–1386. doi: 10.1053/jhep.2001.24431. [DOI] [PubMed] [Google Scholar]
- Li W., Li L., Hui L. Cell plasticity in liver regeneration. Trends Cell Biol. 2020;30(4):329–338. doi: 10.1016/j.tcb.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Li X. The FGF metabolic axis. Front. Med. 2019;13:511–530. doi: 10.1007/s11684-019-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroos P.M., Zarnegar R., Michalopoulos G.K. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991;13:743–750. [PubMed] [Google Scholar]
- López-Luque J., Fabregat I. Revisiting the liver: from development to regeneration- what we ought to know! Int. J. Dev. Biol. 2018;62:441–451. doi: 10.1387/ijdb.170264JL. [DOI] [PubMed] [Google Scholar]
- Lu W., Chen Q., Ying S., Xia X., Yu Z., Lui Y. Evolutionary conserved primary TNF sequences relate to its primitive functions in cell death induction. J. Cell Sci. 2016;129(1):108–120. doi: 10.1242/jcs.175463. [DOI] [PubMed] [Google Scholar]
- Magadum S., Banerjee U., Murugan P., Gangapur D., Ravikesavan R. Gene duplication as a major force in evolution. J. Genet. 2013;92(1):155–161. doi: 10.1007/s12041-013-0212-8. [DOI] [PubMed] [Google Scholar]
- Maeno H., Ono T., Dhar D.K., Sato T., Yamanoi A., Nagasue N. Expression of hypoxia inducible factor-1α during liver regeneration induced by partial hepatectomy in rats. Liver Int. 2005;25(5):1002–1009. doi: 10.1111/j.1478-3231.2005.01144.x. [DOI] [PubMed] [Google Scholar]
- Mao S.A., Glorioso J.M., Nyberg S.L. Liver regeneration. Transl. Res. 2014;163(4):352–362. doi: 10.1016/j.trsl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu F., Marongiu M., Contini A., Serra A., Cadoni E., Murgia R., Laconi E. Hyperplasia vs hypertrophy in tissue regeneration after extensive resection. World J. Gastroenterol. 2017;23(10):1764–1770. doi: 10.3748/wjg.v23.i10.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars W.M., Liu M.L., Kitson R.P., Goldfarb R.H., Gabauer M.K., Michalopoulos G.K. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695–1701. [PubMed] [Google Scholar]
- Merlen G., Ursic-Bedoya J., Jourdainne V., Kahale N., Glenisson M., Doignon I., Rainteau D., Torjdmann T. Bile acids and their receptors during liver regeneration: “Dangerous protectors”. Mol. Aspect. Med. 2017;56:25–33. doi: 10.1016/j.mam.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G.K., DeFrances M.C. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G.K., Barua L., Bowen W.C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41(3):535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulous G.K. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am. J. Pathol. 2010;176(1):2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulous G.K., Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021;18(1):40–55. doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- Min J.S., DeAngelis R.A., Reis E.S., Gupta S., Maurya M.R., Evans C. Subramaniam, S. Systems analysis of the complement-induced priming phase of liver regeneration. J. Immunol. 2016;197:2500–2508. doi: 10.4049/jimmunol.1600628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitaka T., Mikami M., Sattler G.L., Pitot H.C., Mochizuki Y. Small cell colonies appear in the primary culture of adult rat hepatocytes in the presence of nicotinamide and epidermal growth factor. Hepatology. 1992;16(2):440–447. doi: 10.1002/hep.1840160224. [DOI] [PubMed] [Google Scholar]
- Miyaoka Y., Ebato K., Kato H., Arakawa S., Shimizu S., Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie. Liver regeneration. Curr. Biol. 2012;22:1166–1175. doi: 10.1016/j.cub.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Miyaoka Y., Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell Div. 2013;8:8. doi: 10.1186/1747-1028-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschetta A., Xu F., Hagey L.R., van Berge-Henegouwen G.P., van Erpecum K.J., Brouwers J.F. A phylogenetic survey of biliary lipids in vertebrates. J. Lipid Res. 2005;46:2221–2232. doi: 10.1194/jlr.M500178-JLR200. [DOI] [PubMed] [Google Scholar]
- Moura L.R., Santos A.L.Q., Belleti M.E., Vieira L.G., Orpinelli S.R.T., De Simone S.B.S. Morphological aspects of the liver of the freshwater turtle Phrynops geoffroanus Schweigger, 1812 (Testudines, Chelidae) J. Morphol. Sci. 2009;26(3-4):129–134. [Google Scholar]
- Najafpour B., Cardoso J.C.R., Canário A.V.M., Power D.M. Specific evolution and gene family expansion of complement 3 and regulatory factor H in fish. Front. Immunol. 2020;11:568631. doi: 10.3389/fimmu.2020.568631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves F., Abrantes J., Pinheiro A.M., Almeida T., Costa P.P., Esteves P.J. Convergent evolution of IL-6 in two leporids (Oryctolagus and Pentalagus) originated and extended protein. Immunogenetics. 2014;66(9-10):589–595. doi: 10.1007/s00251-014-0787-0. [DOI] [PubMed] [Google Scholar]
- Neves F., Abrantes J., Steinke J.W., Esteves P.J. Maximum-likelihood approaches reveal signatures of positive selection in IL genes in mammals. Innate Immun. 2014;20(2):184–191. doi: 10.1177/1753425913486687. [DOI] [PubMed] [Google Scholar]
- Okihiro M.S., Hinton D.E. Partial hepatectomy and bile duct ligation in rainbow trout (Oncorhynchus mykiss): histologic, immunohistochemical and enzyme histochemical characterization of hepatic regeneration and biliary hyperplasia. Toxicol. Pathol. 2000;28(2):342–356. doi: 10.1177/019262330002800215. [DOI] [PubMed] [Google Scholar]
- Oyashi A., Saito N., Kashimoto R., Furukawa S., Yamamoto S., Satoh A. Axolotl liver regeneration is accomplished via compensatory congestion mechanism regulated by ERK signaling after partial hepatectomy. Dev. Dynam. 2020 doi: 10.1002/dvdy.262. [DOI] [PubMed] [Google Scholar]
- Puglielli L., Amigo L., Arrese M., Núñez L., Rigotti A., Garrido J. Protective role of biliary cholesterol and phospholipid lamellae against bile acid–induced cell damage. Gastroenterology. 1994;107(1):244–254. doi: 10.1016/0016-5085(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Qin X., Gao B. The complement system in liver diseases. Cell. Mol. Immunol. 2006;3(5):333–340. [PubMed] [Google Scholar]
- Quistad S.D., Stotland A., Barott K.L., Smurthwaite C.A., Hilton B.J., Grasis J.A. Evolution of TNF-induced apoptosis reveals 550 My of functional conservation. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111(26):9567–9572. doi: 10.1073/pnas.1405912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racanelli V., Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(S1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Rao K.L., Patnaik B.K. Alterations in the RNA, protein and free amino acid level of the liver of the garden lizard, Calotes versicolor, during aging and partial hepatectomy. Exp. Gerontol. 1971;6(6):397–404. doi: 10.1016/0531-5565(71)90019-2. [DOI] [PubMed] [Google Scholar]
- Rao K.L., Patnaik B.K. Effect of age and hepatectomy on the glycogen content of the liver of the garden lizard, Calotes versicolor. Exp. Gerontol. 1971;6(4):287–291. doi: 10.1016/0531-5565(71)90010-6. [DOI] [PubMed] [Google Scholar]
- Rao K.L., Patnaik B.K. Change in ascorbic acid and water content of the liver of garden lizard during ageing and partial hepatectomy. Exp. Gerontol. 1973;8(1):45–50. doi: 10.1016/0531-5565(73)90050-8. [DOI] [PubMed] [Google Scholar]
- Raven A., Lu W.-Y., Man T.Y., Ferreira-Gonzalez S., O’Duibhir E., Dwyer B. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde F., Schusser B., Hron T., Farkasová H., Plachy J., Härtle S. Characterization of chicken tumor necrosis factor-α, a long missed cytokine in birds. Front. Immunol. 2018;9:605. doi: 10.3389/fimmu.2018.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselló R.A., Chen C.C., Dai R., Howard J.T., Hochgeschwender U., Jarvis E.D. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. eLife. 2013;2 doi: 10.7554/eLife.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler K.C., Krahn K.N., Gaur N.A., Ukomadu C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator uhrf1. Proc. Natl. Acad. Sci. Unit. States Am. 2007;104(5):1570–1575. doi: 10.1073/pnas.0610774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub J.R., Huppert K.A., Kurial S.N.T., Hsu B.Y., Cast A.E., Donnelly B. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature. 2018;557(7704):247–251. doi: 10.1038/s41586-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwolf G.C., Smith J.L. Gastrulation and early mesodermal patterning in vertebrates. Methods Mol. Biol. 2000;135:113–125. doi: 10.1385/1-59259-685-1:113. [DOI] [PubMed] [Google Scholar]
- Sebé-Pedrós A., Degnan B.M., Ruiz-Trillo I. The origin of metazoa: a unicellular perspective. Nat. Rev. Genet. 2017;18:498–512. doi: 10.1038/nrg.2017.21. [DOI] [PubMed] [Google Scholar]
- Secor S.M., Diamond J.M. Evolution of regulatory responses to feeding in snakes. Physiol. Biochem. Zool. 2000;73(2):123–141. doi: 10.1086/316734. [DOI] [PubMed] [Google Scholar]
- Sidorova V.F. Liver regeneration in birds. Bull. Exp. Biol. Med. 1962;52:1426–1429. doi: 10.1007/BF00785312. [DOI] [PubMed] [Google Scholar]
- So J., Kim A., Lee S.-H., Shin D. Liver progenitor cell-driven liver regeneration. Exp. Mol. Med. 2020;52:1230–1238. doi: 10.1038/s12276-020-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger B.Z. Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol. 2015;77:179–200. doi: 10.1146/annurev-physiol-021113-170255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey C.W., Markiewski M., Mastellos D., Tudoran R., Spruce L.A., Greenbaum L.E., Lambris J.D. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 2003;198(6):913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbotin V.M. Arguments on the origin of the vertebrate liver and the amphioxus hepatic diverticulum: a hypothesis on evolutionary novelties. Pisma v Vavilovskii Zhurnal. 2017 http://www.bionet.nsc.ru/vogis/download/hypothesis/appx1.pdf 2017. [Google Scholar]
- Sunyer J.O., Lambris J.D. Evolution and diversity of the complement system of poikilothermic vertebrates. Immunol. Rev. 1998;166:39–57. doi: 10.1111/j.1600-065x.1998.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Tao Y., Wang M., Chen E., Tang H. Liver regeneration: analysis of the main relevant signaling molecules. Mediat. Inflamm. 2017;2017:4256352. doi: 10.1155/2017/4256352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Thorgersen E.B., Barrat- Due A., Haugaa H., Harboe M., Pischke S.E., Nilsson P.H., Mollnes T.E. The role of complement in liver injury, regeneration, and transplantation. Hepatology. 2019;70(2):725–736. doi: 10.1002/hep.30508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissi S., Parto P., Sharifi M. Anatomical and histological study of the liver and pancreas of two closely related mountain newts Neurergus microspilotus and N. kaiseri (Amphibia: Caudata: salamandridae) Zool. 2017;34 [Google Scholar]
- van de Laarschot L.F.M., Jansen P.L.M., Schaap F.G., Olde Damink S.W.M. The role of bile salts in liver regeneration. Hepatol. Int. 2016;10:733–740. doi: 10.1007/s12072-016-9723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M.C., Hamesch K., Lunova M., Kim Y., Weiskirchen R., Strnad P., Friedman S.L. Standard operating procedures in experimental liver research: thioacetamide model in mice and rats. Lab. Anim. 2015;49(S1):21–29. doi: 10.1177/0023677215573040. [DOI] [PubMed] [Google Scholar]
- Wang B., Zhao L., Fish M., Logan C.Y., Nusse R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015;524(7564):180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang C., Hasson D., Desai A., SenBanerjee S., Magnani E. Epigenetic compensation promotes liver regeneration. Dev. Cell. 2019;50(1):43–56. doi: 10.1016/j.devcel.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens G.D., Glenney G.W. Origin and evolution of TNF and TNF receptor superfamilies. Dev. Comp. Immunol. 2011;35(12):1324–1335. doi: 10.1016/j.dci.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Yang J., Cusimano A., Monga J.K., Preziosi M.E., Pullara F., Calero G. WNT5A inhibits hepatocyte proliferation and concludes β-catenin signaling in liver regeneration. Am. J. Pathol. 2015;185(8):2194–2205. doi: 10.1016/j.ajpath.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Lin J., Yang P., Chen Q., Chu X., Gao C., Hu J. Fine structure, enzyme histochemistry, and immunohistochemistry of liver in zebrafish. Anat. Rec. 2012;295(4):567–576. doi: 10.1002/ar.22416. [DOI] [PubMed] [Google Scholar]
- Zaefarian F., Abdollahi M.R., Cowieson A., Ravindran V. Avian liver: the forgotten organ. Animals (Basel) 2019;9:E63. doi: 10.3390/ani9020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A., Qin H., Fu X. What determines the regenerative capacity in animals? Bioscience. 2016;66(9):735–746. [Google Scholar]
- Zheng S., Long J., Liu Z., Tao W., Wang D. Identification and evolution of TGF-ß signaling pathway members in twenty-four animal species and expression in Tilapia. Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19041154. pii: E1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Guo Q., Dai H. Molecular characterization and expression profiles in response to bacterial infection of Chinese soft-shelled turtle interleukin-8 (IL-8), the first reptilian chemokine gene. Dev. Comp. Immunol. 2009;33(7):838–847. doi: 10.1016/j.dci.2009.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.